Hydrothermal Synthesis Optimization of High-Aspect Ratio α-Al2O3 Microfibers for Thermally Conductive Soft Composites
Abstract
1. Introduction
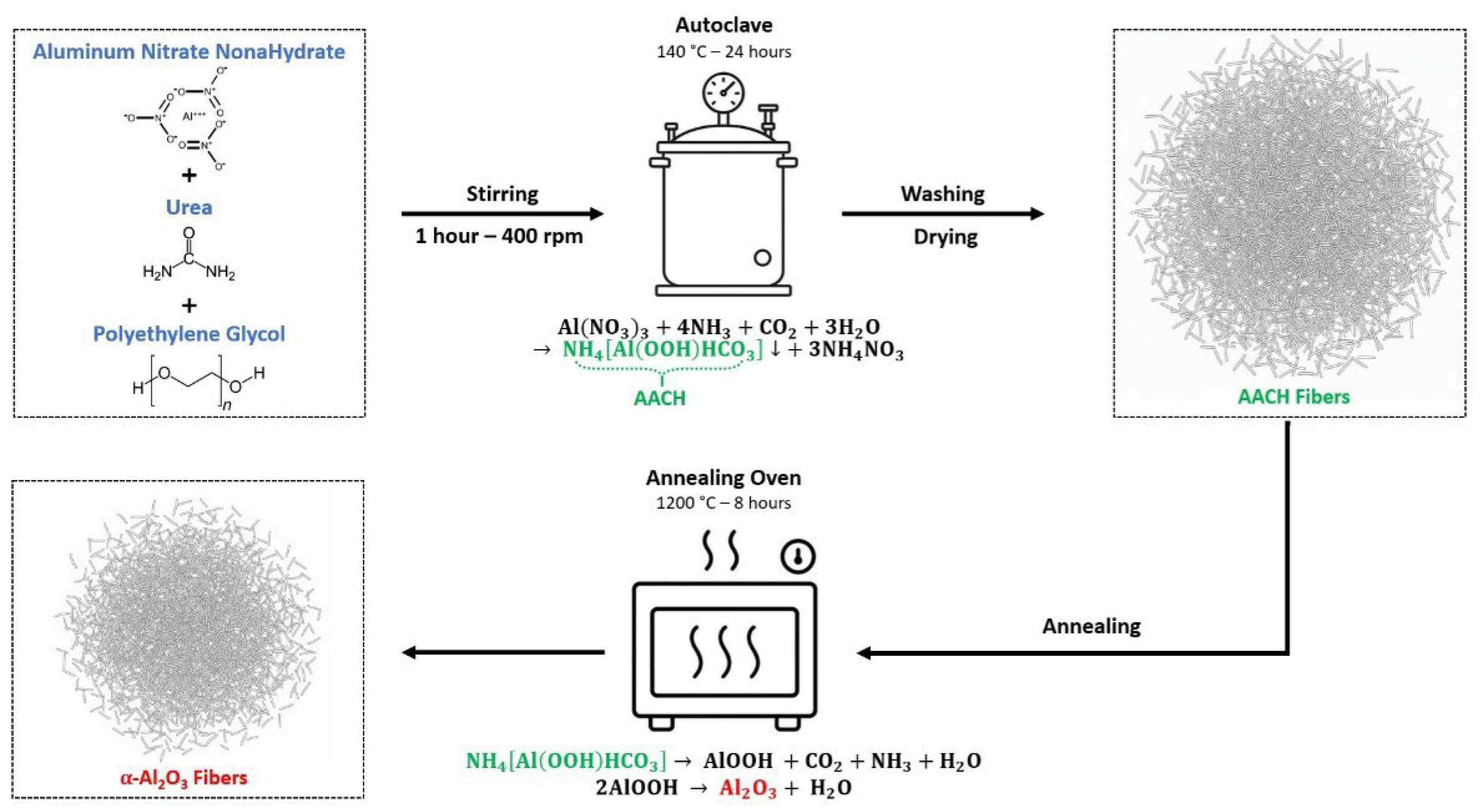
2. Materials and Methods
3. Results and Discussion
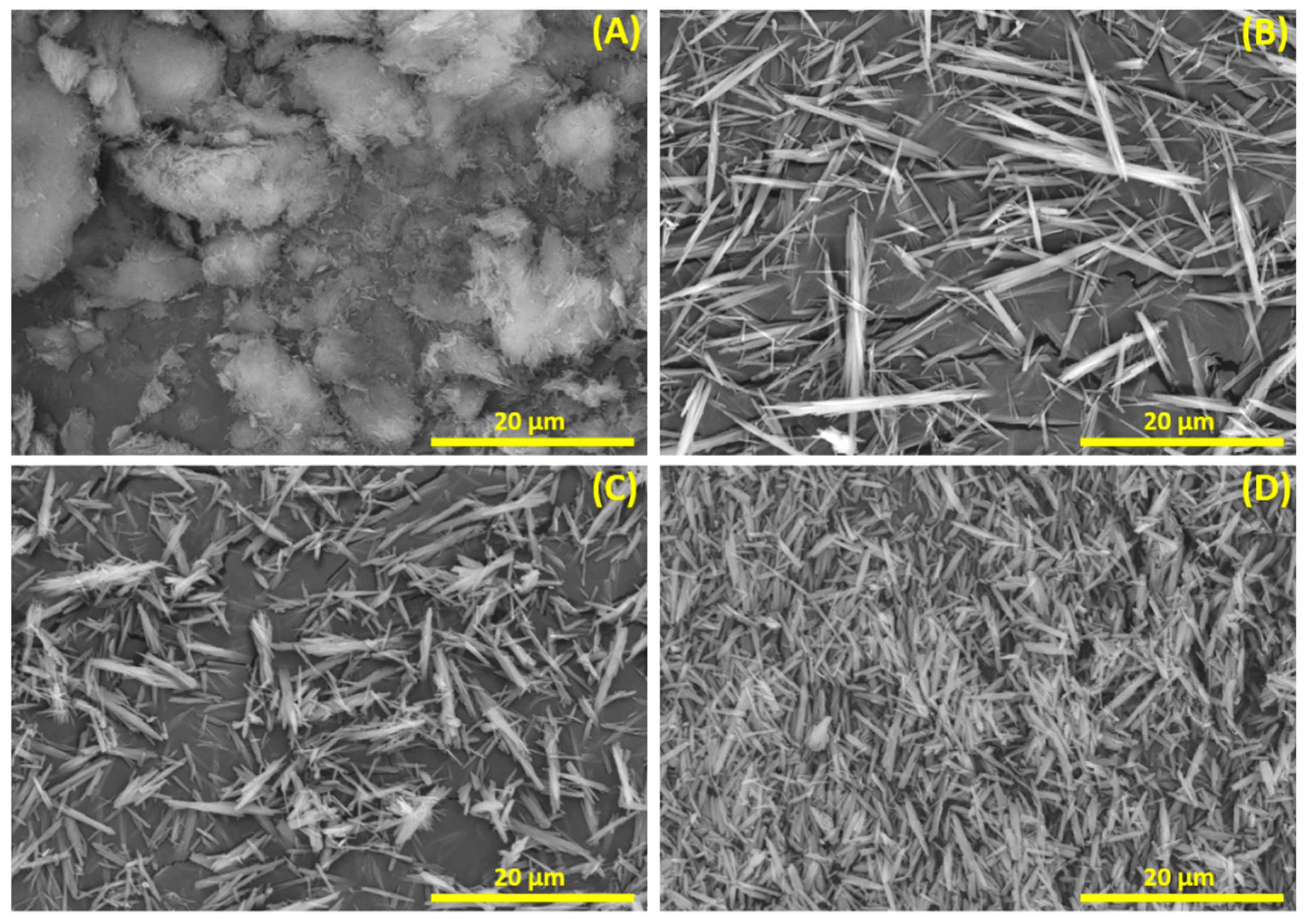
3.1. Improving Solubility Kinetics in the Synthesis Process
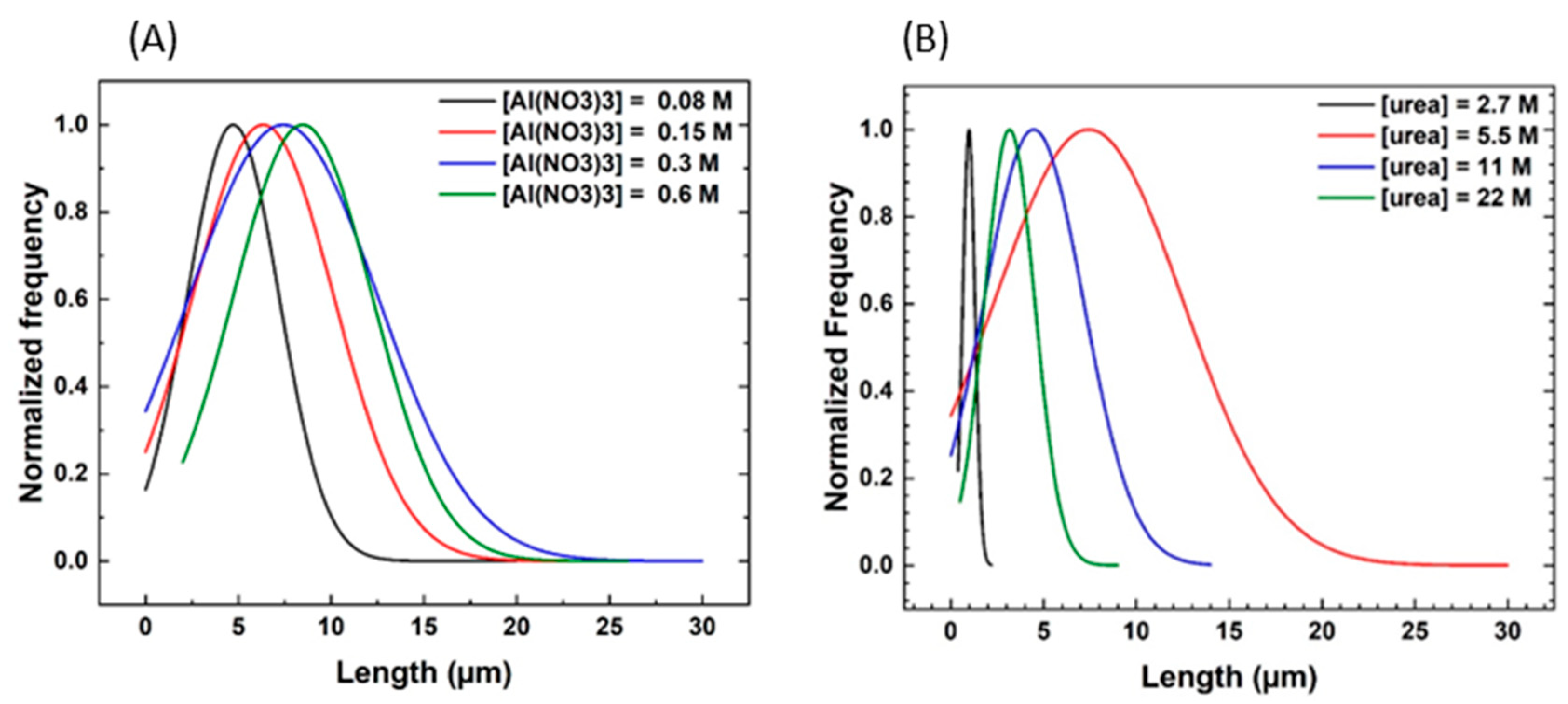
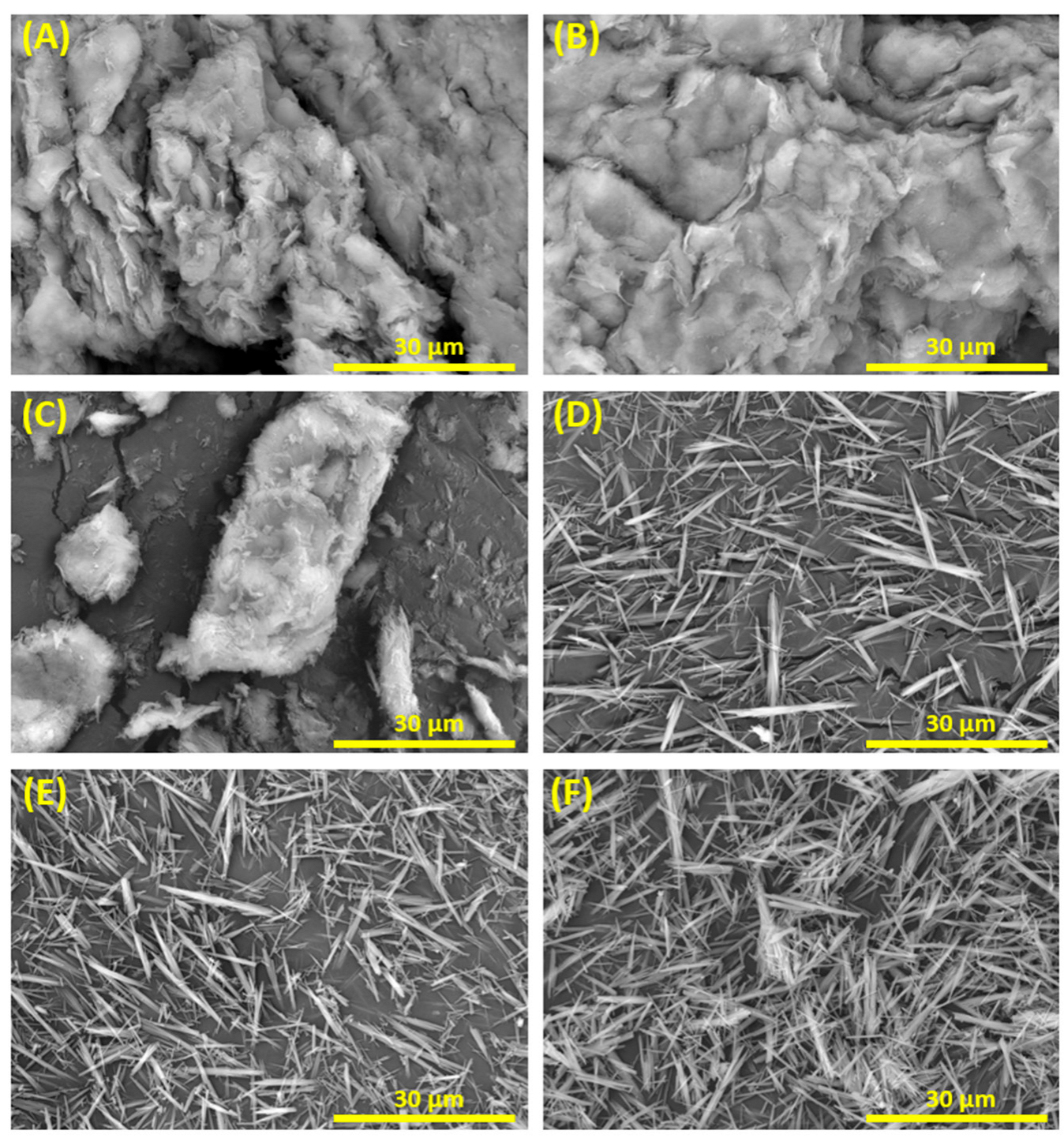
3.2. Influence of the Hydrothermal Synthesis Reagents
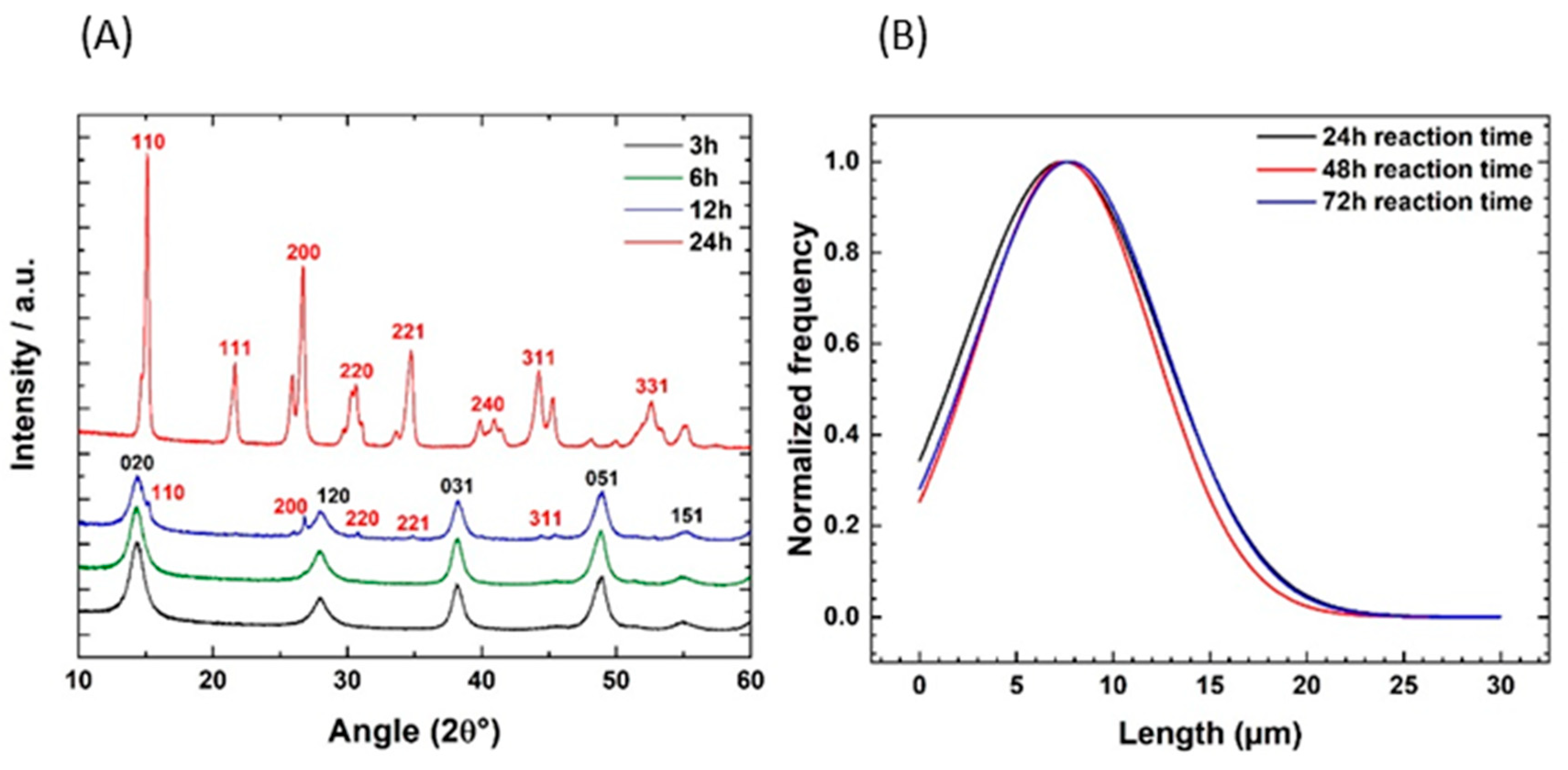
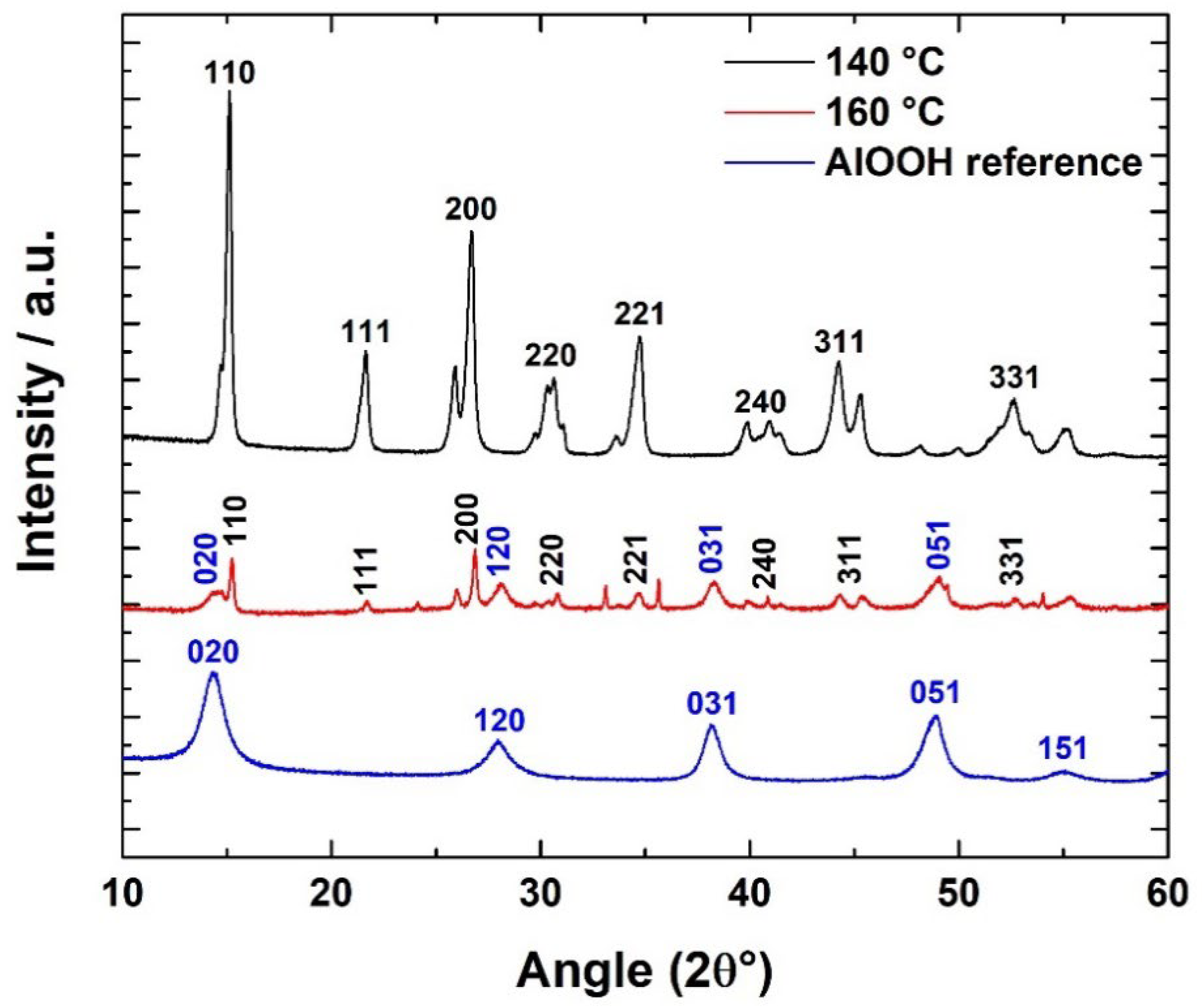
3.3. Time-Temperature Dependence of the Hydrothermal Reaction
3.4. From AACH to α-Al2O3 Fibers
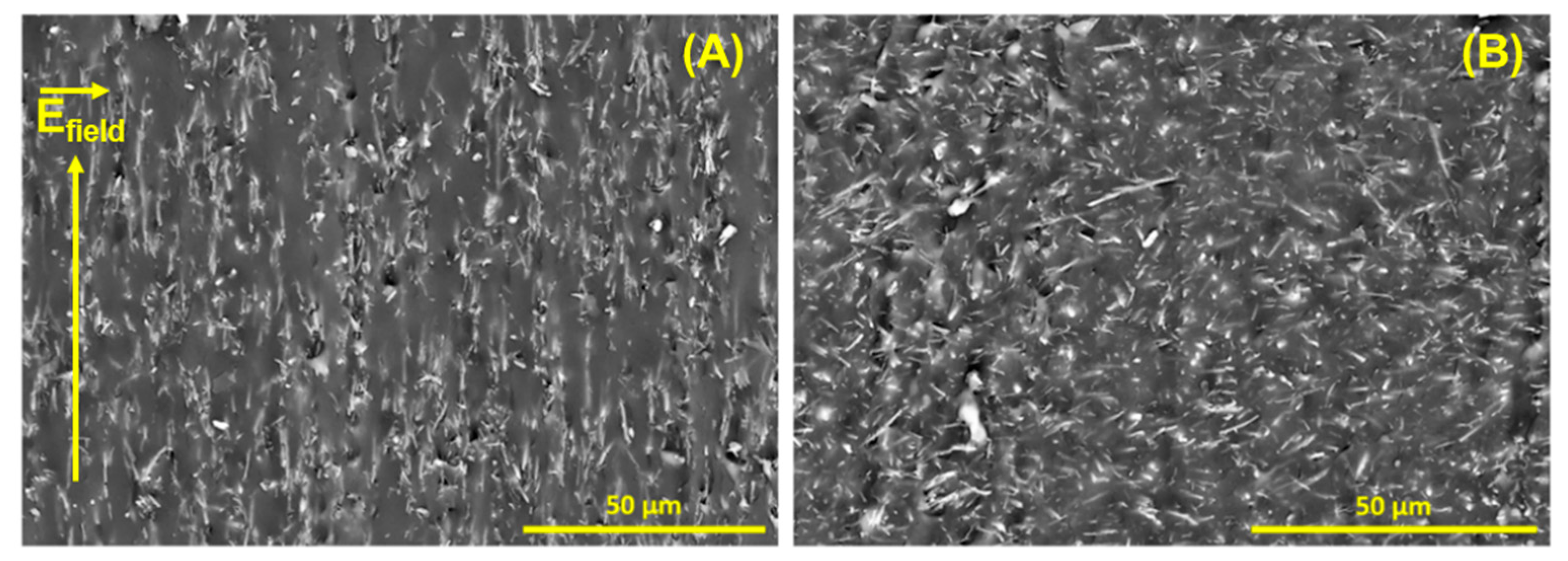
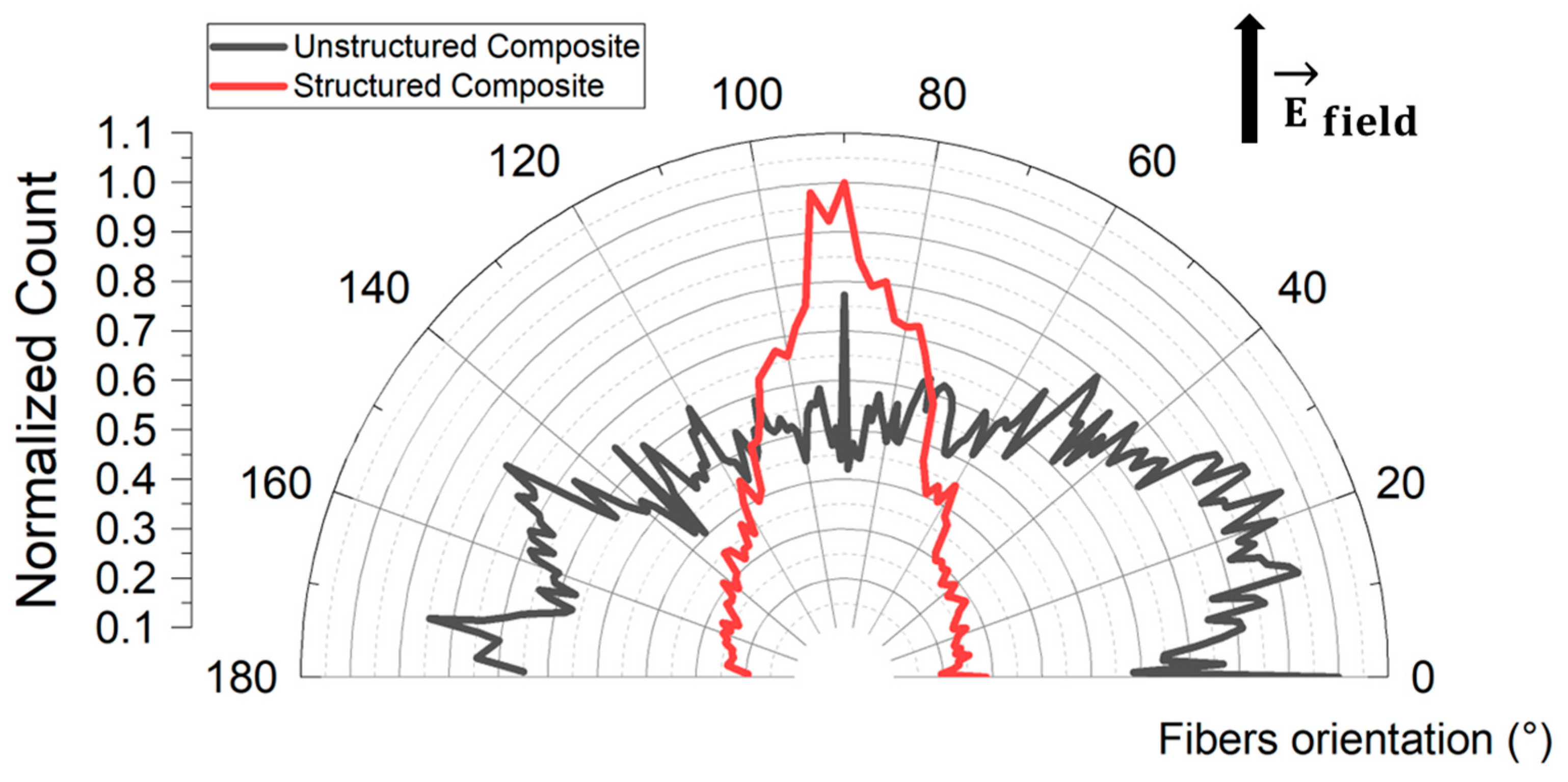
3.5. Development of Hybrid PDMS-Based Composites from α-Al2O3 Fibers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Wu, Z.; Weng, L.; Ge, S.; Jiang, D.; Huang, M.; Mulvihill, D.M.; Chen, Q.; Guo, Z.; Jazzar, A.; et al. A Roadmap Review of Thermally Conductive Polymer Composites: Critical Factors, Progress, and Prospects. Adv. Funct. Mater. 2023, 33, 2301549. [Google Scholar] [CrossRef]
- Fan, P.; Sun, Z.; Wang, Y.; Chang, H.; Zhang, P.; Yao, S.; Lu, C.; Rao, W.; Liu, J. Nano liquid metal for the preparation of a thermally conductive and electrically insulating material with high stability. RSC Adv. 2018, 8, 16232–16242. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhao, Q.; Li, C.; Wang, S.; Dong, L.; Hu, G.-H.; Yang, Q.; Xiong, C. A novel fluid-filler/polymer composite as high-temperature thermally conductive and electrically insulating material. Compos. Sci. Technol. 2017, 150, 128–134. [Google Scholar] [CrossRef]
- Schelling, P.K.; Shi, L.; Goodson, K.E. Managing heat for electronics. Mater. Today 2005, 8, 30–35. [Google Scholar] [CrossRef]
- Ye, M.; Jiang, J.; Zhao, L.; Zhu, H.; Wang, J.; Sun, Z.; Zhang, D.; Li, M.; Zhang, Y. Preparation of Phenolic Epoxy-Based Electronic Packaging Materials with High Thermal Conductivity by Creating an Interfacial Heat Conduction Network. Polymers 2025, 17, 1507. [Google Scholar] [CrossRef]
