Abstract
This study investigated the effect of piranha solution etching duration on the shear bond strength of zirconia ceramics bonded to resin cement, comparing it to conventional sandblasting treatment. Fifty fully sintered zirconia specimens (6.0 mm diameter, 4.0 mm thickness) were prepared and randomly divided into five groups (n = 10): sandblasting control and piranha solution treatment for 1, 2, 3, and 4 min. Piranha solution was prepared by mixing 98% H2SO4 and 35% H2O2 in a 3:1 ratio. All specimens were bonded to resin composite cylinders using dual-cure resin cement. Shear bond strength testing was performed using a universal testing machine at a 0.5 mm/min crosshead speed. Failure modes were analyzed using a stereomicroscope and classified as adhesive, cohesive, or mixed failures. One-way ANOVA revealed significant differences between groups (p < 0.05). Tukey’s post hoc test showed that 1-min piranha treatment produced significantly lower bond strength (7.64 ± 2.02 MPa) compared to all other groups. The 2-min (15.17 ± 2.79 MPa), 3-min (14.99 ± 3.27 MPa), and 4-min (18.34 ± 3.15 MPa) piranha treatments showed no significant differences compared to sandblasting (15.41 ± 2.61 MPa). Failure mode analysis revealed 100% adhesive failures for the 1-min group, while all other groups showed 80% adhesive and 20% mixed failures. Piranha solution treatment duration significantly affected zirconia bonding performance. While 1-min treatment proved inadequate, 2–4 min treatments achieved bond strengths comparable to sandblasting. The findings suggest that piranha solution treatment for 2–4 min represents a viable alternative to sandblasting for zirconia surface preparation, with the 2-min protocol being the most efficient choice for clinical application.
1. Introduction
Restorative esthetic dentistry has depended on the use of ceramic materials. Dental ceramics have evolved from traditional feldspathic porcelains to high-strength ceramics that offer superior mechanical properties and high esthetic outcomes [1]. Among the various ceramic systems available in today’s dentistry, glass ceramics initially dominated the field due to their exceptional translucency and ability to mimic natural tooth structure, making them ideal for anterior restorations where esthetics are required [2]. The success of glass ceramic restorations has been largely attributed to well-established bonding protocols that utilize hydrofluoric acid etching followed by silane application [3]. This two-step process creates reliable micromechanical retention through surface etching and establishes chemical bonding through siloxane bridge formation between the ceramic surface and resin cement [4]. The hydrofluoric acid selectively dissolves the glassy matrix present in feldspathic and leucite-reinforced ceramics, creating a highly retentive surface morphology with increased surface area and enhanced wettability. Subsequently, silane coupling agents form stable chemical bonds with the silica-rich surface, providing a durable interface that has demonstrated excellent clinical longevity over decades of use [5,6].
However, the inherent brittleness of glass ceramics and their susceptibility to catastrophic fracture, particularly in high-stress posterior applications, necessitated the development of stronger ceramic alternatives [2,7]. The introduction of zirconia-based ceramics represented a paradigm shift in restorative dentistry, offering exceptional mechanical properties, including fracture toughness values, which significantly exceed those of conventional glass ceramics [8]. These superior mechanical characteristics, combined with excellent biocompatibility and acceptable esthetic properties, have made zirconia increasingly popular for both single-unit crowns and multi-unit fixed partial dentures, particularly in posterior regions where high occlusal forces are encountered. Despite these advantages, zirconia presents unique challenges that distinguish it fundamentally from glass ceramics. As a polycrystalline ceramic composed entirely of crystalline phases without an amorphous glassy matrix, zirconia exhibits chemical inertness that renders conventional bonding protocols ineffective [9,10]. The absence of a glassy phase means that hydrofluoric acid etching, which is highly effective for glass ceramics, fails to create adequate surface roughness on zirconia surfaces under clinically practical conditions [11,12]. Surface roughness development through chemical etching is controlled by multiple parameters, with treatment time and temperature being critical factors that determine the extent of material modification and topographic changes. Extended etching times generally increase surface roughness through progressive chemical dissolution and material removal, while increased temperatures accelerate reactions and enhance the depth of surface modification. However, optimization of these parameters is important, as insufficient treatment may result in inadequate surface treatment, whereas excessive exposure can lead to uncontrolled material removal and compromised substrate integrity. This chemical inertness, while contributing to zirconia’s excellent biocompatibility and corrosion resistance, creates significant obstacles for achieving predictable adhesive bonding to tooth structure and resin cements.
Recent systematic reviews have comprehensively evaluated various zirconia surface treatment approaches [13,14]. According to Comino-Garayoa et al. (2021), surface treatment methods can be categorized into mechanical approaches such as air-particle abrasion and laser treatments (Er:YAG, CO2 lasers), chemical methods including acid etching solutions, primers containing phosphate monomers (10-MDP), and tribochemical silica coating, and novel approaches encompassing plasma treatments and sol-gel coating methods [14]. Chatterjee and Ghosh (2022) emphasized that while multiple approaches exist, optimal protocols remain controversial, with bond durability being a particular concern [13]. Even with the established clinical protocol of air-particle abrasion followed by phosphate-containing primers, zirconia bonding continues to exhibit suboptimal performance compared to the material’s exceptional mechanical properties. A critical factor contributing to this limitation lies in the core surface chemistry of zirconia, specifically the relatively low oxide content present on the outermost surface layer. Unlike base metals that readily form thick, stable oxide layers upon exposure to atmospheric conditions, zirconia surfaces typically contain only 5.4% oxide content, predominantly in the form of a thin passive zirconium dioxide layer [15]. Sandblasting, also known as air-particle abrasion, has emerged as the gold standard for zirconia surface preparation in everyday dental practice [16]. This mechanical treatment involves spraying abrasive particles—typically aluminum oxide (Al2O3) with particle sizes ranging from 50–110 μm—against the zirconia surface at high velocity [17]. The process creates microscopic surface irregularities that significantly increase surface area and provide essential mechanical retention sites for effective adhesive bonding [18]. Piranha solution, a highly oxidizing mixture of concentrated sulfuric acid and hydrogen peroxide, represents a promising alternative for creating reactive surfaces on zirconia ceramics through simultaneous oxidation and hydroxylation processes.
