Surface Transformation of Ultrahigh-Temperature ZrB2–HfB2–SiC–CCNT Ceramics Under Exposure to Subsonic N2-CH4 Plasma Flow
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Test Facility
2.3. Materials’ Investigation
3. Results and Discussion
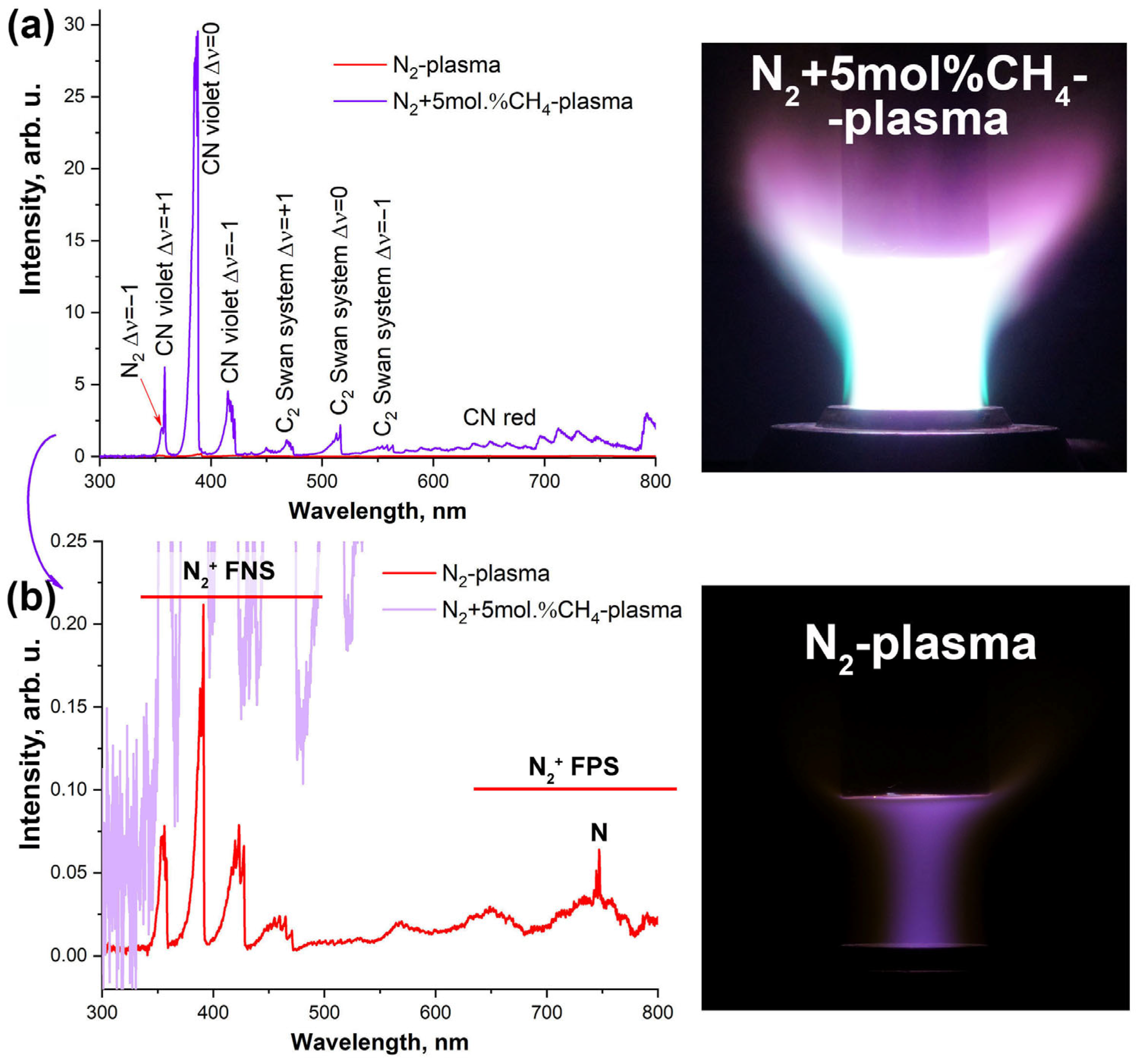
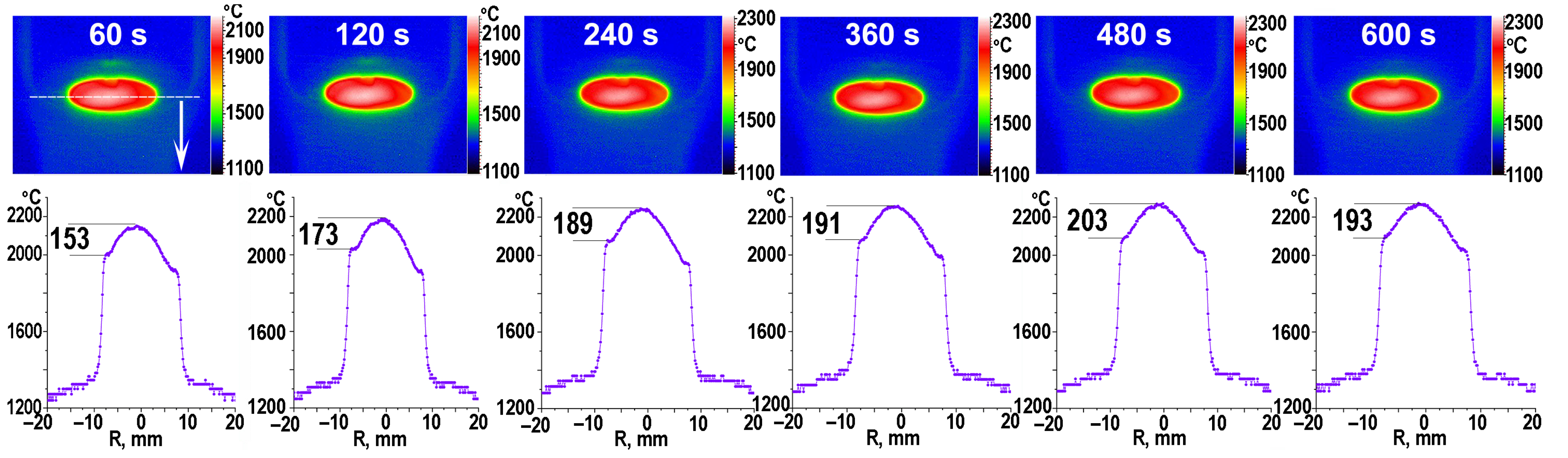