- Rivière, L.; Caussé, N.; Lonjon, A.; Dantras, É.; Lacabanne, C. Specific heat capacity and thermal conductivity of PEEK/Ag nanoparticles composites determined by Modulated-Temperature Differential Scanning Calorimetry. Polym. Degrad. Stab. 2016, 127, 98–104. [Google Scholar] [CrossRef]
- Coulson, M.; Dantras, E.; Olivier, P.; Gleizes, N.; Lacabanne, C. Thermal conductivity and diffusivity of carbon-reinforced polyetherketoneketone composites. J. Appl. Polym. Sci. 2019, 136, 47975. [Google Scholar] [CrossRef]
- Pornea, A.G.; Dinh, D.K.; Hanif, Z.; Yanar, N.; Choi, K.-I.; Kwak, M.S.; Kim, J. Preparations and Thermal Properties of PDMS-AlN-Al2O3 Composites through the Incorporation of Poly(Catechol-Amine)-Modified Boron Nitride Nanotubes. Nanomaterials 2024, 14, 847. [Google Scholar] [CrossRef] [PubMed]
- Zahhaf, O.; D’Ambrogio, G.; Le, M.-Q.; Coativy, G.; Grasland, F.; Cottinet, P.-J.; Capsal, J.-F. Dielectrophoretic alignment of Al2O3/PDMS composites: Enhancement of thermal and dielectric properties through structural sedimentation analysis. Mater. Des. 2021, 211, 110134. [Google Scholar] [CrossRef]
- Sun, Y.; Yin, Y.; Mayers, B.T.; Herricks, T.; Xia, Y. Uniform Silver Nanowires Synthesis by Reducing AgNO3 with Ethylene Glycol in the Presence of Seeds and Poly(Vinyl Pyrrolidone). Chem. Mater. 2002, 14, 4736–4745. [Google Scholar] [CrossRef]
- Liu, P.; Gao, S.; Wang, Y.; Zhou, F.; Huang, Y.; Luo, J. Metal-organic polymer coordination materials derived Co/N-doped porous carbon composites for frequency-selective microwave absorption. Compos. Part B Eng. 2020, 202, 108406. [Google Scholar] [CrossRef]
- Liu, P.; Gao, S.; Liu, X.; Huang, Y.; He, W.; Li, Y. Rational construction of hierarchical hollow CuS@CoS2 nanoboxes with heterogeneous interfaces for high-efficiency microwave absorption materials. Compos. Part B Eng. 2020, 192, 107992. [Google Scholar] [CrossRef]
- Nan, C.-W. Physics of inhomogeneous inorganic materials. Prog. Mater. Sci. 1993, 37, 1–116. [Google Scholar] [CrossRef]
- Newnham, R.E.; Skinner, D.P.; Cross, L.E. Connectivity and piezoelectric-pyroelectric composites. Mater. Res. Bull. 1978, 13, 525–536. [Google Scholar] [CrossRef]