The fundamental difference between sandblasting and piranha solution treatment lies in their distinct bonding mechanisms. Sandblasting creates bonding primarily through micromechanical interlocking, where high-velocity aluminum oxide particles generate microscopic undercuts and surface irregularities that serve as mechanical retention sites [18,19,20,21]. This process significantly increases surface roughness and surface area compared to polished surfaces, with resin cement flowing into these irregularities during application and polymerizing to create physical locks between the cement and the ceramic surface [13,14]. The effectiveness depends on the depth of surface irregularities, surface wettability, and cement flow characteristics, creating a three-dimensional network of interlocking sites that resist tensile and shear forces [18,19].
In contrast, piranha solution treatment creates bonding through chemical modification of the zirconia surface, establishing molecular-level interactions between the ceramic substrate and resin cement [15,22,23]. The dual oxidation-hydroxylation process generates stable zirconium dioxide (ZrO2) layers and reactive Zr–OH groups that can form hydrogen bonds and covalent linkages with functional monomers in resin cements [15,23]. The hydroxylation process is particularly significant because Zr–OH groups serve as reactive sites for adhesion promoters, creating molecular bridges between the inorganic ceramic surface and organic resin matrix [15]. This chemical bonding mechanism offers molecular-level adhesion with more uniform stress distribution and potentially superior long-term stability compared to physical interlocking mechanisms [22,23].
While conventional zirconia surfaces demonstrate limited bonding ability due to their low oxide content, this limitation presents clear opportunities for improvement through innovative surface modification techniques that can enhance chemical bonding potential. Lohbauer et al. mentioned that material treated with piranha solution successfully hydroxylates zirconia surfaces, creating reactive hydroxyl sites [15]. However, the previous study did not find the suitable time to provide the best treatment. Despite this positive potential, the ideal treatment duration for piranha solution to achieve optimal bond strength enhancement on zirconia remains uninvestigated. Based on the identified limitations of current zirconia bonding protocols and the potential advantages of piranha solution treatment, this research aims to investigate whether piranha solution surface modification can significantly improve zirconia bonding performance by addressing the fundamental issue of low oxide content. The null hypothesis (H0) states that there is no significant difference in shear bond strength between zirconia surfaces treated with piranha solution for different time periods (1, 2, 3, and 4 min) compared to conventional sandblasting treatment. The alternative hypothesis (H1) states that piranha solution treatment duration significantly affects the shear bond strength of zirconia–resin cement interfaces, with optimal treatment times producing bond strengths comparable to or superior to sandblasting treatment.
2. Materials and Methods
2.1. Specimen Preparation
Fifty fully sintered zirconia specimens were fabricated from a standardized zirconia block (Ceramill Zolid HT+PS, Amann Girrbach AG, Koblach, Austria) with dimensions of 6.0 mm diameter and 4.0 mm thickness, following the manufacturer’s sintering protocols. The zirconia material had a purity of [≥99.0 wt%] and a density of [6.05 g/cm3] according to the manufacturer specifications.
Each specimen was systematically embedded within polyvinyl chloride (PVC) cylinders and stabilized using epoxy resin. Surface standardization was achieved through controlled polishing protocols utilizing 600-grit silicon carbide abrasive sheets (3M Wetordry, St. Paul, MN, USA) under standardized conditions: 2 kg/cm2 applied pressure, 100 rpm rotational speed, and a 2-min duration with continuous water irrigation using an automated polishing system (ZL-2512 HMP-2A, Dongguan Zhongli Instrument Technology Co., Ltd., Dongguan, China).
Specimens were randomly allocated into five experimental groups (n = 10 per group) according to each surface treatment as shown in Table 1 below.

Table 1.
Experimental groups in this study.
2.2. Sandblast Process
The control group specimens underwent air-particle abrasion using 50 μm aluminum oxide particles (A10723 Base 3, Dental Vision Co., Ltd., Bangkok, Thailand) delivered at 3.8 bar pressure for 15 s from a standardized 10 mm distance [24]. Post-treatment decontamination involved distilled water rinsing followed by a 15-min ultrasonic cleaning in distilled water (P500, Crest Ultrasonics, Trenton, NJ, USA).
2.3. Etching with Piranha Solution
Piranha solution was freshly prepared under strict safety protocols by combining 98% sulfuric acid (H2SO4) and 35% hydrogen peroxide (H2O2) in a precise 3:1 volumetric ratio at room temperature (23 ± 2 °C). The solution was transferred to calibrated droppers for controlled application. Each specimen received exactly one drop of piranha solution for the designated treatment duration according to group assignment.
Following etching, specimens underwent immediate and thorough rinsing with deionized water to neutralize the residual etching solution. Surface drying was accomplished using an oil-free triple syringe at 40–50 pounds/cm2 maintained at 10-mm distance until complete moisture elimination was confirmed. Final decontamination involved a 15-min ultrasonic cleaning in distilled water using the previous protocol.
