Effect of NiO and ZnO Sintering Aids on Sinterability and Electrochemical Performance of BCZY Electrolyte
Abstract
1. Introduction
2. Experimental Details
2.1. Fabrication of Electrolyte Discs/Bars
2.2. Conductivity Measurement
2.3. Archimedes’ Density Measurement
2.4. Dilatometer Shrinkage Studies
2.5. XRD and SEM Characterization
3. Results and Discussion
3.1. Density Measurements and Shrinkage Studies
3.2. XRD and SEM Characterization Results
3.3. Conductivity Studies
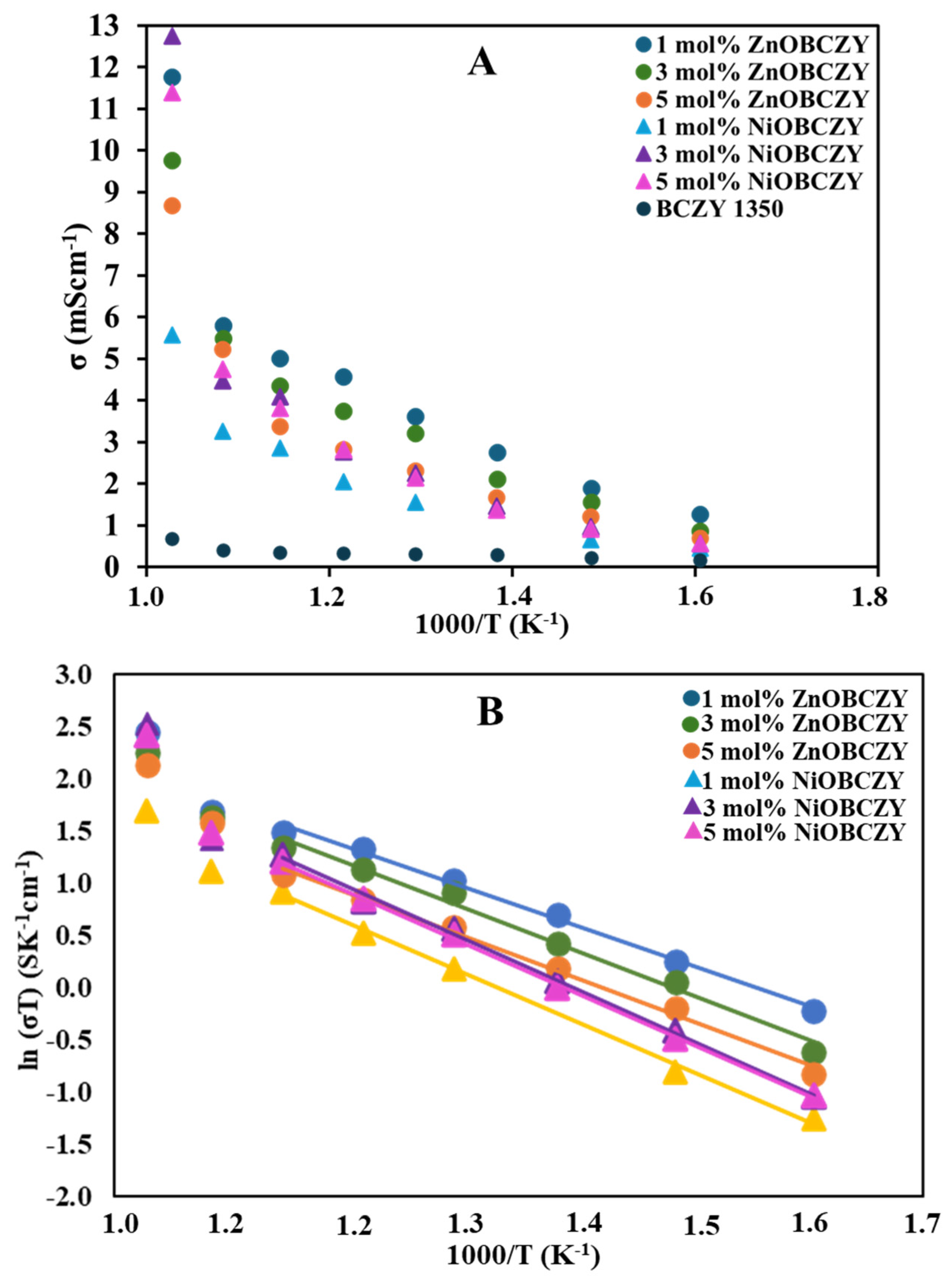
3.3.1. Analysis of ZnOBCZY Conductivity Trends
3.3.2. Analysis of NiOBCZY Conductivity Trends
3.3.3. Comparison of NiOBCZY and ZnOBCZY Conductivity Trends
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iwahara, H.; Esaka, T.; Uchida, H.; Maeda, N. Proton conduction in sintered oxides and its application to steam electrolysis for hydrogen production. Solid State Ion. 1981, 3, 359–363. [Google Scholar] [CrossRef]
- Sailaja, J.M.; Murali, N.; Babu, K.V.; Veeraiah, V. Effect of Strontium the phase structure of Ba1−xSrxCe0.65Zr0.2Y0.15O3−δ (0≤ x ≤ 0.25) proton conductor by citrate–EDTA complexing sol–gel method. J. Asian Ceram. Soc. 2017, 5, 18–30. [Google Scholar] [CrossRef]
- Lv, J.; Wang, L.; Lei, D.; Guo, H.; Kumar, R. Sintering, chemical stability and electrical conductivity of the perovskite proton conductors BaCe0.45Zr0.45M0.1O3−δ (M = In, Y, Gd, Sm). J. Alloys Compd. 2009, 467, 376–382. [Google Scholar] [CrossRef]
- Lyagaeva, J.; Antonov, B.; Dunyushkina, L.; Kuimov, V.; Medvedev, D.; Demin, A.; Tsiakaras, P. Acceptor doping effects on microstructure, thermal and electrical properties of proton-conducting BaCe0.5Zr0.3Ln0.2O3−δ (Ln = Yb, Gd, Sm, Nd, La or Y) ceramics for solid oxide fuel cell applications. Electrochim. Acta 2016, 192, 80–88. [Google Scholar] [CrossRef]
- Lin, B.; Zhang, S.; Zhang, L.; Bi, L.; Ding, H.; Liu, X.; Gao, J.; Meng, G. Prontonic ceramic membrane fuel cells with layered GdBaCO2O5+x cathode prepared by gel-casting and suspension spray. J. Power Sources 2008, 177, 330–333. [Google Scholar] [CrossRef]
- Rashid, N.L.R.M.; Samat, A.A.; Jais, A.A.; Somalu, M.R.; Muchtar, A.; Baharuddin, N.A.; Isahak, W.N.R.W. Review on zirconate-cerate-based electrolytes for proton-conducting solid oxide fuel cell. Ceram. Int. 2019, 45, 6605–6615. [Google Scholar] [CrossRef]
- Choi, S.M.; Lee, J.H.; An, H.; Hong, J.; Kim, H.; Yoon, K.J.; Son, J.W.; Kim, B.K.; Lee, H.W.; Lee, J.H. Fabrication of anode-supported protonic ceramic fuel cell with Ba(Zr0.85Y0.15)O3−δ–Ba(Ce0.9Y0.1)O3−δ dual-layer electrolyte. Int. J. Hydrogen Energy 2014, 39, 12812–12818. [Google Scholar] [CrossRef]