- Wang, J.; Hu, L.; Li, W.; Ouyang, Y.; Bai, L. Development and Perspectives of Thermal Conductive Polymer Composites. Nanomaterials 2022, 12, 3574. [Google Scholar] [CrossRef]
- Lonjon, A.; Caffrey, I.; Carponcin, D.; Dantras, E.; Lacabanne, C. High electrically conductive composites of Polyamide 11 filled with silver nanowires: Nanocomposites processing, mechanical and electrical analysis. J. Non-Cryst. Solids 2013, 376, 199–204. [Google Scholar] [CrossRef]
- Bedel, V.; Lonjon, A.; Dantras, É.; Bouquet, M.; Lacabanne, C. Dynamic electrical and mechanical properties of epoxy/silver nanowires composites. J. Appl. Polym. Sci. 2022, 139, 51710. [Google Scholar] [CrossRef]
- Tan, J.; Zhang, Y. Thermal Conductive Polymer Composites: Recent Progress and Applications. Molecules 2024, 29, 3572. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, Y.; Li, T.; Ma, P.; Zhang, S.; Du, M.; Chen, M.; Dong, W.; Ming, W. Highly thermal conductive and electrically insulating polymer composites based on polydopamine-coated copper nanowire. Compos. Sci. Technol. 2018, 164, 153–159. [Google Scholar] [CrossRef]
- David, C.; Capsal, J.-F.; Laffont, L.; Dantras, E.; Lacabanne, C. Piezoelectric properties of polyamide 11/NaNbO3 nanowire composites. J. Phys. Appl. Phys. 2012, 45, 415305. [Google Scholar] [CrossRef]
- van den Ende, D.A.; Bory, B.F.; Groen, W.A.; van der Zwaag, S. Improving the d33 and g33 properties of 0-3 piezoelectric composites by dielectrophoresis. J. Appl. Phys. 2010, 107, 024107. [Google Scholar] [CrossRef]
- D’Ambrogio, G.; Zahhaf, O.; Le, M.-Q.; Bordet, M.; Lermusiaux, P.; Della Schiava, N.; Liang, R.; Cottinet, P.-J.; Capsal, J.-F. Piezoelectric biosensor for smart cardiovascular grafts based on NaNbO3 fibers/PDMS structured composite. Mater. Des. 2022, 223, 111195. [Google Scholar] [CrossRef]
- Živcová, Z.; Gregorová, E.; Pabst, W.; Smith, D.S.; Michot, A.; Poulier, C. Thermal conductivity of porous alumina ceramics prepared using starch as a pore-forming agent. J. Eur. Ceram. Soc. 2009, 29, 347–353. [Google Scholar] [CrossRef]
