Phase Composition
The XRD pattern of the starting mixture containing 12 wt.% Fe
2O
3 is presented in
Figure 1. The figure confirms the presence of MoO
3, Al
2O
3 and Fe
2O
3 in the form of hematite. As expected, the amorphous SiO
2 was not detected.
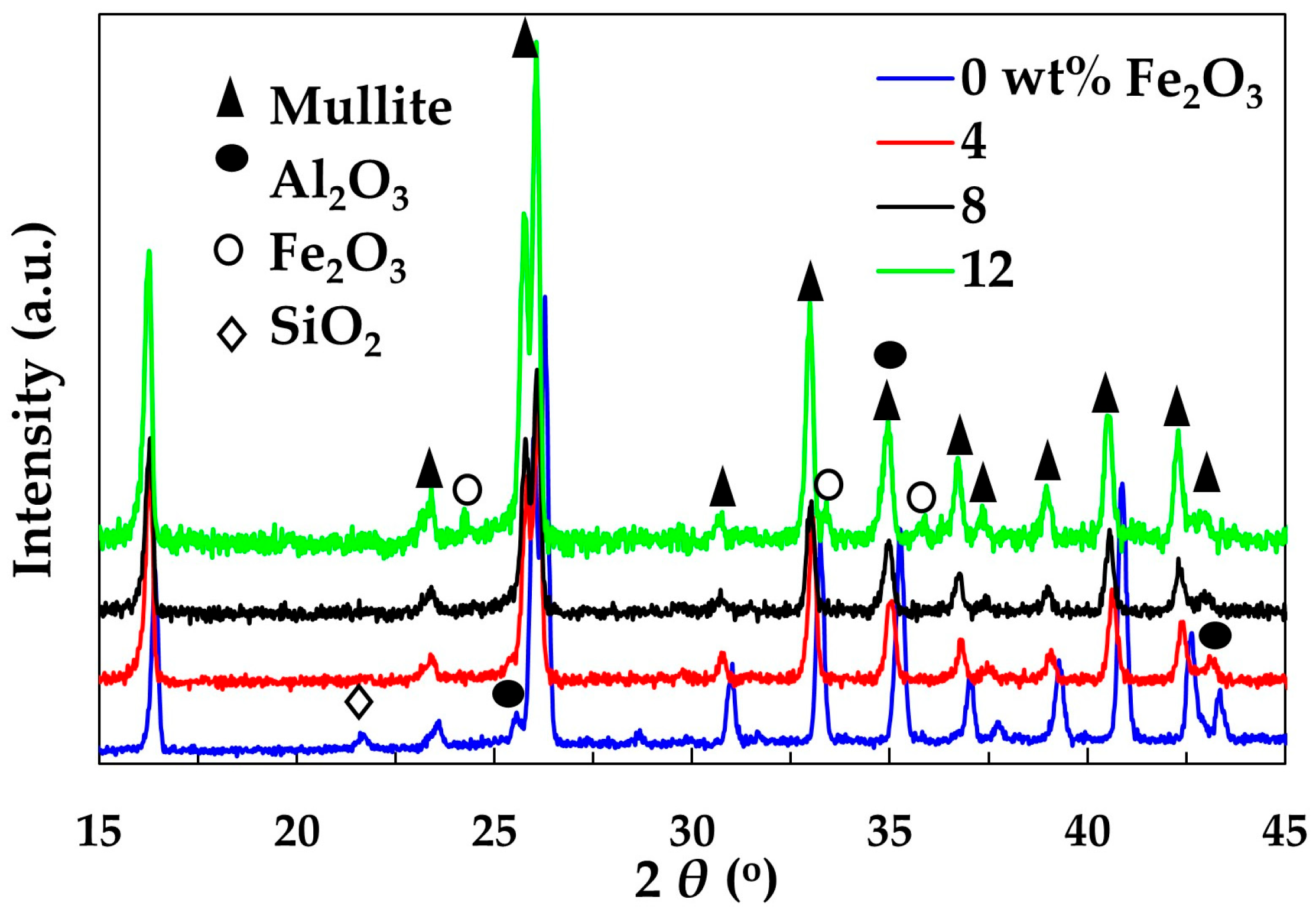
The XRD patterns of samples sintered at 1300 °C are given in
Figure 2. It was shown that mullite is the main crystalline phase in all samples. It has an orthorhombic structure, consisting of octahedral chains of AlO
6 interconnected by tetrahedral chains of aluminum-oxygen and/or silicon-oxygen (AlO
4/SiO
4). It is important to note the presence of small amounts of SiO
2 (cristobalite) and Al
2O
3 (corundum) in the sample without Fe
2O
3, which indicates that amorphous SiO
2 first crystallizes as quartz, then undergoes phase transformation into cristobalite, which subsequently reacts with Al
2O
3 to form mullite. The absence of SiO
2 in samples containing Fe
2O
3 reveals that the reaction between SiO
2 and Al
2O
3 and therefore mullite formation, might be accelerated by the Fe
2O
3 presence. The analysis of the XRD pattern of the sample containing 12 wt.% Fe
2O
3 shows the presence of hematite (Fe
2O
3), indicating that the solubility of Fe
2O
3 in mullite is between 8 wt.% and 12 wt.%.