- He, F.; Song, D.; Peng, R.; Meng, G.; Yang, S. Electrode performance and analysis of reversible solid oxide fuel cells with proton conducting electrolyte of BaCe0.5Zr0.3Y0.2O3−δ. J. Power Sources 2010, 195, 3359–3364. [Google Scholar] [CrossRef]
- Nasani, N.; Ramasamy, D.; Mikhalev, S.; Kovalevsky, A.V.; Fagg, D.P. Fabrication and electrochemical performance of a stable, anode supported thin BaCe0.4Zr0.4Y0.2O3−δ electrolyte Protonic Ceramic Fuel Cell. J. Power Sources 2015, 278, 582–589. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, M.; Zhou, M.; Cao, D.; Liu, P.; Wang, W.; Liu, M.; Huang, J.; Shao, J.; Liu, J. Multiple effects of iron and nickel additives on the properties of proton conducting yttrium-doped barium cerate-zirconate electrolytes for high-performance solid oxide fuel cells. ACS Appl. Mater. Interfaces 2020, 12, 50433–50445. [Google Scholar] [CrossRef]
- Babar, Z.U.D.; Hanif, M.B.; Gao, J.-T.; Li, C.-J.; Li, C.-X. Sintering behavior of BaCe0.7Zr0.1Y0.2O3−δ electrolyte at 1150 °C with the utilization of CuO and Bi2O3 as sintering aids and its electrical performance. Int. J. Hydrogen Energy 2022, 47, 7403–7414. [Google Scholar] [CrossRef]
- Yoo, Y.; Lim, N. Performance and stability of proton conducting solid oxide fuel cells based on yttrium-doped barium cerate-zirconate thin-film electrolyte. J. Power Sources 2013, 229, 48–57. [Google Scholar] [CrossRef]
- Baral, A.K. Reduction in sintering temperature of stable proton conductor BaCe0.35Zr0.5Y0.15O3−δ prepared by sol–gel method and its transport properties. Solid State Ion. 2015, 272, 107–111. [Google Scholar] [CrossRef]
- Tao, S.; Irvine, J.T. A stable, easily sintered proton-conducting oxide electrolyte for moderate-temperature fuel cells and electrolyzers. Adv. Mater. 2006, 18, 1581–1584. [Google Scholar] [CrossRef]
- Lee, K.R.; Tseng, C.J.; Jang, S.C.; Lin, J.C.; Wang, K.W.; Chang, J.K.; Chen, T.C.; Lee, S.W. Fabrication of anode-supported thin BCZY electrolyte protonic fuel cells using NiO sintering aid. Int. J. Hydrogen Energy 2019, 44, 23784–23792. [Google Scholar] [CrossRef]
- Wang, B.; Bi, L.; Zhao, X.S. Exploring the role of NiO as a sintering aid in BaZr0.1Ce0.7Y0.2O3−δ electrolyte for proton-conducting solid oxide fuel cells. J. Power Sources 2018, 399, 207–214. [Google Scholar] [CrossRef]
- Nasani, N.; Shakel, Z.; Loureiro, F.J.; Panigrahi, B.B.; Kale, B.B.; Fagg, D.P. Exploring the impact of sintering additives on the densification and conductivity of BaCe0.3Zr0.55Y0.15O3−δ electrolyte for protonic ceramic fuel cells. J. Alloys Compd. 2021, 862, 158640. [Google Scholar] [CrossRef]
