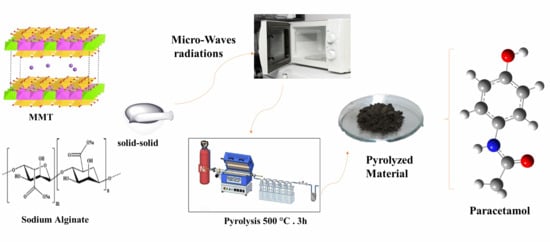
Development and Characterization of Pyrolyzed Sodium Alginate–Montmorillonite Composite for Efficient Adsorption of Emerging Pharmaceuticals: Experimental and Theoretical Insights
Abstract
1. Introduction
2. Materials and Methods
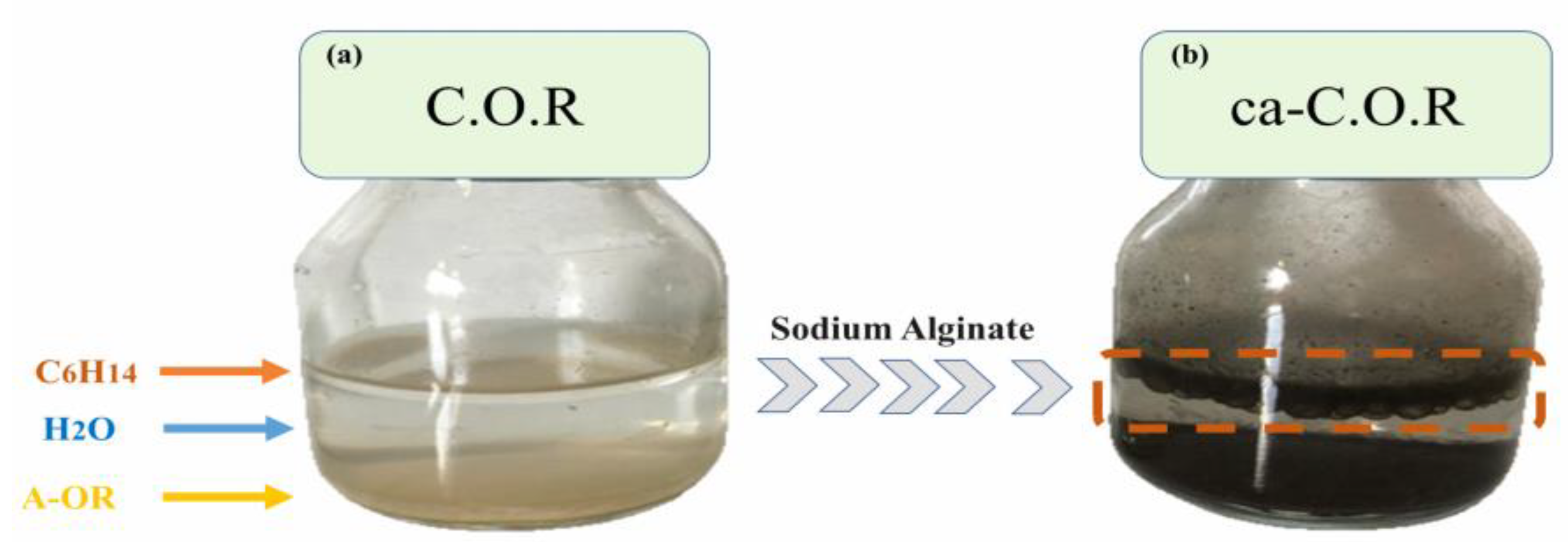
2.1. Preparation of Carbonaceous Composite
2.2. Characterization Techniques
2.3. Adsorption Studies
2.4. Analysis Data
2.5. Computational Details
2.5.1. Quantum Chemical Calculation
2.5.2. Molecular Dynamics
3. Results and Discussion
3.1. Materials Characterization
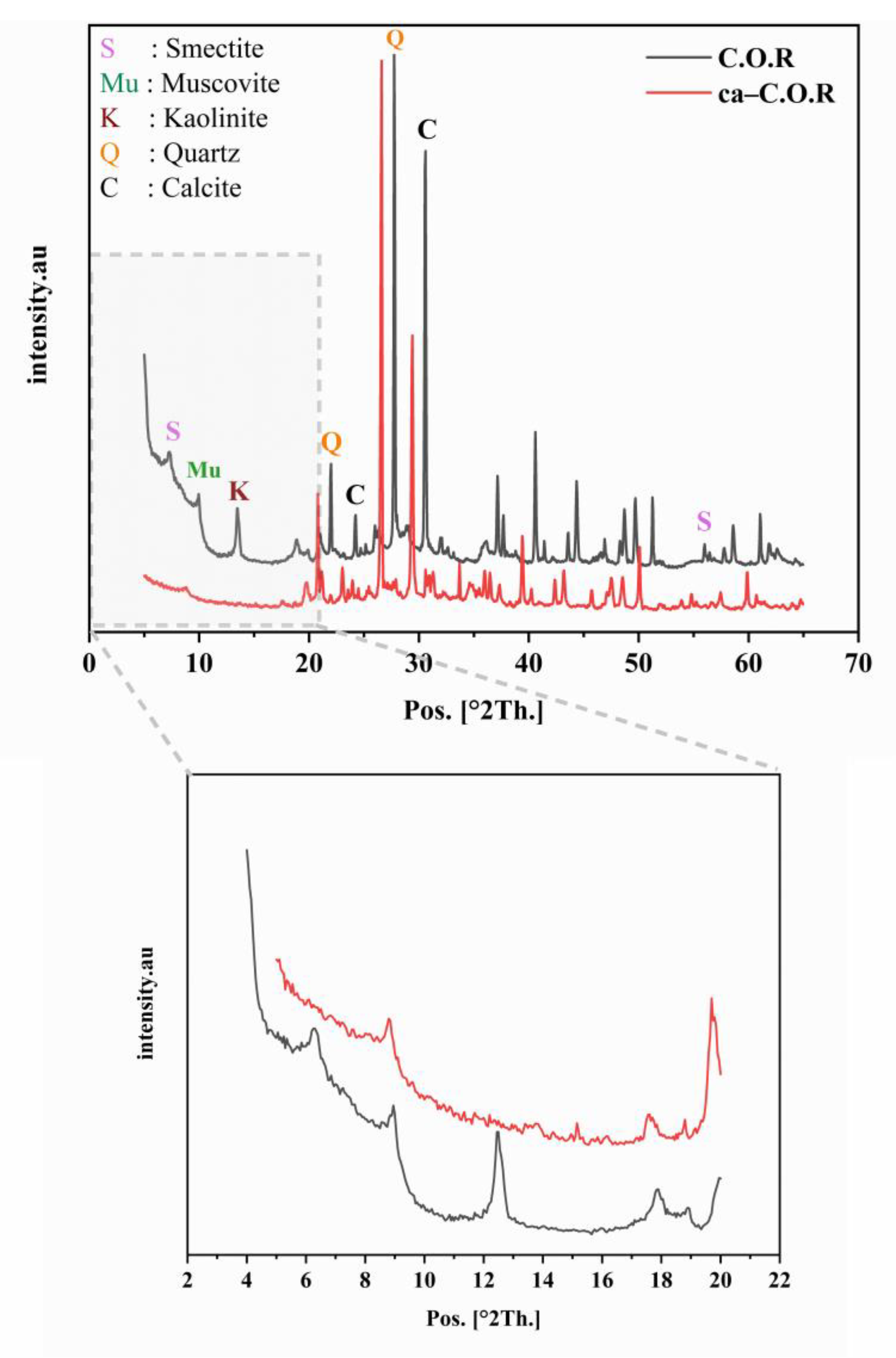
3.1.1. X-Ray Diffraction Analysis
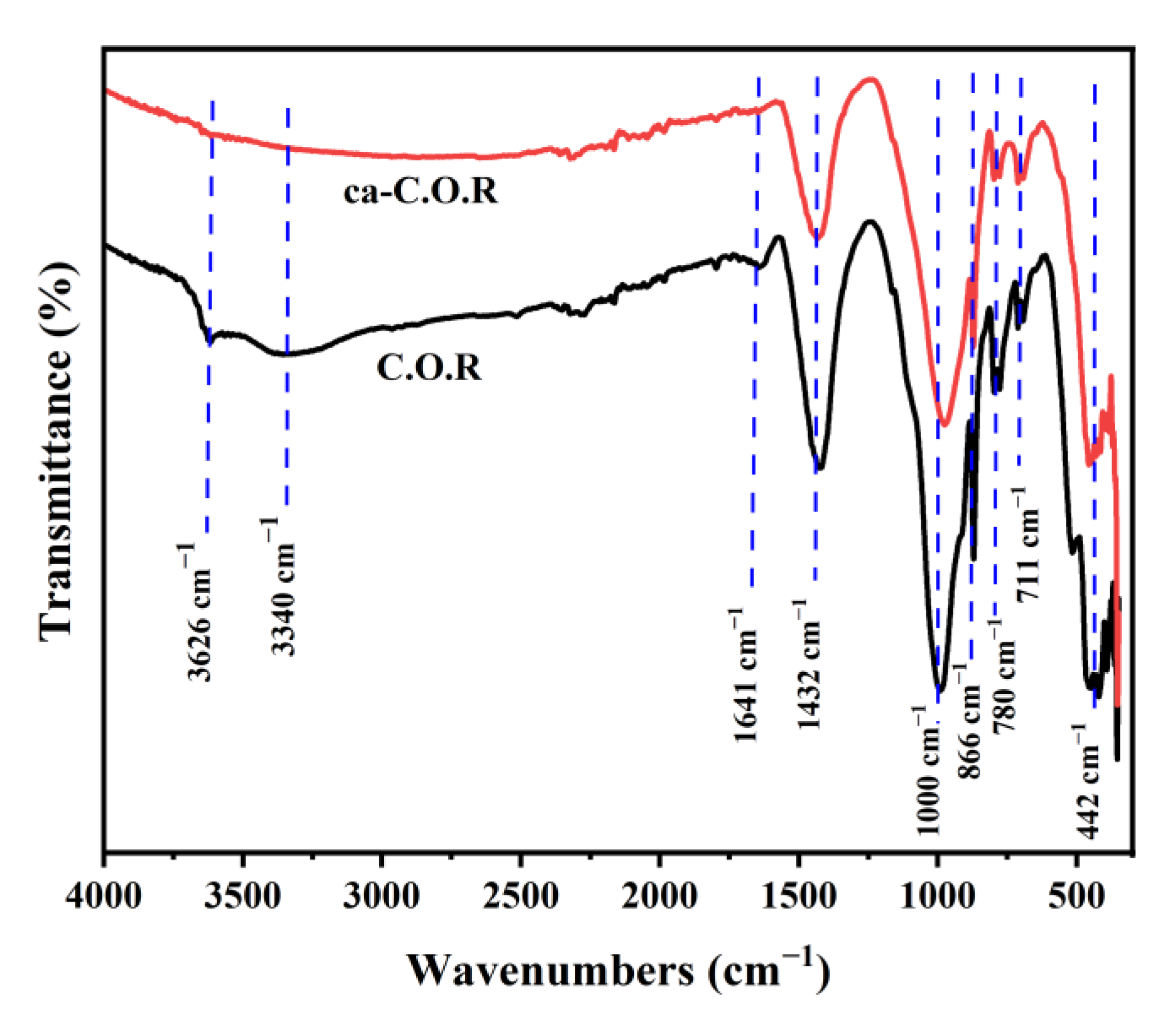
3.1.2. FTIR Analysis
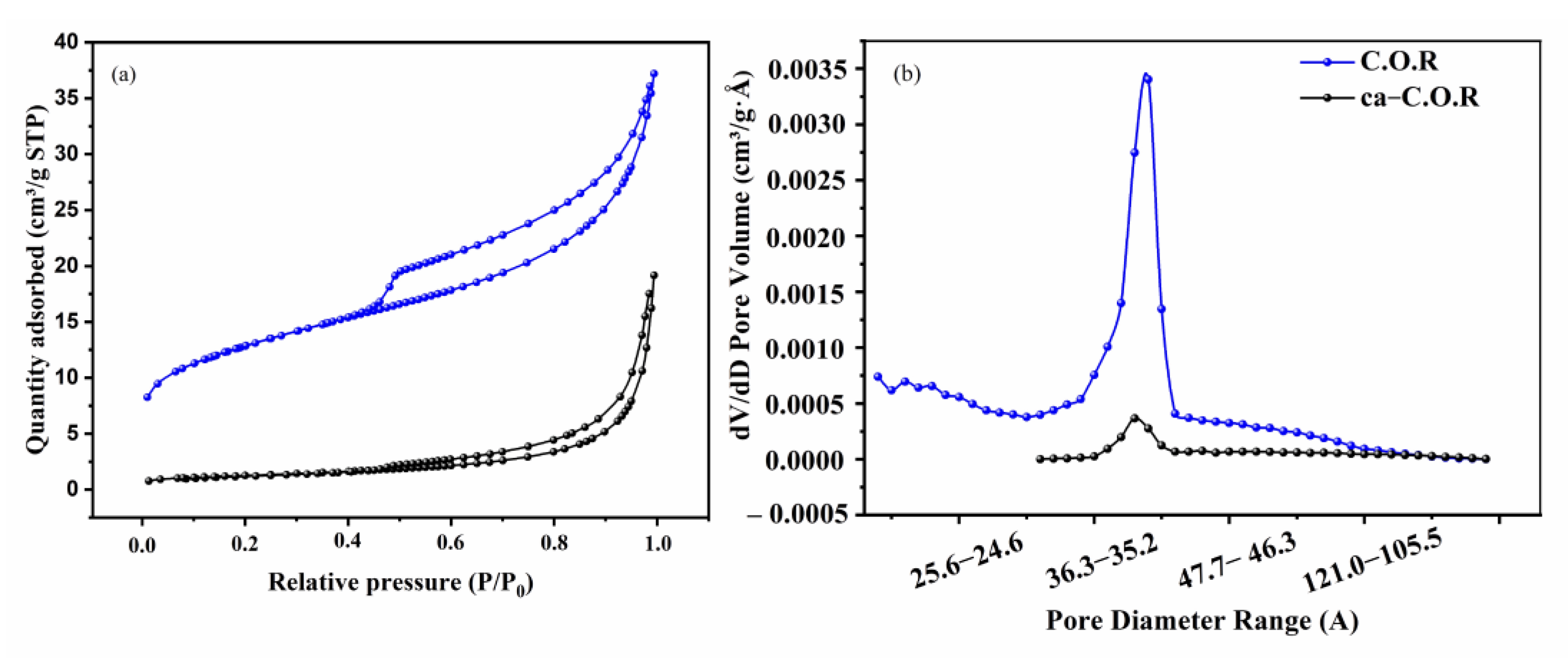
3.1.3. Nitogen Adsorption–Desorption Analysis
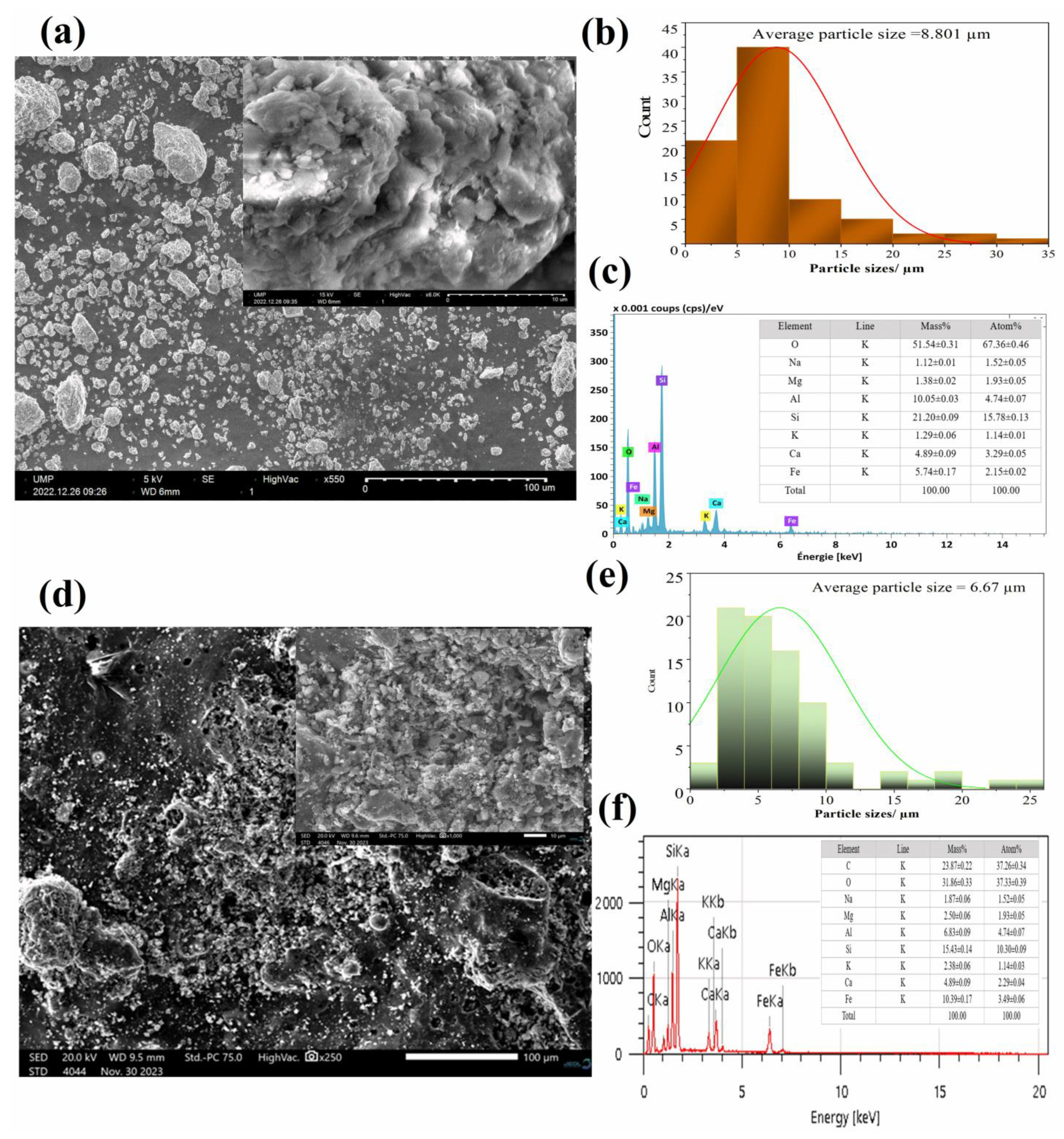
3.1.4. Surface Morphology
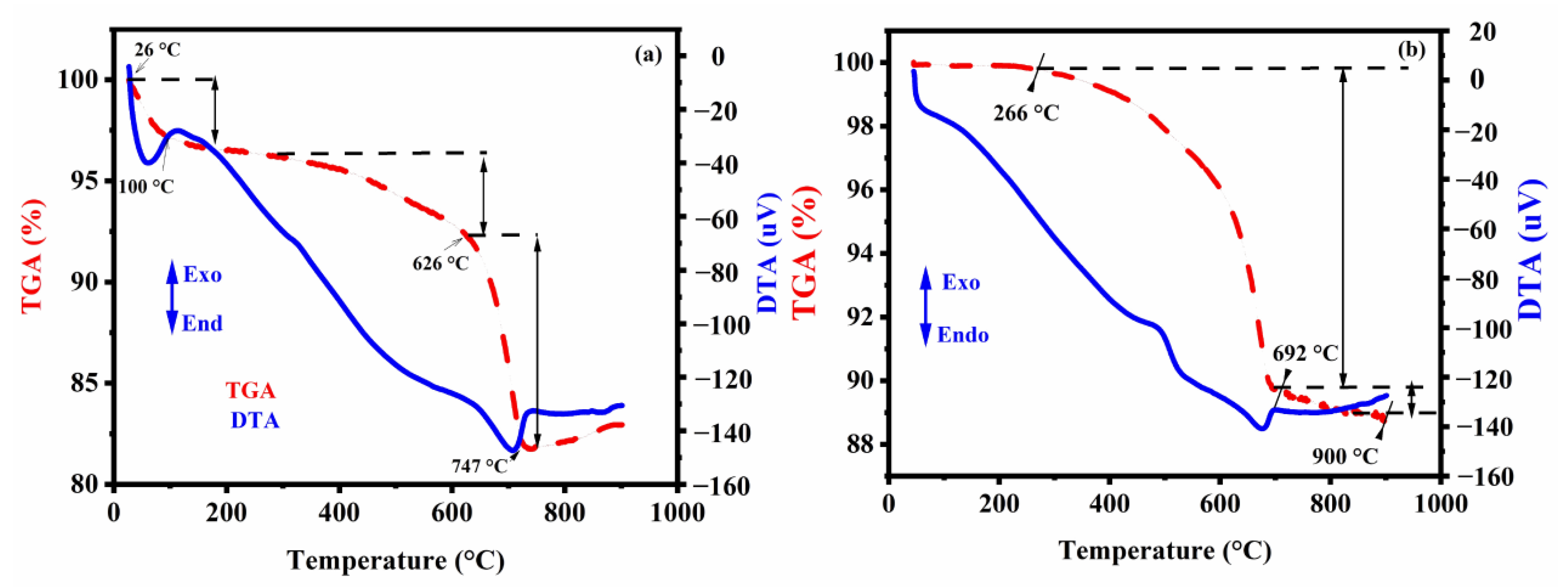
3.1.5. TGA/DTA Thermal Analysis
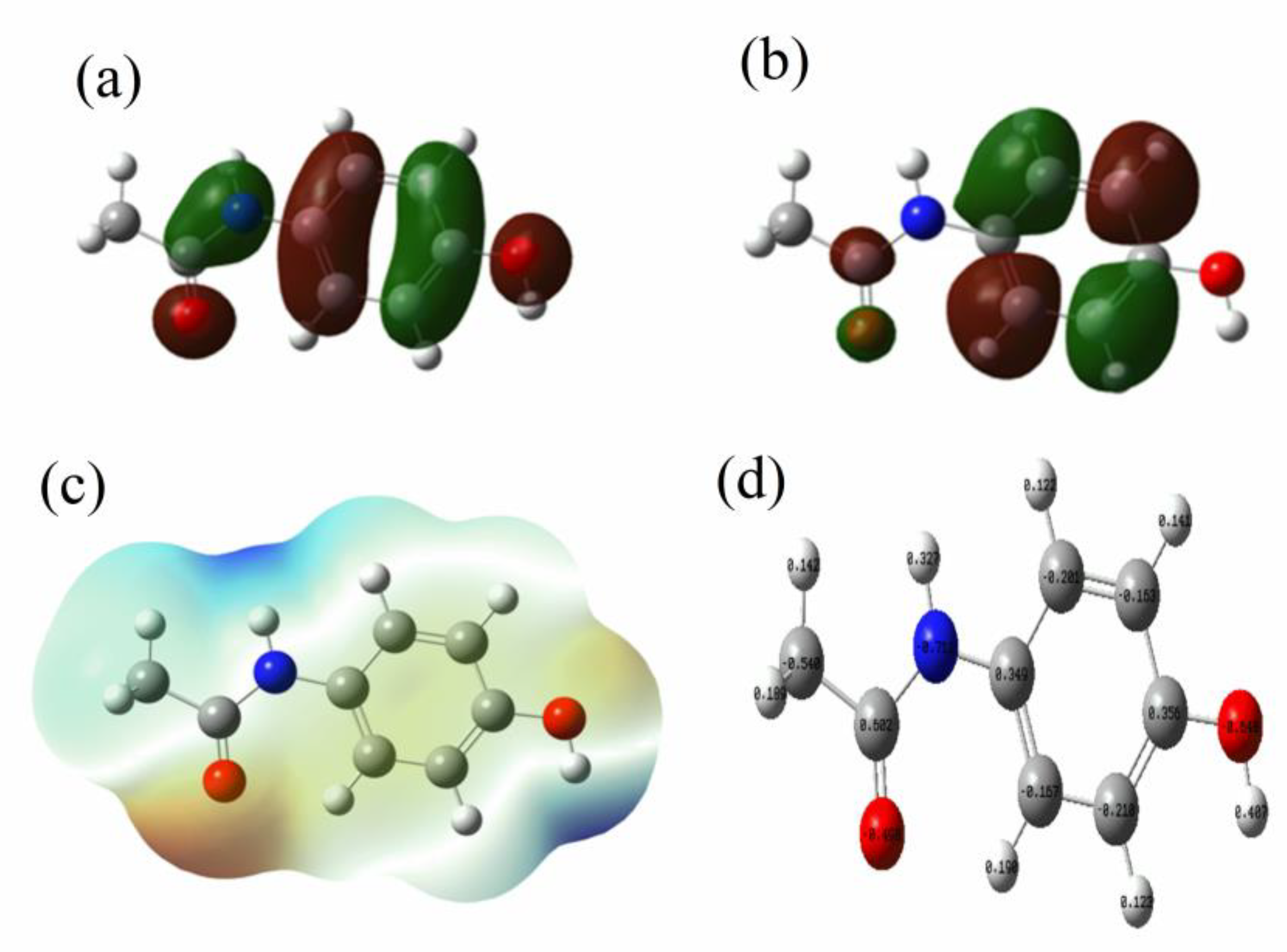
3.1.6. Density Functional Theory
3.2. Batch Study
3.2.1. Selection of the Adsorption
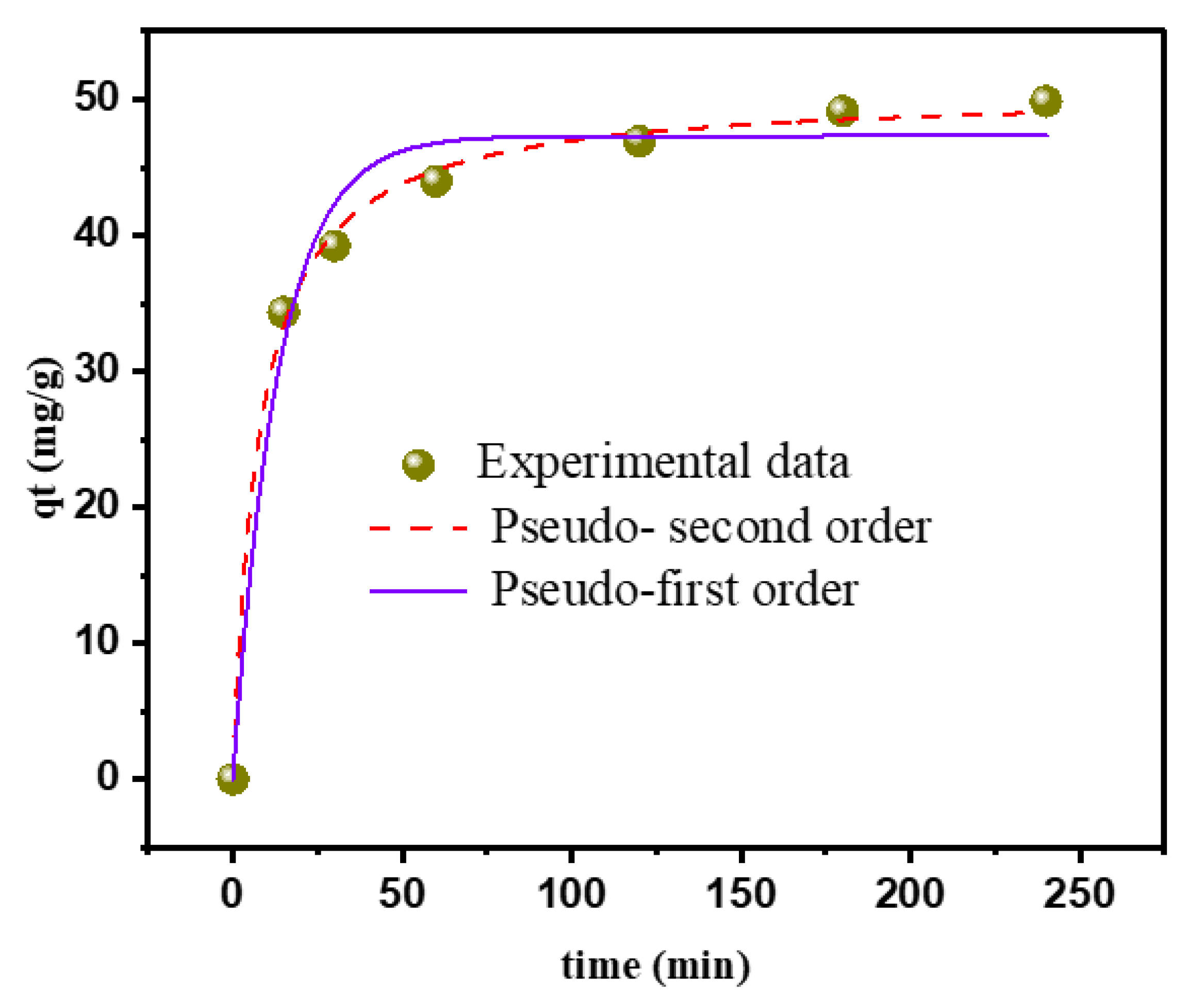
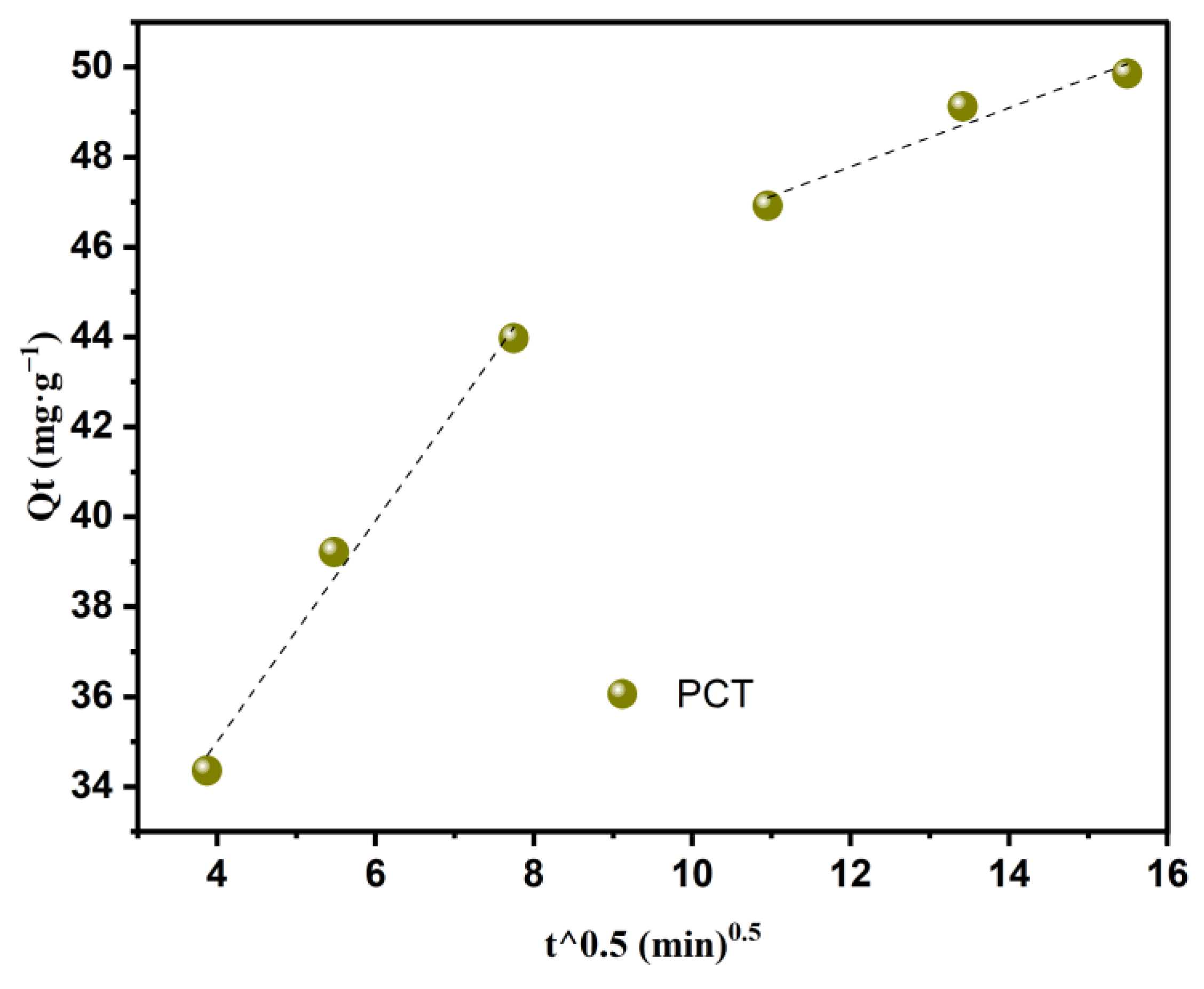
3.2.2. Kinetic Studies
3.2.3. Adsorption Isotherms
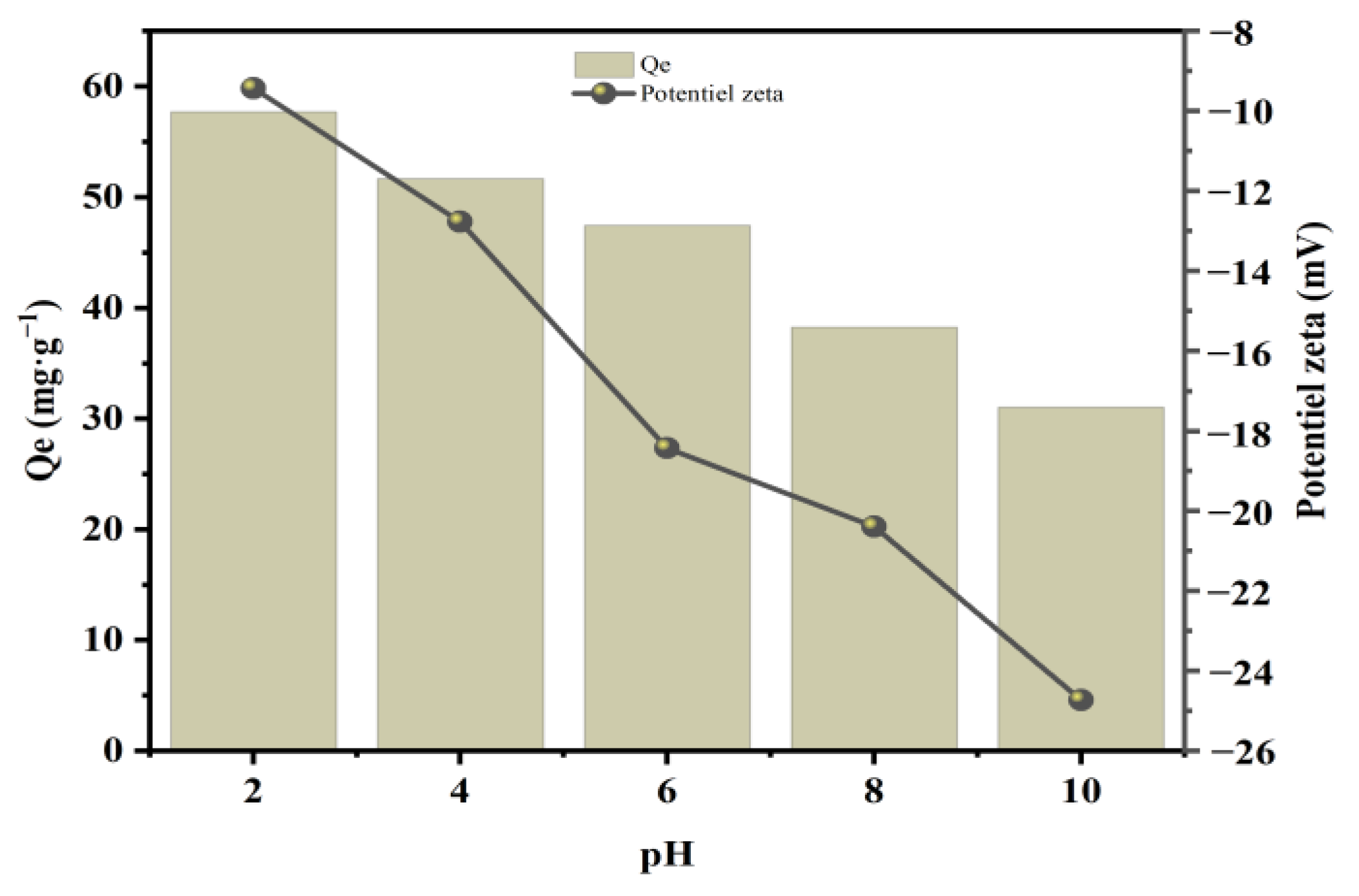
3.2.4. Effect of pH and Zeta Potential
3.2.5. Adsorption Thermodynamics
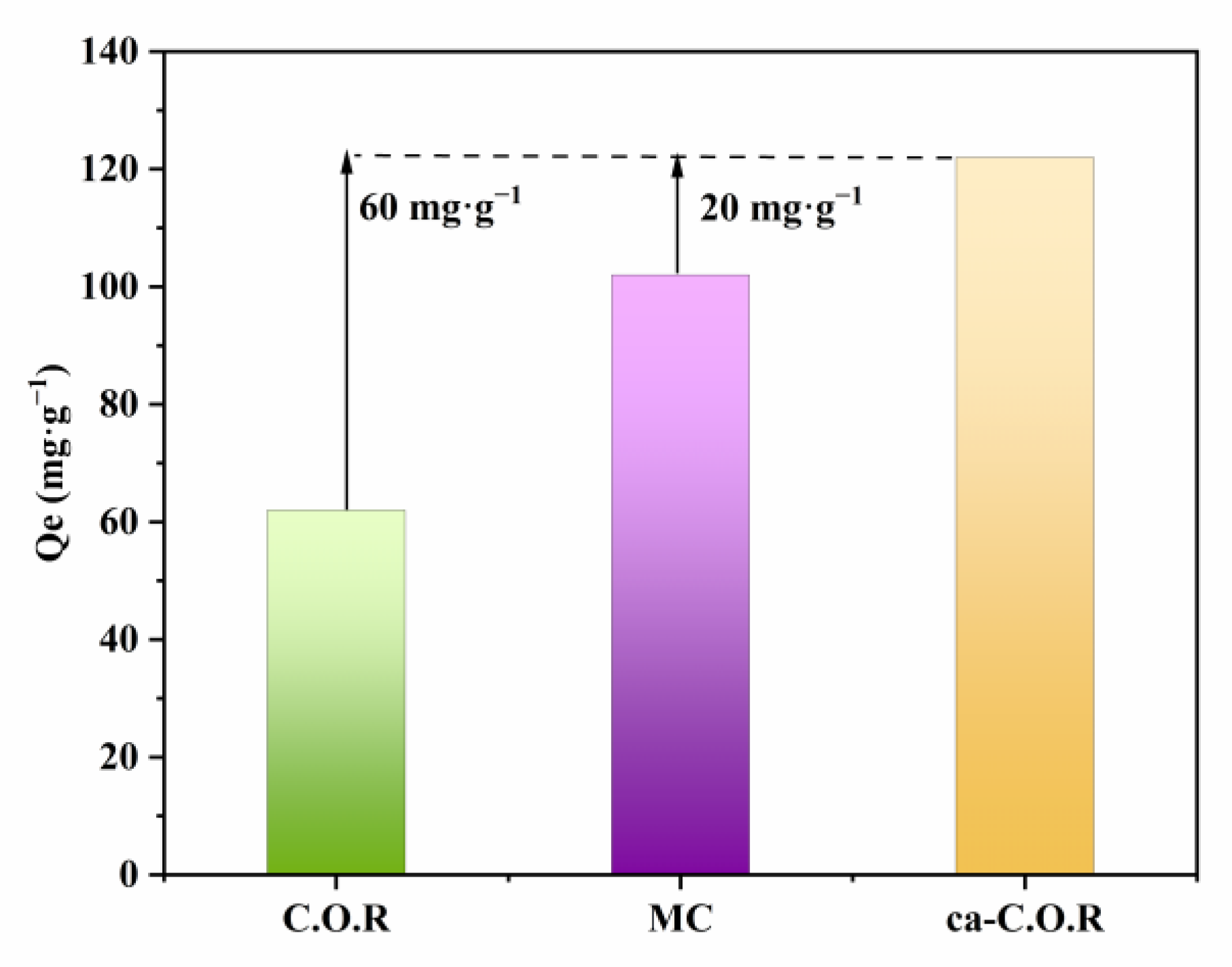
3.3. Hydrophobicity Characteristics of the Various Adsorbents
3.4. Theoretical Study
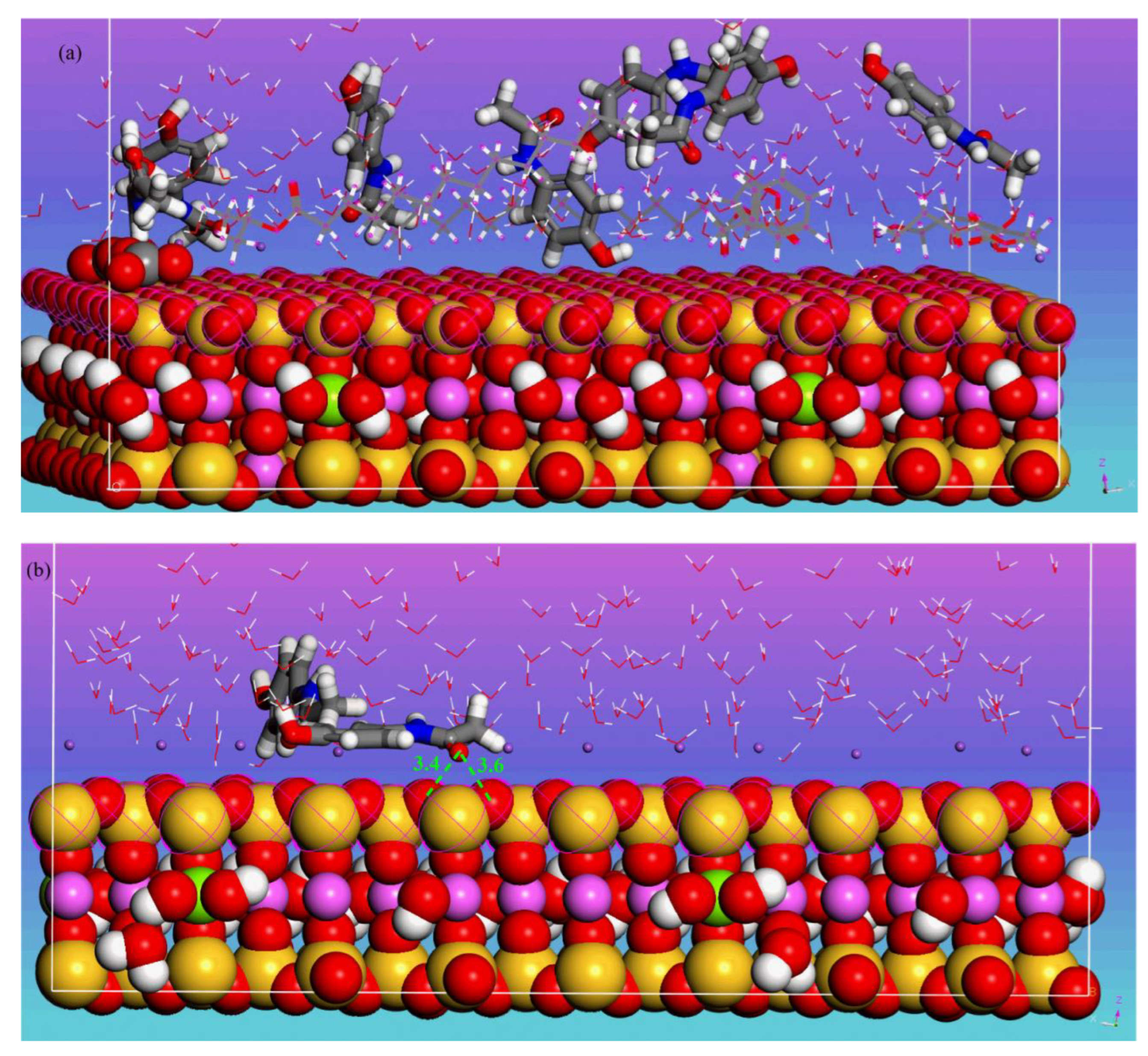
3.4.1. MDS Results
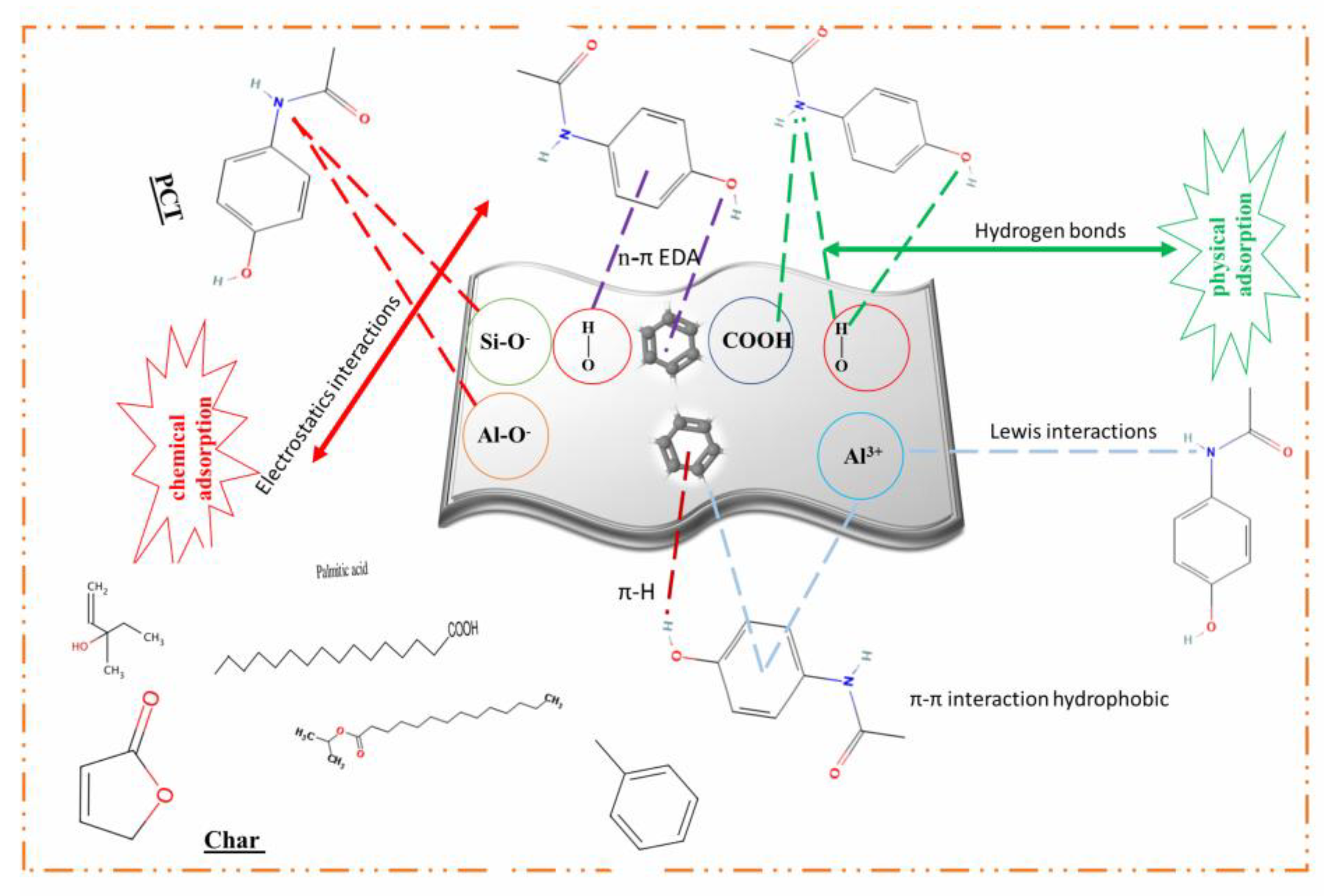
3.4.2. Mechanism Proposal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spessato, L.; Bedin, K.C.; Cazetta, A.L.; Souza, I.P.A.F.; Duarte, V.A.; Crespo, L.H.S.; Silva, M.C.; Pontes, R.M.; Almeida, V.C. KOH-Super Activated Carbon from Biomass Waste: Insights into the Paracetamol Adsorption Mechanism and Thermal Regeneration Cycles. J. Hazard. Mater. 2019, 371, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kumar, R.; Pittman, C.U.; Mohan, D. Ciprofloxacin and Acetaminophen Sorption onto Banana Peel Biochars: Environmental and Process Parameter Influences. Environ. Res. 2021, 201, 111218. [Google Scholar] [CrossRef]
- Sauvé, S.; Desrosiers, M. Una Revisión de Lo Que Es Un Contaminante Emergente. Chem. Cent. J. 2014, 8, 15. [Google Scholar] [CrossRef]
- Wells, P.G. Canadian Aquatic Science and Environmental Legislation under Threat. Mar. Pollut. Bull. 2013, 69, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chu, K.H.; Al-Hamadani, Y.A.J.; Park, C.M.; Jang, M.; Kim, D.H.; Yu, M.; Heo, J.; Yoon, Y. Removal of Contaminants of Emerging Concern by Membranes in Water and Wastewater: A Review. Chem. Eng. J. 2018, 335, 896–914. [Google Scholar] [CrossRef]
- Tran, H.N.; Tomul, F.; Thi Hoang Ha, N.; Nguyen, D.T.; Lima, E.C.; Le, G.T.; Chang, C.T.; Masindi, V.; Woo, S.H. Innovative Spherical Biochar for Pharmaceutical Removal from Water: Insight into Adsorption Mechanism. J. Hazard. Mater. 2020, 39, 122255. [Google Scholar] [CrossRef] [PubMed]
- Harnagea-Theophilus, E.; Gadd, S.L.; Knight-Trent, A.H.; DeGeorge, G.L.; Miller, M.R. Acetaminophen-Induced Proliferation of Breast Cancer Cells Involves Estrogen Receptors. Toxicol. Appl. Pharmacol. 1999, 155, 273–279. [Google Scholar] [CrossRef]
- Hinson, J.A.; Pohl, L.R.; Monks, T.J.; Gillette, J.R. Acetaminophen-Induced Hepatotoxicity. Life Sci. 1981, 29, 107–116. [Google Scholar] [CrossRef]