The comprehensive study on iron solubility in mullite solid solution conducted by Ye et al. confirmed that most iron ions in the mullite are in the form of Fe
3+ due to a smaller atomic radius than that of Fe
2+ [
21]. The effective ionic radius of Fe
2+ (six-fold coordination) is 0.061 nm, notably larger than that of Al
3+ (six-fold coordination, 0.0535 nm), which introduces significant internal strain [
22]. In contrast, the ionic radius of Fe
3+ (six-fold coordination) is 0.055 nm, closely matching that of Al
3+. This similarity enables Fe
3+ ions to partially substitute for Al
3+ ions within the mullite [AlO
6] octahedra. The formation of a solid solution of Fe
2O
3 within the mullite structure, involving the substitution of Al
3+ by Fe
3+, leads to an expansion of the mullite lattice. This is evidenced by a shift in the characteristic mullite XRD peaks toward lower angles, corresponding to increased interplanar spacings (larger
d-values). The shifting is especially pronounced at larger 2
θ values. The addition of 4 and 8 wt.% Fe
2O
3 caused a continuous increase in the shifting of characteristic mullite peaks to the left. However, the shifting caused by the increase of Fe
2O
3 amount from 8 wt.% to 12 wt.% was much smaller and could be detected only at larger values of 2
θ. This finding indicates that the maximum solubility of Fe
2O
3 is slightly higher than 8 wt.%. This is consistent with the results obtained in a study conducted by Ye et al., reporting that iron solubility is between 6 and 9 wt.% [
21]. Zhan et al. [
23] have also found that critical Fe
2O
3 doping threshold is 10 wt.%. The unit-cell parameters of the orthorhombic structure of mullite containing different amounts of Fe
2O
3 are listed in
Table 1. The unit-cell parameters increase with increasing Fe
2O
3 content as the larger Fe
3+ ions substitute for Al
3+ ions.
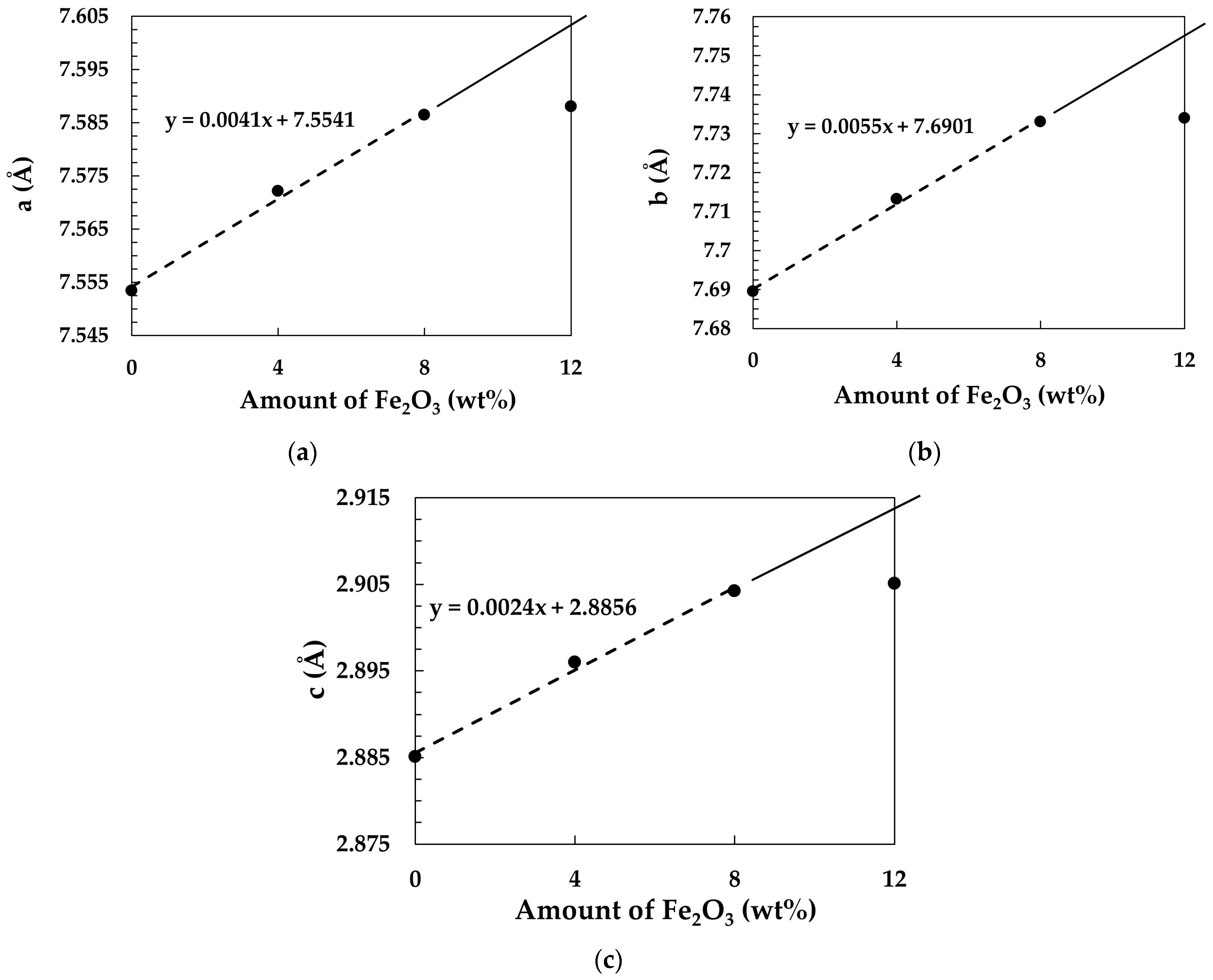
The parameters given in
Table 1 were plotted against Fe
2O
3 content to assess their linear relationship. As shown in
Figure 3, the relationship between Fe
2O
3 content and unit-cell parameters is nearly linear for samples containing 0–8 wt.% Fe
2O
3. However, the unit-cell parameter of the sample with 12 wt.% Fe
2O
3 deviates from linearity, indicating that the maximum Fe ion solubility has been reached in this sample, leaving no scope for further increase in the unit-cell dimensions.
