The Influence of Si(C,N) Layer Composition on the Corrosion of NiCr Prosthetic Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Corrosion Tests
2.2. Adhesion
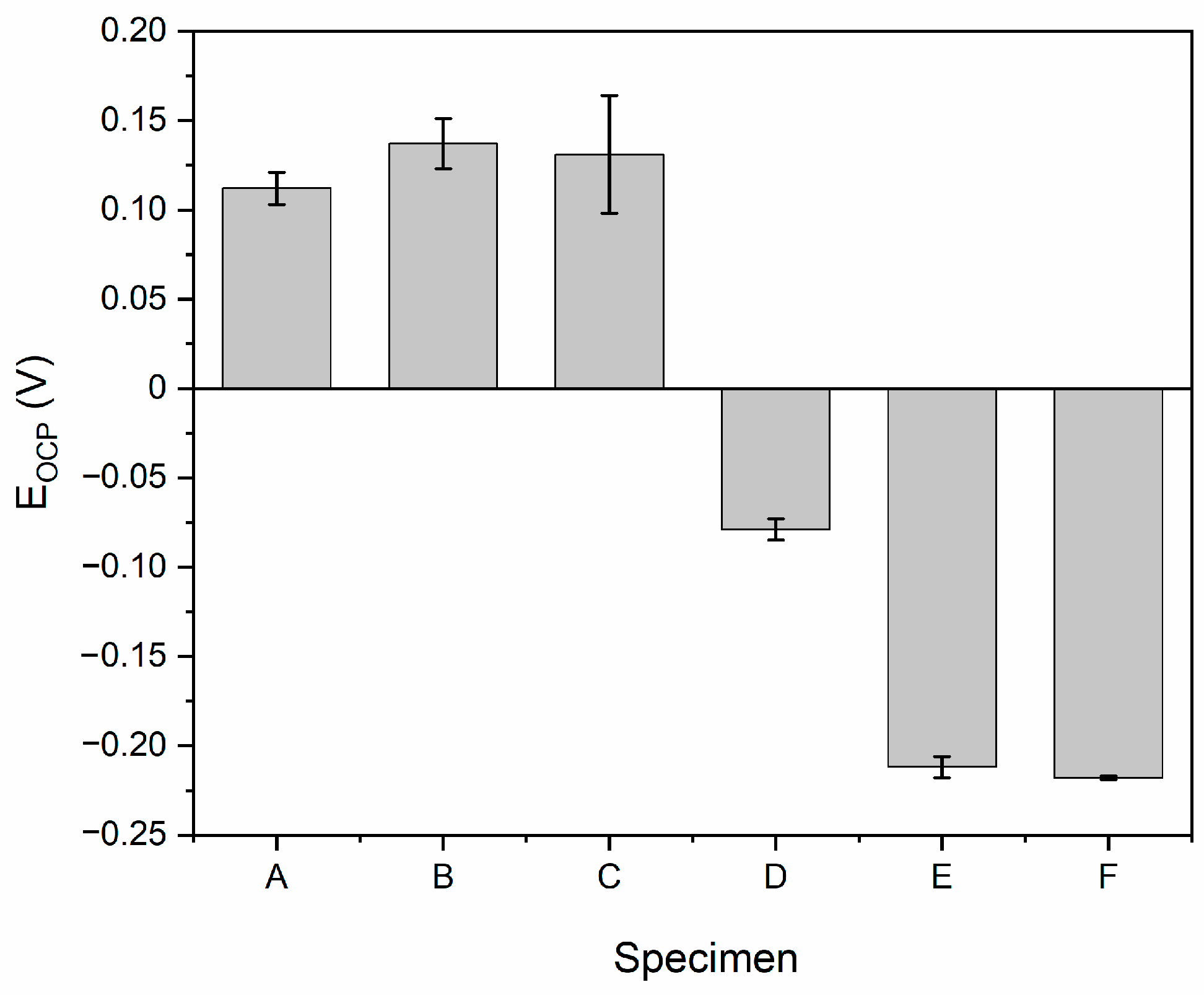
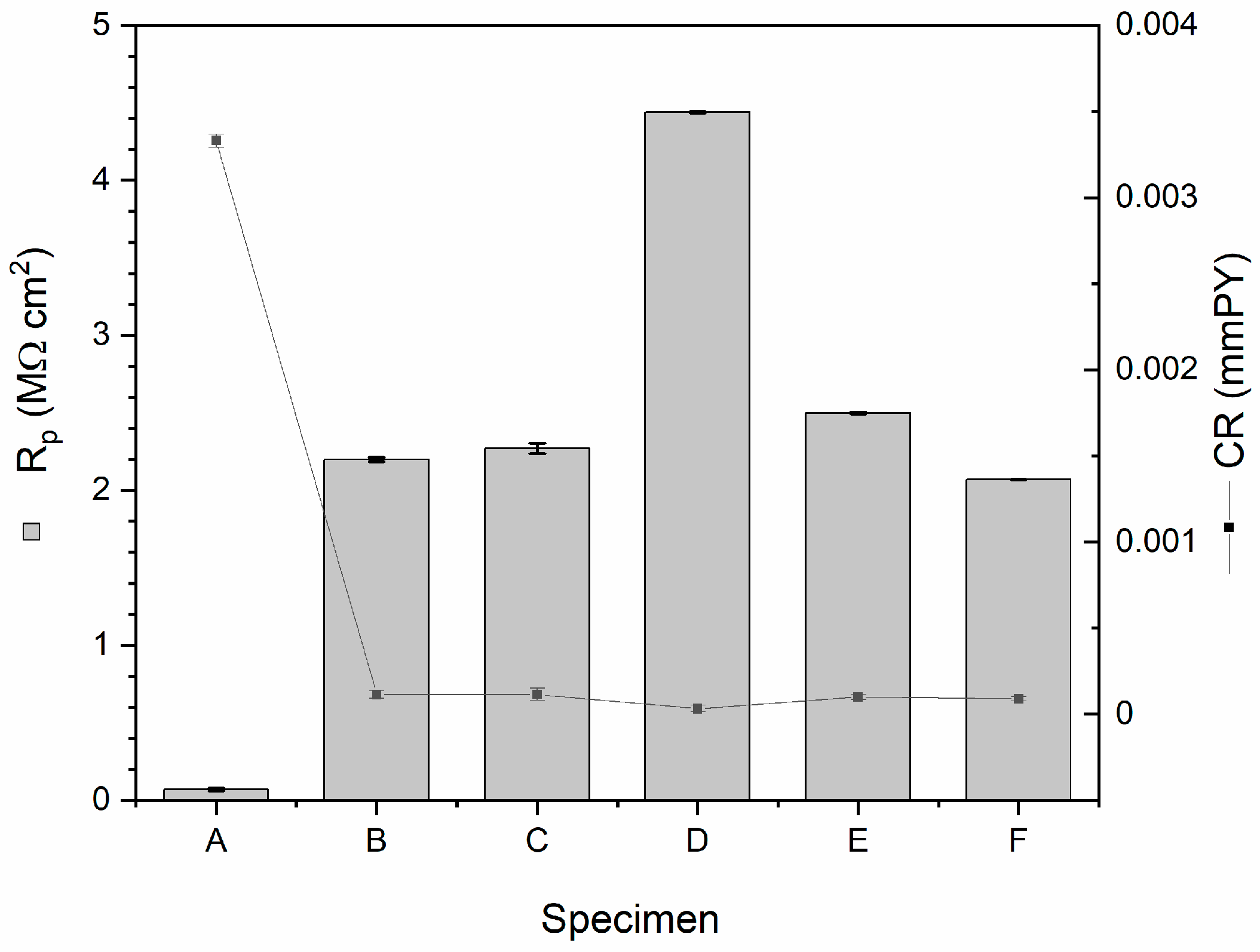
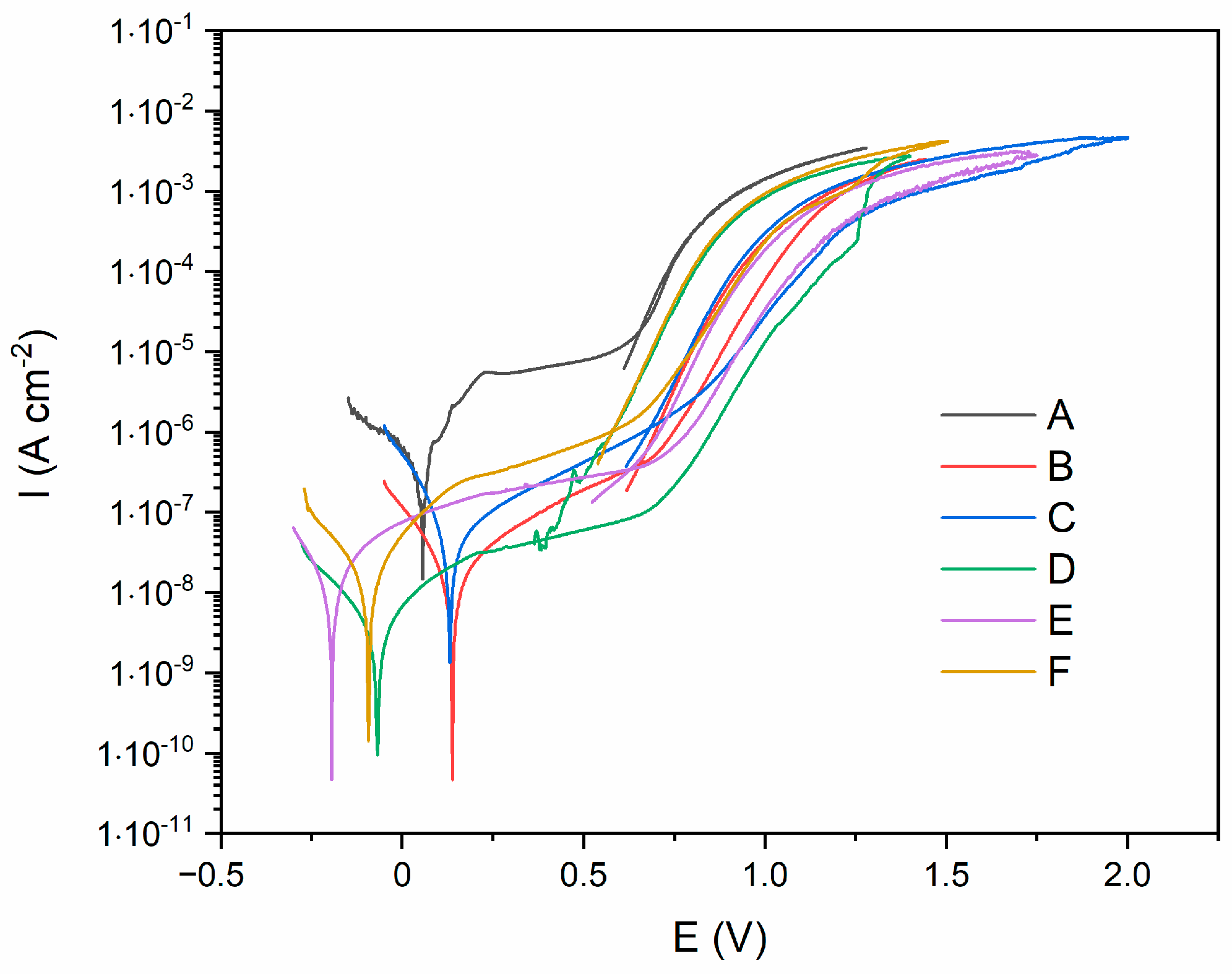
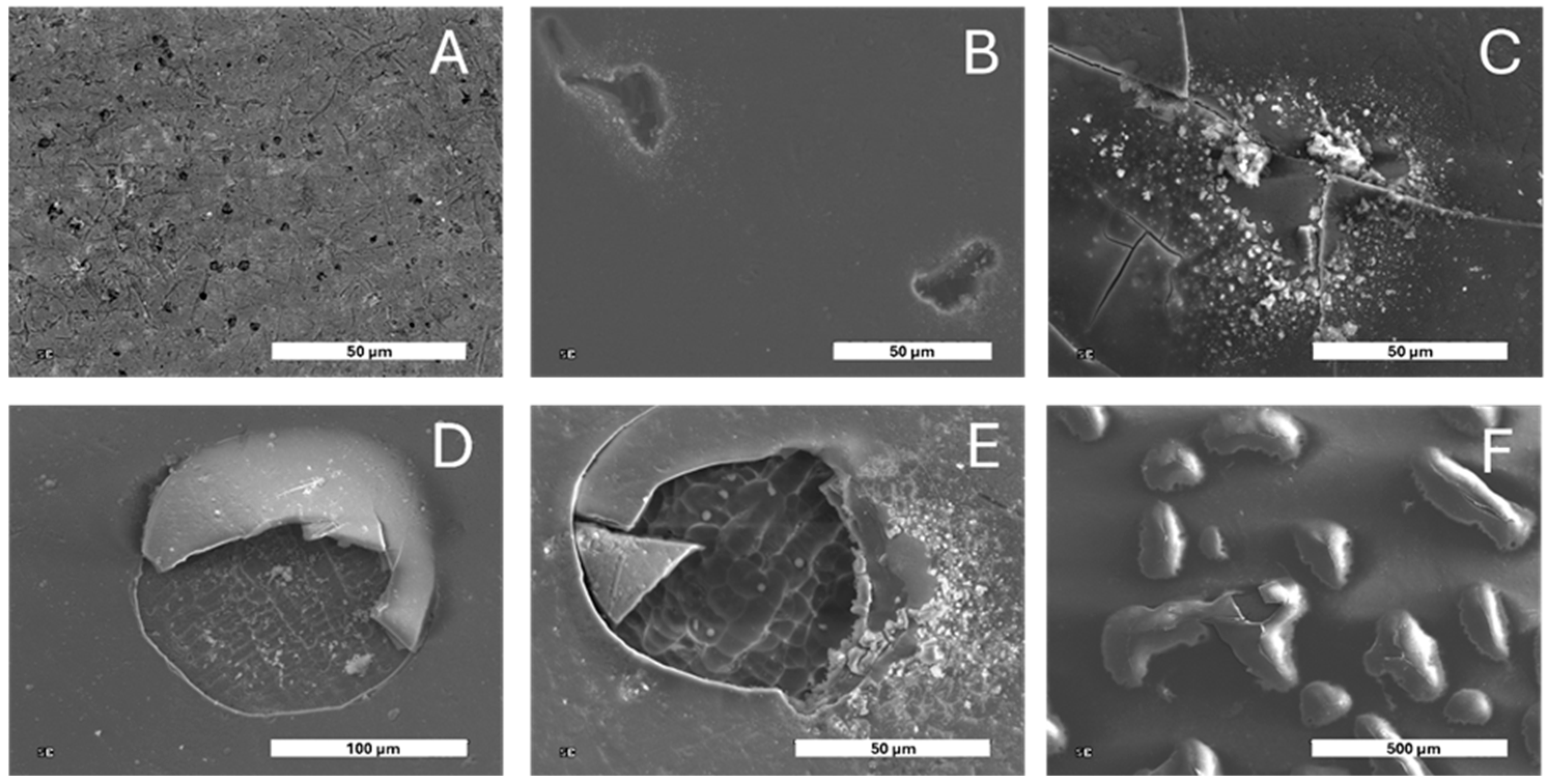
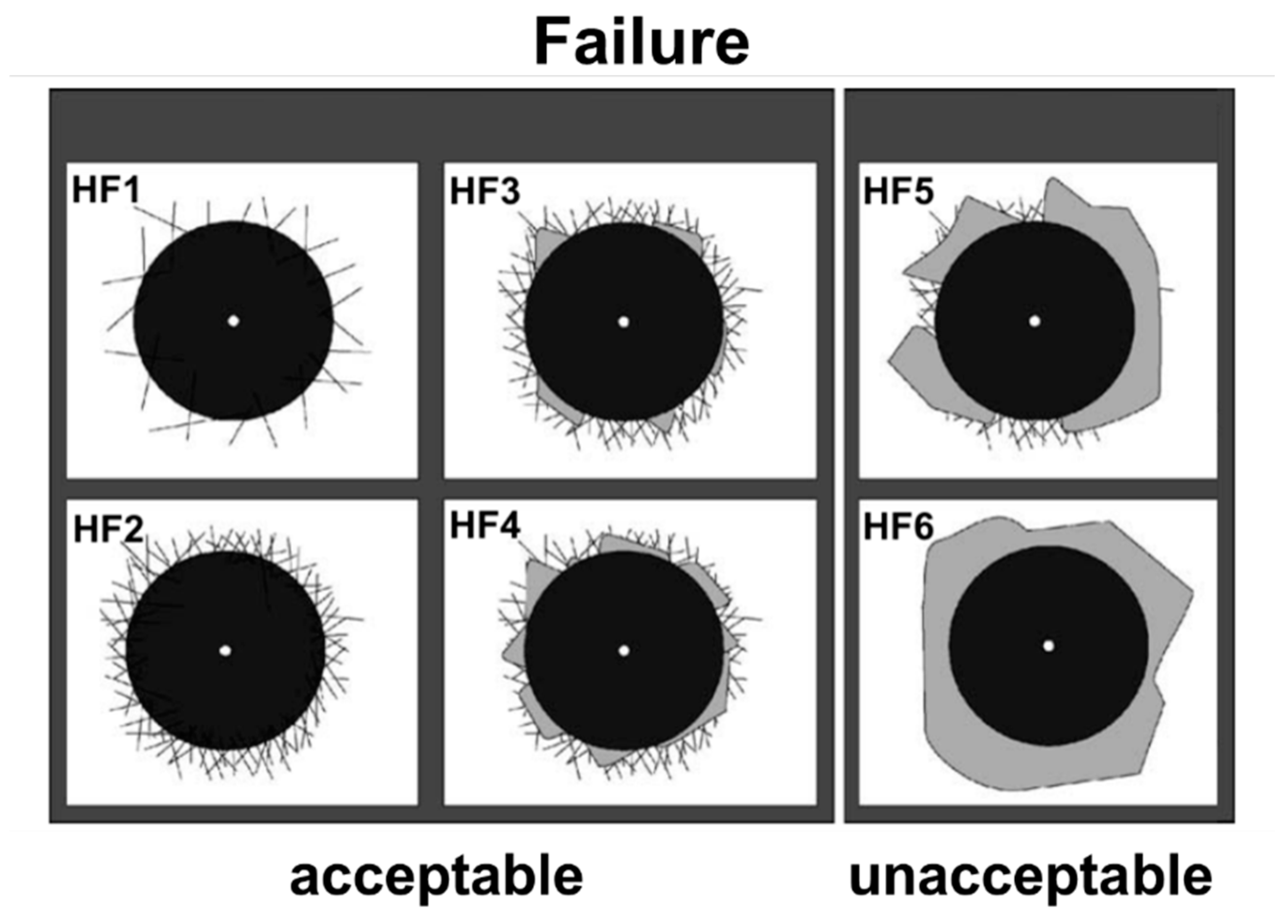
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, W.; Yu, F.; Addison, O.; Zhang, B.; Guan, F.; Zhang, R.; Hou, B.; Sand, W. Microbial corrosion of metallic biomaterials in the oral environment. Acta Biomater. 2024, 184, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Unsal, T.; Xu, D.; Lekbach, Y.; Gu, T. Microbiologically influenced corrosion and current mitigation strategies: A state of the art review. Int. Biodeterior. Biodegrad. 2019, 137, 42–58. [Google Scholar] [CrossRef]
- Fischer, N.G.; Aparicio, C. The salivary pellicle on dental biomaterials. Colloids Surf. B Biointerfaces 2021, 200, 111570. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Xu, F.; Lee, S. Human saliva and model saliva at bulk to adsorbed phases—Similarities and differences. Adv. Colloid Interface Sci. 2019, 273, 102034. [Google Scholar] [CrossRef]
- Proctor, G.B.; Shaalan, A.M. Disease-Induced Changes in Salivary Gland Function and the Composition of Saliva. J. Dent. Res. 2021, 100, 1201–1209. [Google Scholar] [CrossRef]
- Arakelyan, M.; Spagnuolo, G.; Iaculli, F.; Dikopova, N.; Antoshin, A.; Timashev, P.; Turkina, A. Minimization of Adverse Effects Associated with Dental Alloys. Materials 2022, 15, 7476. [Google Scholar] [CrossRef]