- Sivarajasekar, N.; Balasubramani, K.; Baskar, R.; Sivamani, S.; Ganesh Moorthy, I. Eco-Friendly Acetaminophen Sequestration Using Waste Cotton Seeds: Equilibrium, Optimization and Validation Studies. J. Water Chem. Technol. 2018, 40, 334–342. [Google Scholar] [CrossRef]
- Pan, X.-Y.; Qiao, X. chen Influences of Nitrite on Paracetamol Degradation in Dielectric Barrier Discharge Reactor. Ecotoxicol. Environ. Saf. 2019, 180, 610–615. [Google Scholar] [CrossRef]
- Ikehata, K.; Jodeiri Naghashkar, N.; Gamal El-Din, M. Degradation of Aqueous Pharmaceuticals by Ozonation and Advanced Oxidation Processes: A Review. Ozone Sci. Eng. 2006, 28, 353–414. [Google Scholar] [CrossRef]
- Yun, W.C.; Lin, K.Y.A.; Tong, W.C.; Lin, Y.F.; Du, Y. Enhanced Degradation of Paracetamol in Water Using Sulfate Radical-Based Advanced Oxidation Processes Catalyzed by 3-Dimensional Co3O4 Nanoflower. Chem. Eng. J. 2019, 373, 1329–1337. [Google Scholar] [CrossRef]
- Su, C.C.; Bellotindos, L.M.; Chang, A.T.; Lu, M.C. Degradation of Acetaminophen in an Aerated Fenton Reactor. J. Taiwan Inst. Chem. Eng. 2013, 44, 310–316. [Google Scholar] [CrossRef]
- Streit, A.F.M.; Collazzo, G.C.; Druzian, S.P.; Verdi, R.S.; Foletto, E.L.; Oliveira, L.F.S.; Dotto, G.L. Adsorption of Ibuprofen, Ketoprofen, and Paracetamol onto Activated Carbon Prepared from Effluent Treatment Plant Sludge of the Beverage Industry. Chemosphere 2021, 262, 128322. [Google Scholar] [CrossRef]
- Nadour, M.; Boukraa, F.; Benaboura, A. Removal of Diclofenac, Paracetamol and Metronidazole Using a Carbon-Polymeric Membrane. J. Environ. Chem. Eng. 2019, 7, 103080. [Google Scholar] [CrossRef]
- Lima, D.R.; Hosseini-Bandegharaei, A.; Thue, P.S.; Lima, E.C.; de Albuquerque, Y.R.T.; dos Reis, G.S.; Umpierres, C.S.; Dias, S.L.P.; Tran, H.N. Efficient Acetaminophen Removal from Water and Hospital Effluents Treatment by Activated Carbons Derived from Brazil Nutshells. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 583, 123966. [Google Scholar] [CrossRef]
- Turk Sekulic, M.; Boskovic, N.; Slavkovic, A.; Garunovic, J.; Kolakovic, S.; Pap, S. Surface Functionalised Adsorbent for Emerging Pharmaceutical Removal: Adsorption Performance and Mechanisms. Process Saf. Environ. Prot. 2019, 125, 50–63. [Google Scholar] [CrossRef]
- Wong, S.; Lim, Y.; Ngadi, N.; Mat, R.; Hassan, O.; Inuwa, I.M.; Mohamed, N.B.; Low, J.H. Removal of Acetaminophen by Activated Carbon Synthesized from Spent Tea Leaves: Equilibrium, Kinetics and Thermodynamics Studies. Powder Technol. 2018, 338, 878–886. [Google Scholar] [CrossRef]
- Sims, R.A.; Harmer, S.L.; Quinton, J.S. The Role of Physisorption and Chemisorption in the Oscillatory Adsorption of Organosilanes on Aluminium Oxide. Polymers 2019, 11, 410. [Google Scholar] [CrossRef]
- Kerkhoff, C.M.; Martinello, K.d.B.; Franco, D.S.P.; Netto, M.S.; Georgin, J.; Foletto, E.L.; Piccilli, D.G.A.; Silva, L.F.O.; Dotto, G.L. Adsorption of Ketoprofen and Paracetamol and Treatment of a Synthetic Mixture by Novel Porous Carbon Derived from Butia Capitata Endocarp. J. Mol. Liq. 2021, 339, 117184. [Google Scholar] [CrossRef]
