Luminescence of (YxGd3−x)(AlyGa5−y)O12:Ce and (LuxGd3−x)(AlyGa5−y)O12:Ce Radiation-Synthesized Ceramics
Abstract
1. Introduction
2. Materials and Methods
Ceramic Synthesis
3. Results
3.1. Ceramic Luminescence
3.2. X-Ray Diffraction of the Ceramics
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, J.L.; Gundiah, G.; Cheetham, A. Structure–Property Correlations in Ce-Doped Garnet Phosphors for Use in Solid State Lighting. Chem. Phys. Lett. 2007, 441, 250–254. [Google Scholar] [CrossRef]
- Xia, Z.; Meijerink, A. Ce3+-doped garnet phosphors: Composition modification, luminescence properties and applications. Chem. Soc. Rev. 2017, 46, 275–299. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Nikl, M.; Chewpraditkul, W.; Li, J. Development and prospects of garnet ceramic scintillators: A review. J. Adv. Ceram. 2022, 11, 1825–1848. [Google Scholar] [CrossRef]
- Eeckhout, K.V.D.; Poelman, D.; Smet, P.F. Persistent Luminescence in Non-Eu2+-Doped Compounds: A Review. Materials 2013, 6, 2789–2818. [Google Scholar] [CrossRef]
- Ueda, J.; Tanabe, S. (INVITED) Review of luminescent properties of Ce3+-doped garnet phosphors: New insight into the effect of crystal and electronic structure. Opt. Mater. X 2019, 1, 100018. [Google Scholar] [CrossRef]
- Kamada, K.; Endo, T.; Tsutumi, K.; Yanagida, T.; Fujimoto, Y.; Fukabori, A.; Yoshikawa, A.; Pejchal, J.; Nikl, M. Composition Engineering in Cerium-Doped (Lu,Gd)3(Ga,Al)5O12 Single-Crystal Scintillators. Cryst. Growth Des. 2011, 11, 4484–4490. [Google Scholar] [CrossRef]