2.4. Resin Composite Cylinder Fabrication
Standardized resin composite cylinders (3 mm diameter × 2 mm height) were fabricated using precision silicone molds (Honigum putty, DMG GmbH, Hamburg, Germany) and light-activated composite material (Ceram·X SphereTEC™ one, shade A3.5; Dentsply Sirona, Konstanz, Germany). Light curing was performed using a calibrated LED unit (Demi Plus, SDS Kerr, Middleton, WI, USA) with 40-s exposure cycles applied perpendicularly at a minimal distance. Post-fabrication surface finishing employed identical polishing protocols as described for zirconia specimens.
2.5. Resin Cement Application Protocol
A precise 2 mm diameter bonding area was established with a one-sided tape adhesive template (PPM Sticky tape, PPM industries S.p.A., Brembate Sopra, Italy). Templates were cut with dimensions of 10 × 10 mm. Dual-cure resin cement (Panavia SA, Kuraray Noritake, Kyoto, Japan) was applied following the manufacturer’s specifications. The cement was carefully dispensed to fill the template-defined bonding area completely, with excess material removed using microbrushes. Resin composite cylinders were positioned with a standardized 50 N force application using a modified durometer system, ensuring consistent seating pressure across all specimens.
Light activation was performed through a systematic protocol: an initial 40-s cure through the resin composite cylinder, followed by template removal and subsequent 40-s exposures at 90-degree rotational increments to achieve complete 360-degree polymerization. A final 10-min ambient curing period ensured complete polymerization before specimen transfer to controlled storage conditions.
All bonded specimens underwent standardized conditioning in distilled water at 37 °C for 24 h using a calibrated incubator (Incubator 20 L, VEVOR Lab, Rancho Cucamonga, CA, USA) to simulate oral temperature conditions and to allow complete cement polymerization prior to mechanical testing.
2.6. Shear Bond Strength Assessment and Failure Mode Analysis
Bonding area measurements were obtained using precision digital calipers (Mitutoyo CD-6 CS, Mitutoyo Co., Tokyo, Japan) immediately before mechanical testing. Shear bond strength evaluation was conducted using a universal testing machine (AGS-X 500N, Shimadzu Corporation, Kyoto, Japan) with specimens secured in custom fixtures to ensure a consistent loading force (Figure 1).
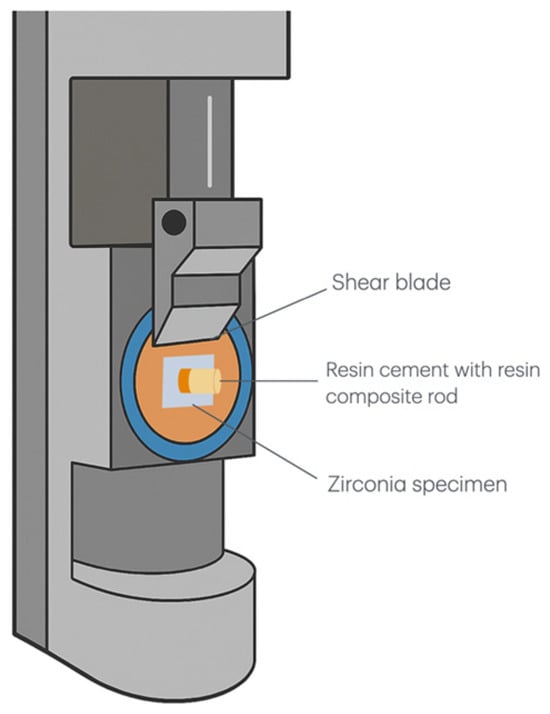
Figure 1.
Schematic diagram of the shear bond strength testing apparatus. The setup shows a zirconia specimen bonded with a resin cement composite rod positioned under a shear blade for bond strength measurement. The shear blade applied force parallel to the bonding interface to determine the maximum load at failure.
The shearing blade was positioned parallel to the zirconia–cement interface, and loads were applied at a standardized crosshead speed of 0.5 mm/min until failure occurrence. Shear bond strength values (MPa) were calculated by dividing the maximum failure load by the measured bonding area for each specimen.
Post-debonding failure surface examination was performed using a stereomicroscope (MDM-5, Meiji Techno Co., Ltd., Saitama, Japan) at 50× magnification. Failure modes were systematically classified according to established criteria [16,17,25]: (1) Adhesive failure occurred when the zirconia surface retained less than 40% of the resin cement, demonstrating failure at the substrate interface. (2) Cohesive failure was present when the zirconia surface showed at least 60% resin cement retention, indicating failure occurred within the cement material. (3) Mixed failure was defined by 40–60% resin cement remaining on the zirconia surface, showing combined adhesive and cohesive failure characteristics.
2.7. Statistical Analysis
Statistical analysis was performed using SPSS 20.0 software (SPSS Inc., Chicago, IL, USA). Results were considered statistically significant at p < 0.05. Data distribution normality was assessed using the Kolmogorov-Smirnov test, while variance homogeneity was evaluated through Levene’s test. Primary outcome analysis employed one-way analysis of variance (ANOVA) to detect significant differences between treatment groups, followed by Tukey’s Honestly Significant Difference (HSD) post hoc testing for multiple pairwise comparisons when significant main effects were detected.
3. Results
A Tukey HSD post hoc test was conducted to determine which treatment groups differed significantly in shear bond strength. The mean shear bond strength values with standard deviations were as follows (Figure 2): SB (15.41 ± 2.61 MPa), Piranha 1 min (7.64 ± 2.02 MPa), Piranha 2 min (15.17 ± 2.79 MPa), Piranha 3 min (14.99 ± 3.27 MPa), and Piranha 4 min (18.34 ± 3.15 MPa).
