On Plasma Activated Acetyl Donors: Comparing the Antibacterial Efficacy of Tetraacetylethylenediamine and Pentaacetate Glucose
Abstract
1. Introduction
2. Experimental Methodology
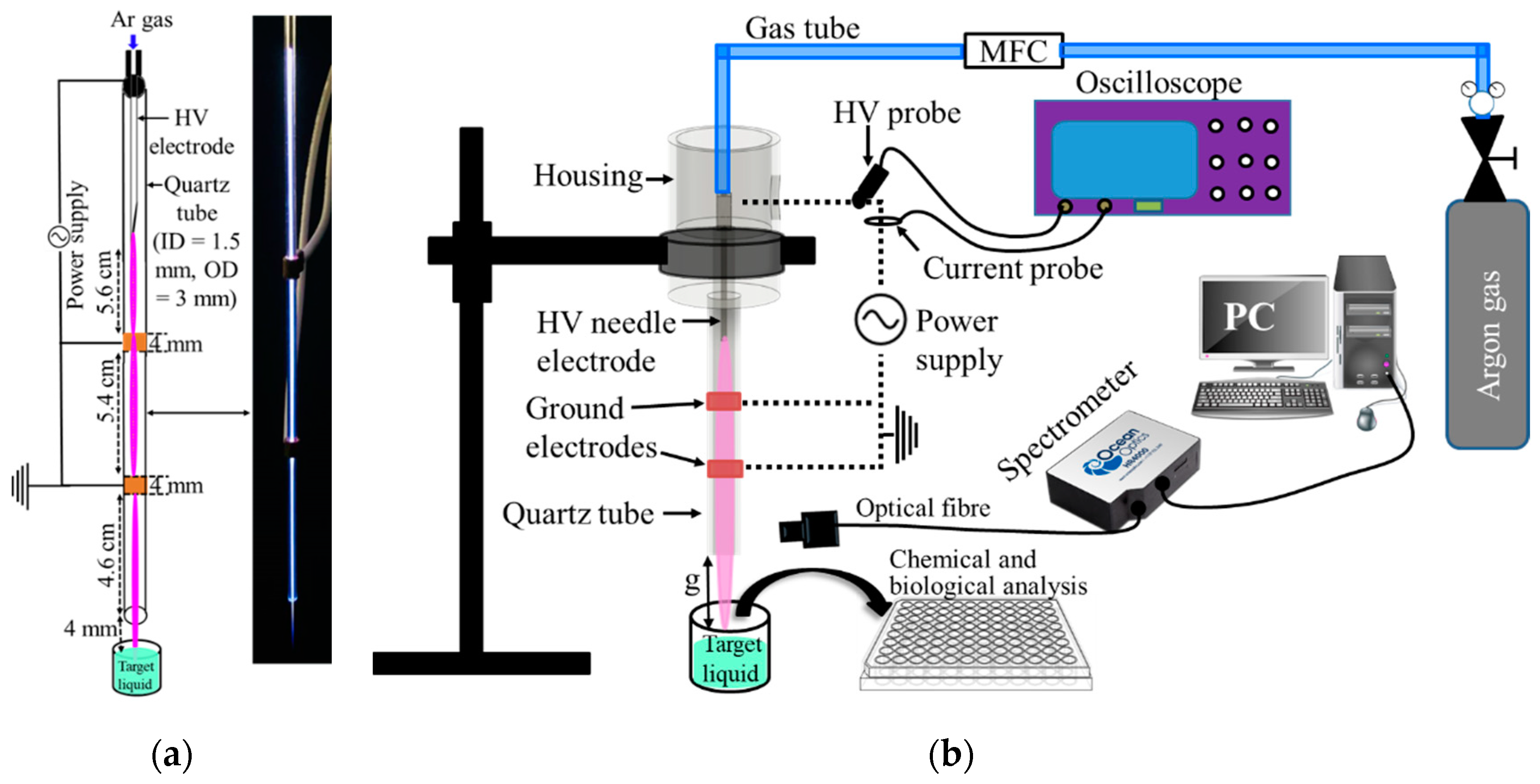