- Kamada, K.; Yanagida, T.; Pejchal, J.; Nikl, M.; Endo, T.; Tsutumi, K.; Fujimoto, Y.; Fukabori, A.; Yoshikawa, A. Scintillator-oriented combinatorial search in Ce-doped (Y,Gd)3(Ga,Al)5O12 multicomponent garnet compounds. J. Phys. D Appl. Phys. 2011, 44, 505104. [Google Scholar] [CrossRef]
- Bartosiewicz, K.; Babin, V.; Kamada, K.; Yoshikawa, A.; Kurosawa, S.; Beitlerova, A.; Kucerkova, R.; Nikl, M.; Zorenko, Y. Ga for Al substitution effects on the garnet phase stability and luminescence properties of Gd3GaxAl5-xO12:Ce single crystals. J. Lumin. 2019, 216, 116724. [Google Scholar] [CrossRef]
- Peng, G.; Zou, Z.; Li, J.; Liao, J.; Wen, H. (Gd1-xTbx)3(Al1-yGay)5O12 green phosphors with high quantum yield and low thermal quenching via modulation the Ga3+ admixture. J. Lumin. 2021, 236, 118066. [Google Scholar] [CrossRef]
- Ermakova, L.V.; Smyslova, V.G.; Dubov, V.V.; Kuznetsova, D.E.; Malozovskaya, M.S.; Saifutyarov, R.R.; Karpyuk, P.V.; Sokolov, P.S.; Komendo, I.Y.; Bondarau, A.G.; et al. Effect of a Phosphorus Additive on Luminescent and Scintillation Properties of Ceramics GYAGG:Ce. Ceramics 2023, 6, 1478–1489. [Google Scholar] [CrossRef]
- Zorenko, T.; Gorbenko, V.; Witkiewicz-Lukaszek, S.; Zorenko, Y. Luminescent properties of (La,Lu,Gd)3(Al,Sc,Ga)5O12:Ce mixed garnets under synchrotron radiation excitation. J. Lumin. 2018, 199, 483–487. [Google Scholar] [CrossRef]
- Fasoli, M.; Vedda, A.; Nikl, M.; Jiang, C.; Uberuaga, B.P.; Andersson, D.A.; McClellan, K.J.; Stanek, C.R. Band-gap engineering for removing shallow traps in rare-earth Lu3Al5O12 garnet scintillators using Ga3+ doping. Phys. Rev. B Condens. Matter. 2011, 84, 081102. [Google Scholar] [CrossRef]
