Spatially-Resolved Spectroscopic Diagnostics of a Miniature RF Atmospheric Pressure Plasma Jet in Argon Open to Ambient Air
Abstract
1. Introduction
2. Experimental Setup and Methods
2.1. Setup Configuration
2.2. Optical Emission Spectroscopy
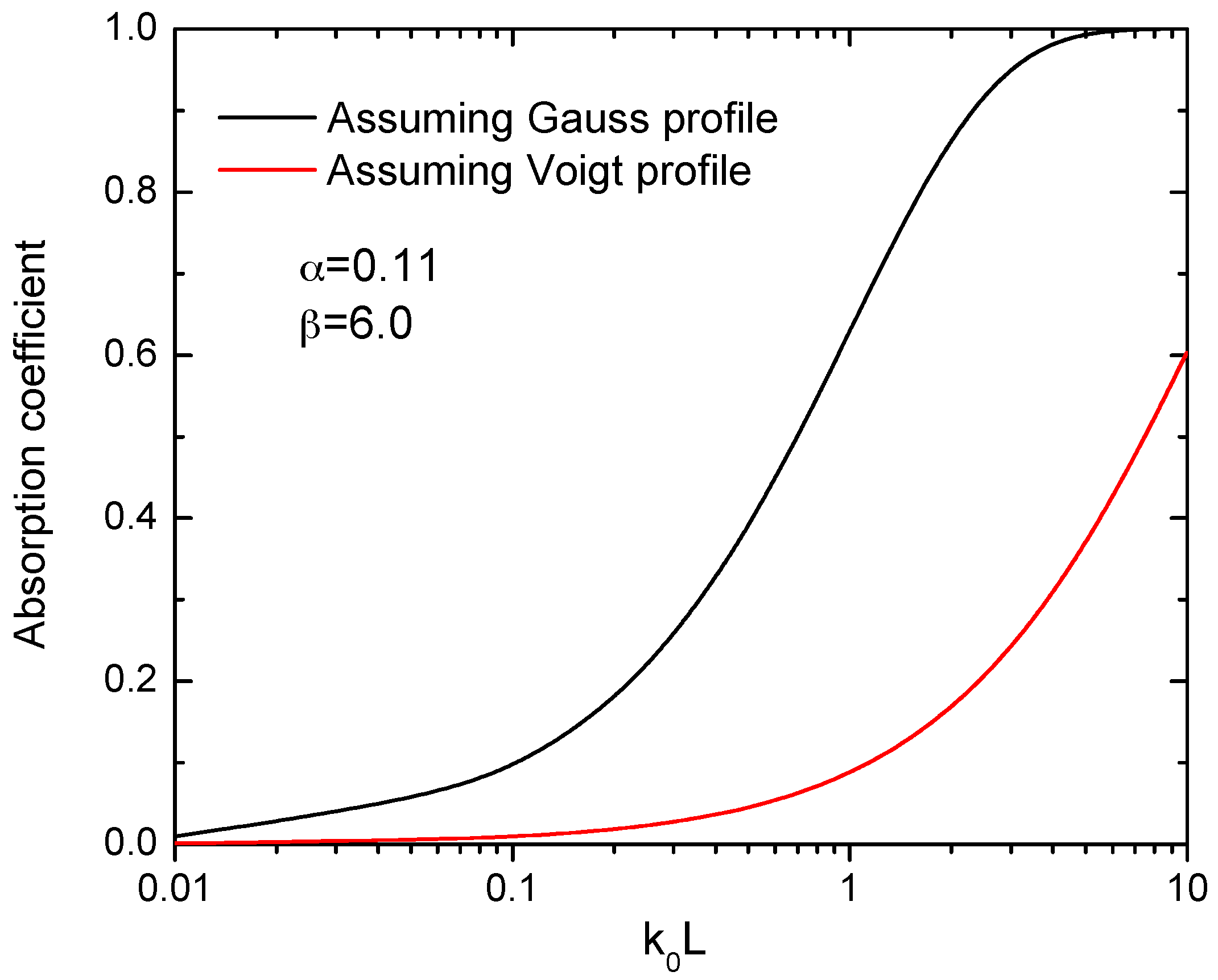
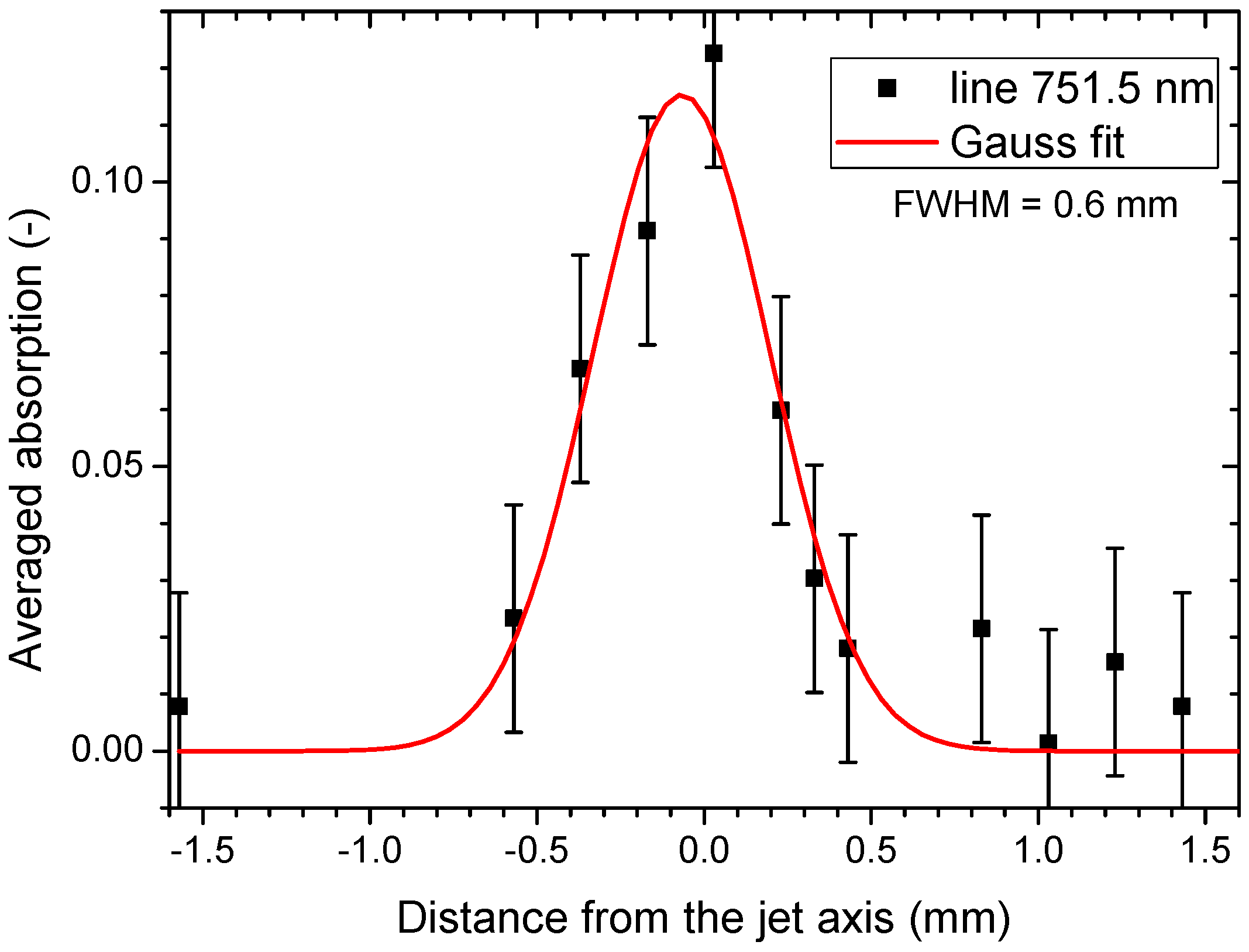
2.3. Optical Absorption Spectroscopy
2.4. Collisional Radiative Model
3. Results and Discussion
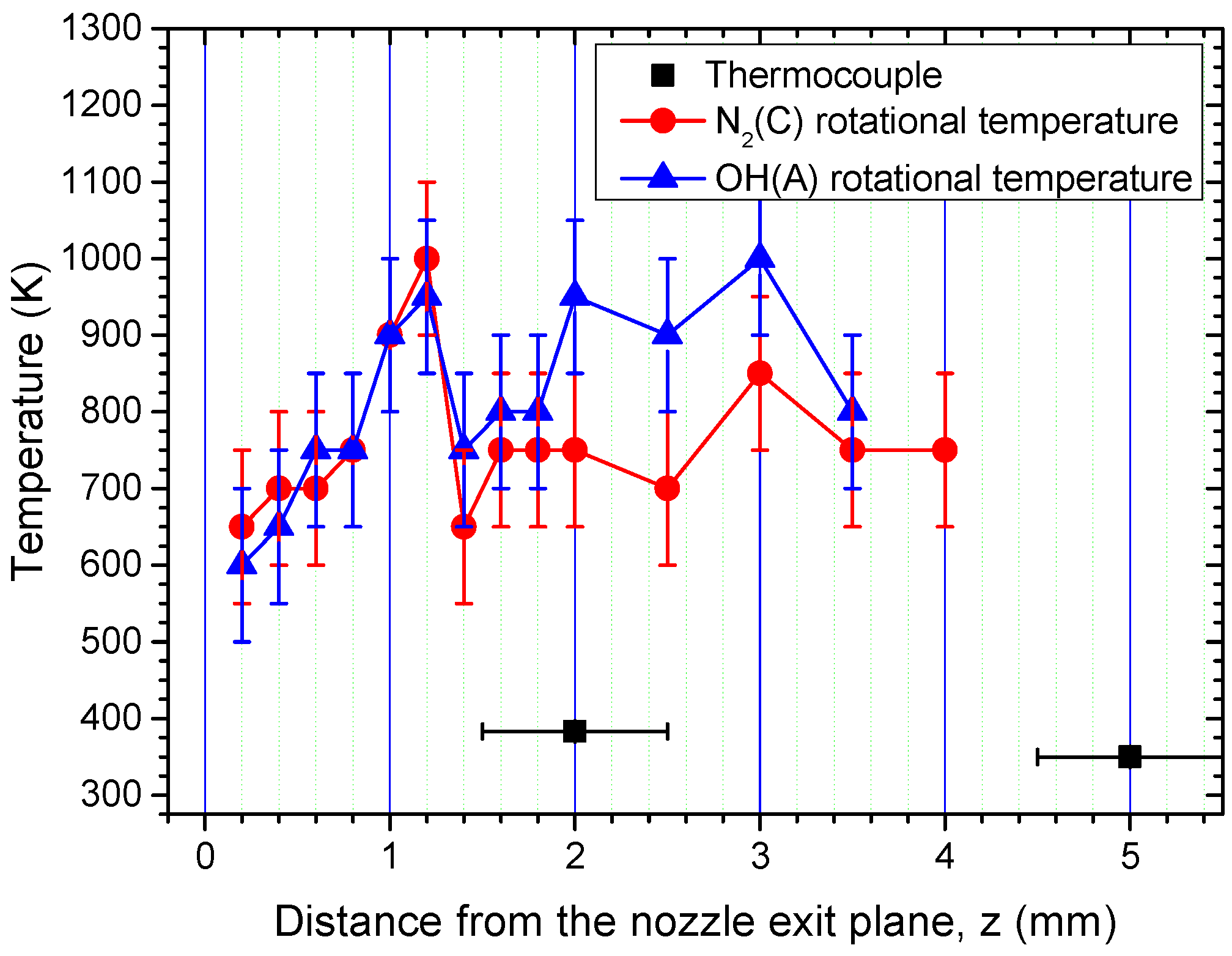
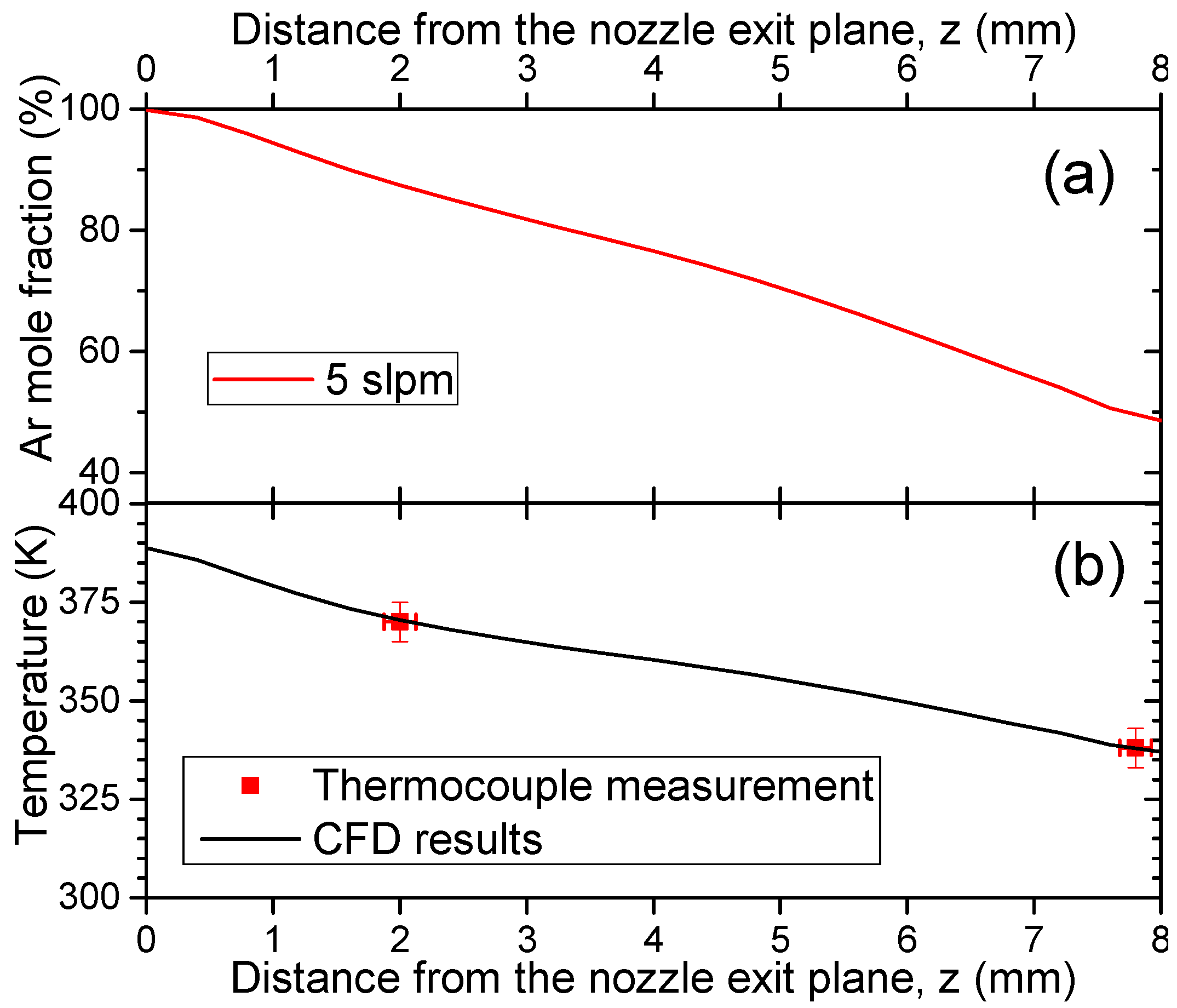
3.1. Gas Temperature and Fluid Flow
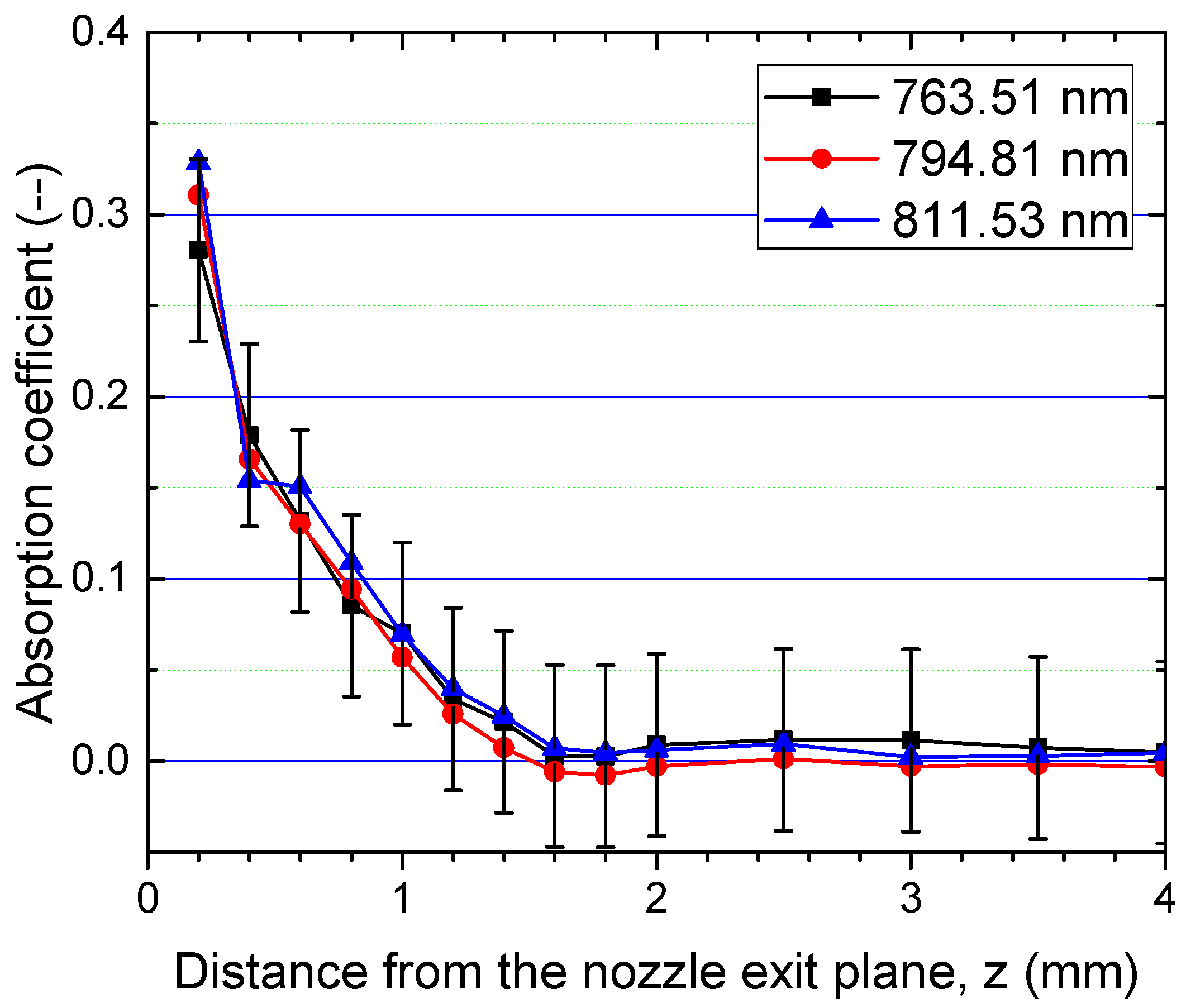
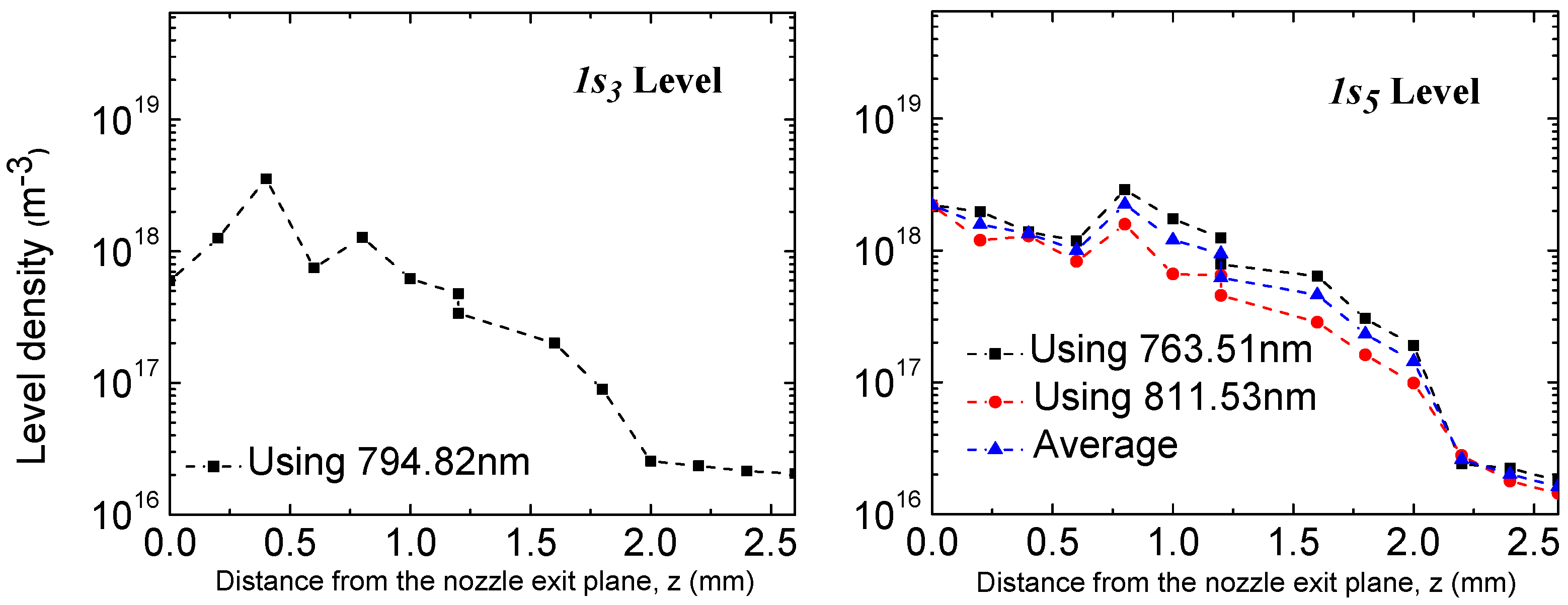
3.2. Spatially-Resolved Ar Level Populations
3.3. Spatially-Resolved Electron Temperature
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Laroussi, M.; Akan, T. Arc-free atmospheric pressure cold plasma jets: A review. Plasma Process. Polym. 2007, 4, 777–788. [Google Scholar] [CrossRef]
- Lacoste, A.; Bourdon, A.; Kuribara, K.; Urabe, K.; Stauss, S.; Terashima, K. Pure air plasma bullets propagating inside microcapillaries and in ambient air. Plasma Sources Sci. Technol. 2014, 23, 062006. [Google Scholar] [CrossRef]
- Van Gessel, B.; Brandenburg, R.; Bruggeman, P.J. Electron properties and air mixing in radio frequency driven argon plasma jets at atmospheric pressure. Appl. Phys. Lett. 2013, 103, 064103. [Google Scholar] [CrossRef]
