Plasma Farming: Non-Thermal Dielectric Barrier Discharge Plasma Technology for Improving the Growth of Soybean Sprouts and Chickens
Abstract
1. Introduction
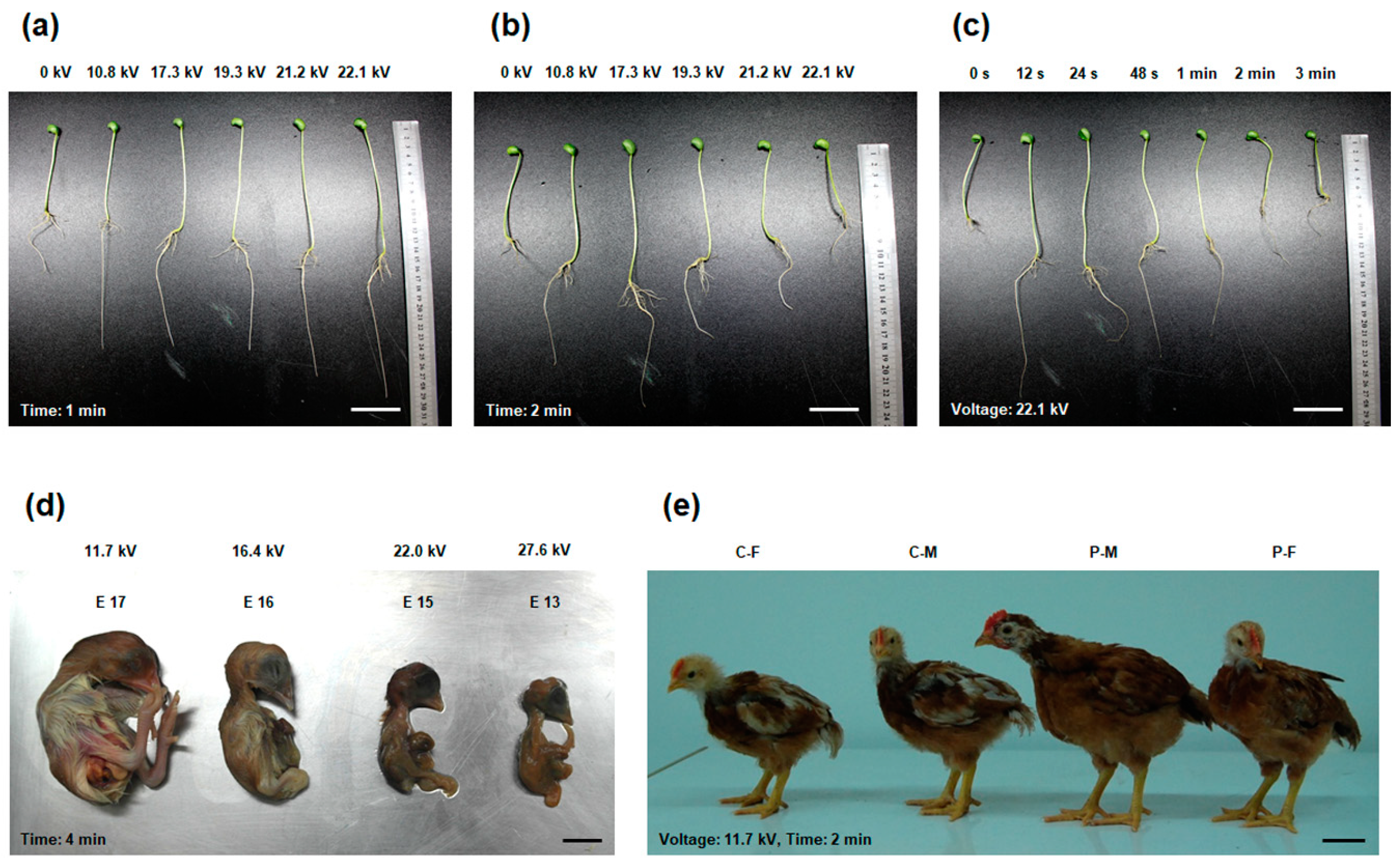
2. Effect of Plasma on the Growth of Soybean Sprouts
3. Plasma Application in Chicken Growth
4. Plasma Improves Male Chicken Fertility
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.D. Plasmas for medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Natalia Yu, B.; Mark, J.K. Intracellular electric fields produced by dielectric barrier discharge treatment of skin. J. Phys. D Appl. Phys. 2010, 43, 185206. [Google Scholar] [CrossRef]
- Liu, D.X.; Yang, A.J.; Wang, X.H.; Rong, M.Z.; Iza, F.; Kong, M.G. Wall fluxes of reactive oxygen species of an rf atmospheric-pressure plasma and their dependence on sheath dynamics. J. Phys. D Appl. Phys. 2012, 45, 305205. [Google Scholar] [CrossRef]
- David, B.G. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012, 45, 263001. [Google Scholar] [CrossRef]
- Beate, H.; Thomas von, W.; Klaus-Dieter, W.; Ulrike, L. Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomol. Ther. 2014, 22, 477–490. [Google Scholar] [CrossRef]
- Conrads, H.; Schmidt, M. Plasma generation and plasma sources. Plasma Sources Sci. Technol. 2000, 9, 441. [Google Scholar] [CrossRef]
- Isbary, G.; Shimizu, T.; Li, Y.F.; Stolz, W.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L. Cold atmospheric plasma devices for medical issues. Expert Rev. Med. Devices 2013, 10, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Yousfi, M.; Merbahi, N.; Pathak, A.; Eichwald, O. Low-temperature plasmas at atmospheric pressure: Toward new pharmaceutical treatments in medicine. Fundam. Clin. Pharmacol. 2013, 28, 123–135. [Google Scholar] [CrossRef]
- Setsuhara, Y. Low-temperature atmospheric-pressure plasma sources for plasma medicine. Arch. Biochem. Biophys. 2016, 605, 3–10. [Google Scholar] [CrossRef]
- Weltmann, K.D.; Metelmann, H.R.; von Woedtke, T. Low temperature plasma applications in medicine. Eur. News 2016, 47, 39–42. [Google Scholar] [CrossRef]
- Begum, A.; Laroussi, M.; Pervez, M.R. Atmospheric pressure He-air plasma jet: Breakdown process and propagation phenomenon. AIP Adv. 2013, 3, 062117. [Google Scholar] [CrossRef]
- Chong, L.; Danil, D.; Alexander, F. Uniform and non-uniform modes of nanosecond-pulsed dielectric barrier discharge in atmospheric air: Fast imaging and spectroscopic measurements of electric fields. J. Phys. D Appl. Phys. 2014, 47, 252003. [Google Scholar] [CrossRef]
- Starikovskaia, S.M.; Allegraud, K.; Guaitella, O.; Rousseau, A. On electric field measurements in surface dielectric barrier discharge. J. Phys. D Appl. Phys. 2010, 43, 124007. [Google Scholar] [CrossRef]
- Obradović, B.M.; Cvetanović, N.; Ivković, S.S.; Sretenović, G.B.; Kovačević, V.V.; Krstić, I.B.; Kuraica, M.M. Methods for spectroscopic measurement of electric field in atmospheric pressure helium discharges. Eur. Phys. J. Appl. Phys. 2017, 77, 30802. [Google Scholar] [CrossRef]