3.1. Exposure of the ZrB2-HfB2-SiC-CCNT Sample Surface to a Subsonic N2+CH4 Plasma Flow
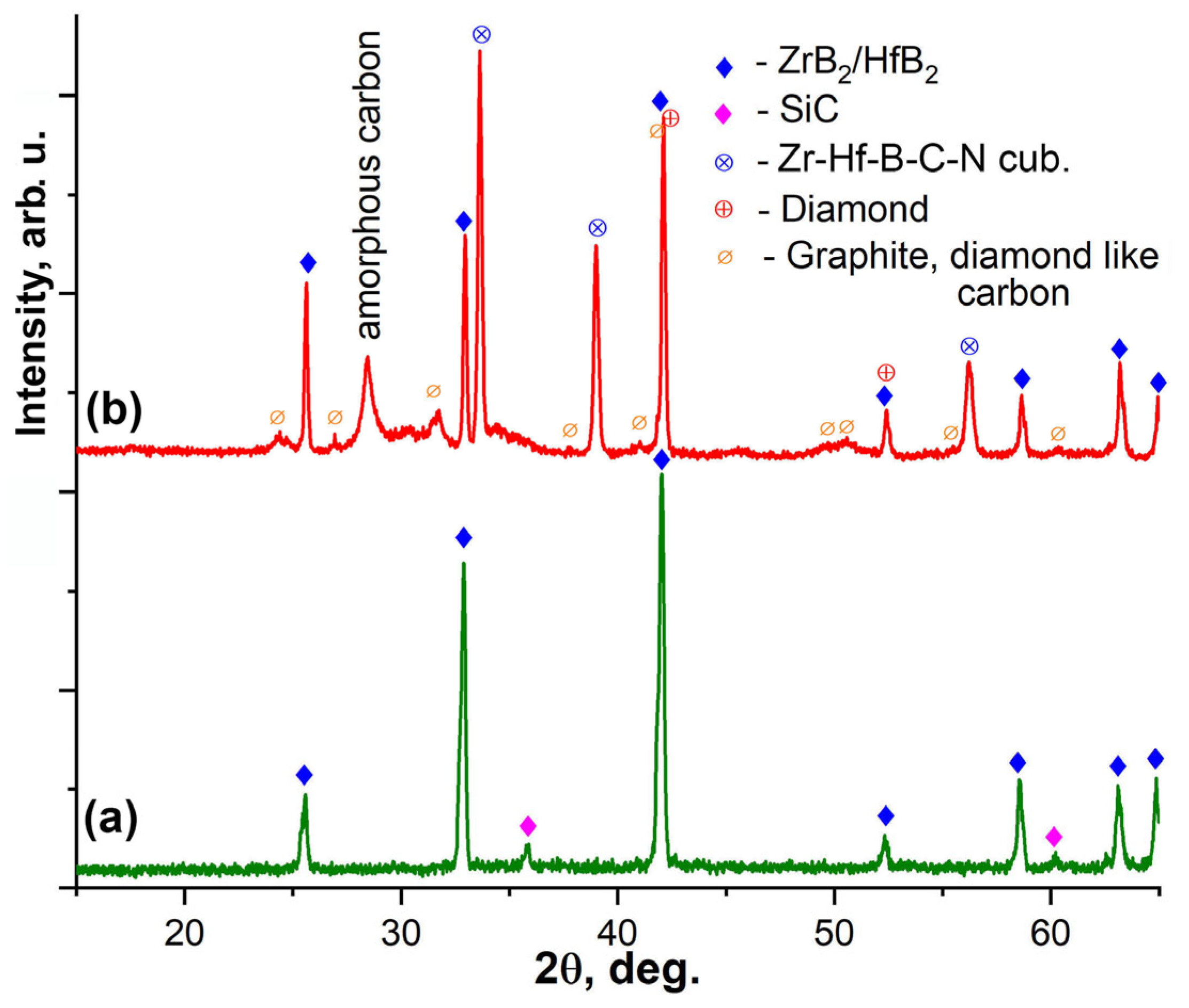
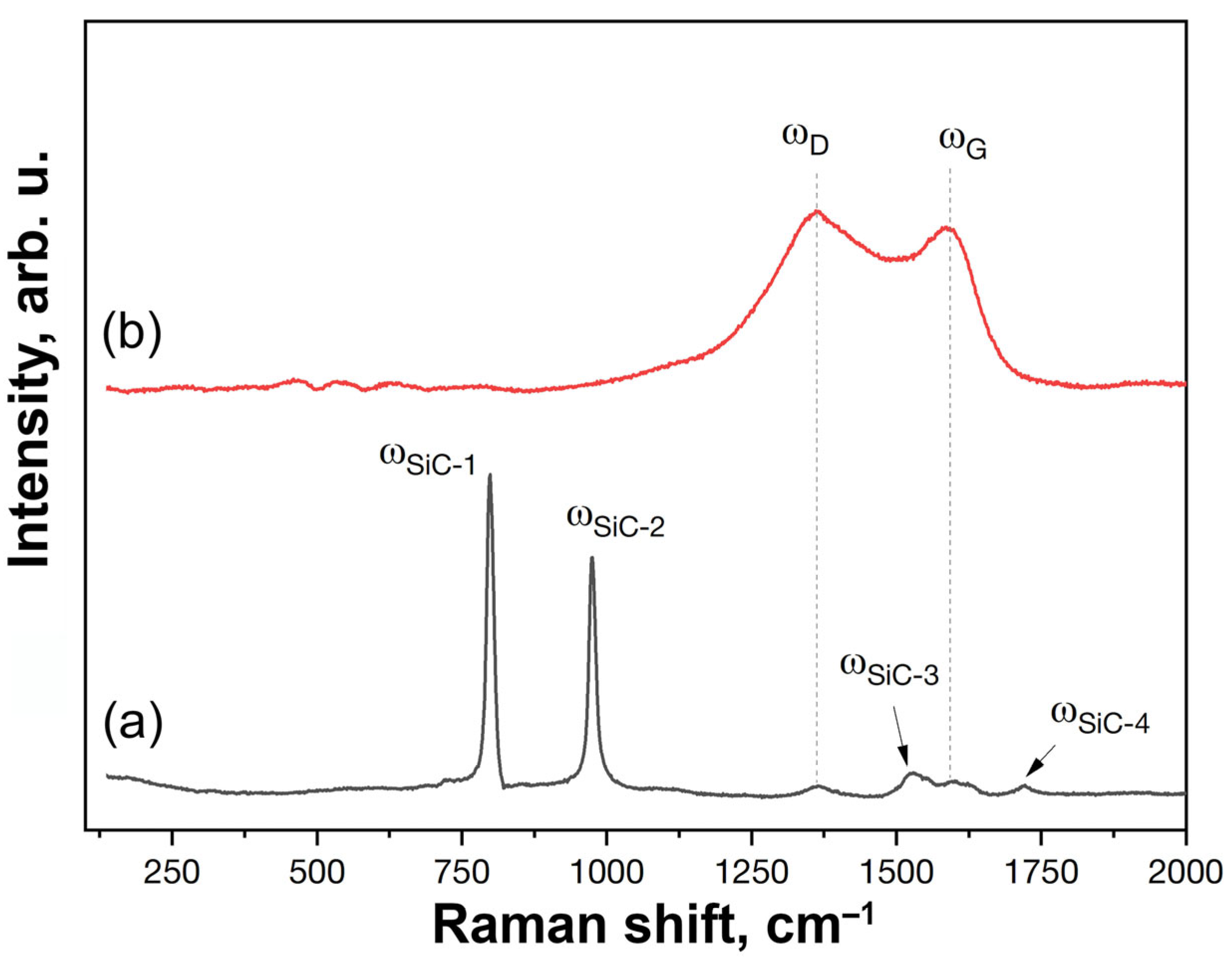
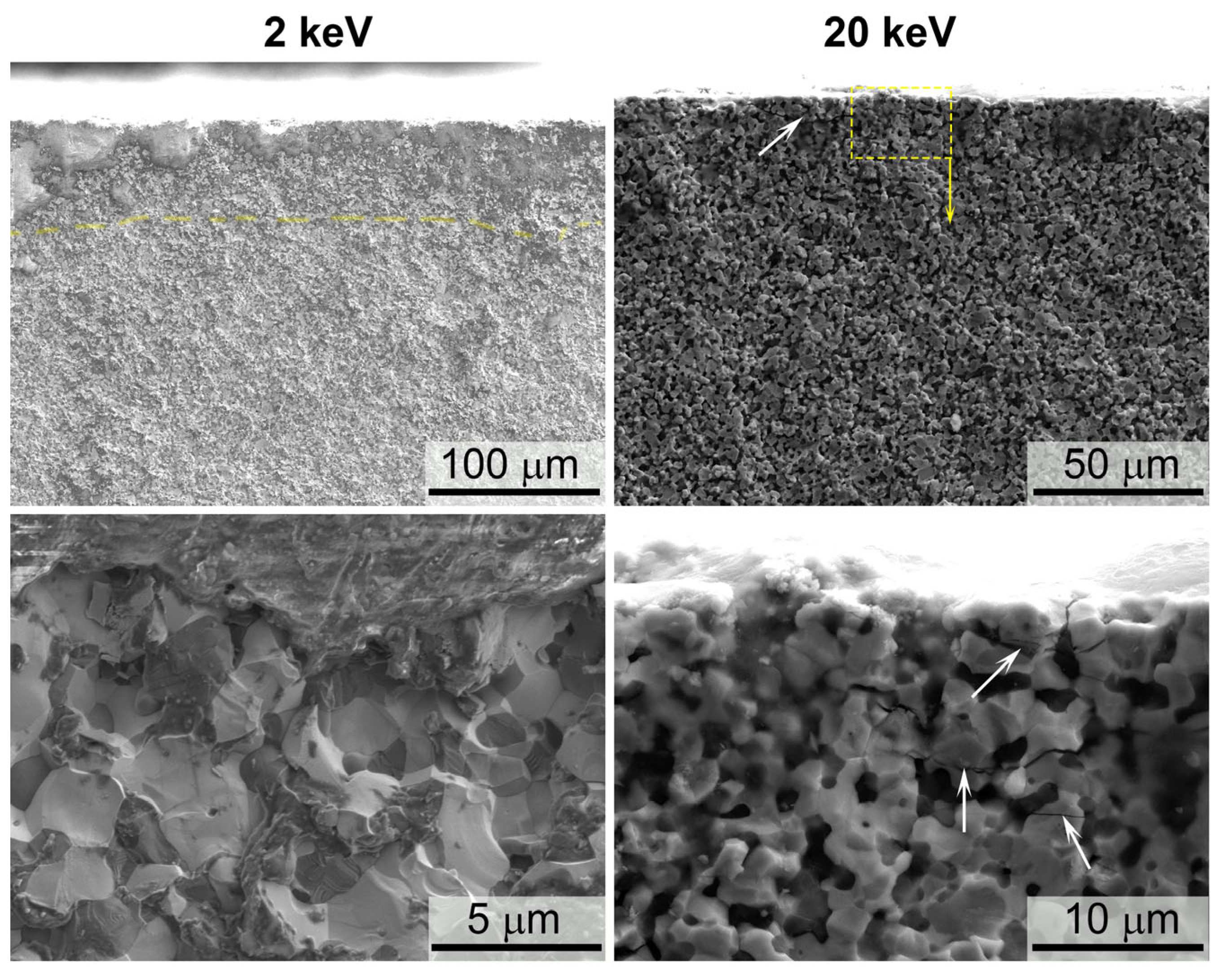
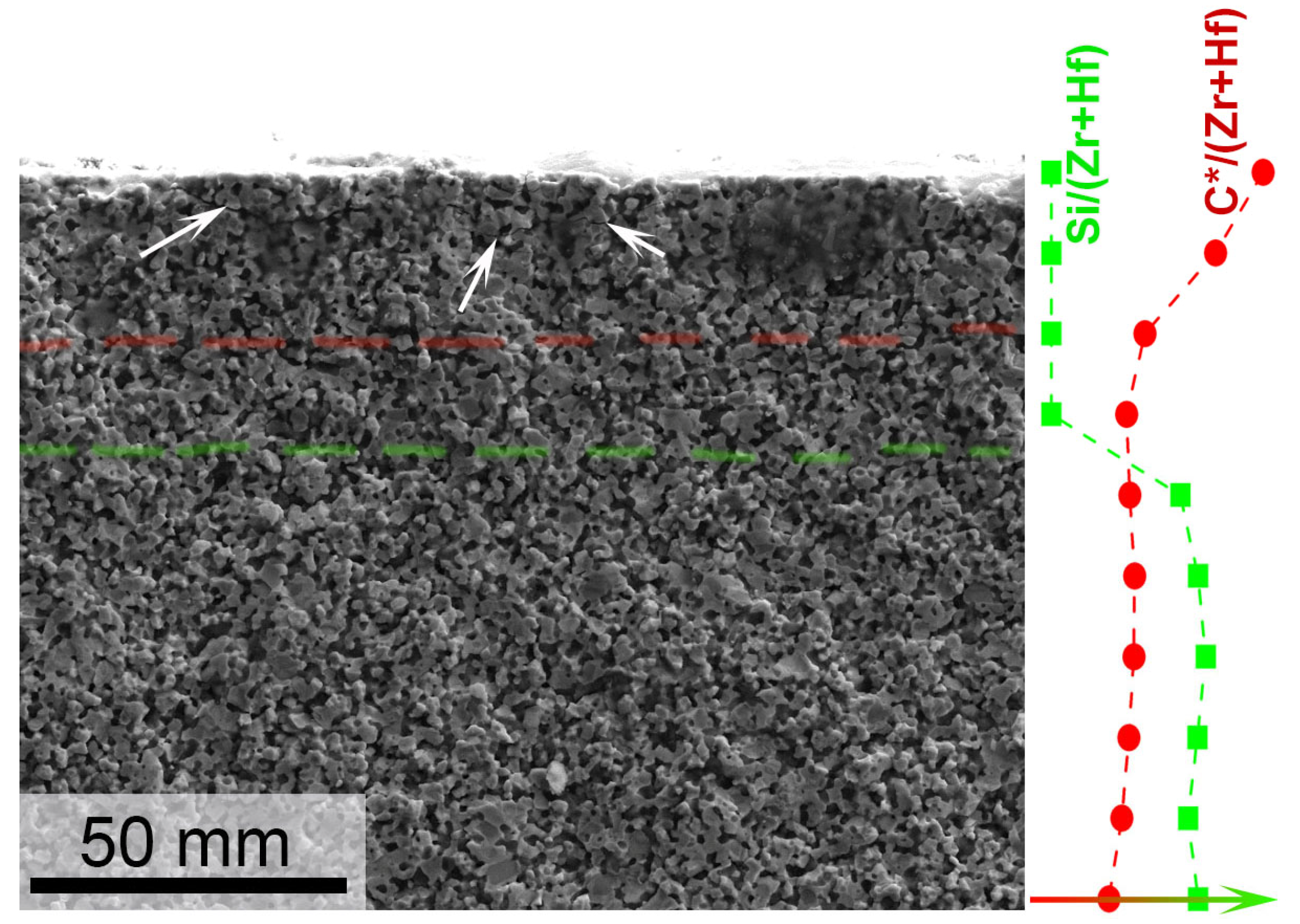
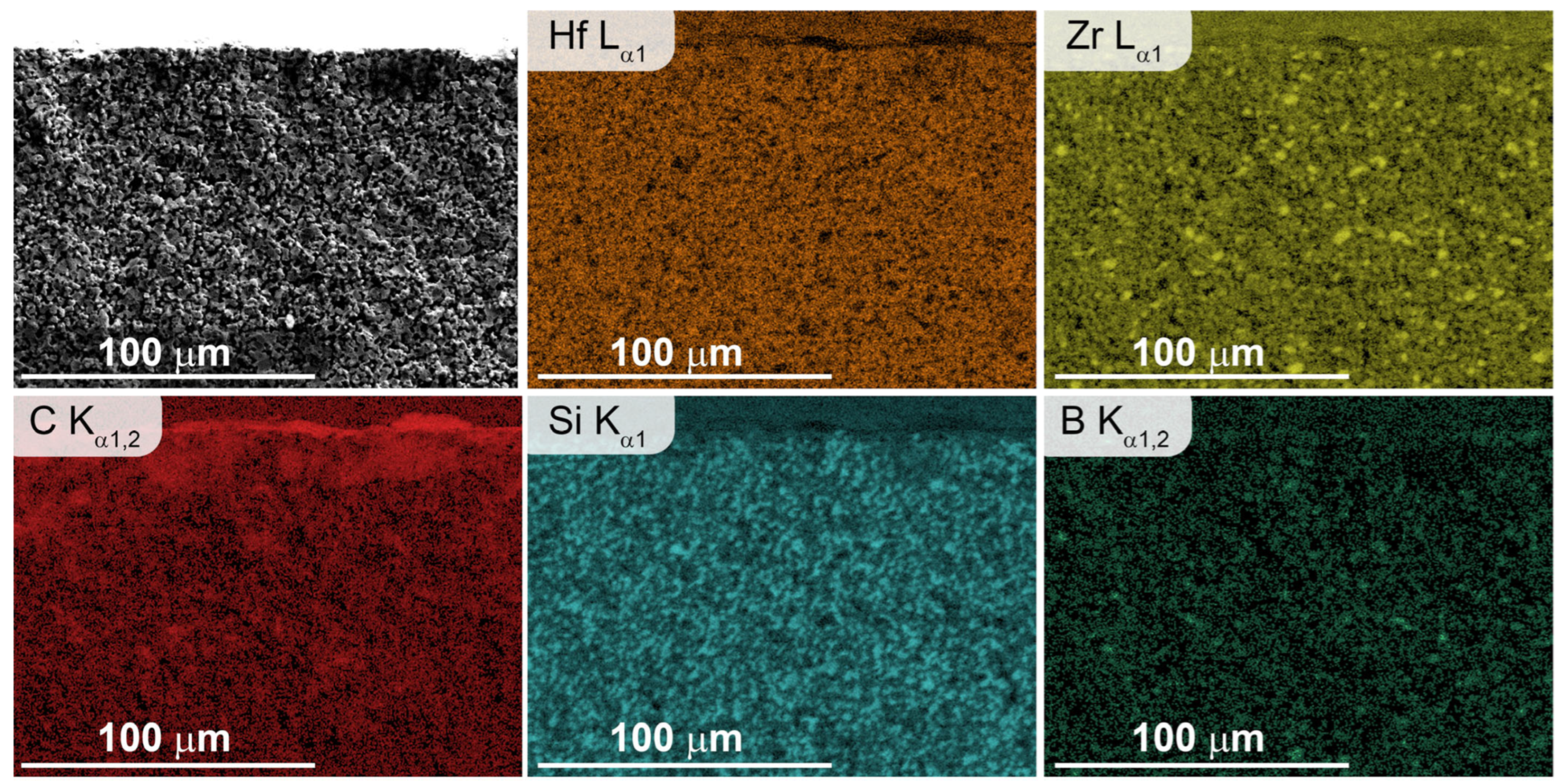
3.2. Investigation of the Surface and Fracture of a ZrB2-HfB2-SiC-CCNT Sample After Exposure to a Subsonic N2+CH4 Flow
4. Conclusions
- The heat flux to a cold copper wall, determined for the plasma based on nitrogen modified with 5 mol% methane, was 315 W/cm2. At the same time, an increase in the plasma radiation intensity was observed compared to the pure nitrogen plasma jet under the same facility parameters (anode power, pressure, and gas flow rate). This increase was also expressed in a significantly higher intensity of the N2+ and CN lines in the emission spectrum of the 95 mol% N2–5 mol% CH4 plasma composition compared to the N2 plasma spectrum. Additionally, emission spectroscopy data revealed the presence of C2 in the composition of the high-enthalpy jet.
