Compositional Effects on Mechanical Performance of Zirconia–Magnesia–Alumina Ceramics
Abstract
1. Introduction
2. Materials and Methods
- P—the load in N;
- υ—Poisson’s ratio (0.305);
- t—the tablet thickness;
- a—the radius of the circle on which the spherical supports are located;
- b—the radius of the pin pressing on the tablet;
- R—the radius of the ceramic tablet.
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, A.G. Perspective on the Development of High-Toughness Ceramics. J. Am. Ceram. Soc. 1990, 73, 187–206. [Google Scholar] [CrossRef]
- Shvydyuk, K.O.; Nunes-Pereira, J.; Rodrigues, F.F.; Silva, A.P. Review of Ceramic Composites in Aeronautics and Aerospace: A Multifunctional Approach for TPS, TBC and DBD Applications. Ceramics 2023, 6, 195–230. [Google Scholar] [CrossRef]
- Kostishin, V.G.; Korovushkin, V.V.; Pokholok, K.V.; Trukhanov, A.V.; Isaev, I.M.; Mironovich, A.Y.; Darwish, M.A. Cation Distribution and Magnetic Properties of Polycrystalline Hexagonal BaFe12 –XSnxO19 Ferrites. Phys. Solid State 2021, 63, 1680–1689. [Google Scholar] [CrossRef]
- Boniecki, M.; Sadowski, T.; Gołębiewski, P.; Węglarz, H.; Piątkowska, A.; Romaniec, M.; Krzyżak, K.; Łosiewicz, K. Mechanical Properties of Alumina/Zirconia Composites. Ceram. Int. 2020, 46, 1033–1039. [Google Scholar] [CrossRef]
- Kelly, J.R.; Denry, I. Stabilized Zirconia as a Structural Ceramic: An Overview. Dent. Mater. 2008, 24, 289–298. [Google Scholar] [CrossRef]
- Cao, X.Q.; Vassen, R.; Stoever, D. Ceramic Materials for Thermal Barrier Coatings. J. Eur. Ceram. Soc. 2004, 24, 1–10. [Google Scholar] [CrossRef]
- Twomey, B.; de Faoite, D.; Doherty, K.A.J.; Stanton, K.T. Ceramics in Space Applications. Encycl. Mater. Tech. Ceram. Glas. 2021, 2, 359–379. [Google Scholar] [CrossRef]
- Sequeira, S.; Fernandes, M.H.; Neves, N.; Almeida, M.M. Development and Characterization of Zirconia–Alumina Composites for Orthopedic Implants. Ceram. Int. 2017, 43, 693–703. [Google Scholar] [CrossRef]
- Meir, S.; Kalabukhov, S.; Frage, N.; Hayun, S. Mechanical Properties of Al2O3\Ti Composites Fabricated by Spark Plasma Sintering. Ceram. Int. 2015, 41, 4637–4643. [Google Scholar] [CrossRef]
- Wahsh, M.M.S.; Khattab, R.M.; Awaad, M. Thermo-Mechanical Properties of Mullite/Zirconia Reinforced Alumina Ceramic Composites. Mater. Des. 2012, 41, 31–36. [Google Scholar] [CrossRef]
- Rittidech, A.; Somrit, R.; Tunkasiri, T. Effect of Adding Y2O3 on Structural and Mechanical Properties of Al2O3–ZrO2 Ceramics. Ceram. Int. 2013, 39, S433–S436. [Google Scholar] [CrossRef]
- Malka, I.E.; Danelska, A.; Kimmel, G. The Influence of Al2O3 Content on ZrO2-Al2O3 Nanocomposite Formation—The Comparison between Sol-Gel and Microwave Hydrothermal Methods. Mater. Today Proc. 2016, 3, 2713–2724. [Google Scholar] [CrossRef]
- Burger, W.; Kiefer, G. Alumina, Zirconia and Their Composite Ceramics with Properties Tailored for Medical Applications. J. Compos. Sci. 2021, 5, 306. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Konuhova, M.; Borgekov, D.B.; Anatoli, P.I. Study of Irradiation Temperature Effect on Radiation-Induced Polymorphic Transformation Mechanisms in ZrO2 Ceramics. Opt. Mater. 2024, 156, 115994. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L.; Virkar, A.V.; Clarke, D.R. The Tetragonal-Monoclinic Transformation in Zirconia: Lessons Learned and Future Trends. J. Am. Ceram. Soc. 2009, 92, 1901–1920. [Google Scholar] [CrossRef]
- Sommer, F.; Landfried, R.; Kern, F.; Gadow, R. Mechanical Properties of Zirconia Toughened Alumina with 10–24 Vol.% 1.5 Mol% Y-TZP Reinforcement. J. Eur. Ceram. Soc. 2012, 32, 3905–3910. [Google Scholar] [CrossRef]
- Łabuz, A.; Lach, R.; Raczka, M.; Wójtowicz, B.; Pyda, W. Processing and Characterization of Ca-TZP Nanoceramics. J. Eur. Ceram. Soc. 2015, 35, 3943–3947. [Google Scholar] [CrossRef]
- Gómez-Rodríguez, C.; García-Quiñonez, L.V.; Aguilar-Martínez, J.A.; Castillo-Rodríguez, G.A.; Rodríguez-Castellanos, E.A.; López-Perales, J.F.; Mendivil-Palma, M.I.; Verdeja, L.F.; Fernández-González, D. MgO–ZrO2 Ceramic Composites for Silicomanganese Production. Materials 2022, 15, 2421. [Google Scholar] [CrossRef]
- Yi, H.; Che, J.; Liang, G.; Liu, X. Effect of Rare Earth Elements on Stability and Sintering Resistance of Tetragonal Zirconia for Advanced Thermal Barrier Coatings. Crystals 2021, 11, 287. [Google Scholar] [CrossRef]
- Wang, J.; Chen, M.; Sun, J.; Hu, M.; Lu, X.; Shu, C.; Zhang, H.; Wang, Y. Phase Stability and Thermophysical Properties of CeO2-Re2O3 (ReEu, Gd, Dy, Y, Er, Yb) Co-Stabilised Zirconia. Ceram. Int. 2023, 49, 21634–21644. [Google Scholar] [CrossRef]
- Ramesh, S.; Sara Lee, K.Y.; Tan, C.Y. A Review on the Hydrothermal Ageing Behaviour of Y-TZP Ceramics. Ceram. Int. 2018, 44, 20620–20634. [Google Scholar] [CrossRef]
- Roy, M.E.; Whiteside, L.A.; Katerberg, B.J.; Steiger, J.A. Phase Transformation, Roughness, and Microhardness of Artificially Aged Yttria- and Magnesia-Stabilized Zirconia Femoral Heads. J. Biomed. Mater. Res. A 2007, 83A, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Rejab, N.A.; Azhar, A.Z.A.; Kian, K.S.; Ratnam, M.M.; Ahmad, Z.A. Effects of MgO Addition on the Phase, Mechanical Properties, and Microstructure of Zirconia-Toughened Alumina Added with CeO2 (ZTA–CeO2) Ceramic Composite. Mater. Sci. Eng. A 2014, 595, 18–24. [Google Scholar] [CrossRef]
- Kosmač, T.; Wallace, J.; Claussen, N. Influence of MgO Additiions on the Microstructure and Mechanical Properties of Al2O3-ZrO2 Composites. J. Am. Ceram. Soc. 1982, 65, c66–c67. [Google Scholar] [CrossRef]
- Ngashangua, S.; Vasanthavel, S.; Ponnilavan, V.; Kannan, S. Effect of MgO Additions on the Phase Stability and Degradation Ability in ZrO2–Al2O3 Composite Systems. Ceram. Int. 2015, 41, 3814–3821. [Google Scholar] [CrossRef]
- Sarkar, M.; Dutta, S.; Chakraborty, S.S.; Mandal, N. Grain Size Distribution Analysis of MgO and VC Inhibited Zirconia Toughened Alumina Ceramics Using Digital Image Processing. Mater. Today Commun. 2024, 39, 108665. [Google Scholar] [CrossRef]
- Azhar, A.Z.A.; Mohamad, H.; Ratnam, M.M.; Ahmad, Z.A. Effect of MgO Particle Size on the Microstructure, Mechanical Properties and Wear Performance of ZTA–MgO Ceramic Cutting Inserts. Int. J. Refract. Met. Hard Mater. 2011, 29, 456–461. [Google Scholar] [CrossRef]
- Meena, K.L.; Karunakar, D.B. Effect of ZrO2 and MgO Added in Alumina on the Physical and Mechanical Properties of Spark Plasma Sintered Nanocomposite. Int. J. Refract. Met. Hard Mater. 2019, 81, 281–290. [Google Scholar] [CrossRef]
- Pavlyuchkov, D.; Savinykh, G.; Fabrichnaya, O. Experimental Investigation and Thermodynamic Modeling of the ZrO2–MgO–Al2O3 System. J. Eur. Ceram. Soc. 2014, 34, 1397–1408. [Google Scholar] [CrossRef]
- ISO 6872:2024; Dentistry—Ceramic Materials. International Organization for Standardization: Geneva, Switzerland, 2024.
