1. Introduction
Implant-associated infections are considered a critical complication in orthopedic, dental, and surgical practice [
1,
2]. Despite advances in surgical asepsis and systemic antibiotic prophylaxis, the incidence of peri-implant infections ranges between 2% and 5% in joint replacements and can be considerably higher in trauma-related cases [
3,
4]. These infections not only compromise implant stability and tissue integration but also lead to prolonged hospitalization, increased healthcare costs, and, in severe cases, implant failure requiring revision surgery [
5]. A significant issue occurs when bacteria adhere to the implant surface, leading to the formation of biofilms. Once biofilms are formed, they create a protective environment for microorganisms, making them resilient against systemic antibiotics and the immune responses of the host [
6].
Currently, infection management mostly relies on the use of systemic antibiotics; however, this method is frequently inadequate. Achieving therapeutic drug concentrations at the implant–tissue interface can be challenging due to poor vascularization in that area [
7]. Also, systemic delivery exposes patients to potential side effects such as microbiota balance alteration, and in time contributes to bacterial antimicrobial resistance [
8]. Advanced drug-delivery systems with high potential for peri-implantitis have therefore emerged as a promising alternative, ensuring high concentrations of therapeutic agents directly at the target site while minimizing systemic exposure [
9]. In this context, composite membranes designed to combine osseointegration and controlled drug release are of increasing scientific and clinical interest [
10,
11,
12].
Chitosan (CS), a naturally derived polysaccharide obtained from chitin deacetylation, has attracted considerable attention as a biomaterial for biomedical applications. Its biocompatibility, biodegradability, and inherent antimicrobial activity make it suitable for wound healing and tissue engineering [
13]. Moreover, chitosan’s film-forming and mucoadhesive properties enable the fabrication of membranes and scaffolds with tunable mechanical and physicochemical characteristics [
14]. Hydroxyapatite (HA), a calcium phosphate ceramic that represents the main mineral component of natural bone, is well known for its osteoconductive behavior and structural similarity to the inorganic phase of bone tissue. Incorporating HA into polymeric matrices not only enhances their mechanical strength but also promotes cellular adhesion, proliferation, and bone regeneration [
15]. Thus, chitosan–hydroxyapatite composites have been widely studied as scaffolds for bone repair [
16], functional coatings for dental or orthopedic implants [
17], and carriers for the controlled delivery of therapeutic agents [
18].
The therapeutic potential of such composites can be further expanded by incorporating antibiotics in their structure to prevent bacterial colonization and biofilm formation. Among available antimicrobials, clindamycin (CL) is of particular relevance for bone-related applications [
19]. Clindamycin exhibits potent activity against
Gram-positive bacteria, especially
Staphylococcus aureus, including strains frequently implicated in osteomyelitis and peri-implant infections [
20]. Its ability to penetrate bone tissue and target anaerobic organisms further strengthens its clinical utility and makes it particularly effective in the treatment of bone disorders [
21]. Nevertheless, the systemic use of clindamycin poses risks of gastrointestinal issues due to the overgrowth of
Clostridium difficile [
22]. A solution to this issue is embedding clindamycin into a biocompatible matrix, such as hydroxyapatite, for the localized delivery of therapeutic antibiotic concentrations, thus minimizing systemic toxicity while preserving significant antibacterial effectiveness at the area of concern. In this context, various drug-delivery systems that integrate chitosan with hydroxyapatite were created for the targeted delivery of clindamycin [
23]. For instance, Wei et al. developed hyperbranched nanoparticle hydrogels made from quaternized chitosan and loaded with clindamycin, which exhibited remarkable antibacterial effectiveness against not only
E. coli and
S. aureus but also
Methicillin-resistant S. aureus [
24]. Additionally, chitosan/alginate polyelectrolyte complex films were explored as mucoadhesive drug-delivery systems for periodontal treatment [
25] or for managing acne [
26].
After a thorough analysis of the available literature data, it was found that there is a lack of studies that explore the combined use of chitosan, hydroxyapatite, and clindamycin in a single composite membrane. Existing approaches focus on either structural scaffolds that enhance osteointegration or on antibiotic-eluting coatings that target infections but rarely achieve both objectives simultaneously [
16]. Therefore, developing a multifunctional system capable of supporting tissue regeneration while providing sustained antimicrobial protection would represent a significant advancement in implantology. The present study addresses this gap by developing and characterizing novel chitosan/hydroxyapatite–clindamycin phosphate (CS/HA-CLY) composite membranes, which could be used as coatings for dental and orthopedic implants, to prevent localized infections at the implantation sites. The physicochemical properties of the membranes, including surface chemistry and morphology, wettability and biomineralization ability, were investigated to ensure their suitability for the intended application. By integrating antimicrobial efficacy with biomineralization and biocompatible features, these composite membranes hold promise as multifunctional biomaterials for improving the safety and success of implantable medical devices.
2. Materials and Methods
2.1. Chemicals and Reagents
Medium molecular weight chitosan powder was supplied by Sigma Aldrich (Burlington, MA, USA). Glacial acetic acid (99%, ChimReactiv, Bucharest, Romania) was used for polymer dissolution. The hydroxyapatite particles were obtained from spongy bone samples according to a method previously established in our research group [
27]. Clindamycin phosphate (Sigma Aldrich Burlington, MA, USA) powder was used as antibacterial agent. Calcium chloride (94%, Roth, Karlsruhe, Germany), hydrochloric acid (37%, Sigma Aldrich, Burlington, MA, USA), tris(hydroxymethyl)aminomethane (99.8%, Sigma Aldrich, Burlington, MA, USA) and anhydrous disodium phosphate (99%, Sigma Aldrich, Burlington, MA, USA) were employed for the mineralization studies. All reagents were analytical grade and were used without further purification. The water utilized in all experiments was distilled water.
2.2. Synthesis of the Composite CS/HA-CLY Membranes
The first step consisted of loading the hydroxyapatite particles with clindamycin phosphate (HA-CLY). 0.1 g of clindamycin phosphate and 0.5 g of hydroxyapatite powder were dispersed in 50 mL of distilled water, and magnetically stirred for 24 h at room temperature. The dispersion was dried for 72 h at 40 °C in a vacuum laboratory oven, and the resulting powder was collected and finely ground using a mortar and pestle prior to further usage.
Both neat and composite were obtained starting from a 1% chitosan solution with different amounts of HA-CLY filler (1, 2 and 4 wt%). First, the chitosan powder (1 wt%) was dissolved in 200 mL of mixed glacial acetic acid/distilled water (10/90) solvent system. In the case of the composite membranes, 1, 2 and 4 wt% HA-CLY powder, reported to the polymer mass, was added in the polymer solution and magnetically stirred until complete homogenization (~6 h, 700 rpm). To disrupt potential aggregates and ensure an even filler dispersion, the polymer solutions were subjected to an ultrasound treatment for 30 min (ultrasonic processor UP100H, 80% amplitude). The final step consisted of casting the membranes in Petri plates and placing them in a vacuum laboratory oven for 72 h at 40 °C for complete solvent evaporation. The resulting membranes were further physicochemically characterized via ATR FT-IR, XPS, SEM, XRD and contact angle measurements, and their biomineralization ability was studied using the Taguchi method.