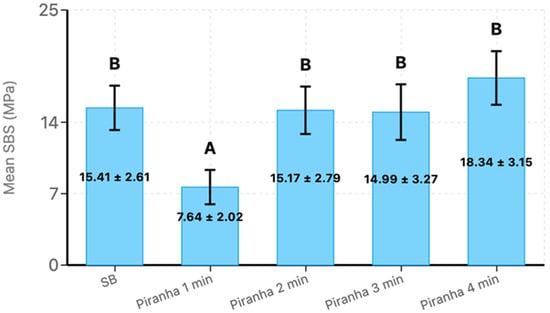
Figure 2.
The mean shear bond strength and standard deviations of the experimental groups, where SB = Sandblast. Bars with the same letter are not significantly different (p > 0.05). Error bars represent standard deviation.
The post hoc analysis revealed significant differences between several treatment pairs (p < 0.05) and identified two distinct statistical groups. Piranha 1 min treatment formed a separate group with significantly lower shear bond strength compared to all other treatments. Specifically, Piranha 1 min demonstrated significantly lower performance compared to SB, Piranha 2 min, Piranha 3 min, and Piranha 4 min. The largest significant difference was observed between Piranha 4 min and Piranha 1 min treatments, with Piranha 4 min demonstrating 10.698 MPa higher shear bond strength. This poor performance of the 1-min treatment was further corroborated by the failure mode analysis, which revealed 100% adhesive failures for this group, indicating complete interfacial debonding between the zirconia and resin cement without any cement remnants remaining on the zirconia surface.
The remaining treatments—SB, Piranha 2 min, Piranha 3 min, and Piranha 4 min—formed a homogeneous group with no statistically significant differences between them (p > 0.05). Among these treatments, Piranha 4 min demonstrated the highest mean shear bond strength (18.34 ± 3.15 MPa), which was numerically higher than the control SB group, though this difference was not statistically significant. Similarly, Piranha 2 min and Piranha 3 min treatments showed comparable performance to the SB control group, with non-significant mean differences of −0.245 MPa and −0.414 MPa, respectively. Importantly, the failure mode analysis supported these bond strength findings, as all effective treatment groups (SB, Piranha 2 min, Piranha 3 min, and Piranha 4 min) exhibited similar failure patterns consisting of 80% adhesive failures and 20% mixed failures. The presence of mixed failures in these groups (Figure 3), characterized by partial cement remnants adhering to the zirconia surface, suggests improved interfacial bonding where some areas achieved bond strengths approaching or exceeding the cohesive strength of the resin cement itself. This contrasts markedly with the exclusively adhesive failure pattern observed in the inadequately treated Piranha 1 min group, confirming that sufficient treatment duration is critical for achieving reliable interfacial modification and optimal bonding performance.
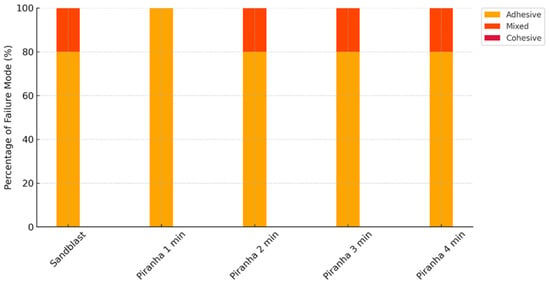
Figure 3.
The failure mode percentages of the experimental groups.
The stereomicroscope examination provided visual confirmation of the failure mode patterns and their correlation with bond strength performance (Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8). The Piranha 1 min group specimens consistently displayed clean zirconia surfaces with minimal resin cement remnants, confirming the 100% adhesive failure classification and providing visual evidence for the significantly lower bond strength (7.64 MPa) observed in this group. The absence of retained cement indicates that debonding occurred entirely at the zirconia–cement interface, demonstrating inadequate interfacial bonding. In contrast, specimens from the effective treatment groups displayed heterogeneous surface patterns characterized by areas of retained resin cement appearing as irregular, darkened regions alongside clean zirconia areas. This mixed surface morphology directly corresponds to the 80% adhesive and 20% mixed failure distribution observed across these groups and supports the improved interfacial bonding achieved by adequate surface treatment duration. The presence of retained cement remnants in the mixed failure areas indicates regions where the interfacial bond strength reached or exceeded the cohesive strength of the resin cement itself, forcing failure to occur partially within the cement rather than purely at the adhesive interface. This pattern was consistently observed across all effective treatment groups, regardless of whether surface modification was achieved through mechanical sandblasting or chemical piranha solution treatment, supporting their equivalent bond strength performance.
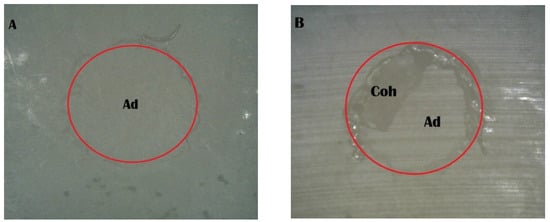
Figure 4.
Stereomicroscope images of the sandblast group: (A) adhesive failure; (B) mixed failure (Ad, adhesive failure; Coh, cohesive failure in resin cement).
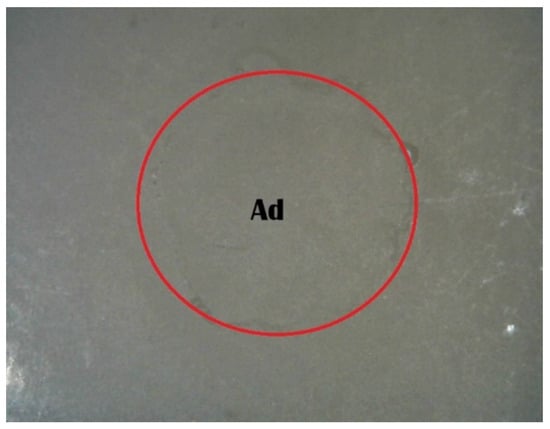
Figure 5.
