Optimizing Sintering Conditions for Y2O3 Ceramics: A Study of Atmosphere-Dependent Microstructural Evolution and Optical Performance
Abstract
1. Introduction
2. Experimental Section
2.1. Ceramic Fabrication
2.2. Characterization
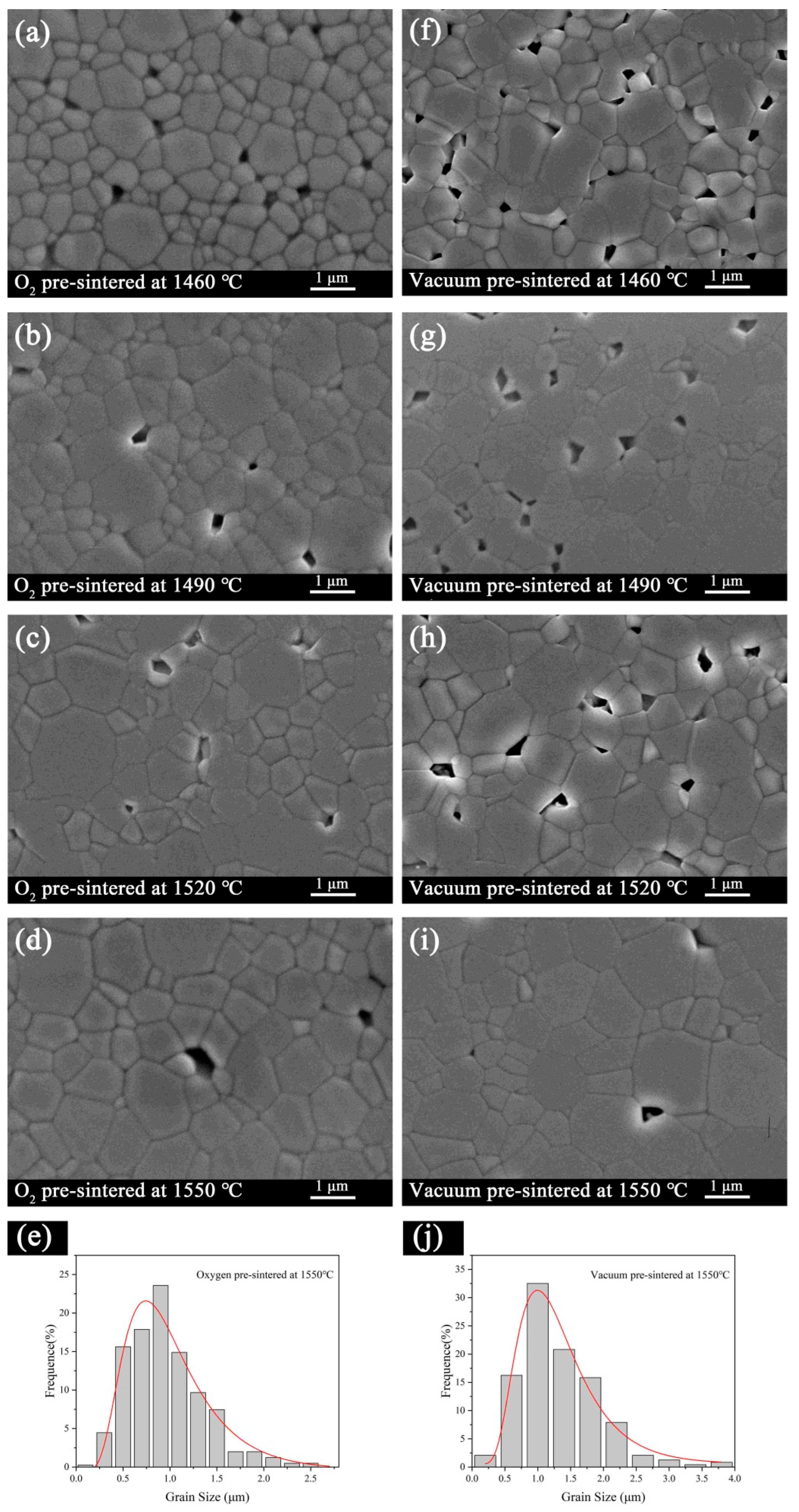
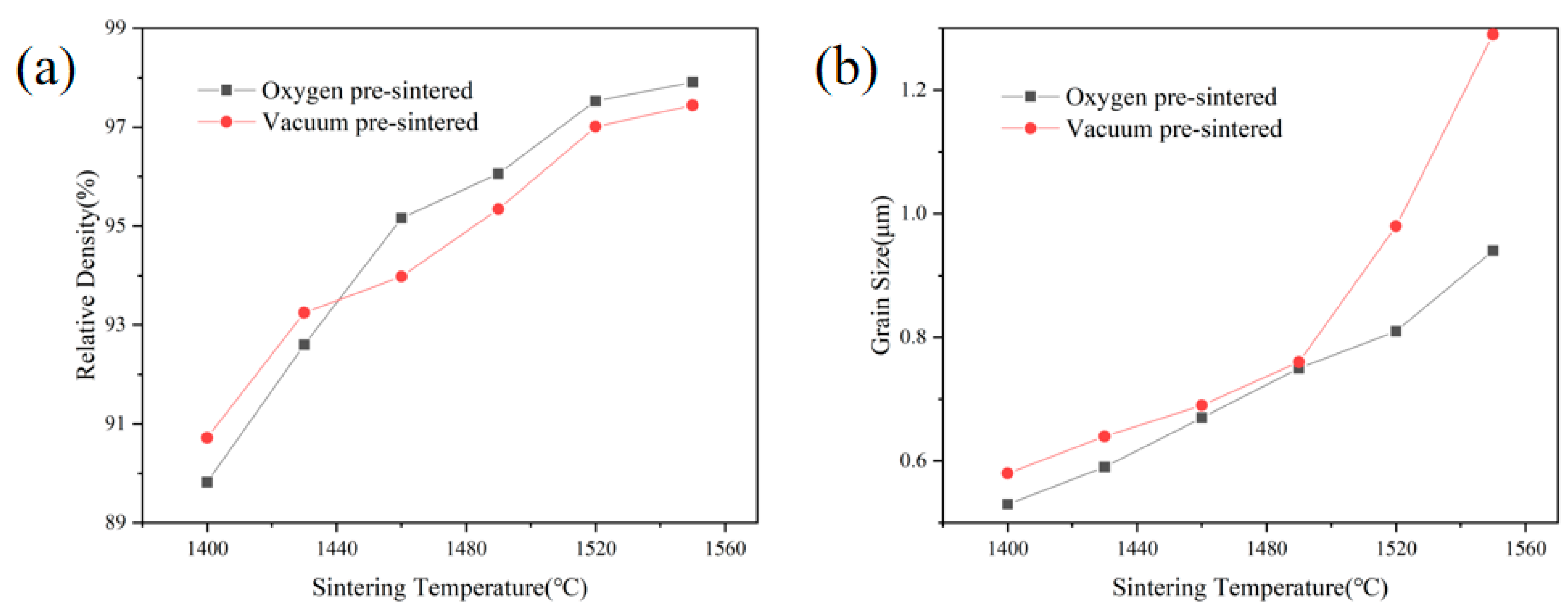
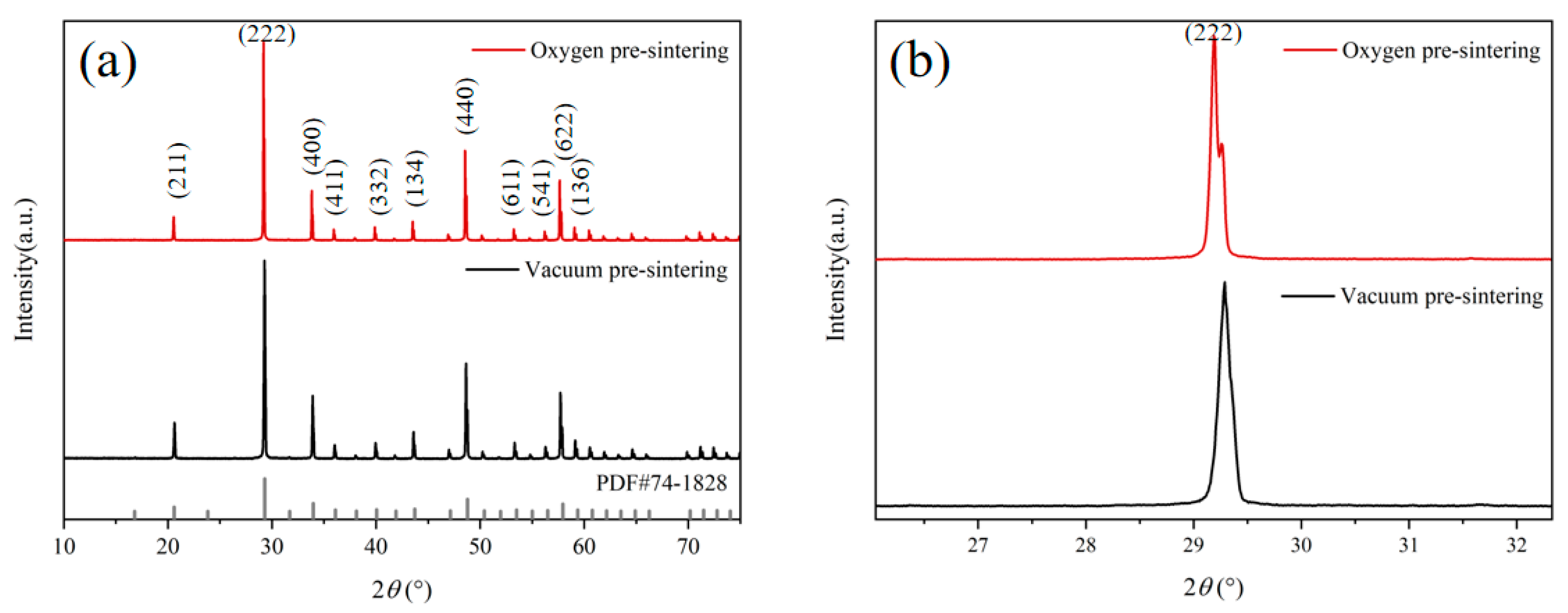
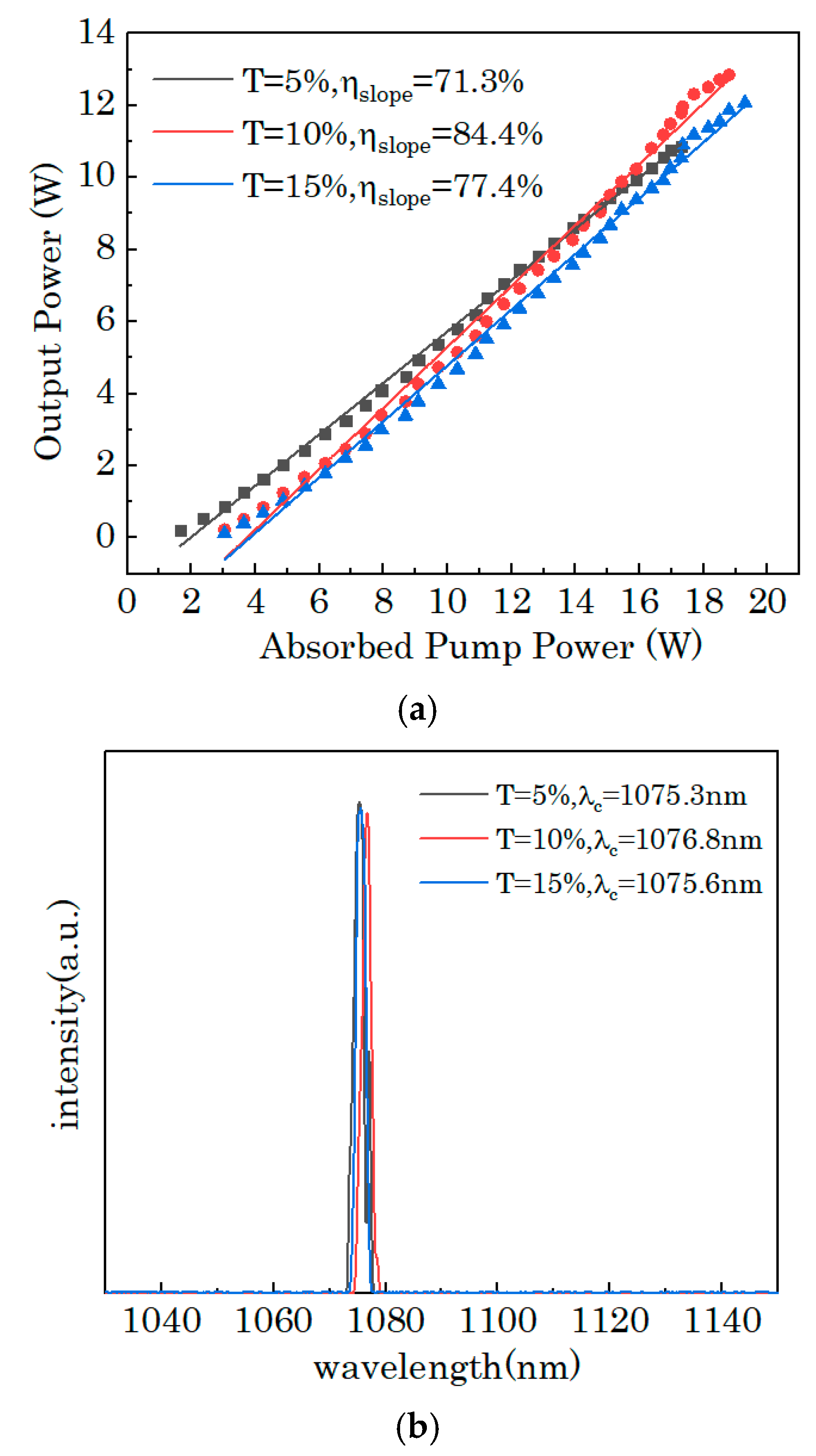
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Ikesue, A.; Li, J. Research progress and prospects of rare-earth doped sesquioxide laser ceramics. J. Eur. Ceram. Soc. 2021, 41, 3895–3910. [Google Scholar] [CrossRef]
- Ikesue, A.; Aung, Y.L. Ceramic laser materials. Nat. Photonics 2008, 2, 721–727. [Google Scholar] [CrossRef]
- Ren, C.; Huang, W.; Xie, H.; Wang, F.; Shen, D.; Wang, J.; Zhu, H.; Tang, D. High power and efficient operation of a Ho:Y2O3 ceramic laser with over 210 W of output power at 2.1 µm. Opt. Express 2022, 30, 31407–31414. [Google Scholar] [CrossRef] [PubMed]
- Ning, K.; Wang, J.; Luo, D.; Zhang, J.; Dong, Z.L.; Kong, L.B.; Tang, D.Y. New double-sintering aid for fabrication of highly transparent ytterbium-doped yttria ceramics. J. Eur. Ceram. Soc. 2016, 36, 253–256. [Google Scholar] [CrossRef]