- Waters, M.J.; Rondinelli, J.M.; Cuevas, J.L.; Courel Piedrahita, M.; Feddi, E.; Buyakova, S.P.; Kalatur, E.S.; Buyakov, A.S.; Kulkov, S.S. Structure and Properties of ZrO2-MgO Powders. IOP Conf. Ser. Mater. Sci. Eng. 2016, 123, 012040. [Google Scholar] [CrossRef]
- Duwez, P.; Odell, F.; Brown, F.H. Stabilization of Zirconia with Calcia and Magnesia. J. Am. Ceram. Soc. 1952, 35, 107–113. [Google Scholar] [CrossRef]
- Toraya, H. Whole-Powder-Pattern Fitting without Reference to a Structural Model: Application to X-Ray Powder Diffraction Data. J. Appl. Crystallogr. 1986, 19, 440–447. [Google Scholar] [CrossRef]
- Echigoya, J.; Sasai, K.S.H. Microstructural Change of 11 Mol% MgO–ZrO2 by Aging. Trans. Jpn. Inst. Met. 1988, 29, 561–569. [Google Scholar] [CrossRef]
- Śniezek, E.; Szczerba, J.; Stoch, P.; Prorok, R.; Jastrzebska, I.; Bodnar, W.; Burkel, E. Structural Properties of MgO–ZrO2 Ceramics Obtained by Conventional Sintering, Arc Melting and Field Assisted Sintering Technique. Mater. Des. 2016, 99, 412–420. [Google Scholar] [CrossRef]
- Keerthana, L.; Sakthivel, C.; Prabha, I. MgO-ZrO2 Mixed Nanocomposites: Fabrication Methods and Applications. Mater. Today Sustain. 2019, 3–4, 100007. [Google Scholar] [CrossRef]
- Bron, V.A.; Perepelitsyn, V.A.; Raeva, I.S.; Adel’, T.A.; Deryavina, V.I. Microhardness of Fused Periclase. Refractories 1984, 25, 198–202. [Google Scholar] [CrossRef]