Stereomicroscope images of adhesive failure in the Piranha 1 min group (Ad, adhesive failure).
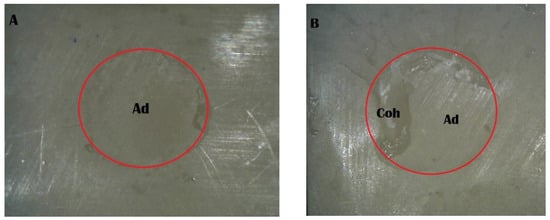
Figure 6.
Stereomicroscope images of the Piranha 2 min group: (A) adhesive failure; (B) mixed failure (Ad, adhesive failure; Coh, cohesive failure in resin cement).
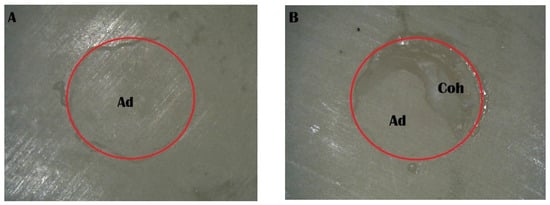
Figure 7.
Stereomicroscope images of the Piranha 3 min group: (A) adhesive failure; (B) mixed failure (Ad, adhesive failure; Coh, cohesive failure in resin cement).
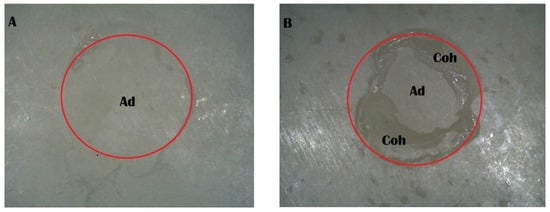
Figure 8.
Stereomicroscope images of the Piranha 4 min group: (A) adhesive failure; (B) mixed failure (Ad, adhesive failure; Coh, cohesive failure in resin cement).
4. Discussion
Based on the outcomes of this study, the null hypothesis (H0) was rejected. The statistical analysis revealed significant differences in shear bond strength between different piranha solution treatment durations, thereby rejecting the null hypothesis that all treatment times would produce equivalent results. Specifically, the 1-min piranha treatment produced significantly lower bond strength compared to all other treatments (p < 0.05), confirming that treatment duration does significantly affect bonding performance.
The alternative hypothesis (H1) was accepted with important clinical implications. While piranha solution treatment duration significantly affected bond strength, the optimal treatment times (2, 3, and 4 min) produced bond strengths that were statistically equivalent to sandblasting treatment. The 4-min treatment showed the highest numerical value compared to sandblasting, but this difference was not statistically significant (p > 0.05). More importantly, the 2-min treatment demonstrated no statistically significant difference from both the 3-min and 4-min treatments (p > 0.05), indicating that the shorter 2-min etching protocol could effectively replace the longer treatment durations without compromising bonding effectiveness.
The results demonstrated that adequate piranha solution treatment duration (≥2 min) was essential for achieving effective surface modification, while insufficient treatment time (1 min) failed to provide clinically acceptable bonding performance. This finding established a clear time-dependent relationship between piranha solution exposure and surface modification effectiveness, validating the research hypothesis regarding the critical importance of treatment duration. The statistical equivalence between 2, 3, and 4-min treatments suggested that once the threshold for effective surface modification was reached at 2 min, extending the treatment time provided no additional bonding advantage, making the 2-min protocol the most efficient choice for clinical application.
The aim of this research was to investigate the effect of piranha solution etching time on the shear bond strength of zirconia bonded with resin cement. The present study demonstrated that piranha solution etching duration significantly influenced the effectiveness of zirconia surface treatment for bonding applications. The results revealed that 1-min piranha etching produced insufficient surface modification, showing no significant difference from untreated controls, while extended etching times of 2, 3, and 4 min achieved surface modifications comparable to sandblasting treatment. This finding indicates that adequate chemical etching required sufficient exposure time to effectively alter the zirconia surface and create optimal conditions for resin bonding. The inadequate etching time of 1 min suggested that brief chemical exposure fails to penetrate and modify the chemically inert zirconia surface to the extent necessary for reliable adhesion.
The failure mode analysis provides crucial insights into the bonding mechanism and quality of adhesion achieved by different surface modifications, supporting the bond strength findings. The predominance of adhesive failures across all effective treatment groups (80% for sandblasting and 2–4 min piranha treatments) indicates that the weakest point in the bonding system remains at the zirconia–resin cement interface. This pattern suggests that despite surface modifications, achieving optimal chemical bonding to zirconia remains challenging due to its polycrystalline structure and absence of a glassy matrix. Notably, the Piranha 1 min group exhibited 100% adhesive failures, which correlated directly with its significantly lower shear bond strength. This complete adhesive failure pattern confirms that insufficient etching time fails to create adequate surface modification for effective bonding, with the bond at the interface being consistently weaker than the cohesive strength of the resin cement itself.
In contrast, the groups with longer piranha etching times and the sandblasting control group showed similar failure patterns, with the presence of 20% mixed failures suggesting improved interfacial bonding, where some areas achieved bond strengths approaching or exceeding the cohesive strength of the resin cement. This mixed failure pattern is clinically favorable, as it indicates more reliable bonding with enhanced resistance to debonding at the interface. The absence of cohesive failures in the resin cement across all groups indicates that the resin cement itself (Panavia SA) demonstrated adequate cohesive strength and was not the limiting factor in bond failure, validating that the observed differences in bond strength are attributable to surface treatment effects rather than cement properties.