- Robert, E.; Darny, T.; Dozias, S.; Iseni, S.; Pouvesle, J.M. New insights on the propagation of pulsed atmospheric plasma streams: From single jet to multi jet arrays. Phys. Plasmas 2015, 22, 122007. [Google Scholar] [CrossRef]
- Bourdon, A.; Darny, T.; Pechereau, F.; Pouvesle, J.-M.; Viegas, P.; Iséni, S.; Robert, E. Numerical and experimental study of the dynamics of a μs helium plasma gun discharge with various amounts of N2 admixture. Plasma Sources Sci. Technol. 2016, 25, 035002. [Google Scholar] [CrossRef]
- Zhang, J.J.; Jo, J.O.; Huynh, D.L.; Mongre, R.K.; Ghosh, M.; Singh, A.K.; Lee, S.B.; Mok, Y.S.; Hyuk, P.; Jeong, D.K. Growth-inducing effects of argon plasma on soybean sprouts via the regulation of demethylation levels of energy metabolism-related genes. Sci. Rep. 2017, 7, 41917. [Google Scholar] [CrossRef]
- Zhang, J.J.; Jo, J.O.; Huynh, D.L.; Ghosh, M.; Kim, N.; Lee, S.B.; Lee, H.K.; Mok, Y.S.; Kwon, T.; Jeong, D.K. Lethality of inappropriate plasma exposure on chicken embryonic development. Oncotarget 2017, 8, 85642–85654. [Google Scholar] [CrossRef]
- Zhang, J.J.; Wang, X.Z.; Kwon, T.; Huynh, D.L.; Chandimali, N.; Kim, N.; Kang, T.Y.; Ghosh, M.; Gera, M.; Lee, S.B.; et al. Innovative approach of non-thermal plasma application for improving the growth rate in chickens. Int. J. Mol. Sci. 2018, 19, 2301. [Google Scholar] [CrossRef]
- Zhang, J.J.; Huynh, D.L.; Chandimali, N.; Kang, T.Y.; Kim, N.; Mok, Y.S.; Kwon, T.; Jeong, D.K. Growth and male reproduction improvement of non-thermal dielectric barrier discharge plasma treatment on chickens. J. Phys. D Appl. Phys. 2018, 51, 205201. [Google Scholar] [CrossRef]
- Zhang, J.J.; Do, H.L.; Chandimali, N.; Lee, S.B.; Mok, Y.S.; Kim, N.; Kim, S.B.; Kwon, T.; Jeong, D.K. Non-thermal plasma treatment improves chicken sperm motility via the regulation of demethylation levels. Sci. Rep. 2018, 8, 7576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Wang, X.Z.; Luong Do, H.; Chandimali, N.; Kang, T.Y.; Kim, N.; Ghosh, M.; Lee, S.B.; Mok, Y.S.; Kim, S.B.; et al. MicroRNA-7450 regulates non-thermal plasma-induced chicken Sertoli cell apoptosis via adenosine monophosphate-activated protein kinase activation. Sci. Rep. 2018, 8, 8761. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.F.; He, X.; Li, L.; Li, J.G.; Shao, H.L.; Xu, Q.L.; Ye, R.H.; Dong, Y.H. Effect of cold plasma treatment on seed germination and growth of wheat. Plasma Sci. Technol. 2014, 16, 54. [Google Scholar] [CrossRef]
- Szili, E.J.; Hong, S.-H.; Oh, J.-S.; Gaur, N.; Short, R.D. Tracking the penetration of plasma reactive species in tissue models. Trends Biotechnol. 2018, 36, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Mraz, I.; Beran, P.; Sera, B.; Gavril, B.; Eugen, H. Effect of low-temperature plasma treatment on the growth and reproduction rate of some plant pathogenic bacteria. J. Plant Pathol. 2014, 96, 1–5. [Google Scholar] [CrossRef]
- Jiang, J.F.; Lu, Y.F.; Li, J.G.; Li, L.; He, X.; Shao, H.L.; Dong, Y.H. Effect of seed treatment by cold plasma on the resistance of tomato to Ralstonia solanacearum (Bacterial Wilt). PLoS ONE 2014, 9, e97753. [Google Scholar] [CrossRef]
