Impact of Thermal Cycling on the Vickers Microhardness of Dental CAD/CAM Materials: Greater Retention in Polymer-Infiltrated Ceramic Networks (PICNs) Compared to Nano-Filled Resin Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Size and Power
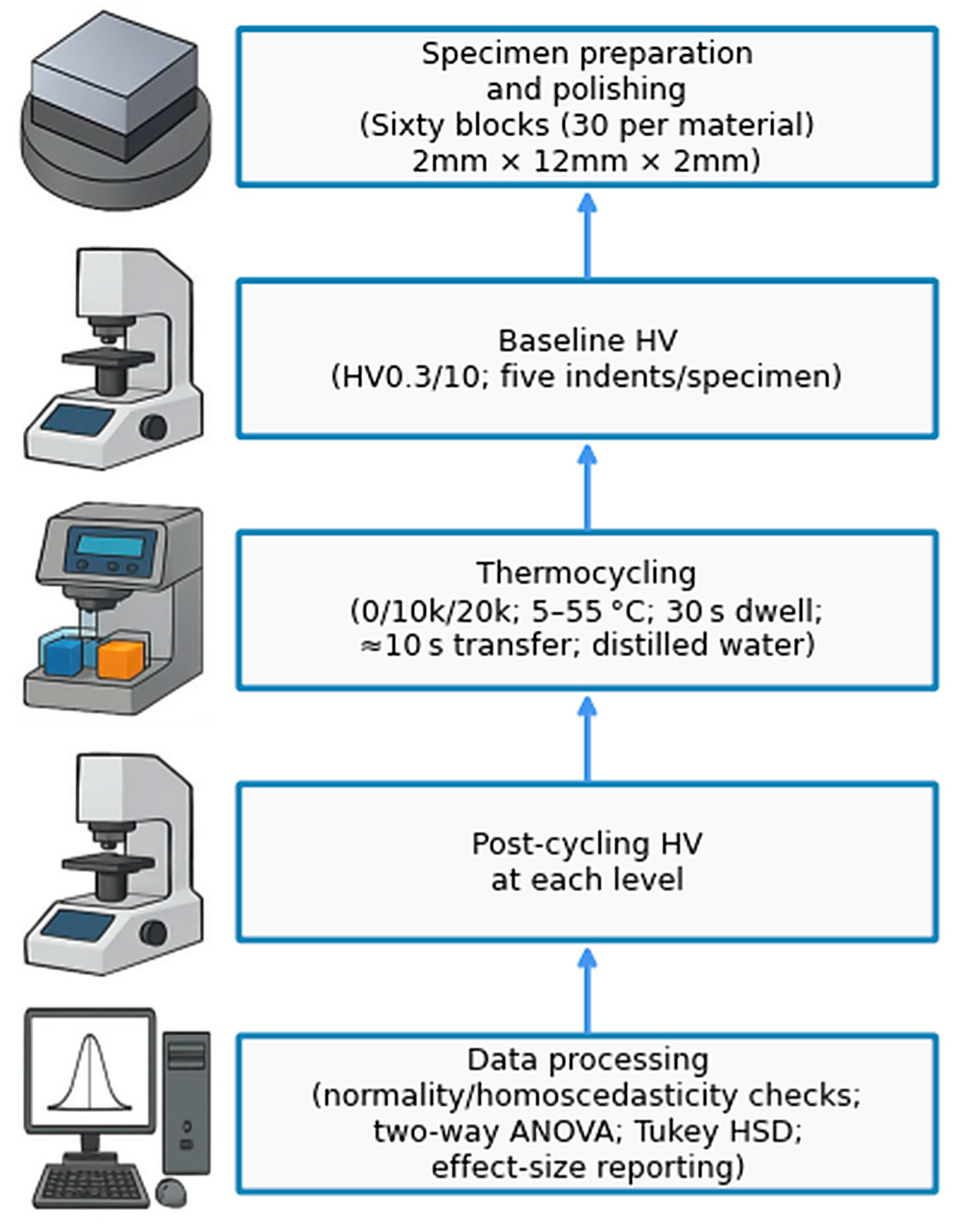
2.3. Specimen Preparation
2.4. Group Assignment and Thermocycling Protocol
2.5. Vickers Microhardness Testing
2.6. Surface Quality Control
2.7. Randomization and Blinding
2.8. Statistical Analysis
3. Results
Limitations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ilie, N.; Hickel, R. Resin Composite Restorative Materials. Aust. Dent. J. 2011, 56 (Suppl. S1), 59–66. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.; Schmidtke, J.; Hahnel, S.; Koenig, A. The Influence of Different Storage Media on Vickers Hardness and Surface Roughness of CAD/CAM Resin Composites. J. Mater. Sci. Mater. Med. 2023, 34, 13. [Google Scholar] [CrossRef]
- Alamoush, R.A.; Sung, R.; Satterthwaite, J.D.; Silikas, N. The Effect of Different Storage Media on the Monomer Elution and Hardness of CAD/CAM Composite Blocks. Dent. Mater. 2021, 37, 1202–1213. [Google Scholar] [CrossRef]
- Alfahed, B.; Alayad, A. The Effect of Sintering Temperature on Vickers Microhardness and Flexural Strength of Translucent Multi-Layered Zirconia Dental Materials. Coatings 2023, 13, 688. [Google Scholar] [CrossRef]
- Dejak, B.D.; Langot, C.; Krasowski, M.; Klich, M. Evaluation of Hardness and Wear of Conventional and Transparent Zirconia Ceramics, Feldspathic Ceramic, Glaze, and Enamel. Materials 2024, 17, 3518. [Google Scholar] [CrossRef]
- Toma, F.R.; Bîrdeanu, M.I.; Uțu, I.-D.; Vasiliu, R.D.; Moleriu, L.C.; Porojan, L. Surface Characteristics of High Translucent Multilayered Dental Zirconia Related to Aging. Materials 2022, 15, 3606. [Google Scholar] [CrossRef]
- Koo, P.-J.; Lee, J.-H.; Ha, S.-R.; Seo, D.-G.; Ahn, J.-S.; Choi, Y.-S. Changes in the Properties of Different Zones in Multilayered Translucent Zirconia Used in Monolithic Restorations During Aging Process. J. Funct. Biomater. 2025, 16, 96. [Google Scholar] [CrossRef]
- Jin, C.; Deng, J.; Pan, P.; Xiong, Y.; Zhu, L.; Gao, S. Comparative Study on the Impact-Sliding Wear Behaviour of CAD/CAM Resin-Ceramic Materials and Tooth Enamel. Dent. Mater. 2023, 39, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Lawson, N.C.; Bansal, R.; Burgess, J.O. Wear, Strength, Modulus and Hardness of CAD/CAM Restorative Materials. Dent. Mater. 2016, 32, e275–e283. [Google Scholar] [CrossRef] [PubMed]
