Abstract
The chemical treatment of wood has been shown to increase its mechanical strength by forming composites with a variety of polymers. Polyethylene glycol diacrylate (PEGDA) has commonly been used as a polymer reinforcement to increase the strength and resistance of spruce wood for various applications, such as protection from weathering. In this study, PEGDA was impregnated into wood samples and polymerized by dielectric barrier discharge (DBD) plasma to form wood–polymer composites (WPCs). The kinetic rate order of PEGDA was explored using FT-IR quantitative analysis and the DBD plasma-initiated polymerization was determined to be second order. The strength of the wood samples was then determined by a three-point flexural test. The PEGDA-treated spruce wood samples showed improved flexural strength versus the untreated wood samples. The WPCs were also made using a UV treatment method and were then compared to the DBD plasma-treated samples. The results showed that the DBD plasma-treated samples yielded superior flexural strength relative to the UV-treated samples. We accredited this difference in strength to the plasma process and its ability to penetrate into the various layers of the wood and initiate polymerization, as opposed to UV light that can only penetrate superficially, initiating polymerization in only the first few layers of the wood surface.
1. Introduction
Wood and wood-based materials are used in many applications because of their abundance and versatility. Spruce is a widely used wood material for structural elements in construction. However, there are several disadvantages to using spruce wood in these structural elements, including dimensional/appearance deformation in response to atmospheric conditions, susceptibility to biological attack, and overall degradation with time [1]. Wood treatment seeks to modify the material to overcome these disadvantages. These modifications can improve the wood’s decay resistance, dimensional stability, and reduce its water absorption [2]. One such treatment method is the use of various monomers to impregnate a wood sample, whereby the monomer species diffuse into the mesopores of the wood and distribute uniformly, followed by their subsequent polymerization [3]. This method forms wood–polymer composites (WPCs), which generally show improved properties compared to untreated wood [4].
Polyethylene glycol (PEG) has been used for decades in the impregnation of wood materials to stabilize wood by preventing cracking, splitting, and shrinking [5]. Poly(ethylene glycol diacrylate) (PEGDA) is a long chain prepolymer—it has been reacted to an intermediate state and is capable of further polymerization into a larger polymer. The polymerization of PEGDA is conventionally conducted using either ultraviolet (UV) radiation [6] or heat [7]. Heat-initiated polymerization for WPCs is not preferred due to the detrimental nature of moderate to high temperatures on the mechanical properties of wood materials [8,9]. For these reasons UV-initiated polymerization is the preferred method for forming WPCs. However, one major drawback to using UV polymerization to form WPCs is its low photon penetration depth, which leads to a low degree of polymerization in non-superficial layers of a sample [10]. Additionally, a photoinitiator is typically necessary for an efficient degree of polymerization to occur.
Recently, we demonstrated that nonthermal dielectric barrier discharge (DBD) plasma can be used to initiate the radical polymerization of solid-state 2,2′-bithiophene to form thin films [11]. We further demonstrated that DBD plasma could be used to polymerize the 2,2′-bithiophene inside of cellulose paper to make paper-based electronics. We wanted to continue our work with DBD plasma on thicker mesoporous materials to determine if plasma could penetrate and initiate polymerization internally in these materials. DBD plasma, unlike other plasmas, is generated at normal atmospheric pressure via a simple device. Unlike thermal plasma, which generates a high degree of heat that would be detrimental to the wood samples, DBD plasma is considered a ‘cold’ plasma (nonthermal) method that generates little to no appreciable heat under ambient atmospheric conditions [12]. Additionally, DBD plasma is an energy-saving and greener approach (requiring neither chemical additives nor solvents) for polymerization in mesoporous materials relative to the other two polymerization methods previously mentioned.
In this study, we aimed to increase the strength of spruce wood samples by first impregnating them with PEGDA and then initiating the polymerization of the prepolymer within the samples using DBD plasma. We characterized and studied the kinetics of the DBD plasma-initiated polymerization of PEGDA using Fourier-transform infrared spectroscopy (FT-IR). We then tested the flexural strength of the samples using a three-point bending test and monitored the flexural stress as a function of flexural strain. This test was repeated for untreated wood samples, which were used as controls, and UV-treated samples, which were used to compare the polymerization methods and their ability to form strong WPCs.