- Sakiyama, Y.; Graves, D.B.; Jarrige, J.; Laroussi, M. Finite element analysis of ring-shaped emission profile in plasma bullet. Appl. Phys. Lett. 2010, 96, 41501. [Google Scholar] [CrossRef]
- Spiekermeier, S.; Schröder, D.; Gathen, V.S.v.D.; Böke, M.; Winter, J. Helium metastable density evolution in a self-pulsing μ-APPJ. J. Phys. D Appl. Phys. 2015, 48, 35203. [Google Scholar] [CrossRef]
- Boffard, J.B.; Jung, R.O.; Lin, C.C.; Wendt, A.E. Measurement of metastable and resonance level densities in rare-gas plasmas by optical emission spectroscopy. Plasma Sources Sci. Technol. 2009, 18, 035017. [Google Scholar] [CrossRef]
- Benedikt, J.; Hofmann, S.; Knake, N.; Böttner, H.; Reuter, R.; von Keudell, A.; Schulz-von der Gathen, V. Phase resolved optical emission spectroscopy of coaxial microplasma jet operated with He and Ar. Eur. Phys. J. D 2010, 60, 539–546. [Google Scholar] [CrossRef]
- Naidis, G.V. Production of active species in cold helium–air plasma jets. Plasma Sources Sci. Technol. 2014, 23, 065014. [Google Scholar] [CrossRef]
- Walsh, J.L.; Kong, M.G. Room-temperature atmospheric argon plasma jet sustained with submicrosecond high-voltage pulses. Appl. Phys. Lett. 2007, 91, 221502. [Google Scholar] [CrossRef]