The comparable bond strengths achieved by both treatments (15.17–18.34 MPa for piranha vs. 15.41 MPa for sandblasting) demonstrate that mechanical and chemical bonding pathways can achieve clinically acceptable adhesion through fundamentally different mechanisms. The similar failure mode patterns (80% adhesive, 20% mixed failures) across effective treatment groups indicate that both treatments create interfacial modifications sufficient to approach the cohesive strength limits of the resin cement. For sandblasted specimens, mixed failures occur where optimal micromechanical interlocking was achieved [26], while in piranha-treated specimens, mixed failures suggest successful chemical bonding in areas with optimal surface hydroxylation. While sandblasting creates retention through surface roughening [27,28,29], piranha solution enhances bonding through surface hydroxylation with complementary oxide layer formation, suggesting potential for synergistic effects if treatments were appropriately combined [23].
Piranha solution, typically composed of concentrated sulfuric acid and hydrogen peroxide (3:1 ratio), creates a highly oxidizing environment that simultaneously cleans and modifies metal surfaces at the molecular level [13]. The hydrogen peroxide acts as a powerful oxidizing agent, simultaneously promoting oxide layer formation (ZrO2) and surface hydroxylation (Zr–OH), while the sulfuric acid component removes organic contaminants and dissolves loosely bound surface materials, exposing fresh surfaces for these chemical modifications [19]. Unlike mechanical treatments, this dual chemical process creates both stable oxide layers and reactive hydroxyl groups, with hydroxylation being particularly critical for bonding, as Zr–OH groups provide reactive sites for chemical interaction with resin cements.
It is important to distinguish that while both oxidation (ZrO2 formation) and hydroxylation (Zr–OH formation) occur simultaneously during piranha treatment, the hydroxylation process is primarily responsible for the enhanced bonding performance observed, as hydroxyl groups provide reactive sites for chemical interaction with resin cement components. The similar failure mode patterns observed between extended piranha treatment groups and sandblasting suggest that both methods achieve comparable interfacial modification, though through different mechanisms. While sandblasting creates mechanical interlocking through surface roughening, piranha solution enhances chemical bonding potential primarily through surface hydroxylation (Zr–OH formation), with complementary oxide layer formation providing structural stability.
Sandblasting primarily relies on mechanical abrasion to increase surface area and remove contaminants [26]. While effective at creating surface roughness, it has several limitations regarding oxide layer formation: The mechanical impact from sandblasting can actually remove existing beneficial oxide layers, and any new oxidation occurs through atmospheric exposure after treatment [27,28,29]. This results in less controlled oxide formation that may vary in thickness and adherence. Additionally, sandblasting can embed abrasive particles in the surface, potentially creating weak points or contamination that interfere with bonding [30]. Studies have shown that piranha-treated surfaces often exhibit comparable durability compared to sandblasted surfaces if sufficient etching time was performed [15]. The surface hydroxylation creates reactive Zr–OH groups that provide optimal bonding sites for resin cement, while simultaneous oxide formation ensures surface stability and maintains bond integrity.
From a clinical perspective, the failure mode analysis has important implications for the longevity and predictability of zirconia restorations. Adhesive failures, while predominant in this study, are generally more favorable clinically than cohesive failures within the tooth structure or restoration material, as they allow for easier repair and retreatment procedures. The mixed failure pattern observed in effectively treated groups suggests improved bonding reliability, which could translate to enhanced clinical performance and reduced restoration failure rates. The consistent failure mode patterns across the effective treatment groups support the clinical equivalence of these approaches, providing practitioners with treatment flexibility while maintaining confidence in achieving reliable bonding outcomes.
However, it is important to note that piranha solution requires careful handling due to its highly corrosive and oxidizing nature, and may not be suitable for all materials or applications where sandblasting might be preferred for cost or safety considerations [31]. From a practical standpoint, the 2–4 min etching protocols offer optimal surface modification for bonding applications while providing process flexibility for clinical implementation. These durations ensure adequate surface treatment that overcomes the limitations observed with brief 1-min exposure, while maintaining the advantages of uniform chemical treatment. The extended treatment time provides practitioners with confidence in achieving consistent surface modification that supports reliable bonding outcomes, as evidenced by both superior bond strength values and more favorable failure mode patterns.
These findings suggest that extended piranha solution etching (2–4 min) represents a scientifically validated alternative to sandblasting for zirconia surface treatment when adequate bond strength is required. The equivalent effectiveness of extended etching protocols, combined with the unique advantages of chemical over mechanical treatment and similar failure mode characteristics, positions prolonged piranha etching as a valuable option in contemporary restorative dentistry. The selection of etching duration should prioritize adequate surface modification over treatment speed, with 2–4 min protocols recommended for achieving reliable bonding outcomes comparable to sandblasting, while avoiding the insufficient surface modification and unfavorable failure patterns associated with brief 1-min treatments.
However, this research has several limitations that should be acknowledged. First, the research was conducted under laboratory conditions using a single type of zirconia material and one specific resin cement system, which may limit the generalizability of the findings to other zirconia brands and cement formulations commonly used in clinical practice. Second, the study evaluated only immediate bond strength after 24-h water storage, which does not reflect the long-term clinical performance under cyclic loading, thermal cycling, and oral environmental conditions that restorations experience over years of service. Third, the piranha solution concentration and composition were standardized, but variations in solution freshness, temperature, and mixing ratios could influence treatment effectiveness in practical applications. Additionally, the study did not include assessment of surface roughness parameters or detailed surface chemical analysis that could provide deeper insights into the mechanisms underlying the observed bonding improvements. Furthermore, this study did not include detailed surface characterization techniques such as profilometry or scanning electron microscopy to quantify and compare the surface topography changes induced by sandblasting versus piranha treatment. Such analysis would provide valuable insights into the relative contributions of micromechanical interlocking versus chemical bonding to the observed adhesion performance, helping to elucidate the mechanistic basis for the equivalent bond strengths achieved by these fundamentally different surface treatment approaches.