- Gopalakrishnan, U.; Felicita, A.S.; Mahendra, L.; Kanji, M.A.; Varadarajan, S.; Raj, A.T.; Feroz, S.M.A.; Mehta, D.; Baeshen, H.A.; Patil, S. Assessing the Potential Association Between Microbes and Corrosion of Intra-Oral Metallic Alloy-Based Dental Appliances Through a Systematic Review of the Literature. Front. Bioeng. Biotechnol. 2021, 9, 631103. [Google Scholar] [CrossRef]
- Dikopova, N.Z.; Volkov, A.G.; Arakelyan, M.G.; Makarenko, N.V.; Soxova, I.A.; Doroshina, V.J.; Arzukanyan, A.V.; Margaryan, E.G. The Study of the Electrochemical Potentials of Metal Structures in the Oral Cavity in Diseases of the Oral Mucosa. New Armen. Med. J. 2020, 14, 54–58. [Google Scholar]
- Mikulewicz, M.; Chojnacka, K.; Woźniak, B.; Downarowicz, P. Release of Metal Ions from Orthodontic Appliances: An in Vitro Study. Biol. Trace Elem. Res. 2012, 146, 272–280. [Google Scholar] [CrossRef]
- Moslehifard, E.; Ghasemzadeh, S.; Nasirpouri, F. Influence of pH Level of Artificial Saliva on Corrosion Behavior and Nickel Ion Release of a Ni-Cr-Mo Alloy: An In Vitro Study. Anti-Corros. Methods Mater. 2019, 66, 746–756. [Google Scholar] [CrossRef]
- Quezada-Castillo, E.; Aguilar-Castro, W.; Quezada-Alván, B. Ion release from non precious dental alloys in the oral cavity. Matéria 2022, 27, e202248593. [Google Scholar] [CrossRef]
- Motoyoshi, M. Current Techniques and Materials in Dentistry; MDPI AG: Basel, Switzerland, 2022; ISBN 3036544135. [Google Scholar]