- Li, B.; Yang, L.; Wang, C.-Q.; Zhang, Q.-P.; Liu, Q.-C.; Li, Y.-D.; Xiao, R. Adsorption of Cd(II) from Aqueous Solutions by Rape Straw Biochar Derived from Different Modification Processes. Chemosphere 2017, 175, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Martins Moreira, W.; Viotti, P.V.; Gurgel Adeodato Vieira, M.; dos Santos Gaudêncio Baptista, C.M.; Neves Olsen Scaliante, M.H.; Gimenes, M.L. Hydrothermal Synthesis of Biobased Carbonaceous Composite from a Blend of Kraft Black Liquor and Tannin and Its Application to Aspirin and Paracetamol Removal. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125597. [Google Scholar] [CrossRef]
- Rossner, A.; Snyder, S.A.; Knappe, D.R.U. Removal of Emerging Contaminants of Concern by Alternative Adsorbents. Water Res. 2009, 43, 3787–3796. [Google Scholar] [CrossRef]
- Elmourabit, M.; Zarki, Y.; Arfoy, B.; Allaoui, I.; Ait Aghzzaf, A.; Raissouni, I.; Bouchta, D.; Chaouket, F.; Draoui, K. Phosphate Sludge Valorization as New Alternative Precursor for Carbonated Hydroxyapatite Nanostructures: Synthesis and Characterization. J. Mater. Cycles Waste Manag. 2024, 26, 602–619. [Google Scholar] [CrossRef]
- Chu, Y.; Khan, M.A.; Xia, M.; Lei, W.; Wang, F.; Zhu, S. Synthesis and Mechanism of Adsorption Capacity of Modified Montmorillonite with Amino Acids for 4-Acetaminophenol Removal from Wastewaters. J. Chem. Eng. Data 2019, 64, 5900–5909. [Google Scholar] [CrossRef]
- Gamoudi, S.; Frini-Srasra, N.; Srasra, E. Influence of Synthesis Method in Preparation of HDTMA+- and HDPy+-Illites/Smectites. Appl. Clay Sci. 2015, 116–117, 78–84. [Google Scholar] [CrossRef]
- Han, H.; Rafiq, M.K.; Zhou, T.; Xu, R.; Mašek, O.; Li, X. A Critical Review of Clay-Based Composites with Enhanced Adsorption Performance for Metal and Organic Pollutants. J. Hazard. Mater. 2019, 369, 780–796. [Google Scholar] [CrossRef]
- Najafi, H.; Farajfaed, S.; Zolgharnian, S.; Mosavi Mirak, S.H.; Asasian-Kolur, N.; Sharifian, S. A Comprehensive Study on Modified-Pillared Clays as an Adsorbent in Wastewater Treatment Processes. Process Saf. Environ. Prot. 2021, 147, 8–36. [Google Scholar] [CrossRef]
- Revellame, E.D.; Lord, D.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption Kinetic Modeling Using Pseudo- Fi Rst Order and Pseudo-Second Order Rate Laws: A Review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Cataldo, S.; Muratore, N.; Orecchio, S.; Pettignano, A. Enhancement of Adsorption Ability of Calcium Alginate Gel Beads towards Pd(II) Ion. A Kinetic and Equilibrium Study on Hybrid Laponite and Montmorillonite-Alginate Gel Beads. Appl. Clay Sci. 2015, 118, 162–170. [Google Scholar] [CrossRef]
- Allaoui, I.; El Mourabit, M.; Arfoy, B.; Hadri, M.; Barhoun, A.; Draoui, K. Adsorption Equilibrium, Kinetic, and Thermodynamic Studies on the Removal of Paracetamol from Wastewater Using Natural and HDTMA-Modified Clay. Desalin. Water Treat. 2024, 318, 100345. [Google Scholar] [CrossRef]
- Fabryanty, R.; Valencia, C.; Soetaredjo, F.E.; Putro, J.N.; Santoso, S.P.; Kurniawan, A.; Ju, Y.H.; Ismadji, S. Removal of Crystal Violet Dye by Adsorption Using Bentonite—Alginate Composite. J. Environ. Chem. Eng. 2017, 5, 5677–5687. [Google Scholar] [CrossRef]
- Engineering, W.R. A Freundlich-Type Multicomponent Isotherm. J. Colloid Interface Sci. 1981, 79, 136–142. [Google Scholar]
- Batool, F.; Akbar, J.; Iqbal, S.; Noreen, S.; Nasir, S.; Bukhari, A. Study of Isothermal, Kinetic, and Thermodynamic Parameters for Adsorption of Cadmium: An Overview of Linear and Nonlinear Approach and Error Analysis. Bioinorg. Chem. Appl. 2018, 2018, 3463724. [Google Scholar] [CrossRef]