2.1. Plasma Jet Setup
2.2. Plasma Activated Solutions
2.3. Chemical Analysis of PA Solutions
2.4. Oxidative Potential of Plasma Activated Solutions
2.5. Antibacterial Efficacy of Plasma Activated Solutions
3. Results
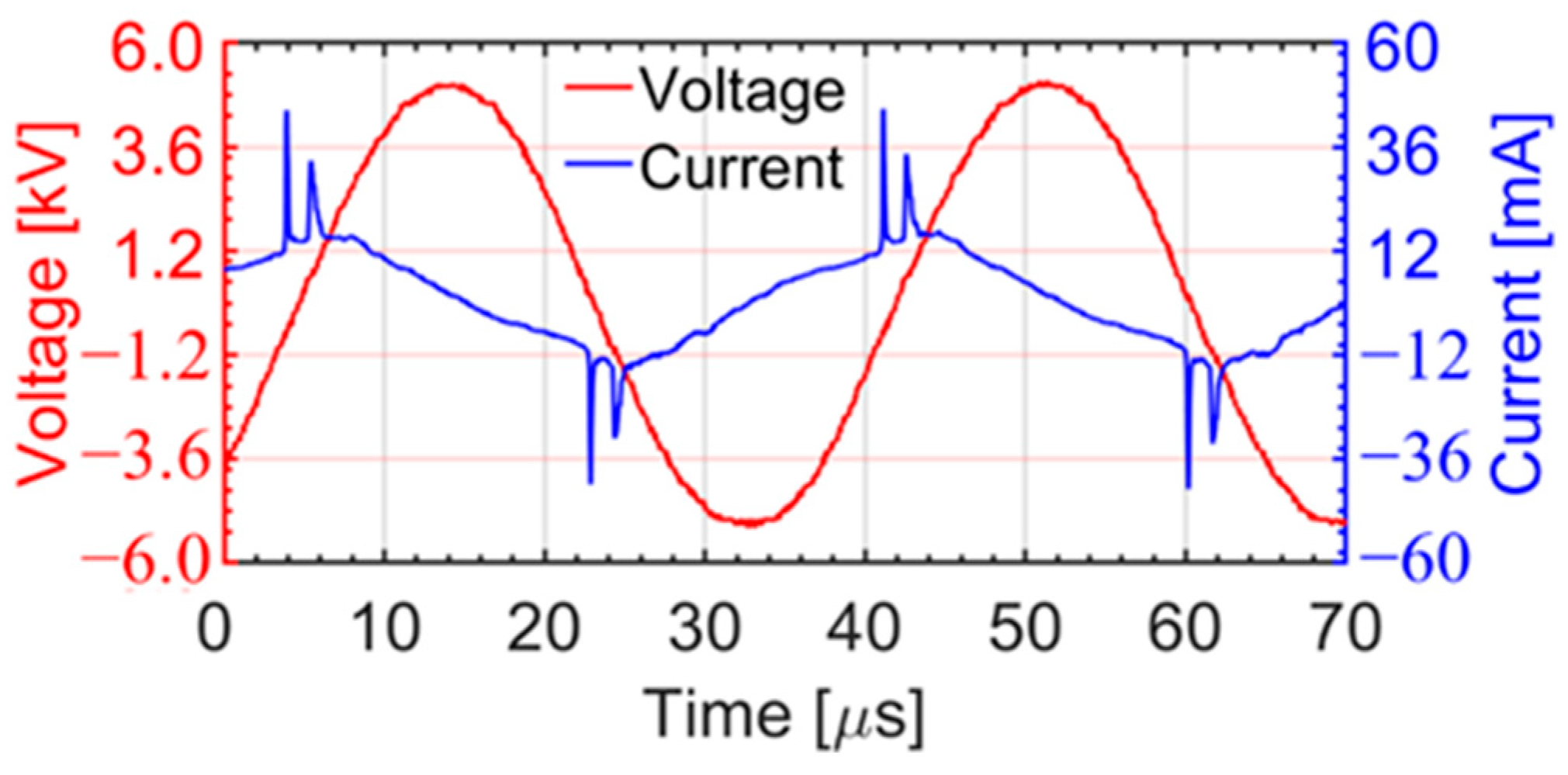
3.1. Current and Voltage Waveforms
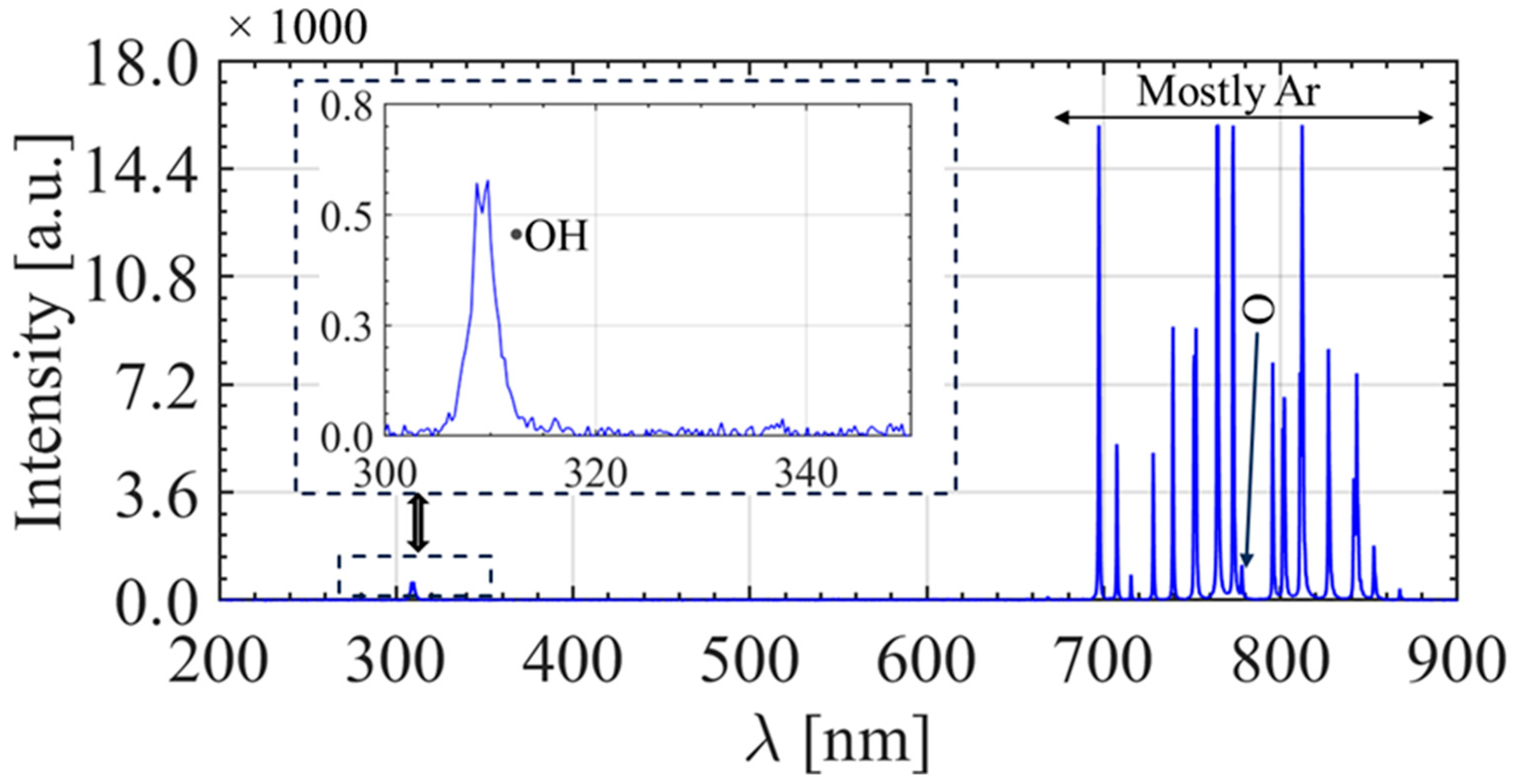
3.2. Optical Emission Characteristics
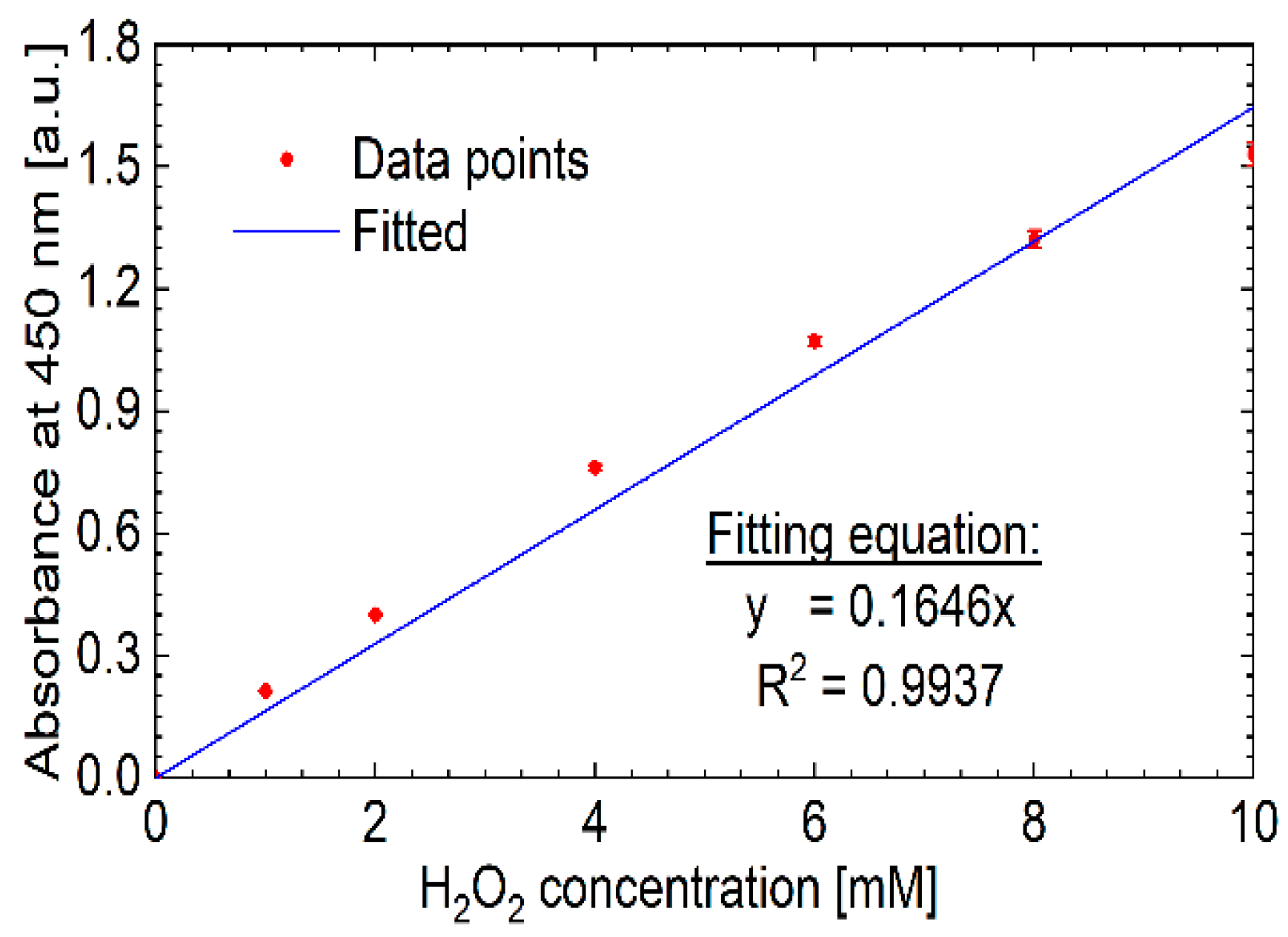
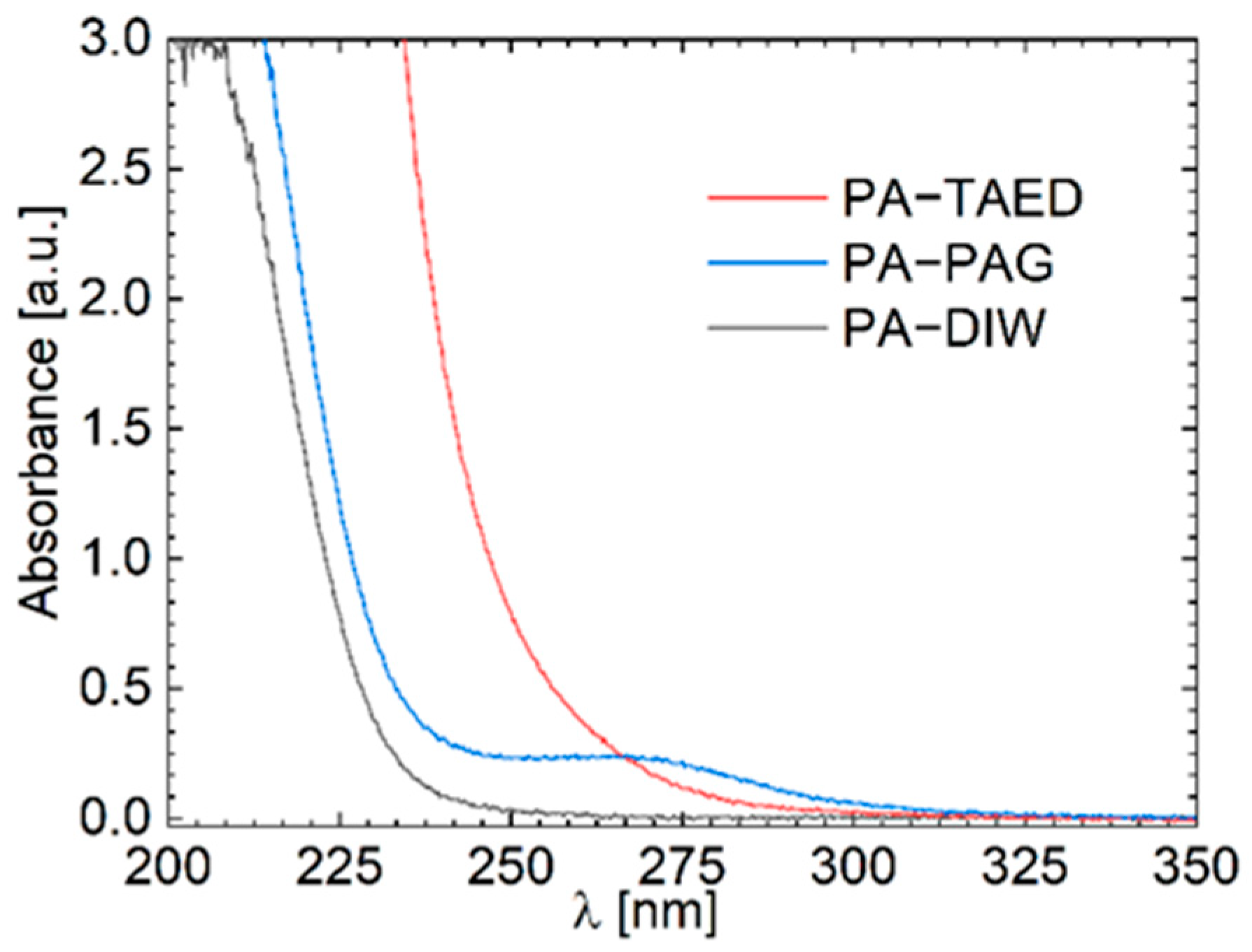