- Łosiewicz, B.; Osak, P.; Kubisztal, J.; Górka-Kulikowska, K. Effect of Artificial Saliva Modification on the Corrosion Resistance and Electronic Properties of Bego Wirobond C Dental Alloys. Appl. Sci. 2023, 13, 12185. [Google Scholar] [CrossRef]
- Schmutz, P.; Quach-Vu, N.-C.; Gerber, I. Metallic Medical Implants: Electrochemical Characterization of Corrosion Processes. Electrochem. Soc. Interface 2020, 17, 35–40. [Google Scholar] [CrossRef]
- Smardz, J.; Skowron, M.; Florjański, W. The use of metals and their alloys in dental prosthetics. Prosthodontics 2016, 66, 461–467. [Google Scholar] [CrossRef]
- Wylie, C.M.; Sheltonb, R.M.; Fleming, G.J.P.; Davenporta, A.J. Corrosion of nickel-based dental casting alloys. Dent. Mater. 2007, 23, 714–723. [Google Scholar] [CrossRef]
- Upadhyaya, D.; Panchal, M.A.; Dubey, R.S.; Srivastava, V.K. Corrosion of alloys used in dentistry: A review. Mater. Sci. Eng. A 2006, 432, 1–11. [Google Scholar] [CrossRef]
- Kula, Z.; Semenov, M.; Klimek, L. Carbon Coatings Deposited on Prosthodontic Ni-Cr Alloy. Appl. Sci. 2021, 11, 4551. [Google Scholar] [CrossRef]
- Olms, C.; Schor, J.; Yahiaoui-Doktor, M. Potential Co-Factors of an Intraoral Contact Allergy—A Cross-Sectional Study. Dent. J. 2020, 8, 83. [Google Scholar] [CrossRef]
- Fletcher, R.; Harrison, W.; Crighton, A. Dental material allergies and oral soft tissue reactions. Br. Dent. J. 2022, 232, 620–625. [Google Scholar] [CrossRef]
- Mercieca, S.; Conti, C.M.; Buhagiar, J.; Camilleri, J. Assessment of corrosion resistance of cast cobalt- and nickel-chromium dental alloys in acidic environments. J. Appl. Biomater. Funct. Mater. 2018, 16, 47–54. [Google Scholar] [CrossRef]
