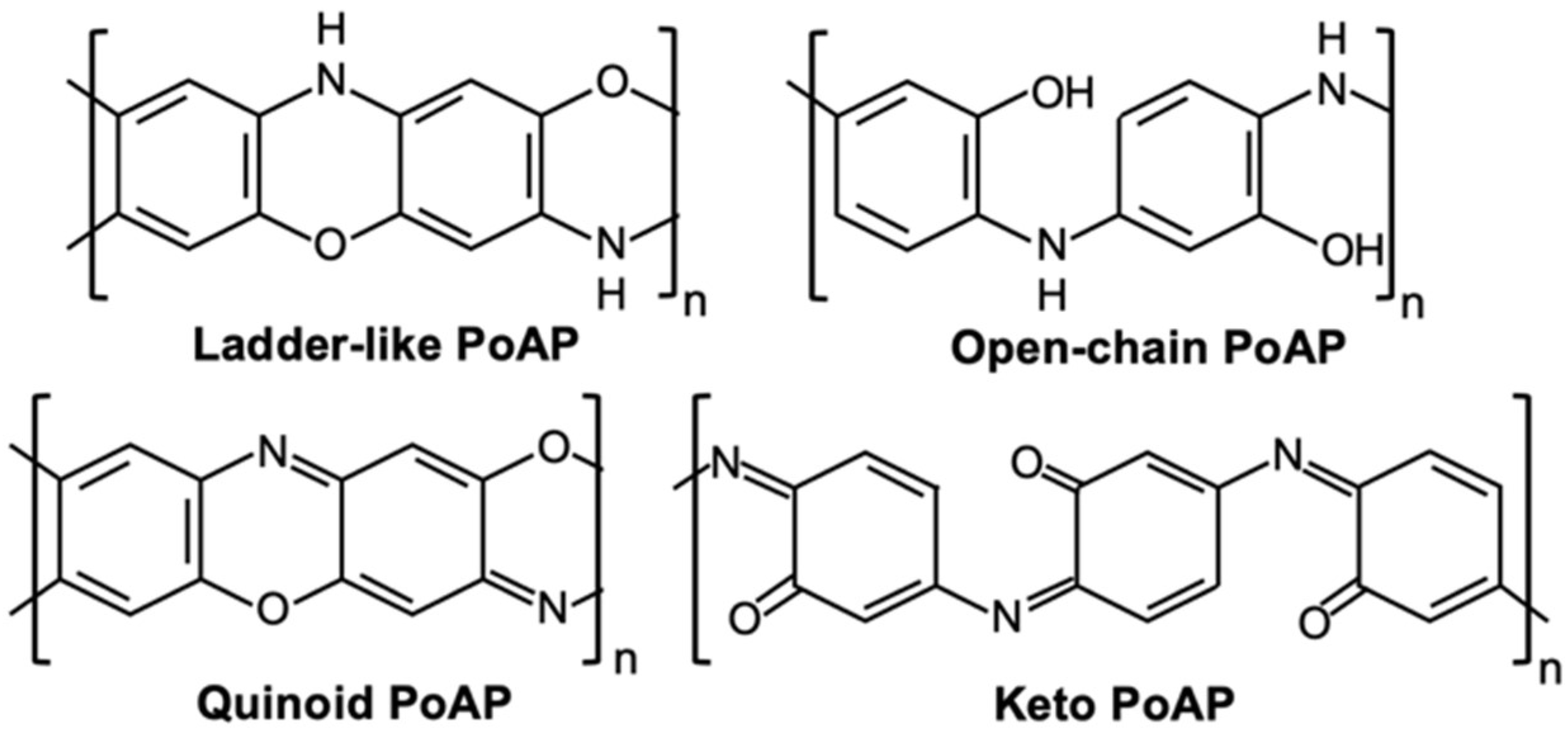
Poly (O-Aminophenol) Produced by Plasma Polymerization Has IR Spectrum Consistent with a Mixture of Quinoid & Keto Structures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Procedure
2.1.1. Digitizing
2.1.2. Optimization
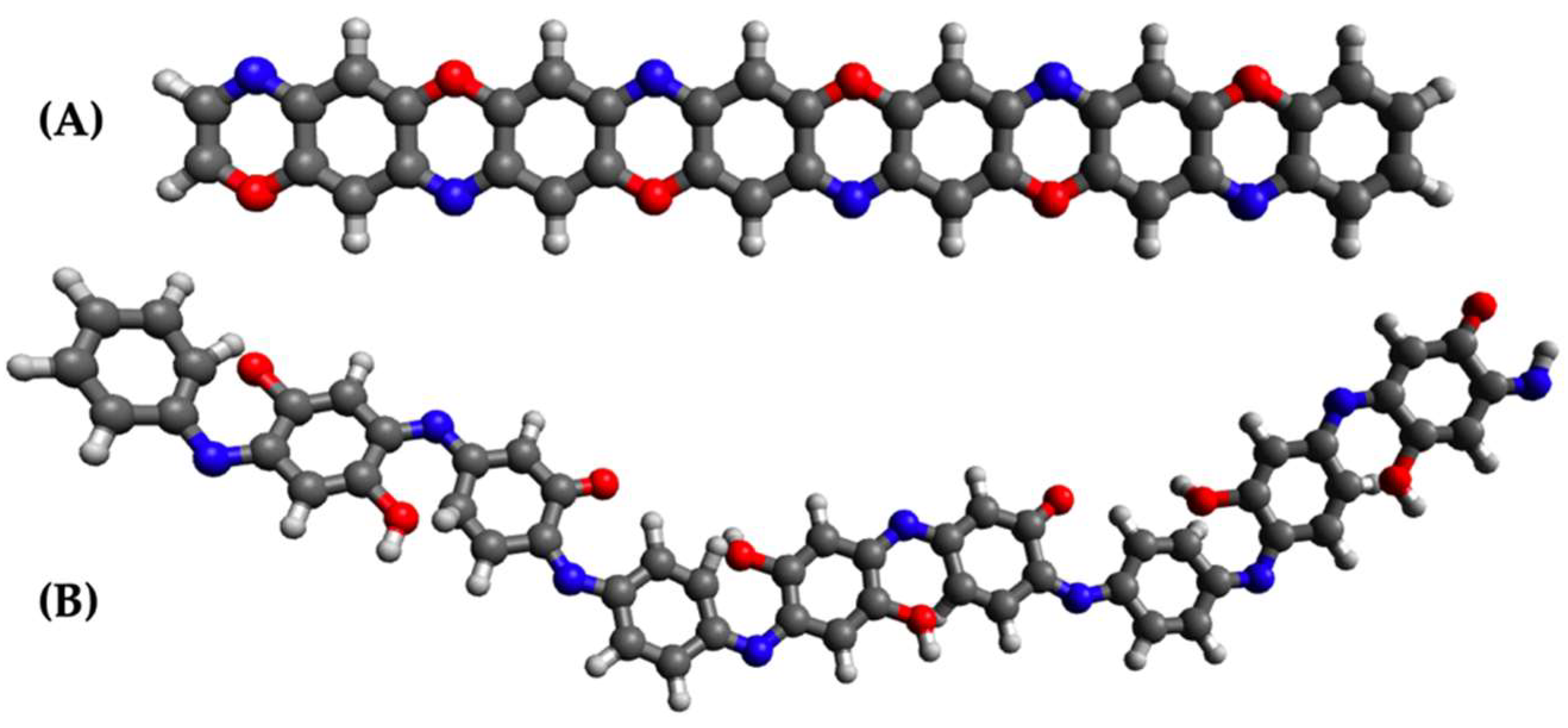
3. Results and Discussion
3.1. Simulated Spectrum
3.2. Packing Efficiency
3.3. Unidentified Peak
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manisankar, P.; Vedhi, C.; Selvanathan, G.; Somasundaram, R. Electrochemical and electrochromic behavior of novel poly (aniline-co-4, 4′-diaminodiphenyl Sulfone). Chem. Mater. 2005, 17, 1722–1727. [Google Scholar] [CrossRef]
- Karami, H.; Mousavi, M.F.; Shamsipur, M. A new design for dry polyaniline rechargeable batteries. J. Power Sources 2003, 117, 255–259. [Google Scholar] [CrossRef]
- Gopalasamy, T.; Gopalswamy, M.; Gopichand, M.; Raj, J. Poly Meta-Aminophenol: Chemical synthesis, characterization and AC impedance study. J. Polym. 2014, 2014, 27043. [Google Scholar] [CrossRef] [Green Version]
- Al-Hossainy, A.; Zoromba, M.S.; Abdel-Aziz, M.; Bassyouni, M.; Attar, A.; Zwawi, M.; Abd-Elmageed, A.; Maddah, H.; Slimane, A.B. Fabrication of heterojunction diode using doped-poly (ortho-aminophenol) for solar cells applications. Phys. B Condens. Matter 2019, 566, 6–16. [Google Scholar] [CrossRef]