- Schneider, S.; Lackmann, J.W.; Ellerweg, D.; Denis, B.; Narberhaus, F.; Bandow, J.E.; Benedikt, J. The role of VUV radiation in the inactivation of bacteria with an atmospheric pressure plasma jet. Plasma Process. Polym. 2012, 9, 561–568. [Google Scholar] [CrossRef]
- Schneider, S.; Lackmann, J.W.; Narberhaus, F.; Bandow, J.E.; Denis, B.; Benedikt, J. Separation of VUV/UV photons and reactive particles in the effluent of a He/O2 atmospheric pressure plasma jet. J. Phys. Appl. Phys. 2011, 44, 379501. [Google Scholar] [CrossRef]
- Nečas, D.; Čudek, V.; Vodák, J.; Ohlídal, M.; Klapetek, P.; Benedikt, J.; Rügner, K.; Zajíčková, L. Mapping of properties of thin plasma jet films using imaging spectroscopic reflectometry. Meas. Sci. Technol. 2014, 25, 115201. [Google Scholar] [CrossRef]
- Joh, H.M.; Choi, J.Y.; Kim, S.J.; Chung, T.H.; Kang, T.H. Effect of additive oxygen gas on cellular response of lung cancer cells induced by atmospheric pressure helium plasma jet. Sci. Rep. 2014, 4, 6638. [Google Scholar] [CrossRef] [PubMed]
- Van Rens, J.F.M.; Schoof, J.T.; Ummelen, F.C.; van Vugt, D.C.; Bruggeman, P.J.; van Veldhuizen, E.M. Induced Liquid Phase Flow by RF Ar Cold Atmospheric Pressure Plasma Jet. IEEE Trans. Plasma Sci. 2014, 42. [Google Scholar] [CrossRef]
- Coulombe, S.; Léveillé, V.; Yonson, S.; Leask, R.L. Miniature atmospheric pressure glow discharge torch (APGD-t) for local biomedical applications. Pure Appl. Chem. 2006, 78, 1147–1156. [Google Scholar] [CrossRef]