2. Materials and Methods
2.1. Materials
PEGDA (>98% Mn = 700, Sigma-Aldrich, St. Louis, MO, USA). Irgacure 2959 (BASF, Vandalia, IL, USA). All chemicals were used as received without further purification. The gold-coated silicon wafers were made in-house [13]. All wood samples were laser cut from a plank of European Spruce (Home Depot, Philadelphia, PA, USA) into rectangular prisms with dimensions of 41 mm × 21 mm × 1.8 mm.
2.2. DBD Plasma Treatment
The DBD plasma was generated by using a microsecond-pulsed power supply (FID Technology, Burbach, Germany) and an electrode−dielectric barrier discharge setup, as reported previously [14]. Briefly, the DBD electrode works by creating a plasma stream between a high voltage 25 mm thick copper plate and a ground. A 1 mm thick quartz dielectric plate is used as an insulating barrier to cover the copper plate. The plasma discharge gap between the bottom of the quartz plate and the surface of the samples was 5 mm. The plasma is generated by using a variable voltage and variable frequency power supply that applies a pulsed alternating polarity voltage of 20 kV (peak to peak) with a 10 ns pulse width and a rise time of 5 V/ns. For all of our experiments, a peak voltage of 11.2 kV and repetition frequency of 690 fHz were used. The input energy used was calculated to be around 10 mJ/pulse. The working area of plasma treatment was equivalent to the copper plate dimensions of 38 mm × 64 mm.
2.3. Drop Casting of PEGDA and Plasma Polymerization
After polymerization of PEGDA has occured to a significant degree it can be said that it is no longer PEGDA and instead just PEG without the diacrylates at each terminal end of the chains. Thus, henceforth, we will occasonally refer to post-polymerization PEGDA as PEG. For the characterization and kinetics study, a thin film of PEGDA was drop casted onto a gold-coated silicon wafer by using an autopipette to deliver 10 µL of the prepolymer. The PEGDA drop distributed uniformly over the 1 cm × 1 cm gold surface yielding an approximate thickness of 100 µm. The gold substrates were used for their IR inactivity, which allowed the PEGDA bands to be isolated and monitored along the plasma treatment process without any IR band interference from the substrate. The PEGDA-coated substrates were then treated with DBD plasma under ambient conditions (normal temperature and pressure) and the relative humidity fluctuated day-by-day between 60–70%. Various plasma treatment times were used to determine the best treatment time for the highest degree of polymerization. Plasma was first applied for 2.5, 5, 10, and 15 min over four different PEGDA films. Afterwards, the samples were allowed to rest for one hour, followed by a second plasma treatment cycle with identical time periods for a total plasma treatment time of 5, 10, 20, and 30 min for each of the four samples. At this point, a solid thin film of PEG could be seen to have formed at the surface.
2.4. FT-IR Characterization and Analysis
A PerkinElmer Spectrum One FT-IR Spectrometer (Waltham, MA, USA) was used to obtain the FT-IR spectra of the samples before and after plasma polymerization. FTIR sampling was performed by attenuated total reflection (ATR) over the range of 500 to 4000 cm−1 with a resolution of 4 cm−1. The background FT-IR spectrum was collected on a blank gold substrate for the kinetics test and in atmospheric air for all other tests. Each sample was pressurized to the same magnitude to ensure good contact between the sample/film and ATR crystal. OriginLab was used to analyze the spectra of the samples after various treatment times.