- Gajdowski, C.; D’Elia, R.; Faderl, N.; Böhmler, J.; Lorgouilloux, Y.; Lemonnier, S.; Leriche, A. Mechanical and Optical Properties of MgAl2O4 Ceramics and Ballistic Efficiency of Spinel Based Armour. Ceram. Int. 2022, 48, 18199–18211. [Google Scholar] [CrossRef]
- Wang, M.; Pan, N. Predictions of Effective Physical Properties of Complex Multiphase Materials. Mater. Sci. Eng. R Rep. 2008, 63, 1–30. [Google Scholar] [CrossRef]
- Patil, R.N.; Subbarao, E.C. Monoclinic–Tetragonal Phase Transition in Zirconia: Mechanism, Pretransformation and Coexistence. Acta Crystallogr. Sect. A 1970, 26, 535–542. [Google Scholar] [CrossRef]
- Seidel, J.; Claussen, N.; Rödel, J. Reliability of Alumina Ceramics: Effect of Grain Size. J. Eur. Ceram. Soc. 1995, 15, 395–404. [Google Scholar] [CrossRef]
- Kambale, K.R.; Mahajan, A.; Butee, S.P. Effect of Grain Size on the Properties of Ceramics. Met. Powder Rep. 2019, 74, 130–136. [Google Scholar] [CrossRef]
- Shakirzyanov, R.I.; Volodina, N.O.; Kozlovskiy, A.L.; Zdorovets, M.V.; Shlimas, D.I.; Borgekov, D.B.; Garanin, Y.A. Study of the Structural, Electrical, and Mechanical Properties and Morphological Features of Y-Doped CeO2 Ceramics with Porous Structure. J. Compos. Sci. 2023, 7, 411. [Google Scholar] [CrossRef]