2.3. Biomineralization Study
After complete drying, the membranes were subjected to
in vitro mineralization to investigate the process of inorganic phase formation on the membrane′s surface under simulated physiological conditions. The biomineralization studies were conducted using the alternate soaking method described by Taguchi et al. and previously employed in our research group [
28,
29]. The samples were first incubated in a 200 mM CaCl
2 solution at 37 °C for 24 h. The pH of the solution was adjusted to 7.4 using HCl and Tris base. Next, the membranes were briefly rinsed with distilled water and incubated for an additional 24 h in a 120 mM Na
2HPO
4 solution at 37 °C. The cycle was repeated two times. Finally, the membranes were rinsed with distilled water and dried for 72 h at 37 °C before characterization.
2.4. Characterization Methods
ATR FT-IR spectra were recorded using a Bruker VERTEX 70 spectrometer (Bruker, Billerica, MA, USA), equipped with a diamond ATR device, in the 4000–600 cm−1 region, at 4 cm−1 resolution. The spectra were recorded as an average of 32 successive measurements for each sample.
The surface chemistry was studied by X-ray Photoelectron Spectroscopy (XPS) using a K-Alpha instrument from Thermo Scientific (Thermo Fisher Scientific, Waltham, MA, USA), with a monochromate Al Kα source (1486.6 eV), at a base pressure of 2 × 10−9 mbar. Charging effects were compensated by a flood gun, and binding energies were calibrated by placing the C1s peak at 284.4 eV as the internal standard. A pass energy of 200 eV and 20 eV was used for survey and high-resolution spectra acquisition, respectively. The deconvolution of C1s, O1s and N1s spectra was performed for both neat and functionalized CA using a Gaussian–Lorentzian function.
The investigations of the surface wettability of the materials were carried out using a Krüss Drop Shape Analyzer DSA100 equipment (A. Krüss Optronic GmbH, Hamburg, Germany). The analysis was performed using distilled water and ethylene glycol as testing liquids. The data obtained were processed and analyzed using the Krüss Vision software version 1.14. The purpose of these determinations was to calculate the free surface energy based on the measured contact angles, in order to evaluate the wettability of the material using the Owens–Wendt equation.
The thermal stability of both pure chitosan and chitosan-based composite materials was studied using thermogravimetric analysis (TGA). The analysis was carried out on a Q500 TA Instruments equipment (TA Instruments, New Castle, DE, USA), under nitrogen atmosphere from room temperature to 800 °C and a heating rate of 20 °C/min.
The morphology of the samples was visualized by scanning electron microscopy (SEM), using a Quanta Inspect F microscope (Hillsboro, OR, USA), equipped with an energy-dispersive X-ray spectrophotometer (EDX), with the accelerating voltage being set at 30 kV.
The phase composition and crystalline structure were investigated by X-ray diffraction using a Shimadzu XRD 6000 instrument (Shimadzu, Kyoto, Japan) with Cu Kα radiation (λ = 1.54 Å), filtered by Ni, with the 2θ angle being varied between 0 and 80°.
The
in vitro release of clindamycin phosphate was studied via UV-Vis spectrophotometry using a Shimadzu UV-3600 UV-Vis spectrophotometer. For this purpose, membrane samples of 2 × 2 cm with a weight of 26 ± 2 mg were placed in a dialysis membrane bag with 4 mL of phosphate-buffer solution (PBS) and then placed in 200 mL PBS and spun at 100 rpm for 72 h at room temperature. The spectra were recorded at a maximum wavelength of 202 nm, and the standard curve was determined for concentrations between 2 and 10 μg/mL [