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, N.A.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. 2017, 77, 1261–1274. [Google Scholar] [CrossRef]
- Rylska, D.; Sokolowski, G.; Sokolowski, K. Ni–Cr dental alloys—Porcelain firing impact on corrosion properties and surface characteristics. Mater. Res. Express 2024, 11, 076501. [Google Scholar] [CrossRef]
- Sampaio, N.A.; Silva, J.W.; Acciari, H.; Nakazato, R.Z.; Codaro, E.N.; Felipe, H. Influence of Ni and Cr Content on Corrosion Resistance of Ni-Cr-Mo Alloys for Fixed Dental Prostheses in 0.05% NaF Aqueous Solution. Mater. Sci. Appl. 2010, 1, 369–372. [Google Scholar] [CrossRef][Green Version]
- Robles, D.; Brizuela, A.; Fernández-Domínguez, M.; Gil, J. Corrosion Resistance and Titanium Ion Release of Hybrid Dental Implants. Materials 2023, 16, 3650. [Google Scholar] [CrossRef] [PubMed]
- Petković Didović, M.; Jelovica Badovinac, I.; Fiket, Ž.; Žigon, J.; Rinčić Mlinarić, M.; Čanadi Jurešić, G. Cytotoxicity of Metal Ions Released from NiTi and Stainless Steel Orthodontic Appliances, Part 1: Surface Morphology and Ion Release Variations. Materials 2023, 16, 4156. [Google Scholar] [CrossRef]
- Klimek, L.; Makówka, M.; Sobczyk-Guzenda, A.; Kula, Z. Characteristics of Si (C, N) Silicon Carbonitride Layers on the Surface of Ni–Cr Alloys Used in Dental Prosthetics. Materials 2024, 17, 2450. [Google Scholar] [CrossRef] [PubMed]
- Rajib, P. Diamond-Like-Carbon Coatings for Advanced Biomedical Applications. Glob. J. Nanomed. 2017, 5, 1–5. [Google Scholar]
