CuNb2O6 Particles Obtained via Solid-State Reaction and Application as Electrocatalyst for Oxygen Evolution Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Structural, Vibrational, and Morphological Characterization
2.2. Electrochemical Characterization
2.3. Synthesis Method
2.4. Synthesis via Solid-State Reaction
3. Results
3.1. XRF Results
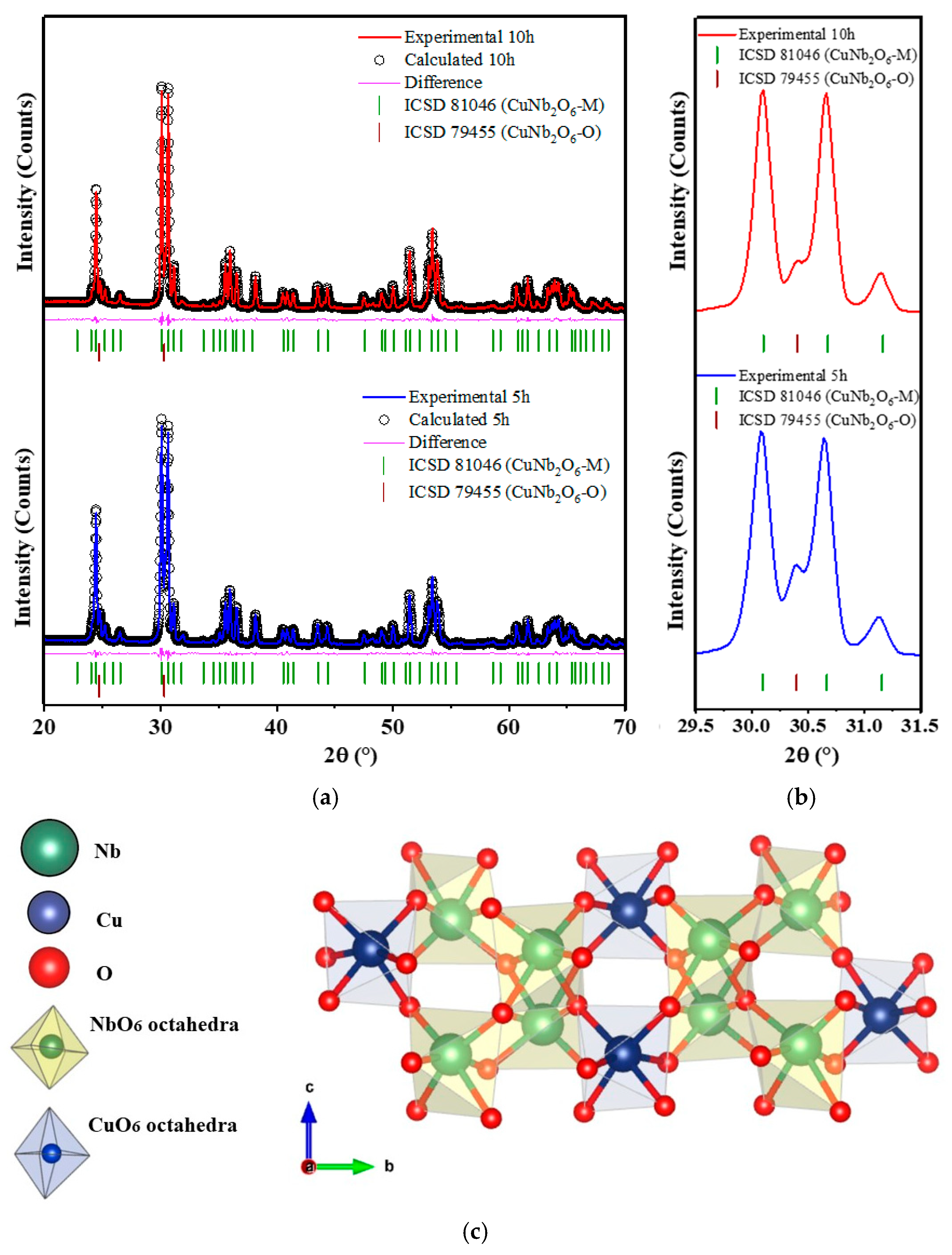
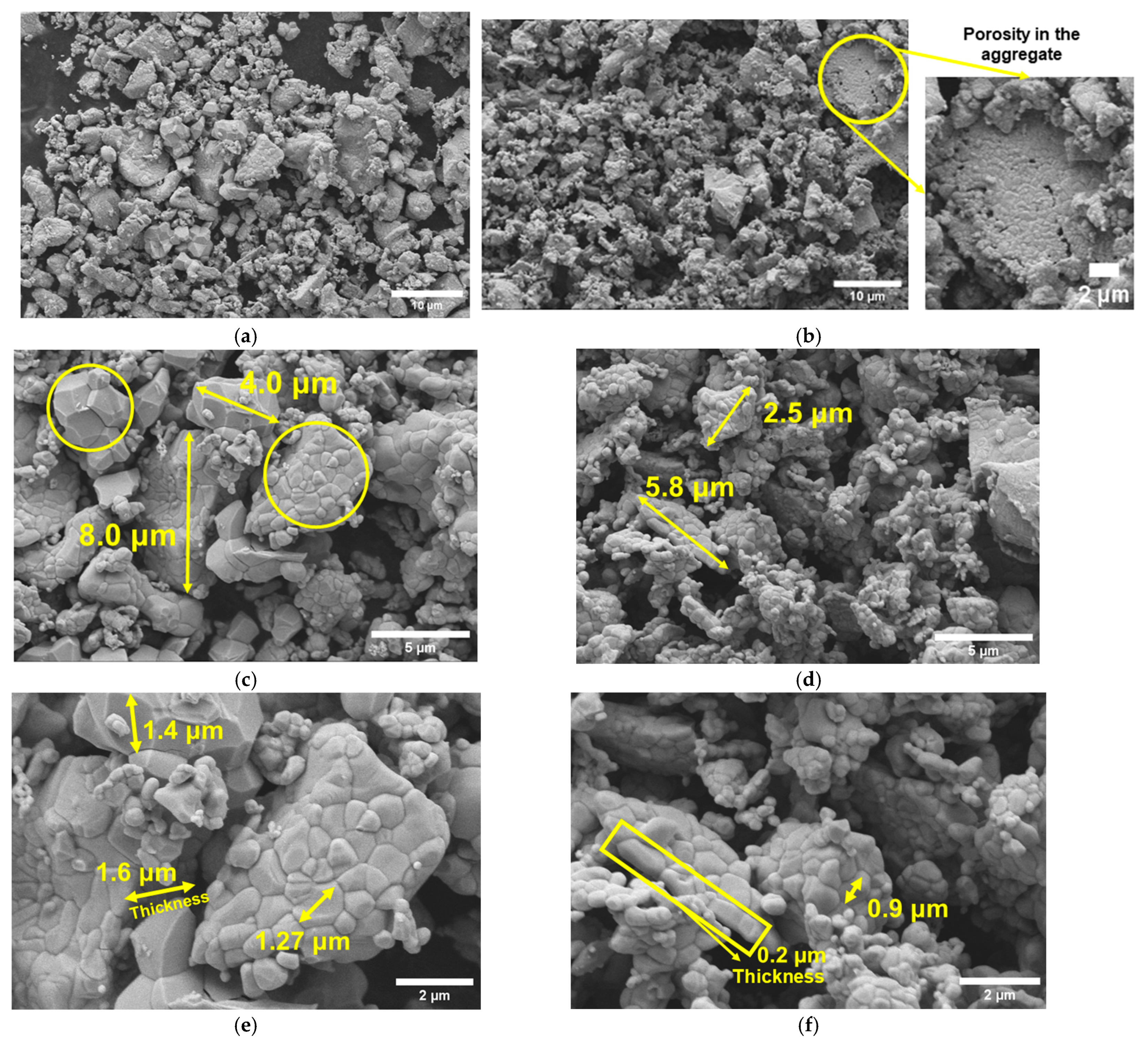
3.2. Structural and Morphological Study
4. Discussion
4.1. Structural, Vibrational, and Morphological
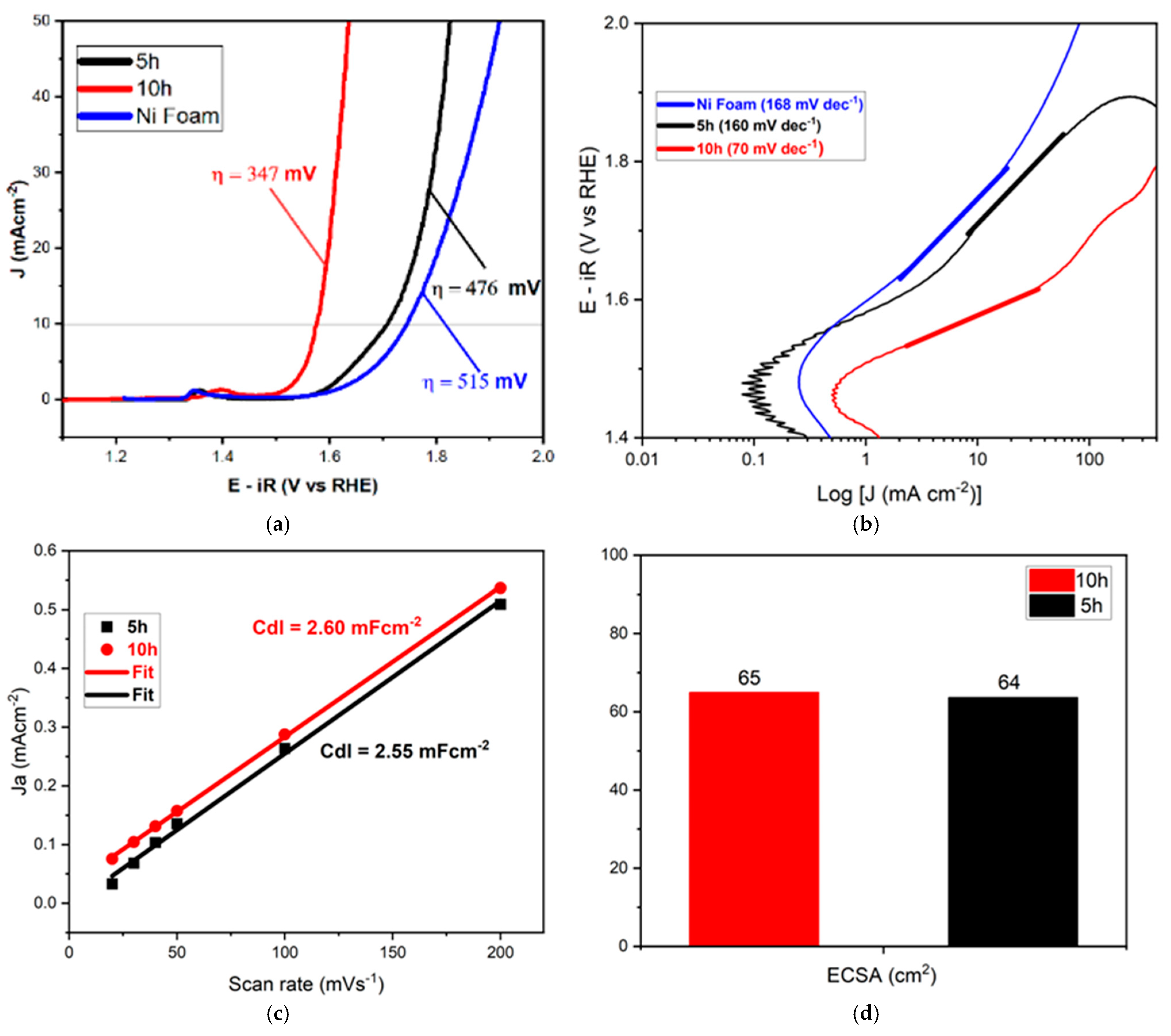
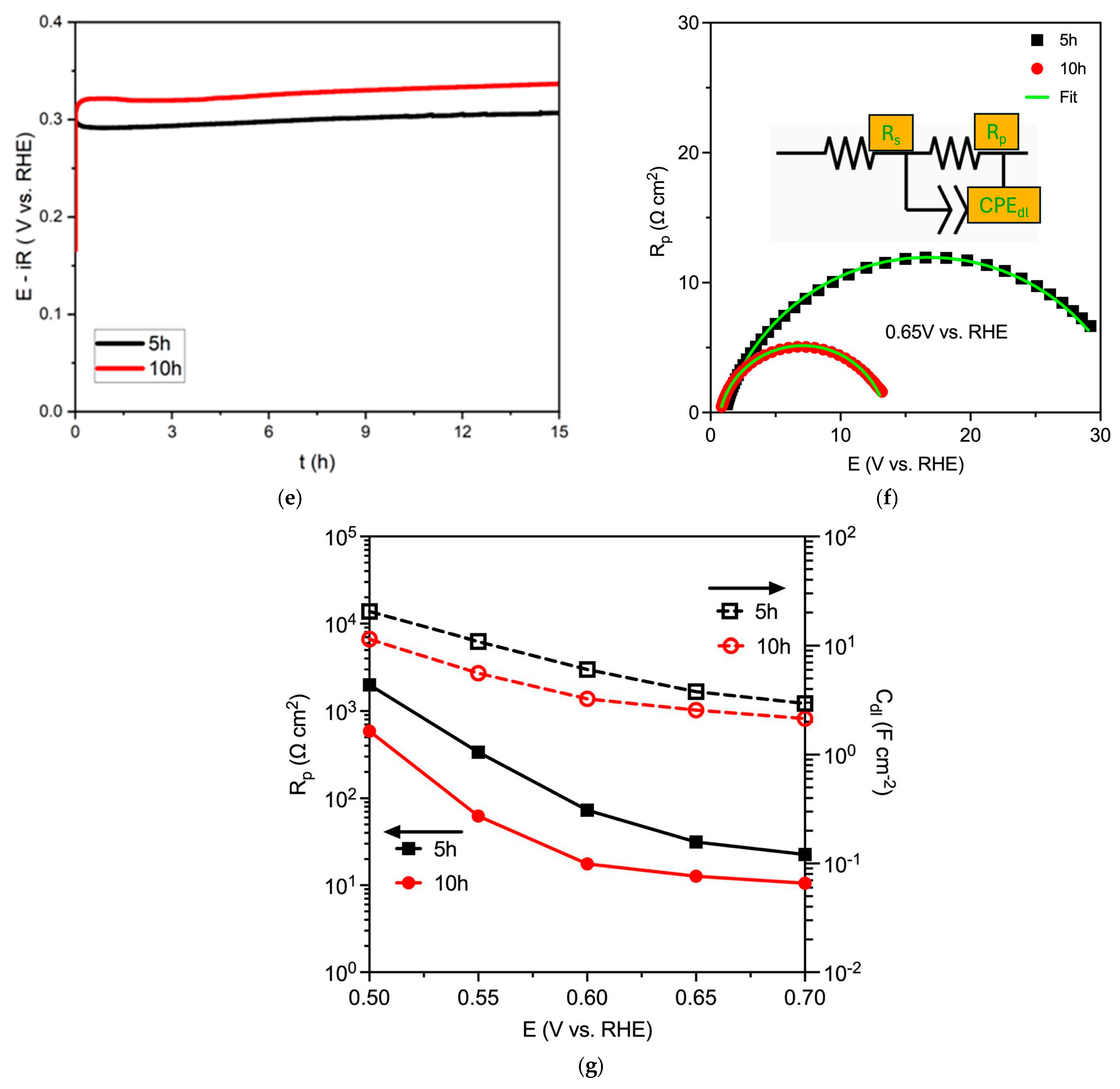
4.2. Electrocatalytic Performances
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, Y.; Zhang, D.; Li, Y.; Huang, B.; Li, H.; Pu, X.; Geng, Y. Fabrication of magnetically recoverable Ag/CuNb2O6/CuFe2O4 ternary heterojunction composite for highly efficient photocatalytic degradation of pollutants. Sep. Purif. Technol. 2019, 220, 78–88. [Google Scholar] [CrossRef]
- Ahmad, N.; Kuo, C.-F.J.; Mustaqeem, M. Synthesis of novel CuNb2O6/g-C3N4 binary photocatalyst towards efficient visible light reduction of Cr (VI) and dyes degradation for environmental remediation. Chemosphere 2022, 298, 134153. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Liu, H.; Gao, Z.; Fornasiero, P.; Wang, F. Nb2O5-based photocatalysts. Adv. Sci. 2021, 8, 2003156. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Khan, F.; Zhang, Y.; Djire, A. Recent development in electrocatalysts for hydrogen production through water electrolysis. Int. J. Hydrogen Energy 2021, 46, 32284–32317. [Google Scholar] [CrossRef]
- Ferreira, L.S.; Silva, T.R.; Santos, J.R.; Silva, V.D.; Raimundo, R.A.; Morales, M.A.; Macedo, D.A. Structure, magnetic behavior and OER activity of CoFe2O4 powders obtained using agar-agar from red seaweed (Rhodophyta). Mater. Chem. Phys. 2019, 237, 121847. [Google Scholar] [CrossRef]
- Dantas, A.P.; Raimundo, R.A.; Neto, P.F.; Lopes, C.M.; Santos, J.R.; Loureiro, F.J.; Pereira, T.O.; Morales, M.A.; Medeiros, E.S.; Macedo, D.A. Copper oxide nanofibers obtained by solution blow spinning as catalysts for oxygen evolution reaction. Ceram. Int. 2024, 50, 13034–13045. [Google Scholar] [CrossRef]
- Chatenet, M.; Pollet, B.G.; Dekel, D.R.; Dionigi, F.; Deseure, J.; Millet, P.; Braatz, R.D.; Bazant, M.Z.; Eikerling, M.; Staffell, I. Water electrolysis: From textbook knowledge to the latest scientific strategies and industrial developments. Chem. Soc. Rev. 2022, 51, 4583–4762. [Google Scholar]