- From the point of view of the effect on the sample, it is necessary to state that there are both similarities and differences in the behavior of the ceramic under the action of dissociated individual nitrogen and the N2+CH4 gas mixture with respect to the pure N2 plasma flow. Namely:
- In both cases, there is no silicon carbide on the surface of the sample, which is likely to be destroyed under the influence of a temperature of 2100–2230 °C and reduced pressure, with transition to the gas phase, or to react with atomic nitrogen or hydrogen-containing particles to form gaseous products.
- Partially, zirconium and hafnium diborides are converted into a solid solution with a cubic structure, most likely, based on metal monoborides with an admixture of monocarbides and mononitrides, which is close to the behavior of ZrB2(HfB2)-SiC-C ceramics under the action of not a subsonic but a supersonic flow of dissociated nitrogen. In a subsonic flow of nitrogen without the addition of methane, it was previously shown that a lower hafnium nitride with the composition Hf3N2 is formed [36].
- The introduction of methane into the subsonic flow of dissociated nitrogen leads to the formation of a carbon layer on the surface, which includes both amorphized carbon and diamond-like coatings, as evidenced by the combined data of XRD, Raman spectroscopy, SEM, and energy-dispersive analysis of the sample. This significant change in the composition and microstructure of the ultrahigh-temperature ceramic surface affects its emissivity and can impact the catalytic component of further sample heating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simonenko, E.P.; Sevast’yanov, D.V.; Simonenko, N.P.; Sevast’yanov, V.G.; Kuznetsov, N.T. Promising Ultra-High-Temperature Ceramic Materials for Aerospace Applications. Russ. J. Inorg. Chem. 2013, 58, 1669–1693. [Google Scholar] [CrossRef]
- Squire, T.H.; Marschall, J. Material Property Requirements for Analysis and Design of UHTC Components in Hypersonic Applications. J. Eur. Ceram. Soc. 2010, 30, 2239–2251. [Google Scholar] [CrossRef]
- Jin, X.; He, R.; Zhang, X.; Hu, P. Ablation Behavior of ZrB2–SiC Sharp Leading Edges. J. Alloys Compd. 2013, 566, 125–130. [Google Scholar] [CrossRef]
- Samsonov, G.V.; Bolgar, A.S.; Guseva, E.A.; Klochkov, L.A.; Kovenskaya, B.A.; Serebryakova, T.I.; Timofeeva, I.I.; Turchanin, A.G.; Fesenko, V.V. Thermophysical Properties of Transition Metal Carbides and Diborides. High Temp.-High Press. 1973, 5, 29–33. [Google Scholar]
- Guria, J.F.; Bansal, A.; Kumar, V. Effect of Additives on the Thermal Conductivity of Zirconium Diboride Based Composites—A Review. J. Eur. Ceram. Soc. 2021, 41, 1–23. [Google Scholar] [CrossRef]
- Harrington, G.J.K.; Hilmas, G.E. Thermal Conductivity of ZrB 2 and HfB 2. In Ultra-High Temperature Ceramics; Wiley: Hoboken, NJ, USA, 2014; pp. 197–235. [Google Scholar]
- Balat-Pichelin, M.; Bêche, E.; Sciti, D.; Alfano, D. Emissivity, Catalycity and Microstructural Characterization of ZrB2–SiCfiber Based UHTC at High Temperature in a Non-Equilibrium Air Plasma Flow. Ceram. Int. 2014, 40, 9731–9742. [Google Scholar] [CrossRef]
- Meng, S.; Zeng, Q.; Jin, H.; Wang, L.; Xu, C. Evaluation of Atomic Oxygen Catalytic Coefficient of ZrB2 –SiC by Laser-Induced Fluorescence up to 1473 K. Meas. Sci. Technol. 2018, 29, 075207. [Google Scholar] [CrossRef]
- Li, N.; Hu, P.; Zhang, X.; Liu, Y.; Han, W. Effects of Oxygen Partial Pressure and Atomic Oxygen on the Microstructure of Oxide Scale of ZrB2–SiC Composites at 1500°C. Corros. Sci. 2013, 73, 44–53. [Google Scholar] [CrossRef]
- Marschall, J.; Chamberlain, A.; Crunkleton, D.; Rogers, B. Catalytic Atom Recombination on ZrB2/SiC and HfB2/SiC Ultrahigh-Temperature Ceramic Composites. J. Spacecr. Rocket. 2004, 41, 576–581. [Google Scholar] [CrossRef]
- Marschall, J.; Pejakovic, D.; Fahrenholtz, W.G.; Hilmas, G.E.; Panerai, F.; Chazot, O. Temperature Jump Phenomenon during Plasmatron Testing of ZrB2-SiC Ultrahigh-Temperature Ceramics. J. Thermophys. Heat Transf. 2012, 26, 559–572. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Jiao, Y.; Wei, B.; Ding, X.; Jin, X.; Ran, S. Reactive Spark Plasma Sintering of an Electrically Conductive B4C-SiC-ZrB2 Composite with Enhanced Mechanical Properties. J. Eur. Ceram. Soc. 2024, 44, 2720–2729. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Shahedi Asl, M.; Zakeri, M.; Farvizi, M. On the Reactive Spark Plasma Sinterability of ZrB2–SiC–TiN Composite. J. Alloys Compd. 2022, 909, 164611. [Google Scholar] [CrossRef]
