Abstract
This study reports the development of electrospun poly(methyl methacrylate) (PMMA) fiber mats incorporating ibuprofen (IBU)-intercalated layered double hydroxides (LDH) for enhanced transdermal drug delivery systems (TDDS). IBU, in its anionic form, was successfully intercalated into LDH, which possesses anion exchange capabilities, and subsequently embedded into PMMA fibers via electrospinning. In vitro drug release experiments demonstrated that UPMMA–LDH–IBU fibers exhibited significantly higher IBU release than PMMA–IBU controls. This enhancement was attributed to the improved hydrophilicity and water absorption imparted by the LDH, as confirmed by contact angle and water uptake measurements. Furthermore, artificial skin permeation tests revealed that the UPMMA–LDH–IBU fibers maintained comparable release rates to those observed during buffer immersion, indicating that the rate-limiting step was the diffusion of IBU within the fiber matrix rather than the interface with the skin or buffer. These findings highlight the critical role of LDH in modulating drug release behavior and suggest that UPMMA–LDH–IBU electrospun fiber mats offer a promising and efficient platform for advanced TDDS applications.
1. Introduction
Drug delivery systems (DDS) represent a critical area in modern pharmacology, aiming to administer therapeutic agents to specific biological targets with precise dosages and controlled release rates [1]. The effectiveness of drug delivery is closely tied to the selection of suitable carriers or vehicles that ensure drug stability, biocompatibility, and efficient transport in complex physiological environments. Given the wide range of drug molecular weights, from small molecules to complex biologics, the choice of carrier materials must be carefully optimized to enhance drug–carrier interactions, binding affinities, and overall stability [2].
Among the diverse array of drug delivery platforms, transdermal drug delivery systems (TDDS) have garnered significant attention owing to their non-invasive nature and ease of application [3]. These systems enable drug absorption through the skin, typically via adhesive patches or topical formulations, and offer several advantages over conventional administration routes. Key benefits include sustained plasma drug concentrations, avoidance of hepatic first-pass metabolism, ease of treatment discontinuation, and improved patient compliance, which are particularly beneficial for elderly individuals and those with swallowing difficulties [4,5].
Despite their advantages, transdermal drug delivery systems (TDDS) continue to face challenges in achieving controlled drug release, particularly owing to the low utilization efficiency of drugs within formulations. Ibuprofen (IBU), a widely used nonsteroidal anti-inflammatory drug (NSAID), exemplifies this issue. Oral administration of IBU is frequently associated with gastrointestinal side effects [6], and its irritant nature necessitates the development of alternative delivery routes [7]. TDDS present a promising solution by enabling the localized or sustained release of IBU, thereby potentially reducing systemic side effects and enhancing therapeutic efficacy. To improve IBU compatibility with transdermal systems and enhance its release profile, recent studies have explored its incorporation into layered double hydroxides (LDHs) [8]. LDHs are biocompatible anionic clays capable of intercalating anionic species, thus providing a promising strategy for regulating drug release [9]. LDHs, often referred to as anionic clays, possess a unique lamellar structure composed of alternating brucite-like hydroxide layers and interlayers that contain water molecules and exchangeable anions. The positive charge of the hydroxide layers results from the isomorphic substitution of divalent metal cations (e.g., Mg2+ and Zn2+) with trivalent cations (e.g., Al3+ and Fe3+) balanced by interlayer anions such as Cl−, NO3−, and CO32−. LDHs are valued for their simple synthesis, environmental friendliness, and broad applicability in drug delivery, nanocomposites, and biomedical materials [9,10,11]. Notably, intercalation of organic anions, including various pharmaceutical agents such as IBU, into LDH interlayers offers advantages such as enhanced drug stability, improved bioavailability, and controlled release [12].
Electrospinning (ES) has emerged as a versatile and scalable technique for fabricating nonwoven polymer fiber mats. This method is particularly attractive for drug delivery because of its ability to produce fibers with a high surface area, interconnected porosity, and tunable morphology [13,14,15,16,17,18,19]. In parallel, poly(methyl methacrylate) (PMMA) has been widely used as a robust polymer matrix in electrospun fibers for therapeutic compound delivery. PMMA-based fibers are attractive owing to their tunable porosity and high surface-to-volume ratio, which support sustained drug release and facilitate easy skin application [20,21,22,23]. The integration of LDH–IBU complexes into electrospun PMMA fibers presents a promising hybrid system that combines the benefits of inorganic drug intercalation with the versatility of polymeric scaffolds, potentially resulting in superior drug release and overall performance.
Building on these principles, the present study aimed to fabricate novel PMMA fiber mats containing IBU-intercalated LDH (LDH–IBU) via electrospinning and evaluate their in vitro drug release and artificial skin permeation performance. It is hypothesized that the inclusion of LDH–IBU significantly enhances drug release rates, primarily owing to improved hydrophilicity, water absorption capacity, and favorable structural modifications. Through a comparative analysis with control fibers containing non-intercalated IBU, this study sought to validate the synergistic effects of LDH intercalation and electrospun architecture in achieving efficient and controlled transdermal drug delivery.