- Maher, N.; Mahmood, A.; Fareed, M.A.; Kumar, N.; Rokaya, D.; Zafar, M.S. An updated review and recent advancements in carbon-based bioactive coatings for dental implant applications. J. Adv. Res. 2024; in press. [Google Scholar] [CrossRef]
- Banaszek, K.; Wiktorowska-Owczarek, A.; Kowalczyk, E.; Klimek, L. Possibilities of applying Ti (C, N) coatings on prosthetic elements—Research with the use of human endothelial cells. Acta Bioeng. Biomater. 2016, 18, 119–126. [Google Scholar]
- Banaszek, K.; Klimek, L.; Zgorzynska, E.; Swarzynska, A.; Walczewska, A. Cytotoxicity of titanium carbonitride coatings for prostodontic alloys with different amounts of carbon and nitrogen. Biomed. Mater. 2018, 13, 045003. [Google Scholar] [CrossRef]
- Banaszek, K.; Klimek, L. Ti (C, N) as Barrier Coatings. Coatings 2019, 9, 432. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Kovrlija, I.; Locs, J.; Loca, D.; Gasik, M. Octacalcium Phosphate-Laden Hydrogels on 3D-Printed Titanium Biomaterials Improve Corrosion Resistance in Simulated Biological Media. Int. J. Mol. Sci. 2023, 24, 13135. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Liu, H.; Zhou, N.; Li, Q.; Yang, G.; Chen, L.; Mou, Y. Surface modified techniques and emerging functional coating of dental implants. Coatings 2020, 10, 1012. [Google Scholar] [CrossRef]
- Ali, M.; Ali, F.; Yang, B.; Abbas, A. A comprehensive account of biomedical applications of CVD diamond coatings. J. Phys. D Appl. Phys. 2021, 54, 443001. [Google Scholar] [CrossRef]
- Saad, K.S.K.; Saba, T.; Rashid, A.B. Application of PVD Coatings in Medical Implantology for Enhanced Performance, Biocompatibility, and Quality of Life. Heliyon 2024, 10, e35541. [Google Scholar]