Since Fe
2O
3 was found to accelerate the mullite formation, a set of samples was sintered at 1200 °C in order to further examine the effect of Fe
2O
3 on mullite formation and grain morphology. The temperature lower than the sintering temperature was convenient due to a lower degree of reaction between SiO
2 and Al
2O
3. These samples are not considered significant from the standpoint of mechanical properties, as they do not comprise pure mullite. The XRD patterns of samples containing a different amount of Fe
2O
3 sintered at 1200 °C are given in
Figure 4. As observed, the sample without Fe
2O
3 contains a large amount of SiO
2 and Al
2O
3. The amount continuously decreases with the increase of Fe
2O
3 content, whereas the amount of mullite increases. This result clearly demonstrates the capability of Fe
2O
3 to promote mullite formation.
The phase composition of samples sintered at 1200 °C was determined using the RIR (Reference Intensity Ratio) method within PDXL2 (version 2.0.3.0) software. Only three crystalline phases; SiO
2, Al
2O
3 and mullite were selected in order to study the change in their content during mullite formation in sample containing different amount of Fe
2O
3. The results are given in
Figure 5, showing the continuous decrease of SiO
2 and Al
2O
3 content and increase in mullite content with the amount of Fe
2O
3, which confirms the ability of Fe
2O
3 to accelerate the mullite formation. The phase composition of samples sintered at 1200 °C with a particular emphasis on the presence of Al
2(MoO
4)
3 will be discussed in detail following SEM analysis, which will be focused on the effect of Fe
2O
3 on microstructural development at 1200 °C.
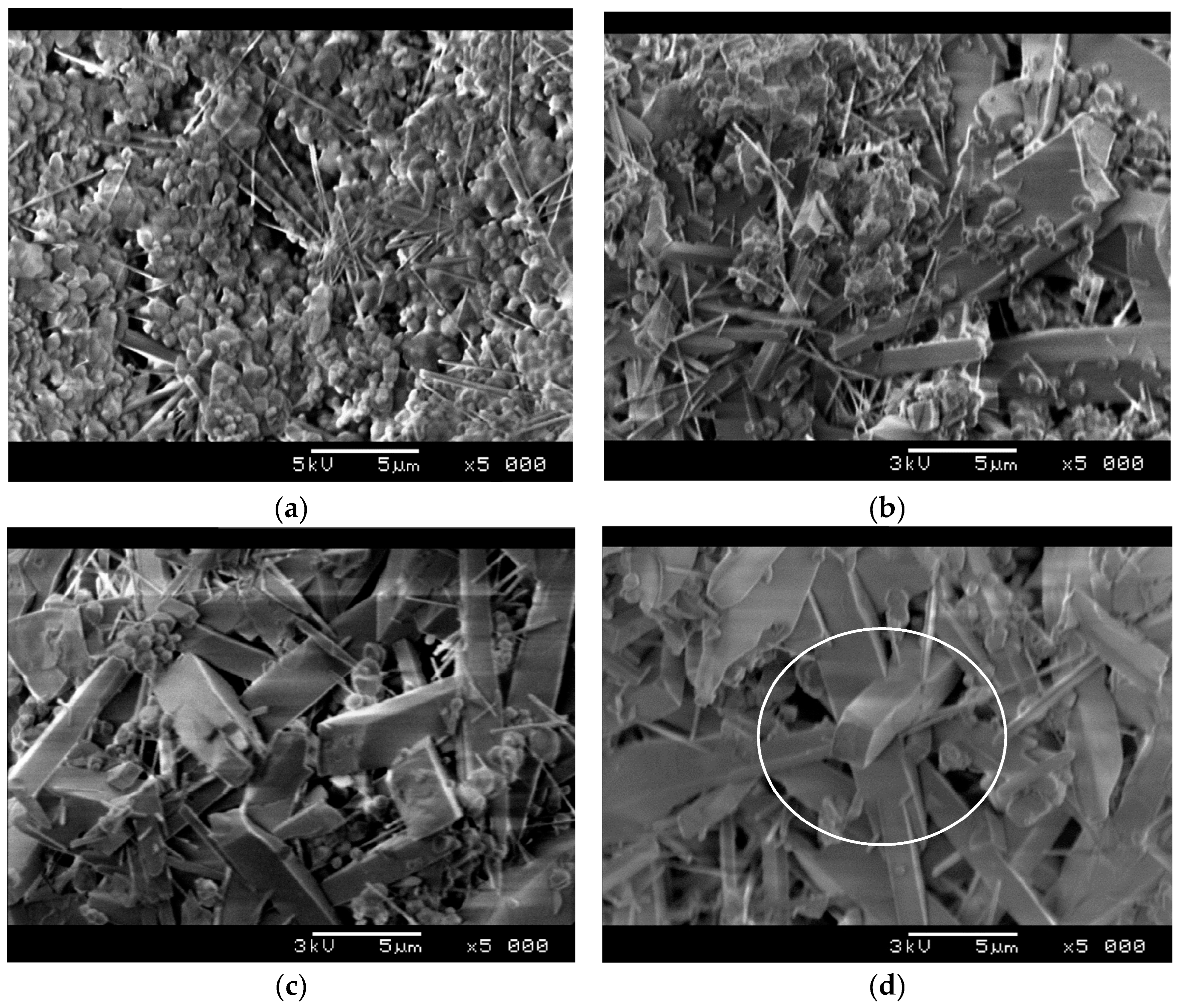
The effect of Fe
2O
3 on the microstructure of samples sintered at 1200 °C is presented in
Figure 6.