17].
The swelling behavior of the synthesized membranes was evaluated by immersing 1 cm
2 of dry membrane samples in distilled water at room temperature. The swelling degree was calculated using the following formula:
where Ws and Wd represent the weights of the swollen and dry membranes, respectively. Measurements were taken at different time intervals to monitor the swelling kinetics [
30].
3. Results
3.1. ATR FT-IR
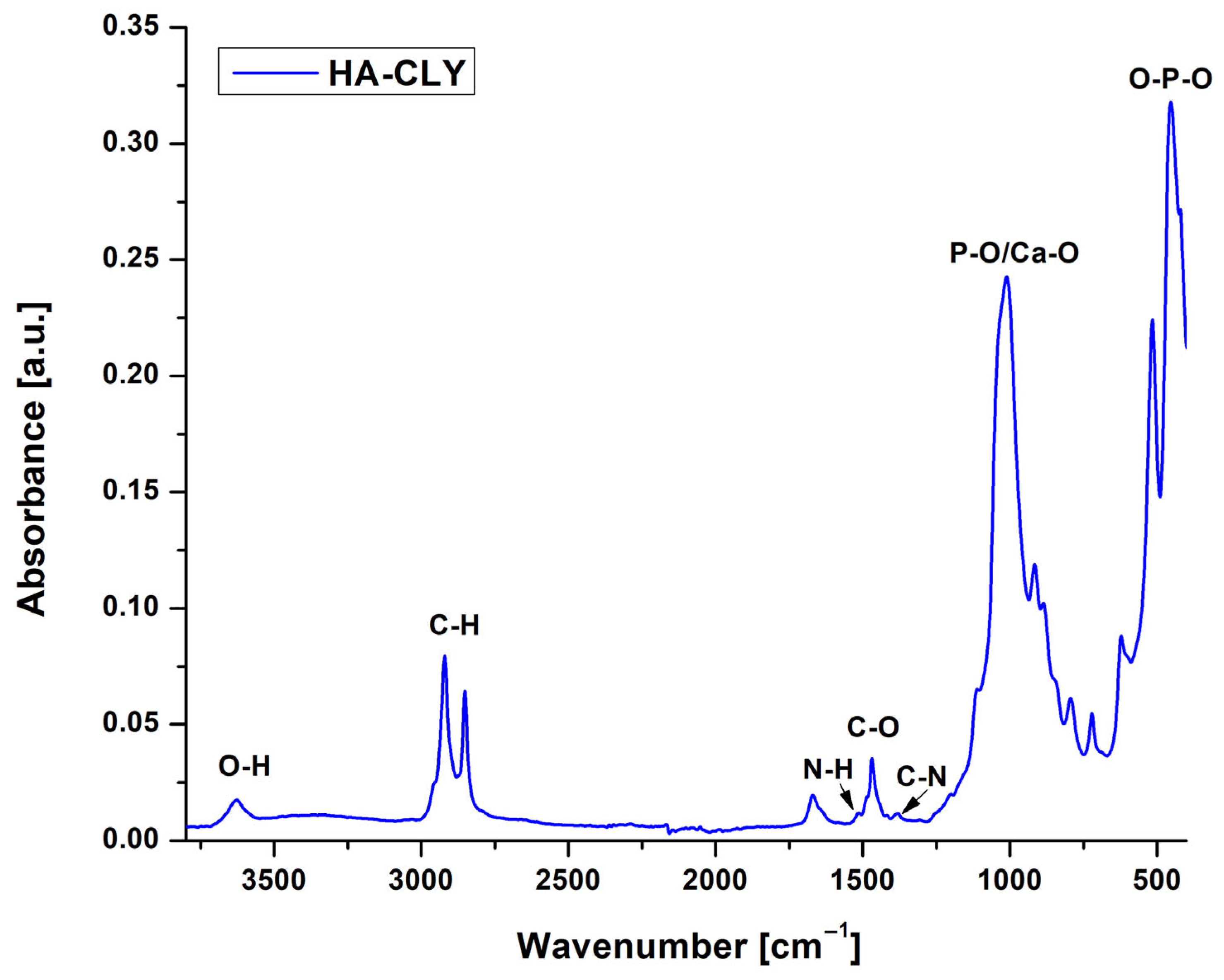
The ATR FT-IR analysis was first performed on the HA-CLY powder to confirm the successful loading of the hydroxyapatite particles with the antibacterial agent; the resulting spectrum is illustrated in
Figure 1. The peaks characteristic of non-stoichiometric hydroxyapatite were observed at 3626 cm
−1 (O–H stretching vibration), 1466 cm
−1 (C-O stretching vibration from the CO
32− groups), 1011, 917 and 515 cm
−1, associated with the symmetrical stretching and deformation vibrations of P-O bonds from phosphate groups (PO
43−) and with the secondary phase vibrations of Ca-O and O-P-O bonds, which characterize hydrated calcium phosphates [
31,
32]. The presence of clindamycin was highlighted by the bands at 2921 cm
−1 and 2856 cm
−1 corresponding to the aliphatic C–H stretching vibrations of the methylene groups and by the N-H and C-N stretching vibrations that generate small peaks at 1664 and 1377 cm
−1, respectively [
33].
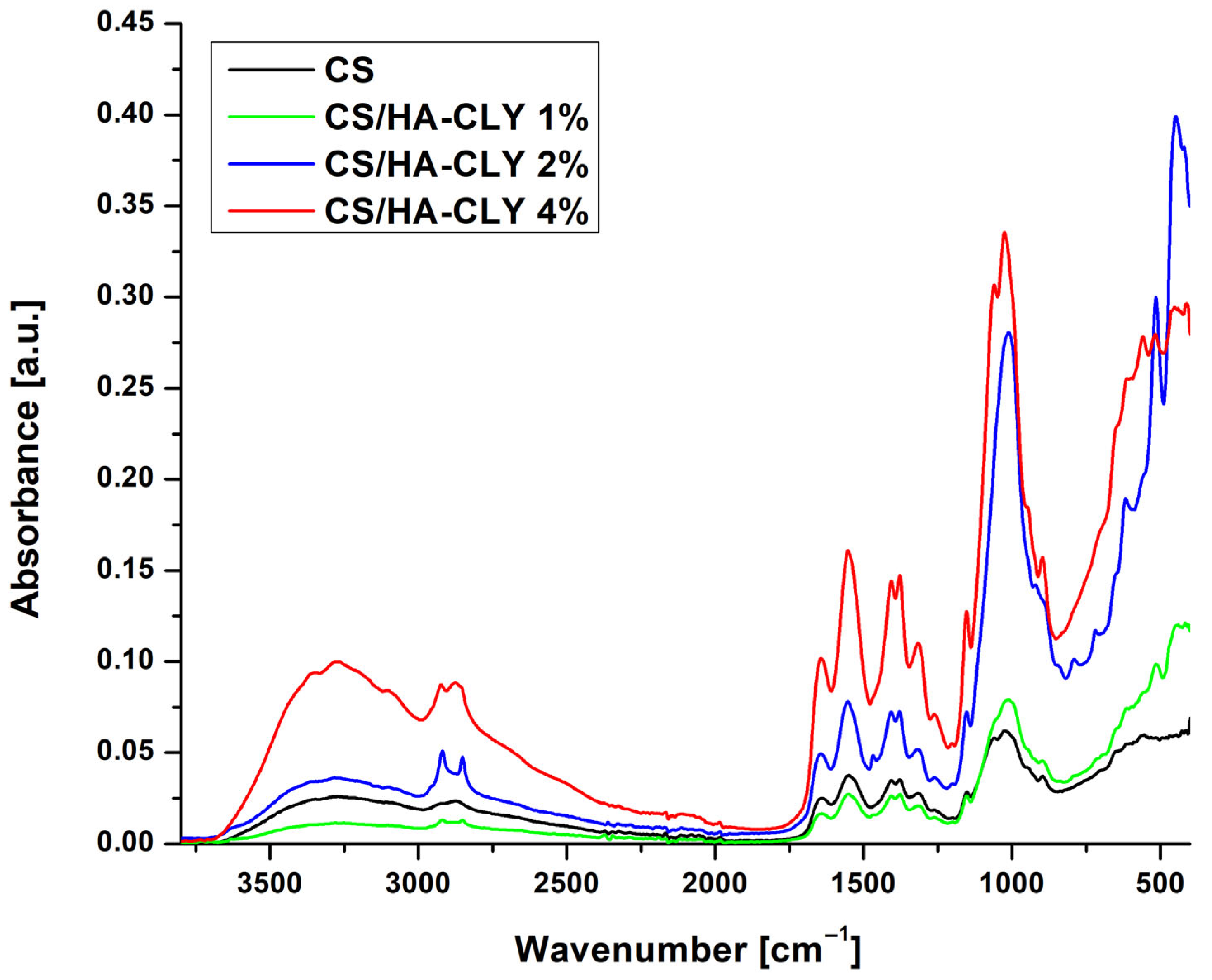
ATR FT-IR was also used to investigate the surface chemistry of the neat and composite chitosan-based membranes and the changes that occur as the filler percentage is increased. As shown in
Figure 2, the spectrum of the neat CS membrane exhibited a broad band in the range of 3500–3000 cm
−1, which was associated with the stretching vibrations of the –OH and –NH groups within the polysaccharide structure, while the bands observed at 2921 cm
−1 and 2856 cm
−1 were linked to the C–H stretching vibrations of methylene groups. The band located at 1647 cm
−1 corresponds to C=O stretching vibrations typical of amide I groups, whereas the weaker signal at 1550 cm
−1 may suggest the presence of N–H vibrations characteristic of amides II. Additionally, in the frequency range of 1378–1398 cm
−1, C–N vibrations or deformations of –CH
3 groups were detected. In the lower frequency range, the peak near 1025 cm
−1 was associated with the C–O–C stretching vibrations, characteristic of glucosamine units [
34]. In comparison to the control membrane, the spectra of the composite membranes displayed intensified and broadened bands in the 1000–1100 cm
−1 range, attributed to the stretching vibrations of phosphate groups from both hydroxyapatite and clindamycin phosphate. With a lower drug loading of 2%, enhanced hydrogen bonding and ionic interactions between the protonated amino groups of chitosan and the phosphate groups may result in a slight shift and modification of band intensity within this region. This finding was further supported by the emergence or intensification of the bands in the 515–560 cm
−1 range, which are characteristic of the deformation vibrations of the PO
43− ion [
35]. In the case of the CS membrane containing 4% HA-CLY, these signals were significantly more intense, indicating a higher degree of inorganic phase loading; therefore, these membranes were selected for further analysis to obtain more visible results.