2. Materials and Methods
2.1. Materials and Chemicals
All chemicals and reagents used in this study were of analytical grade and were employed without further purification unless otherwise specified. Magnesium nitrate hexahydrate (Mg(NO3)2·6H2O) and aluminum nitrate nonahydrate (Al(NO3)3·9H2O) were purchased from Nacalai Tesque, Inc. (Kyoto, Japan) Special-grade potassium hydroxide (KOH) was obtained from FUJIFILM ko Pure Chemical Industries, Ltd. (Osaka, Japan) Ibuprofen (2-(4-isobutylphenyl)propionic acid, C13H18O2) was supplied by Tokyo Kasei Kogyo Co., Ltd. (Tokyo, Japan) Additionally, 1 M Tris-HCl buffer (pH = 7.6) and 2 M hydrochloric acid (HCl) were obtained from Nacalai Tesque Inc. Poly(methyl methacrylate) (PMMA), (C5H8O2)n, was sourced from FUJIFILM Wako Pure Chemical Corporation. The solvents used were acetone (CH3COCH3), N,N-dimethylformamide (DMF, HCON(CH3)2), and tetrahydrofuran (THF, C4H8O), all purchased from Kishida Chemical Co., Ltd. (Osaka, Japan) Ethyl acetate (CH3COOC2H5) was provided by Kanto Chemical Co., Ltd. (Tokyo, Japan) Decarbonated water (DW) was prepared by boiling deionized water, followed by cooling under a nitrogen atmosphere. DW was used throughout all experimental procedures for preparation and purification.
2.2. Equipment
The synthesized samples were characterized using the following instruments. Fourier Transform Infrared Spectroscopy (FTIR) was performed using a JASCO IRT-5200 spectrometer. (Tokyo, Japan) X-ray diffraction (XRD) analysis was conducted using a Rigaku Miniflex600-DX diffractometer (Tokyo, Japan). A JSM-7500FAM scanning electron microscope (JEOL Ltd., Tokyo, Japan) was used for morphological observations. and a VHX-8000 digital microscope (KEYENCE Co., Osaka, Japan) were employed. Contact angle measurements were performed using an AUTO 100 automatic contact angle meter (Excimer, Inc., Kanagawa, Japan). The water absorption rate was measured using a MOC63u moisture analyzer (Shimadzu Co., Kyoto, Japan).
2.3. Experiment
2.3.1. Synthesis of LDH–IBU
Figure 1 illustrates the synthesis procedure of LDH–IBU powder. 1.54 g of magnesium nitrate hexahydrate (Mg(NO3)2·6H2O) and 1.13 g of aluminum nitrate nonahydrate (Al(NO3)3·9H2O) were dissolved in 150 mL of decarbonated water (DW) to prepare a Mg–Al precursor solution with a molar ratio of Mg/Al = 2. A basic IBU solution was prepared by dissolving 2.81 g of potassium hydroxide (KOH) and IBU (1 g of ibuprofen (IBU) in DW (50 mL). The IBU-containing alkaline solution was added dropwise to the metal nitrate solution until the pH reached 10, followed by continuous stirring for 24 h under a nitrogen atmosphere. The resulting suspension was centrifuged and washed with DW until the pH became neutral (pH = 7). The white precipitate obtained was collected and thoroughly dried to yield the LDH–IBU composite. The IBU content in the LDH–IBU was estimated by HPLC analysis after dissolving in diluted HCl solution, where the details of analysis were identical to the procedure mentioned in Section 2.3.5. To prepare the LDH without IBU, the same procedure was followed using an alkaline solution without the addition of ibuprofen.
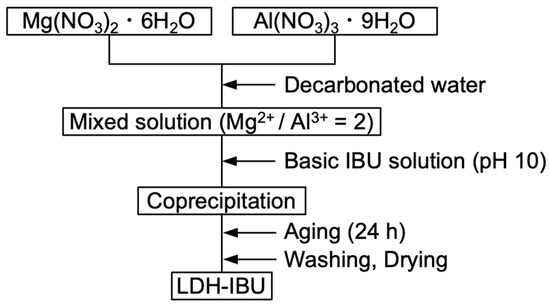
Figure 1.
Synthesis process of LDH–IBU.
2.3.2. Preparation of Spinning Solution
Spinning solutions were prepared for electrospinning both plain PMMA fibers and drug-loaded fibers. First, 0.988 g of PMMA (Wako Pure Chemical Industries, Ltd., Osaka, Japan) was dissolved in 5.00 mL of acetone and continuously stirred for 24 h to ensure complete homogenization. Subsequently, either IBU or LDH–IBU was incorporated into the PMMA–acetone solution. The drug loading was targeted at concentrations equivalent to 5 or 7.5 wt% relative to the PMMA content. Specifically, for 5 wt% IBU-loaded samples, 0.052 g of IBU was added to 0.988 g of PMMA in 5.00 mL of acetone. For 7.5 wt% IBU-loaded samples, 0.080 g of IBU was used. For LDH–IBU-loaded samples, 0.348 g (for 5 wt%) or 0.536 g (for 7.5 wt%) of LDH–IBU was added. All drug-loaded solutions were continuously stirred for 24 h prior to electrospinning.
To optimize the electrospinning process and ensure uniform fiber formation, a screening study was conducted to evaluate the suitability of various organic solvents for dissolving poly(methyl methacrylate) (PMMA) and supporting stable fiber production. The solvents tested included ethyl acetate (0.500 g PMMA in 2.23 mL), dimethylformamide (DMF; 1.18 g PMMA in 5.00 mL), and tetrahydrofuran (THF; 1.11 g PMMA in 5.00 mL). Ibuprofen (IBU) was incorporated into each solvent system, and the mixtures were stirred for 24 h. Based on the preliminary results regarding the formation of uniform IBU-containing nanofibers, acetone was selected as the optimal solvent for subsequent experiments.