- Yi, P.; Song, Y.; Liu, Z.; Xie, P.; Liang, G.; Liu, R.; Chen, L.; Sun, J. Boosting alkaline urea oxidation with a nickel sulfide/cobalt oxide heterojunction catalyst via interface engineering. Adv. Compos. Hybrid Mater. 2023, 6, 228. [Google Scholar] [CrossRef]
- Jamesh, M.-I.; Sun, X. Recent progress on earth abundant electrocatalysts for oxygen evolution reaction (OER) in alkaline medium to achieve efficient water splitting–A review. J. Power Sources 2018, 400, 31–68. [Google Scholar] [CrossRef]
- Zhang, P.; He, H. Rational rope-like CuCo2O4 nanosheets directly on Ni foam as multifunctional electrodes for supercapacitor and oxygen evolution reaction. J. Alloys Compd. 2020, 826, 153993. [Google Scholar] [CrossRef]
- Leng, M.; Huang, X.; Xiao, W.; Ding, J.; Liu, B.; Du, Y.; Xue, J. Enhanced oxygen evolution reaction by Co-OC bonds in rationally designed Co3O4/graphene nanocomposites. Nano Energy 2017, 33, 445–452. [Google Scholar] [CrossRef]
- Xu, X.; Huang, Z.; Zhao, C.; Ding, X.; Liu, X.; Wang, D.; Hui, Z.; Jia, R.; Liu, Y. Interface engineering of copper-cobalt based heterostructure as bifunctional electrocatalysts for overall water splitting. Ceram. Int. 2020, 46, 13125–13132. [Google Scholar] [CrossRef]
- Li, Z.; Hu, J.; Liu, S.; Zhao, B.; Zhu, D.; Song, A. Investigation of p-CuNb2O6 for use as photocathodes for photoelectrochemical water splitting. Int. J. Hydrogen Energy 2024, 51, 713–722. [Google Scholar] [CrossRef]
- Ravi, P.; Sreedhar, A.; Noh, J.-S. S-Scheme TiO2/CuNb2O6 nanocomposite photocatalyst with enhanced H2 evolution activity from aqueous glycerol solution. ACS Appl. Energy Mater. 2023, 6, 6051–6063. [Google Scholar] [CrossRef]
- Alves, H.P.; Pereira, T.O.; Raimundo, R.A.; Alves, R.F.; Junior, R.A.; Campos, L.F.; Macedo, D.A.; Medeiros, E.S. Cu–Nb–O ternary system nanofibers obtained by solution blow spinning as catalysts for oxygen evolution reaction. J. Phys. Chem. Solids 2025, 199, 112525. [Google Scholar] [CrossRef]
- Nunes, B.N.; Lopes, O.F.; Patrocinio, A.O.T.; Bahnemann, D.W. Recent advances in niobium-based materials for photocatalytic solar fuel production. Catalysts 2020, 10, 126. [Google Scholar] [CrossRef]
- Nico, C.; Monteiro, T.; Graça, M.P. Niobium oxides and niobates physical properties: Review and prospects. Prog. Mater. Sci. 2016, 80, 1–37. [Google Scholar] [CrossRef]
