A Novel Approach to Strengthening the Microtensile Bond Between Lithium Disilicate Ceramics Manufactured by CAD/CAM and Dentin Using Coatings of Natural and Synthetic Bio-Modifiers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, and Ethical Approval
2.2. Sample Size Calculation
2.3. Randomization, Grouping, and Blinding
2.4. Preparation of 20% Salvadora persica Extract
2.5. Specimen Preparation
2.6. Digital Impression Fabrication and Cementation of Ceramic Onlays
2.7. Dentin Pretreatment
2.8. Cementation of Ceramic Onlays
2.9. Thermomechanical Cyclic Loading
2.10. Microtensile Bond Strength Test (μTBS)
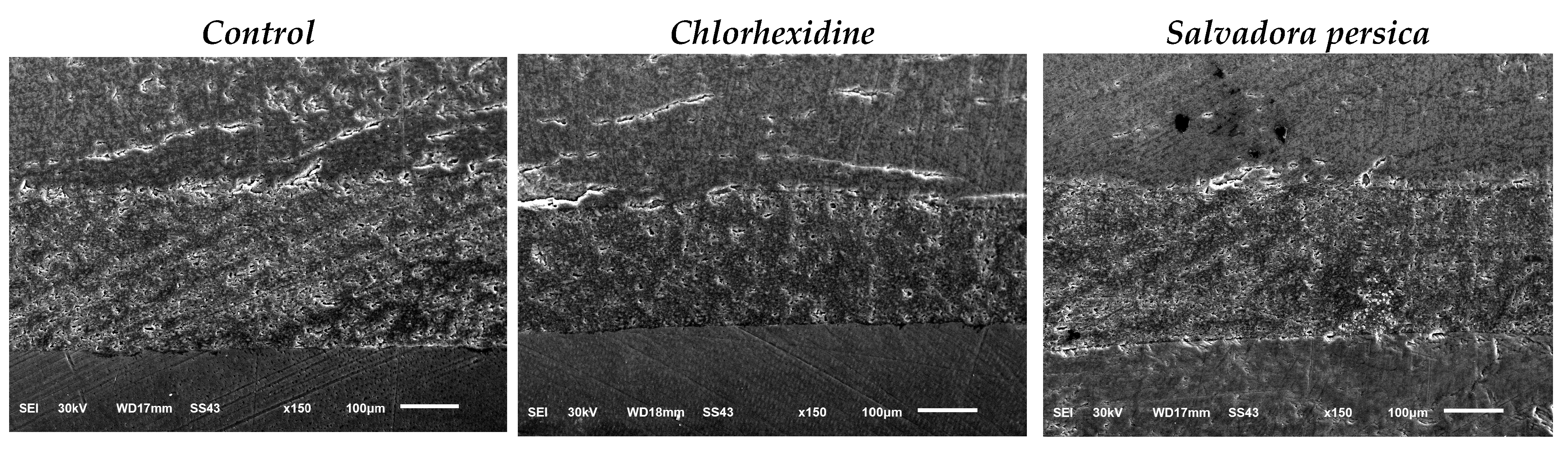
2.11. Nanoleakage Analysis (nL)
2.12. Mode of Failure Analysis
2.13. Statistical Analysis
3. Results
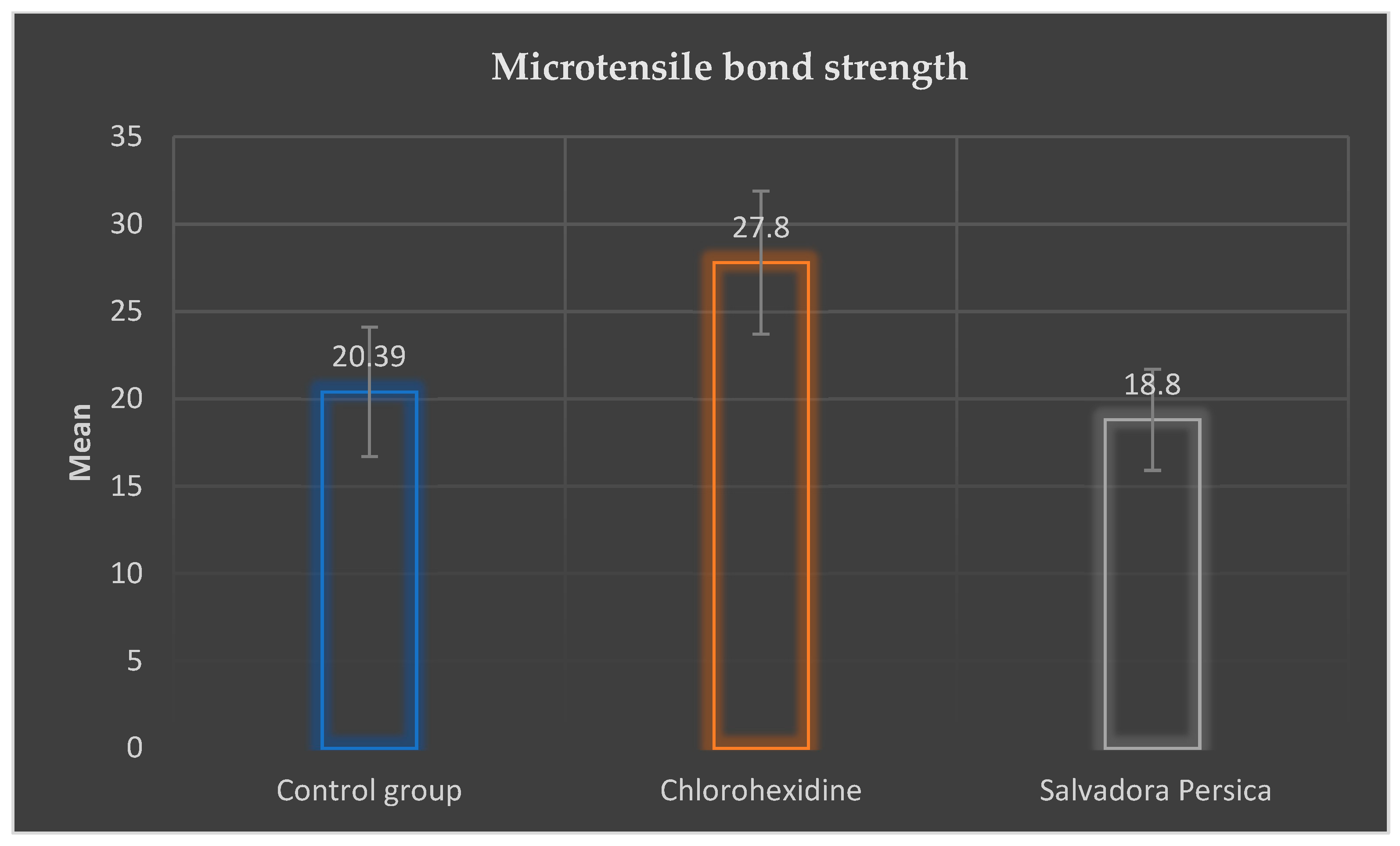
3.1. Microtensile Bond Strength Values
3.2. Nanoleakage Values
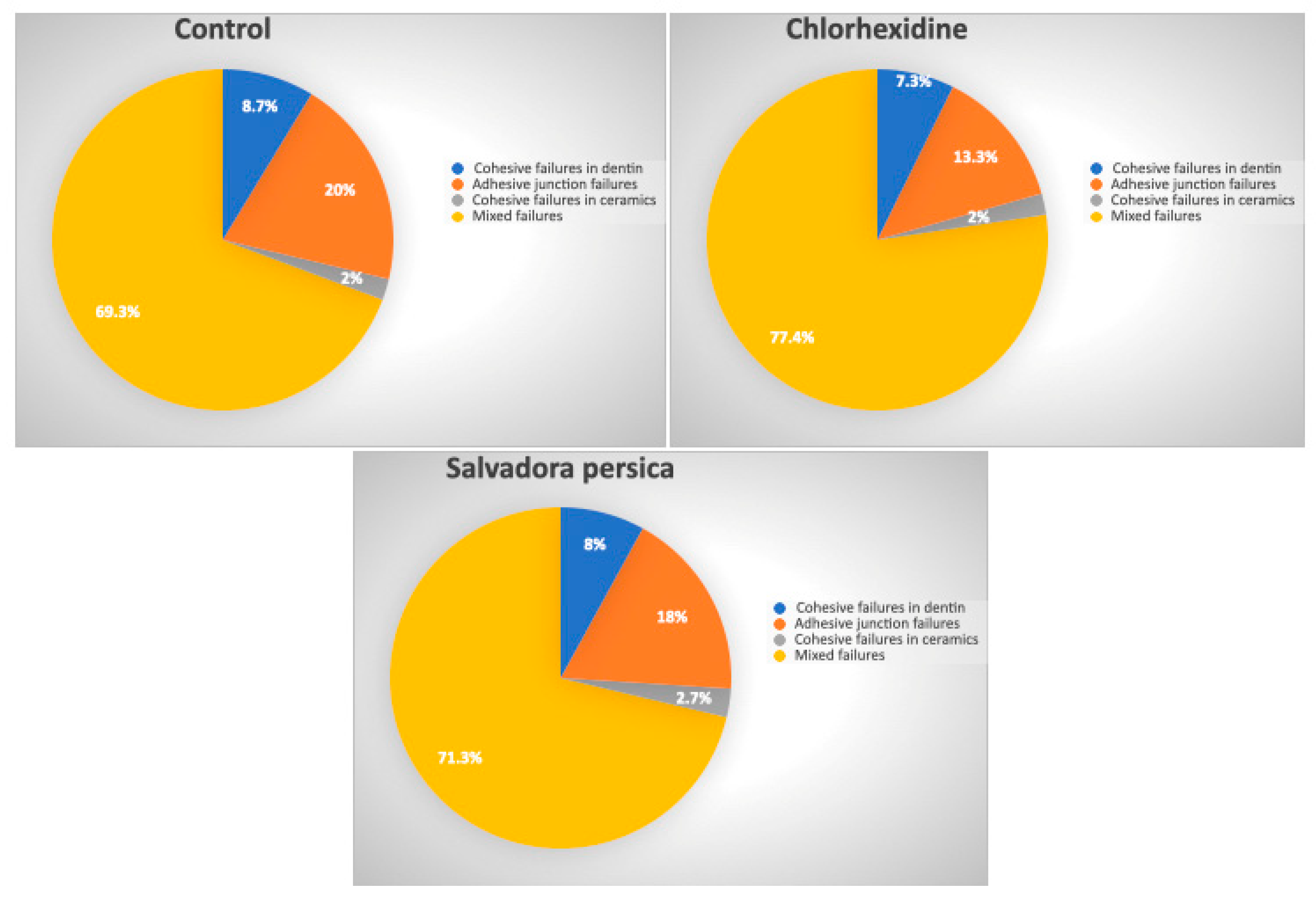
3.3. Distribution of the Failure Mode
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Juntavee, N.; Juntavee, A.; Wongnara, K.; Klomklorm, P.; Khechonnan, R. Shear bond strength of ceramic bracket bonded to different surface-treated ceramic materials. J. Clin. Exp. Dent. 2018, 10, e1167–e1176. [Google Scholar] [PubMed]
- Oztürk, E.; Hickel, R.; Bolay, S.; Ilie, N. Micromechanical properties of veneer luting resins after curing through ceramics. Clin. Oral. Investig. 2012, 16, 139–146. [Google Scholar] [PubMed]