2.3.3. Electrospinning Procedure
The prepared spinning solutions were loaded into a syringe equipped with 18G (internal diameter: ID = 0.838 mm) or 21G (ID = 0.514 mm) needles and connected to a high-voltage power supply. Electrospinning was performed by ejecting the solution through a metal needle onto a grounded collector to form non-woven nanofiber mats. The electrospinning parameters were optimized to produce continuous, bead-free fibers. The typical optimized conditions included a solution concentration of 10 wt%, an applied voltage of 12 kV, a flow rate of 1.0 mL/h, and a working distance of 10 cm between the needle tip and the collector. Hereafter, the samples are denoted as PMMA–IBU(XG, Ywt%) or UPMMA–LDH–IBU(XG, Ywt%), where X and Y are the gauge of the needle used for electrospinning and IBU concentration in wt%, respectively.
2.3.4. Drug Release Experiment
To evaluate the drug release behavior of the electrospun fibers, each fabricated sample (200 mg) in 3 mL of 0.1 M tris-HCl buffer solution (pH = 7.6) was enclosed within a semipermeable membrane, as shown in Figure 2. The ends of the membrane were securely sealed, and the enclosed sample was immersed in 37 mL of the same buffer solution to simulate a release environment. The assembly was then placed in a shaking water bath maintained at 37 °C, providing constant agitation to mimic the physiological conditions. Periodical sampling of the external buffer solution was performed, and the release experiment was conducted for up to 96 h. The collected samples were subsequently analyzed to determine the cumulative release profile of IBU from the mats.
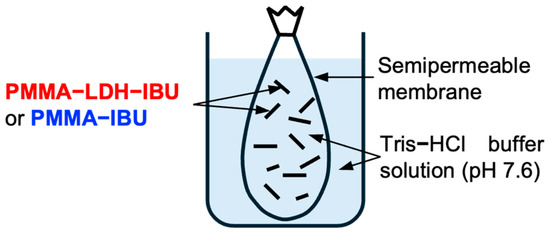
Figure 2.
Schematic diagram of the experimental setup for the IBU release test of UPMMA–LDH–IBU and PMMA–IBU.
2.3.5. Artificial Skin Permeation Experiment
To evaluate the transdermal drug release behavior of the fabricated fibers, a layered assembly was prepared by sequentially placing a fiber mat and sheet of artificial skin (Strat-MTM membrane, Merck Millipore Ltd., Burlington, MA, USA) onto a fixed support platform, as shown in Figure 3. After securing the layers in place, 20 mL of 0.1 M Tris–hydrochloric acid buffer solution (pH = 7.6) was carefully added to the surface to simulate the receiving medium. The entire setup was then transferred to a thermostatically controlled chamber maintained at 37 °C to mimic physiological skin temperature. Buffer solution samples were collected at 1 h intervals, and the release experiment was continued for up to 96 h. The collected samples were subsequently analyzed to determine the cumulative amount of IBU released through the artificial skin, enabling assessment of the transdermal delivery potential of the fiber mats.
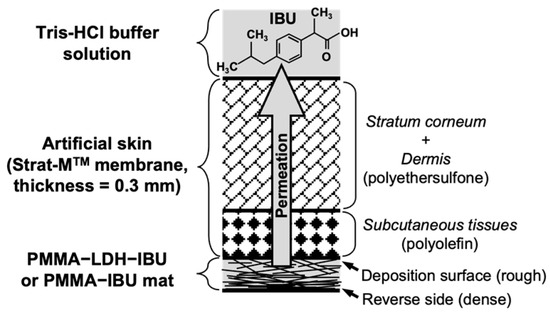
Figure 3.
Schematic diagram of the experimental setup used for the artificial skin permeation test.
The concentration of IBU released into the buffer solution at each predetermined time point during the drug release experiments was quantitatively determined using high-performance liquid chromatography (HPLC) method with a CrestPak C18T-5 column developed in-house. The mobile phase consisted of a mixture of 0.1 mol/L phosphoric acid (37 vol%) and acetonitrile (63 vol%). The mobile phase was delivered through the column to the detector at a constant flow rate of 2 mL/min. Following sample injection, IBU was separated within the column and detected at a retention time of approximately 2.5 min at 313 K using a UV–visible detector set at λ = 220 nm. The amount of IBU released was accurately quantified by integrating the peak areas in the resulting chromatograms.
