Pseudocapacitive Behavior of Protonic Niobate Nanowires in Aqueous Acidic Electrolyte
Abstract
1. Introduction
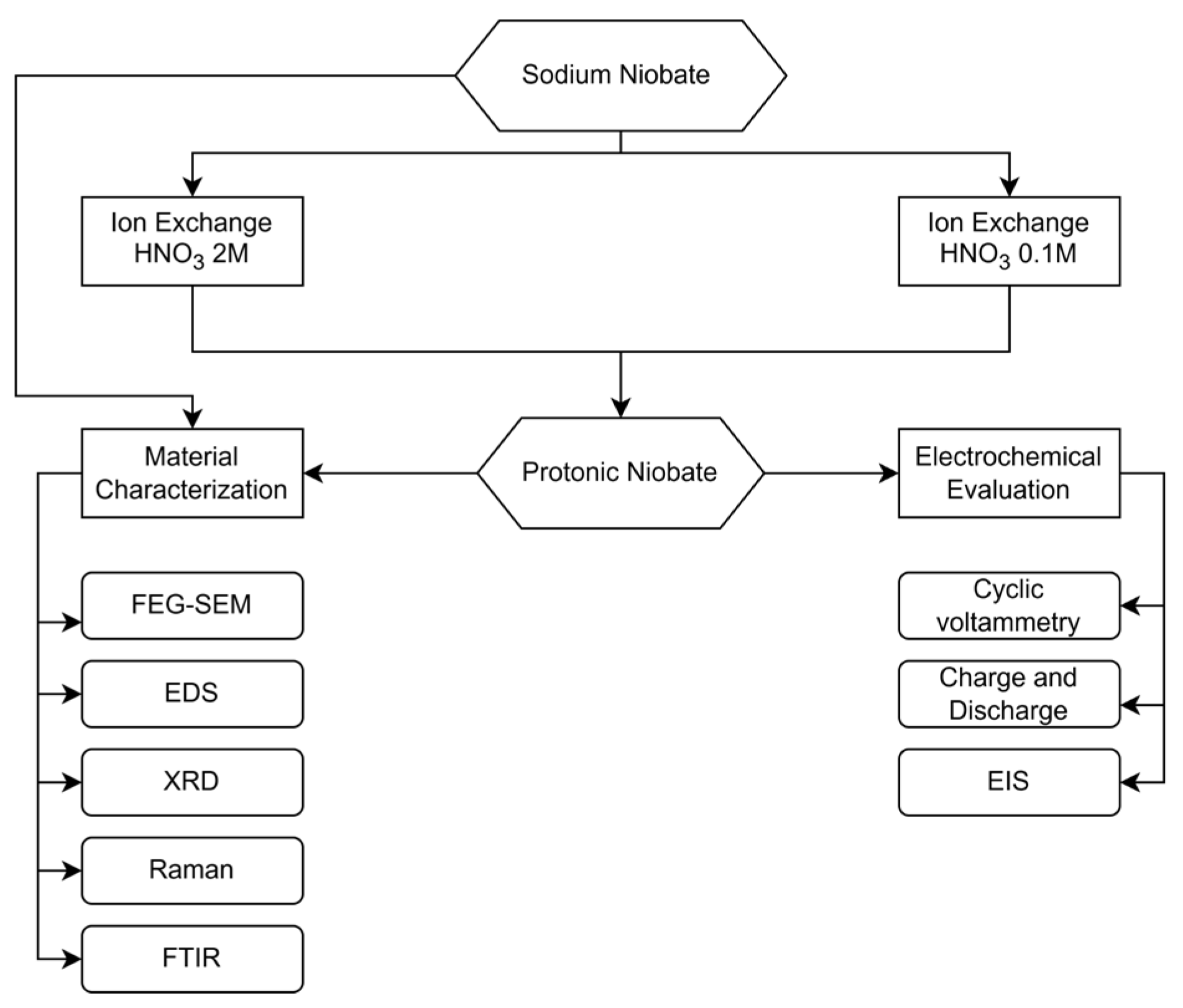
2. Materials and Methods
2.1. Synthesis
2.2. X-Ray Diffraction (XRD)
2.3. Raman Spectroscopy
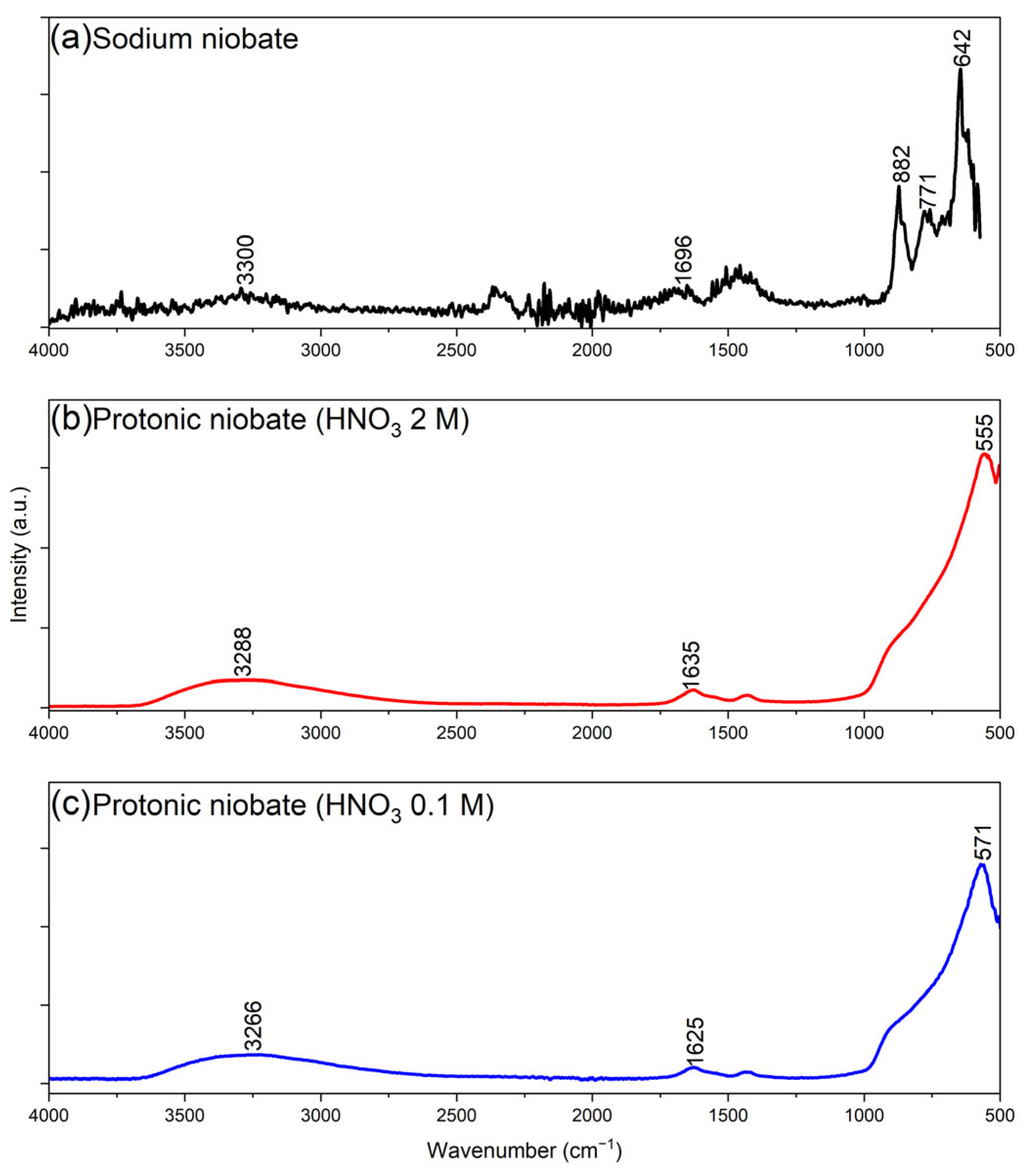
2.4. Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy (ATR-FTIR) Analysis
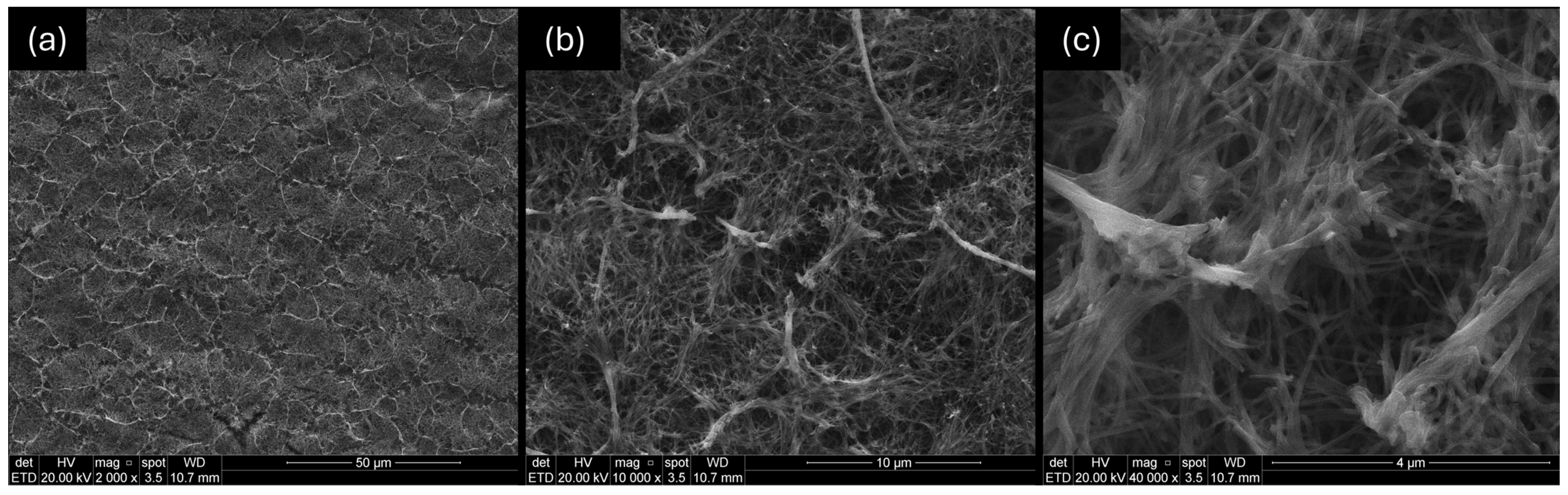
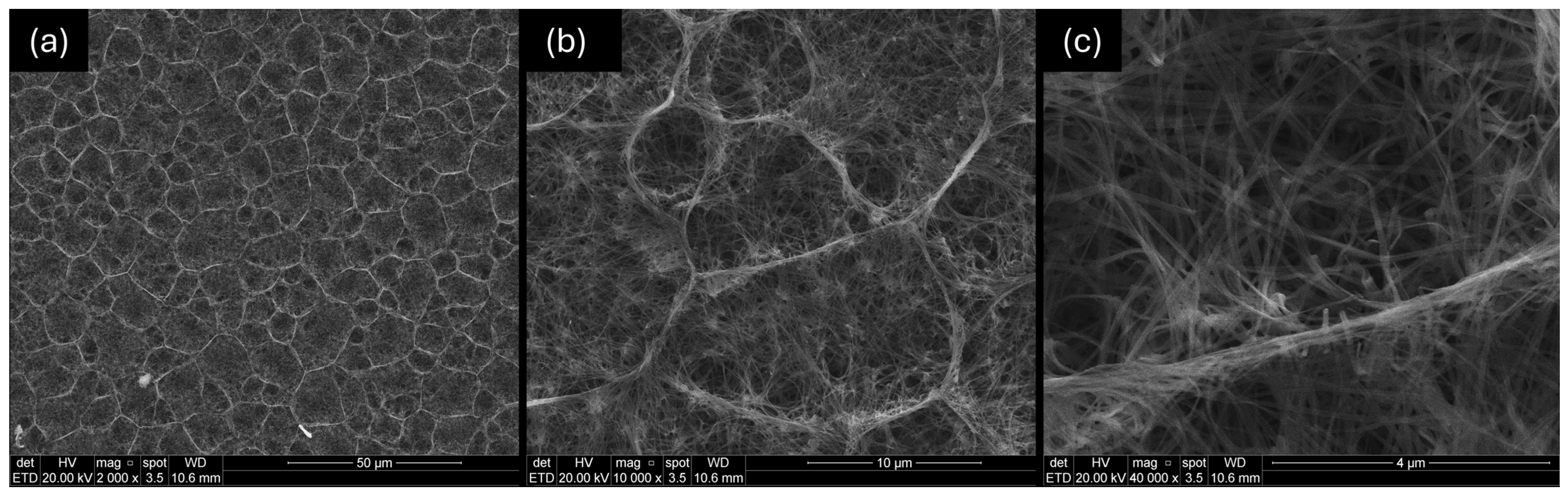
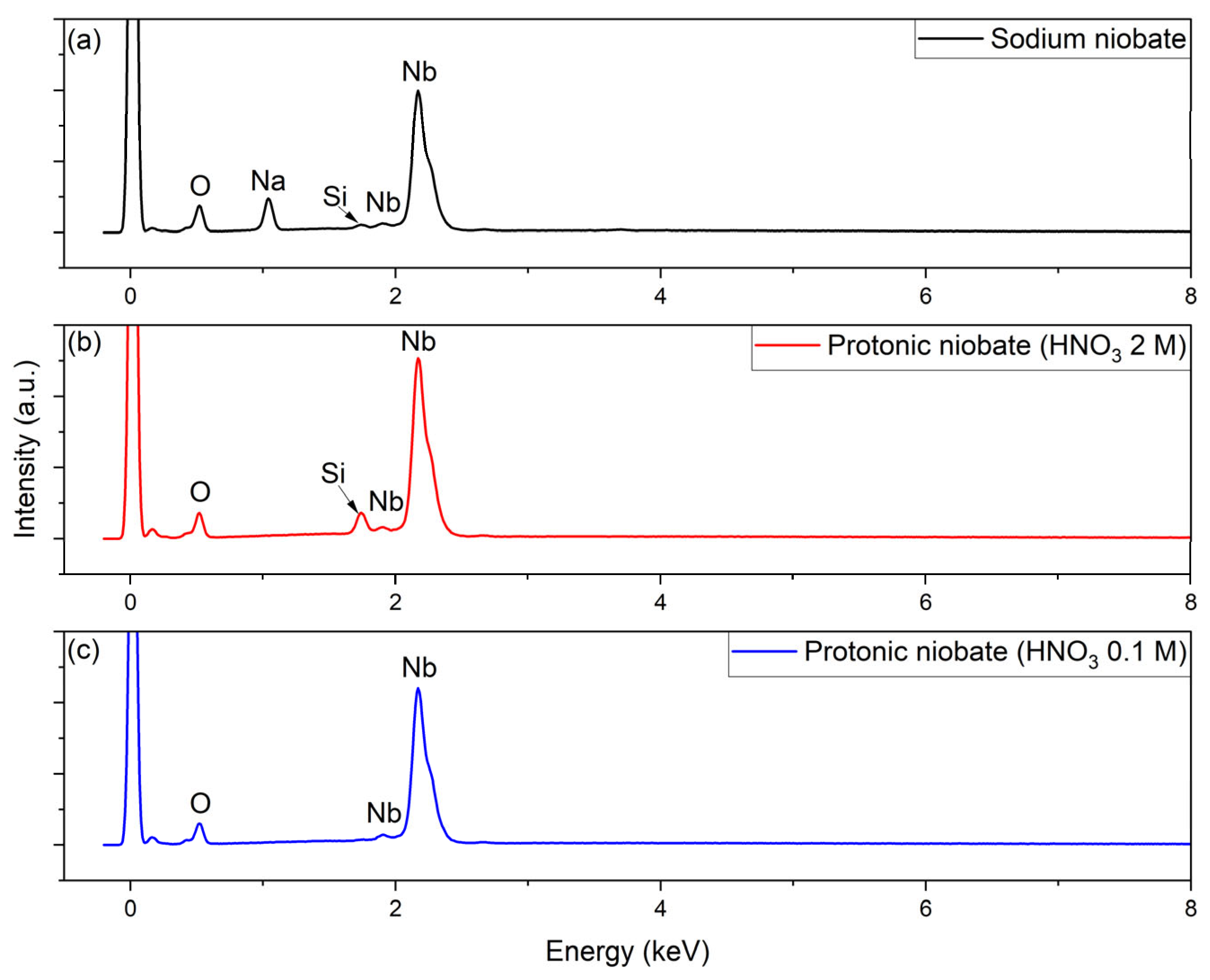
2.5. Morphological Characterization