- Carrilho, M.; Geraldeli, S.; Tay, F.; De Goes, M.; Carvalho, R.M.; Tjäderhane, L.; Reis, A.; Hebling, J.; Mazzoni, A.; Breschi, L. In vivo preservation of the hybrid layer by chlorhexidine. J. Dent. Res. 2007, 86, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Abdeltawab, S.S.; Abu Haimed, T.S.; Bahammam, H.A.; Arab, W.T.; Abou Neel, E.A.; Bahammam, L.A. Biocompatibility and antibacterial action of Salvadora persica extract as intracanal medication (in vitro and ex vivo experiment). Materials 2022, 15, 1373. [Google Scholar] [CrossRef]
- Deshpande, R.R.; Kale, A.A.; Ruikar, A.D.; Panvalkar, P.S.; Kulkarni, A.A.; Deshpande, N.R.; Salvekar, J.P. Antimicrobial activity of different extracts of Juglans regia L. against oral microflora. Int. J. Pharm. Pharm. Sci. 2011, 3, 200–201. [Google Scholar]
- Castellan, C.S.; Bedran-Russo, A.K.; Antunes, A.; Pereira, P.N. Effect of dentin biomodification using naturally derived collagen cross-linkers: One-year bond strength study. Int. J. Dent. 2013, 2013, 918010. [Google Scholar]
- Castellan, C.S.; Bedran-Russo, A.K.; Karol, S.; Pereira, P.N.R. Long-term stability of dentin matrix following treatment with various natural collagen cross-linkers. J. Mech. Behav. Biomed. Mater. 2011, 4, 1343–1350. [Google Scholar]
- El Gindy, A.H.; Sherief, D.I.; El-Korashy, D.I. Effect of dentin biomodification using natural collagen cross-linkers on the durability of the resin-dentin bond and demineralized dentin stiffness. J. Mech. Behav. Biomed. Mater. 2023, 138, 105551. [Google Scholar]