3. Results and Discussion
Figure 4 presents the (Figure 4a) FTIR spectra and (Figure 4b) XRD patterns of LDH and LDH–IBU prepared by the coprecipitation process. Layered double hydroxides (LDHs) are anion-exchangeable materials composed of Mg2Al(OH)6+ host layers with brucite-like structures and interlayer nitrate ions. In the FTIR spectrum of LDH, in addition to the broad stretching vibration of hydroxyl groups around 3500 cm−1, the asymmetric stretching vibration of NO3− ions appears near 1380 cm−1 [24]. The FTIR spectrum of the synthesized LDH–IBU composite reveals characteristic absorption bands corresponding to ibuprofen (IBU), including C–H stretching vibrations around 2900 cm−1 and the asymmetric stretching of the carboxylate group (COO−) near 1600 cm−1, confirming the presence of IBU within the LDH structure. Additionally, a peak corresponding to residual nitrate ions (NO3−), likely due to incomplete ion exchange, is observed around 1400 cm−1 in the LDH–IBU spectrum. The appearance of these distinct IBU-specific absorption bands, along with the structural changes observed in the XRD patterns, provides strong evidence for the successful intercalation of IBU into the LDH matrix. The XRD analysis further supports this conclusion, as shown in Figure 4b. Compared to the pristine LDH, the XRD pattern of LDH–IBU exhibits a noticeable shift in the characteristic basal reflections, specifically the (003) and (006) planes, toward lower 2θ angles, indicating an expansion of the interlayer spacing. Quantitatively, the interlayer distance of the (003) plane, calculated using Bragg’s Law, increased significantly from 0.797 nm in pure LDH to 2.21 nm in LDH–IBU. This substantial expansion (approximately 1.413 nm) strongly confirms that IBU anions, which are bulkier than typical inorganic interlayer anions, were successfully intercalated into the LDH interlayers during synthesis. This was further validated by the fact that IBU exists predominantly in its anionic form at pKa = 4.9 at pH = 10 [25] during the coprecipitation, where IBU anions are stabilized through electrostatic interactions within the interlayer spaces of positively charged LDH (Mg2Al(OH)6+).
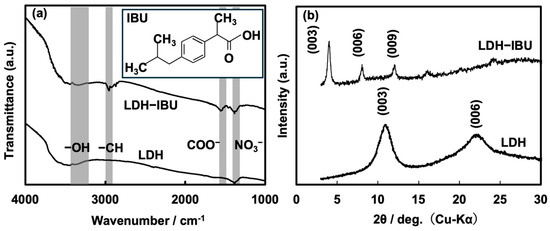
Figure 4.
(a) FTIR spectra and (b) XRD patterns of LDH–IBU and pristine LDH. The inset in (a) shows the molecular structure of IBU.
Polymer fibers were fabricated via electrospinning using an acetone solution containing PMMA and either LDH–IBU or IBU extruded through an 18- or 21-gauge (G) needle. Figure 5 contains digital microscope images of the electrospun PMMA–IBU semitransparent fibers with 7.5 wt% IBU prepared using the needle (18G (Figure 5a) or 21G (Figure 5b)), which were generated at the early stage before the formation of the fiber mat. The images revealed a consistently flattened and compressed morphology of both fibers. This characteristic shape was observed across almost all fibers, regardless of the needle gauge used. The distinctive flattened morphology is primarily attributed to the inherent instability of the Taylor cone formation during electrospinning [26]. Typically, a stable Taylor cone exhibits a well-defined conical geometry at the nozzle tip, which facilitates the formation of uniform cylindrical fibers. However, in these experiments, the Taylor cone adopted a flattened fan-like structure. As a result, the electrospun fibers inherited this pre-existing flattened geometry from the unstable, non-conical jet, leading to the formation of fibers with a notably compressed cross-section rather than a circular one. The average widths of both fibers that were estimated using 30 fibers are indicated in the images. As expected, the average width of the 21G fibers, which are narrower nozzles, is smaller than that of the 18G fibers.
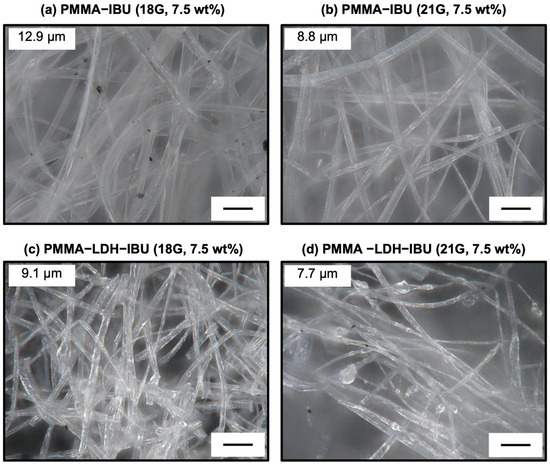
Figure 5.
Digital microscope images of electrospun fibers containing 7.5 wt% IBU: PMMA–IBU (a,b) and UPMMA–LDH–IBU (c,d), prepared using 18G (a,c) and 21G (b,d) needles. Fibers prepared with 21G needles exhibited smaller diameters (average 9.1 μm) compared to those prepared with 18G needles (average 12.9 μm), consistent with the effect of needle gauge on fiber morphology. Incorporation of LDH–IBU did not alter the flattened shape but improved dispersion within the fibers. Scale bars = 40 μm.
Digital microscopy images further elucidated the morphology of PMMA fibers containing 7.5 wt% LDH–IBU fabricated using 18G and 21G needles, as shown in Figure 5c and Figure 5d, respectively. Consistent with the observations for the plain IBU-loaded PMMA fibers, these LDH–IBU-containing fibers also exhibited a flattened morphology. Additionally, a slight reduction in the overall fiber diameter was noted, along with the presence of a few distinct bead-like structures along the fiber length. The formation of these bead-like structures is typically attributed to the onset of Plateau–Rayleigh instability [27]. This fundamental fluid dynamic phenomenon occurs when a liquid jet moving along its longitudinal axis is subjected to surface tension, which causes disturbances of certain wavelengths corresponding to the jet circumference to grow preferentially, ultimately leading to droplet or bead formation. In the context of electrospinning, Plateau–Rayleigh instability can be triggered and influenced by various factors, including the viscosity, concentration, and surface tension of the spinning solution. Because the presence of such bead structures can negatively affect the mechanical strength and overall uniformity of the resulting fibrous mats, it is essential to meticulously optimize the electrospinning parameters to control and minimize their formation.