- Bicak, T.C.; Soylemez, S.; Buber, E.; Toppare, L.; Yagci, Y. Poly (o-aminophenol) prepared by Cu (II) catalyzed air oxidation and its use as a bio-sensing architecture. Polym. Chem. 2017, 8, 3881–3888. [Google Scholar] [CrossRef]
- Pan, D.; Chen, J.; Yao, S.; Tao, W.; Nie, L. An amperometric glucose biosensor based on glucose oxidase immobilized in electropolymerized poly (o-aminophenol) and carbon nanotubes composite film on a gold electrode. Anal. Sci. 2005, 21, 367–371. [Google Scholar] [CrossRef] [Green Version]
- Miland, E.; Ordieres, A.M.; Blanco, P.T.; Smyth, M.; Fagain, C. Poly (o-aminophenol)-modified bienzyme carbon paste electrode for the detection of uric acid. Talanta 1996, 43, 785–796. [Google Scholar] [CrossRef]
- Chen, K.; Cao, M.; Feng, E.; Sohlberg, K.; Ji, H.-F. Polymerization of Solid-State Aminophenol to Polyaniline Derivative Using a Dielectric Barrier Discharge Plasma. Plasma 2020, 3, 187–195. [Google Scholar] [CrossRef]
- Ghanem, M.A.; El-Enany, G. Development of conducting poly (o-aminophenol) film and its capacitance behavior. Int. J. Electrochem. Sci. 2016, 11, 9987–9997. [Google Scholar] [CrossRef]
- Ohsaka, T.; Watanabe, T.; Kitamura, F.; Oyama, N.; Tokuda, K. Electrocatalysis of O2 reduction at poly (o-phenylenediamine)-and poly (o-aminophenol)-coated glassy carbon electrodes. J. Chem. Soc. Chem. Commun. 1991, 16, 1072–1073. [Google Scholar] [CrossRef]