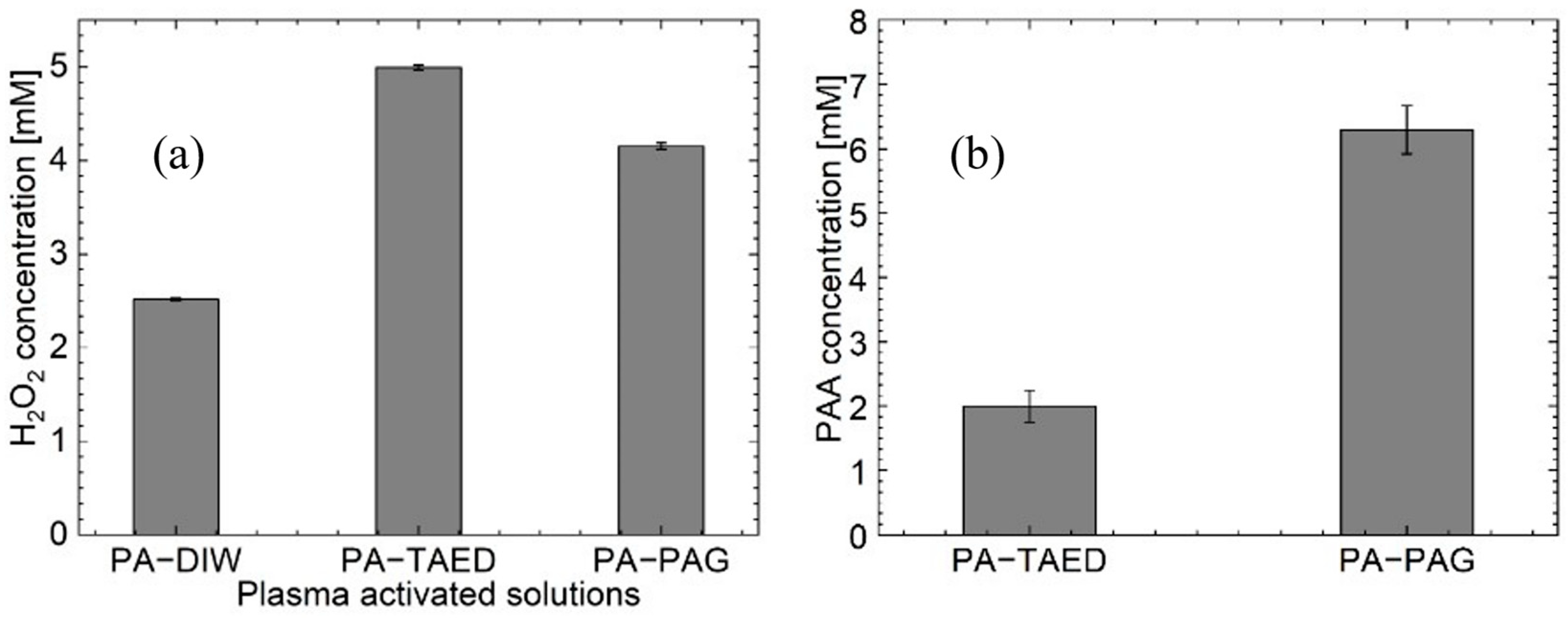
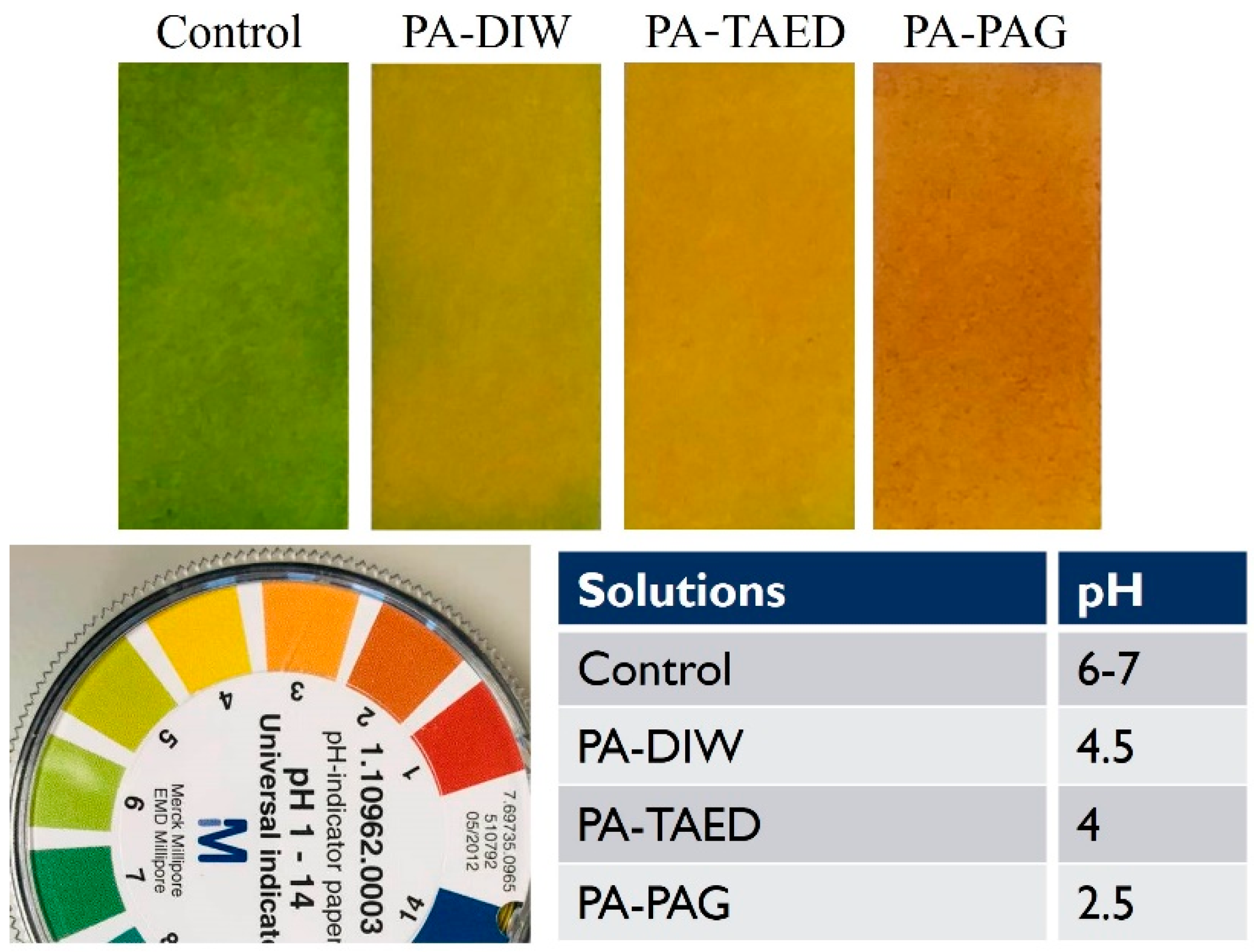
3.3. Chemical Analysis of the Plasma Activated Solutions
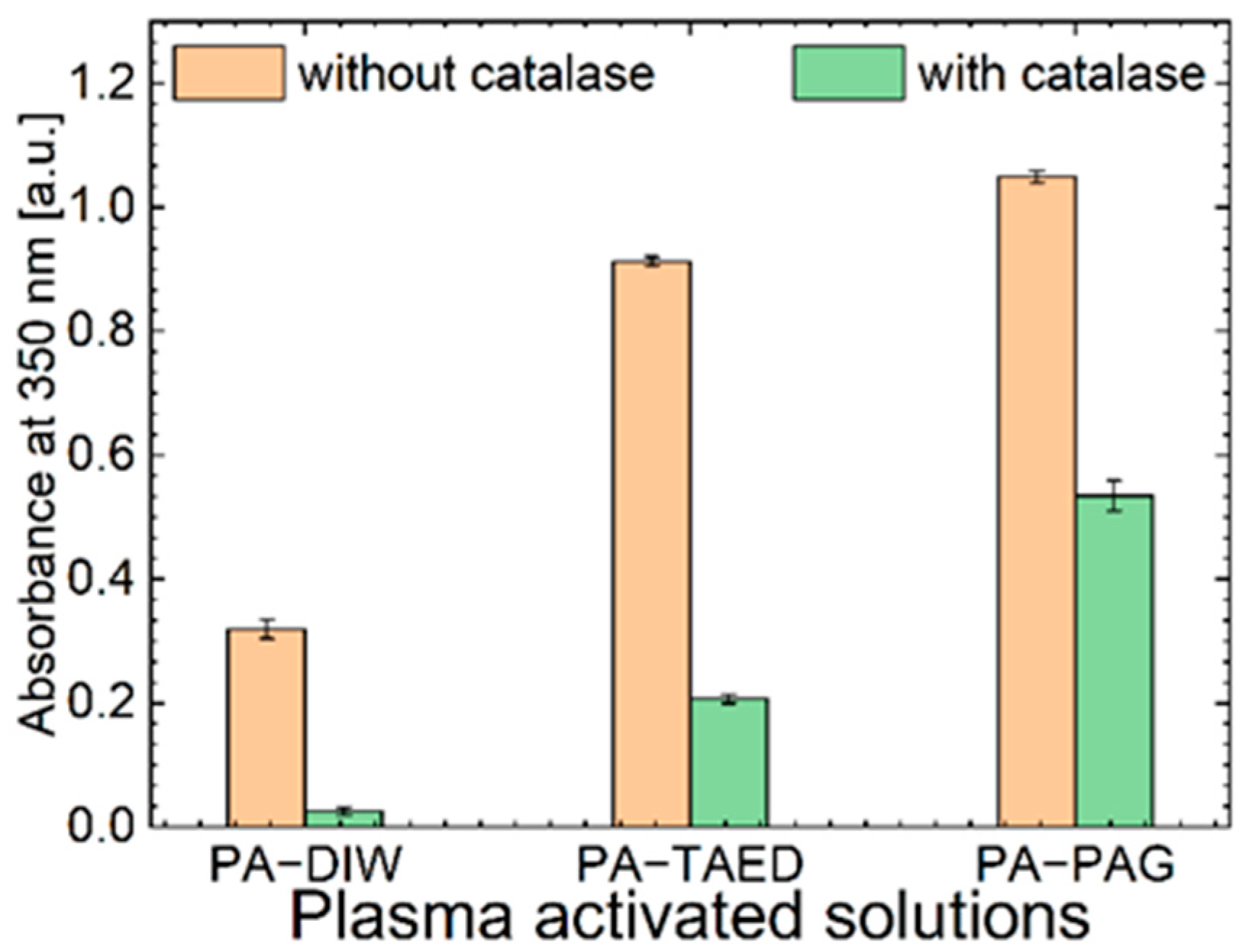
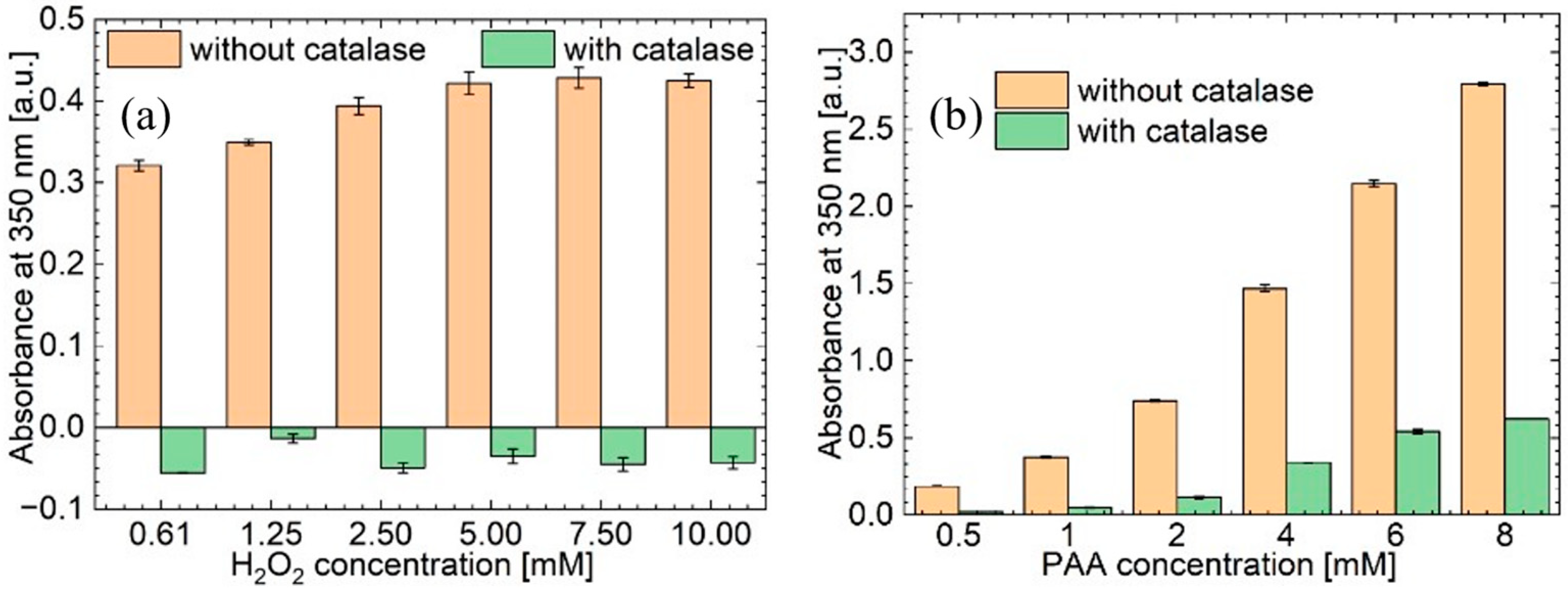