- Dinh, V.P.; Nguyen, P.T.; Tran, M.C.; Luu, A.T.; Hung, N.Q.; Luu, T.T.; Kiet, H.A.T.; Mai, X.T.; Luong, T.B.; Nguyen, T.L.; et al. HTDMA-Modified Bentonite Clay for Effective Removal of Pb(II) from Aqueous Solution. Chemosphere 2022, 286, 131766. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.; Hosseini-bandegharaei, A.; Chao, H. Mistakes and Inconsistencies Regarding Adsorption of Contaminants from Aqueous Solutions: A Critical Review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Ng, J.C.Y.; Cheung, W.H.; Mckay, G. Equilibrium Studies of the Sorption of Cu(II) Ions onto Chitosan. J. Colloid Interface Sci. 2002, 74, 64–74. [Google Scholar] [CrossRef]
- Parimal, S.; Prasad, M.; Bhaskar, U. Prediction of Equillibrium Sorption Isotherm: Comparison of Linear and Nonlinear Methods. Ind. Eng. Chem. Res. 2010, 49, 2882–2888. [Google Scholar] [CrossRef]
- Abdullahi, S.H.; Uzairu, A.; Shallangwa, G.A.; Uba, S.; Umar, A.B.; Srivastava, P.N. In-Silico Design of Some Pyrazolopyridine Analogs as Breast Cancer Inhibitors: QSAR Modeling, Molecular Docking, MD Simulation, and Pharmacological Features Profiling. J. Indian Chem. Soc. 2023, 100, 101053. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision D.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Abdulazeez, I.; Khaled, M.; Al-Saadi, A.A. Impact of Electron-Withdrawing and Electron-Donating Substituents on the Corrosion Inhibitive Properties of Benzimidazole Derivatives: A Quantum Chemical Study. J. Mol. Struct. 2019, 1196, 348–355. [Google Scholar] [CrossRef]
- Diniz, J.E.M.; Borges, R.S.; Alves, C.N. A DFT Study for Paracetamol and 3,5-Disubstituted Analogues. J. Mol. Struct. THEOCHEM 2004, 673, 93–97. [Google Scholar] [CrossRef]
- Ahmed, L.; Omer, R. Omer Computational Study on Paracetamol Drug. J. Phys. Chem. Funct. Mater. 2020, 3, 9–13. [Google Scholar]
- Ustinov, E. Kinetic Monte Carlo Approach for Molecular Modeling of Adsorption. Curr. Opin. Chem. Eng. 2019, 24, 1–11. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.S.; Zhu, P.; Zhao, J.C.; Zhang, C.J.; Guo, Y.; Cui, L. Thermal Degradation Properties of Biobased Iron Alginate Film. J. Anal. Appl. Pyrolysis 2016, 119, 87–96. [Google Scholar] [CrossRef]
- Swai, R.E. Tutorial I: Sodium Montmorillonite (Na+—Mt) Crystal Modeling Process. 2020. Available online: https://www.researchgate.net/publication/340565376_Tutorial_I_Sodium_Montmorillonite_Na_-Mt_Crystal_modeling_Process?channel=doi&linkId=5e90ce884585150839d223e5&showFulltext=true (accessed on 13 May 2025). [CrossRef]
- Achache, M.; Elouilali Idrissi, G.; Chraka, A.; Ben Seddik, N.; Draoui, K.; Bouchta, D.; Mohamed, C. Detection of Paracetamol by a Montmorillonite-Modified Carbon Paste Sensor: A Study Combining MC Simulation, DFT Computation and Electrochemical Investigations. Talanta 2024, 274, 126027. [Google Scholar] [CrossRef]
- Barrak, I.; Ayouch, I.; Kassab, Z.; El Achaby, M.; Barhoun, A.; Draoui, K. Sodium Alginate Encapsulated Moroccan Clay as Eco-Friendly and Efficient Adsorbent for Copper Ions from Aqueous Medium. Mater. Today Proc. 2021, 51, 2040–2046. [Google Scholar] [CrossRef]
- Ouachtak, H.; Guerdaoui, E.; Haouti, E. Dyes on the Eco-Friendly Adsorbent of Activated. RSC Adv. 2023, 13, 5027–5044. [Google Scholar] [CrossRef]