- VDI 3198:1992-08 Coating (CVD, PVD) of Cold Forging Tools. Available online: https://www.dinmedia.de/en/technical-rule/vdi-3198/930369 (accessed on 1 February 2025).
- ASTM G102-89; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM International: West Conshohocken, PA, USA, 2015.
- Baraniak, M.; Ciszewski, A. Aktywność Chemiczna i Elektrochemiczna Pierwiastków w Środowisku Wody; Wydawnictwo Politechniki Poznańskiej: Poznań, Poland, 2006; ISBN 83-7143-222-4. [Google Scholar]
- Ahn, S.H.; Choi, Y.S.; Kim, J.G.; Han, J.G. A study on corrosion resistance characteristics of PVD Cr-N coated steels by electrochemical method. Surf. Coat. Technol. 2002, 150, 319–326. [Google Scholar] [CrossRef]
- Kula, Z.; Dąbrowska, K.; Klimek, L. Structure and Selected Properties of Si(C,N) Coatings on Ni-Cr Prosthetic Alloys. Processes 2025, 13, 624. [Google Scholar] [CrossRef]
- Moslehifard, E.; Moslehifard, M.; Ghasemzadeh, S.; Ghaiour, M.; Nasirpouri, F. Corrosion Behavior of a Nickel-Base Dental Casting Alloy in Artificial Saliva Studied by Weight Loss and Polarization Techniques. Front. Dent. 2019, 16, 13–20. [Google Scholar] [CrossRef]
- Metel, A.; Sotova, C.; Fyodorov, S.; Zhylinski, V.; Chayeuski, V.; Milovich, F.; Seleznev, A.; Bublikov, Y.; Makarevich, K.; Vereschaka, A. Improving the Wear and Corrosion Resistance of Titanium Alloy Parts via the Deposition of DLC Coatings. C 2024, 10, 106. [Google Scholar] [CrossRef]