- Viechineski, F.N.; Ramos, K.; Chinelatto, A.L.; Chinelatto, A.S.A. Optimizing the densification of BaCe0.2Zr0.7Y0.1O3−δ proton conducting electrolyte using Fe2O3, Mn2O3 and ZnO sintering aids. J. Mater. Sci. Mater. Electron. 2023, 34, 2165. [Google Scholar] [CrossRef]
- Wang, H.; Peng, R.; Wu, X.; Hu, J.; Xia, C. Sintering behavior and conductivity study of yttrium-doped BaCeO3–BaZrO3 solid solutions using ZnO additives. J. Am. Ceram. Soc. 2009, 92, 2623–2629. [Google Scholar] [CrossRef]
- Matsuda, R.M.; Nakamura, K.; Mori, M.; Dailly, J. Sintering mechanism and electrical conductivity of ZnO added BaCe0.8Zr0.1Y0.1O3−δ proton conducting perovskites. Solid State Ion. 2023, 403, 116407. [Google Scholar] [CrossRef]
- Peng, C.; Zhao, B.; Meng, X.; Ye, X.; Luo, T.; Xin, X.; Wen, Z. Effect of NiO Addition on the Sintering and Electrochemical Properties of BaCe0.55Zr0.35Y0.1O3−δ Proton-Conducting Ceramic Electrolyte. Membranes 2024, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yu, J.; Tian, N.; Zheng, J.; Qu, Y.; Tan, W.; Luo, Y. Performance of BaCe0.8Y0.2O3−δ Proton Electrolyte Materials for Solid Oxide Fuel Cells by Compositing the Transition Metal Oxide NiO. Coatings 2022, 12, 1692. [Google Scholar] [CrossRef]
- Ge, L.; Jiao, J.; Zhang, Q.; Zheng, Y.; Chen, H.; Guo, L. A facile method to fabricate proton-conducting BaZr0.85Y0.15O3−δ electrolyte with a large grain size and high conductivity. Ceram. Int. 2019, 45, 24946–24952. [Google Scholar] [CrossRef]
- Hakim, M.; Joo, J.H.; Yoo, C.-Y.; Kim, B.-K.; Yu, J.H. Enhanced chemical stability and sinterability of refined proton-conducting perovskite: Case study of BaCe0.5Zr0.3Y0.2O3−δ. J. Eur. Ceram. Soc. 2015, 35, 1855–1863. [Google Scholar] [CrossRef]
- Chien, R.; Tu, C.-S.; Schmidt, V.H.; Lee, S.-C.; Huang, C.-C. Synthesis and characterization of proton-conducting Ba(Zr0.8−xCexY0.2)O2.9 ceramics. Solid State Ion. 2010, 181, 1251–1257. [Google Scholar] [CrossRef]
- Amsif, M.; Marrero-López, D.; Ruiz-Morales, J.; Savvin, S.; Núñez, P. The effect of Zn addition on the structure and transport properties of BaCe0.9−xZrxY0.1O3−δ. J. Eur. Ceram. Soc. 2014, 34, 1553–1562. [Google Scholar] [CrossRef]
- Hagy, L.; Ramos, K.; Gelfuso, M.; Chinelatto, A.; Chinelatto, A. Effects of ZnO addition and microwave sintering on the properties of BaCe0.2Zr0.7Y0.1O3−δ proton conductor electrolyte. Ceram. Int. 2023, 49, 17261–17270. [Google Scholar] [CrossRef]
- Reddy, G.S.; Bauri, R. A novel route to enhance the sinterability and its effect on microstructure, conductivity and chemical stability of BaCe0.4Zr0.4Y0.2O3−δ proton conductors. Mater. Chem. Phys. 2018, 216, 250–259. [Google Scholar] [CrossRef]