- Kelly, S.; Turner, M.M. Power modulation in an atmospheric pressure plasma jet. Plasma Sources Sci. Technol. 2014, 23, 065012. [Google Scholar] [CrossRef]
- Leduc, M.; Coulombe, S.; Leask, R.L. Atmospheric Pressure Plasma Jet Deposition of Patterned Polymer Films for Cell Culture Applications. IEEE Trans. Plasma Sci. 2009, 37, 927–933. [Google Scholar] [CrossRef]
- Walsh, J.L.; Iza, F.; Janson, N.B.; Law, V.J.; Kong, M.G. Three distinct modes in a cold atmospheric pressure plasma jet. J. Phys. D Appl. Phys. 2010, 43, 075201. [Google Scholar] [CrossRef]
- Laroussi, M.; Lu, X. Room-temperature atmospheric pressure plasma plume for biomedical applications. Appl. Phys. Lett. 2005, 87, 28–30. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ballato, J.; Kim, S.O. Intense and energetic atmospheric pressure plasma jet arrays. Plasma Process. Polym. 2012, 9, 253–260. [Google Scholar] [CrossRef]
- Léveillé, V.; Coulombe, S. Design and preliminary characterization of a miniature pulsed RF APGD torch with downstream injection of the source of reactive species. Plasma Sources Sci. Technol. 2005, 14, 467–476. [Google Scholar] [CrossRef]