- Singh, A.K.; Drunka, R.; Smits, K.; Vanags, M.; Iesalnieks, M.; Joksa, A.A.; Blumbergs, I.; Steins, I. Nanomechanical and Electrochemical Corrosion Testing of Nanocomposite Coating Obtained on AZ31 via Plasma Electrolytic Oxidation Containing TiN and SiC Nanoparticles. Crystals 2023, 13, 508. [Google Scholar] [CrossRef]
- Mirzayev, M.N.; Parau, A.C.; Slavov, L.; Dinu, M.; Neov, D.; Slavkova, Z.; Popov, E.P.; Belova, M.; Hasanov, K.; Aliyev, F.A.; et al. TiSiCN as Coatings Resistant to Corrosion and Neutron Activation. Materials 2023, 16, 1835. [Google Scholar] [CrossRef] [PubMed]
- Mirzayev, M.N.; Hasanov, K.M.; Parau, A.C.; Demir, E.; Abiyev, A.S.; Karaman, T.; Jabarov, S.H.; Dinu, M.; Popov, E.P.; Vladescu, A.D. Effect of the C/N ratio modification on the corrosion behavior and performance of carbonitride coatings prepared by cathodic arc deposition. J. Mater. Res. Technol. 2023, 27, 1724–1738. [Google Scholar] [CrossRef]
- Xia, X.; Chiang, C.-C.; Gopalakrishnan, S.K.; Kulkarni, A.V.; Ren, F.; Ziegler, K.J.; Esquivel-Upshaw, J.F. Properties of SiCN Films Relevant to Dental Implant Applications. Materials 2023, 16, 5318. [Google Scholar] [CrossRef] [PubMed]

| Specimen | Element | ||||||
|---|---|---|---|---|---|---|---|
| Si | N | C | at. C/N | ||||
| at. [%] | wt. [%] | at. [%] | wt. [%] | at. [%] | wt. [%] | ||
| B | 24.8 | 38.5 | - | - | 75.2 | 61.5 | - |
| C | 29.6 | 46.7 | 15.9 | 12.5 | 54.5 | 40.8 | 3.4 |
| D | 35.2 | 53.3 | 25.2 | 19.0 | 39.6 | 27.7 | 1.6 |
| E | 42.9 | 61.0 | 35.3 | 25.0 | 21.8 | 14.0 | 0.6 |
| F | 47.7 | 64.7 | 52.3 | 35.3 | - | - | - |
| Specimen | Rp (MOhm cm2) | Icor (nA cm−2) | CR (mmPY) | MR (g m−2 d−1) |
|---|---|---|---|---|