- Azad, A.K.; Savaniu, C.; Tao, S.; Duval, S.; Holtappels, P.; Ibberson, R.M.; Irvine, J.T. Structural origins of the differing grain conductivity values in BaZr0.9Y0.1O2.95 and indication of novel approach to counter defect association. J. Mater. Chem. 2008, 18, 3414–3418. [Google Scholar] [CrossRef]
- Han, D.; Otani, Y.; Noda, Y.; Onishi, T.; Majima, M.; Uda, T. Strategy to improve phase compatibility between proton conductive BaZr0.8Y0.2O3−δ and nickel oxide. RSC Adv. 2016, 6, 19288–19297. [Google Scholar] [CrossRef]
- Loureiro, F.J.; Nasani, N.; Reddy, G.S.; Munirathnam, N.; Fagg, D.P. A review on sintering technology of proton conducting BaCeO3-BaZrO3 perovskite oxide materials for Protonic Ceramic Fuel Cells. J. Power Sources 2019, 438, 226991. [Google Scholar] [CrossRef]
- Rabiei, M.; Palevicius, A.; Monshi, A.; Nasiri, S.; Vilkauskas, A.; Janusas, G. Comparing methods for calculating nano crystal size of natural hydroxyapatite using X-Ray diffraction. Nanomaterials 2020, 10, 1627. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhou, M.; Liu, Z.; Liu, J. A comparative investigation on protonic ceramic fuel cell electrolytes BaZr0.8Y0.2O3−δ and BaZr0.1Ce0.7Y0.2O3−δ with NiO as sintering aid. Ceram. Int. 2022, 48, 17208–17216. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Liu, M.; Liu, J. Enhancing sinterability and electrochemical properties of Ba(Zr0.1Ce0.7Y0.2)O3−δ proton conducting electrolyte for solid oxide fuel cells by addition of NiO. Int. J. Hydrogen Energy 2018, 43, 13501–13511. [Google Scholar] [CrossRef]
- Zamudio-García, J.; Porras-Vázquez, J.M.; dos Santos-Gómez, L.; Losilla, E.R.; Marrero-López, D. Effect of Zn addition on the structure and electrochemical properties of co-doped BaCe0.6Zr0.2Ln0.2O3−δ (Ln = Y, Gd, Yb) proton conductors. Ceram. Int. 2018, 44, 14113–14121. [Google Scholar] [CrossRef]
- Huang, Y. Proton Conducting Electrolytes for Ceramic Fuel Cells. Doctoral Dissertation, University of Stuttgart, Stuttgart, Germany, 2020. [Google Scholar]
- An, H.; Shin, D.; Ji, H.-I. Effect of nickel addition on sintering behavior and electrical conductivity of BaCe0.35Zr0.5Y0.15O3−δ. J. Korean Ceram. Soc. 2019, 56, 91–97. [Google Scholar] [CrossRef]
- Tong, J.; Clark, D.; Bernau, L.; Sanders, M.; O’Hayre, R. Solid-state reactive sintering mechanism for large-grained yttrium-doped barium zirconate proton conducting ceramics. J. Mater. Chem. 2010, 20, 6333–6341. [Google Scholar] [CrossRef]
- Xu, P.; Wang, H.; Zheng, K.; Chen, B.; Yang, M.; Chen, Q.; Wang, B.; Tu, B.; Wang, W.; Fu, Z. Novel transparent ZnO·3Al2O3 ceramics prepared by reactive hot isostatic pressing. J. Eur. Ceram. Soc. 2022, 42, 724–728. [Google Scholar] [CrossRef]