- Bouna, L.; El Fakir, A.A.; Benlhachemi, A.; Draoui, K.; Villain, S.; Guinneton, F. Physico-Chemical Characterization of Clays from Assa-Zag for Valorization in Cationic Dye Methylene Blue Adsorption. Mater. Today Proc. 2020, 22, 22–27. [Google Scholar] [CrossRef]
- Luo, Z.; Gao, M.; Ye, Y.; Yang, S. Modification of Reduced-Charge Montmorillonites by a Series of Gemini Surfactants: Characterization and Application in Methyl Orange Removal. Appl. Surf. Sci. 2015, 324, 807–816. [Google Scholar] [CrossRef]
- Loutou, M.; Hajjaji, M.; Mansori, M.; Favotto, C.; Hakkou, R. Phosphate Sludge: Thermal Transformation and Use as Lightweight Aggregate Material. J. Environ. Manag. 2013, 130, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Ayouch, I.; Barrak, I.; Kassab, Z.; El Achaby, M.; Barhoun, A.; Draoui, K. Improved Recovery of Cadmium from Aqueous Medium by Alginate Composite Beads Filled by Bentonite and Phosphate Washing Sludge. Colloids Surf. A Physicochem. Eng. Asp. 2020, 604, 125305. [Google Scholar] [CrossRef]
- Salerno, P.; Asenjo, M.B.; Mendioroz, S. Influence of Preparation Method on Thermal Stability and Acidity of Al-PILCs. Thermochim. Acta 2001, 379, 101–109. [Google Scholar] [CrossRef]
- Das, L.; Saha, N.; Ganguli, A.; Das, P.; Bhowal, A.; Bhattacharjee, C. Calcium Alginate–Bentonite/Activated Biochar Composite Beads for Removal of Dye and Biodegradation of Dye-Loaded Composite after Use: Synthesis, Removal, Mathematical Modeling and Biodegradation Kinetics. Environ. Technol. Innov. 2021, 24, 101955. [Google Scholar] [CrossRef]
- Largo, F.; Akbal, F.; Benlhachemi, A.; Jada, A.; Addi, A. Elaboration and Properties of a New SDS/CTAB@Montmorillonite Organoclay Compositeas a Superb Adsorbent for the Removal of Malachite Green from Aqueous Solutions. Sep. Purif. Technol. 2020, 225, 117335. [Google Scholar] [CrossRef]
- Azaryouh, L.; Abara, H.; Kassab, Z.; Ablouh, E.H.; Aboulkas, A.; El Achaby, M.; Draoui, K. Hybrid Carbonaceous Adsorbents Based on Clay and Cellulose for Cadmium Recovery from Aqueous Solution. RSC Adv. 2023, 13, 6954–6965. [Google Scholar] [CrossRef]
- Bounab, L.; Draoui, K.; Ahrouch, M.; Hadri, M.; Bouchta, D.; Barhoun, A. An Effective Functionalized Moroccan Bentonite: Application for a Green Remediation of m-Cresol. J. Mater. Environ. Sci. 2017, 8, 244–256. [Google Scholar]
- Jara, G.E.; Solis, C.A.; Gsponer, N.S.; Torres, J.J.; Glusko, C.A.; Previtali, C.M.; Pierini, A.B.; Vera, D.M.A.; Chesta, C.A.; Montejano, H.A. An Experimental and TD-DFT Theoretical Study on the Photophysical Properties of Methylene Violet Bernthsen. Dye. Pigment. 2015, 112, 341–351. [Google Scholar] [CrossRef]
- Bentahar, S.; Dbik, A.; El Khomri, M.; El Messaoudi, N.; Lacherai, A. Adsorption of Methylene Blue, Crystal Violet and Congo Red from Binary and Ternary Systems with Natural Clay: Kinetic, Isotherm, and Thermodynamic. J. Environ. Chem. Eng. 2017, 5, 5921–5932. [Google Scholar] [CrossRef]
- Kausar, A.; Sher, F.; Hazafa, A.; Javed, A.; Sillanpää, M.; Iqbal, M. International Journal of Biological Macromolecules Biocomposite of Sodium-Alginate with Acidi Fi Ed Clay for Wastewater Treatment: Kinetic, Equilibrium and Thermodynamic Studies. Int. J. Biol. Macromol. 2020, 161, 1272–1285. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Mittal, A.; Ahmad, R.; Hasan, I. Iron oxide-impregnated dextrin nanocomposite: Synthesis and its application for the biosorption of Cr(VI) ions from aqueous solution. Desalin. Water Treat. 2016, 57, 15133–15145. [Google Scholar] [CrossRef]