- Bharthasaradhi, R.; Nehru, L.C. Structural and phase transition of α-Al2O3 powders obtained by co-precipitation method. Phase Transit. 2016, 89, 77–83. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, H.; Sun, H.; Yang, D. PEG-directed hydrothermal synthesis of multilayered alumina microfibers with mesoporous structures. Microporous Mesoporous Mater. 2009, 123, 39–44. [Google Scholar] [CrossRef]
- Wu, C.; Zhan, L.; Liu, H.; Wang, J.; Liu, W.; Yao, S.; Ma, Y. The oriented growth behavior of α-Al2O3 grains in alumina-mullite biphasic fibers. J. Eur. Ceram. Soc. 2024, 44, 319–327. [Google Scholar] [CrossRef]
- Gai, K.; Wang, Q.; Li, J.; Lu, B.; Liang, L.; Tai, J.; Cheng, Y.; Guan, B.; Zhao, T. Studies on the Crystallization Behavior of Al2O3-HfO2 Ceramic Fibers Prepared by Melt-spinning of Polymer Precursors. J. Eur. Ceram. Soc. 2023, 43, 5587–5595. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, J.; Liu, H. Urea controlled hydrothermal synthesis of ammonium aluminum carbonate hydroxide rods. AIP Adv. 2018, 8, 035103. [Google Scholar] [CrossRef]
- Salzmann, B.B.V.; Van Der Sluijs, M.M.; Soligno, G.; Vanmaekelbergh, D. Oriented Attachment: From Natural Crystal Growth to a Materials Engineering Tool. Acc. Chem. Res. 2021, 54, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Sun, H.; Liu, H.; Yang, D. PEG-directed hydrothermal synthesis of alumina nanorods with mesoporous structure via AACH nanorod precursors. J. Mater. Sci. 2010, 45, 46–50. [Google Scholar] [CrossRef]
- Muhammad, A. An Overview of Mesoporous Alumina Synthesis with Controlled Particle Size Distribution and Improved Textural Morphology. Iran. J. Chem. Chem. Eng. 2024, 43, 522–543. [Google Scholar] [CrossRef]
- Sun, Z.-X.; Zheng, T.-T.; Bo, Q.-B.; Du, M.; Forsling, W. Effects of calcination temperature on the pore size and wall crystalline structure of mesoporous alumina. J. Colloid Interface Sci. 2008, 319, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.Y.; Riches, J.D.; Barry, J.C. γ-Alumina Nanofibers Prepared from Aluminum Hydrate with Poly(ethylene oxide) Surfactant. Chem. Mater. 2002, 14, 2086–2093. [Google Scholar] [CrossRef]