- Klym, H.; Karbovnyk, I.; Piskunov, S.; Popov, A.I. Positron Annihilation Lifetime Spectroscopy Insight on Free Volume Conversion of Nanostructured MGAL2O4 Ceramics. Nanomaterials 2021, 11, 3373. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, X. Kinetics and Thermodynamics of Mg-Al Disorder in MgAl2O4-Spinel: A Review. Molecules 2019, 24, 1704. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, B.; Wang, Y.; Hong, H.; Li, H.; Cheng, B.; Yang, J.; Li, B.; Wang, X. Preparation of High-Performance Zirconia Toughened Alumina Doped with Cr2O3 and SrO for Artificial Joints by Spray Granulation. Ceram. Int. 2024, 50, 28641–28651. [Google Scholar] [CrossRef]
- Saggioro, A.C.B.; Fernandes, L.; De Oliveira Carlos Villas-Bôas, M.; Salomão, R.; Pinelli, L.A.P. Processing and Characterization of a Novel ZTA-MGO for Dental Applications. Mater. Res. 2025, 28, e20240404. [Google Scholar] [CrossRef]
- Cai, H.; Lee, M.-Y.; Jiang, H.B.; Kwon, J.-S. Influence of Various Cleaning Solutions on the Geometry, Roughness, Gloss, Hardness, and Flexural Strength of 3D-Printed Zirconia. Sci. Rep. 2024, 14, 22551. [Google Scholar] [CrossRef] [PubMed]
- Kulyk, V.; Vasyliv, B.; Duriagina, Z.; Lyutyy, P.; Vavrukh, V.; Kostryzhev, A. The Effect of Sintering Temperature on Phase-Related Peculiarities of the Microstructure, Flexural Strength, and Fracture Toughness of Fine-Grained ZrO2–Y2O3–Al2O3–CoO–CeO2–Fe2O3 Ceramics. Crystals 2024, 14, 175. [Google Scholar] [CrossRef]
- Zenthöfer, A.; Ilani, A.; Schmitt, C.; Rammelsberg, P.; Hetzler, S.; Rues, S. Biaxial Flexural Strength of 3D-Printed 3Y-TZP Zirconia Using a Novel Ceramic Printer. Clin. Oral Investig. 2024, 28, 145. [Google Scholar] [CrossRef]