- Jarrige, J.; Laroussi, M.; Karakas, E. Formation and dynamics of plasma bullets in a non-thermal plasma jet: Influence of the high-voltage parameters on the plume characteristics. Plasma Sources Sci. Technol. 2010, 19. [Google Scholar] [CrossRef]
- Chauvet, L.; Therese, L.; Caillier, B.; Guillot, P. Characterization of an asymmetric DBD plasma jet source at atmospheric pressure. J. Anal. At. Spectrom. 2014, 29, 2050–2057. [Google Scholar] [CrossRef]
- Yonemori, S.; Nakagawa, Y.; Ono, R.; Oda, T. Measurement of OH density and air–helium mixture ratio in an atmospheric-pressure helium plasma jet. J. Phys. D Appl. Phys. 2012, 45, 225202. [Google Scholar] [CrossRef]
- Knake, N.; Reuter, S.; Niemi, K.; Schulz-von der Gathen, V.; Winter, J. Absolute atomic oxygen density distributions in the effluent of a microscale atmospheric pressure plasma jet. J. Phys. D Appl. Phys. 2008, 41, 194006. [Google Scholar] [CrossRef]
- Sarani, A.; De Geyter, N.; Nikiforov, A.Y.; Morent, R.; Leys, C.; Hubert, J.; Reniers, F. Surface modification of PTFE using an atmospheric pressure plasma jet in argon and argon+CO2. Surf. Coat. Technol. 2012, 206, 2226–2232. [Google Scholar] [CrossRef]
- Massines, F.; Sarra-Bournet, C.; Fanelli, F.; Naudé, N.; Gherardi, N. Atmospheric Pressure Low Temperature Direct Plasma Technology: Status and Challenges for Thin Film Deposition. Plasma Process. Polym. 2012, 9, 1041–1073. [Google Scholar] [CrossRef]