- Eilers, H. Fabrication, optical transmittance, and hardness of IR-transparent ceramics made from nanophase yttria. J. Eur. Ceram. Soc. 2007, 27, 4711–4717. [Google Scholar] [CrossRef]
- Pirri, A.; Toci, G.; Patrizi, B.; Vannini, M. An overview on Yb-Doped transparent polycrystalline sesquioxide laser ceramics. IEEE J. Sel. Top. Quantum Electron. 2018, 24, 1602108. [Google Scholar] [CrossRef]
- Ding, M.; Li, X.; Wang, F.; Shen, D.; Wang, J.; Tang, D.; Zhu, H. Power scaling of diode-pumped Er:Y2O3 ceramic laser at 2.7 μm. Appl. Phys. Express 2022, 15, 062004. [Google Scholar] [CrossRef]
- Yavetskiy, R.P.; Balabanov, A.E.; Parkhomenko, S.V.; Kryzhanovska, O.S.; Doroshenko, A.G.; Mateychenko, P.V.; Tolmachev, A.V.; Li, J.; Jiang, N.; Gheorghe, L.; et al. Effect of starting materials and sintering temperature on microstructure and optical properties of Y2O3:Yb3+ 5 at% transparent ceramics. J. Adv. Ceram. 2021, 10, 49–61. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, D.; Zhang, J.; Lin, Q.; Huang, Z. Sintering of transparent yttria ceramics in oxygen atmosphere. J. Am. Ceram. Soc. 2010, 93, 2964–2967. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, J.; Yu, H.; Pan, W. High performance of La-doped Y2O3 transparent ceramics. J. Adv. Ceram. 2020, 9, 493–502. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Wang, J.; Ma, J.; Ni, M.; Lin, H.; Zhang, J.; Liu, P.; Xu, X.; Tang, D. High transparency Pr:Y2O3 ceramics: A promising gain medium for red emission solid-state lasers. J. Adv. Ceram. 2022, 11, 874–881. [Google Scholar] [CrossRef]
- Chen, P.L.; Chen, I.W. Grain boundary mobility in Y2O3: Defect mechanism and dopant effects. J. Am. Ceram. Soc. 1996, 79, 1801–1809. [Google Scholar] [CrossRef]
- Seaverson, L.M.; Luo, S.Q.; Chien, P.L.; McClelland, J.F. Carbonate Associated with Hydroxide Sol-Gel Processing of Yttria: An Infrared Spectroscopic Study. J. Am. Ceram. Soc. 1986, 69, 423–429. [Google Scholar] [CrossRef]
- Brindley, G.W.; Nakahira, M. Kinetics of dehydroxylation of kaolinite and halloysite. J. Am. Ceram. Soc. 1957, 40, 346–350. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, Q.; Zhu, Q.; Jiang, B.; Feng, M.; Zhang, L. Fabrication of fine-grained undoped Y2O3 transparent ceramic using nitrate pyrogenation synthesized nanopowders. Ceram. Int. 2019, 45, 5339–5345. [Google Scholar] [CrossRef]