- Ouachtak, H.; El, R.; El, A.; Haounati, R.; Amaterz, E.; Ait, A.; Akbal, F.; Labd, M. Experimental and Molecular Dynamics Simulation Study on the Adsorption of Rhodamine B Dye on Magnetic Montmorillonite Composite γ-Fe2O3 @ Mt. J. Mol. Liq. 2020, 309, 113142. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Goscianska, J.; Ejsmont, A.; Olejnik, A.; Ludowicz, D.; Stasiłowicz, A.; Cielecka-Piontek, J. Design of Paracetamol Delivery Systems Based on Functionalized Ordered Mesoporous Carbons. Materials 2020, 13, 4151. [Google Scholar] [CrossRef]
- Popa, V.T.; Schwarz, J.A. Two-step sequences with extended temkin nonuniform reactivity—Rate-determining step revisited. Chem. Eng. Sci. 1996, 51, 3657–3664. [Google Scholar] [CrossRef]
- Saadi, R.; Saadi, Z.; Fazaeli, R.; Fard, N.E. Monolayer and Multilayer Adsorption Isotherm Models for Sorption from Aqueous Media Monolayer and Multilayer Adsorption Isotherm Models for Sorption. Korean J. Chem. Eng. 2015, 32, 787–799. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Gopal, S.S.; Sandeep, S. Applied Clay Science Synthesis and Characterization of Montmorillonite/N-(Carboxyacyl) Chitosan Coated Magnetic Particle Nanocomposites for Controlled Delivery of Paracetamol. Appl. Clay Sci. 2014, 88–89, 151–158. [Google Scholar] [CrossRef]
- Sajid, M.; Bari, S.; Saif Ur Rehman, M.; Ashfaq, M.; Guoliang, Y.; Mustafa, G. Adsorption Characteristics of Paracetamol Removal onto Activated Carbon Prepared from Cannabis Sativum Hemp. Alexandria Eng. J. 2022, 61, 7203–7212. [Google Scholar] [CrossRef]
- Chauhan, M.; Saini, V.K.; Suthar, S. Ti-Pillared Montmorillonite Clay for Adsorptive Removal of Amoxicillin, Imipramine, Diclofenac-Sodium, and Paracetamol from Water. J. Hazard. Mater. 2020, 399, 122832. [Google Scholar] [CrossRef]
- Pathania, D.; Sharma, S.; Singh, P. Removal of Methylene Blue by Adsorption onto Activated Carbon Developed from Ficus Carica Bast. Arab. J. Chem. 2017, 10, S1445–S1451. [Google Scholar] [CrossRef]
- Lung, I.; Soran, M.L.; Stegarescu, A.; Opris, O.; Gutoiu, S.; Leostean, C.; Lazar, M.D.; Kacso, I.; Silipas, T.D.; Porav, A.S. Evaluation of CNT-COOH/MnO2/Fe3O4 Nanocomposite for Ibuprofen and Paracetamol Removal from Aqueous Solutions. J. Hazard. Mater. 2021, 403, 123528. [Google Scholar] [CrossRef] [PubMed]
- González-Hourcade, M.; Simões dos Reis, G.; Grimm, A.; Dinh, V.M.; Lima, E.C.; Larsson, S.H.; Gentili, F.G. Microalgae Biomass as a Sustainable Precursor to Produce Nitrogen-Doped Biochar for Efficient Removal of Emerging Pollutants from Aqueous Media. J. Clean. Prod. 2022, 348, 131280. [Google Scholar] [CrossRef]
- Başar, C.A. Applicability of the Various Adsorption Models of Three Dyes Adsorption onto Activated Carbon Prepared Waste Apricot. J. Hazard. Mater. 2006, 135, 232–241. [Google Scholar] [CrossRef]
- Milonjić, S.K. A Consideration of the Correct Calculation of Thermodynamic Parameters of Adsorption. J. Serbian Chem. Soc. 2007, 72, 1363–1367. [Google Scholar] [CrossRef]
- Sun, C.; Wang, C. Estimation on the Intramolecular Hydrogen-Bonding Energies in Proteins and Peptides by the Analytic Potential Energy Function. J. Mol. Struct. THEOCHEM 2010, 956, 38–43. [Google Scholar] [CrossRef]