- Castaños Martínez, E.; Moisan, M. Absorption spectroscopy measurements of resonant and metastable atom densities in atmospheric-pressure discharges using a low-pressure lamp as a spectral-line source and comparison with a collisional-radiative model. Spectrochim. Acta Part B At. Spectrosc. 2010, 65, 199–209. [Google Scholar] [CrossRef]
- Moussounda, P.S.; Ranson, P. Pressure broadening of argon lines emitted by a high-pressure microwave discharge (Surfatron). J. Phys. B At. Mol. Phys. 1987, 20, 949. [Google Scholar] [CrossRef]
- Kramida, A.; Ralchenko, Y.; Reader, J.; NIST ASD Team. NIST Atomic Spectra Database (ver. 5.0); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012.
- Durocher-Jean, A.; Desjardins, E.; Stafford, L. Characterization of a microwave argon plasma column at atmospheric pressure by optical emission and absorption spectroscopy coupled with collisional-radiative modelling. Phys. Plasmas 2019, 26, 1–13. [Google Scholar] [CrossRef]
- Malyshev, M.V.; Donnelly, V.M. Trace rare gases optical emission spectroscopy: Nonintrusive method for measuring electron temperatures in low-pressure, low-temperature plasmas. Phys. Rev. E 1999, 60, 6016–6029. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Sadeghi, N. Rate coefficients for collisional population transfer between 3p54p argon levels at 300 K. Phys. Rev. A 1978, 18, 1388–1395. [Google Scholar] [CrossRef]