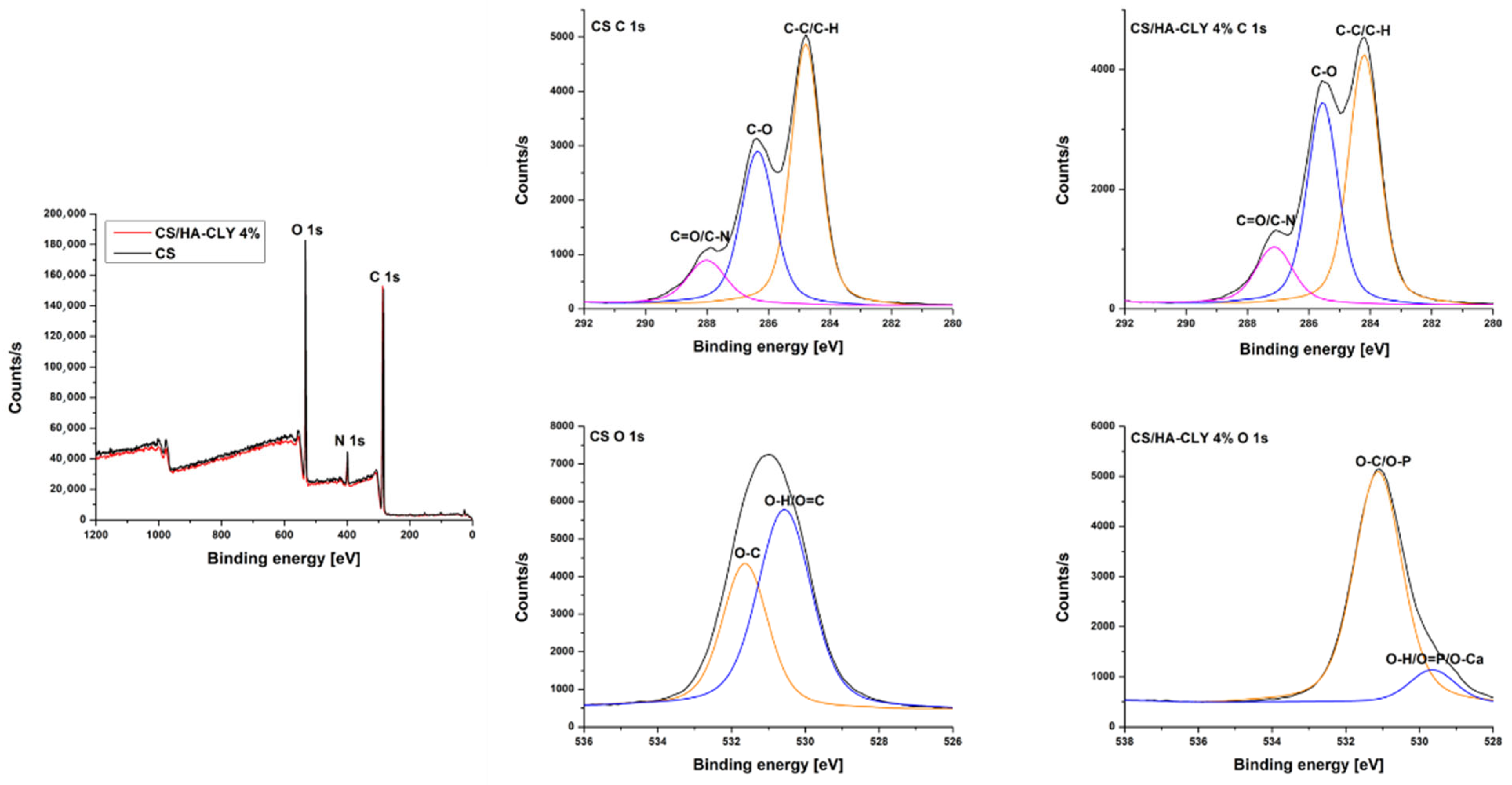
3.2. XPS
The survey XPS spectrum of the neat chitosan membrane highlighted the peaks characteristic of the basic structure of the polymer, namely carbon (C 1s) at 284.78 eV, oxygen (O 1s) at 531.81 eV, and nitrogen (N 1s) at 398.82 eV. These elements reflect the presence of chitosan-specific functional groups, such as hydroxyl (–OH) and primary amine (–NH
2) groups, resulting from chitin deacetylation. The survey XPS spectrum of the composite exhibited the same characteristic peaks, with similar peak positioning for carbon (284.78 eV), oxygen (531.31 eV), and nitrogen (398.43 eV) [
36]. In this case, the nitrogen signal was maintained, confirming the presence of clindamycin phosphate in the structure and the increase in the proportion of oxygen that may be associated with the additional intake of phosphate groups and oxides from hydroxyapatite (
Figure 3,
Table 1).
For a detailed analysis of the chemical species present on the surface of the membranes, high-resolution spectra were recorded for the C 1s and O 1s regions. In the case of the CS membrane, the high-resolution spectrum for C1s had three major components: At 284.78 eV, corresponding to C–C/C–H bonds; at 286.22 eV, associated with C–O bonds (from hydroxyl groups); and at 287.89 eV, assigned to C=O or C–N groups. These values were consistent with the usual structure of chitosan previously reported in the literature. The O1s spectrum showed two peaks: one centered at 531.66 eV, specific to O–C bonds, and a second around 530.2 eV, which can be associated with O–H or O=C bonds [
37].