- Fu, Z.; Li, X.; Zhang, M.; Zhu, Q.; Li, J.G.; He, J.; Wang, X.A.; Sun, X. Achieving fabrication of highly transparent Y2O3 ceramics via air pre-sintering by deionization treatment of suspension. J. Am. Ceram. Soc. 2021, 104, 2689–2701. [Google Scholar] [CrossRef]
- Li, X.; Mao, X.; Feng, M.; Xie, J.; Jiang, B.; Zhang, L. Optical absorption and mechanism of vacuum-sintered ZrO2-doped Y2O3 ceramics. J. Eur. Ceram. Soc. 2016, 36, 4181–4184. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Ma, J.; Ni, M.; Yang, F.; Liu, P.; Lee, K.Y.; Hsiang, H.I.; Shen, D.; Tang, D. Fabrication of high-efficiency Yb:Y2O3 laser ceramics without photodarkening. J. Am. Ceram. Soc. 2022, 105, 3375–3381. [Google Scholar] [CrossRef]



| Sample | Sintering Method | Sintering Additive | Transmittance | Ref. |
|---|---|---|---|---|
| Y2O3 | VS + HIP | None | 83.0%@3–5 μm | [15] |
| Y2O3 | AS + HIP | None | 81.8%@800 nm | [16] |
| Y2O3 | VS | ZrO2 | 80%@3–6 μm | [17] |
| Y2O3 | OS | ZrO2 | 80%@800 nm | [9] |
| Y2O3 | VS + HIP | None | 81.6%@800 nm 84.0%@3.3 μm | This work |