- Kazak, M.; Koymen, S.; Yurdan, R.; Tekdemir, K.; Donmez, N. Effect of Thermal Aging Procedure on the Microhardness and Surface Roughness of Fluoride Ion Containing Materials. Ann. Med. Res. 2020, 27, 888. [Google Scholar] [CrossRef]
- Mainjot, A.K.; Dupont, N.M.; Oudkerk, J.C.; Dewael, T.Y.; Sadoun, M.J. From Artisanal to CAD-CAM Blocks. J. Dent. Res. 2016, 95, 487–495. [Google Scholar] [CrossRef]
- Pot, G.J.; Van Overschelde, P.A.; Keulemans, F.; Kleverlaan, C.J.; Tribst, J.P.M. Mechanical Properties of Additive-Manufactured Composite-Based Resins for Permanent Indirect Restorations: A Scoping Review. Materials 2024, 17, 3951. [Google Scholar] [CrossRef]
- Coldea, A.; Swain, M.V.; Thiel, N. Mechanical Properties of Polymer-Infiltrated-Ceramic-Network Materials. Dent. Mater. 2013, 29, 419–426. [Google Scholar] [CrossRef]
- Alharbi, N.; Teerakanok, S.; Satterthwaite, J.D.; Giordano, R.; Silikas, N. Quantitative Nano-Mechanical Mapping AFM-Based Method for Elastic Modulus and Surface Roughness Measurements of Model Polymer Infiltrated Ceramics. Dent. Mater. 2022, 38, 935–945. [Google Scholar] [CrossRef]
- Eldafrawy, M.; Karevan, Y.; Nguyen, J.-F.; Mainjot, A. Interblock and Intrablock Homogeneity of CAD-CAM Composites Mechanical Properties. Dent. Mater. J. 2023, 42, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, G.; Camurri Piloni, A.; Nicolin, V.; Turco, G.; Di Lenarda, R. Chairside CAD/CAM Materials: Current Trends of Clinical Uses. Biology 2021, 10, 1170. [Google Scholar] [CrossRef] [PubMed]
- Temizci, T.; Bozoğulları, H.N. Effect of Thermocycling on the Mechanical Properties of Permanent Composite-Based CAD-CAM Restorative Materials Produced by Additive and Subtractive Manufacturing Techniques. BMC Oral Health 2024, 24, 334. [Google Scholar] [CrossRef]
- Ciocan, L.T.; Ghitman, J.; Vasilescu, V.G.; Iovu, H. Mechanical Properties of Polymer-Based Blanks for Machined Dental Restorations. Materials 2021, 14, 7293. [Google Scholar] [CrossRef]
- Alamoush, R.A.; Salim, N.A.; Elraggal, A.; Satterthwaite, J.D.; Silikas, N. The Effect of Water Storage on Nanoindentation Creep of Various CAD-CAM Composite Blocks. BMC Oral Health 2023, 23, 543. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Derchi, G.; Thorat, S.; Ceseracciu, L.; Ruffilli, R.; Barone, A.C. Surface Morphology and Mechanical Properties of New-Generation Flowable Resin Composites for Dental Restoration. Dent. Mater. 2011, 27, 1221–1228. [Google Scholar] [CrossRef]