- Hetzler, S.; Hinzen, C.; Rues, S.; Schmitt, C.; Rammelsberg, P.; Zenthöfer, A. Biaxial Flexural Strength and Vickers Hardness of 3D-Printed and Milled 5Y Partially Stabilized Zirconia. J. Funct. Biomater. 2025, 16, 36. [Google Scholar] [CrossRef]
- Hajjaj, M.S.; Alamoudi, R.a.A.; Babeer, W.A.; Rizg, W.Y.; Basalah, A.A.; Alzahrani, S.J.; Yeslam, H.E. Flexural Strength, Flexural Modulus and Microhardness of Milled vs. Fused Deposition Modeling Printed Zirconia; Effect of Conventional vs. Speed Sintering. BMC Oral Health 2024, 24, 38. [Google Scholar] [CrossRef] [PubMed]
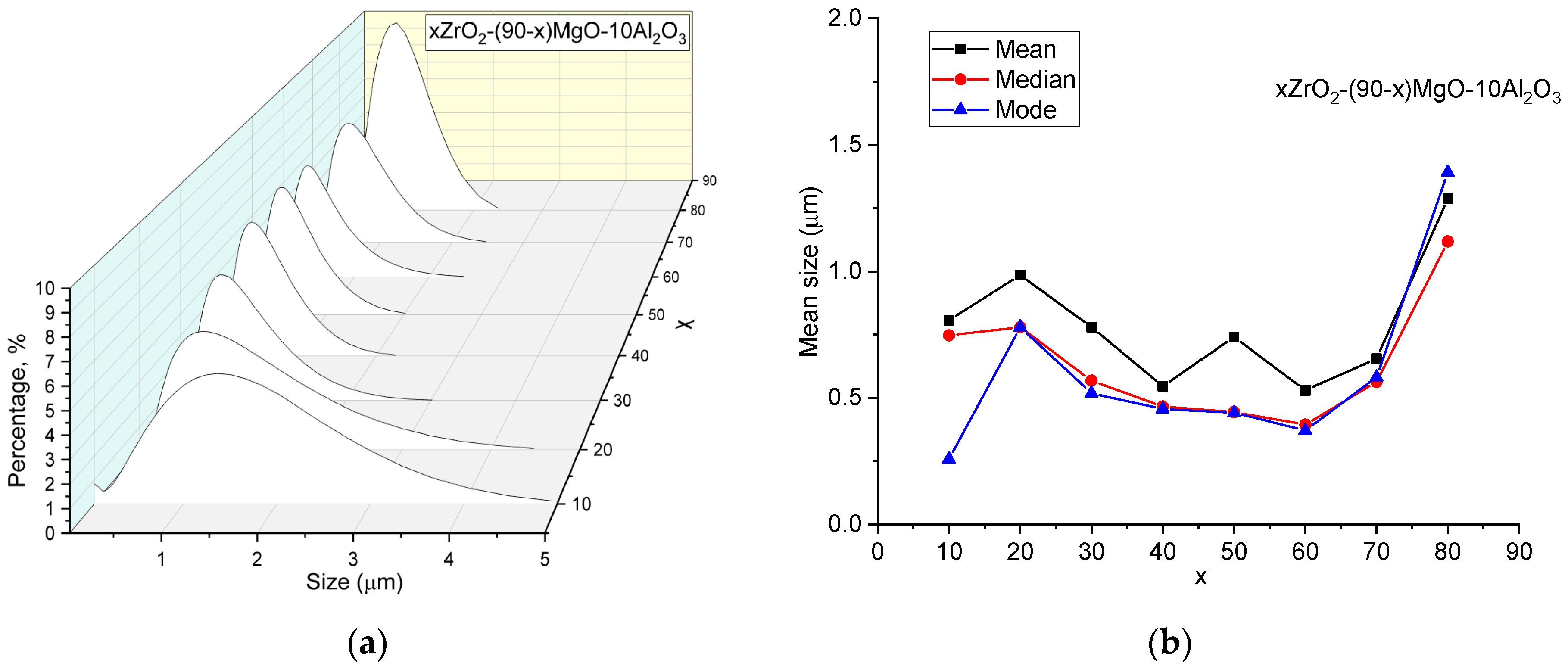
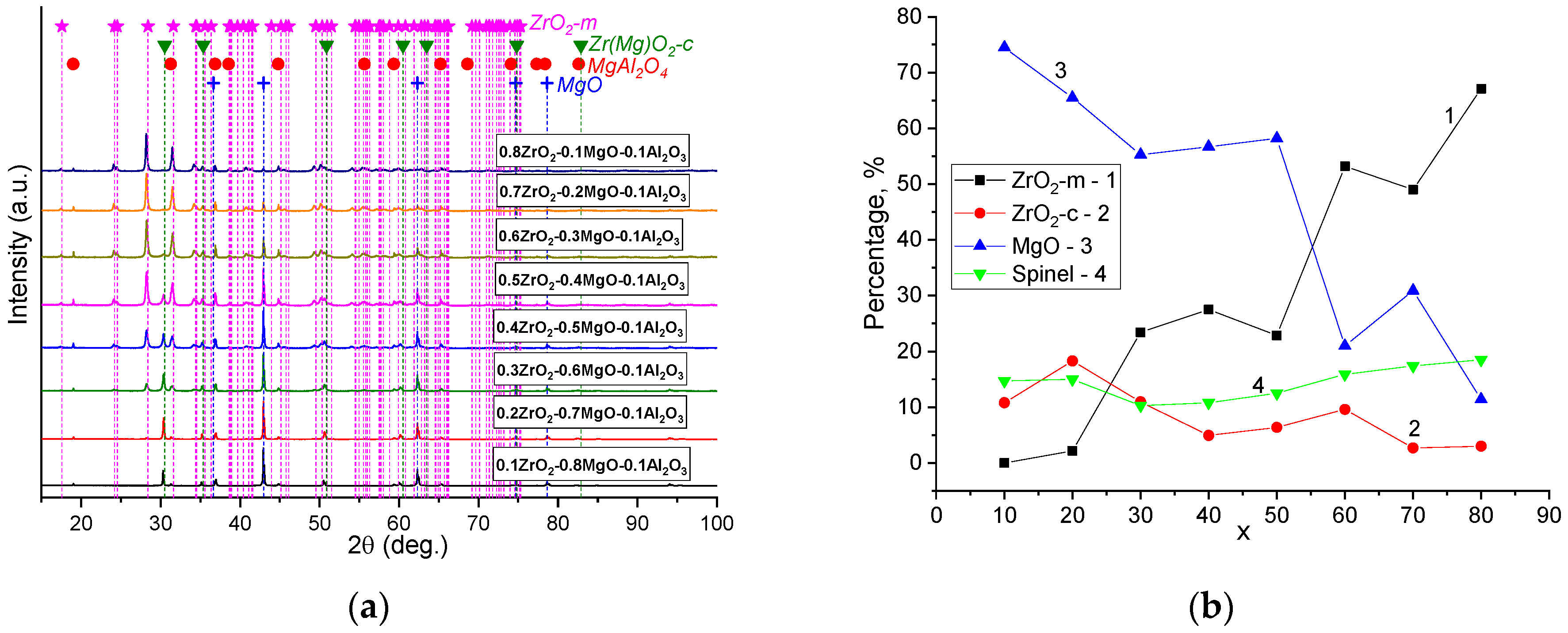
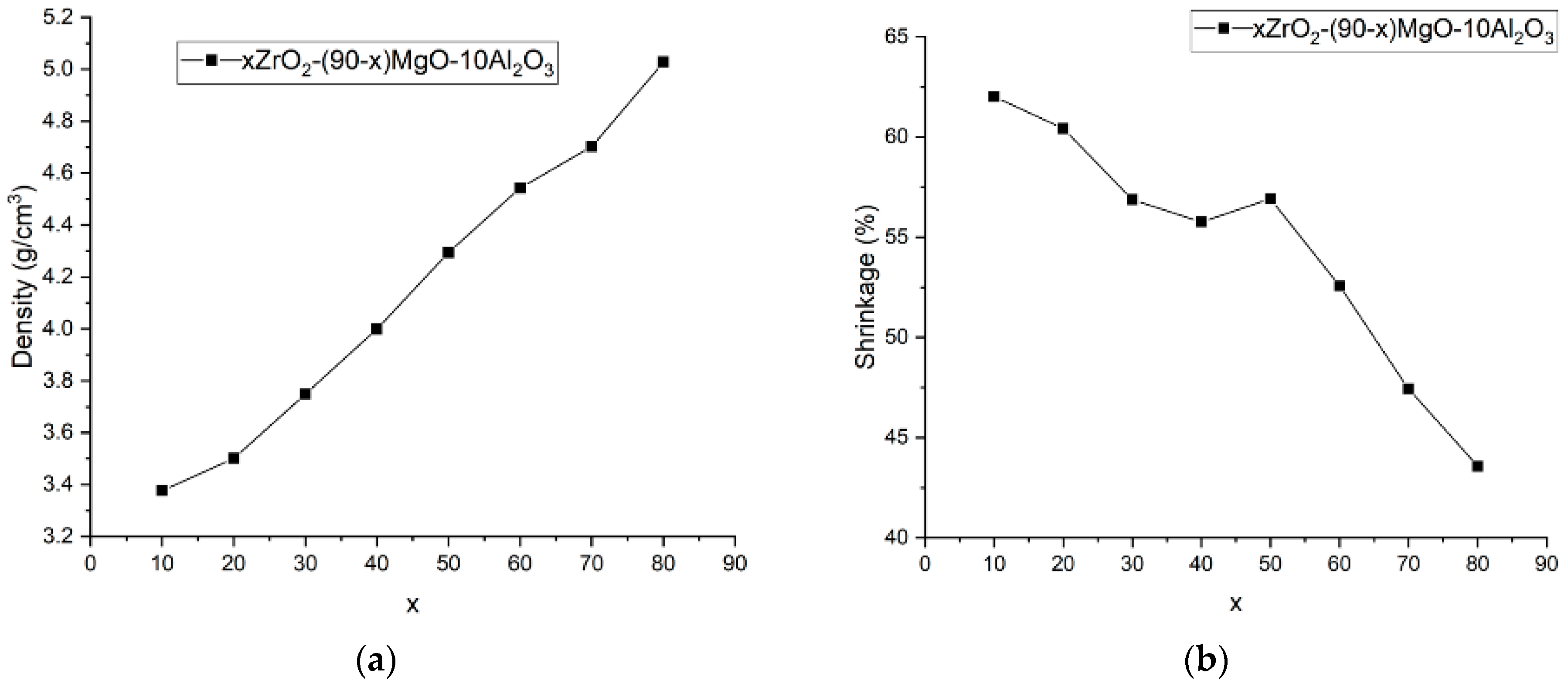
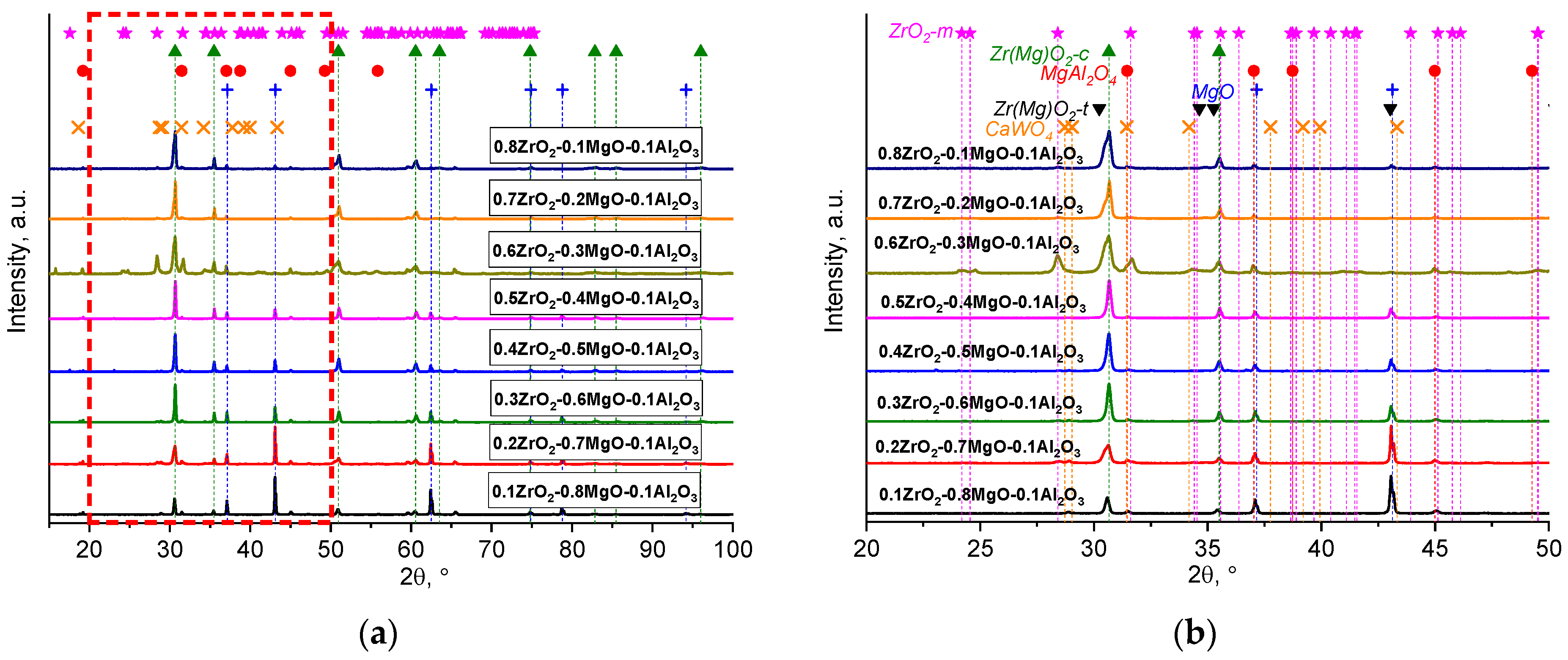
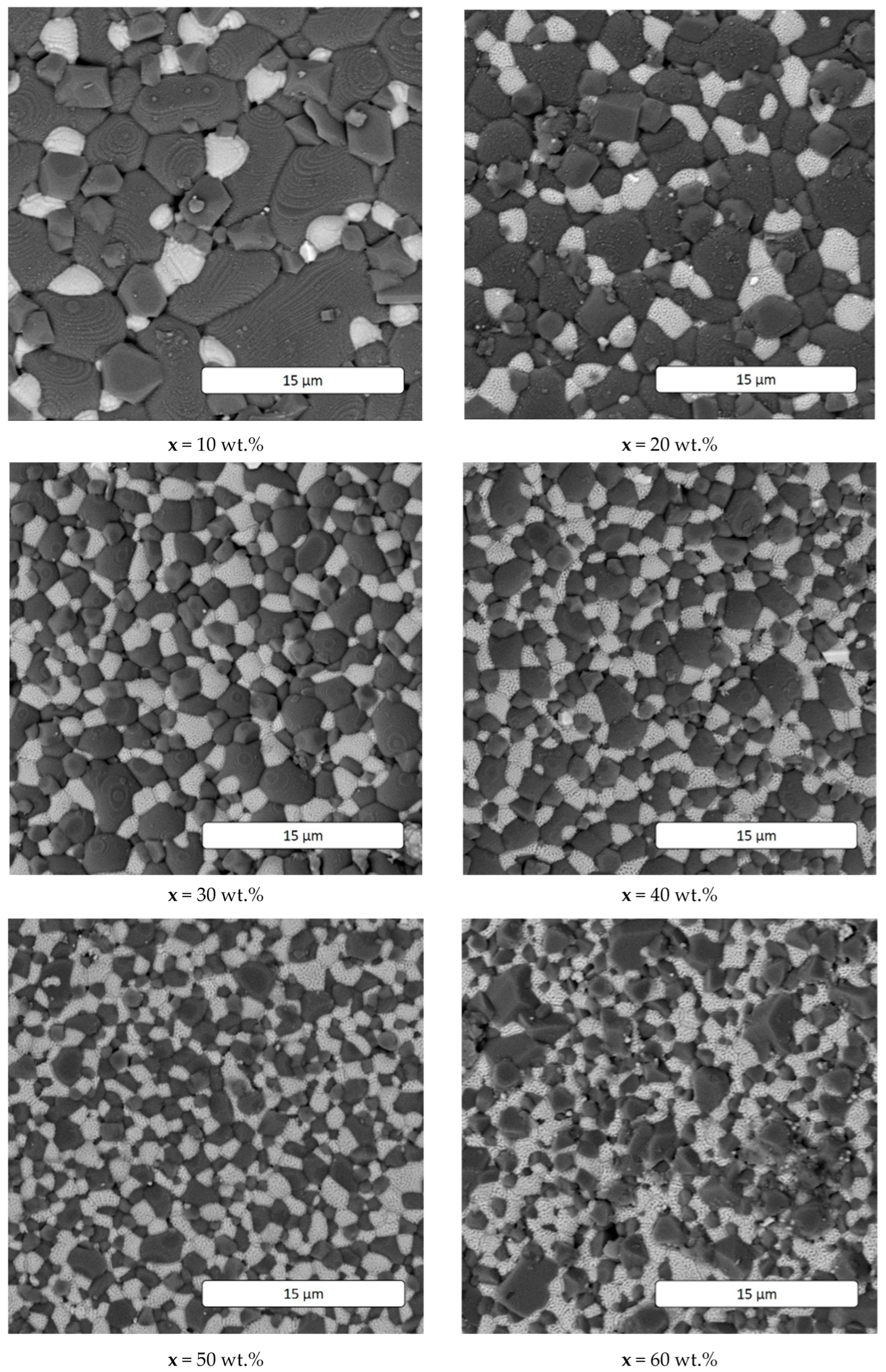
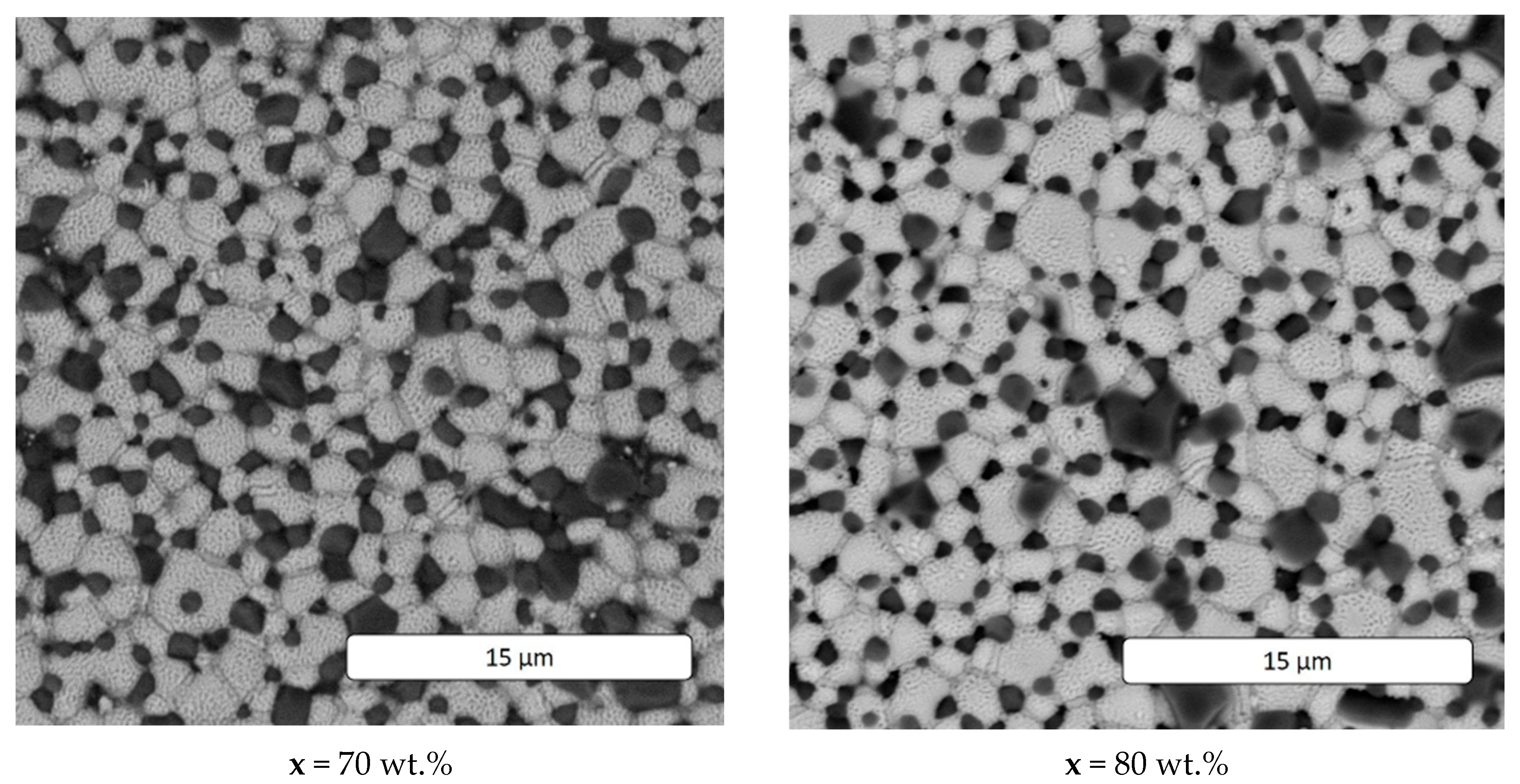
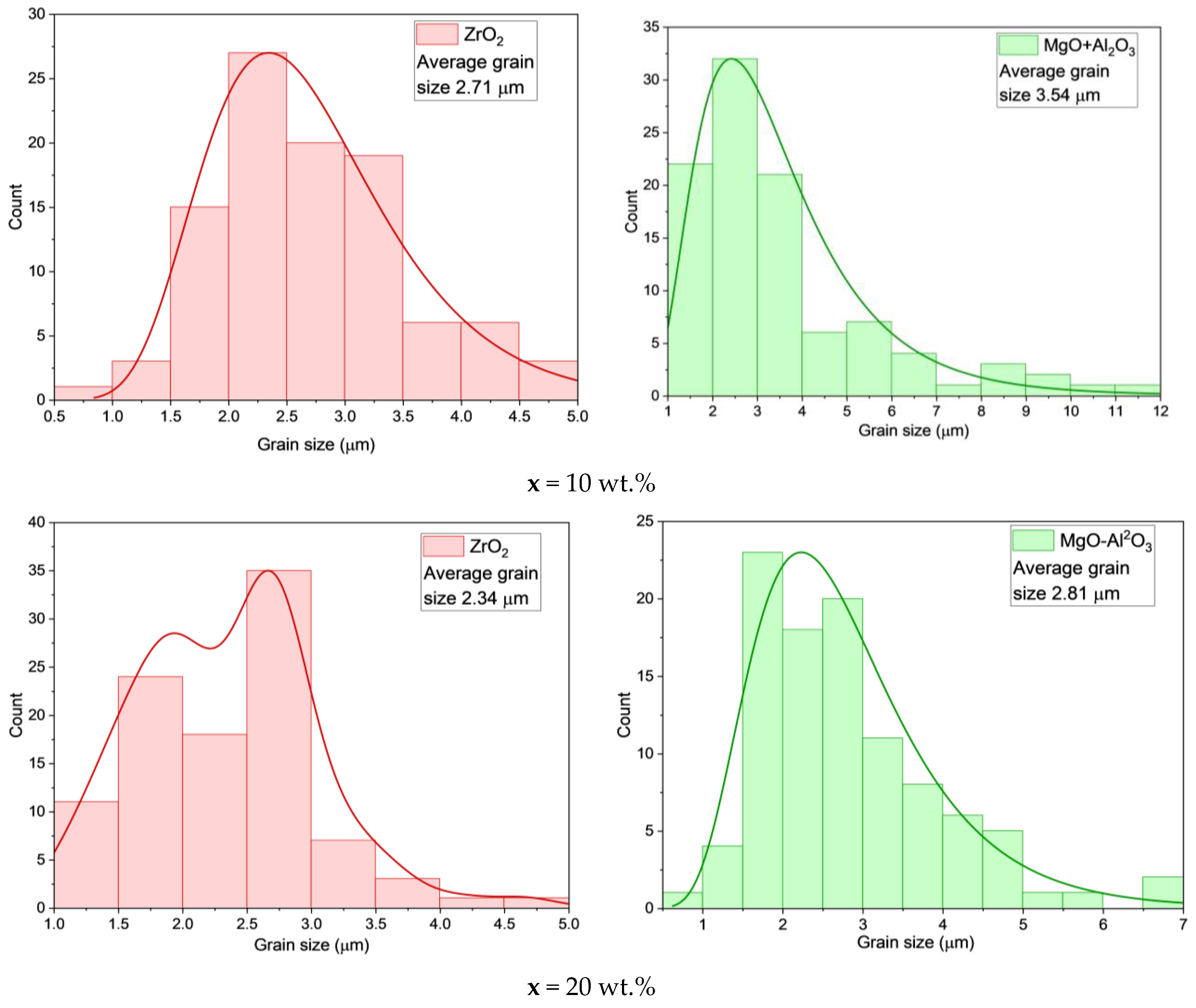
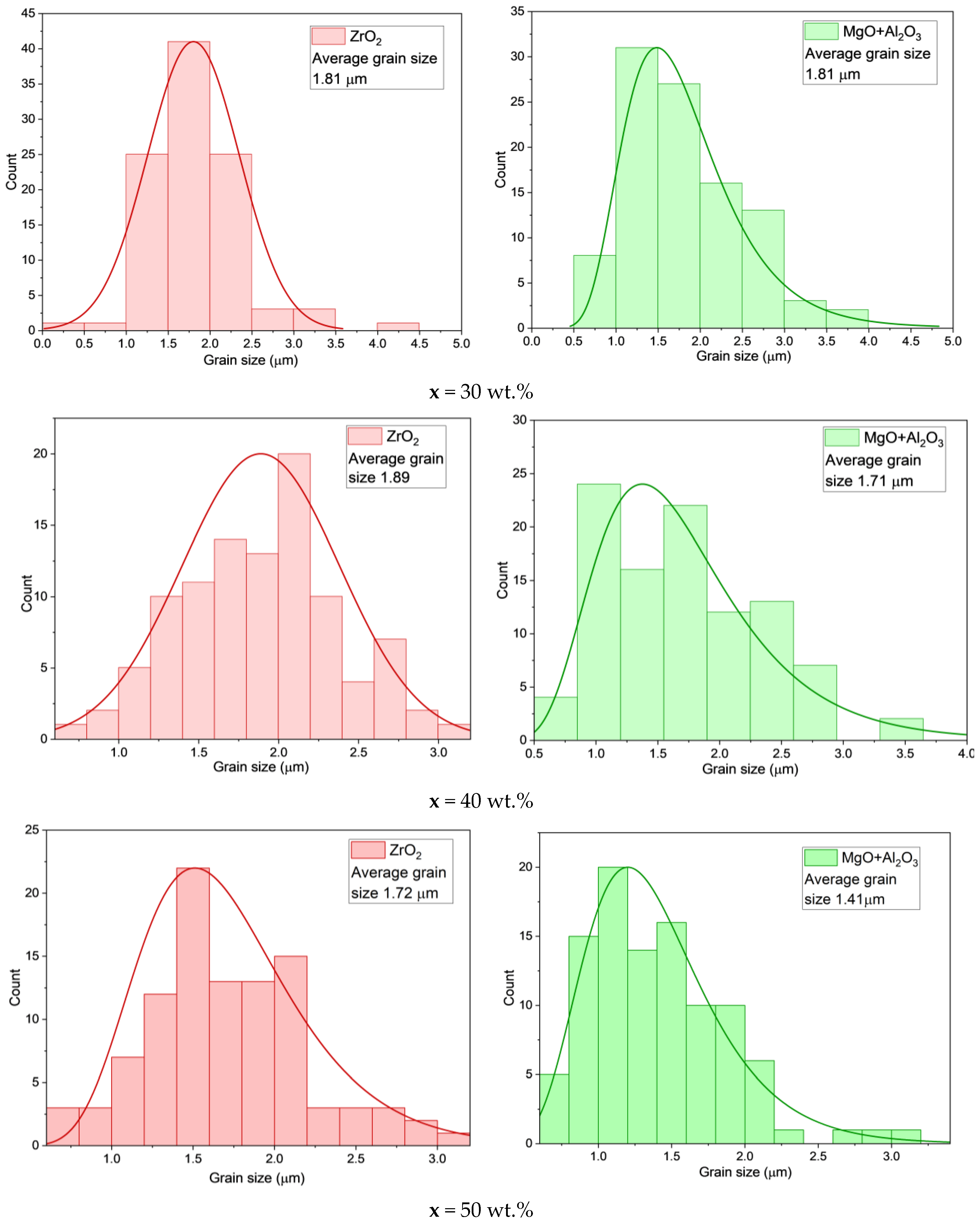
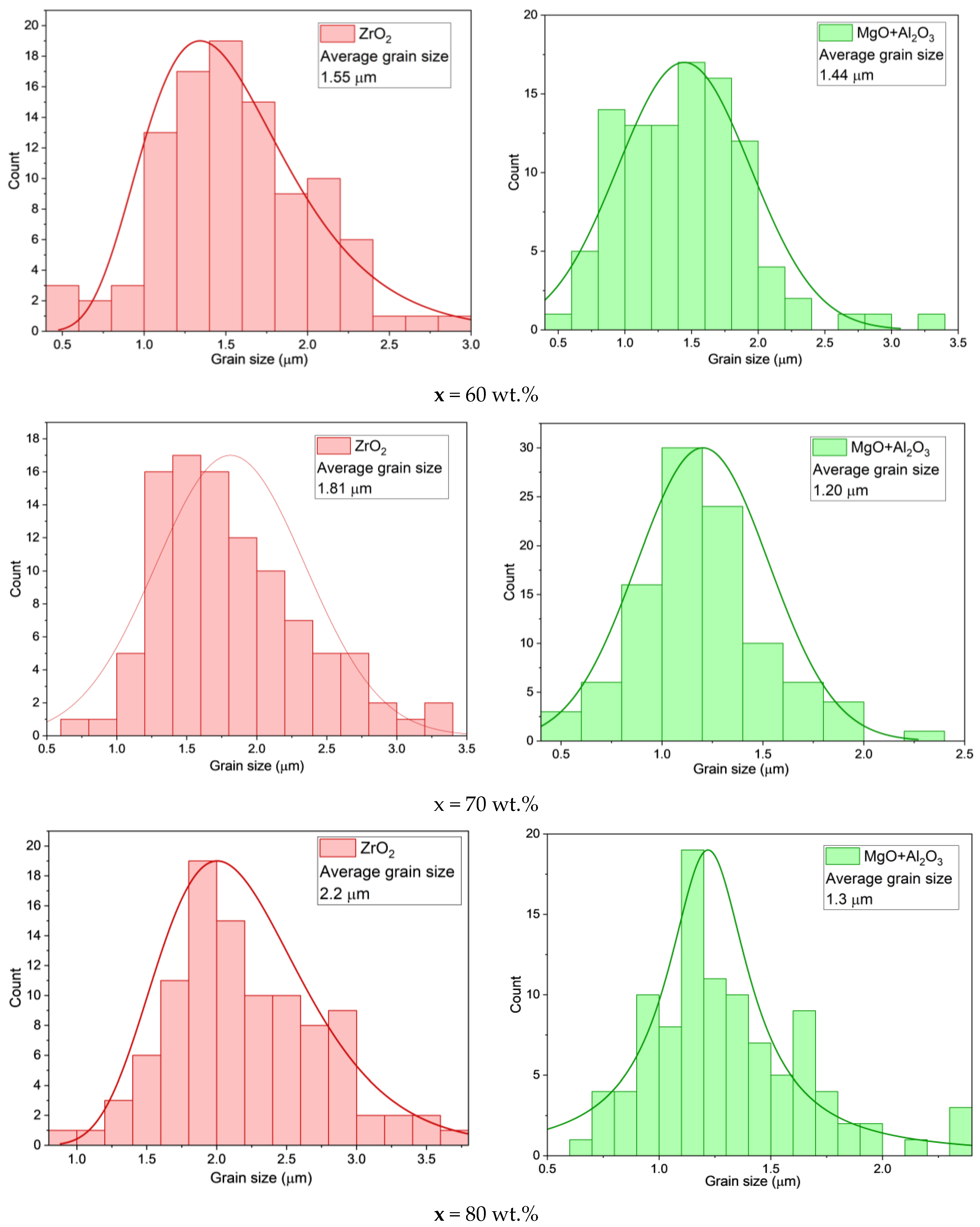
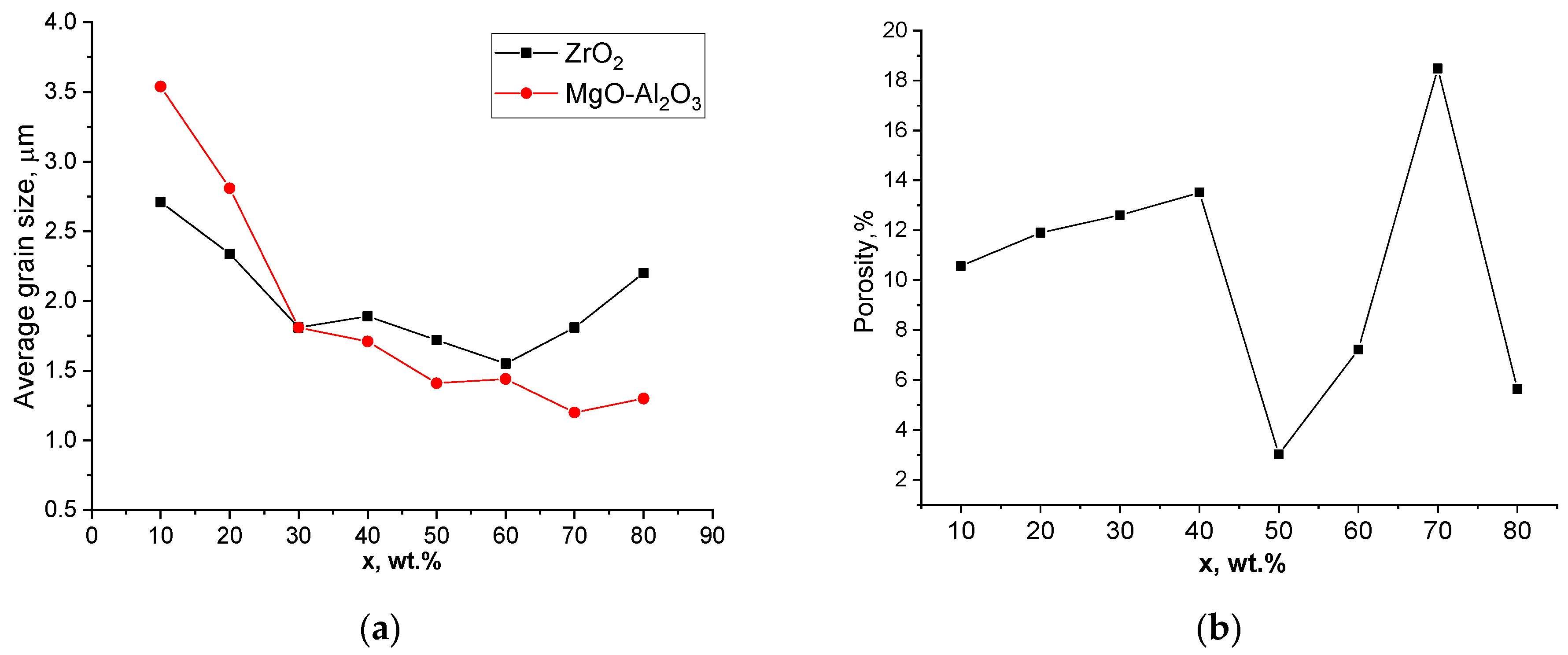
| x, wt.% | Phase Name | Phase Content, % | Statistical Parameters | ρexp, g/cm3 | Open Porosity, % | HV1 | ϭflex, MPa | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Rwp, % | Rp, % | Re, % | Χ2 | |||||||
| 10 | c-ZrMgO2 | 11.53 ± 0.09 | 8.04 | 6.02 | 7.3 | 1.2106 | 3.3368 | 6.86 | 830 ± 38 | 122 ± 4.76 |
| MgO | 74.05 ± 0.02 | |||||||||
| MgAl2O4 | 13.09 ± 0.17 | |||||||||