2.6. Electrochemical Evaluation
3. Results and Discussion
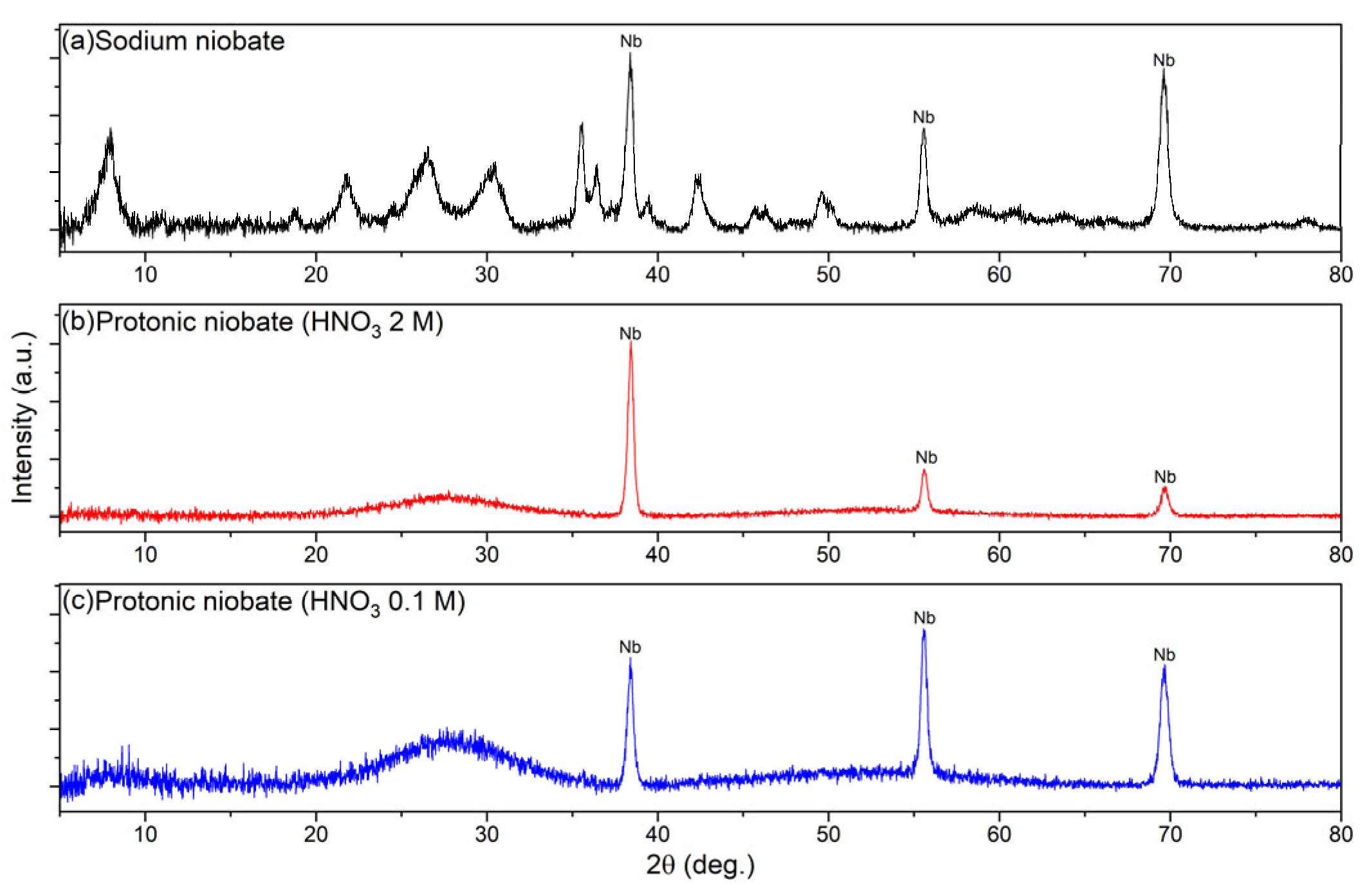
3.1. X-Ray Diffraction (XRD)
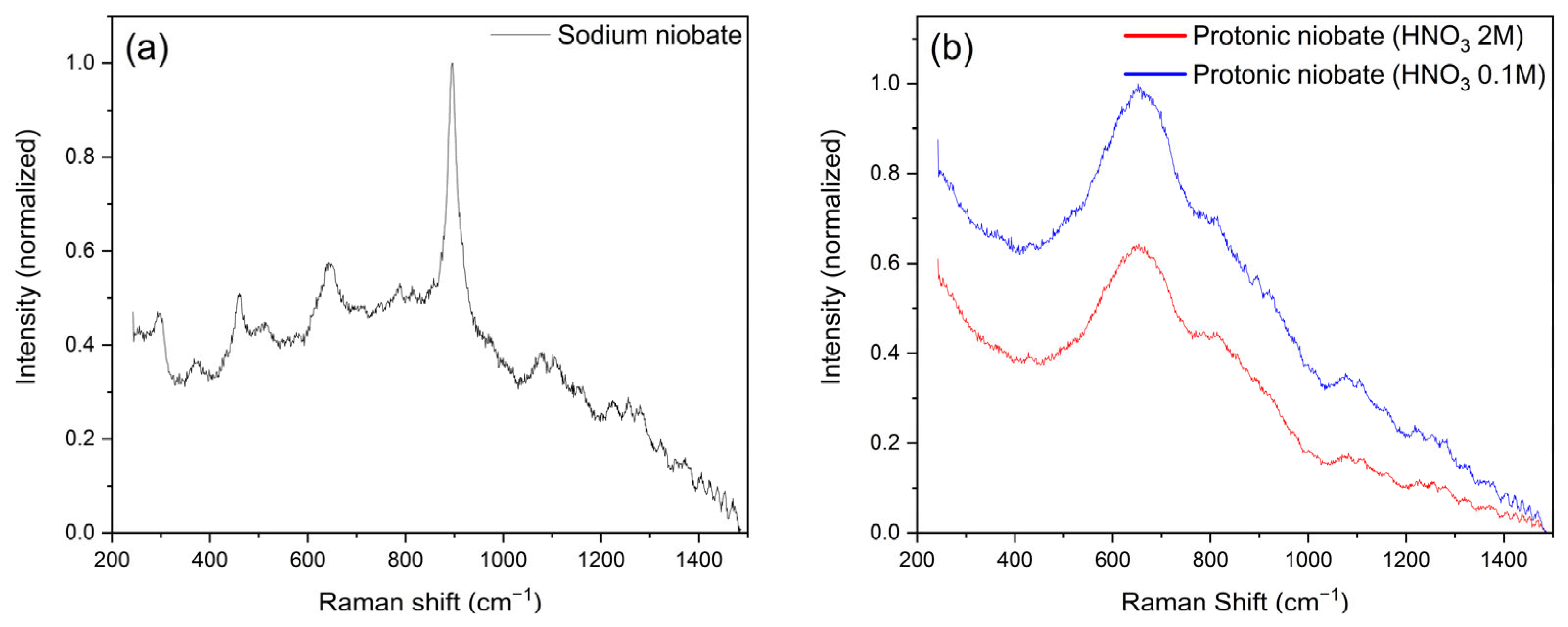
3.2. Raman Spectroscopy
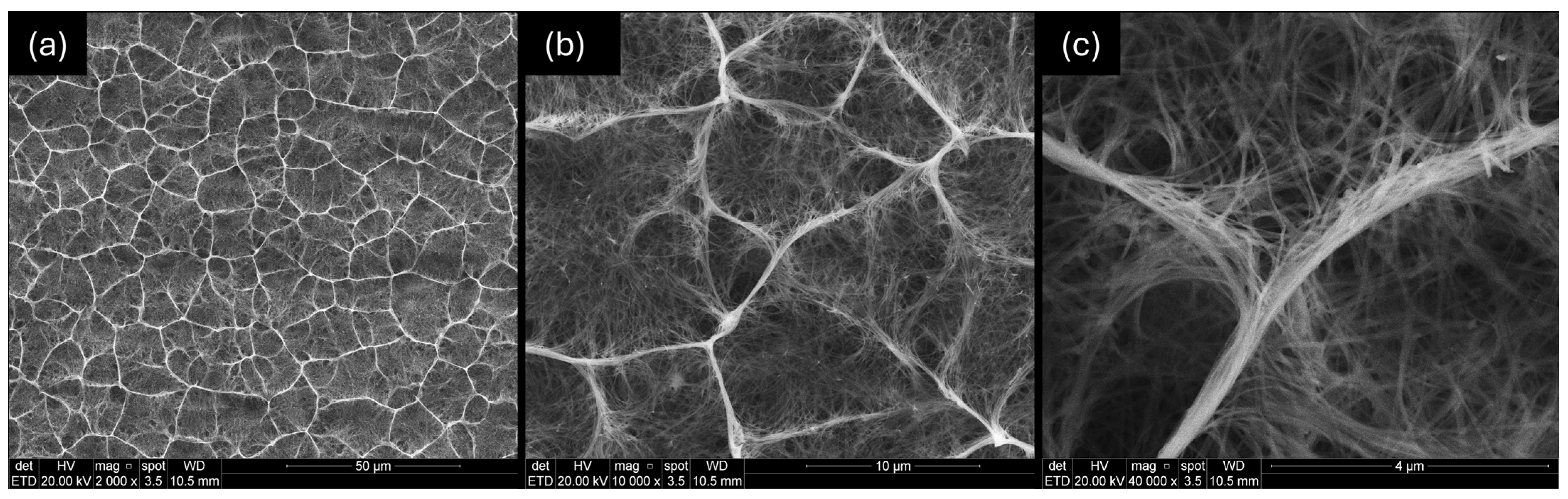
3.3. Morphological Characterization
3.4. Fourier-Transform Infrared Spectroscopy (FTIR)
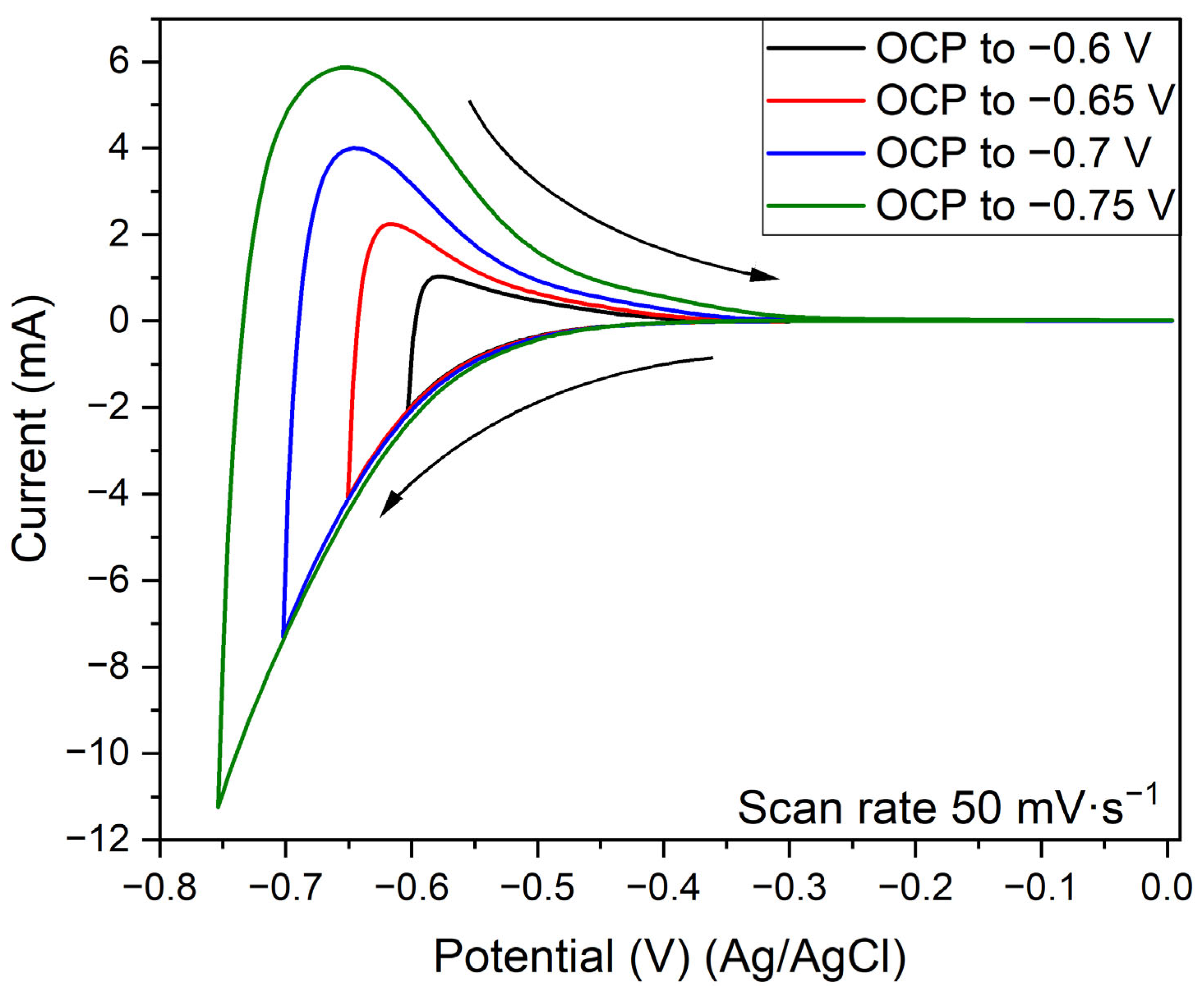
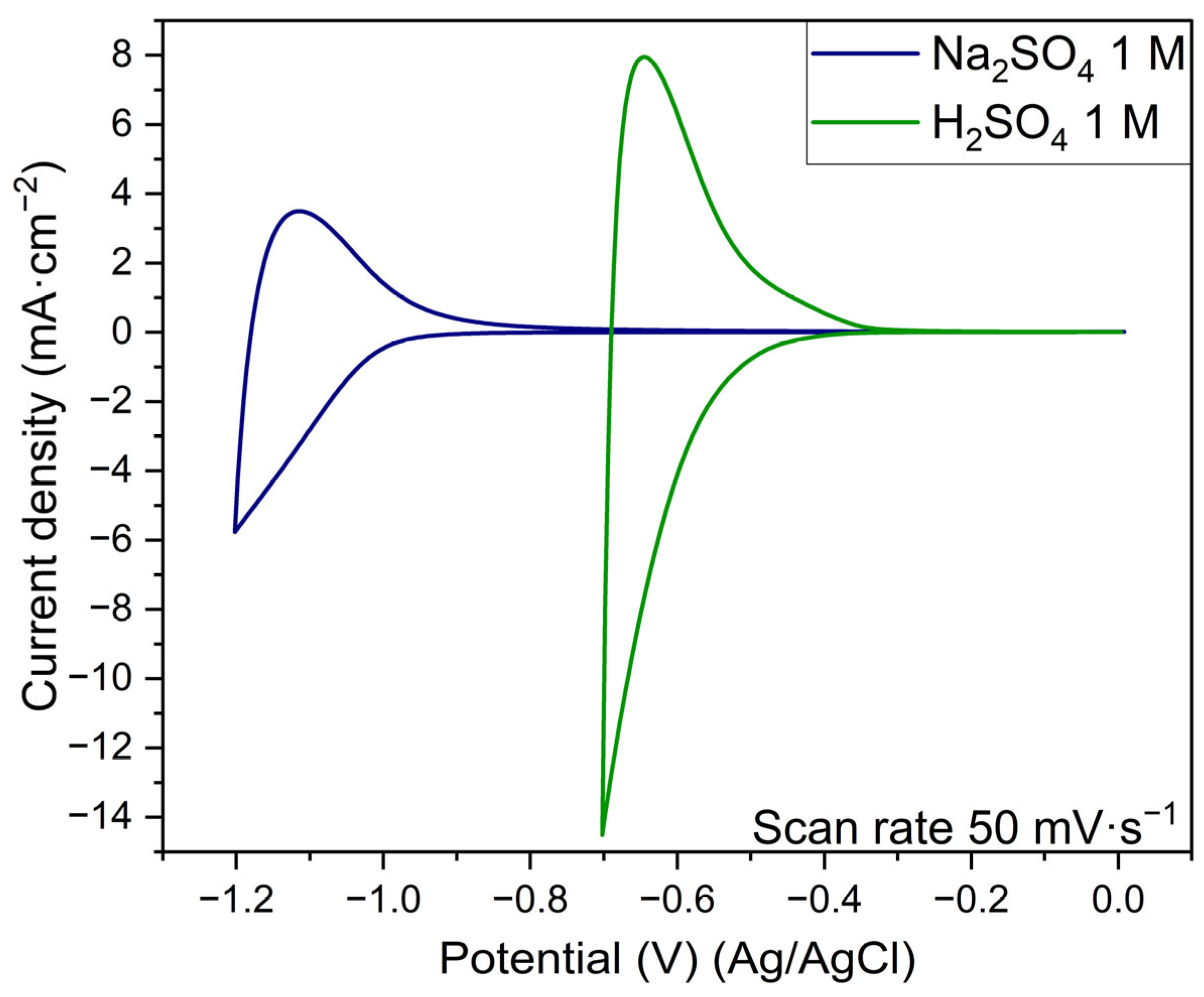
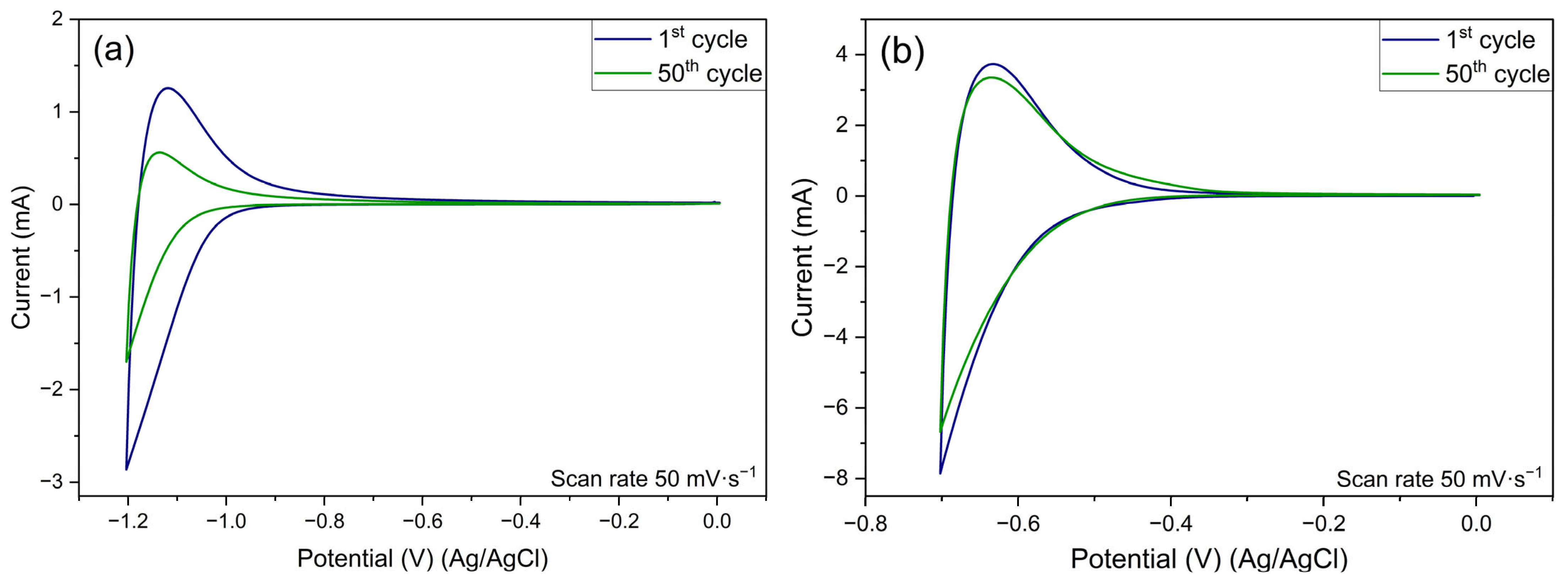
3.5. Electrochemical Evaluation
4. Conclusions