3.4. Oxidative Capacity of the Plasma Activated Solutions
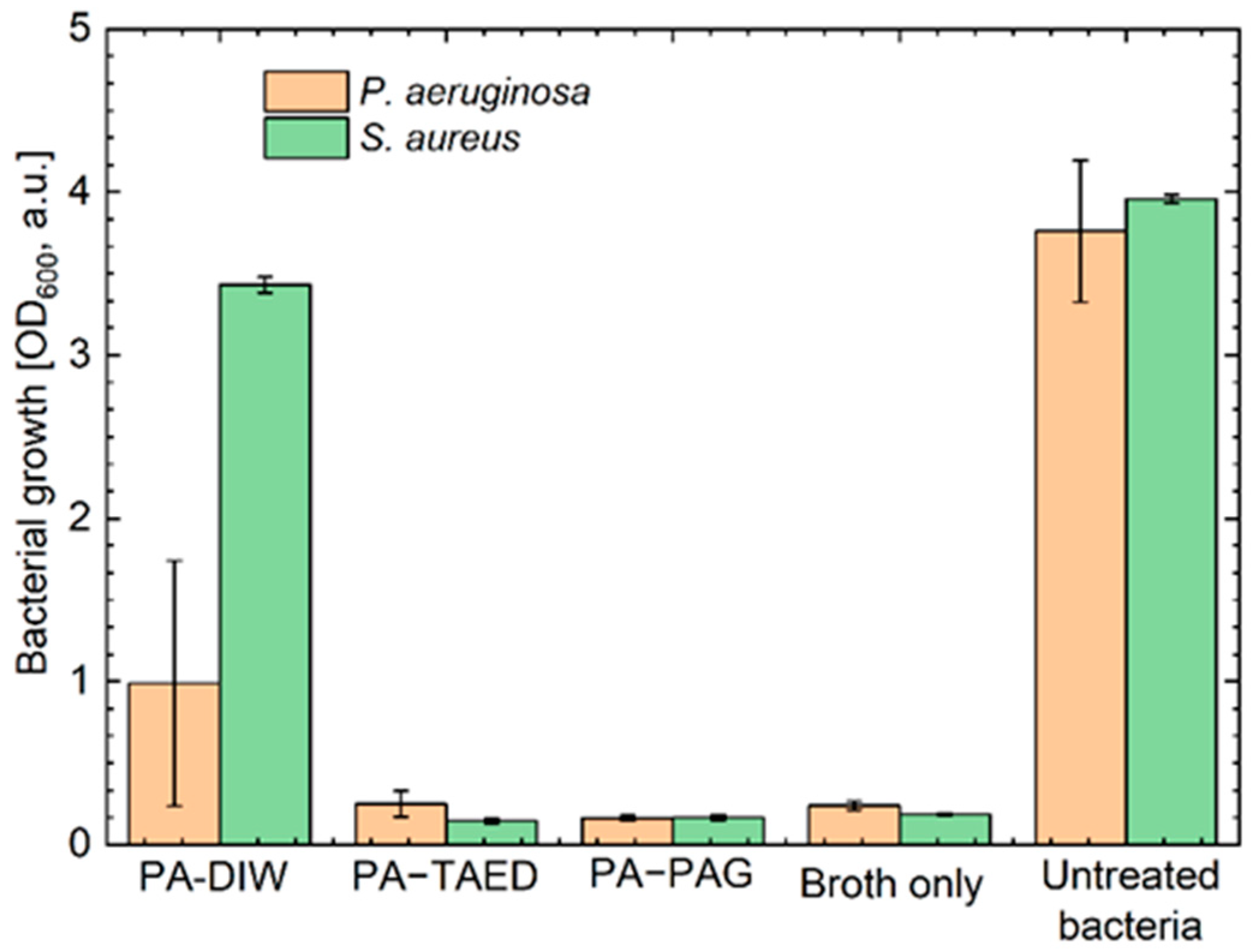
3.5. Antibacterial Efficacy of the Plasma Activated Solutions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Da Cunha, B.R.; Fonseca, L.P.; Calado, C.R.C. Antibiotic Discovery: Where Have We Come from, Where Do We Go? Antibiotics 2019, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. 2016. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 13 October 2017).