The C 1s spectrum of the composite CS/HA-CLY membrane retained the same three main components, but with a redistribution of intensities. In addition to the peak at 284.78 eV (C–C/C–H), a more pronounced peak at 285.99 eV was noticed and attributed to clindamycin-specific C–N bonds, which indicated the interaction between the antibiotic and the polymer matrix [
38]. The interactions between clindamycin phosphate and the chitosan–hydroxyapatite matrix are mainly electrostatic and hydrogen bonding in nature. The protonated amino groups of chitosan interact with the anionic phosphate groups and polar functionalities (e.g., –OH, –NH–) of clindamycin phosphate, as supported by the intensified C–N peak in the C 1s XPS spectrum. These non-covalent interactions may increase the affinity of the antibiotic for the polymer matrix, resulting in a slower and more controlled release due to the need to overcome ionic and hydrogen bonding forces during diffusion. Similar interactions have been reported in chitosan-based drug-delivery systems containing phosphate-bearing drugs [
39]. Also, the peak in the 287.2 eV area was maintained, suggesting the presence of carbonyl groups. The O 1s spectrum showed a band centered at 531.31 eV, representing O–C or O–P bonds, and a secondary component around 529.9 eV, associated with O=P or O–Ca bonds, specific to HA [
40].
In terms of atomic percentages, the CS membrane predominantly contained carbon (71.34%), followed by oxygen (23.25%) and nitrogen (5.41%), consistent with the chemical structure of chitosan. In the composite membrane, only slight variations were observed: oxygen increased to 23.41% and nitrogen to 5.60%, while carbon decreased marginally to 70.99%. Although these variations are small and within the experimental uncertainty of XPS measurements, their direction is consistent with the expected incorporation of oxygen- and nitrogen-containing species from hydroxyapatite and clindamycin phosphate. Overall, the elemental composition confirms that the chitosan matrix remains the dominant component while reflecting minor contributions from the inorganic and drug phases.
3.3. Contact Angle Measurements
Wettability and surface free energy are essential parameters in understanding the membrane behavior in biological environments and have a direct impact on the properties of osseointegration and controlled release [
41]. To summarize the essential data of the measurements, the average values of the contact angle for each membrane with the two model liquids (water and ethylene glycol), as well as the values of the total free energy of the surface and its components, dispersive and polar, are presented in
Table 2.
When ethylene glycol was used as the testing liquid, the neat CS membrane displayed a contact angle of 52.07° and the CS/HA-CLY 4% membrane was 42.58°. Both values were well below 90°, confirming hydrophilic surfaces due to hydrogen bonding with the polymer’s polar groups, although the difference between the membranes was not statistically significant. In contrast, water contact angles were higher, with the neat CS membrane showing 100.68°, indicating a predominantly hydrophobic surface, while the CS/HA-CLY 4% membrane had a slightly lower water contact angle of 97.15°, reflecting a modest increase in hydrophilicity, likely attributable to the presence of hydroxyapatite. These results demonstrate that the membranes’ wettability is strongly liquid-dependent and highlight the contribution of hydroxyapatite to increased interaction with polar liquids such as water. It can be concluded that the incorporation of hydroxyapatite into the chitosan matrix resulted in a slight hydrophilicity improvement compared to neat chitosan. This behavior can be interpreted within the framework of the Wenzel model, which describes how surface roughness amplifies the wettability of a material [
42]. For both chitosan and hydroxyapatite, the increased roughness introduced by hydroxyapatite particles facilitates water penetration into the surface asperities (observed in the SEM images), thereby decreasing the contact angle and improving wettability [
43].
Furthermore, the neat chitosan (CS) membrane exhibited a total surface free energy of 86.53 ± 11.44 mN/m, as determined using the OWRK model, with a predominant dispersive component measured at 83.24 ± 9.38 mN/m and a reduced polar component of 3.29 ± 2.06 mN/m, indicating a surface that is primarily non-polar in nature. The total free energy of the CS/HA-CLY 4% membrane surface was found to be 100.14 ± 17.63 mN/m, characterized by a strong dispersive component of 96.32 ± 14.87 mN/m and a minor polar component of 3.82 ± 2.76 mN/m. This property indicated an enhanced capability of the composite membrane to interact with aqueous environments, which is crucial for its intended biomedical uses, as well as a favorable balance of hydrophilicity, making it suitable for drug-delivery applications by providing adequate interaction with biological fluids while minimizing excessive absorption.
3.4. TGA, DTG
According to the TGA results (
Figure 4), pure chitosan (CS) exhibits a maximum degradation temperature (T
max) of 272 °C and a residual mass of 40%. In contrast, the CS/HA-CLY composites show enhanced thermal stability and higher residual masses. Specifically, the composites containing 1, 2, and 4 wt.% HA-CLY display residual masses of 61%, 57%, and 46%, respectively, with a consistent T
max of 284 °C for all formulations. The increase in T
max from 272 °C to 284 °C after incorporating HA-CLY demonstrates a clear improvement in the thermal stability of the chitosan matrix. This enhancement can be attributed to the inorganic and thermally stable nature of hydroxyapatite, which acts as a barrier to heat and mass transfer during polymer decomposition. Additionally, strong intermolecular interactions between chitosan and HA—such as hydrogen bonding and electrostatic attraction—likely restrict the mobility of polymer chains, further improving stability. The variation in residual mass with increasing HA-CLY content supports these findings. The highest residue (61%) was observed for the CS/HA-CLY 1 wt.% composite, suggesting optimal interfacial compatibility between the organic and inorganic components at this concentration. At higher HA-CLY loadings (2 and 4 wt.%), the decrease in residue (to 57% and 46%, respectively) may result from particle agglomeration and reduced dispersion, which weaken matrix–filler interactions and lower the effectiveness of the thermally stable phase.
In summary, TGA confirms that the incorporation of HA-CLY significantly improves the thermal stability of chitosan composites, with the most pronounced effect observed at lower HA-CLY contents, where the organic–inorganic interfacial interactions are optimized.