- Protonic niobates were successfully obtained by the ion exchange of Na+ for H3O+ from sodium niobate samples immersed in 2 M and 0.1 M HNO3.
- The protonic niobate obtained with 2 M HNO3 had a higher fraction of amorphous material than the protonic niobate obtained with 0.1 M HNO3.
- The resultant morphology of the ionic exchange treatment was concentration-dependent, since the treatment with 0.1 M HNO3 produced negligible changes in the oxide, whereas the treatment with 2 M HNO3 caused the partial collapse of the structure.
- The ATR-FTIR results demonstrated that the protonic niobates exhibited less-defined and lower-energy peaks, with a reduction in the vibrational modes, compared to sodium niobate. The sample obtained with 2 M HNO3 had a lower chemical bond strength compared to the sample obtained in 0.1 M HNO3.
- The protonic niobate presented a current density response that was suitable for a cathode material in both 1 M Na2SO4 and 1 M H2SO4 electrolytes.
- The results in Na2SO4 1M electrolyte did not present suitable reversibility for application as an electrochemical capacitor electrode. On the other hand, the protonic niobate presented good reversibility in the H2SO4 1 M electrolyte.
- Protonic niobate obtained with HNO3 2 M presented better specific capacitance than the sample obtained with HNO3 0.1 M. This result suggests that the structure of the protonic niobate is more important than the morphology in terms of energy storage.
- The protonic niobate obtained with 2 M HNO3 2 showed excellent reversibility of the specific capacitance up to approximately 350 cycles.