Future research should address these limitations through several avenues of investigation. Long-term studies incorporating thermocycling and mechanical fatigue testing are essential to evaluate the durability of piranha-treated zirconia bonds under simulated clinical conditions. Comparative studies using different zirconia formulations (3Y-TZP, 4Y-TZP, 5Y-TZP) and various resin cement systems would help establish the broader applicability of piranha solution treatment across different material combinations. Surface characterization studies using X-ray photoelectron spectroscopy, atomic force microscopy, and contact angle measurements could elucidate the specific surface modifications responsible for improved bonding. Additionally, clinical trials comparing piranha solution treatment to conventional sandblasting in actual restorative procedures would provide valuable real-world evidence of treatment effectiveness. Investigation of optimal piranha solution compositions and concentrations, as well as the development of standardized protocols for clinical application, would further enhance the practical utility of this surface treatment method. Future investigations should include comprehensive surface characterization studies combining profilometry, X-ray photoelectron spectroscopy, and scanning electron microscopy to quantify both topographical and chemical changes induced by different surface treatments. Comparative studies evaluating the durability of mechanical versus chemical bonding mechanisms under various aging conditions (thermocycling, mechanical fatigue, hydrolytic challenge) would provide crucial insights into the long-term performance characteristics of each bonding pathway. Additionally, research into combined treatment protocols that leverage both micromechanical and chemical bonding mechanisms could potentially yield superior adhesion performance that exceeds the effectiveness of either approach alone.
5. Conclusions
This study demonstrates that piranha solution etching time significantly affects the bonding performance of zirconia ceramics. While 1-min piranha treatment proved inadequate, resulting in significantly lower bond strength and 100% adhesive failures, extended etching times of 2–4 min achieved bond strengths and failure mode patterns comparable to the gold standard sandblasting treatment. The findings indicate that piranha solution treatment for 2–4 min represents a viable alternative to sandblasting for zirconia surface preparation, offering equivalent bonding effectiveness with the potential advantages of uniform surface hydroxylation and controlled oxidation. These results provide valuable guidance for clinicians seeking alternative surface treatment methods for zirconia restorations, emphasizing that adequate treatment duration is critical for achieving reliable bonding outcomes in restorative dentistry.
Author Contributions
Conceptualization, A.M. and A.K.; methodology, A.M., N.K., T.R., and A.K.; formal analysis, A.M. and A.K.; investigation, A.M. and A.K.; data curation, A.M. and A.K.; writing—original draft preparation, A.M., N.K., T.R., and A.K.; writing—review and editing, A.M. and A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Faculty of Dentistry Thammasat University Research Fund, Contract No. DTGG 2/2568.
Institutional Review Board Statement
This research does not include human or animal participants. This study follows institutional protocols for research concerning dental materials.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors gratefully acknowledge Natee Sirisit from the Department of Chemistry, Faculty of Science and Technology, Thammasat University, for the expert preparation of piranha solution and adherence to safety protocols throughout the chemical processing procedures, which were essential for the surface treatment described in this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gracis, S.; Thompson, V.P.; Ferencz, J.L.; Silva, N.R.; Bonfante, E.A. A new classification system for all-ceramic and ceramic-like restorative materials. Int. J. Prosthodont. 2015, 28, 227–235. [Google Scholar] [CrossRef]
- Denry, I.; Kelly, J.R. State of the art of zirconia for dental applications. Dent. Mater. 2008, 24, 299–307. [Google Scholar] [CrossRef]
- Matinlinna, J.P.; Lung, C.Y.K.; Tsoi, J.K.H. Silane adhesion mechanism in dental applications and surface treatments: A review. Dent. Mater. 2018, 34, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Blatz, M.B.; Sadan, A.; Kern, M. Resin-ceramic bonding: A review of the literature. J. Prosthet. Dent. 2003, 89, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Klaisiri, A.; Krajangta, N.; Sriamporn, T.; Phumpatrakom, P.; Thamrongananskul, N. The Use of Silane Coupling Agents on Lithium Disilicate Glass Ceramic Repaired with Resin Composite. J. Int. Dent. Med. Res. 2021, 14, 169–172. [Google Scholar]
- Leelaponglit, S.; Maneenacarith, A.; Wutikhun, T.; Klaisiri, A. The Various Silane Agents in Universal Adhesives on Repair Strength of Resin Composite to Resin Composite. J. Compos. Sci. 2023, 7, 7. [Google Scholar] [CrossRef]