- Kaushik, N.K.; Ghimire, B.; Li, Y.; Adhikari, M.; Veerana, M.; Kaushik, N.; Jha, N.; Adhikari, B.; Lee, S.-J.; Masur, K.; et al. Biological and medical applications of plasma-activated media, water and solutions. Biol. Chem. 2018, 400, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, P.; Aadhikari, B.C.; Nguyen, L.N.; Paneru, R.; Ghimire, B.; Mumtaz, S.; Lim, J.S.; Hong, Y.J.; Choi, E.H. Sustainable nitrogen fixation from synergistic effect of photo-electrochemical water splitting and atmospheric pressure N2 plasma. Plasma Sources Sci. Technol. 2020, 29, 045026. [Google Scholar] [CrossRef]
- Traylor, M.J.; Pavlovich, M.J.; Karim, S.; Hait, P.; Sakiyama, Y.; Clark, D.S.; Graves, D.B. Long-term antibacterial efficacy of air plasma-activated water. J. Phys. D Appl. Phys. 2011, 44, 47. [Google Scholar] [CrossRef]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Naítali, M.; Kamgang-Youbi, G.; Herry, J.-M.; Bellon-Fontaine, M.-N.; Brisset, J.-L. Combined Effects of Long-Living Chemical Species during Microbial Inactivation Using Atmospheric Plasma-Treated Water. Appl. Environ. Microbiol. 2010, 76, 7662–7664. [Google Scholar] [CrossRef] [PubMed]
- Busco, G.; Robert, E.; Chettouh-Hammas, N.; Pouvesle, J.-M.; Grillon, C. The emerging potential of cold atmospheric plasma in skin biology. Free Radic. Biol. Med. 2020, 161, 290–304. [Google Scholar] [CrossRef]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma–liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Ikawa, S.; Tani, A.; Nakashima, Y.; Kitano, K. Physicochemical properties of bactericidal plasma-treated water. J. Phys. D Appl. Phys. 2016, 49, 425401. [Google Scholar] [CrossRef]
- Ghimire, B.; Szili, E.J.; Patenall, B.L.; Lamichhane, P.; Gaur, N.; Robson, A.J.; Trivedi, D.; Thet, N.T.; Jenkins, A.T.A.; Choi, E.H.; et al. Enhancement of hydrogen peroxide production from an atmospheric pressure argon plasma jet and implications to the antibacterial activity of plasma activated water. Plasma Sources Sci. Technol. 2021, 30, 035009. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016, 6, 38610. [Google Scholar] [CrossRef]
- Hathaway, H.J.; Patenall, B.L.; Thet, N.T.; Sedgwick, A.C.; Williams, G.T.; Jenkins, A.T.A.; Allinson, S.L.; Short, R.D. Delivery and quantification of hydrogen peroxide generated via cold atmospheric pressure plasma through biological material. J. Phys. D Appl. Phys. 2019, 52, 505203. [Google Scholar] [CrossRef]
- Khlyustova, A.; Labay, C.; Machala, Z.; Ginebra, M.-P.; Canal, C. Important parameters in plasma jets for the production of RONS in liquids for plasma medicine: A brief review. Front. Chem. Sci. Eng. 2019, 13, 238–252. [Google Scholar] [CrossRef]
- Ghimire, B.; Szili, E.J.; Patenall, B.L.; Mistry, D.; Fellows, A.; Jenkins, A.T.A.; Short, R.D. Cold Plasma Generation of Peracetic Acid for Antimicrobial Applications. Plasma Med. 2021, 11, 4. [Google Scholar] [CrossRef]
- Szili, E.J.; Ghimire, B.; Patenall, B.L.; Rohaim, M.; Mistry, D.; Fellows, A.; Munir, M.; Jenkins, A.T.A.; Short, R.D. On-demand cold plasma activation of acetyl donors for bacteria and virus decontamination. Appl. Phys. Lett. 2021, 119, 054104. [Google Scholar] [CrossRef]
- Ghimire, B.; Patenall, B.L.; Szili, E.J.; Gaur, N.; Lamichhane, P.; Thet, N.T.; Trivedi, D.; Jenkins, A.T.A.; Short, R.D. The influence of a second ground electrode on hydrogen peroxide production from an atmospheric pressure argon plasma jet and correlation to antibacterial efficacy and mammalian cell cytotoxicity. J. Phys. D Appl. Phys. 2022, 55, 125207. [Google Scholar] [CrossRef]
- Humphries, R.M.; Ambler, J.; Mitchell, S.L.; Castanheira, M.; Dingle, T.; Hindler, J.A.; Koeth, L.; Sei, K. CLSI methods development and standardization working group best practices for evaluation of antimicrobial susceptibility tests. J. Clin. Microbiol. 2018, 56, e01934-17. [Google Scholar] [CrossRef]
- Ghimire, B.; Sornsakdanuphap, J.; Hong, Y.J.; Uhm, H.S.; Weltmann, K.-D.; Choi, E.H. The effect of the gap distance between an atmospheric-pressure plasma jet nozzle and liquid surface on OH and N2 species concentrations. Phys. Plasmas 2017, 24, 073502. [Google Scholar] [CrossRef]