- The EIS measurements indicated that the material had to be excited to present good conductivity.
- The good energy storage of the protonic niobate can be attributed to the nanometric morphology and the structural arrangement of the material, which allow the energy to be stored by the double electrochemical layer, surface oxidation–-reduction reactions, and ion intercalation/deintercalation.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Energy Agency. Global Energy Review 2025. 2025. Available online: https://www.iea.org/reports/global-energy-review-2025 (accessed on 8 May 2025).
- Yin, T.; Guo, Y.; Huang, X.; Yang, X.; Qin, L.; Ning, T.; Tan, L.; Li, L.; Zou, K. Heteroatom Doping Strategy of Advanced Carbon for Alkali Metal-Ion Capacitors. Batteries 2025, 11, 69. [Google Scholar] [CrossRef]
- Yan, L.; Rui, X.; Chen, G.; Xu, W.; Zou, G.; Luo, H. Recent advances in nanostructured Nb-based oxides for electrochemical energy storage. Nanoscale 2016, 8, 8443–8465. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Sun, Z.; He, Q.; Sun, J.; Kaner, R.B.; Shao, Y. Niobium pentoxide based materials for high rate rechargeable electrochemical energy storage. Mater. Horiz. 2021, 8, 1130–1152. [Google Scholar] [CrossRef]
- Liu, Y.; Russo, P.A.; Montoro, L.A.; Pinna, N. Recent developments in Nb-based oxides with crystallographic shear structures as anode materials for high-rate lithium-ion energy storage. Batter. Energy 2023, 2, 20220037. [Google Scholar] [CrossRef]
- Ding, H.; Song, Z.; Zhang, H.; Li, X. Niobium-based oxide anodes toward fast and safe energy storage: A review. Mater. Today Nano 2020, 11, 100082. [Google Scholar] [CrossRef]
- Sampaio, E.J.P.; Júnior, A.G.d.S.; Araújo, C.C.; Malfatti, C.d.F. A Review on Pseudocapacitors: Advances in Nb Oxide—Sodium-Ion Systems. Adv. Mater. Sci. Technol. 2023, 5, 1. [Google Scholar] [CrossRef]
- Gomes, M.A.B.; Bulhoes, L.O.S.; DE Castro, S.C.; Damiao, A.J. ChemInform Abstract: The Electrochromic Process at Nb2O5 Electrodes Prepared by Thermal Oxidation of Niobium. ChemInform 1990, 21, 3067. [Google Scholar] [CrossRef]
- Upadhyay, K.K.; Cha, G.; Hildebrand, H.; Schmuki, P.; Silva, T.M.; Montemor, M.F.; Altomare, M. Capacitance response in an aqueous electrolyte of Nb2O5 nanochannel layers anodically grown in pure molten o-H3PO4. Electrochim. Acta 2018, 281, 725–737. [Google Scholar] [CrossRef]
- Beknalkar, S.; Teli, A.; Bhat, T.; Amate, R.; Morankar, P.; Baik, S.; Wu, Y.; Lee, J.; Kim, H.; Shin, J. A brief review on niobium oxide for supercapacitors: Unveiling fundamentals, recent breakthroughs, and promising future horizons. J. Alloys Compd. 2024, 1010, 177473. [Google Scholar] [CrossRef]
- Yi, T.-F.; Sari, H.M.K.; Li, X.; Wang, F.; Zhu, Y.-R.; Hu, J.; Zhang, J.; Li, X. A review of niobium oxides based nanocomposites for lithium-ion batteries, sodium-ion batteries and supercapacitors. Nano Energy 2021, 85, 105955. [Google Scholar] [CrossRef]
- Yuan, T.; Soule, L.; Zhao, B.; Zou, J.; Yang, J.; Liu, M.; Zheng, S. Recent Advances in Titanium Niobium Oxide Anodes for High-Power Lithium-Ion Batteries. Energy Fuels 2020, 34, 13321–13334. [Google Scholar] [CrossRef]