- Chang, R.S.F.; Setser, D.W. Radiative lifetimes and two-body deactivation rate constants for Ar(3p5, 4p) and Ar(3p5,4p’) states. J. Chem. Phys. 1978, 69, 3885–3897. [Google Scholar] [CrossRef]
- Zhu, X.M.; Pu, Y.K. A simple collisional–radiative model for low-temperature argon discharges with pressure ranging from 1 Pa to atmospheric pressure: Kinetics of Paschen 1s and 2p levels. J. Phys. D Appl. Phys. 2009, 43, 015204. [Google Scholar] [CrossRef]
- Van Gaens, W.; Bogaerts, A. Corrigendum: Kinetic modelling for an atmospheric pressure argon plasma jet in humid air. J. Phys. D Appl. Phys. 2014, 47, 079502. [Google Scholar] [CrossRef]
- Laux, C.O.; Spence, T.G.; Kruger, C.H.; Zare, R.N. Optical diagnostics of atmospheric pressure air plasmas. Plasma Sources Sci. Technol. 2003, 12, 125–138. [Google Scholar] [CrossRef]
- Specair Software. Available online: www.specair-radiation.net (accessed on 3 October 2019).
- Poirier, J.S.; Bérubé, P.M.; Muñoz, J.; Margot, J.; Stafford, L.; Chaker, M. On the validity of neutral gas temperature by N 2 rovibrational spectroscopy in low-pressure inductively coupled plasmas. Plasma Sources Sci. Technol. 2011, 20. [Google Scholar] [CrossRef]
- ANSYS FLUENT 14.5, Theory Guide; ANSYS, Inc.: Canonsburg, PA, USA, 2012.