Figure 6 presents low-magnification SEM views of the PMMA–IBU and UPMMA–LDH–IBU fiber mats containing 5 wt% IBU, along with magnified images focusing on individual fibers. A notable observation was the distinct morphological difference between the deposition surface and the reverse side of both fiber mats. This phenomenon is typically attributed to the continuous deposition of fibers during the electrospinning process, wherein the initially collected fiber layers on the collector become compressed and densified by the subsequent layers. Further detailed SEM analysis of the individual fibers from these mats revealed that both the PMMA–IBU and UPMMA–LDH–IBU fibers had an approximate diameter of 10 µm. In addition, according to the elemental distribution maps of the fibers (Figure S1), LDH and LDH–IBU were homogeneously distributed over the entire fiber because the elemental distributions of Mg, Al, and C almost overlapped each other. Importantly, the surfaces of these individual fibers exhibited observable pores. This porous morphology is generally attributed to the delayed evaporation of the residual solvent from within the stretched polymer jet during electrospinning, often caused by factors such as an excessively high applied voltage or an uncontrolled solution flow rate during fiber formation [28]. However, the porous structure formed on the fiber surfaces may be beneficial for enhancing the release efficiency and rate of IBU [29].
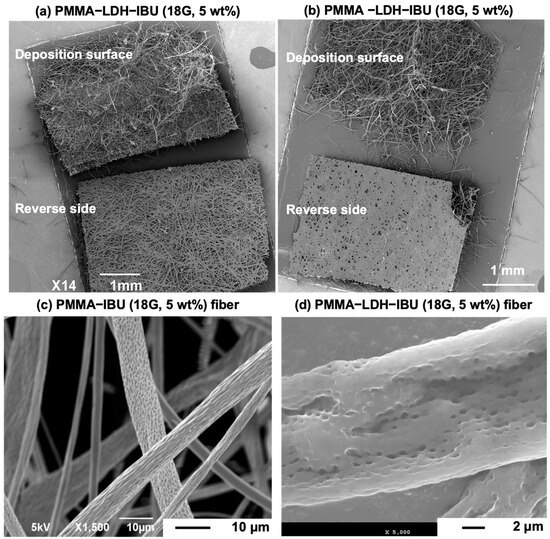
Figure 6.
SEM images of fiber mats containing 5 wt% IBU: PMMA–IBU (a,c) and UPMMA–LDH–IBU (b,d), both prepared with 18G needles. The mats exhibited different morphologies between top and bottom surfaces, attributed to compaction of the initial deposition layer during electrospinning. Individual fibers had diameters of ~10 μm with visible surface pores, which are likely formed by delayed solvent evaporation. The inclusion of LDH–IBU enhanced fiber uniformity and improved surface features compared to PMMA–IBU.
The drug release performance of ibuprofen (IBU) from electrospun fiber mats fabricated using both 18G and 21G needles was thoroughly investigated by immersion in a 0.1 M Tris-HCl buffer solution at pH = 7.6. Figure 7a presents the release profiles of IBU from PMMA–IBU and UPMMA–LDH–IBU fiber mats containing 5 wt% IBU prepared using 18G needles. To avoid confusion between the IBU concentration (wt%) in the fibers and the release efficiency (%), the latter is hereafter denoted as %R. The total amount of IBU in the mats exposed to the solution prior to its release was 5 mg. In both cases, the overall release kinetics exhibited a characteristic two-phase profile: an initial burst release attributed to the rapid dissolution of IBU near or on the fiber surface, followed by a more sustained, diffusion-controlled release of IBU from within the fiber structure over an extended period [30]. Focusing on %R from the mats, it was confirmed that the incorporation of LDH–IBU into the PMMA fiber matrix significantly enhanced the cumulative release of IBU and accelerated its release rate compared to fibers containing only free IBU. In the case of PMMA–IBU, because IBU is likely present in its anionic form, strong electrostatic interactions (such as hydrogen bonding) with PMMA, which contains carboxyl groups, are unlikely. However, the aromatic rings of IBU may interact with the PMMA main chain (–CH2–C(CH3)(COOH)–) via hydrophobic interactions, potentially hindering the outward diffusion of IBU from the PMMA matrix. In contrast, the pronounced increase in the IBU release rate is considered to result from alterations in the diffusivity of IBU throughout the fiber structure. In this context, the release of IBU anions from the UPMMA–LDH–IBU mat is thought to occur through ion exchange with chloride anions contained in the external Tris-HCl buffer solution. The contact angle measurements for PMMA–IBU and UPMMA–LDH–IBU with water droplets (Figure S2) revealed that the hydrophilicity of the former is higher than that of the latter. That is, water-soluble IBU anions could migrate more smoothly through the interlayer space of the hydrophilic LDH, resulting in a high releasing efficiency as schematically illustrated in the inset of Figure 7a. This was further supported by the following observation: after immersing the fiber mats in pure water for 24 h and subsequently drying them at 70 °C to evaluate water absorption (Figure S3), UPMMA–LDH–IBU exhibited approximately 1.5 times higher moisture loss (water uptake) per unit mass compared to PMMA–IBU. Thus, it was demonstrated that the intercalation of IBU into the LDH interlayer space significantly enhanced its release rate from the PMMA fiber matrix. However, despite the incorporation of LDH, the release rate of UPMMA–LDH–IBU remained below 8%R after 24 h.