- Hu, L.; Luo, L.; Tang, L.; Lin, C.; Li, R.; Chen, Y. Ti2Nb2xO4+5x anode materials for lithium-ion batteries: A comprehensive review. J. Mater. Chem. A 2018, 6, 9799–9815. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, G.; Qiao, W.; Wang, J.; Ling, L. Oxygen vacancies enhance the lithium ion intercalation pseudocapacitive properties of orthorhombic niobium pentoxide. J. Colloid Interface Sci. 2020, 562, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Augustyn, V.; Dunn, B. The Effect of Crystallinity on the Rapid Pseudocapacitive Response of Nb2O5. Adv. Energy Mater. 2012, 2, 141–148. [Google Scholar] [CrossRef]
- Fic, K.; Lota, G.; Meller, M.; Frackowiak, E. Novel insight into neutral medium as electrolyte for high-voltage supercapacitors. Energy Environ. Sci. 2012, 5, 5842–5850. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]
- Júnior, A.G.d.S.; Antonini, L.M.; Sampaio, E.J.P.; de Andrade, A.M.H.; Aguzzoli, C.; Malfatti, C.d.F. Sodium niobates and protonic niobates nanowires obtained from hydrothermal synthesis: Electrochemical behavior in aqueous electrolyte. Ceram. Int. 2022, 48, 1522–1531. [Google Scholar] [CrossRef]
- Sampaio, E.J.P.; Antonini, L.M.; Júnior, A.G.d.S.; de Andrade, A.M.H.; Malfatti, C.d.F.; Hubler, R.; Aguzzoli, C. Highly ordered nanotubular niobium oxide obtained by self-organizing anodization: A study of capacitive behavior. Ceram. Int. 2022, 48, 25424–25430. [Google Scholar] [CrossRef]
- Sharma, A.; Bhardwaj, U.; Jain, D.; Kushwaha, H.S. NaNbO3 Nanorods: Photopiezocatalysts for Elevated Bacterial Disinfection and Wastewater Treatment. ACS Omega 2022, 7, 7595–7605. [Google Scholar] [CrossRef]
- Haring, M.M.M.; McDonald, A.M. Franconite, NaNb2O5(OH)·3H2O: Structure determination and the role of H bonding, with comments on the crystal chemistry of franconite-related minerals. Miner. Mag. 2014, 78, 591–607. [Google Scholar] [CrossRef]
- Yang, J.-L.; Chen, H.-Y.; Wang, Z. Synthesis of sodium-potassium niobate (K, Na)NbO3 lead-free piezoelectric powders using solvothermal and hydrothermal processing. In Proceedings of the 2010 Symposium on Piezoelectricity, Acoustic Waves, and Device Applications (SPAWDA 2010), Xiamen, China, 10–13 December 2010; pp. 249–253. [Google Scholar]
- Da, L.; Zhu, J.; He, J.; Hu, L.; Zhang, P.; Li, Q.; Zhou, P. Construction and Photocatalytic Properties of TiO2@HNb3O8-NS. J. Mol. Struct. 2020, 1212, 128126. [Google Scholar] [CrossRef]
- Mannino, G.; Condorelli, M.; Compagnini, G.; Faraci, G. Raman amplification for trapped radiation in crystalline single Si nanoparticle. Sci. Rep. 2023, 13, 1014. [Google Scholar] [CrossRef] [PubMed]
- Colomban, P.; Slodczyk, A. Raman Intensity: An Important Tool in the Study οf Nanomaterials and Nanostructures. Acta Phys. Pol. A 2009, 116, 7–12. [Google Scholar] [CrossRef]
- Júnior, A.G.d.S.; Gotardi, J.; Pilger, D.A.; Gnoatto, S.C.B.; Hubler, R.; Pitthan, E.; Etcheverry, L.P.; Bernardi, F.; Malfatti, C.d.F. Free-standing sodium niobate nanowires film: Morphological and chemical surface characterization. Surf. Interfaces 2024, 54, 105223. [Google Scholar] [CrossRef]
- Wang, S.-L.; Wang, P.-C. In situ XRD and ATR-FTIR study on the molecular orientation of interlayer nitrate in Mg/Al-layered double hydroxides in water. Colloids Surf. A 2007, 292, 131–138. [Google Scholar] [CrossRef]