- Islam, A.N.; Pieper, O.; Lake, B.; Siemensmeyer, K. Unconventional Growth Mechanism in Optical Traveling Solvent Floating Zone Growth of Large β-CuNb2O6 Single Crystals. Cryst. Growth Des. 2011, 11, 154–157. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, H.; Tang, Q.; Lu, S.; Zhang, H.; Zheng, X. Z-scheme Ag-loaded g-C3N4/CuNb2O6 composite photocatalyst for RhB dye degradation. Res. Chem. Intermed. 2022, 48, 4163–4182. [Google Scholar] [CrossRef]
- Priyadarshani, N.; Vinitha, G.; Girisun, T.S. Third order nonlinear optical properties of monoclinic and orthorhombic CuNb2O6 under CW laser illumination. Opt. Laser Technol. 2018, 108, 287–294. [Google Scholar] [CrossRef]
- Priyadarshani, N.; Girisun, T.S.; Rao, S.V. Influence of sintering time on switching of the femtosecond nonlinear optical properties of CuNb2O6. Opt. Mater. 2017, 66, 534–541. [Google Scholar] [CrossRef]
- Weiss, M.; Marschall, R. Syntheses and characterisation of p-type copper niobium oxides for photocatalytic hydrogen generation. Appl. Catal. A Gen. 2023, 661, 119234. [Google Scholar] [CrossRef]
- Guz, R.; Tiburtius, E.R.L.; Pessôa, C.A. Association of adsorption and heterogeneous photocatalysis in the degradation of Tartrazine yellow dye with CuNb2O6 synthesized and immobilized on chitosan membranes. Inorg. Chem. Commun. 2023, 152, 110645. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Lim, A.S.; Kwon, Y.M.; Cho, K.Y.; Yoon, S. Copper, zinc, and manganese niobates (CuNb2O6, ZnNb2O6, and MnNb2O6 Structural characteristics, Li+ storage properties, and working mechanisms. Inorg. Chem. Front. 2020, 7, 3176–3183. [Google Scholar] [CrossRef]
- Girisun, T.S.; Priyadarshani, N.; Panda, S.K.; Subramanian, B. Pulsed laser deposited CuNb2O6 polymorph photocathode-An alternate to NiO p-DSSCs. Mater. Sci. Semicond. Process. 2021, 121, 105404. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Li, H.; Gao, Y.; Yan, J.; Zhu, K.; Ye, K.; Cheng, K.; Wang, G.; Cao, D. Copper niobate nanowires immobilized on reduced graphene oxide nanosheets as rate capability anode for lithium ion capacitor. J. Colloid Interface Sci. 2021, 583, 652–660. [Google Scholar] [CrossRef]
- Kamimura, S.; Abe, S.; Tsubota, T.; Ohno, T. Solar-driven H2 evolution over CuNb2O6: Effect of two polymorphs (monoclinic and orthorhombic) on optical property and photocatalytic activity. J. Photochem. Photobiol. A Chem. 2018, 356, 263–271. [Google Scholar] [CrossRef]