- Lima, E.C.; Gomes, A.A.; Nguyen, H. Comparison of the Nonlinear and Linear Forms of the van ’ t Hoff Equation for Calculation of Adsorption Thermodynamic Parameters (ΔS° and ΔH°). J. Mol. Liq. 2020, 311, 113315. [Google Scholar] [CrossRef]
- Nourmoradi, H.; Moghadam, K.F.; Jafari, A.; Kamarehie, B. Removal of Acetaminophen and Ibuprofen from Aqueous Solutions by Activated Carbon Derived from Quercus Brantii (Oak) Acorn as a Low-Cost Biosorbent. J. Environ. Chem. Eng. 2018, 6, 6807–6815. [Google Scholar] [CrossRef]
- Nur, H.; Faizal, A.; Abdul, N.; Wei, L.K.; Nazlan, M.; Muhid, M.; Hamdan, H. Simultaneous Adsorption of a Mixture of Paraquat and Dye by NaY Zeolite Covered with Alkylsilane. J. Hazard. Mater. 2005, 117, 35–40. [Google Scholar] [CrossRef]
- Benhouria, A.; Zaghouane-Boudiaf, H.; Bourzami, R.; Djerboua, F.; Hameed, B.H.; Boutahala, M. Cross-Linked Chitosan-Epichlorohydrin/Bentonite Composite for Reactive Orange 16 Dye Removal: Experimental Study and Molecular Dynamic Simulation. Int. J. Biol. Macromol. 2023, 242, 124786. [Google Scholar] [CrossRef]
- Liu, L.; Luo, X.; Ding, L.; Luo, S. 4—Application of Nanotechnology in the Removal of Heavy Metal From Water; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128148372. [Google Scholar]
- Abidi, N.; Duplay, J.; Errais, E.; Jada, A.; Trabelsi-ayadi, M. Discoloration of Textile Effluent by Natural Clay Improved through the Presence of Dyeing Additives. Desalination Water Treat. 2016, 3994, 27954–27968. [Google Scholar] [CrossRef]
- Spessato, L.; Cazetta, A.L.; Melo, S.; Pezoti, O.; Tami, J.; Ronix, A.; Fonseca, J.M.; Martins, A.F.; Silva, T.L.; Almeida, V.C. Synthesis of Superparamagnetic Activated Carbon for Paracetamol Removal from Aqueous Solution. J. Mol. Liq. 2020, 300, 112282. [Google Scholar] [CrossRef]
- Pauletto, P.S.; Lütke, S.F.; Dotto, G.L.; Salau, N.P.G. Adsorption Mechanisms of Single and Simultaneous Removal of Pharmaceutical Compounds onto Activated Carbon: Isotherm and Thermodynamic Modeling. J. Mol. Liq. 2021, 336, 116203. [Google Scholar] [CrossRef]


| Isothermal or Kinetic | Model Equations | Reference | |
|---|---|---|---|
| Pseudo-first-order | Qt = Qe (1 − | Equation (3) | [29] |
| Pseudo-second-order | Qt = | Equation (4) | [30] |
| Intraparticle diffusion | Equation (5) | [31] | |
| Langmuir isotherm | Qe = | Equation (6) | [32] |
| Freundlich isotherm | Qe = | Equation (7) | [33] |
| Temkin | Qe = | Equation (8) | [34] |
| Sips | Qe = | Equation (9) | [35] |
| Error functions | |||
| Chi-squared X2 | X2 = | Equation (10) | [36] |
| Residual root mean square error | RMSE = | Equation (11) | [37] |
| Coefficient of determination | R2 = 1 − | Equation (12) | [38] |
| Adjusted cofficient | R2adj = | Equation (13) | [39] |
| Sample | Surface Area m2·g−1 | Pore Volume Differential cm3·g−1·Å−1 | Size of Pores Å |
|---|---|---|---|
| C.O.R | 44.74 | 0.0034 | 42.70 |
| ca-C.O.R | 4.38 | 0.00036 | 146.01 |
| Molecule | EHOMO (eV) | ELUMO (eV) | Eg (eV) | η (eV) | X (eV) | Dipole Moment | Et (eV) |
|---|---|---|---|---|---|---|---|
| Paracetamol | −0.2013 | −0.0025 | 0.1988 | 0.0994 | 0.1019 | 2.0458 | −515 |
| Kinetic Model | Param. | ca-C.O.R |
|---|---|---|
| Pseudo-first-order | k2 (g·mg−1·min−1) | 0.075 |
| Qe,cal (mg·g−1) | 47.31 | |
| ꭓ2 | 6.64 | |
| RMSE | 2.18 | |
| R2 | 0.982 | |