- Tong, J.; He, R.; Zhang, X.; Zhan, R.; Chen, W.; Yang, S. Effects of atmospheric pressure air plasma pretreatment on the seed germination and early growth of andrographis paniculata. Plasma Sci. Technol. 2014, 16, 260. [Google Scholar] [CrossRef]
- Ling, L.; Jiafeng, J.; Jiangang, L.; Minchong, S.; Xin, H.; Hanliang, S.; Yuanhua, D. Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci. Rep. 2014, 4, 5859. [Google Scholar] [CrossRef]
- Sarinont, T.; Amano, T.; Kitazaki, S.; Koga, K.; Uchida, G.; Shiratani, M.; Hayashi, N. Growth enhancement effects of radish sprouts: Atmospheric pressure plasma irradition vs. heat shock. J. Phys. Conf. Ser. 2014, 518, 12–17. [Google Scholar] [CrossRef]
- Satoshi, K.; Kazunori, K.; Masaharu, S.; Nobuya, H. Growth enhancement of radish sprouts induced by low pressure O2 radio frequency discharge plasma irradiation. Jpn. J. Appl. Phys. 2012, 51, 01AE01. [Google Scholar] [CrossRef]
- Bormashenko, E.; Shapira, Y.; Grynyov, R.; Whyman, G.; Bormashenko, Y.; Drori, E. Interaction of cold radiofrequency plasma with seeds of beans (Phaseolus vulgaris). J. Exp. Bot. 2015, 66, 4013–4021. [Google Scholar] [CrossRef] [PubMed]
- Sera, B.; Sery, M.; Stranak, V.; Spatenka, P.; Tichy, M. Does cold plasma affect breaking dormancy and seed germination? A study on seeds of lamb’s quarters (Chenopodium album agg.). Plasma Sci. Technol. 2009, 11, 750. [Google Scholar] [CrossRef]
- Volin, J.C.; Denes, F.S.; Young, R.A.; Park, S.M.T. Modification of seed germination performance through cold plasma chemistry technology. Crop Sci. 2000, 40, 1706–1718. [Google Scholar] [CrossRef]
- Zhang, B.; Li, R.; Yan, J. Study on activation and improvement of crop seeds by the application of plasma treating seeds equipment. Arch. Biochem. Biophys. 2018, 655, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Sarinont, T.; Amano, T.; Koga, K.; Shiratani, M.; Hayashi, N. Multigeneration effects of plasma irradiation to seeds of Arabidopsis Thaliana and Zinnia on their growth. MRS Online Proc. Libr. 2015, 1723, mrsf14-1723-g03-04. [Google Scholar] [CrossRef]
- Ji, S.-H.; Choi, K.-H.; Pengkit, A.; Im, J.S.; Kim, J.S.; Kim, Y.H.; Park, Y.; Hong, E.J.; Jung, S.K.; Choi, E.-H.; et al. Effects of high voltage nanosecond pulsed plasma and micro DBD plasma on seed germination, growth development and physiological activities in spinach. Arch. Biochem. Biophys. 2016, 605, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Huang, Y.; Yang, S.; Chen, W. Introduction of a new atmospheric pressure plasma device and application on tomato seeds. Agric. Sci. 2011, 2, 23–27. [Google Scholar] [CrossRef]
- Henselova, M.; Slovakova, L.; Martinka, M.; Zahoranova, A. Growth, anatomy and enzyme activity changes in maize roots induced by treatment of seeds with low-temperature plasma. Biologia 2012, 67, 490–497. [Google Scholar] [CrossRef]
- Dhayal, M.; Lee, S.-Y.; Park, S.-U. Using low-pressure plasma for Carthamus tinctorium L. seed surface modification. Vacuum 2006, 80, 499–506. [Google Scholar] [CrossRef]