- Tassin, M.; Bonte, E.; Loison-Robert, L.S.; Nassif, A.; Berbar, T.; Goff, S.L.; Berdal, A.; Sadoun, M.; Fournier, B.P.J. Effects of High-Temperature-Pressure Polymerized Resin-Infiltrated Ceramic Networks on Oral Stem Cells. PLoS ONE 2016, 11, e0155450. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.F.; Ruse, D.; Phan, A.C.; Sadoun, M.J. High-Temperature-Pressure Polymerized Resin-Infiltrated Ceramic Networks. J. Dent. Res. 2013, 93, 62–67. [Google Scholar] [CrossRef]
- Albero, A.; Pascual, A.; Camps, I.; Grau-Benitez, M. Comparative Characterization of a Novel Cad-Cam Polymer-Infiltrated-Ceramic-Network. J. Clin. Exp. Dent. 2015, 7, e495–e500. [Google Scholar] [CrossRef]
- Kaizer, M.R.; Gierthmuehlen, P.C.; dos Santos, M.B.; Cava, S.S.; Zhang, Y. Speed Sintering Translucent Zirconia for Chairside One-Visit Dental Restorations: Optical, Mechanical, and Wear Characteristics. Ceram. Int. 2017, 43, 10999–11005. [Google Scholar] [CrossRef]
- Spitznagel, F.A.; Boldt, J.; Gierthmuehlen, P.C. CAD/CAM Ceramic Restorative Materials for Natural Teeth. J. Dent. Res. 2018, 97, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Porto, T.S.; Roperto, R.C.; Teich, S.T.; Faddoul, F.F.; Rizzante, F.A.P.; Porto-Neto Sde, T.; Campos, E.A. de Brittleness Index and Its Relationship with Materials Mechanical Properties: Influence on the Machinability of CAD/CAM Materials. Braz. Oral res. 2019, 33, e026. [Google Scholar] [CrossRef] [PubMed]
- Güleç, C.; Sarıkaya, I. The Influence of Aging on the Fracture Load of Milled Monolithic Crowns. BMC Oral Health 2022, 22, 516. [Google Scholar] [CrossRef]
- Souza, J.; Fuentes, M.V.; Baena, E.; Ceballos, L. One-Year Clinical Performance of Lithium Disilicate versus Resin Composite CAD/CAM Onlays. Odontology 2021, 109, 259–270. [Google Scholar] [CrossRef]
- Almohammed, S.N.; Alshorman, B.; Abu-Naba’a, L.A. Mechanical Properties of Five Esthetic Ceramic Materials Used for Monolithic Restorations: A Comparative In Vitro Study. Ceramics 2023, 6, 1031–1049. [Google Scholar] [CrossRef]
- Baroudi, K.; Rodrigues, J.C. Flowable Resin Composites: A Systematic Review and Clinical Considerations. J. Clin. Diagn. Res. 2015, 9, ZE18–ZE24. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Hahnel, S.; König, A.; Brambilla, E. Resin Composite Blocks for Dental CAD/CAM Applications Reduce Biofilm Formation in Vitro. Dent. Mater. 2020, 36, 603–616. [Google Scholar] [CrossRef]
- Lin-Gibson, S.; Sung, L.; Forster, A.M.; Hu, H.; Cheng, Y.; Lin, N.J. Effects of Filler Type and Content on Mechanical Properties of Photopolymerizable Composites Measured across Two-Dimensional Combinatorial Arrays. Acta Biomater. 2009, 5, 2084–2094. [Google Scholar] [CrossRef]
- Martin, N.; Jedynakiewicz, N.M.; Fisher, A.C. Hygroscopic Expansion and Solubility of Composite Restoratives. Dent. Mater. 2003, 19, 77–86. [Google Scholar] [CrossRef]