- Li, M.; Su, A.; Qin, Q.; Qin, Y.; Dou, A.; Zhou, Y.; Su, M.; Liu, Y. High-rate capability of columbite CuNb2O6 anode materials for lithium-ion batteries. Mater. Lett. 2021, 284, 128915. [Google Scholar] [CrossRef]
- Lourenço, C.S.; Raimundo, R.A.; Silva, A.S.; Morales, M.A.; de Oliveira Vitoriano, J.; Lima, M.J.S.; Filgueira, M.; Gomes, U.U.; da Costa, F.A. Influence of the high-energy milling and spark plasma sintering on the formation of Cu-Graphite composite with enhanced properties. J. Alloys Compd. Commun. 2025, 6, 100063. [Google Scholar] [CrossRef]
- Silva, T.Q.; Vieira, P.S.; Raimundo, R.A.; Marques, A.C.; Vasconcelos, G.D.S.; Mashhadikarimi, M.; de OR Senos, A.M.; Gomes, U.U. Modulating the microstructural and mechanical characteristics of copper-tungsten carbide composite powders by WC content and milling time. Mater. Today Commun. 2025, 42, 111321. [Google Scholar] [CrossRef]
- Koch, C. Synthesis of nanostructured materials by mechanical milling: Problems and opportunities. Nanostructured Mater. 1997, 9, 13–22. [Google Scholar] [CrossRef]
- Burmeister, C.F.; Kwade, A. Process engineering with planetary ball mills. Chem. Soc. Rev. 2013, 42, 7660–7667. [Google Scholar] [CrossRef] [PubMed]
- Drew, M.G.; Hobson, R.J.; Padayatchy, V.T. Synthesis, structure and magnetic properties of monoclinic CuNb2O6 and the electronic spectra of both polymorphs of CuNb2O6. J. Mater. Chem. 1995, 5, 1779–1783. [Google Scholar] [CrossRef]
- Norwig, J.; Weitzel, H.; Paulus, H.; Lautenschläger, G.; Rodriguez-Carvajal, J.; Fuess, H. Structural relations in mixed oxides CuxZn1−x Nb2O6. J. Solid State Chem. 1995, 115, 476–483. [Google Scholar] [CrossRef]
- Arroyo y de Dompablo, M.; Lee, Y.-L.; Morgan, D. First principles investigation of oxygen vacancies in columbite MNb2O6 (M= Mn, Fe, Co, Ni, Cu). Chem. Mater. 2010, 22, 906–913. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Al-Joubori, A.A.; Wang, Z. Nanostructured materials and nanocomposites by mechanical alloying: An overview. Met. Mater. Int. 2022, 28, 41–53. [Google Scholar] [CrossRef]
- Eisen, W.; Ferguson, B.; German, R.; Iacocca, R.; Lee, P.; Madan, D.; Moyer, K.; Sanderow, H.; Trudel, Y. ASM Handbook, Volume 07, Powder Metal Technologies and Applications, 2nd ed.; ASM International: Almere, The Netherlands, 1998. [Google Scholar]