| CaWO4 | 1.33 ± 0.09 | |||||||||
| 20 | c-ZrMgO2 | 12.2 ± 0.03 | 8.05 | 6.03 | 7.3 | 1.2123 | 3.5012 | 5.59 | 832 ± 51 | 146 ± 11.41 |
| MgO | 56.7 ± 0.05 | |||||||||
| MgAl2O4 | 18.5 ± 0.02 | |||||||||
| m-ZrO2 | 5.2 ± 0.02 | |||||||||
| t-ZrO2 | 6.21 ± 0.03 | |||||||||
| CaWO4 | 1.19 ± 0.07 | |||||||||
| 30 | c-ZrMgO2 | 40.1 ± 0.03 | 10.49 | 7.54 | 6.82 | 2.3663 | 3.7488 | 5.39 | 898 ± 70 | 150 ± 6.57 |
| MgO | 32.2 ± 0.03 | |||||||||
| MgAl2O4 | 26.4 ± 0.04 | |||||||||
| m-ZrO2 | 0.0 | |||||||||
| CaWO4 | 1.30 ± 0.16 | |||||||||
| 40 | c-ZrMgO2 | 46.3 ± (5) | 11.15 | 7.68 | 6.69 | 2.7734 | 3.9999 | 5.91 | 1037 ± 12 | 208 ± 11.07 |
| MgO | 28.2 ± (4) | |||||||||
| MgAl2O4 | 13.2 ± (3) | |||||||||
| CaWO4 | 7.2 ± (6) | |||||||||
| Mg2SiO4 | 5.1 ± (6) | |||||||||
| 50 | c-ZrMgO2 | 47.2 ± 0.04 | 7.62 | 5.78 | 6.64 | 1.3124 | 4.2942 | 2.78 | 1037 ± 12 | 250 ± 15.16 |
| MgO | 25.6 ± 0.05 | |||||||||
| MgAl2O4 | 26.8 ± 0.03 | |||||||||
| CaWO4 | 0.4 ± 0.03 | |||||||||
| 60 | c-ZrMgO2 | 38.5 ± 0.03 | 10.38 | 7.74 | 6.57 | 2.4932 | 4.5432 | 0.34 | 736 ± 19 | 113 ± 3.73 |
| MgO | 10.1 ± 0.02 | |||||||||
| MgAl2O4 | 18.3 ± 0.03 | |||||||||
| m-ZrO2 | 26.5 ± 0.02 | |||||||||
| Si192O384 | 6.6 ± 0.03 | |||||||||
| 70 | c-ZrMgO2 | 42.9 ± 0.03 | 8.78 | 6.94 | 6.54 | 1.7977 | 4.7018 | 11.93 | 777 ± 7 | 151 ± 6.47 |
| MgO | 0.46 ± 0.07 | |||||||||
| MgAl2O4 | 5.34 ± 0.04 | |||||||||
| m-ZrO2 | 4.3 ± 0.03 | |||||||||