3.5. Biomineralization Study
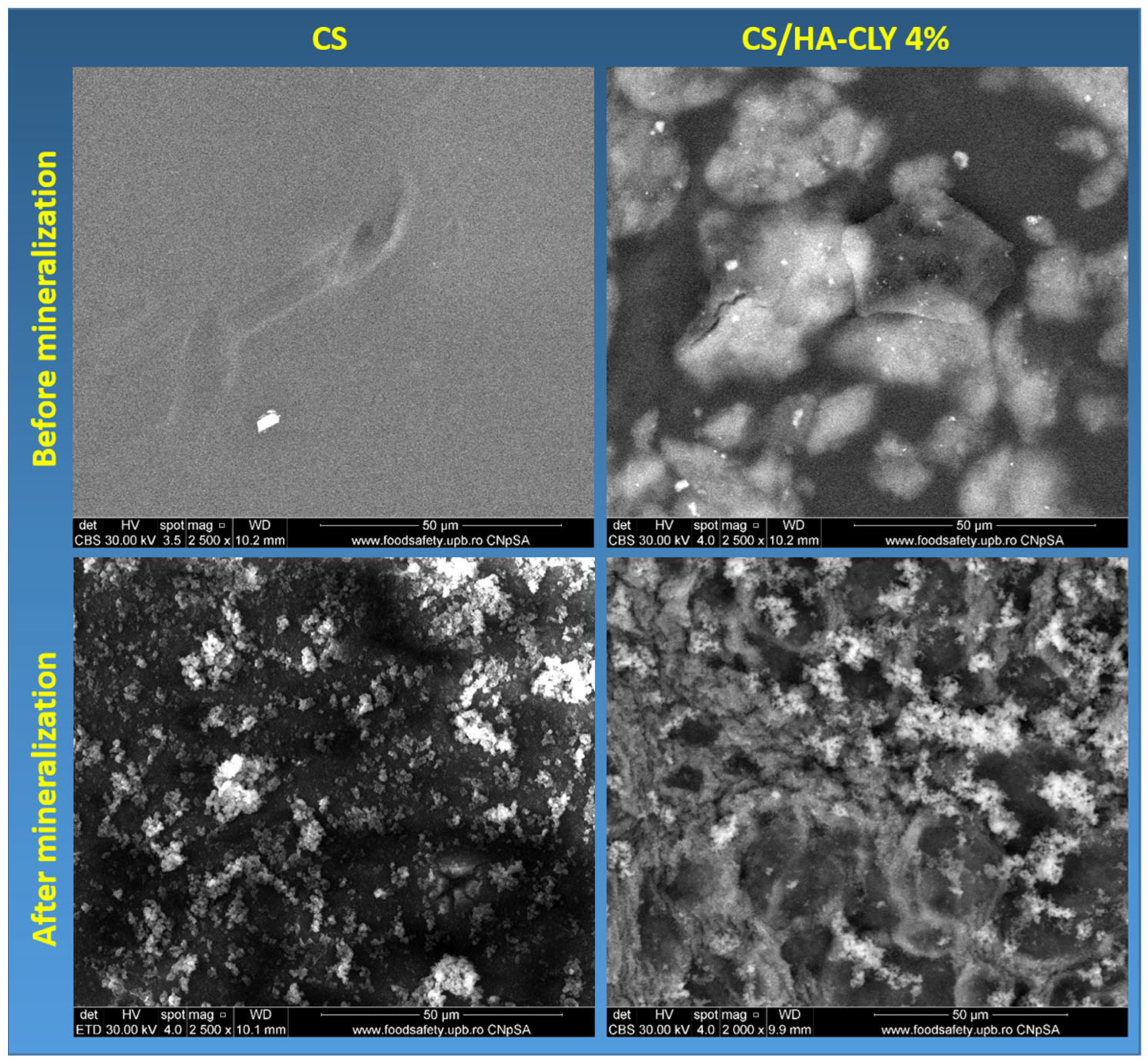
3.5.1. SEM
Figure 5 illustrates the morphology of the neat and 4% HA-CLY CS membranes before and after mineralization. The neat membrane had a smooth, compact surface, lacking visible porosity, with a dark homogeneous contrast, indicating the exclusive presence of the polymer phase. Scattered particles with irregular morphology were also observed and were considered residual aggregates of chitosan that resulted from incomplete polymer homogenization or impurities attached to the membrane surface during the drying process [
44]. The right-side images provide an overview of the surface of the composite membrane consisting of chitosan, hydroxyapatite and clindamycin phosphate. A relatively uniform distribution of hydroxyapatite particles was observed in the form of light aggregates of varying sizes, indicating inefficient incorporation of the inorganic phase into the polymer matrix, without areas of excessive accumulation or absence. In addition, a central area with a more intense contrast, of larger dimensions, with a different morphology than the H A particles was distinguished. This was associated with the presence of clindamycin phosphate, visible even from this scale as a separate formation [
45].
After mineralization, the neat CS membrane presented an uneven coating of fine, irregularly shaped particles distributed over the entire surface. Some striations or traces of fibrous structure were also observed in the substrate, suggesting a residual orientation of the chitosan network. This coating may be associated with the formation of a homogeneous inorganic phase on the surface of chitosan following the mineralization process. The SEM images of the mineralized composite membrane highlighted a complex microstructure, consisting of conglomerates of particles with well-defined edges, indicating a granular integration of hydroxyapatite particles into the network. The very bright areas may correspond to local antibiotic accumulations or crystalline agglomerations rich in calcium and phosphorus, which denote effective mineralization. The matrix appeared to be completely covered, and the particle distribution was relatively uniform.
Comparing the results, it was observed that the mineralized composite membrane presented a denser and more uniform mineralization, with a homogeneous particle distribution and a well-defined granular morphology, in contrast to the mineralized control membrane, where mineral deposits are rarer and unevenly distributed. This indicates that the presence of the HA-CLY filler in the membrane structure favors the mineralization process on the polymer support.
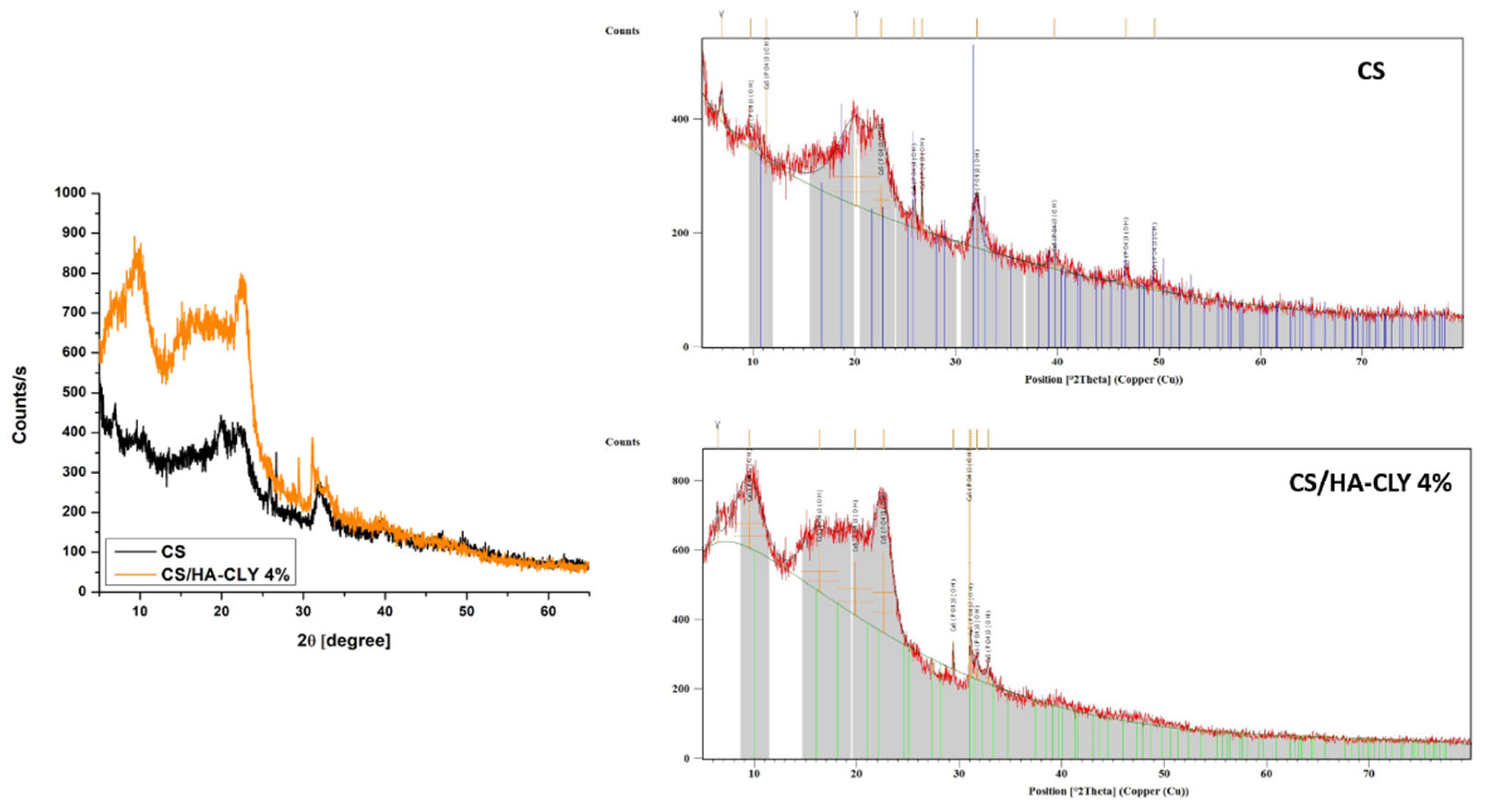
3.5.2. XRD
XRD analysis (
Figure 6) was employed to identify the nature of the inorganic compounds deposited on the membranes after Taguchi biomineralization. The diffractogram of the biomineralized CS membrane revealed a broad, low-intensity peak, located around 2θ ≈ 20°, attributed to the plane (020) of chitosan. This peak is characteristic of the semi-crystalline nature of chitosan, reflecting the presence of polymer chain domains with a low degree of organization. Moreover, a series of low-intensity peaks corresponding to the crystallographic planes of hydroxyapatite, such as 2θ ≈ 25.8° (002), 31.8° (211), 32.9° (112), 34.0° (300), and 39.8° (310), were observed. The peaks that were not in agreement with the JCPDS 09-0432 standard sheet, specific to the hexagonal structure of HA, were attributed to secondary phases, such as calcium phosphate (β-TCP), or to decomposition products of clindamycin phosphate, which may occur as a result of reactions in an acidic environment or interactions with the organic matrix. Compared to neat chitosan, the biomineralized composite membrane had a significantly higher degree of crystallinity, noticeable by the more intense and sharper peaks in the diffractogram.
The general conclusion of the biomineralization study was that the presence of hydroxyapatite in the membranes’ structure led to the deposition of higher quantities of calcium phosphates on the membranes’ surface and enhanced the crystallinity of the deposited compounds. The results were in good agreement with previously reported studies [