- Yin, M.Q.; Huang, M.J.; Ma, B.Z.; Ma, T.C. Stimulating effects of seed treatment by magnetized plasma on tomato growth and yield. Plasma Sci. Technol. 2005, 7, 3143–3147. [Google Scholar] [CrossRef]
- Perl, M. ATP synthesis and utilization in the early stage of seed germination in relation to seed dormancy and quality. Physiol. Plant. 1986, 66, 177–182. [Google Scholar] [CrossRef]
- Deprost, D.; Yao, L.; Sormani, R.; Moreau, M.; Leterreux, G.; Nicolai, M.; Bedu, M.; Robaglia, C.; Meyer, C. The Arabidopsis TOR kinase links plant growth, yield, stress resistance and mRNA translation. EMBO Rep. 2007, 8, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Omidbakhshfard, M.A.; Proost, S.; Fujikura, U.; Mueller-Roeber, B. Growth-regulating factors (GRFs): A small transcription factor family with important functions in plant biology. Mol. Plant 2015, 8, 998–1010. [Google Scholar] [CrossRef] [PubMed]
- Kavlock, R.J.; Daston, G.P. Drug Toxicity in Embryonic Development I: Advances in Understanding Mechanisms of Birth Defects: Morphogenesis and Processes at Risk, 1st ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1997; pp. 519–548. ISBN 978-3-642-60445-4. [Google Scholar]
- Kalghatgi, S.; Kelly, C.M.; Cerchar, E.; Torabi, B.; Alekseev, O.; Fridman, A.; Friedman, G.; Azizkhan-Clifford, J. Effects of non-thermal plasma on mammalian cells. PLoS ONE 2011, 6, e16270. [Google Scholar] [CrossRef] [PubMed]
- Kalghatgi, S.; Friedman, G.; Fridman, A.; Clyne, A.M. Endothelial cell proliferation is enhanced by low dose non-thermal plasma through fibroblast growth factor-2 release. Ann. Biomed. Eng. 2010, 38, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Chernets, N.; Zhang, J.; Steinbeck, M.J.; Kurpad, D.S.; Koyama, E.; Friedman, G.; Freeman, T.A. Nonthermal atmospheric pressure plasma enhances mouse limb bud survival, growth, and elongation. Tissue Eng. Part A 2015, 21, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Kieft, I.E.; Kurdi, M.; Stoffels, E. Reattachment and apoptosis after plasma-needle treatment of cultured cells. IEEE Trans. Plasma Sci. 2006, 34, 1331–1336. [Google Scholar] [CrossRef]
- Nakai, N.; Fujita, R.; Kawano, F.; Takahashi, K.; Ohira, T.; Shibaguchi, T.; Nakata, K.; Ohira, Y. Retardation of C2C12 myoblast cell proliferation by exposure to low-temperature atmospheric plasma. J. Physiol. Sci. 2014, 64, 365–375. [Google Scholar] [CrossRef]
- Balzer, J.; Heuer, K.; Demir, E.; Hoffmanns, M.A.; Baldus, S.; Fuchs, P.C.; Awakowicz, P.; Suschek, C.V.; Opländer, C. Non-thermal dielectric barrier discharge (DBD) effects on proliferation and differentiation of human fibroblasts are primary mediated by hydrogen peroxide. PLoS ONE 2015, 10, e0144968. [Google Scholar] [CrossRef]
- Haertel, B.; Volkmann, F.; von Woedtke, T.; Lindequist, U. Differential sensitivity of lymphocyte subpopulations to non-thermal atmospheric-pressure plasma. Immunobiology 2012, 217, 628–633. [Google Scholar] [CrossRef]
- Jia, H.; Fujiwara, H.; Kondo, M.; Kuraseko, H. Optical emission spectroscopy of atmospheric pressure microwave plasmas. J. Appl. Phys. 2008, 104, 054908. [Google Scholar] [CrossRef]