| t-ZrO2 | 47 ± 0.02 | |||||||||
| 80 | c-ZrMgO2 | 44.6 ± 0.02 | 8.05 | 6.07 | 6.34 | 1.6058 | 5.0280 | 1.61 | 1312 ± 14 | 211 ± 10.24 |
| MgO | 6.7 ± 0.04 | |||||||||
| MgAl2O4 | 11.9 ± 0.07 | |||||||||
| m-ZrO2 | 7.4 ± 0.05 | |||||||||
| t-ZrO2 | 29.4 ± 0.10 | |||||||||
| Composition of Ceramics | Synthesis Method | Starting Powder Size, μm | ϭflex, MPa | Microhardness | Porosity, % | Reference |
|---|---|---|---|---|---|---|
| Al2O3:ZrO2 = 3:1 with SrO, Cr2O3 doping | Conventional processes with pressing and sintering, 1600 °C | 0.15–0.20 | 501 ± 21 | HV1658 ± 28 | Low, fully dense | [46] |
| ZTA-Cr2O3 | Conventional processes with pressing and sintering, 1450 °C, 2 °C/min heating rate, 3 h hold, 10 °C/min cooling rate | - | 546 ± 59 | - | 4.09 | [47] |
| ZTA-MgO | Conventional processes with pressing and sintering, 1450 °C, 2 °C/min heating rate, 3 h hold, 10 °C/min cooling rate | - | 915 ± 41 | - | 6.17 | [47] |
| ZrO2 92.5; Al2O3 0.07; SiO2 0.58; MgO 0.14; Na2O 0.14; K2O 0.07; SnO2 0.15; Y2O2 4.67; HfO2 1.64. | Printed via digital light processing, Ramp-up rate 0.2–0.5 °C/min, 1500 °C, 2 h sintering | - | 650 ± 76 | HV1359 ± 35 | - | [48] |
| 1 mol% Y2O3; 12 mol% Al2O3; 1 mol% CoO; 7 mol% CeO2; 1 mol% Fe2O3 | Conventional processes with pressing and sintering 1550–1620 °C | 0.01–0.04 | 606 ± 148 | - | - | [49] |
| Zirconia | DLP printed, 30 h of debinding 1100 °C, 1500 °C with dwell time 5 h of sintering | - | 1238 ± 327 | - | Fully dense | [50] |