- Gribchenkova, N.A.; Steblevsky, A.V.; Alikhanyan, A.S. Vaporization thermodynamics of the ZnO–SnO2 system. J. Chem. Thermodyn. 2014, 70, 203–206. [Google Scholar] [CrossRef]
- Ji, H.-I.; Kim, B.-K.; Son, J.-W.; Yoon, K.J.; Lee, J.-H. Influence of sintering activators on electrical property of BaZr0.85Y0.15O3−δ proton-conducting electrolyte. J. Power Sources 2021, 507, 230296. [Google Scholar] [CrossRef]
- Bi, L.; Shafi, S.P.; Traversa, E. Y-doped BaZrO3 as a chemically stable electrolyte for proton-conducting solid oxide electrolysis cells (SOECs). J. Mater. Chem. A 2015, 3, 5815–5819. [Google Scholar] [CrossRef]
- Zhao, L.; Tan, W.; Zhong, Q. The chemical stability and conductivity improvement of protonic conductor BaCe0.8−xZrxY0.2O3−δ. Ionics 2013, 19, 1745–1750. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, X.; Luo, Y.; He, Y.; Liu, H.; Geng, S.; Yu, K.; Dong, Y. Ionic conduction mechanism of a nanostructured BCY electrolyte for low-temperature SOFC. Int. J. Hydrogen Energy 2020, 45, 24108–24115. [Google Scholar] [CrossRef]
- Liang, K.; Nowick, A. High-temperature protonic conduction in mixed perovskite ceramics. Solid State Ion. 1993, 61, 77–81. [Google Scholar] [CrossRef]
- He, F.; Wu, T.; Peng, R.; Xia, C. Cathode reaction models and performance analysis of Sm0.5Sr0.5CoO3−δ–BaCe0.8Sm0.2O3−δ composite cathode for solid oxide fuel cells with proton conducting electrolyte. J. Power Sources 2009, 194, 263–268. [Google Scholar] [CrossRef]
- Wu, T.; Zhao, Y.; Peng, R.; Xia, C. Nano-sized Sm0.5Sr0.5CoO3−δ as the cathode for solid oxide fuel cells with proton-conducting electrolytes of BaCe0.8Sm0.2O2.9. Electrochim. Acta 2009, 54, 4888–4892. [Google Scholar] [CrossRef]
- Dailly, J.; Mauvy, F.; Marrony, M.; Pouchard, M.; Grenier, J.-C. Electrochemical properties of perovskite and A2MO4-type oxides used as cathodes in protonic ceramic half cells. J. Solid State Electrochem. 2011, 15, 245–251. [Google Scholar] [CrossRef]
- Toriumi, H.; Jeong, S.; Kitano, S.; Habazaki, H.; Aoki, Y. Enhanced performance of protonic solid oxide steam electrolysis cell of Zr-rich side BaZr0.6Ce0.2Y0.2O3−δ electrolyte with an anode functional layer. ACS Omega 2022, 7, 9944–9950. [Google Scholar] [CrossRef]
- Nikodemski, S.; Tong, J.; O’Hayre, R. Solid-state reactive sintering mechanism for proton conducting ceramics. Solid State Ion. 2013, 253, 201–210. [Google Scholar] [CrossRef]
- Sawant, P.; Varma, S.; Wani, B.; Bharadwaj, S. Synthesis, stability and conductivity of BaCe0.8−xZrxY0.2O33−δ as electrolyte for proton conducting SOFC. Int. J. Hydrogen Energy 2012, 37, 3848–3856. [Google Scholar] [CrossRef]
- Ricote, S.; Bonanos, N.; Manerbino, A.; Coors, W.G. Conductivity study of dense BaCexZr(0.9−x)Y0.1O(3−δ) prepared by solid state reactive sintering at 1500 °C. Int. J. Hydrogen Energy 2012, 37, 7954–7961. [Google Scholar] [CrossRef]