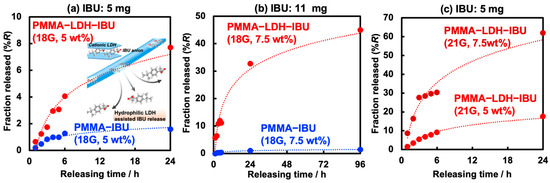
Figure 7.
Release profiles of IBU from PMMA–IBU and UPMMA–LDH–IBU, including (a) 5% and (b) 7.5 wt% IBU formed using 18G needles, respectively. The profiles of UPMMA–LDH–IBU (21G, 5 or 7.5 wt%) are also displayed in (c). The initial amount of IBU in the fibers in (a,c) was ~5 mg, and that in (b) was ~11 mg.
Figure 7b shows the release profiles from the fiber mats, in which a higher IBU concentration (7.5%) and greater total amount of IBU (11 mg) were used than in Figure 7a. For fibers loaded solely with IBU (PMMA–IBU), varying the drug content (5 or 7.5 wt%) did not result in a noticeable change in the release profile, with the release efficiencies remaining less than 2%R at 24 h. This suggests a limited influence of drug concentration on release efficiency in the absence of LDH. Conversely, the release efficiency was significantly improved for UPMMA–LDH–IBU, reaching 32 and 43%R after 24 and 96 h, respectively, highlighting the crucial role of LDH in modulating drug release. Such a dramatic improvement in the release rate of UPMMA–LDH–IBU upon increasing the IBU content from 5 to 7.5 wt% is considered to result from the formation of a percolated network of LDH, which provides diffusion pathways for IBU anions, similar to the percolation phenomenon observed when conductive materials are dispersed in insulators, thereby enhancing the diffusivity. The %R was also evaluated for UPMMA–LDH–IBU fiber mats prepared with 21G needles (Figure 7c). As expected, because the fibers fabricated using 21G needles were thinner and had a larger surface area than those prepared with 18G needles, the %R increased regardless of the IBU loading amount and exceeded 60%R after 24 h, which is a more than two-fold increase in IBU release with 5 wt% drug content. Thus, it was demonstrated that both the reduction in fiber diameter and intercalation into the LDH effectively facilitated the diffusion of IBU within the hydrophobic PMMA matrix. The release of IBU from UPMMA–LDH–IBU is considered to be governed by diffusion within the fiber. Therefore, the data for UPMMA–LDH–IBU in Figure 7a,c were fitted using two diffusion-controlled drug release models: the Higuchi and the Korsmeyer–Peppas model (See Table S1). As a result, the latter model showed a higher correlation coefficient, suggesting that a combination of factors such as diffusion and polymer swelling is involved in the diffusion of IBU. In the future, a detailed analysis of the IBU release mechanism is expected to enable control over the release rate.
Considering the potential applications in transdermal drug delivery systems (TDDS), the release behavior of IBU through artificial skin from the fiber mats was investigated. The fiber mat was placed at the bottom with its rough deposition surface (see Figure 3) facing upward and in contact with the artificial skin. The upper part was immersed in 0.1 M Tris-HCl buffer solution (pH = 7.6), and the amounts of IBU permeated at various time intervals were quantified by HPLC (Figure 8). Consistent with the results shown in Figure 7, the release experiment using artificial skin confirmed that the intercalation of IBU into the LDH interlayer significantly increased the amount of drug released. Despite the hydrophobic and porous solid artificial skin membrane being as thick as 0.3 mm, no lag time was observed in the initial stage of IBU release. Furthermore, the release efficiency of UPMMA–LDH–IBU reached approximately 18%R after 24 h, which was nearly equivalent to the rate observed when the mats were directly immersed in the buffer solution (17%R in Figure 7c). These results suggest that IBU can rapidly diffuse through the solid–solid interface between the fiber mat and artificial skin and subsequently across the membrane, implying that the rate-limiting step in the release of IBU from the fiber mats is not within the artificial skin but rather the diffusion of IBU anions within the PMMA fibers.
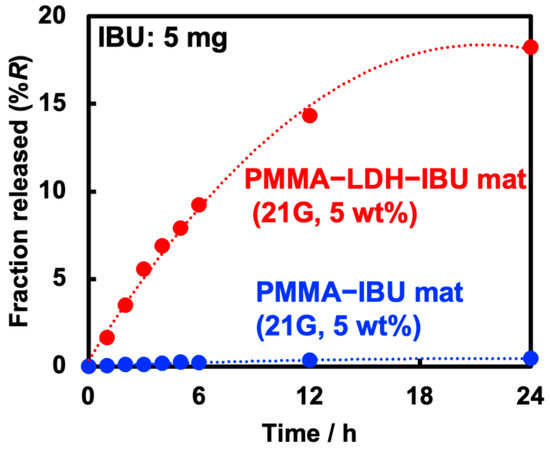
Figure 8.
IBU release behavior of PMMA–IBU and UPMMA–LDH–IBU (21G, 5 wt%), where the total IBU amount in the mats was 5 mg.