- Duan, X.; Wei, C.; Geng, X.; Meng, F.; Zhou, L.; Wang, P.; Dong, Z.; Dong, S. Effects of Adding Si3N4 Whiskers to ZrB2–SiC Ceramics on Microstructure, Mechanical Properties and Oxidation Resistance. Ceram. Int. 2024, 50, 43153–43164. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Sevastyanov, V.G.; Kuznetsov, N.T. ZrB2/HfB2–SiC Ultra-High-Temperature Ceramic Materials Modified by Carbon Components: The Review. Russ. J. Inorg. Chem. 2018, 63, 1772–1795. [Google Scholar] [CrossRef]
- Yang, B.; Kuang, C.; Liu, Z.; Yu, C.; Deng, C.; Ding, J. Effect of Nano-Graphite on Mechanical Properties and Oxidation Resistance of ZrB2–SiC–Graphite Electrode Ceramics. J. Iron Steel Res. Int. 2024, 31, 1502–1513. [Google Scholar] [CrossRef]
- Asl, M.S.; Balak, Z. Fabrication and Characterization of ZrB2 Ceramic in Presence of Graphite Platelet and SiC. Silicon 2023, 15, 6911–6919. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Kolesnikov, A.F.; Chaplygin, A.V.; Lysenkov, A.S.; Nagornov, I.A.; Mokrushin, A.S.; Kuznetsov, N.T. Investigation of the Effect of Supersonic Flow of Dissociated Nitrogen on ZrB2–HfB2–SiC Ceramics Doped with 10 Vol.% Carbon Nanotubes. Materials 2022, 15, 8507. [Google Scholar] [CrossRef]
- Tripathi, S.; Bhadauria, A.; Tiwari, A.; Tiwari, A.K. Effect of Carbonaceous Reinforcements on Mechanical Properties of ZrB2-SiC Composites via Nanoindentation Study. Diam. Relat. Mater. 2023, 140, 110537. [Google Scholar] [CrossRef]
- He, L.; Wu, J.; Meng, Q.; Sun, Y.; Pan, J.; Tian, Y.; Wu, F. Study on Low Content Short Carbon Fibers to Enhance Mechanical Properties of ZrB2-SiC Composites with Improved Damage Tolerance. Mater. Today Commun. 2025, 42, 111391. [Google Scholar] [CrossRef]
- Chen, Y. ULTRA-HIGH-TEMPERATURE CERAMIC MATERIALS MODIFIED BY GRAPHENE: AN OVERVIEW. Ceram.-Silikaty 2023, 67, 260–296. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Kolesnikov, A.F.; Chaplygin, A.V.; Lysenkov, A.S.; Nagornov, I.A.; Simonenko, T.L.; Gubin, S.P.; Sevastyanov, V.G.; Kuznetsov, N.T. Oxidation of Graphene-Modified HfB2-SiC Ceramics by Supersonic Dissociated Air Flow. J. Eur. Ceram. Soc. 2022, 42, 30–42. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Kolesnikov, A.F.; Chaplygin, A.V.; Lysenkov, A.S.; Nagornov, I.A.; Lukomskii, I.V.; Galkin, S.S.; Mokrushin, A.S.; Simonenko, N.P.; Kuznetsov, N.T. Transformation of the Surface of HfB2–SiC–C(Graphene) Ultrahigh-Temperature Ceramics in a High-Velocity Flow of Dissociated Nitrogen. Russ. J. Inorg. Chem. 2024, 69, 517–527. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Kolesnikov, A.F.; Chaplygin, A.V.; Kotov, M.A.; Yakimov, M.Y.; Lukomskii, I.V.; Galkin, S.S.; Shemyakin, A.N.; Solovyov, N.G.; Lysenkov, A.S.; et al. Oxidation of Ceramic Materials Based on HfB2-SiC under the Influence of Supersonic CO2 Jets and Additional Laser Heating. Int. J. Mol. Sci. 2023, 24, 13634. [Google Scholar] [CrossRef] [PubMed]
- Simonenko, E.P.; Simonenko, N.P.; Kolesnikov, A.F.; Chaplygin, A.V.; Sakharov, V.I.; Lysenkov, A.S.; Nagornov, I.A.; Kuznetsov, N.T. Effect of 2 Vol % Graphene Additive on Heat Transfer of Ceramic Material in Underexpanded Jets of Dissociated Air. Russ. J. Inorg. Chem. 2022, 67, 2050–2061. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, Y.; An, Y.; Hu, N. High Thermal-Conductivity RGO/ZrB2-SiC Ceramics Consolidated from ZrB2-SiC Particles Decorated GO Hybrid Foam with Enhanced Thermal Shock Resistance. J. Eur. Ceram. Soc. 2020, 40, 2760–2767. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, X.; Hong, C.; Qiu, Y.; Zhang, J.; Han, J.; Hu, P. Electrostatic Assembly Preparation of High-Toughness Zirconium Diboride-Based Ceramic Composites with Enhanced Thermal Shock Resistance Performance. ACS Appl. Mater. Interfaces 2016, 8, 11675–11681. [Google Scholar] [CrossRef]
- Wang, A.; Hu, P.; Zhang, X.; Zhang, D.; Fang, C. Thermal Shock Behavior of ZrB2-Based Sharp Leading Edges Evaluated by a Novel Water Spraying Method. Ceram. Int. 2018, 44, 2376–2382. [Google Scholar] [CrossRef]
- Boulos, M.I.; Auweter-Kurtz, M.; Fauchais, P.L.; Pfender, E. Plasma in the Aerospace Industry. In Handbook of Thermal Plasmas; Springer International Publishing: Cham, Germany, 2023; pp. 1509–1580. [Google Scholar]
- Chinnaraj, R.K.; Hong, S.M.; Kim, H.S.; Oh, P.Y.; Choi, S.M. Ablation Experiments of Ultra-High-Temperature Ceramic Coating on Carbon–Carbon Composite Using ICP Plasma Wind Tunnel. Int. J. Aeronaut. Space Sci. 2020, 21, 889–905. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Papynov, E.K.; Shichalin, O.O.; Belov, A.A.; Nagornov, I.A.; Simonenko, T.L.; Gorobtsov, P.Y.; Teplonogova, M.A.; Mokrushin, A.S.; Simonenko, N.P.; et al. Reactive Spark Plasma Sintering and Oxidation of ZrB2-SiC and ZrB2-HfB2-SiC Ceramic Materials. Ceramics 2024, 7, 1566–1584. [Google Scholar] [CrossRef]