- Guo, Y.; Ran, R.; Shao, Z. A novel way to improve performance of proton-conducting solid-oxide fuel cells through enhanced chemical interaction of anode components. Int. J. Hydrogen Energy 2011, 36, 1683–1691. [Google Scholar] [CrossRef]
- Nasani, N.; Pukazhselvan, D.; Kovalevsky, A.V.; Shaula, A.L.; Fagg, D.P. Conductivity recovery by redox cycling of yttrium doped barium zirconate proton conductors and exsolution of Ni-based sintering additives. J. Power Sources 2017, 339, 93–102. [Google Scholar] [CrossRef]
- Tao, S.; Irvine, J.T. Conductivity studies of dense yttrium-doped BaZrO3 sintered at 1325 °C. J. Solid State Chem. 2007, 180, 3493–3503. [Google Scholar] [CrossRef]
- Lin, B.; Hu, M.; Ma, J.; Jiang, Y.; Tao, S.; Meng, G. Stable, easily sintered BaCe0.5Zr0.3Y0.16Zn0.04O3−δ electrolyte-based protonic ceramic membrane fuel cells with Ba0.5Sr0.5Zn0.2Fe0.8O3−δ perovskite cathode. J. Power Sources 2008, 183, 479–484. [Google Scholar] [CrossRef]
- Khan, K.; Qayyum, S.; Hanif, M.B.; Rauf, S.; Sultan, A.; Mosiałek, M.; Motola, M.; Lin, B. Design of efficient and durable symmetrical protonic ceramic fuel cells at intermediate temperatures via B-site doping of Ni in BaCe0.56Zr0.2Ni0.04Y0.2O3−δ. Ceram. Int. 2023, 49, 16826–16835. [Google Scholar] [CrossRef]
- Lim, D.-K.; Park, C.-J.; Choi, M.-B.; Park, C.-N.; Song, S.-J. Partial conductivities of mixed conducting BaCe0.65Zr0.2Y0.15O3–δ. Int. J. Hydrogen Energy 2010, 35, 10624–10629. [Google Scholar] [CrossRef]
- Lybye, D.; Bonanos, N. Proton and oxide ion conductivity of doped LaScO3. Solid State Ion. 1999, 125, 339–344. [Google Scholar] [CrossRef]
- Bonanos, N. Transport properties and conduction mechanism in high-temperature protonic conductors. Solid State Ion. 1992, 53, 967–974. [Google Scholar] [CrossRef]
- Uchida, H.; Maeda, N.; Iwahara, H. Relation between proton and hole conduction in SrCeO3-based solid electrolytes under water-containing atmospheres at high temperatures. Solid State Ion. 1983, 11, 117–124. [Google Scholar] [CrossRef]
- Basbus, J.F.; Arce, M.D.; Napolitano, F.R.; Troiani, H.E.; Alonso, J.A.; Saleta, M.E.; Gonzaález, M.A.; Cuello, G.J.; Fernaá;ndez-Díaz, M.T.; Sainz, M.P.; et al. Revisiting the crystal structure of BaCe0.4Zr0.4Y0.2O3−δ proton conducting perovskite and its correlation with transport properties. ACS Appl. Energy Mater. 2020, 3, 2881–2892. [Google Scholar] [CrossRef]
- Azimova, M.A.; McIntosh, S. Transport properties and stability of cobalt doped proton conducting oxides. Solid State Ion. 2009, 180, 160–167. [Google Scholar] [CrossRef]