4. Conclusions
In this study, electrospinning (ES) was successfully employed to fabricate drug-loaded poly(methyl methacrylate) (PMMA) fiber mats that could be used in transdermal drug delivery systems (TDDS), where ibuprofen, commonly used as an analgesic, was employed as a model drug. Morphological characterization revealed that the fibers exhibited a flattened shape, and the widths varied depending on the inner diameter of the needle, reflecting the difference in the internal diameters of the needles used. IBU-intercalated layered double hydroxide (LDH–IBU) was successfully synthesized by co-precipitating IBU during the LDH synthesis process at a basic pH. Since IBU exists as an anion in neutral–basic solutions, this anionic form was intercalated into the interlayer spaces of LDH, which exhibits anion exchange capabilities, and then incorporated into PMMA fibers. All fabricated fiber samples immersed in neutral buffer solutions consistently showed a characteristic two-phase drug release pattern: an initial burst release, attributed to the rapid dissolution of IBU located near the fiber surface, followed by a sustained release phase, which was governed by the slower diffusion of IBU from the interior of the fiber. Based on the presence of hydrophilic LDHs within the hydrophobic PMMA matrix (UPMMA–LDH–IBU), the release efficiency of IBU was significantly improved compared to of that PMMA containing IBU not intercalated into LDH (PMMA–IBU). In addition to the release of IBU from fiber mats immersed in a buffer solution, the release of IBU from the solid–solid interface formed by attaching the mats to artificial skin was evaluated. The results showed a release rate comparable to that of the former. This finding suggests that the rate-limiting step in the desorption of IBU from UPMMA–LDH–IBU is the migration of IBU within the fibers. The desorption or ion exchange of guest molecules such as IBU from layered materials such as LDH is considered to be further enhanced by orienting the layered planes outward. Therefore, by controlling the orientation of anisotropic LDH–IBU crystals within the fibers, it may be possible to further increase (or regulate) the release rate, which is regarded as a subject for future investigation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ceramics8040124/s1, Figure S1: SEM image and elemental distribution maps of a UPMMA–LDH–IBU (18G, 5 wt%) fiber with a characteristic X-ray spectrum, Figure S2: Contact angle measurements of PMMA–IBU (5 wt%) and UPMMA–LDH–IBU (5 wt%) films with water droplets, Figure S3: Water evaporation behavior of PMMA–IBU (18G, 5 wt%) and UPMMA–LDH–IBU (18G, 5 wt%) after immersion in pure water for 24 h. Table S1: Fitting parameters of release profiles of UPMMA–LDH–IBU in Figure 7a and c for the Higuchi and Korsmeyer–Peppas model.
Author Contributions
Conceptualization, Methodology, and Writing—review and editing, K.K.; Investigation and Writing—original draft, V.T.T.T. and S.Y.; Analysis, O.N., H.S., S.T. The authors contributed to all phases of the research, from conception to experimental development, analysis of results, and writing of the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly funded by JST SPRING (Grant Number JPMJSP2172) and JSPS KAKENHI (Grant Number JP25K01861).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Jamrozy, M.; Kudlacik-Kramarczyk, S.; Drabczyk, A.; Krzan, M. Advanced Drug Carriers: A Review of Selected Protein, Polysaccharide, and Lipid Drug Delivery Platforms. Int. J. Mol. Sci. 2024, 25, 786. [Google Scholar] [CrossRef]
- Sanjivani, A.C.; Vikas, K.G.; Shivshankar, D.M.; Rutuja, G.W.; Tejas, J.S. Transdermal Drug Delivery System: A Review of Current Advances and Challenges. J. Ayurveda Integr. Med. Sci. 2025, 9, 203–212. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; Nasereddin, J.; Hamed, R.; Nimrawi, S.; Hussein, G.; Abo-Zour, H.; Donnelly, R.F. Beneath the Skin: A Review of Current Trends and Future Prospects of Transdermal Drug Delivery Systems. Pharmaceutics 2022, 14, 1152. [Google Scholar] [CrossRef]
- Shankhapal, S.; Pandav, V.; Jaiswal, R.; Kalam, S. Transdermal Drug Delivery System. Int. J. Pharm. Sci. 2024, 2, 3570–3580. [Google Scholar]
- Tai, F.W.D.; McAlindon, M.E. Non-steroidal anti-inflammatory drugs and the gastrointestinal tract. Clin. Med. 2021, 21, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Bushra, R.; Aslam, N. An overview of clinical pharmacology of Ibuprofen. Oman Med. J. 2010, 25, 155–1661. [Google Scholar] [CrossRef]
- Elsie, E.; Gaskell, T.H.; Hamilton, A.R. Ibuprofen intercalation and release from different layered double hydroxides. Ther. Deliv. 2018, 9, 653–666. [Google Scholar] [CrossRef]