2.5. Wood Treatment to Form WPCs
WPCs were formed by submerging the spruce wood samples into one of three impregnation solutions. These three solutions were pure PEGDA, 60% (v/v) PEGDA in distilled water, and pure PEGDA with 0.1% (w/w) Irgacure 2959. Five wood samples were incubated in each beaker for 24 h for complete impregnation of the prepolymer into the wood. Another five samples were immersed in distilled water for 24 h as controls. The wood samples were then left to dry under vacuum for 24 h. Once dry, any excess PEGDA was dabbed off the surface of the wood using a Kimwipe. DBD plasma was then applied to each wood sample for 15 min, which was left to rest for one hour, after which the opposite side was then exposed to plasma for an additional 15 min. At this point, the wood samples displayed a glossy sheen on the surface. The WPCs were then considered to be formed and ready for testing. This process was repeated for the UV-treated samples, apart from the last step, in which a UV lamp (Blak-Ray, 100 W power, 365 nm wavelength), instead of plasma, was used to irradiate the wood samples for the same treatment time intervals on each side. This process is depicted in Figure 1 below.
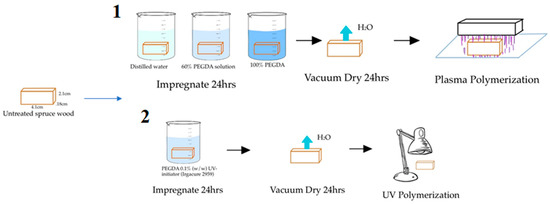
Figure 1.
Wood modification process to form the WPCs. Method 1 is for the DBD plasma treatment and method 2 is for the UV treatment.
2.6. Flexural Test
The flexural test was conducted on the WPCs using a 3-point flexural setup on an MTS Criterion Model 43 instrument. A load cell with a capacity of 500 N and sensitivity of 2.154 mV/V was used. The span distance was 24.5 mm and the strain rate chosen was 0.1 mm s−1. Each wood sample had dimensions as previously mentioned and these dimensions were used to compute the cross-sectional area needed in the calculation of the flexural strength, which is defined as:
where F is the load at fracture in Newtons (N), L is the length of the span in mm, b is the width of sample in mm, and d is the thickness of the sample in mm. The flexural strength (σ) is then computed in units of Pascals (Pa).
3. Results and Discussion
3.1. FT-IR Analysis
After polymerization of PEGDA on the gold substrates using DBD plasma was completed, they were characterized using FT-IR. In Figure 2 below we highligted four bands that represent vibrational modes assosiated with the carbon–carbon double bond (C=C) of the acrylate group. The band at 1618 cm−1 that is present in PEGDA but decreases as a function of plasma treatment time (eventually completely disappearing after 30 min.) corresponds to the C=C asymmetric stretch [15]. The decrease in absorption bands at 988 cm−1 and 810 cm−1 are also related to the C=C vibrational modes of bending and twisting modes, respectively [16]. Furthermore, the decrease in the degree of C=C is also made more evident by the decrease in the band centered at 1408 cm−1, which corresponds to the C=CH2 bending vibration [15,17].
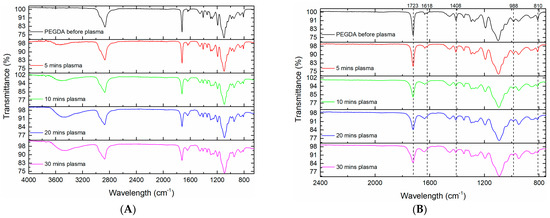
Figure 2.
FT-IR spectra of PEGDA before and after several DBD plasma treatment times. (A) The full range of the FT-IR spectra taken. (B) The x-axis has been truncated to better show the annotated bands of interest.
3.2. Kinetics Study
To determine the conversion rate of the polymerization reaction we used the peak area ratio of two bands in the FT-IR spectra and plotted this value as a function of plasma treatment time. This quantitative analysis method for determining the reaction rate order using FT-IR was adapted from Pintar et al. [18].The carbonyl band at 1723 cm−1 was chosen as the reference peak since it stays virtually constant throughout the polymerization process. The band at 1408 cm−1 was chosen as the other peak of interest as it noticeably decreases proportionately with increasing polymerization time as a result of the loss of the C=C in the acrylate group. The peak areas of these two bands at 1723 and 1408 cm−1 were denoted as A0 and A1, respectively. The ratio of these values (A1/A0) was then used to determine the degree of conversion of the acrylate groups to form PEG from PEGDA.