- Qian, L.; Zhao, H. Nanoindentation of Soft Biological Materials. Micromachines 2018, 9, 654. [Google Scholar] [CrossRef]
- Nguyen, J.-F.; Migonney, V.; Ruse, N.D.; Sadoun, M. Resin Composite Blocks via High-Pressure High-Temperature Polymerization. Dent. Mater. 2012, 28, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Vasiliu, R.-D.; Porojan, S.-D.; Bîrdeanu, M.-I.; Uțu, I.-D.; Porojan, L. The Effect of Thermocycling and Surface Treatments on the Surface Roughness and Microhardness of Three Heat-Pressed Ceramics Systems. Crystals 2020, 10, 160. [Google Scholar] [CrossRef]
- Vasiliu, R.D.; Uțu, I.-D.; Rusu, L.; Boloș, A.; Porojan, L. Fractographic and Microhardness Evaluation of All-Ceramic Hot-Pressed and CAD/CAM Restorations after Hydrothermal Aging. Materials 2022, 15, 3987. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Bae, H.-J.; Lee, H.-H.; Lee, J.-H.; Kim, Y.-J.; Choi, Y.-S.; Lee, J.-H.; Shin, S.-Y. The Effects of Thermocycling on the Physical Properties and Biocompatibilities of Various CAD/CAM Restorative Materials. Pharmaceutics 2023, 15, 2122. [Google Scholar] [CrossRef]
- Limpuangthip, N.; Poosanthanasarn, E.; Salimee, P. Surface Roughness and Hardness of CAD/CAM Ceramic Materials after Polishing with a Multipurpose Polishing Kit: An In Vitro Study. Eur. J. Dent. 2022, 17, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Çakmak, G.; Donmez, M.B.; de Paula, M.S.; Akay, C.; Fonseca, M.; Kahveci, Ç.; Abou-Ayash, S.; Yilmaz, B. Surface Roughness, Optical Properties, and Microhardness of Additively and Subtractively Manufactured CAD-CAM Materials after Brushing and Coffee Thermal Cycling. J. Prosthodont. 2025, 34, 68–77. [Google Scholar] [CrossRef]
- Colombo, M.; Poggio, C.; Lasagna, A.; Chiesa, M.; Scribante, A. Vickers Micro-Hardness of New Restorative CAD/CAM Dental Materials: Evaluation and Comparison after Exposure to Acidic Drink. Materials 2019, 12, 1246. [Google Scholar] [CrossRef]
- Oliveira, J.R.; Cruz, M.E.M.D.; Dovigo, L.N.; Fonseca, R.G. Long-Term Effects of Simulated Gastric Juice Alternated with Brushing on Hardness, Substance Loss, Flexural Strength and Reliability of CAD-CAM Monolithic Materials. J. Appl. Oral Sci. 2022, 30, e20210536. [Google Scholar] [CrossRef]
- Farahat, D.S.; El-Wassefy, N.A. Effects of Food-Simulating Solutions on the Surface Properties of Two CAD/CAM Resin Composites. J. Clin. Exp. Dent. 2022, 14, e782–e790. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, W.S.; Labban, N.; Maawadh, A.; Alsayed, H.D.; Alshehri, H.; Alrahlah, A.; Alnafaiy, S.M. Influence of Acidic Environment on the Hardness, Surface Roughness and Wear Ability of CAD/CAM Resin-Matrix Ceramics. Materials 2022, 15, 6146. [Google Scholar] [CrossRef]