- Zhang, D. Processing of advanced materials using high-energy mechanical milling. Prog. Mater. Sci. 2004, 49, 537–560. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, H.; Zhu, Z.; Chen, X.; Qiu, M.; Fan, Y. Synthesis of lead-free piezoelectric potassium sodium niobates for the preparation of anti-fouling KNN porous membrane. Ceram. Int. 2024, 50, 27255–27264. [Google Scholar] [CrossRef]
- Ali, W.A.; Richards, S.E.; Alzard, R.H. Unlocking the potential of ball milling for nanomaterial Synthesis: An overview. J. Ind. Eng. Chem. 2025, in press. [Google Scholar] [CrossRef]
- Nwanna, E.C.; Imoisili, P.E.; Bitire, S.; Jen, T.-C. Green synthesis of CuNb2O6 thin film using allium cepa as catalyst. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 3610–3627. [Google Scholar] [CrossRef]
- Radamson, H.H. Raman Spectroscopy, Fourier Transform Infrared Spectroscopy (FTIR) and X-Ray Photoelectron Spectroscopy (XPS). In Analytical Methods and Instruments for Micro-and Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2023; pp. 87–114. [Google Scholar]
- Ismail, A.A.; van de Voort, F.R.; Sedman, J. Fourier transform infrared spectroscopy: Principles and applications. In Techniques and Instrumentation in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 1997; Volume 18, pp. 93–139. [Google Scholar]
- Bacsik, Z.; Mink, J.; Keresztury, G. FTIR spectroscopy of the atmosphere. I. Principles and methods. Appl. Spectrosc. Rev. 2004, 39, 295–363. [Google Scholar] [CrossRef]
- Kormányos, A.; Thomas, A.; Huda, M.; Sarker, P.; Liu, J.P.; Poudyal, N.; Janáky, C.; Rajeshwar, K. Solution combustion synthesis, characterization, and photoelectrochemistry of CuNb2O6 and ZnNb2O6 nanoparticles. J. Phys. Chem. C 2016, 120, 16024–16034. [Google Scholar] [CrossRef]
- Kamimura, S.; Beppu, N.; Sasaki, Y.; Tsubota, T.; Ohno, T. Platinum and indium sulfide-modified Cu3BiS3 photocathode for photoelectrochemical hydrogen evolution. J. Mater. Chem. A 2017, 5, 10450–10456. [Google Scholar] [CrossRef]
- Cheng, C.; Yan, Y.; Jia, M.; Liu, Y.; Hou, L.; Yuan, C. Insights into Formation and Li-Storage Mechanisms of Hierarchical Accordion-Shape Orthorhombic CuNb2O6 toward Lithium-Ion Capacitors as an Anode-Active Material. Energy Environ. Mater. 2024, 7, e12583. [Google Scholar] [CrossRef]