- Zhang, A.; Cui, C.; Chen, Y.; Lee, J. Synthesis and electrochromic properties of poly-o-aminophenol. J. Electroanal. Chem. 1994, 373, 115–121. [Google Scholar] [CrossRef]
- Tucceri, I.R. Poly (o-aminophenol) as material of biosensors. Res. Open Access 2014, 2014, 884. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self—Consistent molecular orbital methods. XII. Further extensions of Gaussian—Type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Barca, G.M.J.; Bertoni, C.; Carrington, L.; Datta, D.; De Silva, N.; Deustua, J.E.; Fedorov, D.G.; Gour, J.R.; Gunina, A.O.; Guidez, E.; et al. Recent developments in the general atomic and molecular electronic structure system. J. Chem. Phys. 2020, 152, 154102. [Google Scholar] [CrossRef] [Green Version]
- Scott, A.P.; Radom, L. Harmonic vibrational frequencies: An evaluation of Hartree−Fock, Møller−Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors. J. Phys. Chem. 1996, 100, 16502–16513. [Google Scholar] [CrossRef]
- Suenaga, M. Facio. Available online: http://zzzfelis.sakura.ne.jp/index.html (accessed on 10 March 2021).
- Rohatgi, A. WebPlotDigitizer. Available online: https://automeris.io/WebPlotDigitizer (accessed on 18 October 2021).