- Xingfu, Z.; Zhaolin, H.; Yiqun, F.; Su, C.; Weiping, D.; Nanping, X. Microspheric Organization of Multilayered ZnO Nanosheets with Hierarchically Porous Structures. J. Phys. Chem. C 2008, 112, 11722–11728. [Google Scholar] [CrossRef]
- Orel, B.; Maček, M.; Grdadolnik, J.; Meden, A. In situ UV-Vis and ex situ IR spectroelectrochemical investigations of amorphous and crystalline electrochromic Nb2O5 films in charged/discharged states. J. Solid State Electrochem. 1998, 2, 221–236. [Google Scholar] [CrossRef]
- Hossain, S.; Ahmed, S. FTIR spectrum analysis to predict the crystalline and amorphous phases of hydroxyapatite: A comparison of vibrational motion to reflection. RSC Adv. 2023, 13, 14625–14630. [Google Scholar] [CrossRef]
- Tossici, R.; Marassi, R.; Berrettoni, M.; Stizza, S.; Pistoia, G. Study of amorphous and crystalline Li1+xV3O8 by FTIR, XAS and electrochemical techniques. Solid State Ion. 1992, 57, 227–234. [Google Scholar] [CrossRef]
- Shaner, S.E.; Stone, K.L. Determination of Stretching Frequencies by Isotopic Substitution Using Infrared Spectroscopy: An Upper-Level Undergraduate Experiment for an In-Person or Online Laboratory. J. Chem. Educ. 2023, 100, 2347–2352. [Google Scholar] [CrossRef]
- Nedjar, R.; Borel, M.; Raveau, B. H3ONb3O8 and HNb3O8: New protonic oxides with a layer structure involving ion exchange properties. Mater. Res. Bull. 1985, 20, 1291–1296. [Google Scholar] [CrossRef]
- Gao, B.; Xiao, X.; Su, J.; Zhang, X.; Peng, X.; Fu, J.; Chu, P.K. Synthesis of mesoporous niobium nitride nanobelt arrays and their capacitive properties. Appl. Surf. Sci. 2016, 383, 57–63. [Google Scholar] [CrossRef]


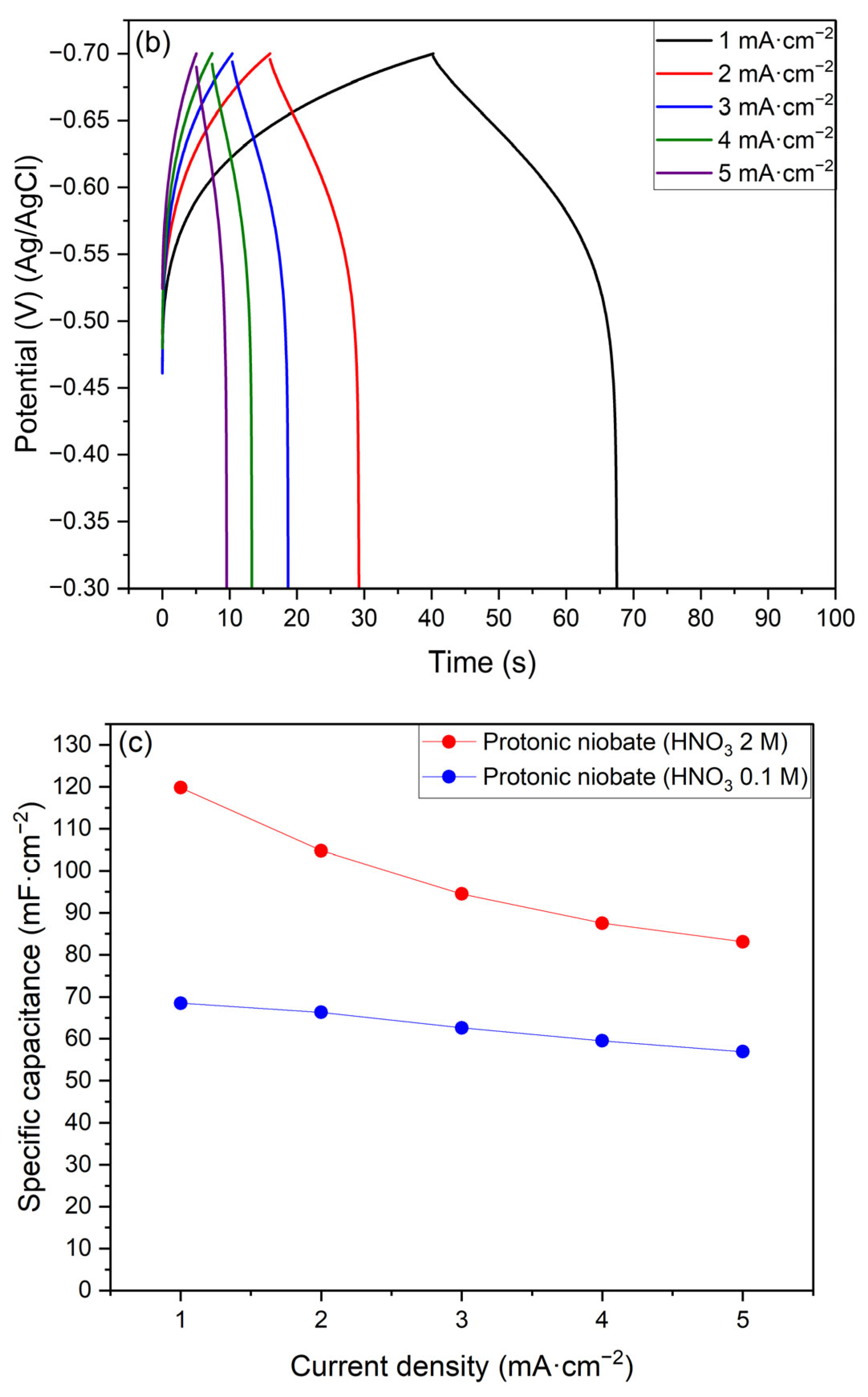
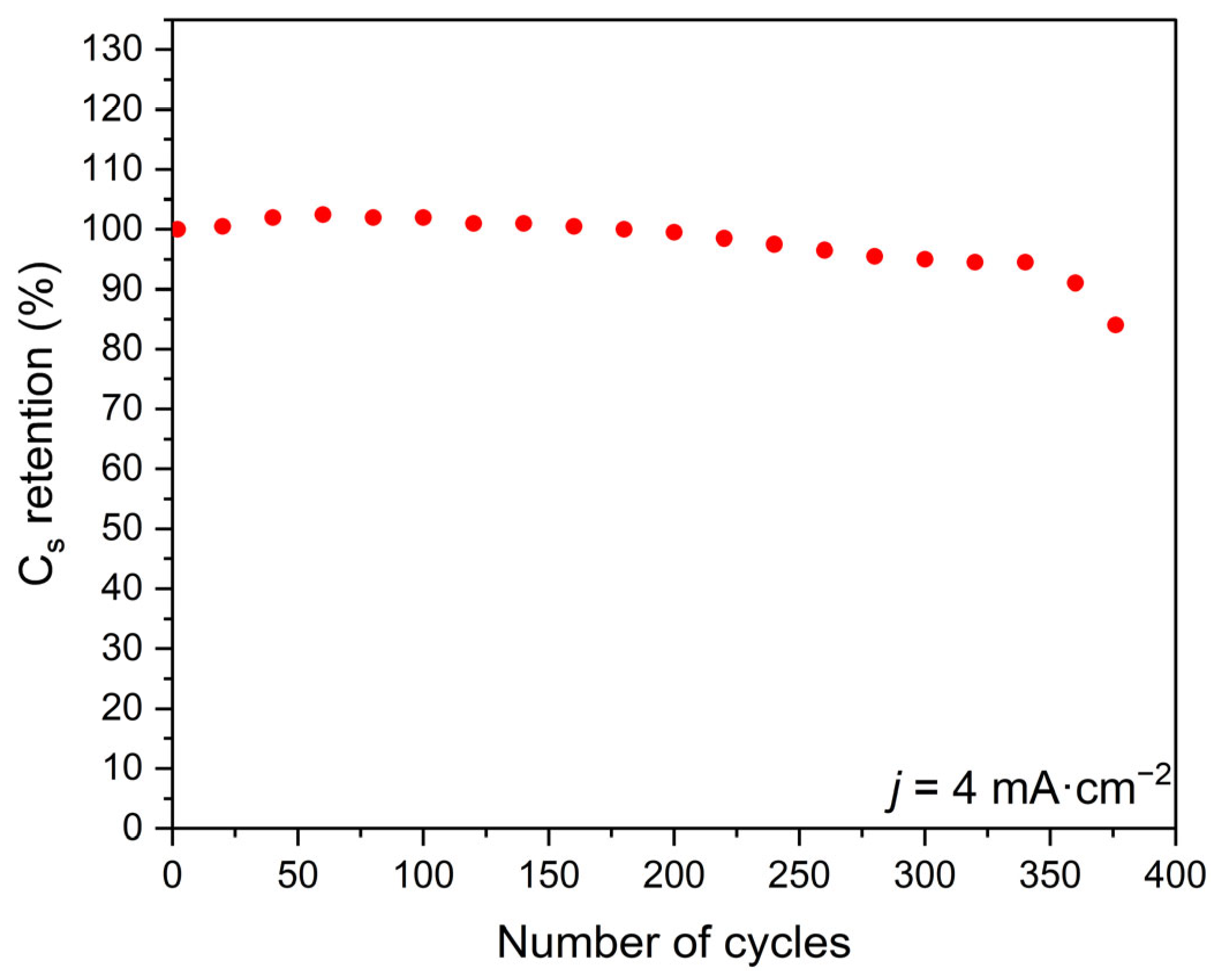
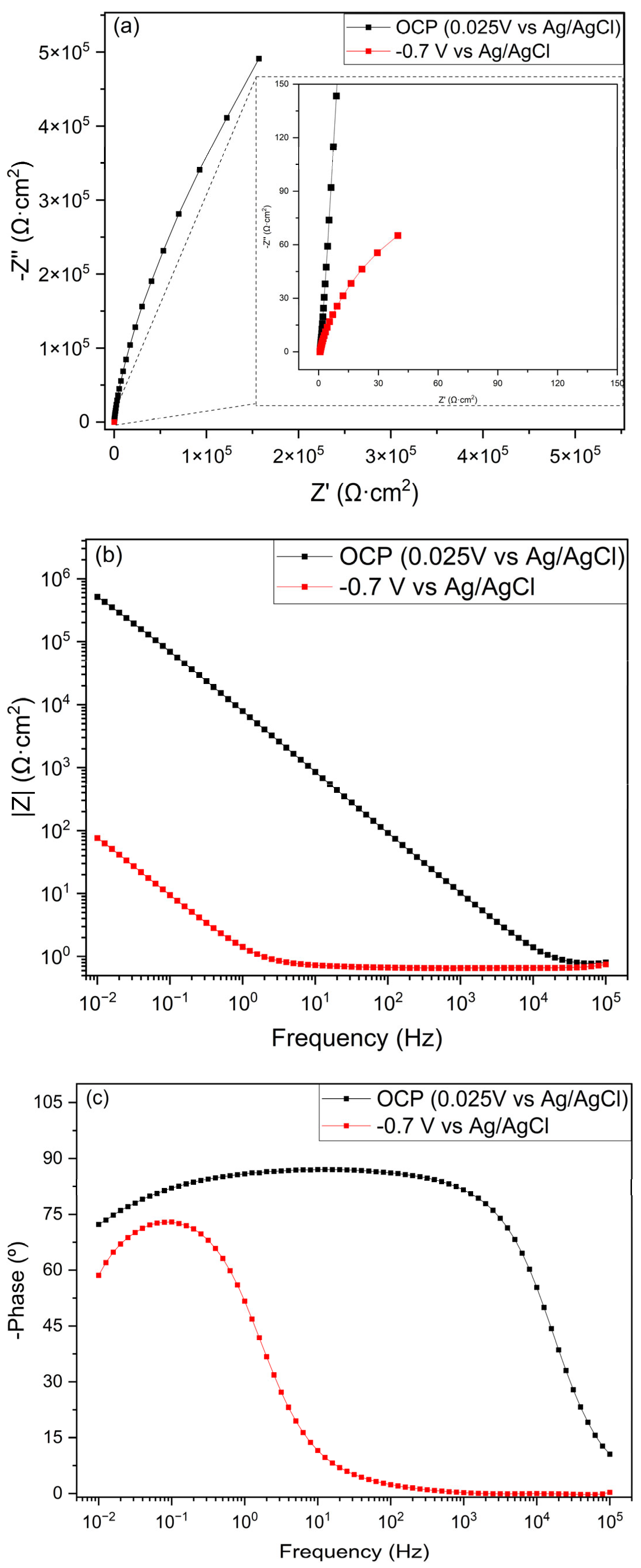
| Current Density (j): (mA·cm−2) | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Specific capacitance (CS) (mF·cm−2) HNO3 2 M | 119.8 | 104.8 | 94.5 | 87.5 | 83.1 |
| Specific capacitance (CS) (mF·cm−2) HNO3 0.1 M | 68.5 | 66.3 | 62.6 | 59.5 | 56.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos Júnior, A.G.d.; Gotardi, J.; Sampaio, E.J.P.; Araújo, C.C.; Rasch, G.L.; de Andrade, A.M.H.; Hübler, R.; Suárez, A.C.; Malfatti, C.d.F. Pseudocapacitive Behavior of Protonic Niobate Nanowires in Aqueous Acidic Electrolyte. Ceramics 2025, 8, 59. https://doi.org/10.3390/ceramics8020059
Santos Júnior AGd, Gotardi J, Sampaio EJP, Araújo CC, Rasch GL, de Andrade AMH, Hübler R, Suárez AC, Malfatti CdF. Pseudocapacitive Behavior of Protonic Niobate Nanowires in Aqueous Acidic Electrolyte. Ceramics. 2025; 8(2):59. https://doi.org/10.3390/ceramics8020059
Chicago/Turabian StyleSantos Júnior, Adilar Gonçalves dos, Jessica Gotardi, Edna Jerusa Pacheco Sampaio, Cristiano Campos Araújo, Gabriel Luiz Rasch, Antonio Marcos Helgueira de Andrade, Roberto Hübler, Andrés Cuña Suárez, and Célia de Fraga Malfatti. 2025. "Pseudocapacitive Behavior of Protonic Niobate Nanowires in Aqueous Acidic Electrolyte" Ceramics 8, no. 2: 59. https://doi.org/10.3390/ceramics8020059
APA StyleSantos Júnior, A. G. d., Gotardi, J., Sampaio, E. J. P., Araújo, C. C., Rasch, G. L., de Andrade, A. M. H., Hübler, R., Suárez, A. C., & Malfatti, C. d. F. (2025). Pseudocapacitive Behavior of Protonic Niobate Nanowires in Aqueous Acidic Electrolyte. Ceramics, 8(2), 59. https://doi.org/10.3390/ceramics8020059