- Hincke, M.T.; Nys, Y.; Gautron, J.; Mann, K.; Rodriguez-Navarro, A.B.; McKee, M.D. The eggshell: Structure, composition and mineralization. Front. Biosci. 2012, 17, 1266–1280. [Google Scholar] [CrossRef]
- Kuchenbecker, M.; Bibinov, N.; Kaemlimg, A.; Wandke, D.; Awakowicz, P.; Viol, W. Characterization of DBD plasma source for biomedical applications. J. Phys. D Appl. Phys. 2009, 42, 045212. [Google Scholar] [CrossRef]
- Fridman, A.; Chirokov, A.; Gutsol, A. Non-thermal atmospheric pressure discharges. J. Phys. D Appl. Phys. 2005, 38, R1–R24. [Google Scholar] [CrossRef]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied plasma medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Lin, A.; Truong, B.; Patel, S.; Kaushik, N.; Choi, E.H.; Fridman, G.; Fridman, A.; Miller, V. Nanosecond-pulsed DBD plasma-generated reactive oxygen species trigger immunogenic cell death in A549 lung carcinoma cells through intracellular oxidative stress. Int. J. Mol. Sci. 2017, 18, 966. [Google Scholar] [CrossRef]
- Yan, X.; Xiong, Z.; Zou, F.; Zhao, S.; Lu, X.; Yang, G.; He, G.; Ostrikov, K. Plasma-induced death of HepG2 cancer cells: Intracellular effects of reactive species. Plasma Process. Polym. 2011, 9, 59–66. [Google Scholar] [CrossRef]
- Steinbeck, M.J.; Chernets, N.; Zhang, J.; Kurpad, D.S.; Fridman, G.; Fridman, A.; Freeman, T.A. Skeletal cell differentiation is enhanced by atmospheric dielectric barrier discharge plasma treatment. PLoS ONE 2013, 8, e82143. [Google Scholar] [CrossRef]
- Blackert, S.; Haertel, B.; Wende, K.; von Woedtke, T.; Lindequist, U. Influence of non-thermal atmospheric pressure plasma on cellular structures and processes in human keratinocytes (HaCaT). J. Dermatol. Sci. 2013, 70, 173–181. [Google Scholar] [CrossRef]
- Ma, Y.; Ha, C.S.; Hwang, S.W.; Lee, H.J.; Kim, G.C.; Lee, K.W.; Song, K. Non-thermal atmospheric pressure plasma preferentially induces apoptosis in p53-mutated cancer cells by activating ROS stress-response pathways. PLoS ONE 2014, 9, e91947. [Google Scholar] [CrossRef]
- Kim, K.C.; Piao, M.J.; Hewage, S.R.K.M.; Han, X.I.A.; Kang, K.A.; Jo, J.O.; Mok, Y.S.; Shin, J.H.; Park, Y.; Yoo, S.J.; et al. Non-thermal dielectric-barrier discharge plasma damages human keratinocytes by inducing oxidative stress. Int. J. Mol. Med. 2016, 37, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Schröder, K.; Zhang, M.; Benkhoff, S.; Mieth, A.; Pliquett, R.; Kosowski, J.; Kruse, C.; Luedike, P.; Michaelis, U.R.; Weissmann, N.; et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res. 2012, 110, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, J.; Ago, T.; Matsushima, S.; Zhai, P.; Schneider, M.D.; Sadoshima, J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc. Natl. Acad. Sci. USA 2010, 107, 15565–15570. [Google Scholar] [CrossRef] [PubMed]
- Ershova, E.S.; Sergeeva, V.A.; Chausheva, A.I.; Zheglo, D.G.; Nikitina, V.A.; Smirnova, T.D.; Kameneva, L.V.; Porokhovnik, L.N.; Kutsev, S.I.; Troshin, P.A.; et al. Toxic and DNA damaging effects of a functionalized fullerene in human embryonic lung fibroblasts. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 805, 46–57. [Google Scholar] [CrossRef]