Figure 6a shows that the formation of prismatic mullite grains is preceded by the formation of mullite whiskers from the equiaxed particles of starting powder. As the figure evidences, a bunch of mullite whiskers start to grow at one point, and after that, they spread out. The above made conclusion that the presence of Fe
2O
3 promotes mullite formation is also confirmed by
Figure 6b,c.
Figure 6b shows the microstructure of a sample having 4 wt.% of Fe
2O
3. The comparison of the microstructure of the sample without Fe
2O
3 (
Figure 6a) and the microstructure of the sample containing 4 wt.% Fe
2O
3 shows that the elongated mullite grains are much larger in the sample with 4 wt.% Fe
2O
3. To be more precise, the tine mullite whiskers grew into prismatic, rectangular grains. At the same time the amount of starting, equiaxed particles decreased due to the reaction between SiO
2 and Al
2O
3. This effect is even more pronounced in samples containing 8 wt.% Fe
2O
3 (
Figure 6c) and 12 wt.% Fe
2O
3 (
Figure 6d). As
Figure 6d shows, the microstructure of sample containing 12 wt.% Fe
2O
3 predominantly consists of mullite grains with small amount of unreacted starting powder located between them. It would be of great importance to point out the interlocking of acicular mullite grains that is denoted in
Figure 6d. The strong bonding between interlocked grains provides a skeleton for a rigid structure that possesses high compressive strength and high porosity at the same time. This kind of microstructure normally causes a relatively small pressure drop when it comes to gas filtration, as the gas can flow along the elongated grains.
It is evident from
Figure 6 that the presence of Fe
2O
3 accelerates mullite formation and promotes the grain growth in an elongated fashion, which is related to its crystal structure. Mullite has an orthorhombic structure, consisting of octahedral chains of AlO
6 located at the vertices and in the center of the unit cell, with edges aligned in parallel along the “c” crystallographic axis. The AlO
6 chains are interconnected by double tetrahedral chains of aluminum–oxygen and/or silicon–oxygen (AlO
4/SiO
4), also aligned in parallel with the c axis [
24,
25,
26]. Ye et al. [
20] made a calculation which showed that the presence of Fe
2O
3 causes a decrease in surface energy of the (001) mullite plane, which subsequently causes an increase in the growth tendency on the (001) plane and therefore preferential growth along the c-axis. A similar effect was observed in boron-dopped mullite [
27]. The addition of ~6 wt.% of B
2O
3 caused a considerable increase in the aspect ratio of mullite grains as well as the reduction in mullite formation temperature. It was found that the presence of boron reduces the viscosity of the SiO
2 containing amorphous phase and enhances atomic diffusion facilitating mullite nucleation and anisotropic grain growth. Similarly to boron, it was documented that iron also participates in the formation of the liquid phase, which promotes mullite formation. To be more precise, the presence of iron triggers the formation of an iron–silicate liquid phase which subsequently reacts with Al
2O
3 to form mullite. As previously mentioned, the presence of a liquid phase enhances diffusion, thereby facilitating nucleation of mullite grains, which is essential for reducing the mullite formation temperature and promoting the growth of elongated grains.
It can be concluded that the literature data ascribes the elongation of mullite grains and lowering of mullite formation temperature to the presence of dopants, i.e., iron. While this study confirms that iron can lower the temperature required for mullite formation, it does not verify iron’s critical role in the development of elongated mullite grains.
Figure 7. shows samples with different amounts of Fe
2O
3 sintered at 1300 °C. The images were taken at two different magnifications in order to provide a comprehensive insight into the grain size and morphology.