- Chakrapani, K.; Bendt, G.; Hajiyani, H.; Schwarzrock, I.; Lunkenbein, T.; Salamon, S.; Landers, J.; Wende, H.; Schlögl, R.; Pentcheva, R. Role of composition and size of cobalt ferrite nanocrystals in the oxygen evolution reaction. ChemCatChem 2017, 9, 2988–2995. [Google Scholar] [CrossRef]
- Broicher, C.; Klingenhof, M.; Frisch, M.; Dresp, S.; Kubo, N.M.; Artz, J.; Radnik, J.; Palkovits, S.; Beine, A.K.; Strasser, P. Particle size-controlled synthesis of high-performance MnCo-based materials for alkaline OER at fluctuating potentials. Catal. Sci. Technol. 2021, 11, 7278–7286. [Google Scholar] [CrossRef]
- Roy, C.; Sebok, B.; Scott, S.; Fiordaliso, E.; Sørensen, J.; Bodin, A.; Trimarco, D.; Damsgaard, C.; Vesborg, P.; Hansen, O. Impact of nanoparticle size and lattice oxygen on water oxidation on NiFeOxHy. Nat. Catal. 2018, 1, 820–829. [Google Scholar] [CrossRef]
- Wu, S.-H.; Hsiao, C.-H.; Hsieh, P.-L.; Huang, X.-F.; Huang, M.H. Growth of CeO2 nanocubes showing size-dependent optical and oxygen evolution reaction behaviors. Dalton Trans. 2021, 50, 15170–15175. [Google Scholar] [CrossRef]
- Priyadarshani, N.; Girisun, T.S.; Ravidhas, C. Enhanced electrical behaviour of monoclinic p-CuNb2O6. Mater. Res. Bull. 2016, 84, 39–45. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, H.; Kang, Q.; Ma, H.; Tong, Y.; Gao, F.; Lu, Q. Rapid solvent-evaporation strategy for three-dimensional cobalt-based complex hierarchical architectures as catalysts for water oxidation. Sci. Rep. 2019, 9, 15681. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, C.; Lee, C.; Cho, K.; Kim, J.; Park, B.; Kim, W.; Lim, N.; Kim, H.; Pak, Y.; Chae, K.H. High-performance rechargeable metal–air batteries enabled by efficient charge transport in multielement random alloy electrocatalyst. Appl. Catal. B Environ. 2023, 330, 122631. [Google Scholar] [CrossRef]
- De, A.; Rambaran, M.A.; Ohlin, C.A. Fabrication and Electrocatalytic Evolution of Alkali-Free CoNb2O6 Thin Films for Water Oxidation via Polyoxoniobates. ACS Appl. Energy Mater. 2024, 7, 8333–8341. [Google Scholar] [CrossRef]
- Lourenço, A.A.; Silva, V.D.; Da Silva, R.; Silva, U.; Chesman, C.; Salvador, C.; Simões, T.A.; Macedo, D.A.; da Silva, F.F. Metal-organic frameworks as template for synthesis of Mn3+/Mn4+ mixed valence manganese cobaltites electrocatalysts for oxygen evolution reaction. J. Colloid Interface Sci. 2021, 582, 124–136. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, J.; Zhong, M.; Guo, S. Co3O4 nanosheet arrays on Ni foam as electrocatalyst for oxygen evolution reaction. Electrocatalysis 2018, 9, 653–661. [Google Scholar] [CrossRef]