- Reidelbach, C.; Swoboda, M.; Spraul, M.; Vach, K.; Patzelt, S.B.M.; Hellwig, E.; Polydorou, O. Effects of Erosion and Abrasion on Resin-Matrix Ceramic CAD/CAM Materials: An in Vitro Investigation. Eur. J. Oral Sci. 2024, 132, e12967. [Google Scholar] [CrossRef]
- Vulović, S.; Blatz, M.B.; Todorović, M.; Milić Lemić, A.; Todorović, A. Impact of Erosive and Abrasive Wear on the Surface Characteristics of Hybrid Ceramic-Polymer Dental Materials. J. Oral Sci. 2025, 67, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Komagata, Y.; Nagamatsu, Y.; Soh, I.; Ikeda, H. Impact of 1,500 Ppm NaF Solution at Different pH Levels on the Mechanical and Physicochemical Properties of CAD-CAM Materials. Dent. Mater. J. 2025, 44, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Goujat, A.; Abouelleil, H.; Colon, P.; Jeannin, C.; Pradelle, N.; Seux, D.; Grosgogeat, B. Mechanical Properties and Internal Fit of 4 CAD-CAM Block Materials. J. Prosthet. Dent. 2018, 119, 384–389. [Google Scholar] [CrossRef]
- Swain, M.V.; Coldea, A.; Bilkhair, A.; Guess, P.C. Interpenetrating Network Ceramic-Resin Composite Dental Restorative Materials. Dent. Mater. 2016, 32, 34–42. [Google Scholar] [CrossRef]
- Ling, L.; Ma, Y.; Malyala, R. A Novel CAD/CAM Resin Composite Block with High Mechanical Properties. Dent. Mater. 2021, 37, 1150–1155. [Google Scholar] [CrossRef]
- Alamoush, R.A.; Silikas, N.; Salim, N.A.; Al-Nasrawi, S.; Satterthwaite, J.D. Effect of the Composition of CAD/CAM Composite Blocks on Mechanical Properties. BioMed Res. Int. 2025, 2018, 4893143. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Choi, Y.-S.; Kang, K.-H.; Att, W. Effects of Thermal and Mechanical Cycling on the Mechanical Strength and Surface Properties of Dental CAD-CAM Restorative Materials. J. Prosthet. Dent. 2022, 128, 79–88. [Google Scholar] [CrossRef]
- Soh, G.; Selwyn, M.J. An Evaluation of Exposure Time and Temperature in the Thermocycling of Dental Restorative Materials. Clin. Mater. 1992, 9, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Hampe, R.; Theelke, B.; Lümkemann, N.; Stawarczyk, B. Impact of Artificial Aging by Thermocycling on Edge Chipping Resistance and Martens Hardness of Different Dental CAD-CAM Restorative Materials. J. Prosthet. Dent. 2021, 125, 326–333. [Google Scholar] [CrossRef] [PubMed]
- E384-22; ASTM Standard Test Method for Microindentation Hardness of Materials. ASTM International: West Conshohocken, PA, USA, 2022.