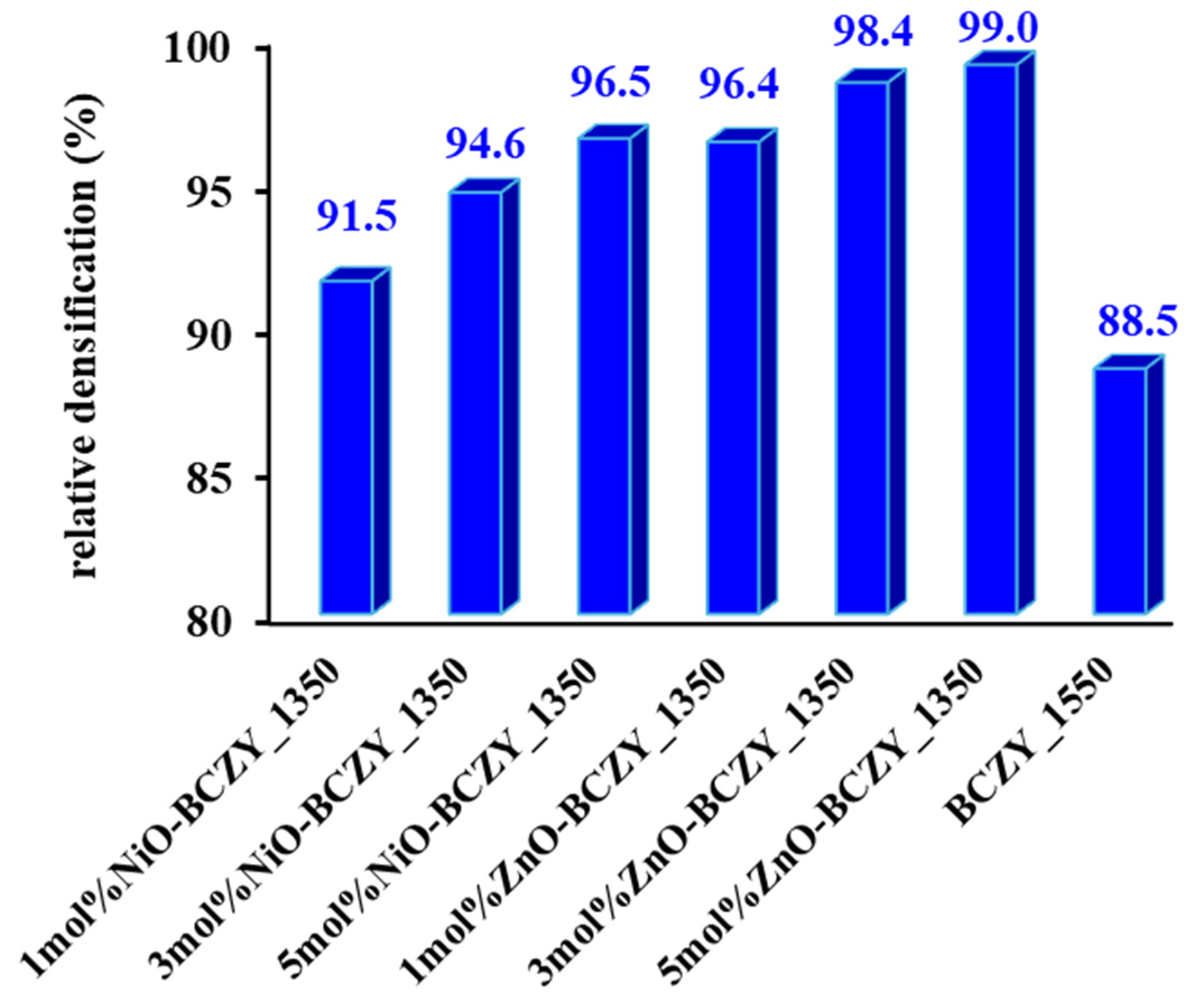

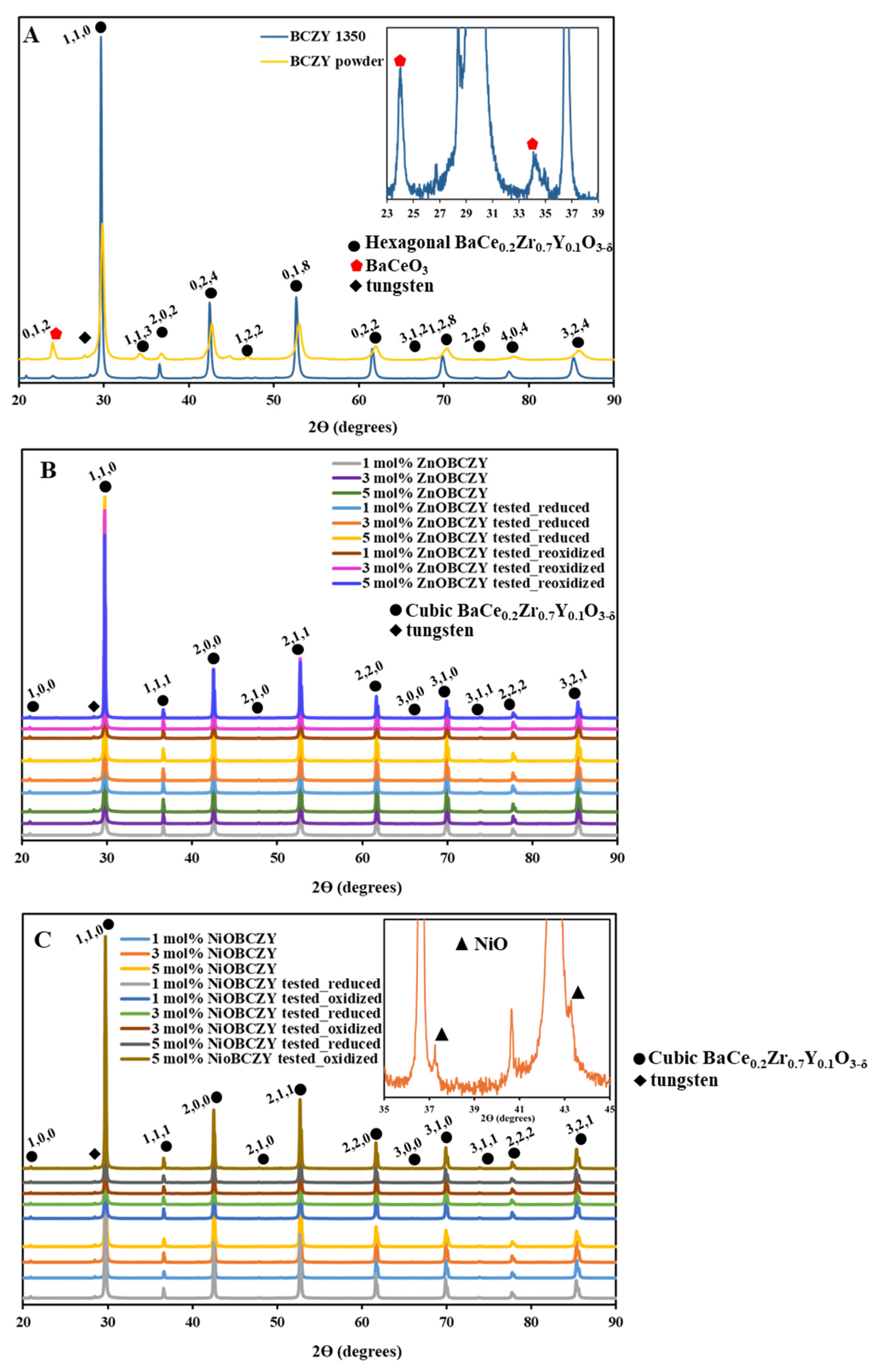

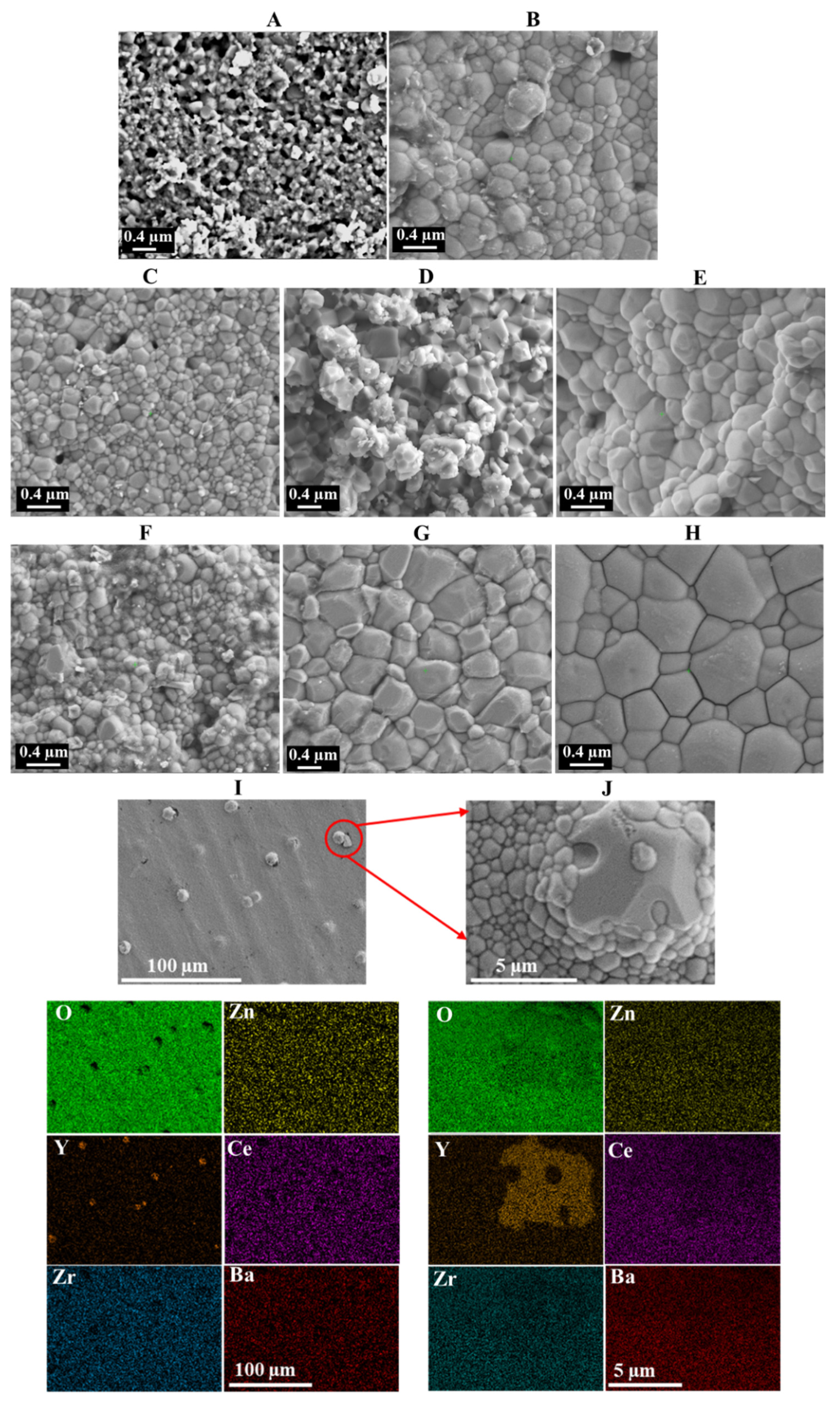

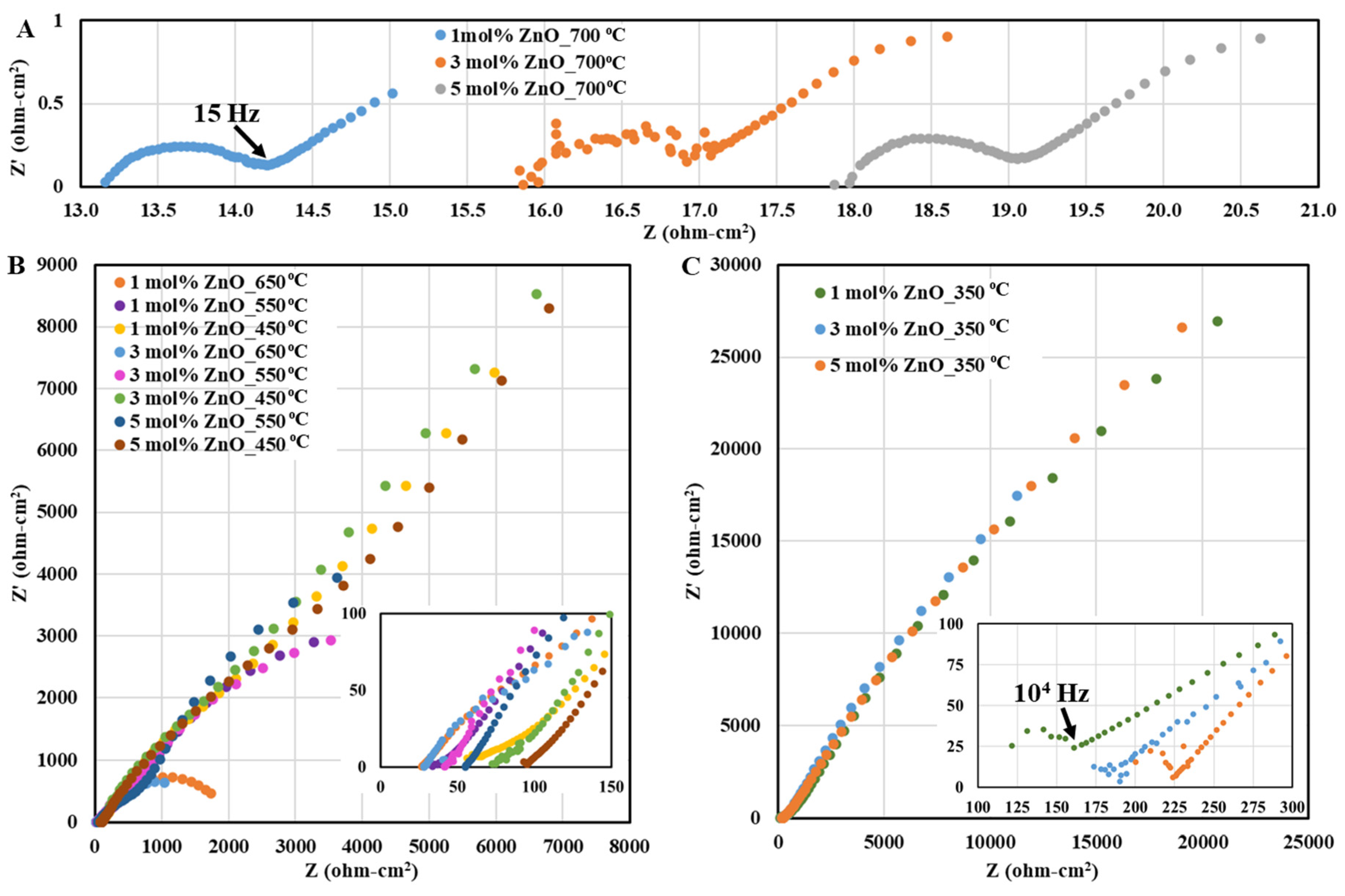
| Sample | Phases | Lattice Structure | Lattice Parameter (nm) |
|---|---|---|---|
| 1 mol% ZnOBCZY | BaCe0.2Zr0.7Y0.1O2.95 | cubic | 0.4255 |
| 3 mol% ZnOBCZY | BaCe0.2Zr0.7Y0.1O2.95 | cubic | 0.4254 |
| 5 mol% ZnOBCZY | 99.5% BaCe0.2Zr0.7Y0.1O2.95 | cubic | 0.4253 |
| 0.5% Y2O3 | cubic | N/A | |
| 1 mol% ZnOBCZY tested_reduced * | BaCe0.2Zr0.7Y0.1O2.95 | cubic | 0.4255 |
| 3 mol% ZnOBCZY tested_reduced * | BaCe0.2Zr0.7Y0.1O2.95 | cubic | 0.4254 |
| 5 mol% ZnOBCZY tested_reduced * | 99.6% BaCe0.2Zr0.7Y0.1O2.95 | cubic | 0.4253 |
| 0.4% Y2O3 | cubic | N/A | |
| 1 mol% ZnOBCZY tested_oxidized ** | BaCe0.2Zr0.7Y0.1O2.95 | cubic | 0.4254 |
| 3 mol% ZnOBCZY tested_oxidized ** | BaCe0.2Zr0.7Y0.1O2.95 | cubic | 0.4253 |
| 5 mol% ZnOBCZY tested_oxidized ** | 99.6% BaCe0.2Zr0.7Y0.1O2.95 | cubic | 0.4252 |
| 0.4% Y2O3 | cubic | N/A | |
| BCZY sintered at 1350 °C | 98% BaCe0.2Zr0.7Y0.1O2.95 | hexagonal | 0.6025, 1.4715 |
| 2% BaCO3 | orthorhombic | N/A |
| Sample | Phases | Lattice Structure | Lattice Parameter (nm) |
|---|---|---|---|
| 1 mol% NiOBCZY | BaCe0.2Zr0.7Y0.1O2.95 | cubic | 0.4255 |
| 3 mol% NiOBCZY | BaCe0.2Zr0.7Y0.1O2.95 | cubic | 0.4254 |
| 0.9% NiO | cubic | N/A | |
| 0.3% BaCO3 | orthorhombic | N/A | |
| 5 mol% NiOBCZY | 99.5% BaCe0.2Zr0.7Y0.1O2.95 | cubic | 0.4253 |
| 1.6% NiO | cubic | N/A | |
| 0.5% BaCO3 | orthorhombic | N/A | |
| 1 mol% NiOBCZY tested_reduced * | BaCe0.2Zr0.7Y0.1O2.95 | cubic | 0.4255 |
| 3 mol% NiOBCZY tested_reduced * | BaCe0.2Zr0.7Y0.1O2.95 | cubic | 0.4254 |
| 0.5% NiO | cubic | N/A | |
| 0.2% BaCO3 | orthorhombic | N/A | |
| 5 mol% NiOBCZY tested_reduced * | 99.6% BaCe0.2Zr0.7Y0.1O2.95 | cubic | 0.4253 |
| 1.3% NiO | cubic | N/A | |
| 0.3% BaCO3 | orthorhombic | N/A | |
| 1 mol% NiOBCZY tested_oxidized ** | BaCe0.2Zr0.7Y0.1O2.95 | cubic | 0.4255 |
| 3 mol% NiOBCZY tested_oxidized ** | BaCe0.2Zr0.7Y0.1O2.95 | Cubic | 0.4253 |
| 0.6% NiO | cubic | N/A | |
| 0.1% BaCO3 | orthorhombic | N/A | |
| 5 mol% NiOBCZY tested_oxidized ** | 99.6% BaCe0.2Zr0.7Y0.1O2.95 | cubic | 0.4252 |
| 1.7% NiO | cubic | N/A | |
| 0.3% BaCO3 | orthorhombic | N/A | |
| BCZY sintered at 1350 °C | 98% BaCe0.2Zr0.7Y0.1O2.95 | hexagonal | 0.6025, 1.4715 |
| 2% BaCO3 | orthorhombic | N/A |
| Electrolyte Composition | Sintering Aid/Incorporation Method | Sintering Temperature (°C), Duration (h) | Total Conductivity (mS/cm) |
|---|---|---|---|
| BaCe0.3Zr0.55Y0.15O3−δ [17] | 1 wt% NiO, citrate–nitrate combustion method | 1400, 8 | 0.91 (500 °C) |