| 5Y-PSZ | DLP printed, 30 h of debinding 1100 °C, 1500 °C with dwell time 5 h of sintering | - | 1059 ± 178 | HV1590 ± 24 | Fully dense | [51] |
| Zirconia | FDM printed and milled, conventional sintering at 1500 °C with dwell time 2 h | 0.4–0.6 | 1241 ± 200 | HV1622 ± 216 | - | [52] |
| x·ZrO2-(90−x)·MgO-10·Al2O3 | Conventional processes with pressing and sintering, 10 °C/min, 1500 °C, 5 h | 0.8–1.25 | 250 ± 15.16 | HV1312 ± 14 | 3–18.5 | This Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakirzyanov, R.; Maznykh, S.; Garanin, Y.; Kaliyekperov, M. Compositional Effects on Mechanical Performance of Zirconia–Magnesia–Alumina Ceramics. Ceramics 2025, 8, 114. https://doi.org/10.3390/ceramics8030114
Shakirzyanov R, Maznykh S, Garanin Y, Kaliyekperov M. Compositional Effects on Mechanical Performance of Zirconia–Magnesia–Alumina Ceramics. Ceramics. 2025; 8(3):114. https://doi.org/10.3390/ceramics8030114
Chicago/Turabian StyleShakirzyanov, Rafael, Sofiya Maznykh, Yuriy Garanin, and Malik Kaliyekperov. 2025. "Compositional Effects on Mechanical Performance of Zirconia–Magnesia–Alumina Ceramics" Ceramics 8, no. 3: 114. https://doi.org/10.3390/ceramics8030114
APA StyleShakirzyanov, R., Maznykh, S., Garanin, Y., & Kaliyekperov, M. (2025). Compositional Effects on Mechanical Performance of Zirconia–Magnesia–Alumina Ceramics. Ceramics, 8(3), 114. https://doi.org/10.3390/ceramics8030114