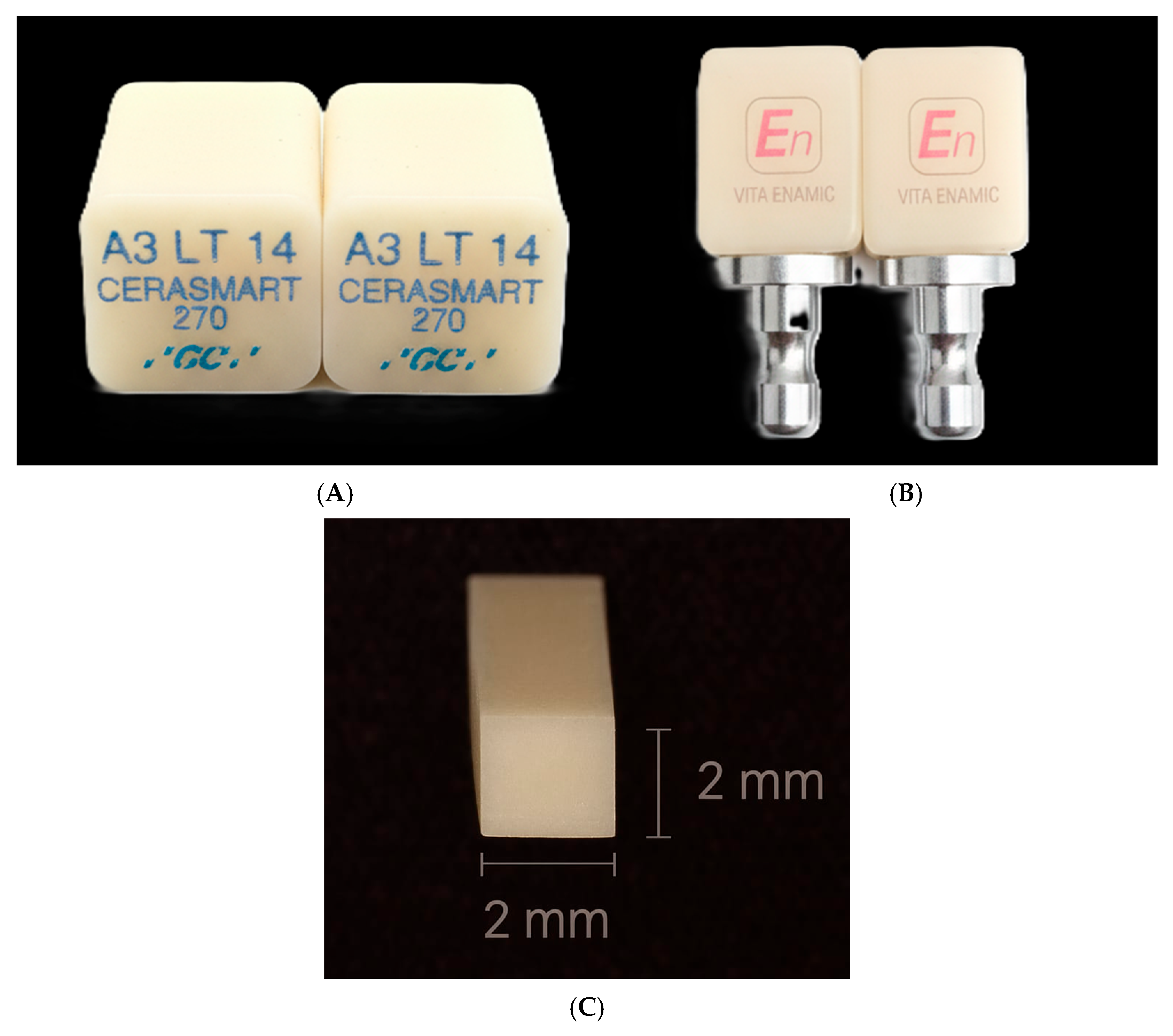

| Material | Cycles | n | Mean HV | SD | IC95% Lower | IC95% Upper |
|---|---|---|---|---|---|---|
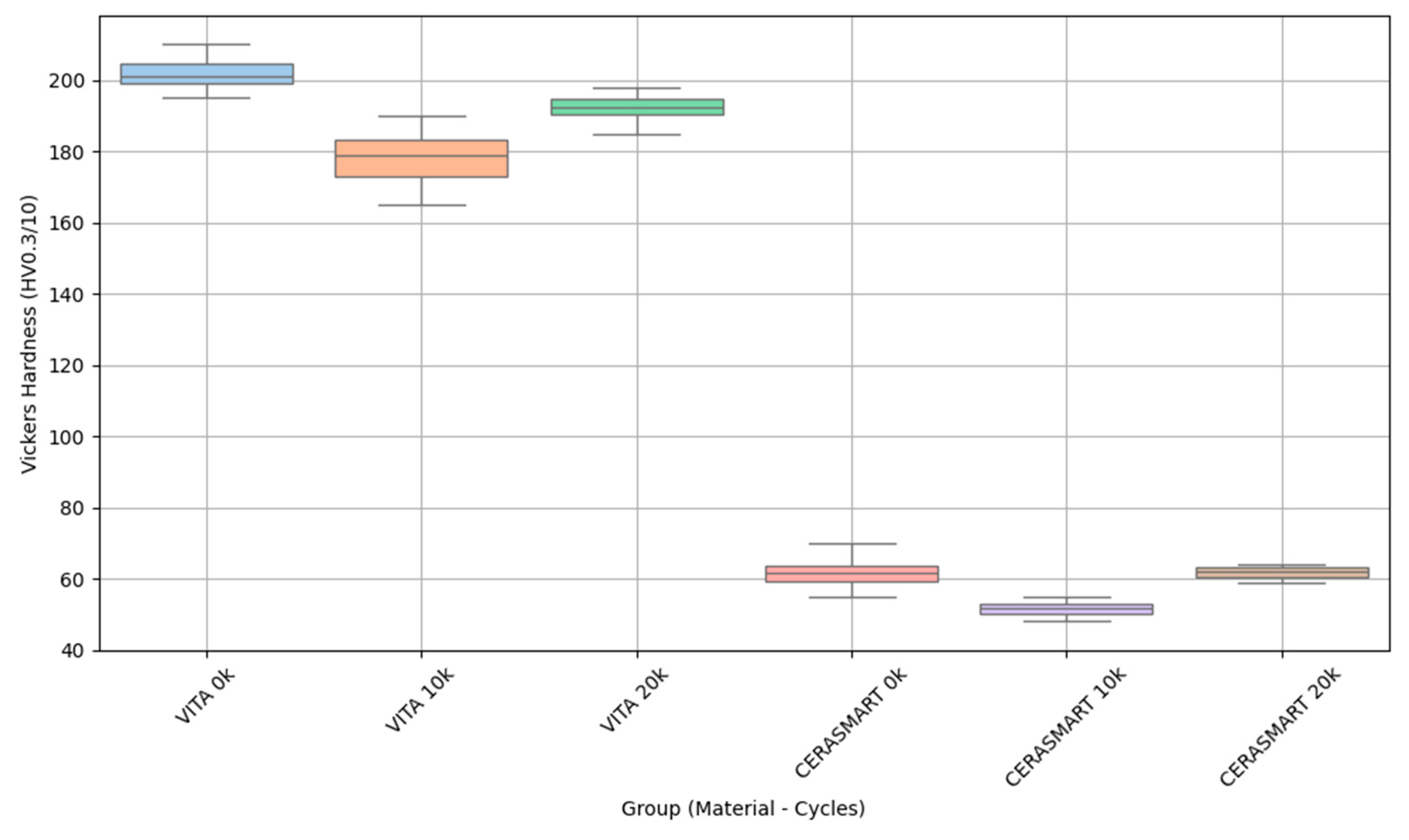
| VITA ENAMIC | 0 | 10 | 200.2 | 10.8 | 192.5 | 208.0 |
| VITA ENAMIC | 10,000 | 10 | 192.4 | 13.9 | 182.4 | 202.3 |
| VITA ENAMIC | 20,000 | 10 | 196.7 | 9.3 | 190.1 | 203.3 |
| CERASMART | 0 | 10 | 60.8 | 6.1 | 56.4 | 65.1 |
| CERASMART | 10,000 | 10 | 53.4 | 4.7 | 50.0 | 56.8 |
| CERASMART | 20,000 | 10 | 62.1 | 3.8 | 59.4 | 64.8 |
| Effect | gl | Sum of Squares | F | p | η2 Partial |
|---|---|---|---|---|---|
| Material | 1 | 426,000 | 1870.4 | <0.0001 | 0.972 |
| Cycles | 2 | 5700 | 12.5 | <0.001 | 0.316 |
| Material × Cycles | 2 | 3100 | 6.8 | 0.002 | 0.201 |
| Residual | 54 | 12,312 | — | — | — |
| Comparison | Diff of Means (HV) | IC95% Lower | IC95% Upper | p Adjusted | Method |
|---|---|---|---|---|---|
| VITA ENAMIC 10,000 vs. 0 | −7.8 | −20.4 | 4.8 | 0.050 | Tukey HSD |
| VITA ENAMIC 20,000 vs. 0 | −3.5 | −13.7 | 6.7 | 0.190 | Tukey HSD |
| VITA ENAMIC 20,000 vs. 10,000 | 4.3 | −7.7 | 16.3 | 0.310 | Tukey HSD |