- Cullinan, S.B.; Zhang, D.; Hannink, M.; Arvisais, E.; Kaufman, R.J.; Diehl, J.A. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell. Boil. 2003, 23, 7198–7209. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Boil. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Kalghatgi, S.; Fridman, A.; Azizkhan-Clifford, J.; Friedman, G. DNA damage in mammalian cells by non-thermal atmospheric pressure microsecond pulsed dielectric barrier discharge plasma is not mediated by ozone. Plasma Process. Polym. 2012, 9, 726–732. [Google Scholar] [CrossRef]
- Leduc, M.; Guay, D.; Coulombe, S.; Leask, R.L. Effects of non-thermal plasmas on DNA and mammalian cells. Plasma Process. Polym. 2010, 7, 899–909. [Google Scholar] [CrossRef]
- Yasuda, H.; Miura, T.; Kurita, H.; Takashima, K.; Mizuno, A. Biological evaluation of DNA damage in bacteriophages inactivated by atmospheric pressure cold plasma. Plasma Process. Polym. 2010, 7, 301–308. [Google Scholar] [CrossRef]
- O’Connell, D.; Cox, L.J.; Hyland, W.B.; McMahon, S.J.; Reuter, S.; Graham, W.G.; Gans, T.; Currell, F.J. Cold atmospheric pressure plasma jet interactions with plasmid DNA. Appl. Phys. Lett. 2011, 98, 043701. [Google Scholar] [CrossRef]
- Kim, G.J.; Kim, W.; Kim, K.T.; Lee, J.K. DNA damage and mitochondria dysfunction in cell apoptosis induced by nonthermal air plasma. Appl. Phys. Lett. 2010, 96, 021502. [Google Scholar] [CrossRef]
- Bahnev, B.; Bowden, M.D.; Stypczyńska, A.; Ptasińska, S.; Mason, N.J.; Braithwaite, N.S.J. A novel method for the detection of plasma jet boundaries by exploring DNA damage. Eur. Phys. J. D 2014, 68, 140. [Google Scholar] [CrossRef]
- Kalghatgi, S.; Azizkhan-Clifford, J. DNA damage in mammalian cells by non-thermal atmospheric pressure microsecond pulsed dielectric barrier discharge plasma is not mediated via lipid peroxidation. Plasma Med. 2011, 1, 167–177. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schmidt, A.; Kramer, A.; Metelmann, H.-R.; Adler, F.; von Woedtke, T.; Niessner, F.; Weltmann, K.-D.; Wende, K. High throughput image cytometry micronucleus assay to investigate the presence or absence of mutagenic effects of cold physical plasma. Environ. Mol. Mutagen. 2018, 59, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Strassenburg, S.; Greim, U.; Bussiahn, R.; Haertel, B.; Wende, K.; von Woedtke, T.; Lindequist, U. Comparison of biological effects on human keratinocytes using different plasma treatment regimes. Plasma Med. 2013, 3, 57–69. [Google Scholar] [CrossRef]
- Wende, K.; Straßenburg, S.; Haertel, B.; Harms, M.; Holtz, S.; Barton, A.; Masur, K.; von Woedtke, T.; Lindequist, U. Atmospheric pressure plasma jet treatment evokes transient oxidative stress in HaCaT keratinocytes and influences cell physiology. Cell Boil. Int. 2013, 38, 412–425. [Google Scholar] [CrossRef]
- Wende, K.; Bekeschus, S.; Schmidt, A.; Jatsch, L.; Hasse, S.; Weltmann, K.D.; Masur, K.; von Woedtke, T. Risk assessment of a cold argon plasma jet in respect to its mutagenicity. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 798–799, 48–54. [Google Scholar] [CrossRef]