As illustrated in
Figure 7a,a’, the sample without Fe
2O
3 contains elongated prism-like mullite grains, which means that the growth of acicular mullite grains was initiated without the presence of iron. The morphology of mullite grains in the sample with 12 wt.% Fe
2O
3 sintered at 1200 °C (
Figure 6d) was quite similar to that of the sample without Fe
2O
3 sintered at 1300 °C. It would be important to point out the sharp edges of rectangular mullite prisms. As shown in
Figure 7b,b’, these edges stay sharp in the sample containing 4 wt.% Fe
2O
3 sintered at 1300 °C. However, the edges become rounded in samples containing 8 wt.% (
Figure 7c,c’) and 12 wt.% Fe
2O
3 (
Figure 7d,d’) indicating that dissolved iron accelerates diffusion and therefore material transport. The FeO–SiO
2–Al
2O
3 phase diagram [
28] shows that the reaction between Fe ion and SiO
2 can produce a liquid phase at temperatures as low as 1185 °C. The liquid phase then reacts with Al
2O
3 to form an iron-containing solid solution, which aligns well with the earlier conclusion that the presence of iron promotes mullite formation at 1200 °C (
Figure 6). As shown in
Figure 2, iron ions dissolve into the mullite structure, suggesting that their concentration in the liquid phase decreases during sintering as well as mullite grain growth. Consequently, it can be inferred that the edge rounding observed in samples with 8 and 12 wt.% Fe
2O
3 is primarily the result of rapid solid-state diffusion occurring at 1300 °C, facilitated by the significant amount of iron ions dissolved in mullite grains. A similar conclusion was reached by Tu et al., [
29] who observed that substituting Al
3+ with Gd
3+ in MgAl
2O
4 markedly enhanced mass transport, resulting in an almost fully densified material.
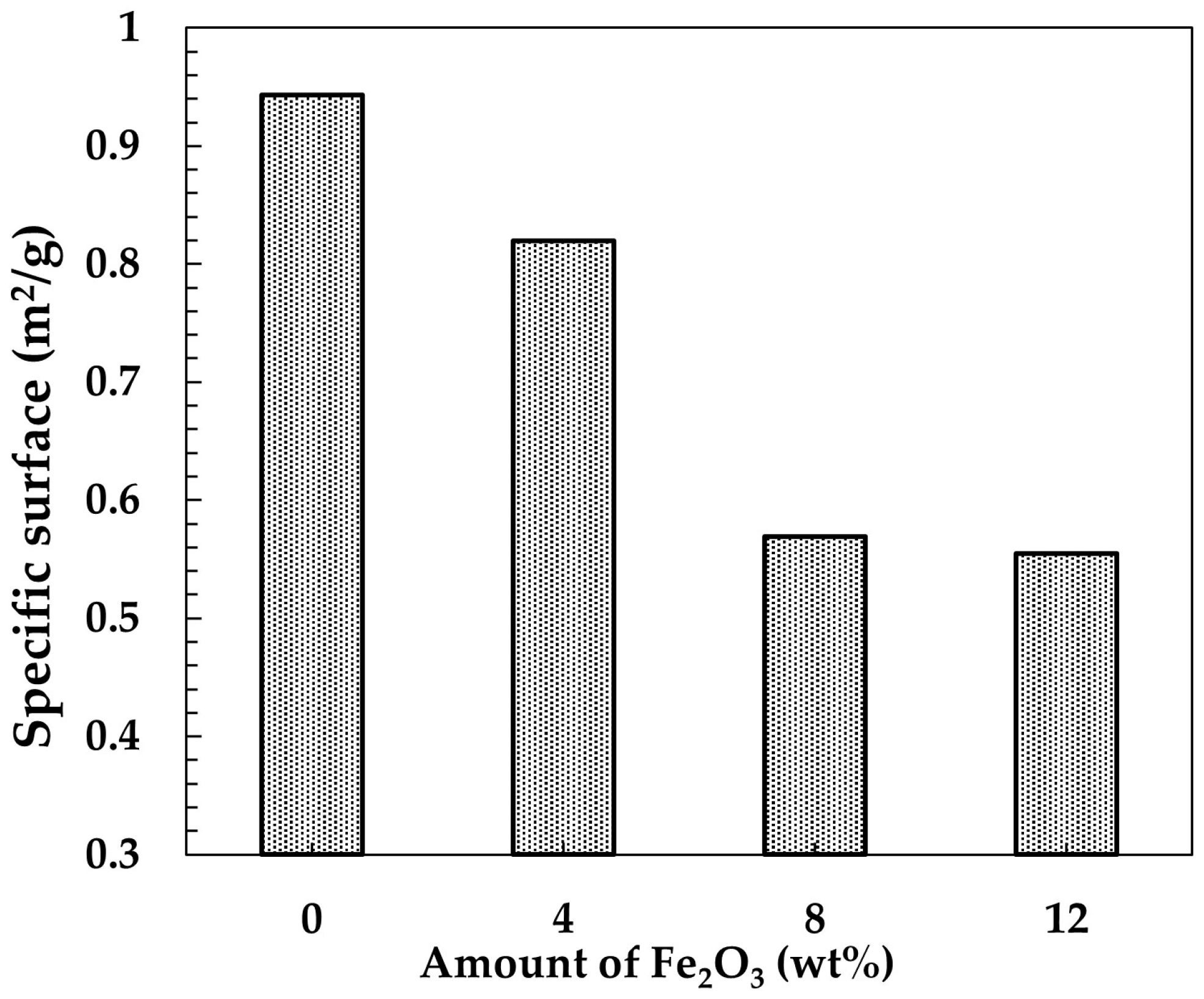
As a consequence, the grain edges become rounded as a result of material’s tendency to reduce its specific surface. This is confirmed by specific surface analysis results presented in
Figure 8 showing that the specific surface continuously decreases from 0.95 m
2/g in the sample without Fe
2O
3 to 0.55 m
2/g in the sample containing 12 wt.% Fe
2O
3. As revealed in
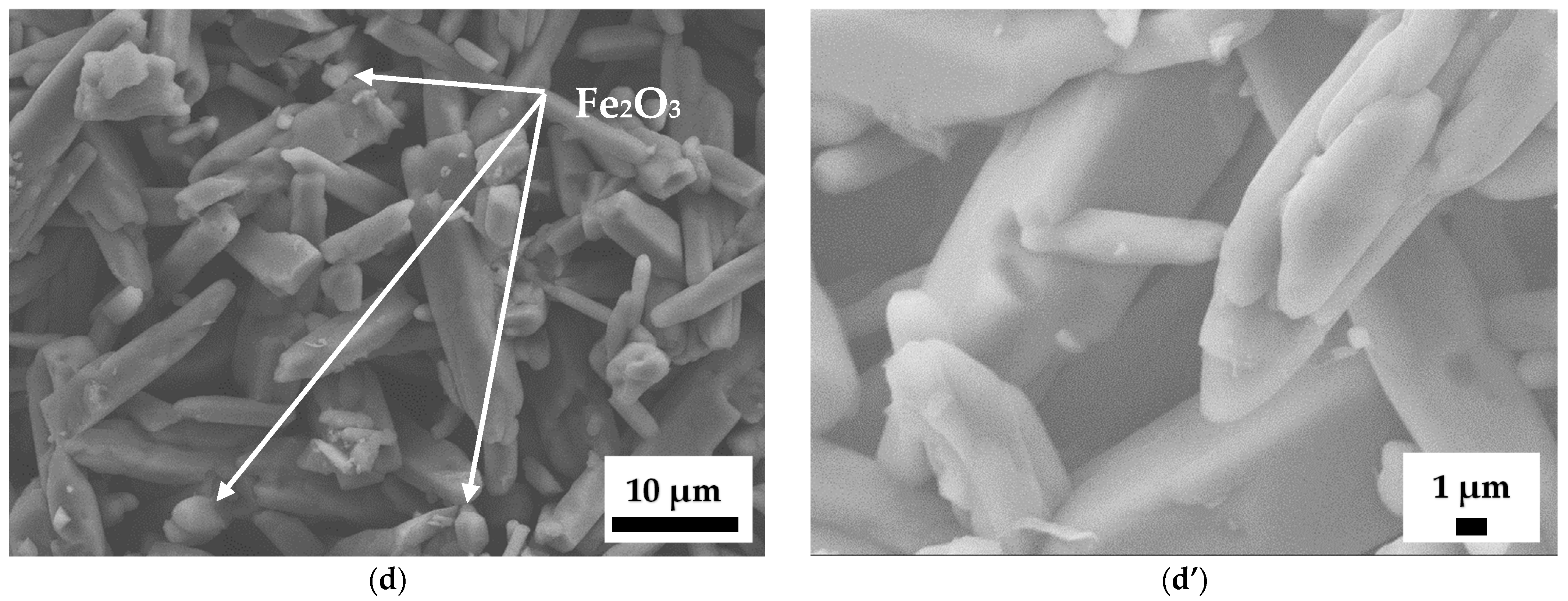
Figure 7d,d’, the grains in the sample containing 12 wt.% Fe
2O
3 predominantly exhibit rounded edges, making it difficult to identify grains with a rectangular cross-section. In addition, the Fe
2O
3 phase present in the samples containing 12 wt.% Fe
2O
3 was in the form of equiaxed grains embedded between elongated mullite grains, as marked in