- Khanin, V.; Venevtsev, I.; Chernenko, K.; Pankratov, V.; Klementiev, K.; van Swieten, T.; van Bunningen, A.J.; Vrubel, I.; Shendrik, R.; Ronda, C.; et al. Exciton interaction with Ce3+ and Ce4+ ions in (LuGd)3(Ga,Al)5O12 ceramics. J. Lumin. 2021, 237, 118150. [Google Scholar] [CrossRef]
- Ding, S.; Ren, H.; Zou, Y.; Liu, W.; Zhang, Q. Single crystal growth and property investigation of Dy3+ and Tb3+ co-doped Gd3Sc2Al3O12 (GSAG): Multiple applications for GaN blue LD pumped all-solid-state yellow lasers and UV or blue light chip excited solid-state lighting. J. Mater. Chem. C 2021, 9, 9532–9538. [Google Scholar] [CrossRef]
- Xu, Y.-N.; Ching, W.Y.; Brickeen, B.K. Electronic structure and bonding in garnet crystals. Phys. Rev. B 2000, 61, 1817–1824. [Google Scholar] [CrossRef]
- Sarukura, N.; Nawata, T.; Ishibashi, H.; Ishii, M.; Fukuda, T. 4—Czochralski Growth of Oxides and Fluorides. In Handbook of Crystal Growth, 2nd ed.; Rudolph, P., Ed.; Elsevier: Boston, MA, USA, 2015; pp. 131–168. [Google Scholar] [CrossRef]
- Grazenaite, E.; Garskaite, E.; Stankeviciute, Z.; Raudonyte-Svirbutaviciene, E.; Zarkov, A.; Kareiva, A. Ga-Substituted Cobalt-Chromium Spinels as Ceramic Pigments Produced by Sol–Gel Synthesis. Crystals 2020, 10, 1078. [Google Scholar] [CrossRef]
- McDonald, K.A.; Schweitzer, G.K. Synthesis of GAGG:Ce3+ powder for ceramics using mechanochemical and solution combustion methods. J. Am. Ceram. Soc. 2018, 101, 3741. [Google Scholar] [CrossRef]
- Wu, J.; Wu, X.; Gao, Y.; Yan, Z. Innovations in Electric Current-Assisted Sintering for SOFC: A Review of Advances in Flash Sintering and Ultrafast High-Temperature Sintering. Appl. Sci. 2024, 14, 3953. [Google Scholar] [CrossRef]