For the quantitative analysis of the IR bands we used the peak analysis feature of the Origin software program; there was no need for any deconvolution of peaks as each band of interest was sharp and unconvoluted. Using this tool we were able to determine the peak area for both bands for each plasma treatment time and the inverse ratio ((A1/A0)−1) values can be seen in the table inset in Figure 3B below.
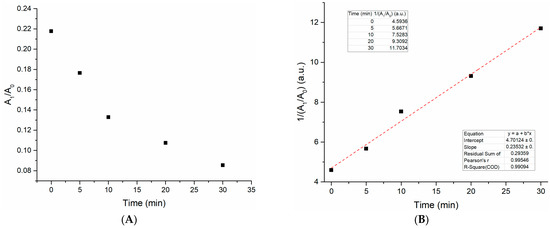
Figure 3.
(A) Plot of the FT-IR peak area ratio (A1/A0) as a function of plasma treatment time. (B) A second-order treatment of the A1/A0 ratio was plotted by graphing the inverse of this ratio as a function of treatment time. The first inset is a table with the inverse ratio values at each treatment time. The second inset shows several relevant parameters for the linear fit and, most importantly, the R-squared value of 0.99094.
The plot in Figure 3A shows that as plasma treatment time increases, the A1/A0 ratio decreases non-proportionally. By applying a second-order treatment to the data and replotting this as a function of time, a straight line with a high R-squared value was obtained, indicating that the kinetic model was a satisfactory fit to the data. The DBD plasma-initiated polymerization reaction can therefore be said to have a second overall order. It is worth noting that this rate order is in reference to how the concentration of the acrylate end groups in PEGDA affect the conversion rate to PEG and does not necessarily describe the kinetics for the polymerization of PEG.
3.3. Characterization of the Wood–Polymer Composites (WPCs)
We first formed the WPCs according to the procedure discussed in the experimental section. A plasma treatment time of 15 min per each side of the wood samples, for a total treatment time of 30 min, was chosen based on the kinetic study results, to allow for the highest degree of polymerization to occur. To confirm that PEGDA was able to penetrate into the mesopores of the spruce wood and then become remotely polymerized by the plasma treatment, we collected before and after FT-IR spectra of both the surface and the cross-section of the WPCs for comparison. The spectra in Figure 4A show both the surface and cross-section showed the signature bands for PEGDA before plasma treatment, confirming the successful impregnation into the wood. After plasma treatment, the signature band at 1408 cm−1 is seen to completely disappear (Figure 4B) at the surface and almost completely disappears at the cross-section as well. This confirms that the plasma treatment is capable of penetrating into the mesopores of the wood samples and initiating polymerization internally. It should be mentioned that several cross-sectional areas were analyzed with FT-IR and not every section showed complete disappearance of the 1408 cm−1 band. Its peak area ratio relative to the carbonyl peak varied across the different cross-sections analyzed. This may imply that DBD plasma does not uniformly initiate the polymerization of PEGDA internally and future work may investigate characterizing the locality of this process across a sample.
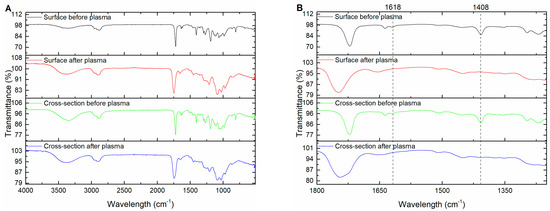
Figure 4.
FTIR spectra for the surface and cross-section of the WPCs before and after plasma treatment. (A) Full range FT-IR spectra. (B) Truncated FT-IR spectra with signature bands annotated.