- Carandente, V.; Savino, R.; Esposito, A.; Zuppardi, G.; Caso, V. Experimental and Numerical Simulation, by an Arc-Jet Facility, of Hypersonic Flow in Titan’s Atmosphere. Exp. Therm. Fluid Sci. 2013, 48, 97–101. [Google Scholar] [CrossRef]
- Savino, R.; De Stefano Fumo, M.; Silvestroni, L.; Sciti, D. Arc-Jet Testing on HfB2 and HfC-Based Ultra-High Temperature Ceramic Materials. J. Eur. Ceram. Soc. 2008, 28, 1899–1907. [Google Scholar] [CrossRef]
- Silvestroni, L.; Mungiguerra, S.; Sciti, D.; Di Martino, G.D.; Savino, R. Effect of Hypersonic Flow Chemical Composition on the Oxidation Behavior of a Super-Strong UHTC. Corros. Sci. 2019, 159, 108125. [Google Scholar] [CrossRef]
- Kolesnikov, A.F.; Kuznetsov, N.T.; Murav’eva, T.I.; Nagornov, I.A.; Sakharov, V.I.; Sevastyanov, V.G.; Simonenko, E.P.; Simonenko, N.P.; Chaplygin, A.V.; Shcherbakova, O.O. Investigation of Heat Transfer to HfB2-SiC-Based Ceramics in Underexpanded Dissociated-Nitrogen Jets and Analysis of the Surface. Fluid Dyn. 2022, 57, 513–523. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Chaplygin, A.V.; Lukomskii, I.V.; Galkin, S.S.; Lysenkov, A.S.; Nagornov, I.A.; Mokrushin, A.S.; Kolesnikov, A.F.; Kuznetsov, N.T. Surface Degradation of Ultrahigh-Temperature Ceramics Based on HfB2–30vol%SiC in Subsonic Nitrogen Plasma Flow. Int. J. Refract. Met. Hard Mater. 2025, 130, 107139. [Google Scholar] [CrossRef]
- Nixon, C.A. The Composition and Chemistry of Titan’s Atmosphere. ACS Earth Space Chem. 2024, 8, 406–456. [Google Scholar] [CrossRef] [PubMed]
- Owen, T. The Composition and Origin of Titan’s Atmosphere. Planet. Space Sci. 1982, 30, 833–838. [Google Scholar] [CrossRef]
- Chaplygin, A.V.; Vasil’evskii, S.A.; Galkin, S.S.; Kolesnikov, A.F. Thermal State of Uncooled Quartz Discharge Channel of Powerful High-Frequency Induction Plasmatron. Phys. Kinet. Gas Dyn. 2022, 23, 38–56. [Google Scholar] [CrossRef]
- Gordeev, A. Overview of Characteristics and Experiments in IPM Plasmatrons. In VKI, RTO AVT/VKI Special Course on Measurement Techniques for High Enthalpy Plasma Flows; RTO EN-8; Defense Technical Information Center: Fort Belvoir, VA, USA, 1999; Available online: https://apps.dtic.mil/sti/citations/ADP010736 (accessed on 1 June 2025).
- Chaplygin, A.; Simonenko, E.; Simonenko, N.; Kotov, M.; Yakimov, M.; Lukomskii, I.; Galkin, S.; Kolesnikov, A.; Vasil’evskii, S.; Shemyakin, A.; et al. Heat Transfer and Behavior of Ultra High Temperature Ceramic Materials under Exposure to Supersonic Carbon Dioxide Plasma with Additional Laser Irradiation. Int. J. Therm. Sci. 2024, 201, 109005. [Google Scholar] [CrossRef]
- Chaplygin, A.; Yakimov, M.; Vasil’evskii, S.; Kotov, M.; Lukomskii, I.; Galkin, S.; Shemyakin, A.; Solovyov, N.; Kolesnikov, A. Combined Plasma and Laser Heating of Graphite. Plasma 2025, 8, 9. [Google Scholar] [CrossRef]
- Roeck, W.; Auweter-Kurtz, M. Experimental Investigation of the Huygens Entry into the Titan Atmosphere within a Plasma Wind Tunnel. In Proceedings of the 30th Thermophysics Conference, San Diego, CA, USA, 19–22 June 1995; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 1995. [Google Scholar]
- Playez, M.; Vancrayenest, B.; Zuber, M.E.; Fletcher, D.G. Titan Atmosphere Plasma Investigation Using Spectroscopic Techniques. In Proceedings of the Fifth European Symposium on Aerothermodynamics for Space Vehicles, Cologne, Germany, 8–11 November 2005; pp. 1–6. [Google Scholar]
- Kouakou, P.; Brien, V.; Assouar, B.; Hody, V.; Belmahi, M.; Migeon, H.N.; Bougdira, J. Preliminary Synthesis of Carbon Nitride Thin Films by N2/CH4 Microwave Plasma Assisted Chemical Vapour Deposition: Characterisation of the Discharge and the Obtained Films. Plasma Process. Polym. 2007, 4, S210–S214. [Google Scholar] [CrossRef]
- Bulou, S.; Le Brizoual, L.; Hugon, R.; De Poucques, L.; Belmahi, M.; Migeon, H.; Bougdira, J. Characterization of a N2/CH4 Microwave Plasma With a Solid Additive Si Source Used for SiCN Deposition. Plasma Process. Polym. 2009, 6, S576–S581. [Google Scholar] [CrossRef]
- Vacher, D.; Dudeck, M.; André, P.; Lino da Silva, M.; Faure, G.; Dubois, M.; Hamwi, A. Radiation from an Inductively Coupled Plasma in the Near-UV to near-IR Spectral Region for a Titan-Type N2-CH4 Mixture. In Proceedings of the EUCASS, the European Conference for AeroSpace Sciences; p. 184. Available online: https://www.eucass.eu/component/docindexer/?task=download&id=6310 (accessed on 1 June 2025).