- Veldhuis, J.D.; Roemmich, J.N.; Richmond, E.J.; Rogol, A.D.; Lovejoy, J.C.; Sheffield-Moore, M.; Mauras, N.; Bowers, C.Y. Endocrine control of body composition in infancy, childhood, and puberty. Endocr. Rev. 2005, 26, 114–146. [Google Scholar] [CrossRef]
- Kita, K.; Nagao, K.; Okumura, J. Nutritional and tissue specificity of IGF-I and IGFBP-2 gene expression in growing chickens. Asian Australas. J. Anim. Sci. 2005, 18, 747–754. [Google Scholar] [CrossRef]
- Bassett, J.H.D.; Williams, G.R. Role of thyroid hormones in skeletal development and bone maintenance. Endocr. Rev. 2016, 37, 135–187. [Google Scholar] [CrossRef] [PubMed]
- Kadi, F. Cellular and molecular mechanisms responsible for the action of testosterone on human skeletal muscle. A basis for illegal performance enhancement. Br. J. Pharmacol. 2008, 154, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, I.Y.; Lebedev, V.A.; Grossmann, R.; Parvizi, N. Age-dependent role of steroids in the regulation of growth of the hen follicular wall. Reprod. Boil. Endocrinol. 2010, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Wistedt, A.; Ridderstrale, Y.; Wall, H.; Holm, L. Exogenous estradiol improves shell strength in laying hens at the end of the laying period. Acta Vet. Scand. 2014, 56, 34. [Google Scholar] [CrossRef] [PubMed]
- Bacon, W.L.; Liu, H.-K. Progesterone injection and egg production in Turkey Hens. Boil. Reprod. 2004, 71, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Janczak, A.M.; Torjesen, P.; Palme, R.; Bakken, M. Effects of stress in hens on the behaviour of their offspring. Appl. Anim. Behav. Sci. 2007, 107, 66–77. [Google Scholar] [CrossRef]
- Dixon, L.M.; Sparks, N.H.C.; Rutherford, K.M.D. Early experiences matter: A review of the effects of prenatal environment on offspring characteristics in poultry. Poult. Sci. 2016, 95, 489–499. [Google Scholar] [CrossRef]
- Khan, R.U. Antioxidants and poultry semen quality. World’s Poult. Sci. J. 2011, 67, 297–308. [Google Scholar] [CrossRef]
- Misro, M.M.; Ramya, T. Fuel/Energy Sources of Spermatozoa. In Male Infertility: Contemporary Clinical Approaches, Andrology, ART & Antioxidants, 1st ed.; Parekattil, S.J., Agarwal, A., Eds.; Springer: New York, NY, USA, 2012; pp. 209–223. ISBN 978-1-4614-3335-4. [Google Scholar]
- Nguyen, T.M.; Alves, S.; Grasseau, I.; Metayer-Coustard, S.; Praud, C.; Froment, P.; Blesbois, E. Central role of 5′-AMP-activated protein kinase in chicken sperm functions. Biol. Reprod. 2014, 91, 121. [Google Scholar] [CrossRef]
- Bansal, A.K.; Bilaspuri, G.S. Impacts of oxidative stress and antioxidants on semen functions. Vet. Med. Int. 2011, 2011, 686137. [Google Scholar] [CrossRef]
- Tuncer, P.B.; Bucak, M.N.; Sarıözkan, S.; Sakin, F.; Yeni, D.; Çiğerci, İ.H.; Ateşşahin, A.; Avdatek, F.; Gündoğan, M.; Büyükleblebici, O. The effect of raffinose and methionine on frozen/thawed Angora buck (Capra hircus ancryrensis) semen quality, lipid peroxidation and antioxidant enzyme activities. Cryobiology 2010, 61, 89–93. [Google Scholar] [CrossRef]