- Babar, P.; Lokhande, A.; Karade, V.; Pawar, B.; Gang, M.G.; Pawar, S.; Kim, J.H. Towards highly efficient and low-cost oxygen evolution reaction electrocatalysts: An effective method of electronic waste management by utilizing waste Cu cable wires. J. Colloid Interface Sci. 2019, 537, 43–49. [Google Scholar] [CrossRef]
- Silva, V.D.; Raimundo, R.A.; Simões, T.A.; Loureiro, F.J.; Fagg, D.P.; Morales, M.A.; Macedo, D.A.; Medeiros, E.S. Nonwoven Ni–NiO/carbon fibers for electrochemical water oxidation. Int. J. Hydrogen Energy 2021, 46, 3798–3810. [Google Scholar] [CrossRef]
- Angulo, A.; van der Linde, P.; Gardeniers, H.; Modestino, M.; Rivas, D.F. Influence of bubbles on the energy conversion efficiency of electrochemical reactors. Joule 2020, 4, 555–579. [Google Scholar] [CrossRef]
- Lyons, M.E.; Brandon, M.P. The significance of electrochemical impedance spectra recorded during active oxygen evolution for oxide covered Ni, Co and Fe electrodes in alkaline solution. J. Electroanal. Chem. 2009, 631, 62–70. [Google Scholar] [CrossRef]
- Alves, R.F.; Raimundo, R.A.; Lima, B.A.; Loureiro, F.J.; Fagg, D.P.; Macedo, D.A.; Gomes, U.U.; Morales, M.A. The effect of particle size on structural and catalysts for oxygen evolution reaction of (CoFeNiMnCr)3O4 prepared by controlled synthesis with polyvinylpyrrolidone (PVP). J. Colloid Interface Sci. 2025, 680, 818–831. [Google Scholar] [CrossRef]
- Da Silva, L.; Alves, V.; Da Silva, M.; Trasatti, S.; Boodts, J.F.C. Oxygen evolution in acid solution on IrO2+ TiO2 ceramic films. A study by impedance, voltammetry and SEM. Electrochim. Acta 1997, 42, 271–281. [Google Scholar] [CrossRef]
- Li, G.; Chuang, P.-Y.A. Identifying the forefront of electrocatalytic oxygen evolution reaction: Electronic double layer. Appl. Catal. B Environ. 2018, 239, 425–432. [Google Scholar] [CrossRef]



| Sample | CuO (mol.%) | Nb2O5 (mol.%) |
|---|---|---|
| CuNb2O6-5 h | 47.4 | 52.6 |
| CuNb2O6-10 h | 48.1 | 51.9 |
| Crystal Structure Parameters | Quality of Fit | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Phase | β (°) | Lattice Parameters (Å) | Phase (wt.%) | Cell Volume (Å3) | Cryst. Size (nm) | Rwp | Rexp | χ2 | ||
| a | b | c | |||||||||