- Kim, D.; Jung, J.H.; Ihm, J. Theoretical Study of Aluminum Hydroxide as a Hydrogen-Bonded Layered Material. Nanomaterials 2018, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Liu, H.; Sun, H.; Yang, D. Surfactant assisted hydrothermal and thermal decomposition synthesis of alumina microfibers with mesoporous structure. Chem. Eng. J. 2009, 155, 925–930. [Google Scholar] [CrossRef]
- Shi, X.; Yang, C.; Zhang, L.; Lu, Z.; Zhu, Y.; Tang, D.; Cui, C.; Zeng, H. Mesoporous alumina microfibers in situ transformation from AACH fibers and the adsorption performance. J. Nanomater. 2014, 2014, 381235. [Google Scholar] [CrossRef]
- Bai, P.; Su, F.; Wu, P.; Wang, L.; Lee, F.Y.; Lv, L.; Yan, Z.F.; Zhao, X.S. Copolymer-controlled homogeneous precipitation for the synthesis of porous microfibers of alumina. Langmuir 2007, 23, 4599–4605. [Google Scholar] [CrossRef]
- Schott, C.M.; Schneider, P.M.; Song, K.-T.; Yu, H.; Götz, R.; Haimerl, F.; Gubanova, E.; Zhou, J.; Schmidt, T.O.; Zhang, Q.; et al. How to Assess and Predict Electrical Double Layer Properties. Implications for Electrocatalysis. Chem. Rev. 2024, 124, 12391–12462. [Google Scholar] [CrossRef]
- Xue, X.; Penn, R.L.; Leite, E.R.; Huang, F.; Lin, Z. Crystal growth by oriented attachment: Kinetic models and control factors. CrystEngComm 2014, 16, 1419. [Google Scholar] [CrossRef]
- Van Westen, T.; Groot, R.D. Effect of Temperature Cycling on Ostwald Ripening. Cryst. Growth Des. 2018, 18, 4952–4962. [Google Scholar] [CrossRef]
- Ouyang, R.; Liu, J.-X.; Li, W.-X. Atomistic Theory of Ostwald Ripening and Disintegration of Supported Metal Particles under Reaction Conditions. J. Am. Chem. Soc. 2013, 135, 1760–1771. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yang, Z.; Zhao, X.; Ji, H.; Peng, C.; Sui, B.; Chen, W.; Zhang, J.; Qian, G.; Zhou, X.; et al. Kinetics and mechanistic insights into the hydrothermal synthesis of alumina microrods. Chem. Eng. Sci. 2021, 244, 116817. [Google Scholar] [CrossRef]
- Atkins, P.; de Paula, J. Physical Chemistry for the Life Sciences; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Liu, H.; Sun, H.; Li, J.; He, X.; Zhu, Z. PH-dependent formation of AACH fibers with tunable diameters and their in situ transformation to alumina nanocrystals with mesoporous structure. Adv. Powder Technol. 2012, 23, 164–169. [Google Scholar] [CrossRef]