- Aljarbou, F.; Almobarak, A.; Binrayes, A.; Alamri, H.M. Salvadora persica’s biological properties and applications in different dental specialties: A narrative review. J. Evid. Based. Complementary. Altern. Med. 2022, 2022, 8667687. [Google Scholar]
- Khunkar, S.; Hariri, I.; Alsayed, E.; Linjawi, A.; Khunkar, S.; Islam, S.; Bakhsh, T.A.; Nakashima, S. Inhibitory effect of Salvadora persica extract (Miswak) on collagen degradation in demineralized dentin: In vitro study. J. Dent. Sci. 2021, 16, 208–213. [Google Scholar]
- World Health Organization. Consensus statement on oral hygiene. Int. Dent. J. 2000, 50, 139. [Google Scholar]
- Hassona, Y. An Interview with ChatGPT About Special Care Dentistry. In Special Care in Dentistry; American Association of Hospital Dentists, The Academy of Dentistry for the Handicapped, The American Society for Geriatric Dentistry: Chicago, IL, USA, 2024. [Google Scholar]
- Al Bratty, M.; Makeen, H.A.; Alhazmi, H.A.; Syame, S.M.; Abdalla, A.N.; Homeida, H.E.; Sultana, S.; Ahsan, W.; Khalid, A. Phytochemical, cytotoxic, and antimicrobial evaluation of the fruits of miswak plant, Salvadora persica L. J. Chemi. 2020, 2020, 4521951. [Google Scholar] [CrossRef]