| A | 0.07 ± 0.01 | 355.6 ± 4.1 | (3.33 ± 0.04)·10−3 | (7.57 ± 0.09)·10−2 |
| B | 2.20 ± 0.45 | 12.2 ± 2.2 | (1.14 ± 0.21)·10−4 | (2.59 ± 0.48)·10−3 |
| C | 2.27 ± 0.69 | 12.3 ± 3.9 | (1.15 ± 0.36)·10−4 | (2.61 ± 0.83)·10−3 |
| D | 4.44 ± 0.20 | 5.9 ± 2.1 | (3.24 ± 1.97)·10−5 | (7.36 ± 4.47)·10−4 |
| E | 2.50 ± 0.39 | 10.6 ± 1.6 | (9.92 ± 1.49)·10−5 | (2.25 ± 0.34)·10−3 |
| F | 2.07 ± 0.21 | 9.6 ± 1.3 | (8.96 ± 1.23)·10−5 | (2.04 ± 0.28)·10−3 |
| Element | Mass Fraction | Valence | Atomic Weight | EW |
|---|---|---|---|---|
| Ni | 0.6423 | 2 | 58.69 | 23.77 |
| Cr | 0.2436 | 3 | 52.00 | |
| Mo | 0.0891 | 4 | 95.94 | |
| Si | 0.0143 | 4 | 28.09 | |
| Fe | 0.0107 | 2 | 55.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kula, Z.; Burnat, B.; Dąbrowska, K.; Klimek, L. The Influence of Si(C,N) Layer Composition on the Corrosion of NiCr Prosthetic Alloy. Ceramics 2025, 8, 50. https://doi.org/10.3390/ceramics8020050
Kula Z, Burnat B, Dąbrowska K, Klimek L. The Influence of Si(C,N) Layer Composition on the Corrosion of NiCr Prosthetic Alloy. Ceramics. 2025; 8(2):50. https://doi.org/10.3390/ceramics8020050
Chicago/Turabian StyleKula, Zofia, Barbara Burnat, Katarzyna Dąbrowska, and Leszek Klimek. 2025. "The Influence of Si(C,N) Layer Composition on the Corrosion of NiCr Prosthetic Alloy" Ceramics 8, no. 2: 50. https://doi.org/10.3390/ceramics8020050
APA StyleKula, Z., Burnat, B., Dąbrowska, K., & Klimek, L. (2025). The Influence of Si(C,N) Layer Composition on the Corrosion of NiCr Prosthetic Alloy. Ceramics, 8(2), 50. https://doi.org/10.3390/ceramics8020050