- Holleck, H. Legierungsverhalten von HfB2 Mit Uran- Und Übergangsmetalldiboriden. J. Nucl. Mater. 1967, 21, 14–20. [Google Scholar] [CrossRef]
- Kugai, L.N. Chemical Stability of Borides of Transition Metals of Groups IV-VI Inalkaline Solutions. Izv. Akad. Nauk Neorg. Mater. 1972, 8, 669–670. (In Russian) [Google Scholar]
- Braekken, H. Zur Kristallstruktur Des Kubischen Karborunds. Z. Krist. 1930, 75, 572–573. [Google Scholar]
- Shulishova, O.I.; Shcherbak, I.A. Superconductivity of the Borides of Transition and Rare-Earth Metals. Inorg. Mater. 1967, 3, 1304–1306. [Google Scholar]
- Wyckoff, R.W.G. Second Edition. Interscience Publishers, New York, New Yorkrocksalt Structure. Cryst. Struct. 1963, 1, 85–237. [Google Scholar]
- Becker, K.; Ebert, F. Die Kristallstruktur Einiger Binärer Carbide Und Nitride. Z. Phys. 1925, 31, 268–272. [Google Scholar] [CrossRef]
- Rudy, E. The Crystal Structures of Hf3N2 and Hf4N3. Metall. Mater. Trans. B 1970, 1, 1249–1252. [Google Scholar] [CrossRef]
- Li, Y.; Mu, L.; Hu, Y.-S.; Li, H.; Chen, L.; Huang, X. Pitch-Derived Amorphous Carbon as High Performance Anode for Sodium-Ion Batteries. Energy Storage Mater. 2016, 2, 139–145. [Google Scholar] [CrossRef]
- Chen, P.; Huang, F.; Yun, S. Characterization of the Condensed Carbon in Detonation Soot. Carbon N. Y. 2003, 41, 2093–2099. [Google Scholar] [CrossRef]
- TRUCANO, P.; CHEN, R. Structure of Graphite by Neutron Diffraction. Nature 1975, 258, 136–137. [Google Scholar] [CrossRef]
- Fayos, J. Possible 3D Carbon Structures as Progressive Intermediates in Graphite to Diamond Phase Transition. J. Solid State Chem. 1999, 148, 278–285. [Google Scholar] [CrossRef]
- Larichev, Y.V.; Yeletsky, P.M.; Yakovlev, V.A. Study of Silica Templates in the Rice Husk and the Carbon–Silica Nanocomposites Produced from Rice Husk. J. Phys. Chem. Solids 2015, 87, 58–63. [Google Scholar] [CrossRef]
- Bechelany, M.; Brioude, A.; Cornu, D.; Ferro, G.; Miele, P. A Raman Spectroscopy Study of Individual SiC Nanowires. Adv. Funct. Mater. 2007, 17, 939–943. [Google Scholar] [CrossRef]
- Burton, J.C.; Sun, L.; Long, F.H.; Feng, Z.C.; Ferguson, I.T. First- and Second-Order Raman Scattering from Semi-Insulating 4-H SiC. Phys. Rev. B 1999, 59, 7282–7284. [Google Scholar] [CrossRef]
- Jiang, C.; Zhao, J.; Therese, H.A.; Friedrich, M.; Mews, A. Raman Imaging and Spectroscopy of Heterogeneous Individual Carbon Nanotubes. J. Phys. Chem. B 2003, 107, 8742–8745. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on Carbon Nanotubes and Graphene Raman Spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J. Diamond-like Amorphous Carbon. Mater. Sci. Eng. R Rep. 2002, 37, 129–281. [Google Scholar] [CrossRef]
- Argibay, N.; Babuska, T.F.; Curry, J.F.; Dugger, M.T.; Lu, P.; Adams, D.P.; Nation, B.L.; Doyle, B.L.; Pham, M.; Pimentel, A.; et al. In-Situ Tribochemical Formation of Self-Lubricating Diamond-like Carbon Films. Carbon N. Y. 2018, 138, 61–68. [Google Scholar] [CrossRef]
- Das, S.; Mojumder, S.; Saha, D.; Pal, M. Influence of Major Parameters on the Sensing Mechanism of Semiconductor Metal Oxide Based Chemiresistive Gas Sensors: A Review Focused on Personalized Healthcare. Sens. Actuators B Chem. 2022, 352, 131066. [Google Scholar] [CrossRef]
- Ashfold, M.N.R.; Mankelevich, Y.A. Self-Consistent Modeling of Microwave Activated N2/CH4/H2 (and N2/H2) Plasmas Relevant to Diamond Chemical Vapor Deposition. Plasma Sources Sci. Technol. 2022, 31, 035005. [Google Scholar] [CrossRef]
- Almeida, L.S.; Souza, A.R.M.; Costa, L.H.; Rangel, E.C.; Manfrinato, M.D.; Rossino, L.S. Effect of Nitrogen in the Properties of Diamond-like Carbon (DLC) Coating on Ti 6 Al 4 V Substrate. Mater. Res. Express 2020, 7, 065601. [Google Scholar] [CrossRef]
- Badzian, A.; Badzian, T.; Lee, S.-T. Synthesis of Diamond from Methane and Nitrogen Mixture. Appl. Phys. Lett. 1993, 62, 3432–3434. [Google Scholar] [CrossRef]
- Carandente, V.; Caso, V.; Esposito, A.; Savino, R.; Zuppardi, G. Simulation of Titan Atmosphere by an Arc-Heated Facility. In Proceedings of the Ntervento Presentato al Convegno 9th International Planetary Probe Workshop, Toulouse, France, 18–22 June 2012; pp. 1–6. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonenko, E.P.; Chaplygin, A.V.; Simonenko, N.P.; Lukomskii, I.V.; Galkin, S.S.; Lysenkov, A.S.; Nagornov, I.A.; Mokrushin, A.S.; Kolesnikov, A.F.; Kuznetsov, N.T. Surface Transformation of Ultrahigh-Temperature ZrB2–HfB2–SiC–CCNT Ceramics Under Exposure to Subsonic N2-CH4 Plasma Flow. Ceramics 2025, 8, 67. https://doi.org/10.3390/ceramics8020067
Simonenko EP, Chaplygin AV, Simonenko NP, Lukomskii IV, Galkin SS, Lysenkov AS, Nagornov IA, Mokrushin AS, Kolesnikov AF, Kuznetsov NT. Surface Transformation of Ultrahigh-Temperature ZrB2–HfB2–SiC–CCNT Ceramics Under Exposure to Subsonic N2-CH4 Plasma Flow. Ceramics. 2025; 8(2):67. https://doi.org/10.3390/ceramics8020067
Chicago/Turabian StyleSimonenko, Elizaveta P., Aleksey V. Chaplygin, Nikolay P. Simonenko, Ilya V. Lukomskii, Semen S. Galkin, Anton S. Lysenkov, Ilya A. Nagornov, Artem S. Mokrushin, Anatoly F. Kolesnikov, and Nikolay T. Kuznetsov. 2025. "Surface Transformation of Ultrahigh-Temperature ZrB2–HfB2–SiC–CCNT Ceramics Under Exposure to Subsonic N2-CH4 Plasma Flow" Ceramics 8, no. 2: 67. https://doi.org/10.3390/ceramics8020067
APA StyleSimonenko, E. P., Chaplygin, A. V., Simonenko, N. P., Lukomskii, I. V., Galkin, S. S., Lysenkov, A. S., Nagornov, I. A., Mokrushin, A. S., Kolesnikov, A. F., & Kuznetsov, N. T. (2025). Surface Transformation of Ultrahigh-Temperature ZrB2–HfB2–SiC–CCNT Ceramics Under Exposure to Subsonic N2-CH4 Plasma Flow. Ceramics, 8(2), 67. https://doi.org/10.3390/ceramics8020067