- Lisitsyn, V.M.; Golkovsky, M.G.; A Musakhanov, D.; Tulegenova, A.T.; Abdullin, K.A.; Aitzhanov, M.B. YAG based phosphors, synthesized in a field of radiation. J. Phys. Conf. Ser. 2018, 1115, 052007. [Google Scholar] [CrossRef]
- Lisitsyn, V.; Lisitsyna, L.; Dauletbekova, A.; Golkovskii, M.; Karipbayev, Z.; Musakhanov, D.; Akilbekov, A.; Zdorovets, M.; Kozlovskiy, A.; Polisadova, E. Luminescence of the tungsten-activated MgF2 ceramics synthesized under the electron beam. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2018, 435, 263–267. [Google Scholar] [CrossRef]
- Lisitsyn, V.; Tulegenova, A.; Kaneva, E.; Mussakhanov, D.; Gritsenko, B. Express Synthesis of YAG:Ce Ceramics in the High-Energy Electrons Flow Field. Materials 2023, 16, 1057. [Google Scholar] [CrossRef]
- Lisitsyn, V.; Mussakhanov, D.; Tulegenova, A.; Kaneva, E.; Lisitsyna, L.; Golkovski, M.; Zhunusbekov, A. The Optimization of Radiation Synthesis Modes for YAG:Ce Ceramics. Materials 2023, 16, 3158. [Google Scholar] [CrossRef] [PubMed]
- Lisitsyn, V.; Tulegenova, A.; Golkovski, M.; Polisadova, E.; Lisitsyna, L.; Mussakhanov, D.; Alpyssova, G. Radiation Synthesis of High-Temperature Wide-Bandgap Ceramics. Micromachines 2023, 14, 2193. [Google Scholar] [CrossRef] [PubMed]
- Lisitsyn, V.M.; Karipbayev, Z.T.; Zhilgildinov, Z.S.; Zhunusbekov, A.M.; Tulegenova, A.T.; Golkovski, M.G. Effect of Precursor Prehistory on the Efficiency of Radiation-Assisted Synthesis and Luminescence of YAG:Ce Ceramics. Photonics 2023, 10, 494. [Google Scholar] [CrossRef]