| CERASMART 10,000 vs. 0 | −7.4 | −12.9 | −1.9 | <0.001 | Tukey HSD |
| CERASMART 20,000 vs. 0 | 1.3 | −3.8 | 6.4 | 0.410 | Tukey HSD |
| CERASMART 20,000 vs. 10,000 | 8.7 | 4.4 | 13.0 | <0.001 | Tukey HSD |
| Inter-material (0 ciclos) VE vs. CS | 139.4 | 130.5 | 148.3 | <0.001 | Tukey HSD |
| Inter-material (10,000) VE vs. CS | 139.0 | 128.5 | 149.5 | <0.001 | Tukey HSD |
| Inter-material (20,000) VE vs. CS | 134.6 | 127.4 | 141.8 | <0.001 | Tukey HSD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fajardo, J.I.; Paltán, C.A.; León, M.; Matute, A.Y.; Armas-Vega, A.; Puratambi, R.H.; Delgado-Gaete, B.A.; Requena, S.; Benalcazar, A. Impact of Thermal Cycling on the Vickers Microhardness of Dental CAD/CAM Materials: Greater Retention in Polymer-Infiltrated Ceramic Networks (PICNs) Compared to Nano-Filled Resin Composites. Ceramics 2025, 8, 125. https://doi.org/10.3390/ceramics8040125