Figure 7d. Now the question arises: what causes the acicular grain growth? According to our previous research on mullite without an additive [
11], the key component for acicular grain growth is MoO
3 which reacts with Al
2O
3 above 500 °C to form Al
2(MoO
4)
3 according to the following reaction [
30]:
The newly created Al
2(MoO
4)
3 is unstable above 800 °C and therefore it decomposed into Al
2O
3 and MoO
3 during heating. The positive outcome of this transformation is grain refinement, which yields very fine, highly reactive MoO
3 and Al
2O
3 particles. The melting point of MoO
3 is 795 °C; however, it exhibits volatile behavior near the melting point, which was confirmed by thermogravimetric analysis conducted in our previous study [
11]. A considerable weight loss due to MoO
3 evaporation was measured in the temperature range from 800 to 900 °C. It was estimated that about 65 wt.% of the total amount of MoO
3 evaporated during heating to 900 °C. The residual liquid MoO
3 kept evaporating in the temperature interval from 900 °C to 1350 °C which was the highest temperature of thermogravimetric analysis. It is important to note that the evaporation rate significantly decreased at temperatures above 900 °C, suggesting that molten MoO
3 forms a liquid mixture in which its partial pressure is reduced. The XRD analysis results, shown in
Figure 4, confirm the presence of Al
2(MoO
4)
3 in the samples sintered at 1200 °C. The characteristic peaks are located between 22 and 24°. Further increase in temperature to 1300 °C led to complete decomposition of Al
2(MoO
4)
3. As
Figure 2 evidences characteristic peaks of Al
2(MoO
4)
3 phase were not detected in samples sintered at 1300 °C. It is believed that the presence of MoO
3 favors the formation of silica-rich glassy phase, which promotes dissolution of Al
2O
3 and accelerates mullitization reaction [
31]. EDS analysis of mullite grains in samples sintered at 1300 °C did not detect the presence of Mo, suggesting that Mo ions are not incorporated into the mullite lattice. The substitution of Al
3+ by Mo
6+ is unlikely due to differences in their charges and ionic radii [
22]. The ionic radius of Mo
6+ in six-fold coordination is 0.059 nm, which is larger than that of both Al
3+ (0.0535 nm) and Fe
3+ (0.055 nm). For this reason, the sintering process in samples without Fe
2O
3 is not as fast as that in samples containing Fe ions dissolved into the mullite lattice. Consequently, the sharp edges of elongated prismatic grains in samples without Fe
2O
3 are preserved even at temperatures as high as 1300 °C.