- Bárta, J.; Pestovich, K.; Valdez, J.; Wiggins, B.; Richards, C.; Smith, E.; Clayton, J.; Smalley, D.; McClellan, K. Compositional screening of Ce-doped (Gd,Lu,Y)3(Al,Ga)5O12 ceramics prepared by quenching from melt and their luminescence properties. J. Alloy. Compd. 2021, 889, 161687. [Google Scholar] [CrossRef]
- Bárta, J.; Müllerová, E.; Kuzár, M.; Procházková, L.; Kučerková, R.; Jakubec, I.; Čuba, V. Photochemical synthesis and characterization of multi-component (Lu,Gd)3(Al,Ga)5O12:Ce garnet powders. Radiat. Meas. 2019, 124, 98–102. [Google Scholar] [CrossRef]
- Zorenko, Y.; Gorbenko, V.; Vasylkiv, J.; Strzyżewski, T.; Fedorov, A.; Kucerkova, R.; Mares, J.; Nikl, M.; Bilski, P.; Twardak, A. Growth and luminescent properties of scintillators based on the single crystalline films of (Lu,Gd)3(Al,Ga)5O12:Ce garnets. J. Lumin. 2016, 169, 828–837. [Google Scholar] [CrossRef]
- Dai, P.; Ji, C.; Shen, L.; Qian, Q.; Guo, G.; Zhang, X.; Bao, N. Photoluminescence properties of YAG:Ce3+,Pr3+ nano-sized phosphors synthesized by a modified co-precipitation method. J. Rare Earths 2017, 35, 341–346. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, K.; Li, Y.; Wang, Z.; Feng, Q. Luminescent properties and energy transfer of Y3Al5O12:Ce3+, Ln3+ (Ln = Tb, Pr) prepared by polymer-assisted sol–gel method. J. Lumin. 2012, 132, 3004–3009. [Google Scholar] [CrossRef]
- Cremers, D.A.; Radziemski, L.J. Handbook of Laser-Induced Breakdown Spectroscopy, 2nd ed.; Wiley: Chichester, UK, 2013. [Google Scholar]