- Guthrie, H.D.; Welch, G.R. Effects of reactive oxygen species on sperm function. Theriogenology 2012, 78, 1700–1708. [Google Scholar] [CrossRef]
- Rui, B.R.; Shibuya, F.Y.; Kawaoku, A.J.T.; Losano, J.D.A.; Angrimani, D.S.R.; Dalmazzo, A.; Nichi, M.; Pereira, R.J.G. Impact of induced levels of specific free radicals and malondialdehyde on chicken semen quality and fertility. Theriogenology 2017, 90, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Montjean, D.; Zini, A.; Ravel, C.; Belloc, S.; Dalleac, A.; Copin, H.; Boyer, P.; McElreavey, K.; Benkhalifa, M. Sperm global DNA methylation level: Association with semen parameters and genome integrity. Andrology 2015, 3, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Cassuto, N.G.; Montjean, D.; Siffroi, J.P.; Bouret, D.; Marzouk, F.; Copin, H.; Benkhalifa, M. Different levels of DNA methylation detected in human sperms after morphological selection using high magnification microscopy. Biomed. Res. Int. 2016, 2016, 6372171. [Google Scholar] [CrossRef] [PubMed]
- Houshdaran, S.; Cortessis, V.K.; Siegmund, K.; Yang, A.; Laird, P.W.; Sokol, R.Z. Widespread epigenetic abnormalities suggest a broad DNA methylation erasure defect in abnormal human sperm. PLoS ONE 2007, 2, e1289. [Google Scholar] [CrossRef] [PubMed]
- Montjean, D.; De La Grange, P.; Gentien, D.; Rapinat, A.; Belloc, S.; Cohen-Bacrie, P.; Menezo, Y.; Benkhalifa, M. Sperm transcriptome profiling in oligozoospermia. J. Assist. Reprod. Genet. 2012, 29, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Aston, K.I.; Uren, P.J.; Jenkins, T.G.; Horsager, A.; Cairns, B.R.; Smith, A.D.; Carrell, D.T. Aberrant sperm DNA methylation predicts male fertility status and embryo quality. Fertil. Steril. 2015, 104, 1388–1397. [Google Scholar] [CrossRef]
- Barzideh, J.; Scott, R.J.; Aitken, R.J. Analysis of the global methylation status of human spermatozoa and its association with the tendency of these cells to enter apoptosis. Andrologia 2013, 45, 424–429. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.J.; Kwon, T.; Kim, S.B.; Jeong, D.K. Plasma Farming: Non-Thermal Dielectric Barrier Discharge Plasma Technology for Improving the Growth of Soybean Sprouts and Chickens. Plasma 2018, 1, 285-296. https://doi.org/10.3390/plasma1020025
Zhang JJ, Kwon T, Kim SB, Jeong DK. Plasma Farming: Non-Thermal Dielectric Barrier Discharge Plasma Technology for Improving the Growth of Soybean Sprouts and Chickens. Plasma. 2018; 1(2):285-296. https://doi.org/10.3390/plasma1020025
Chicago/Turabian StyleZhang, Jiao Jiao, Taeho Kwon, Seong Bong Kim, and Dong Kee Jeong. 2018. "Plasma Farming: Non-Thermal Dielectric Barrier Discharge Plasma Technology for Improving the Growth of Soybean Sprouts and Chickens" Plasma 1, no. 2: 285-296. https://doi.org/10.3390/plasma1020025
APA StyleZhang, J. J., Kwon, T., Kim, S. B., & Jeong, D. K. (2018). Plasma Farming: Non-Thermal Dielectric Barrier Discharge Plasma Technology for Improving the Growth of Soybean Sprouts and Chickens. Plasma, 1(2), 285-296. https://doi.org/10.3390/plasma1020025