- Mirzajany, R.; Alizadeh, M.; Rahimipour, M.R.; Saremi, M. Seed-assisted hydrothermally synthesized AACH as the alumina precursors. Mater. Chem. Phys. 2019, 221, 188–196. [Google Scholar] [CrossRef]
- Hou, H.; Xie, Y.; Yang, Q.; Guo, Q.; Tan, C. Preparation and characterization of γ-AlOOH nanotubes and nanorods. Nanotechnology 2005, 16, 741–745. [Google Scholar] [CrossRef]
- Li, G.; Liu, Y.; Guan, L.; Hu, X.; Liu, C. Meso/macroporous γ-Al2O3 fabricated by thermal decomposition of nanorods ammonium aluminium carbonate hydroxide. Mater. Res. Bull. 2012, 47, 1073–1079. [Google Scholar] [CrossRef]
- Parida, K.M.; Pradhan, A.C.; Das, J.; Sahu, N. Synthesis and characterization of nano-sized porous gamma-alumina by control precipitation method. Mater. Chem. Phys. 2009, 113, 244–248. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Yan, Z. Facile route to prepare bimodal mesoporous γ-Al2O3 as support for highly active CoMo-based hydrodesulfurization catalyst. Appl. Catal. B Environ. 2012, 121–122, 50–56. [Google Scholar] [CrossRef]
- Prins, R. On the structure of γ-Al2O3. J. Catal. 2020, 392, 336–346. [Google Scholar] [CrossRef]
- Cheng, J.P.; Liu, T.; Zhang, J.; Wang, B.B.; Ying, J.; Liu, F.; Zhang, X.B. Influence of phase and morphology on thermal conductivity of alumina particle/silicone rubber composites. Appl. Phys. Mater. Sci. Process. 2014, 117, 1985–1992. [Google Scholar] [CrossRef]
- Sadabadi, H.; Aftabtalab, A.; Zafarian, S.; Shaker, S.; Ahmadipour, M.; Venkateswara, K. High purity Alpha Alumina nanoparticle: Synthesis and characterization. Int. J. Sci. Eng. Res. 2013, 4, 1593–1595. [Google Scholar]
- Morinaga, K.; Torikai, T.; Nakagawa, K.; Fujino, S. Fabrication of fine α -alumina powders by thermal decomposition of ammonium aluminum carbonate hydroxide (AACH). Acta Mater. 2000, 48, 4735–4741. [Google Scholar] [CrossRef]
- Wang, W.; Bi, J.; Qi, Y.; Zhang, Z.; Xing, Z.; Zhu, H.; Guo, D.; Xu, J.; Bai, Y. Preparation of micrometer-sized α-Al2O3 platelets by thermal decomposition of AACH. Powder Technol. 2010, 201, 273–276. [Google Scholar] [CrossRef]