- Kelly, J.R.; Rungruanganunt, P.; Hunter, B.; Vailati, F. Development of a clinically validated bulk failure test for ceramic crowns. J. Prosthet. Dent. 2010, 104, 228–238. [Google Scholar] [CrossRef]
- Thompson, J.Y.; Stoner, B.R.; Piascik, J.R.; Smith, R. Adhesion/cementation to zirconia and other non-silicate ceramics: Where are we now? Dent. Mater. 2011, 27, 71–82. [Google Scholar] [CrossRef]
- Kern, M.; Wegner, S.M. Bonding to zirconia ceramic: Adhesion methods and their durability. Dent. Mater. 1998, 14, 64–71. [Google Scholar] [CrossRef]
- Özcan, M.; Bernasconi, M. Adhesion to zirconia used for dental restorations: A systematic review and meta-analysis. J. Adhes. Dent. 2015, 17, 7–26. [Google Scholar] [CrossRef]
- Atsu, S.S.; Kilicarslan, M.A.; Kucukesmen, H.C.; Aka, P.S. Effect of zirconium-oxide ceramic surface treatments on the bond strength to adhesive resin. J. Prosthet. Dent. 2006, 95, 430–436. [Google Scholar] [CrossRef]
- Sriamporn, T.; Thamrongananskul, N.; Busabok, C.; Poolthong, S.; Uo, M.; Tagami, J. Dental zirconia can be etched by hydrofluoric acid. Dent. Mater. J. 2014, 33, 79–85. [Google Scholar] [CrossRef]
- Chatterjee, N.; Ghosh, A. Current scenario on adhesion to zirconia; surface pretreatments and resin cements: A systematic review. J. Indian Prosthodont. Soc. 2022, 22, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Comino-Garayoa, R.; Peláez, J.; Tobar, C.; Rodríguez, V.; Suárez, M.J. Adhesion to Zirconia: A Systematic Review of Surface Pretreatments and Resin Cements. Materials 2021, 14, 2751. [Google Scholar] [CrossRef] [PubMed]
- Lohbauer, U.; Zipperle, M.; Rischka, K.; Petschelt, A.; Müller, F.A. Hydroxylation of dental zirconia surfaces: Characterization and bonding potential. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 87, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Akrawatcharawittaya, P.; Sriamporn, T.; Vuddhakanok, S.; Thamrongananskul, N.; Klaisiri, A. The Effect of a Dual Cure Activator on Self-Adhesive Resin Cements and Zirconia Shear Bond Strength. Ceramics 2024, 7, 1237–1246. [Google Scholar] [CrossRef]
- Klaisiri, A.; Suebnukarn, S.; Krajangta, N.; Rakmanee, T.; Sriamporn, T.; Thamrongananskul, N. The Effect of Morpholine on Composite-to-Composite Repair Strength Contaminated with Saliva. Polymers 2022, 14, 4718. [Google Scholar] [CrossRef]
- He, M.; Zhang, Z.; Zheng, D.; Ding, N.; Liu, Y. Effect of sandblasting on surface roughness of zirconia-based ceramics and shear bond strength of veneering porcelain. Dent. Mater. J. 2014, 33, 778–785. [Google Scholar] [CrossRef]
- Su, N.; Yue, L.; Liao, Y.; Liu, W.; Zhang, H.; Li, X.; Wang, H.; Shen, J. The effect of various sandblasting conditions on surface changes of dental zirconia and shear bond strength between zirconia core and indirect composite resin. J. Adv. Prosthodont. 2015, 7, 214–223. [Google Scholar] [CrossRef]
- Sun, L.; Hong, G. Surface Modifications for Zirconia Dental Implants: A Review. Front. Dent. Med. 2021, 2, 733242. [Google Scholar] [CrossRef]
- Zhang, Y.; Lawn, B.R. Novel Zirconia Materials in Dentistry. J. Dent. Res. 2018, 97, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.-S.; Chin, J.; Chia, J.; Chiang, C.-L. Quantitative Studies on PDMS-PDMS Interface Bonding with Piranha Solution and its Swelling Effect. Micromachines 2012, 3, 427–441. [Google Scholar] [CrossRef]
- Targhan, H.; Evans, P.; Bahrami, K. A review of the role of hydrogen peroxide in organic transformations. J. Ind. Eng. Chem. 2021, 104, 295–332. [Google Scholar] [CrossRef]
- Maneenacarith, A.; Rakmanee, T.; Klaisiri, A. The influence of resin cement thicknesses on shear bond strength of the cement-zirconia. J. Stomatol. 2022, 75, 7–12. [Google Scholar] [CrossRef]
- Matinlinna, J.P.; Lassila, L.V. Enhanced resin-composite bonding to zirconia framework after pretreatment with selected silane monomers. Dent. Mater. 2011, 27, 273–280. [Google Scholar] [CrossRef]
- Yang, B.; Wolfart, S.; Scharnberg, M.; Ludwig, K.; Adelung, R.; Kern, M. Influence of contamination on zirconia ceramic bonding. J. Dent. Res. 2007, 86, 749–753. [Google Scholar] [CrossRef]
- Nowak, W.J.; Ochał, K.; Wierzba, P.; Gancarczyk, K.; Wierzba, B. Effect of Substrate Roughness on Oxidation Resistance of an Aluminized Ni-Base Superalloy. Metals 2019, 9, 782. [Google Scholar] [CrossRef]
- Nowak, W.J.; Serafin, D.; Wierzba, B. Effect of surface mechanical treatment on the oxidation behavior of FeAl-model alloy. J. Mater. Sci. 2019, 54, 9185–9196. [Google Scholar] [CrossRef]
- Serafin, D.; Nowak, W.J.; Wierzba, B. The effect of surface preparation on high temperature oxidation of Ni, Cu and Ni-Cu alloy. Appl. Surf. Sci. 2019, 476, 442–451. [Google Scholar] [CrossRef]
- Karakoca, S.; Yılmaz, H. Influence of surface treatments on surface roughness, phase transformation, and biaxial flexural strength of Y-TZP ceramics. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 930–937. [Google Scholar] [CrossRef]
- Alsmael, M.A.; Mohammed Al-Khafaji, A. Improving Surface Properties of PEEK for Dental Applications by Using Piranha Solution. Int. J. Dent. 2023, 2023, 7840601. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).