| R2adj | 0.978 | |
| Pseudo-second-order | k2 (g·mg−1·min−1) | 0.002 |
| Qe,cal (mg·g−1) | 50.59 | |
| ꭓ2 | 0.82 | |
| RMSE | 0.77 | |
| R2 | 0.997 | |
| R2adj | 0.997 | |
| Intraparticle diffusion | k1 | 2.46 |
| C1 | 25.17 | |
| R2 | 0.989 | |
| R2adj | 0.977 | |
| k2 | 0.66 | |
| C2 | 39.91 | |
| R2 | 0.947 | |
| R2adj | 0.894 | |
| Qexp = 48.75 mg·g−1 |
| Isotherm Model | Parameters | ca-C.O.R |
|---|---|---|
| Langmuir | KL (L·mg−1) | 0.00003 |
| qm (mg·g−1) | 606 | |
| ꭓ2 | 3.33 | |
| RMSE | 6.41 | |
| R2 | 0.98 | |
| R2adj | 0.97 | |
| Freundlich | KF | 0.29 |
| 1/nF | 0.89 | |
| ꭓ2 | 3.73 | |
| RMSE | 7.12 | |
| R2 | 0.96 | |
| R2adj | 0.94 | |
| Temkin | BT | 64 |
| KT | 0.007 | |
| ꭓ2 | 3.08 | |
| RMSE | 5.53 | |
| R2 | 0.98 | |
| R2adj | 0.96 | |
| Sips | ks (L·mg−1) | 0.002 |
| qs (mg·g−1) | 162 | |
| ns | 1.93 | |
| ꭓ2 | 0.99 | |
| RMSE | 3.09 | |
| R2 | 0.99 | |
| R2adj | 0.99 |
| Adsorbent | Capacities (mg·g−1) | Reference |
|---|---|---|
| Activated carbon from Cannabis sativum hemp | 26.31 | [71] |
| Natural montmorillonite (Ti-PILC) | 20.83 | [72] |
| Activated carbon from Ficus carica bast | 47.62 | [73] |
| Banana peel biochar (PBC750) | 49.43 | [2] |
| CNT-COOH/MnO2/Fe3O4 composit | 80.64 | [74] |
| Porous carbon derived from Butia capitata endocar | 100.6 | [20] |
| N-doped biochar-600 | 105.6 | [75] |
| Activated carbon from waste apricot | 102.00 | [76] |
| Carbonaceous composite | 122 | This work |
| ∆G°(kJ·mol−1) | ∆H° (kJ·mol−1) | ∆S° (J·K−1·mol−1) | ||||
|---|---|---|---|---|---|---|
| 298 K | 303 K | 308 K | 313 K | |||
| ca-C.O.R | −12.159 | −12.808 | −11.510 | −11.023 | −34.805 | −75.782 |
| Structure | Etot | Eads | Erigid | Edef | dEads/dNi |
|---|---|---|---|---|---|
| MMT | −193.115 | −120.309 | −96.170 | −24.139 | −58.377 |
| ca-C.O.R | −547.346 | −292.524 | −202.291 | −90.233 | −33.118 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allaoui, I.; Et-Tanteny, R.; Barhdadi, I.; Elmourabit, M.; Arfoy, B.; Draoui, Y.; Hadri, M.; Draoui, K. Development and Characterization of Pyrolyzed Sodium Alginate–Montmorillonite Composite for Efficient Adsorption of Emerging Pharmaceuticals: Experimental and Theoretical Insights. Ceramics 2025, 8, 60. https://doi.org/10.3390/ceramics8020060
Allaoui I, Et-Tanteny R, Barhdadi I, Elmourabit M, Arfoy B, Draoui Y, Hadri M, Draoui K. Development and Characterization of Pyrolyzed Sodium Alginate–Montmorillonite Composite for Efficient Adsorption of Emerging Pharmaceuticals: Experimental and Theoretical Insights. Ceramics. 2025; 8(2):60. https://doi.org/10.3390/ceramics8020060
Chicago/Turabian StyleAllaoui, Ibrahim, Rachid Et-Tanteny, Imane Barhdadi, Mohammad Elmourabit, Brahim Arfoy, Youssef Draoui, Mohamed Hadri, and Khalid Draoui. 2025. "Development and Characterization of Pyrolyzed Sodium Alginate–Montmorillonite Composite for Efficient Adsorption of Emerging Pharmaceuticals: Experimental and Theoretical Insights" Ceramics 8, no. 2: 60. https://doi.org/10.3390/ceramics8020060
APA StyleAllaoui, I., Et-Tanteny, R., Barhdadi, I., Elmourabit, M., Arfoy, B., Draoui, Y., Hadri, M., & Draoui, K. (2025). Development and Characterization of Pyrolyzed Sodium Alginate–Montmorillonite Composite for Efficient Adsorption of Emerging Pharmaceuticals: Experimental and Theoretical Insights. Ceramics, 8(2), 60. https://doi.org/10.3390/ceramics8020060