- Yong-Taeg, O.; Kim, S.; Shin, D. Fabrication and synthesis of α-alumina nanopowders by thermal decomposition of ammonium aluminum carbonate hydroxide (AACH). Colloids Surf. A Physicochem. Eng. Asp. 2008, 314, 415–418. [Google Scholar] [CrossRef]
- Zhang, H.J.; Wang, X.J.; Jia, Q.L.; Sun, H.W. Artificial neural network analysis of preparation of nano α-Al2O3 powders by thermal decomposition of ammonium aluminium carbonate hydroxide. Mater. Sci. Technol. 2007, 23, 1021–1027. [Google Scholar] [CrossRef]
- Huang, Y.; Xia, Y.; Liao, S.; Tong, Z.; Liu, G.; Li, Y.; Chen, Z. Synthesis of α-Al2O3 platelets and kinetics study for thermal decomposition of its precursor in molten salt. Ceram. Int. 2014, 40, 8071–8079. [Google Scholar] [CrossRef]
- He, H.; Wang, Y.; Farkas, B.; Nagy, Z.K.; Molnar, K. Analysis and prediction of the diameter and orientation of AC electrospun nanofibers by response surface methodology. Mater. Des. 2020, 194, 108902. [Google Scholar] [CrossRef]
- Tsekmes, I.A.; Kochetov, R.; Morshuis, P.H.F.; Smit, J.J. Thermal conductivity of polymeric composites: A review. In Proceedings of the 2013 IEEE International Conference on Solid Dielectrics (ICSD), Bologna, Italy, 30 June–4 July 2013; pp. 678–681. [Google Scholar] [CrossRef]
- Hill, R.F.; Supancic, P.H. Thermal Conductivity of Platelet-Filled Polymer Composites. J. Am. Ceram. Soc. 2002, 85, 851–857. [Google Scholar] [CrossRef]
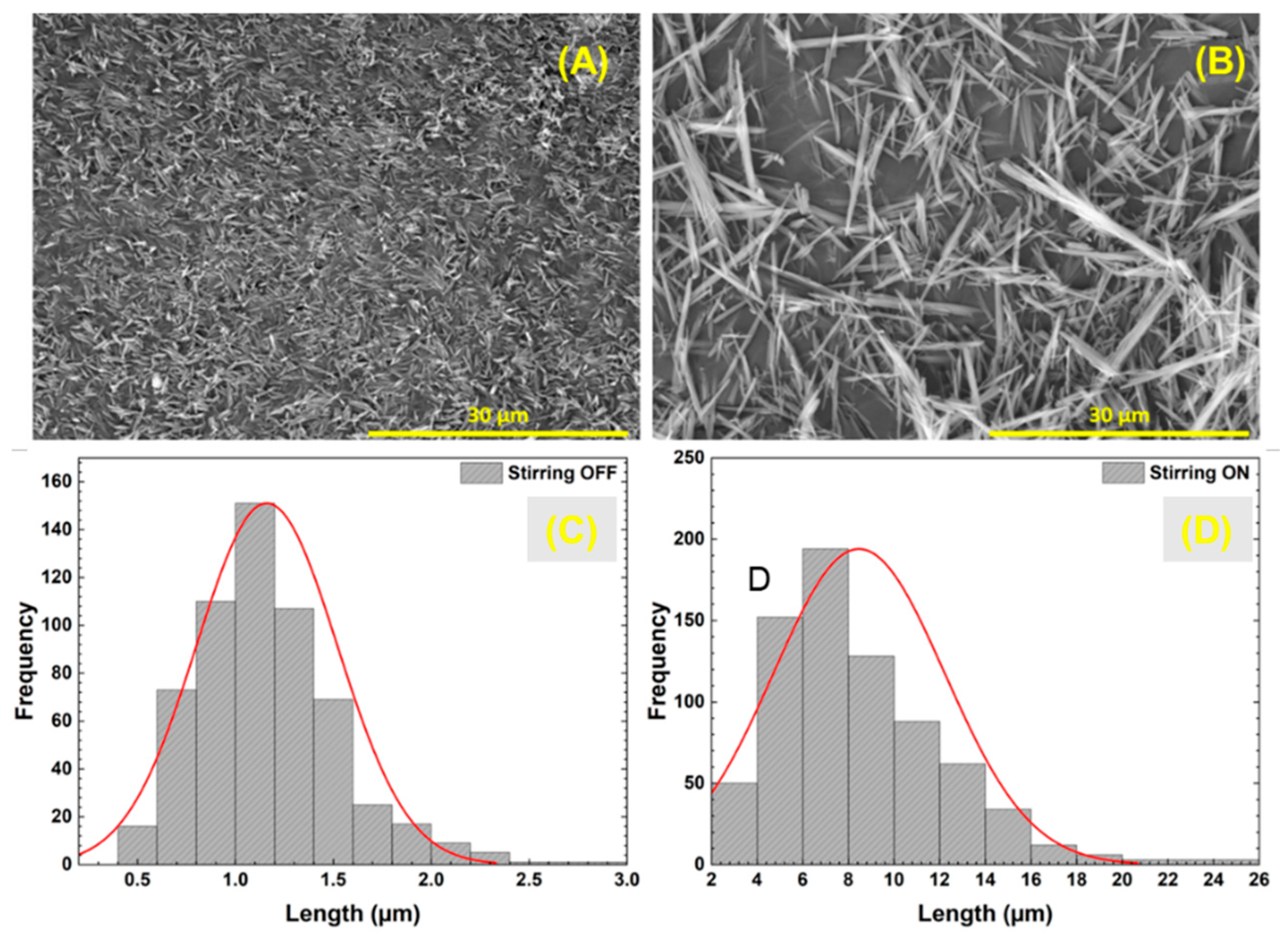
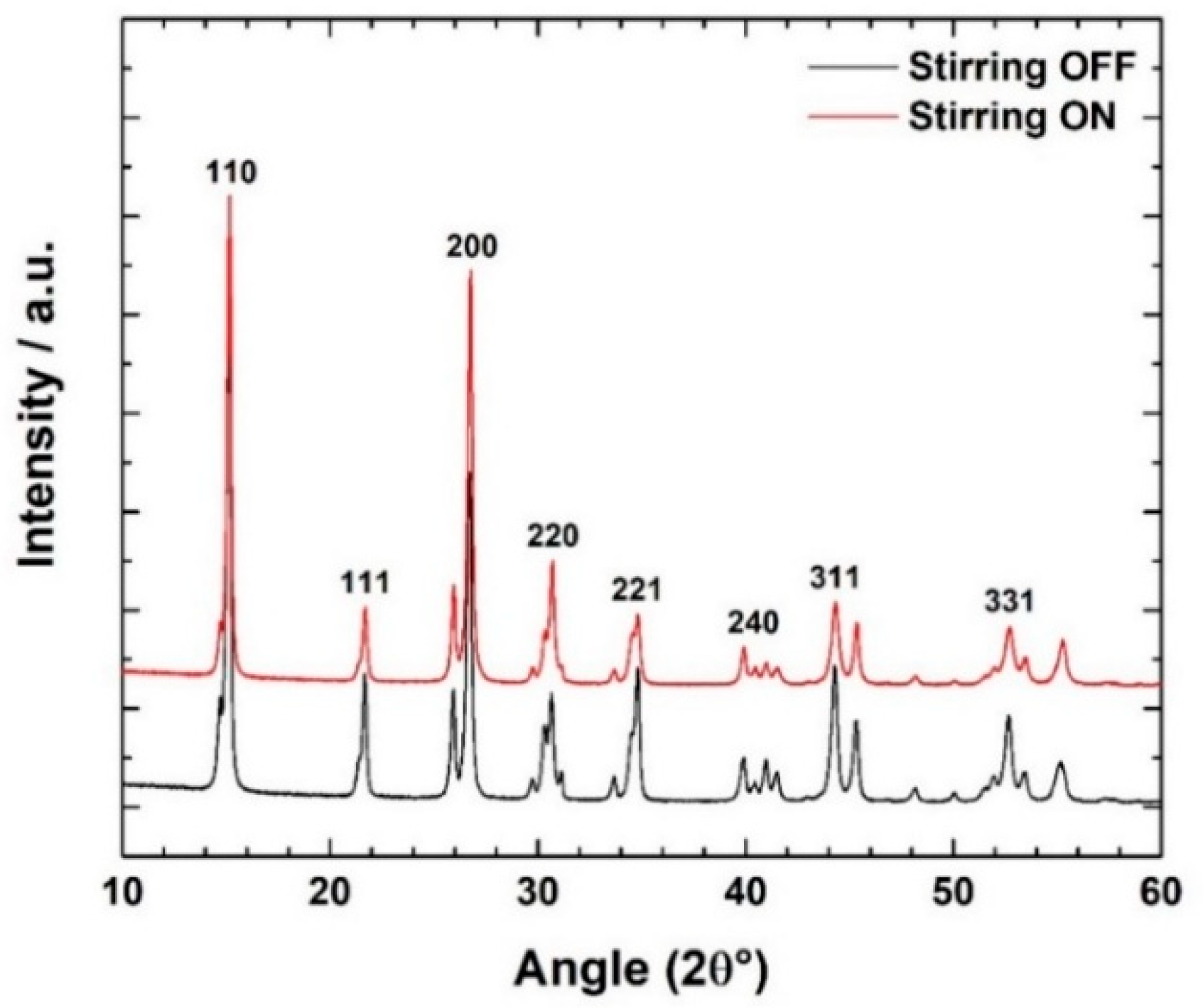





| Time | Temperature | [Al(NO3)3] | [urea] | [PEG] | Stirring | Parameter Studied | |
|---|---|---|---|---|---|---|---|
| Synthesis 1 | 24 h | 140 °C | 0.6 M | 5.5 M | 1.5 mM | OFF | Solubility Kinetics |
| Synthesis 2 | 24 h | 140 °C | 0.6 M | 5.5 M | 1.5 mM | 250 rpm | |
| Synthesis A | 24 h | 140 °C | 0.08 M | 5.5 M | 1.5 mM | 250 rpm | Influence of Al(NO3)3 |
| Synthesis B | 24 h | 140 °C | 0.15 M | 5.5 M | 1.5 mM | 250 rpm | |
| Synthesis C | 24 h | 140 °C | 0.3 M | 5.5 M | 1.5 mM | 250 rpm | |
| Synthesis D | 24 h | 140 °C | 0.6 M | 5.5 M | 1.5 mM | 250 rpm | |
| Synthesis E | 24 h | 140 °C | 0.3 M | 2.7 M | 1.5 mM | 250 rpm | Influence of urea |