- Lisitsyn, V.M.; Korepanov, V.I.; Yakovlev, V.Y. Evolution of primary radiation defects in ionic crystals. Russ. Phys. J. 1996, 39, 1009–1028. [Google Scholar] [CrossRef]


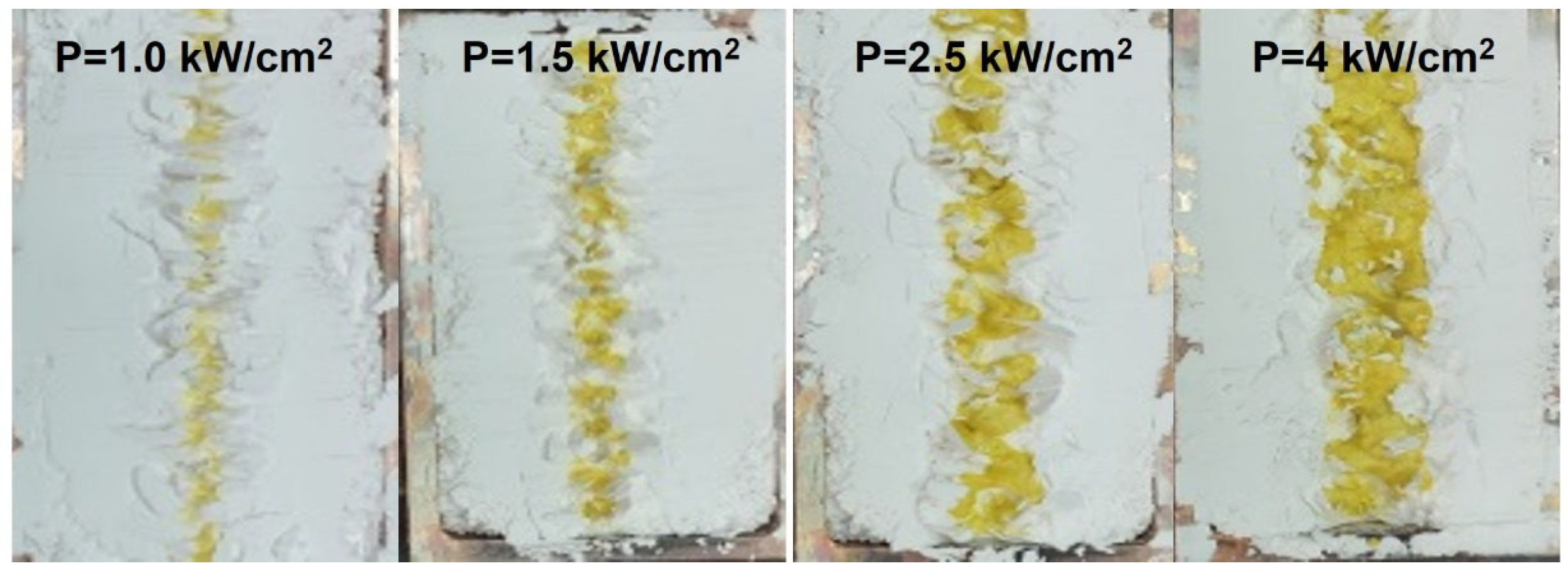




| N | Sample, Description | Power Density, kW/cm2 | Yield, % Weight Mm/Mo |
|---|---|---|---|
| 740 | GdY2 GaAl4O12:Ce (Gd10.257, Y2 0.32) (Ga10.133,Al4 0.29) O12: Ce2O3 (0.5%) | 18 | 97.0 |
| 741 | Gd2YGaAl4O12:Ce (Gd20.469, Y10.145) (Ga10.121,Al40.264) O12: Ce2O3 (0.5%) | 18 | 99.2 |
| 742 | GdY2Ga3Al2O12:Ce (Gd10.229, Y20.286) (Ga30.356,Al20.129) O12: Ce2O3 (0.5%) | 18 | 95.7 |
| 743 | GdY2Ga2Al3O12:Ce (Gd10.242, Y20.302) (Ga20.251,Al30.205) O12: Ce2O3 (0.5%) | 18 | 98.2 |
| 744 | GdLu2 GaAl4O12:Ce (Gd1 0.206, Lu2 0.32) (Ga10.107,Al4 0.232) O12: Ce2O3 (0.5%) | 18 | 96.6 |
| 745 | Gd2LuGaAl4O12:Ce (Gd20.422, Lu10.232) (Ga10.109,Al40.237) O12: Ce2O3 (0.5%) | 18 | 99.5 |
| Power Density P, kW/cm2 | Weight Gd3Al5O12:Ce, g | Weight GdLu2Ga3Al2O12:Ce, g | Weight GdLu2Ga2Al3O12:Ce, g |
|---|---|---|---|
| 0.5 | 0 | 0 | 0 |
| 1 | 4.53 | 2.18 | 1.74 |
| 1.5 | 7.14 | 4.51 | 4.22 |
| 2.5 | 11.17 | 10 | 9.03 |
| 4 | 17.93 | 16.85 |
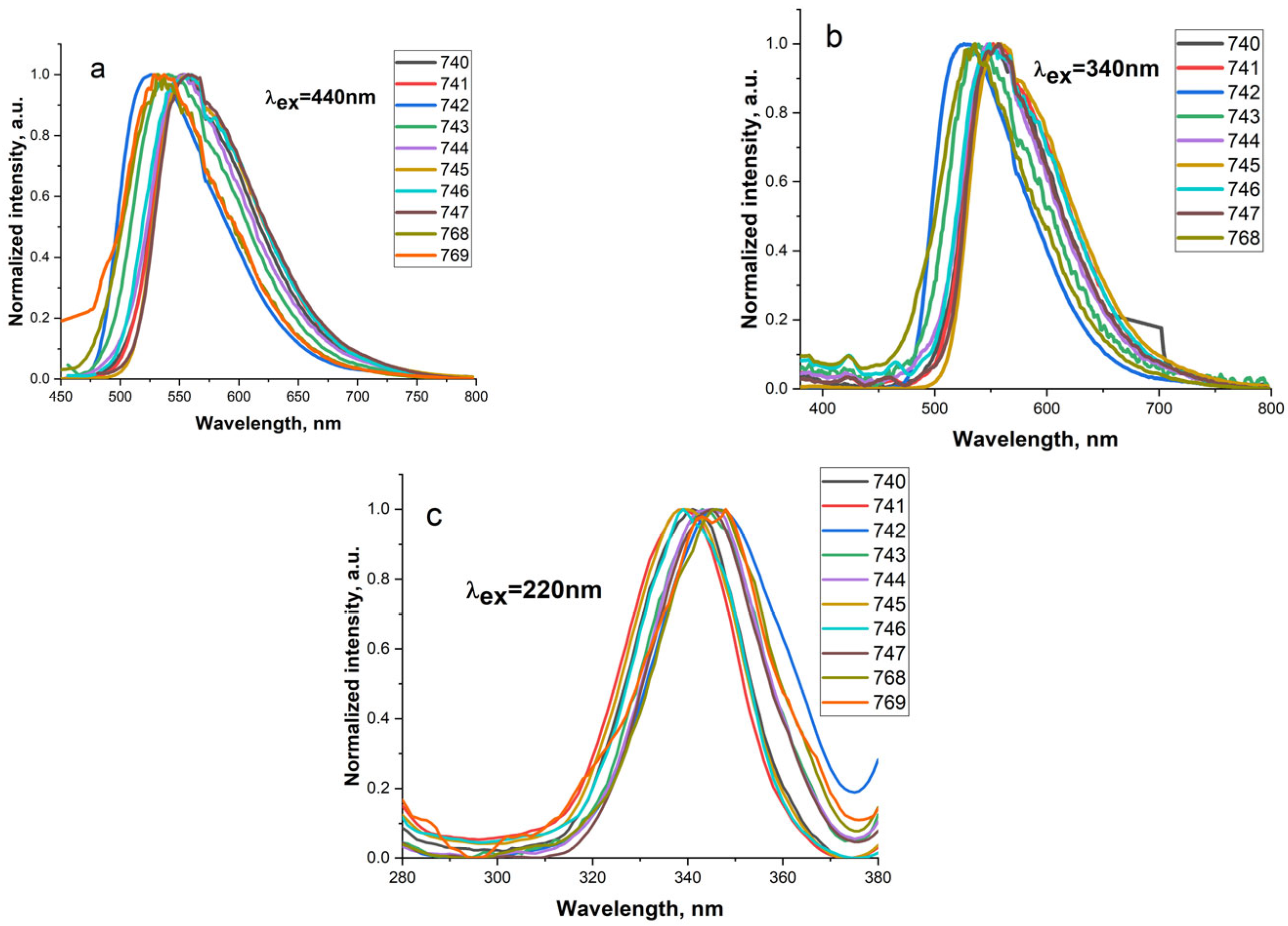
| N Sample | Luminescence Band Maximum Position λem, nm | ||
|---|---|---|---|
| λex = 450 nm | λex = 340 nm | λex = 230 nm | |
| 740 | 553 | 548 | 530 |
| 741 | 555 | 552 | 555 |
| 742 | 525 | 525 | 525 |
| 743 | 540 | 536 | 540 |
| 744 | 555 | 552 | 555 |
| 745 | 557 | 558 | 555 |
| 746 | 560 | 548 | 555 |
| 747 | 557 | 556 | 555 |
| 766 | - | - | 530 |
| 768 | 532 | 529 | 530 |
| 769 | 537 | - | 540 |
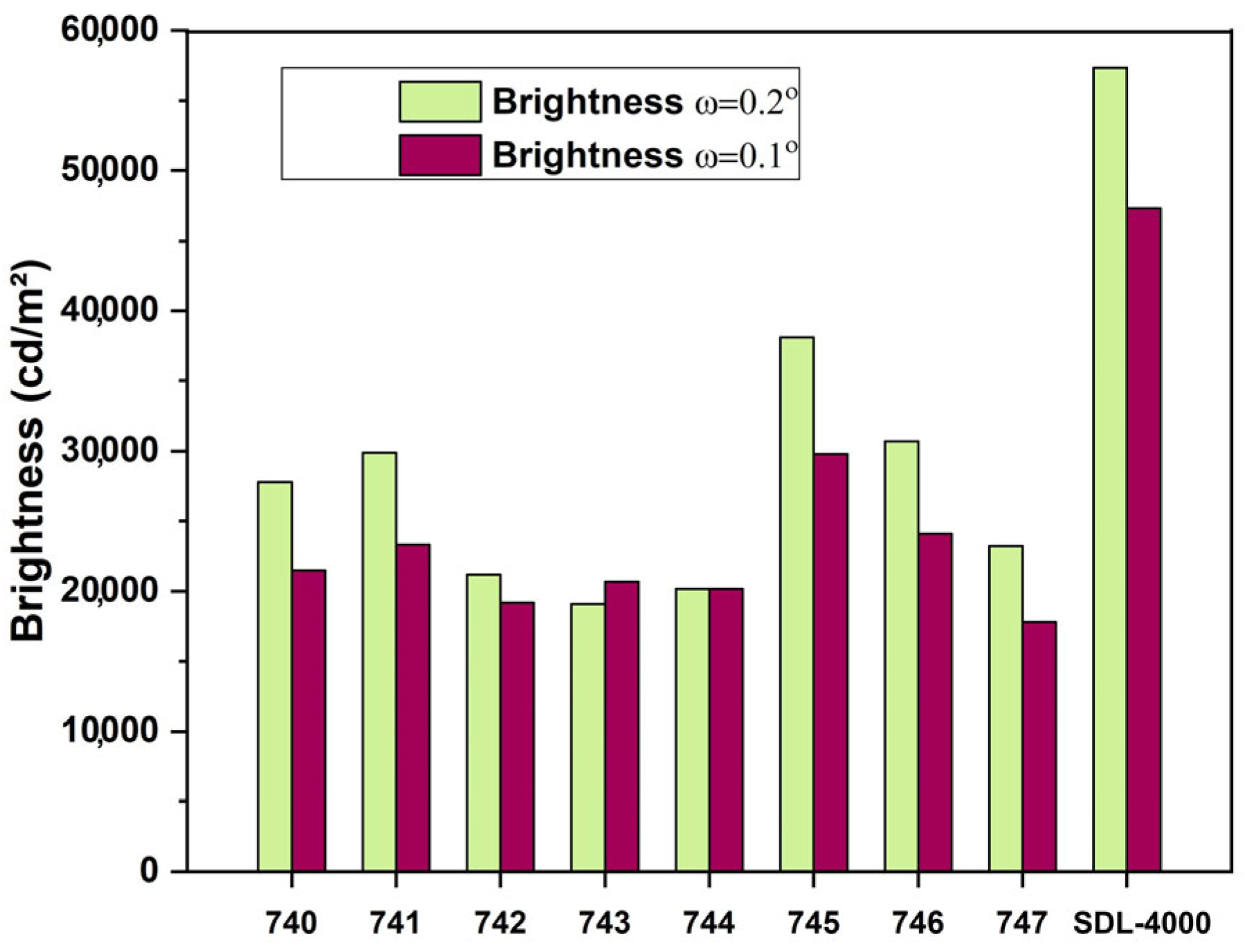