46]. This can be explained by the fact that HA particles provide calcium- and phosphate-rich sites that act as heterogeneous nucleation centers for new apatite growth when the membrane is immersed in simulated body fluid (SBF) or other mineralizing solutions. This accelerates and directs apatite formation compared with neat chitosan [
47]. Moreover, HA increases surface polarity (e.g., PO
43−, OH
−, Ca
2+) and raises surface roughness and wettability—all of which are factors that favor ion adsorption and subsequent crystal growth on the membrane surface [
48].
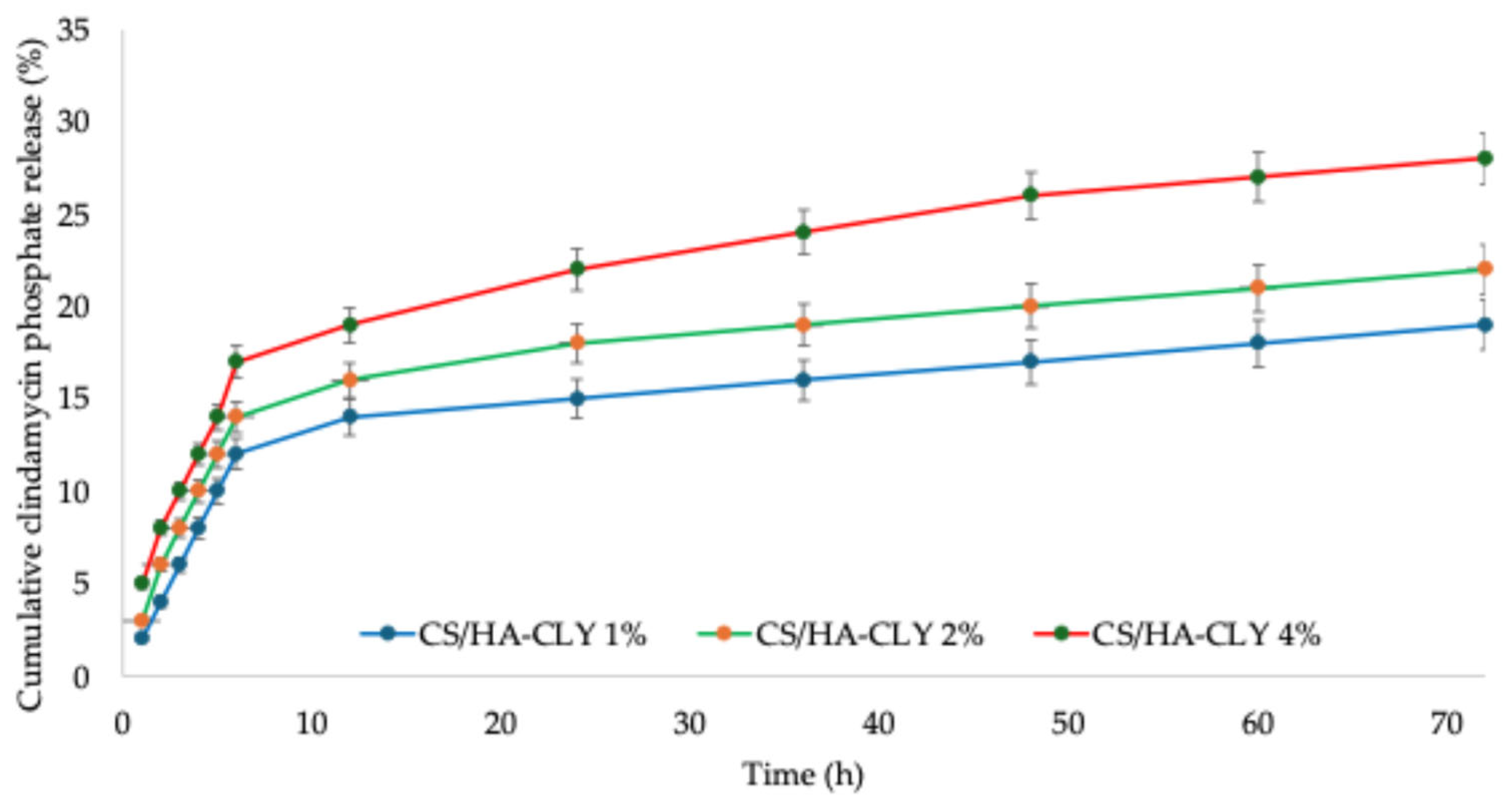
3.6. Clindamycin Phosphate Release and Swelling Behavior
Figure 7 illustrates the cumulative release profile of clindamycin phosphate from the CS/HA composite membranes, demonstrating the typical biphasic pattern often observed in chitosan-based composites: A significant initial burst during the first few hours, followed by a gradual, diffusion-controlled sustained release lasting up to 72 h. This initial burst is typically attributed to the rapid desorption or diffusion of the drug positioned at or near the membrane surface, along with a weakly bound fraction that escapes from the porous structure [
49]. The sustained second phase likely results from a combination of (i) diffusion through the swollen chitosan matrix, (ii) gradual erosion/dissolution of chitosan, and (iii) drug–matrix interactions, which together retain part of the payload and slow its release. These processes have been outlined in previous studies for chitosan/HA composites and are associated with release profiles that stay significantly below the total release point even after multiple days [
50]. The hydroxyapatite content affects the release kinetics in two significant ways: (i) HA offers additional sites for adsorption that can hold onto the phosphate-containing drug, thus slowing down their release, and (ii) the particle size, dispersion pattern, and the interface of HA with chitosan alter membrane porosity and diffusion routes, encouraging a more stable and extended release. Consequently, the morphology of HA and the ratio of CS to HA are practical factors to adjust the burst and sustained release phases [
51,
52]. From the intended application perspective, the observed initial burst followed by sustained release is advantageous for peri-implant infection prophylaxis: the burst delivers an immediate antibiotic concentration at the surgical site, while the sustained release helps prevent recolonization during the critical first days. Nonetheless, if a prolonged release is required, the formulation variables can be adjusted by increasing membrane porosity, altering the CS deacetylation degree, reducing HA percentage, or using layered coatings or nanoparticulate carriers, as shown in the specialty literature [
53,
54]. Moreover, considering that the effective dose of clindamycin phosphate for dental applications has been reported to be 20 µg/mL, the release behavior governed by the membrane’s structural properties indicates that a sufficient amount of the antibiotic is released over an extended period, enabling effective prophylaxis against peri-implant infections at the surgical site [
55].
Due to the synthesis method and the inherent properties of chitosan membranes, the neat membrane exhibited a relatively narrow variation in swelling degree over time (
Figure 8), ranging from 223% to 260%. In the case of the composite membranes, the swelling degree decreased, as part of the membrane volume was occupied by hydroxyapatite particles. A similar swelling behavior was observed for CS/HA-CLY 1% and CS/HA-CLY 2% membranes, while a more pronounced reduction was seen for CS/HA-CLY 4%, corresponding to the higher filler content.
4. Discussion