- Silver, A.; Dong, H.; Sun, Z.; Sohlberg, K. Semiempirical study of low molecular weight polymers of 3-phenyl-1-ureidonitrile. J. Mol. Struct. THEOCHEM 2005, 731, 149–155. [Google Scholar] [CrossRef]
- Avogadro: An Open-Source Molecular Builder and Visualization Tool, Version 1.2.0; Available online: http://avogadro.cc/ (accessed on 18 October 2021).
- Kunimura, S.; Ohsaka, T.; Oyama, N. Preparation of thin polymeric films on electrode surfaces by electropolymerization of o-aminophenol. Macromolecules 1988, 21, 894–900. [Google Scholar] [CrossRef]
- Molinspiration Chemoinformatic Software. Available online: https://www.molinspiration.com (accessed on 10 February 2022).
- Smith, A.L. The Coblentz Society Desk Book of Infrared Spectra, 2nd ed.; Coblentz Society: Kirkwood, MO, USA, 1982; pp. 1–24. [Google Scholar]
- James, C.; Ravikumar, C.; Sundius, T.; Krishnakumar, V.; Kesavamoorthy, R.; Jayakumar, V.; Joe, I.H. FT-Raman and FTIR spectra, normal coordinate analysis and ab initio computations of (2-methylphenoxy) acetic acid dimer. Vib. Spectrosc. 2008, 47, 10–20. [Google Scholar] [CrossRef]
- Shipman, S.T.; Douglass, P.C.; Yoo, H.S.; Hinkle, C.E.; Mierzejewski, E.L.; Pate, B.H. Vibrational dynamics of carboxylic acid dimers in gas and dilute solution. Phys. Chem. Chem. Phys. 2007, 9, 4572–4586. [Google Scholar] [CrossRef] [PubMed]
- Dubis, A.T.; Grabowski, S.J.; Romanowska, D.B.; Misiaszek, T.; Leszczynski, J. Pyrrole-2-carboxylic acid and its dimers: Molecular structures and vibrational spectrum. J. Phys. Chem. A 2002, 106, 10613–10621. [Google Scholar] [CrossRef]
- Grimme, S. Accurate description of van der Waals complexes by density functional theory including empirical corrections. J. Comput. Chem. 2004, 25, 1463–1473. [Google Scholar] [CrossRef]