| N Sample | SDL-4000 | 740 | 741 | 742 | 743 | 744 | 745 | 746 | 747 |
|---|---|---|---|---|---|---|---|---|---|
| Brightness at ω = 0.2° (S ≈ 1 mm2) | 57,300 | 27,800 | 29,900 | 21,200 | 19,100 | 21,300 | 38,100 | 30,700 | 23,200 |
| Brightness at ω = 1.0° (S ≈ 17 mm2) | 47,300 | 21,500 | 23,300 | 19,200 | 20,700 | 20,200 | 29,800 | 24,100 | 17,800 |
| Color coordinates (x; y) | 0.4274 0.5462 | 0.4430 0.5327 | 0.4650 0.5165 | 0.3684 0.5549 | 0.4044 0.5118 | 0.4344 0.5310 | 0.4685 0.5109 | 0.4653 0.5135 | 0.4388 0.5337 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tulegenova, A.; Lisitsyn, V.; Nogaibekova, G.; Nemkayeva, R.; Markhabayeva, A. Luminescence of (YxGd3−x)(AlyGa5−y)O12:Ce and (LuxGd3−x)(AlyGa5−y)O12:Ce Radiation-Synthesized Ceramics. Ceramics 2025, 8, 112. https://doi.org/10.3390/ceramics8030112
Tulegenova A, Lisitsyn V, Nogaibekova G, Nemkayeva R, Markhabayeva A. Luminescence of (YxGd3−x)(AlyGa5−y)O12:Ce and (LuxGd3−x)(AlyGa5−y)O12:Ce Radiation-Synthesized Ceramics. Ceramics. 2025; 8(3):112. https://doi.org/10.3390/ceramics8030112
Chicago/Turabian StyleTulegenova, Aida, Victor Lisitsyn, Gulnur Nogaibekova, Renata Nemkayeva, and Aiymkul Markhabayeva. 2025. "Luminescence of (YxGd3−x)(AlyGa5−y)O12:Ce and (LuxGd3−x)(AlyGa5−y)O12:Ce Radiation-Synthesized Ceramics" Ceramics 8, no. 3: 112. https://doi.org/10.3390/ceramics8030112
APA StyleTulegenova, A., Lisitsyn, V., Nogaibekova, G., Nemkayeva, R., & Markhabayeva, A. (2025). Luminescence of (YxGd3−x)(AlyGa5−y)O12:Ce and (LuxGd3−x)(AlyGa5−y)O12:Ce Radiation-Synthesized Ceramics. Ceramics, 8(3), 112. https://doi.org/10.3390/ceramics8030112