The total amount of MoO
3 in the starting mixture was 23 wt.%. After intensive evaporation at temperatures between 800 and 900 °C, the residual amount of MoO
3 was estimated at 8 wt.%. This is very close to the 7 wt.% identified by Chen et al. [
30] as the optimal concentration for the longitudinal growth of mullite whiskers. The presence of MoO
3 lowered the melting temperature of silica-rich glassy liquid and decreased the high-temperature viscosity of the liquid system which resulted in a decrease in activation energy for mullite whisker growth. To be more precise, the activation energy of longitudinal grain growth was reduced, suggesting a stronger catalytic effect on longitudinal than transverse grain growth.
As mentioned, the source of SiO
2 in this study was pulverized and calcined MoSi
2, which consisted of amorphous SiO
2 and MoO
3. The starting mixture contained 23 wt.% of MoO
3 in order to ensure the correct stoichiometric ratio of components for mullite formation. This is a relatively large amount when compared to other studies investigating the effect of MoO
3 presence [
30]. The advantage of a large amount of MoO
3 is the empty space in samples that is formed in the temperature range 800–900 °C due to MoO
3 evaporation. Since evaporation takes place prior to mullite formation, the resulting empty space allows for the unconstrained growth of elongated mullite grains.
Figure 9 illustrates the formation of a mullite whisker, which is expected to develop further into a rectangular prism-like grain. It is evident that the length of the whisker is limited by the space available for its growth. The growth of the whisker is ultimately obstructed by the neighboring grains, suggesting that large pores in the green samples contribute to the high aspect ratio of mullite grains.
Figure 10 presents the effect of Fe
2O
3 amount on open porosity and compressive strength. The decrease in porosity is expected, knowing that iron dissolved in the mullite lattice enhances mass transport and therefore accelerates the sintering/densification process. It is important to note that the reduction in porosity is relatively minor from a filtration performance perspective. The addition of 12 wt.% of Fe
2O
3 caused a decrease in open porosity of only 3.6%, from 55.4% in pure mullite to 51.8% in the sample containing 12 wt.% Fe
2O
3. Despite the large amount of additives, the porosity of the sample containing 12 wt.% of Fe
2O
3, reaching a value of ~52%, is considered sufficiently high for filtering of diesel engine exhaust gases.
Unlike the relatively small change in porosity, the change in compressive strength was substantial. As shown in
Figure 10, increasing the Fe
2O
3 content corresponded with a rise in strength, reaching 76 MPa in the sample containing 12 wt.% Fe
2O
3. This value was three times as high as that of mullite without Fe
2O
3. The improved compressive strength of samples containing Fe
2O
3 could be ascribed to the accelerated mass transport, which promotes sintering and therefore reinforces the contact between elongated mullite grains. As revealed in
Figure 11, the two mullite grains in the sample containing 12 wt.% Fe
2O
3 are strongly bonded at the contact point, which reinforces the mullite skeleton and enhances its resistance to deformation under compression. However, this type of reinforcement does not significantly impact porosity because of the initially high porosity of the samples and the substantial gaps between grains formed during MoO
3 evaporation before sintering and densification.