- Bi, X.; Zhang, H.; Dou, L. Layered double hydroxide-based nanocarriers for drug delivery. Pharmaceutics 2014, 6, 298–332. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, M.; Szerlauth, A.; Murath, S.; Varga, G.; Szilagyi, I. Surface modification of two-dimensional layered double hydroxide nanoparticles with biopolymers for biomedical applications. Adv. Drug Deliv. Rev. 2022, 191, 114590. [Google Scholar] [CrossRef]
- Arrabito, G.; Pezzilli, R.; Prestopino, G.; Medaglia, P.G. Layered Double Hydroxides in Bioinspired Nanotechnology. Crystals 2020, 10, 602. [Google Scholar] [CrossRef]
- Rives, V.; del Arco, M.; Martín, C. Intercalation of drugs in layered double hydroxides and their controlled release: A review. Appl. Clay Sci. 2014, 88–89, 239–269. [Google Scholar] [CrossRef]
- Wang, C.; Su, Y.; Xie, J. Advances in Electrospun Nanofibers: Versatile Materials and Diverse Biomedical Applications. Acc. Mater. Res. 2024, 5, 987–999. [Google Scholar] [CrossRef]
- Franco, R.F.; Jimenez, P.C. Pharmacological Applications of Electrospun Nanofibers Loaded with Bioactive Natural Compounds and Extracts: A Systematic Review. Drugs Drug Candidates 2025, 4, 8. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Fast Dissolving Oral Drug Delivery System Based on Electrospun Nanofibrous Webs of Cyclodextrin/Ibuprofen Inclusion Complex Nanofibers. Mol. Pharm. 2019, 16, 4387–4398. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Liu, J.; Tang, R.; Liu, Z.; Wu, J. Chitosan and ibuprofen functionalized electrospun nanofiber membrane modulates inflammatory response and promotes full-thickness abdominal wall repair. Int. J. Biol. Macromol. 2025, 321, 146380. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, D.; Zhang, Z.; Pan, J.; Cui, Z.; Yu, D.-G.; Annie Bligh, S.-W. Testing of fast dissolution of ibuprofen from its electrospun hydrophilic polymer nanocomposites. Polym. Test. 2021, 93, 106872. [Google Scholar] [CrossRef]
- Immich, A.P.; Arias, M.L.; Carreras, N.; Boemo, R.L.; Tornero, J.A. Drug delivery systems using sandwich configurations of electrospun poly(lactic acid) nanofiber membranes and ibuprofen. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4002–4008. [Google Scholar] [CrossRef] [PubMed]
- Riggin, C.N.; Qu, F.; Kim, D.H.; Huegel, J.; Steinberg, D.R.; Kuntz, A.F.; Soslowsky, L.J.; Mauck, R.L.; Bernstein, J. Electrospun PLGA Nanofiber Scaffolds Release Ibuprofen Faster and Degrade Slower After In Vivo Implantation. Ann. Biomed. Eng. 2017, 45, 2348–2359. [Google Scholar] [CrossRef]
- Bae, H.-S.; Haider, A.; Selim, K.M.K.; Kang, D.-Y.; Kim, E.-J.; Kang, I.-K. Fabrication of highly porous PMMA electrospun fibers and their application in the removal of phenol and iodine. J. Polym. Res. 2013, 20, 158. [Google Scholar] [CrossRef]
- Abdulhussain, R.; Adebisi, A.; Conway, B.R.; Asare-Addo, K. Electrospun nanofibers: Exploring process parameters, polymer selection, and recent applications in pharmaceuticals and drug delivery. J. Drug Deliv. Sci. Technol. 2023, 90, 105156. [Google Scholar] [CrossRef]
- Philip, P.; Tomlal Jose, E.; Chacko, J.K.; Philip, K.C.; Thomas, P.C. Preparation and characterisation of surface roughened PMMA electrospun nanofibers from PEO-PMMA polymer blend nanofibers. Polym. Test. 2019, 74, 257–265. [Google Scholar] [CrossRef]
- Sivakumar, M.; Rao, K.P. Synthesis and characterization of poly(methyl methacrylate) functional microspheres. React. Funct. Polym. 2000, 46, 29–37. [Google Scholar] [CrossRef]
- Cardinale, A.M.; Carbone, C.; Molinari, S.; Salviulo, G.; Ardini, F. MgAl-NO3 LDH: Adsorption Isotherms and Multivariate Optimization for Cr(VI) Removal. Chemistry 2023, 5, 633–645. [Google Scholar] [CrossRef]
- Yamin, M.; Ghouri, Z.K.; Rohman, N.; Syed, J.A.; Skelton, A.; Ahmed, K. Unravelling pH/pKa influence on pH-responsive drug carriers: Insights from ibuprofen-silica interactions and comparative analysis with carbon nanotubes, sulfasalazine, and alendronate. J. Mol. Graph. Model. 2024, 128, 108720. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Q.; Huang, F.; Wei, Q. The morphology of Taylor cone influenced by different coaxial composite nozzle structures. Fibers Polym. 2016, 17, 624–629. [Google Scholar] [CrossRef]
- Gabbard, C.T.; Bostwick, J.B. Scaling analysis of the Plateau–Rayleigh instability in thin film flow down a fiber. Exp. Fluids 2021, 62, 141. [Google Scholar] [CrossRef]
- Li, L.; Jiang, Z.; Li, M.; Li, R.; Fang, T. Hierarchically structured PMMA fibers fabricated by electrospinning. RSC Adv. 2014, 4, 52973–52985. [Google Scholar] [CrossRef]
- Ma, M.; Sun, R.; Kang, H.; Wang, S.; Jia, P.; Song, Y.; Sun, J. Direct writing concave structure on viscoelastic substrate for loading and releasing liquid on skin surface. Colloids Surf. B. Biointerfaces 2023, 231, 113571. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wei, Z.; Zhao, H.; Liu, T.; Dong, A.; Zhang, J. Electrospinning of Ibuprofen-Loaded Composite Nanofibers for Improving the Performances of Transdermal Patches. J. Nanosci. Nanotechnol. 2013, 13, 3855–3863. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).