| CuNb2O6-5 h | Mono | 91.7 | 5.005 | 14.172 | 5.761 | 87.4 | 408.5 | 59.9 | 2.72 | 0.84 | 3.19 |
| Ortho | 90.0 | 14.087 | 5.604 | 5.116 | 12.6 | 403.9 | 54.1 | ||||
| CuNb2O6-10 h | Mono | 91.7 | 5.019 | 14.211 | 5.776 | 87.1 | 411.8 | 58.3 | 2.88 | 0.83 | 3.47 |
| Ortho | 90.0 | 14.154 | 5.625 | 5.136 | 12.9 | 408.9 | 42.6 | ||||
| Electrocatalyst | Electrolyte (1.0 M) | ղ10 (mV) | Tafel (mV dec−1) | Reference |
|---|---|---|---|---|
| CuNb2O6-10 h | KOH | 347 | 70 | This work |
| CuNb2O6-5 h | KOH | 476 | 160 | This work |
| CuO/CuNb2O6 | KOH | 380 | 102 | [15] |
| CoNb2O6@Ni | KOH | 220 | 50 | [55] |
| CoNb2O6@Ag | KOH | 250 | 54 | [55] |
| CoNb2O6 | KOH | 330 | 100 | [55] |
| CoNb2O6@Ag0.6Ni0.4 | KOH | 400 * | 40 | [55] |
| CuO-N | KOH | 385 | 76 | [55] |
| CoNb2O6-600 °C | KOH | 503 | 82 | [56] |
| CoNb2O6-1000 °C | KOH | 559 | 122 | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Araújo, K.F.G.; Lourenço, C.S.; Souza, V.M.S.F.; da Silva, M.D.; Vasconcelos, G.D.S.; Lima, M.J.S.; Santos, J.R.D.; Gomes, K.C.; Loureiro, F.J.A.; Morales, M.A.; et al. CuNb2O6 Particles Obtained via Solid-State Reaction and Application as Electrocatalyst for Oxygen Evolution Reaction. Ceramics 2025, 8, 55. https://doi.org/10.3390/ceramics8020055
de Araújo KFG, Lourenço CS, Souza VMSF, da Silva MD, Vasconcelos GDS, Lima MJS, Santos JRD, Gomes KC, Loureiro FJA, Morales MA, et al. CuNb2O6 Particles Obtained via Solid-State Reaction and Application as Electrocatalyst for Oxygen Evolution Reaction. Ceramics. 2025; 8(2):55. https://doi.org/10.3390/ceramics8020055
Chicago/Turabian Stylede Araújo, Kívia F. G., Cleber S. Lourenço, Vitor M. S. F. Souza, Matheus D. da Silva, Gabriel D. S. Vasconcelos, Maria J. S. Lima, Jakeline R. D. Santos, Kelly C. Gomes, Francisco J. A. Loureiro, Marco A. Morales, and et al. 2025. "CuNb2O6 Particles Obtained via Solid-State Reaction and Application as Electrocatalyst for Oxygen Evolution Reaction" Ceramics 8, no. 2: 55. https://doi.org/10.3390/ceramics8020055
APA Stylede Araújo, K. F. G., Lourenço, C. S., Souza, V. M. S. F., da Silva, M. D., Vasconcelos, G. D. S., Lima, M. J. S., Santos, J. R. D., Gomes, K. C., Loureiro, F. J. A., Morales, M. A., & Gomes, U. U. (2025). CuNb2O6 Particles Obtained via Solid-State Reaction and Application as Electrocatalyst for Oxygen Evolution Reaction. Ceramics, 8(2), 55. https://doi.org/10.3390/ceramics8020055