3.4. Flexural Test of the WPCs
A three-point flexural test was performed to measure the flexural strength of the WPC samples. The controls for this test were untreated wood (pristine spruce wood) and wood that had been soaked in only distilled water for 24 h and exposed to plasma for the same time interval as the treated samples. The flexural stress at breakage for the samples was recorded and the data are summarized in Table 1 below. The pristine wood samples and water-soaked samples had similar average strengths, of 60.02 MPa and 61.43 MPa, respectively. This would imply that neither soaking in water for 24 h nor DBD plasma application for a total time of 30 min have a detrimental effect on the wood’s flexural strength. Thus, any changes in the strength should be attributed to the polymer reinforcement and the degree of polymerization due to the treatment method. Additionally, this also demonstrates that DBD plasma is a non-destructive treatment method for forming WPCs.

Table 1.
Average flexural strengths for various sample types. All samples were treated with DBD plasma except the last sample in the table, which was treated with UV light. Sample size: N = 5.
For the polymer solution concentrations, it seemed that the pure PEGDA samples performed marginally better than the 60% PEGDA soaked samples. Pure PEGDA samples yielded an average flexural strength of 80.31 MPa, while the 60% PEGDA samples yielded a slightly lower strength of 77.50 MPa. The difference is too small to be fully attributed to the differences in concentration; however, what can be inferred from these data is that both performed better than either of the two types of controls. Finally, the UV-treated samples performed the poorest, with an average strength of 54.95 MPa. This value was not only much lower than the plasma-treated samples, but was even lower than both types of controls. It can then be said that the UV treatment process is not only less efficient at polymerizing the PEGDA internally in the wood samples, as evidenced by FT-IR cross-sections taken after UV treatment that showed almost identical signature peak areas (e.g., 1408 cm−1) to before treatment, but that UV light is also damaging to some degree to the spruce wood itself, as its strength decreased even from that of the controls. This decrease in flexural strength is most likely due to the high UV light intensity from the Blak-Ray lamp, which is a commonly used light source in photopolymerization research [19,20].
4. Conclusions
From our data, we were able to conclude that the DBD plasma treatment method is a fast and efficient process to polymerize even prepolymers such as PEGDA, achieving a high degree of polymerization after only 10 min of plasma treatment. This was evident by analyzing the FT-IR spectra, which showed the disappearance of several bands associated with the C=C of the acrylate end groups in PEGDA. After forming several WPCs of PEG in spruce wood using two methods (DBD plasma and UV treatment) and then determining their strength with a three-point flexural test, it was evident that the plasma-treated samples performed exceptionally compared with the UV-treated samples. Overall, it can be said that DBD plasma is effective at polymerizing monomers/prepolymers in mesoporous materials with thicknesses up to 2 mm and specifically within wood samples. This polymerization method provides a novel method to form WPCs that can improve the mechanical properties and weathering resistance of wood-based materials.
Author Contributions
Conceptualization, H.-F.J.; Data curation, C.S.; Writing–review & editing, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
CS was supported by the Maryanoff Scholarship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Temiz, A.; Terziev, N.; Eikenes, M.; Hafren, J. Effect of accelerated weathering on surface chemistry of modified wood. Appl. Surf. Sci. 2007, 253, 5355–5362. [Google Scholar] [CrossRef]
- Ozdemir, T.; Temiz, A.; Aydin, I. Effect of Wood Preservatives on Surface Properties of Coated Wood. Adv. Mater. Sci. Eng. 2015, 2015, 631835. [Google Scholar] [CrossRef]