| BaCe0.3Zr0.55Y0.15O3−δ [17] | 1 wt% ZnO, citrate–nitrate combustion method | 1400, 8 | 1.11(500 °C) |
| BaZr0.1Ce0.66Ni0.04Y0.2O3−δ [16] | 4 mol% NiO, sol–gel method | 1400, 5 | 6.30 (600 °C) |
| BaCe0.5Zr0.3Y0.16Zn0.04O3−δ [14] | 1 wt% ZnO, ball-milling | 1325, 10 | 10 (600 °C) |
| BaCe0.2Zr0.7Y0.1O3−δ [18] | 4 wt% ZnO, ball-milling | 1500, 4 | 8.64 (600 °C) |
| BaCe0.5Zr0.3Y0.2O2.9 [19] | 4 mol% ZnO, ball-milling | NA | 13.5 (600 °C) |
| 4 mol% ZnO, wet impregnation | 11.2 (600 °C) | ||
| BaCe0.35Zr0.5Y0.15O3−δ [13] | 2 wt% ZnO, sol–gel method | 1100, 4 | 4.02 (700 °C) |
| BaCe0.7Zr0.1Y0.2O3−δ [10] | 2 mol % Ni0.5Fe0.5, ball-milling | 1400, 5 | 3.80 (600 °C) |
| BaCe0.5Zr0.3Y0.16Zn0.04O3−δ [56] | 2 wt% ZnO, sol–gel method | 1200, 5 | 2.73 (700 °C) |
| BaCe0.5Zr0.3Y0.2O2.9 [19] | 4 mol% ZnO, solid state reaction | 1300, 10 | 13.50 (600 °C) |
| BaZr0.6Ce0.2Y0.2O3−δ [49] | Zn(NO3)2 to get Zn/Ba molar ratio 0.03, solid state reaction | 1400, 12 | 3.00 (600 °C) |
| BaCe0.6Zr0.2Y0.2O3−δ [15] | 3 wt% NiO, ball-milling | 1500, 10 | 1.4 (600 °C) |
| BaCe0.56Zr0.2Ni0.04Y0.2O3−δ [57] | 4 mol% NiO, combustion method | 1300, NA | 11.00 (650 °C) |
| BaCe0.35Zr0.5Y0.15O3−δ [37] | 4 mol% NiO, solid state reaction | 1400, 6 | 2.00 (600 °C) |
| 4 mol% NiO, ball-milling | 1400, 6 | 4.80 (600 °C) | |
| BaCe0.8Zr0.1Y0.1O3−δ [20] | 0.1 wt% ZnO, ball-milling | 1400, 6 | 13.00 (600 °C) |
| This work | 1 mol% ZnO, wet ball-milling | 1350, 2 | 5.02 (600 °C) |
| This work | 3 mol% NiO, wet ball-milling | 1350, 2 | 4.08 (600 °C) |
| This work | 1 mol% ZnO, wet ball-milling | 1350, 2 | 11.78 (700 °C) |
| This work | 3 mol% NiO, wet ball-milling | 1350, 2 | 12.76 (700 °C) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biswas, S.; Vafakhah, S.; Kaur, G.; Seeber, A.; Giddey, S. Effect of NiO and ZnO Sintering Aids on Sinterability and Electrochemical Performance of BCZY Electrolyte. Ceramics 2025, 8, 78. https://doi.org/10.3390/ceramics8020078
Biswas S, Vafakhah S, Kaur G, Seeber A, Giddey S. Effect of NiO and ZnO Sintering Aids on Sinterability and Electrochemical Performance of BCZY Electrolyte. Ceramics. 2025; 8(2):78. https://doi.org/10.3390/ceramics8020078
Chicago/Turabian StyleBiswas, Saheli, Sareh Vafakhah, Gurpreet Kaur, Aaron Seeber, and Sarbjit Giddey. 2025. "Effect of NiO and ZnO Sintering Aids on Sinterability and Electrochemical Performance of BCZY Electrolyte" Ceramics 8, no. 2: 78. https://doi.org/10.3390/ceramics8020078
APA StyleBiswas, S., Vafakhah, S., Kaur, G., Seeber, A., & Giddey, S. (2025). Effect of NiO and ZnO Sintering Aids on Sinterability and Electrochemical Performance of BCZY Electrolyte. Ceramics, 8(2), 78. https://doi.org/10.3390/ceramics8020078