Another important characteristic of material for diesel engine exhaust filtration is thermal conductivity [
32,
33]. Since the captured soot will eventually clog the filter, it is essential to periodically burn off the accumulated soot. The soot burnout generates local overheating which commonly causes filter cracking due to thermal shock [
34]. Therefore, it is important to design a porous filter material with adequate thermal conductivity (>0.5 W/m·K) to ensure more uniform heat distribution. As presented in
Figure 11, the addition of 4 wt.% Fe
2O
3 does not change the thermal conductivity. It is the same as the conductivity of the sample without Fe
2O
3. The porosity (
Figure 10), phase composition (
Figure 2) and microstructure (
Figure 7) of the samples with 0 wt.% and 4 wt.% Fe
2O
3 are very similar, as is the thermal conductivity. The increase in thermal conductivity was recorded for samples containing 8 and 12 wt.% Fe
2O
3. The coalescence of mullite grains in these samples provides paths for heat transfer, causing an increase in conductivity [
35]. In addition, the thermal conductivity of Fe
2O
3 at 300 °C is about 6 W/m·K, which is higher than that of mullite. Therefore, the presence of Fe
2O
3 secondary phase in samples containing 12 wt.% Fe
2O
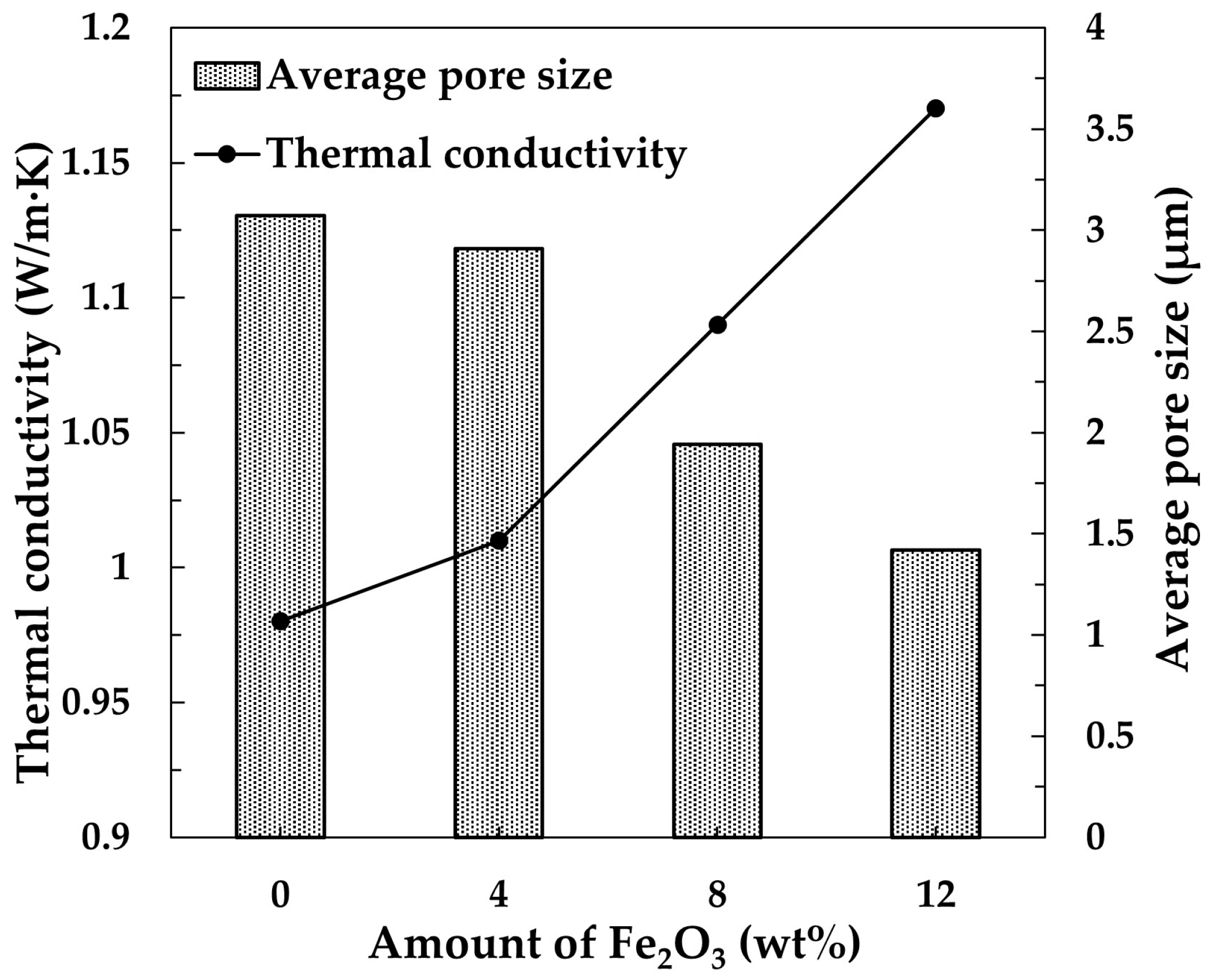
3 is likely responsible for the high thermal conductivity of these samples. The thermal conductivity is inversely proportional to the pore size. As shown in
Figure 12, the average pore sizes in samples containing 0 wt.% and 4 wt.% Fe
2O
3 are nearly identical, which aligns well with the observation that their thermal conductivities are also similar. The increase in Fe
2O
3 content led to a reduction in pore size, which, as expected, resulted in an increase in thermal conductivity of samples containing 8 and 12 wt.% Fe
2O
3. Considering that diesel particulate sizes range from 10 to 200 nm, it is likely that the significant reduction in pore size observed in samples containing 12 wt.% Fe
2O
3 could increase the risk of particulate clogging [
36].