- Launder, B.E.; Spalding, D.B. Lectures in Mathematical Models of Turbulence; Academic Press: London, UK, 1972. [Google Scholar]
- Niermann, B.; Reuter, R.; Kuschel, T.; Benedikt, J.; Böke, M.; Winter, J. Argon metastable dynamics in a filamentary jet micro-discharge at atmospheric pressure. Plasma Sources Sci. Technol. 2012, 21, 034002. [Google Scholar] [CrossRef]
- Niermann, B.; Böke, M.; Sadeghi, N.; Winter, J. Space resolved density measurements of argon and helium metastable atoms in radio-frequency generated He-Ar micro-plasmas. Eur. Phys. J. D 2010, 60, 489–495. [Google Scholar] [CrossRef]
- Sands, B.L.; Leiweke, R.J.; Ganguly, B.N. Spatiotemporally resolved Ar (1s5) metastable measurements in a streamer-like He/Ar atmospheric pressure plasma jet. J. Phys. D Appl. Phys. 2010, 43, 282001. [Google Scholar] [CrossRef]
- Zhu, X.M.; Pu, Y.K. Optical emission spectroscopy in low-temperature plasmas containing argon and nitrogen: Determination of the electron temperature and density by the line-ratio method. J. Phys. D Appl. Phys. 2010, 43, 403001. [Google Scholar] [CrossRef]
- Förster, S.; Mohr, C.; Viöl, W. Investigations of an atmospheric pressure plasma jet by optical emission spectroscopy. Surf. Coat. Technol. 2005, 200, 827–830. [Google Scholar] [CrossRef]
- Sismanoglu, B.N.; Amorim, J.; Souza-Corrêa, J.A.; Oliveira, C.; Gomes, M.P. Optical emission spectroscopy diagnostics of an atmospheric pressure direct current microplasma jet. Spectrochim. Acta Part B At. Spectrosc. 2009, 64, 1287–1293. [Google Scholar] [CrossRef]
- Li, S.Z.; Lim, J.P.; Uhm, H.S. Discharge characteristics of an atmospheric-pressure capacitively coupled radio-frequency argon plasmas. Phys. Lett. Sect. A Gen. At. Solid State Phys. 2006, 360, 304–308. [Google Scholar] [CrossRef]
- Palomares, J.M.; Iordanova, E.I.; Gamero, A.; Sola, A.; Mullen, J.J.A.M.V.D. Mixtures Studied With a Combination of Passive and Active Spectroscopic Methods. J. Phys. D Appl. Phys. 2010, 43, 395202. [Google Scholar] [CrossRef]
- Iordanova, E.; Palomares, J.M.; Gamero, A.; Sola, A.; van der Mullen, J.J.A.M. A novel method to determine the electron temperature and density from the absolute intensity of line and continuum emission: Application to atmospheric microwave induced Ar plasmas. J. Phys. D Appl. Phys. 2009, 42, 155208. [Google Scholar] [CrossRef]
- Hübner, S.; Hofmann, S.; van Veldhuizen, E.M.; Bruggeman, P.J. Electron densities and energies of a guided argon streamer in argon and air environments. Plasma Sources Sci. Technol. 2013, 22, 65011–650118. [Google Scholar] [CrossRef]
- Farouk, T.; Farouk, B.; Gutsol, A.; Fridman, A. Atmospheric pressure radio frequency glow discharges in argon: Effects of external matching circuit parameters. Plasma Sources Sci. Technol. 2008, 17, 35015. [Google Scholar] [CrossRef]





| Ref. | Gas | Power (W) | (m) | (m) |
|---|---|---|---|---|
| [42] | Ar | n.a. | - | 10 |
| [44] | He/Ar | n.a. | - | 10 |
| [43] | He/Ar | 23 | - | 10 |
| [36] | Ar | 6.5 | 10 | 10 |
| This work | Ar | 40 | 10 | 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sainct, F.P.; Durocher-Jean, A.; Gangwar, R.K.; Mendoza Gonzalez, N.Y.; Coulombe, S.; Stafford, L. Spatially-Resolved Spectroscopic Diagnostics of a Miniature RF Atmospheric Pressure Plasma Jet in Argon Open to Ambient Air. Plasma 2020, 3, 38-53. https://doi.org/10.3390/plasma3020005
Sainct FP, Durocher-Jean A, Gangwar RK, Mendoza Gonzalez NY, Coulombe S, Stafford L. Spatially-Resolved Spectroscopic Diagnostics of a Miniature RF Atmospheric Pressure Plasma Jet in Argon Open to Ambient Air. Plasma. 2020; 3(2):38-53. https://doi.org/10.3390/plasma3020005
Chicago/Turabian StyleSainct, Florent P., Antoine Durocher-Jean, Reetesh Kumar Gangwar, Norma Yadira Mendoza Gonzalez, Sylvain Coulombe, and Luc Stafford. 2020. "Spatially-Resolved Spectroscopic Diagnostics of a Miniature RF Atmospheric Pressure Plasma Jet in Argon Open to Ambient Air" Plasma 3, no. 2: 38-53. https://doi.org/10.3390/plasma3020005
APA StyleSainct, F. P., Durocher-Jean, A., Gangwar, R. K., Mendoza Gonzalez, N. Y., Coulombe, S., & Stafford, L. (2020). Spatially-Resolved Spectroscopic Diagnostics of a Miniature RF Atmospheric Pressure Plasma Jet in Argon Open to Ambient Air. Plasma, 3(2), 38-53. https://doi.org/10.3390/plasma3020005