Fajardo JI, Paltán CA, León M, Matute AY, Armas-Vega A, Puratambi RH, Delgado-Gaete BA, Requena S, Benalcazar A. Impact of Thermal Cycling on the Vickers Microhardness of Dental CAD/CAM Materials: Greater Retention in Polymer-Infiltrated Ceramic Networks (PICNs) Compared to Nano-Filled Resin Composites. Ceramics. 2025; 8(4):125. https://doi.org/10.3390/ceramics8040125
Chicago/Turabian StyleFajardo, Jorge I., César A. Paltán, Marco León, Annie Y. Matute, Ana Armas-Vega, Rommel H. Puratambi, Bolívar A. Delgado-Gaete, Silvio Requena, and Alejandro Benalcazar. 2025. "Impact of Thermal Cycling on the Vickers Microhardness of Dental CAD/CAM Materials: Greater Retention in Polymer-Infiltrated Ceramic Networks (PICNs) Compared to Nano-Filled Resin Composites" Ceramics 8, no. 4: 125. https://doi.org/10.3390/ceramics8040125
APA StyleFajardo, J. I., Paltán, C. A., León, M., Matute, A. Y., Armas-Vega, A., Puratambi, R. H., Delgado-Gaete, B. A., Requena, S., & Benalcazar, A. (2025). Impact of Thermal Cycling on the Vickers Microhardness of Dental CAD/CAM Materials: Greater Retention in Polymer-Infiltrated Ceramic Networks (PICNs) Compared to Nano-Filled Resin Composites. Ceramics, 8(4), 125. https://doi.org/10.3390/ceramics8040125