The working hypotheses of this study were that loading the hydroxyapatite particles with clindamycin and further embedding them within the chitosan matrix would (1) modulate the release profile of clindamycin phosphate (e.g., reduce burst release, prolong release time, etc.), (2) enhance hydrophilicity (via HA’s polar groups introduced on the polymer surface), and (3) enhance the membranes’ bioactivity/biomineralization ability for improved performances in implantology and peri-implantitis prevention. Consistent with hypothesis (2), the contact angle test results showed a marked decrease in both water and ethylene glycol contact angles with increasing HA content. The results were in good agreement with previous studies where the addition of 60 wt% HA in CS coatings resulted in a water contact angle of ~43° versus ~65–70° for lower HA content [
56]. Similarly, Jiang et al. found that the incorporation of hybrid nano-hydroxyapatite/carboxymethyl cellulose/phytic acid particles in the structure of CS membranes led to significantly better wettability compared to neat CS [
57]. In terms of drug release, to the best of our knowledge, no recent studies combine CS with HA-CLY in this exact configuration [
58]. However, Zhan et al. studied the sequential release of vancomycin/BMP-2 from CS/nano-HA hydrogels and observed that HA inclusion slows the release rate and extends the release duration [
59]. Furthermore, Podar et al. modified PCL-based scaffolds with CS/alendronate-modified HA composite coatings and showed that HA governs the bioactive drug release and enhances cellular adhesion, proliferation, and differentiation into osteocytes. In this study, during the initial 6 h of release, the percentage of antibiotic varied between 12% for CS/HA-CLY 1% and 17% for CS/HA-CLY 4%, which was attributed to the higher amount of antibiotic present as the HA percentage increases. When compared to the values obtained after 72 h (17% for CS/HA-CLY 1%, 20% for CS/HA-CLY 2%, and 26% for CS/HA-CLY 4%), the membranes demonstrate a prolonged release profile, making them ideal candidates for potential treatment of infections at the site of implantation. Moreover, the results related to antibiotic release in this study indicate that the developed system is an appropriate option for sustained drug delivery at the implantation site, especially in comparison to other release profiles reported in the literature for clindamycin phosphate, such as wound-healing hydrogels that show complete release within the first 6 h [
33] or calcium-orthophosphate porous particles that attain 100% release within the initial 72 h [
60].
5. Conclusions
The purpose of this study was the development of a novel generation of chitosan-based membranes loaded with hydroxyapatite-clindamycin particles with applications as multifunctional coatings for dental or orthopedic implants. It was found that the HA content in the formulated membranes resulted in (i) a more hydrophilic membrane surface due to the presence of exposed hydroxyl and phosphate groups; (ii) an increase in roughness or microtopography, which was potentially involved in the enhancement of surface wettability through the Wenzel mechanism; (iii) a physical barrier to clindamycin diffusion, which contributed to reduced burst release and prolonged release time; (iv) an enhancement of the in vitro biomineralization capability resulting from the presence of heterogeneous nucleation sites and the availability of calcium and phosphate ions, which, along with the functional groups of chitosan, promoted local supersaturation and encouraged apatite formation. These results demonstrate that chitosan-based membranes loaded with hydroxyapatite–clindamycin may be promising candidates for localized drug-delivery systems with dual functions: promoting implant integration while providing sustained antibacterial activity.
Although the present findings highlight the potential of chitosan–hydroxyapatite composite membranes as localized drug-delivery systems for clindamycin phosphate, several avenues for further research remain open. Future trends involve comprehensive in vitro evaluations that move beyond basic release kinetics such as dynamic cell culture systems that mimic the implantation environment. Another key direction is the systematic assessment of antibacterial efficacy against clinically relevant pathogens, particularly Staphylococcus aureus and Staphylococcus epidermidis, which are the main causative agents of implant-associated infections.