| Y2O3 | OS + HIP | None | 81.9%@800 nm 80.8%@3.3 μm | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Xing, D.; Wang, Y.; Wang, J.; Ma, J.; Liu, P.; Zhang, J.; Tang, D. Optimizing Sintering Conditions for Y2O3 Ceramics: A Study of Atmosphere-Dependent Microstructural Evolution and Optical Performance. Ceramics 2025, 8, 66. https://doi.org/10.3390/ceramics8020066
Wang X, Xing D, Wang Y, Wang J, Ma J, Liu P, Zhang J, Tang D. Optimizing Sintering Conditions for Y2O3 Ceramics: A Study of Atmosphere-Dependent Microstructural Evolution and Optical Performance. Ceramics. 2025; 8(2):66. https://doi.org/10.3390/ceramics8020066
Chicago/Turabian StyleWang, Xueer, Dongliang Xing, Ying Wang, Jun Wang, Jie Ma, Peng Liu, Jian Zhang, and Dingyuan Tang. 2025. "Optimizing Sintering Conditions for Y2O3 Ceramics: A Study of Atmosphere-Dependent Microstructural Evolution and Optical Performance" Ceramics 8, no. 2: 66. https://doi.org/10.3390/ceramics8020066
APA StyleWang, X., Xing, D., Wang, Y., Wang, J., Ma, J., Liu, P., Zhang, J., & Tang, D. (2025). Optimizing Sintering Conditions for Y2O3 Ceramics: A Study of Atmosphere-Dependent Microstructural Evolution and Optical Performance. Ceramics, 8(2), 66. https://doi.org/10.3390/ceramics8020066