- Hill, C.A.S. Wood Modification: Chemical, Thermal and Other Processes; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Manrich, S.; Agnelli, J.A.M. The effect of chemical treatment of wood and polymer characteristics on the properties of wood–polymer composites. J. Appl. Polym. Sci. 1989, 37, 1777–1790. [Google Scholar] [CrossRef]
- Ermeydan, M.A. Modification of spruce wood by UV-crosslinked PEG hydrogels inside wood cell walls. React. Funct. Polym. 2018, 131, 100–106. [Google Scholar] [CrossRef]
- Smoak, E.M.; Henricus, M.M.; Banerjee, I.A. In situ photopolymerization of PEGDA-protein hydrogels on nanotube surfaces. J. Appl. Polym. Sci. 2010, 118, 2562–2571. [Google Scholar] [CrossRef]
- Das, D.; Pham, T.T.H.; Noh, I. Characterizations of hyaluronate-based terpolymeric hydrogel synthesized via free radical polymerization mechanism for biomedical applications. Colloids Surf. B Biointerfaces 2018, 170, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Korkut, S.; Hiziroglu, S. Effect of heat treatment on mechanical properties of hazelnut wood (Corylus colurna L.). Mater. Des. 2009, 30, 1853–1858. [Google Scholar] [CrossRef]
- Srinivas, K.; Pandey, K.K. Effect of Heat Treatment on Color Changes, Dimensional Stability, and Mechanical Properties of Wood. J. Wood Chem. Technol. 2012, 32, 304–316. [Google Scholar] [CrossRef]
- Kataoka, Y.; Kiguchi, M.; Williams, R.S.; Evans, P.D. Violet light causes photodegradation of wood beyond the zone affected by ultraviolet radiation. Wood Res. Technol. 2007, 61, 23–27. [Google Scholar] [CrossRef]
- Chen, K.; Cao, M.; Qiao, Z.; He, L.; Wei, Y.; Ji, H.-F. Polymerization of Solid-State 2,2′-Bithiophene Thin Film or Doped in Cellulose Paper Using DBD Plasma and Its Applications in Paper-Based Electronics. ACS Appl. Polym. Mater. 2020, 2, 1518–1527. [Google Scholar] [CrossRef]
- Hegemann, D.; Nisol, B.; Gaiser, S.; Watson, S.; Wertheimer, M.R. Energy conversion efficiency in low- and atmospheric-pressure plasma polymerization processes with hydrocarbons. Phys. Chem. Chem. Phys. 2019, 21, 8698–8708. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ji, H.-F. Electric field-directed assembly of gold and platinum nanowires from an electrolysis process. Electrochem. Commun. 2008, 10, 222–224. [Google Scholar] [CrossRef]
- Li, Y.; Kojtari, A.; Friedman, G.; Brooks, A.D.; Fridman, A.; Ji, H.F. Decomposition of L-valine under nonthermal dielectric barrier discharge plasma. J. Phys. Chem. B 2014, 118, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Pullagura, B.K.; Amarapalli, S.; Gundabala, V. Coupling electrohydrodynamics with photopolymerization for microfluidics-based generation of polyethylene glycol diacrylate (PEGDA) microparticles and hydrogels. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125586. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Song, L.; Niu, G.; Cao, H.; Wang, G.; Yang, H.; Zhu, S. Controllable properties and microstructure of hydrogels based on crosslinked poly(ethylene glycol) diacrylates with different molecular weights. J. Appl. Polym. Sci. 2011, 121, 531–540. [Google Scholar] [CrossRef]
- Brokken-Zijp, J.C.M.; van Asselen, O.L.J.; Kleinjan, W.E.; van de Belt, R.; de With, G. Photocatalysed (Meth)acrylate Polymerization by (Antimony-Doped) Tin Oxide Nanoparticles and Photoconduction of Their Crosslinked Polymer Nanoparticle Composites. J. Nanotechnol. 2010, 2010, 579708. [Google Scholar] [CrossRef][Green Version]
- Pintar, A.; Batista, J.; Levec, J. In situ Fourier transform infrared spectroscopy as an efficient tool for determination of reaction kinetics. Analyst 2002, 127, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.-M.; Liou, S.-J.; Chang, Y.-W. Polyacrylamide-clay nanocomposite materials prepared by photopolymerization with acrylamide as an intercalating agent. J. Appl. Polym. Sci. 2004, 91, 3489–3496. [Google Scholar] [CrossRef]
- Sawhney, A.S.; Pathak, C.P.; Van Rensburg, J.J.; Dunn, R.C.; Hubbell, J.A. Optimization of photopolymerized bioerodible hydrogel properties for adhesion prevention. J. Biomed. Mater. Res. 1994, 28, 831–838. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).