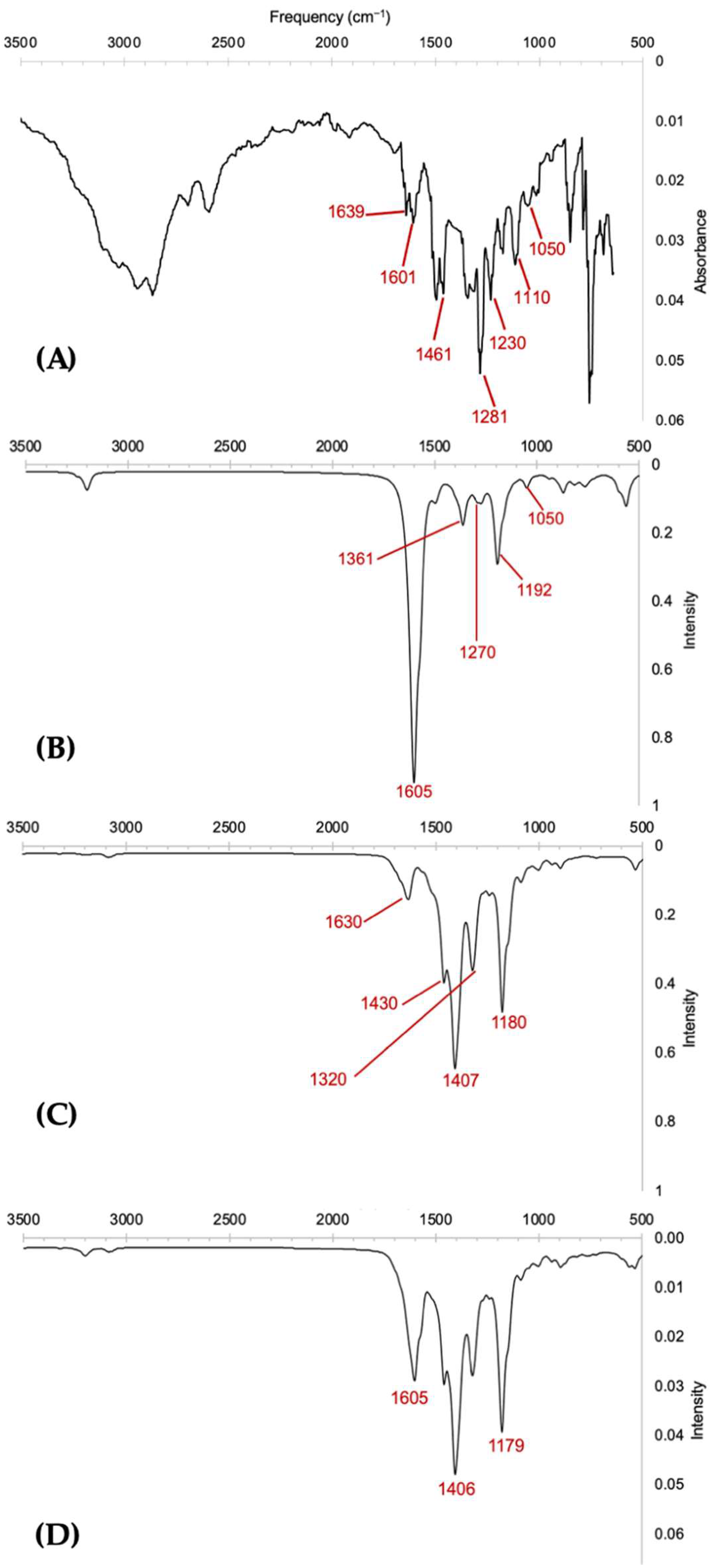


| Wavenumber, cm−1 | |||||
|---|---|---|---|---|---|
| Vibration Assignments | Chen et al. [8] | Theo. Quinoid | Theo. Keto | Lit. Quinoid | Lit. Keto |
| C=O stretching | n/a | n/a | 1669, 1697 | n/a | 1670–1690 [23] |
| C=N stretching | 1639 | 1613, 1615, 1622, 1636 | 1620, 1625, 1637 | 1645 [23] | n/a |
| C=C stretching | 1461, 1601 | 1397, 1491, 1572, 1595, 1600, 1605 | 1378, 1392, 1403, 1411, 1431, 1455, 1563 | 1430–1613 [9,23] | 1450, 1590 [5,23] |
| C–N stretching of aromatic ring | 1281 | 1289, 1297 | 1170, 1298, 1300, 1307 | 1284 [9] | n/a |
| C–O–C stretching | 1050, 1110, 1230 | 1049, 1161, 1193, 1270 | 1036, 1047, 1072, 1078, 1082, 1237 | 1050, 1112 [5] 1235 [23] | 1050, 1235 [23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuart, N.M.; Sohlberg, K. Poly (O-Aminophenol) Produced by Plasma Polymerization Has IR Spectrum Consistent with a Mixture of Quinoid & Keto Structures. Plasma 2022, 5, 196-205. https://doi.org/10.3390/plasma5020015
Stuart NM, Sohlberg K. Poly (O-Aminophenol) Produced by Plasma Polymerization Has IR Spectrum Consistent with a Mixture of Quinoid & Keto Structures. Plasma. 2022; 5(2):196-205. https://doi.org/10.3390/plasma5020015
Chicago/Turabian StyleStuart, Natalie M., and Karl Sohlberg. 2022. "Poly (O-Aminophenol) Produced by Plasma Polymerization Has IR Spectrum Consistent with a Mixture of Quinoid & Keto Structures" Plasma 5, no. 2: 196-205. https://doi.org/10.3390/plasma5020015
APA StyleStuart, N. M., & Sohlberg, K. (2022). Poly (O-Aminophenol) Produced by Plasma Polymerization Has IR Spectrum Consistent with a Mixture of Quinoid & Keto Structures. Plasma, 5(2), 196-205. https://doi.org/10.3390/plasma5020015