- Karakas, E.; Laroussi, M. Experimental studies on the plasma bullet propagation and its inhibition. J. Appl. Phys. 2010, 108, 063305. [Google Scholar] [CrossRef]
- Ghimire, B.; Szili, E.J.; Lamichhane, P.; Short, R.D.; Lim, J.S.; Attri, P.; Masur, K.; Weltmann, K.-D.; Hong, S.-H.; Choi, E.H. The role of UV photolysis and molecular transport in the generation of reactive species in a tissue model with a cold atmospheric pressure plasma jet. Appl. Phys. Lett. 2019, 114, 093701. [Google Scholar] [CrossRef]
- Sarani, A.; Nikiforov, A.Y.; Leys, C. Atmospheric pressure plasma jet in Ar and Ar/H2O mixtures: Optical emission spectroscopy and temperature measurements. Phys. Plasmas 2010, 17, 063504. [Google Scholar] [CrossRef]
- Oh, J.-S.; Szili, E.J.; Ogawa, K.; Short, R.D.; Ito, M.; Furuta, H.; Hatta, A. UV–vis spectroscopy study of plasma-activated water: Dependence of the chemical composition on plasma exposure time and treatment distance. Jpn. J. Appl. Phys. 2017, 57, 0102B9. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, M.; Pang, Z.; Dai, L.; Lu, J.; Zou, J. Simultaneous spectrophotometric determination of peracetic acid and the coexistent hydrogen peroxide using potassium iodide as the indicator. Anal. Methods 2019, 11, 1930–1938. [Google Scholar] [CrossRef]
- Gott, R.P.; Xu, K.G. OH Production and Jet Length of an Atmospheric-Pressure Plasma Jet for Soft and Biomaterial Treatment. IEEE Trans. Plasma Sci. 2019, 47, 4988–4999. [Google Scholar] [CrossRef]
- Sofokleous, P.; Ali, S.; Wilson, P.; Buanz, A.; Gaisford, S.; Mistry, D.; Fellows, A.; Day, R.M. Sustained antimicrobial activity and reduced toxicity of oxidative biocides through biodegradable microparticles. Acta Biomater. 2017, 64, 301–312. [Google Scholar] [CrossRef]
- Szili, E.J.; Harding, F.J.; Hong, S.-H.; Herrmann, F.; Voelcker, N.H.; Short, R.D. The hormesis effect of plasma-elevated intracellular ROS on HaCaT cells. J. Phys. D Appl. Phys. 2015, 48, 495401. [Google Scholar] [CrossRef]
- Thomas, D.C.; Tsu, C.L.; Nain, R.A.; Arsat, N.; Fun, S.S.; Lah, N.A.S.N. The role of debridement in wound bed preparation in chronic wound: A narrative review. Ann. Med. Surg. 2021, 71, 102876. [Google Scholar] [CrossRef]
- Patenall, B.L.; Williams, G.T.; Gwynne, L.; Stephens, L.J.; Lampard, E.V.; Hathaway, H.J.; Thet, N.T.; Young, A.E.; Sutton, M.J.; Short, R.D.; et al. Reaction-based indicator displacement assay (RIA) for the development of a triggered release system capable of biofilm inhibition. Chem. Commun. 2019, 55, 15129–15132. [Google Scholar] [CrossRef]
- Loo, A.E.K.; Wong, Y.T.; Ho, R.; Wasser, M.; Du, T.; Ng, W.T.; Halliwell, B. Effects of Hydrogen Peroxide on Wound Healing in Mice in Relation to Oxidative Damage. PLoS ONE 2012, 7, e49215. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szili, E.J.; Patenall, B.L.; Fellows, A.; Mistry, D.; Jenkins, A.T.A.; Short, R.D.; Ghimire, B. On Plasma Activated Acetyl Donors: Comparing the Antibacterial Efficacy of Tetraacetylethylenediamine and Pentaacetate Glucose. Plasma 2022, 5, 423-435. https://doi.org/10.3390/plasma5040031
Szili EJ, Patenall BL, Fellows A, Mistry D, Jenkins ATA, Short RD, Ghimire B. On Plasma Activated Acetyl Donors: Comparing the Antibacterial Efficacy of Tetraacetylethylenediamine and Pentaacetate Glucose. Plasma. 2022; 5(4):423-435. https://doi.org/10.3390/plasma5040031
Chicago/Turabian StyleSzili, Endre J., Bethany L. Patenall, Adrian Fellows, Dharmit Mistry, A. Toby A. Jenkins, Robert D. Short, and Bhagirath Ghimire. 2022. "On Plasma Activated Acetyl Donors: Comparing the Antibacterial Efficacy of Tetraacetylethylenediamine and Pentaacetate Glucose" Plasma 5, no. 4: 423-435. https://doi.org/10.3390/plasma5040031
APA StyleSzili, E. J., Patenall, B. L., Fellows, A., Mistry, D., Jenkins, A. T. A., Short, R. D., & Ghimire, B. (2022). On Plasma Activated Acetyl Donors: Comparing the Antibacterial Efficacy of Tetraacetylethylenediamine and Pentaacetate Glucose. Plasma, 5(4), 423-435. https://doi.org/10.3390/plasma5040031