| Synthesis F | 24 h | 140 °C | 0.3 M | 5.5 M | 1.5 mM | 250 rpm | |
| Synthesis G | 24 h | 140 °C | 0.3 M | 11 M | 1.5 mM | 250 rpm | |
| Synthesis H | 24 h | 140 °C | 0.3 M | 22 M | 1.5 mM | 250 rpm | |
| Synthesis I | 3 h | 140 °C | 0.3 M | 5.5 M | 1.5 mM | 250 rpm | Influence of reaction time |
| Synthesis J | 6 h | 140 °C | 0.3 M | 5.5 M | 1.5 mM | 250 rpm | |
| Synthesis K | 12 h | 140 °C | 0.3 M | 5.5 M | 1.5 mM | 250 rpm | |
| Synthesis L | 24 h | 140 °C | 0.3 M | 5.5 M | 1.5 mM | 250 rpm | |
| Synthesis M | 48 h | 140 °C | 0.3 M | 5.5 M | 1.5 mM | 250 rpm | |
| Synthesis N | 72 h | 140 °C | 0.3 M | 5.5 M | 1.5 mM | 250 rpm | |
| Synthesis O | 24 h | 120 °C | 0.3 M | 5.5 M | 1.5 mM | 250 rpm | Influence of reaction Temperature |
| Synthesis P | 24 h | 140 °C | 0.3 M | 5.5 M | 1.5 mM | 250 rpm | |
| Synthesis Q | 24 h | 160 °C | 0.3 M | 5.5 M | 1.5 mM | 250 rpm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahhaf, O.; D’Ambrogio, G.; Grasland, F.; Rival, G.; Le, M.-Q.; Cottinet, P.-J.; Capsal, J.-F. Hydrothermal Synthesis Optimization of High-Aspect Ratio α-Al2O3 Microfibers for Thermally Conductive Soft Composites. Ceramics 2025, 8, 127. https://doi.org/10.3390/ceramics8040127
Zahhaf O, D’Ambrogio G, Grasland F, Rival G, Le M-Q, Cottinet P-J, Capsal J-F. Hydrothermal Synthesis Optimization of High-Aspect Ratio α-Al2O3 Microfibers for Thermally Conductive Soft Composites. Ceramics. 2025; 8(4):127. https://doi.org/10.3390/ceramics8040127
Chicago/Turabian StyleZahhaf, Omar, Giulia D’Ambrogio, François Grasland, Guilhem Rival, Minh-Quyen Le, Pierre-Jean Cottinet, and Jean-Fabien Capsal. 2025. "Hydrothermal Synthesis Optimization of High-Aspect Ratio α-Al2O3 Microfibers for Thermally Conductive Soft Composites" Ceramics 8, no. 4: 127. https://doi.org/10.3390/ceramics8040127
APA StyleZahhaf, O., D’Ambrogio, G., Grasland, F., Rival, G., Le, M.-Q., Cottinet, P.-J., & Capsal, J.-F. (2025). Hydrothermal Synthesis Optimization of High-Aspect Ratio α-Al2O3 Microfibers for Thermally Conductive Soft Composites. Ceramics, 8(4), 127. https://doi.org/10.3390/ceramics8040127