- Ghahri, S.; Chen, X.; Pizzi, A.; Hajihassani, R.; Papadopoulos, A.N. Natural tannins as new cross-linking materials for soy-based adhesives. Polymers 2021, 13, 595. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Nascimento, F.; Carrilho, M.; Tersariol, I.; Papa, V.; Tjäderhane, L.; Di Lenarda, R.; Tay, F.; Pashley, D.H.; Breschi, L. MMP activity in the hybrid layer detected with in situ zymography. J. Dent. Res. 2012, 91, 467–472. [Google Scholar] [CrossRef]
- Tjäderhane, L.; Nascimento, F.D.; Breschi, L.; Mazzoni, A.; Tersariol, I.L.; Geraldeli, S.; Tezvergil-Mutluay, A.; Carrilho, M.R.; Carvalho, R.M.; Tay, F.R. Optimizing dentin bond durability: Control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dent. Mater. 2013, 29, 116–135. [Google Scholar] [CrossRef]
- Nishitani, Y.; Yoshiyama, M.; Wadgaonkar, B.; Breschi, L.; Mannello, F.; Mazzoni, A.; Carvalho, R.M.; Tjäderhane, L.; Tay, F.R.; Pashley, D.H. Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur. J. Oral. Sci. 2006, 114, 160–166. [Google Scholar] [CrossRef]
- Mazzoni, A.; Pashley, D.H.; Nishitani, Y.; Breschi, L.; Mannello, F.; Tjäderhane, L.; Toledano, M.; Pashley, E.L.; Tay, F.R. Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials 2006, 27, 4470–4476. [Google Scholar] [CrossRef]
- Carrilho, M.R.; Tay, F.R.; Donnelly, A.M.; Agee, K.A.; Tjäderhane, L.; Mazzoni, A.; Breschi, L.; Foulger, S.; Pashley, D.H. Host-derived loss of dentin matrix stiffness associated with solubilization of collagen. J. Biomed. Mater. Res. A Appl. Biomater. 2009, 90, 373–380. [Google Scholar] [CrossRef]
- De Munck, J.; Van den Steen, P.; Mine, A.; Van Landuyt, K.; Poitevin, A.; Opdenakker, G.; Van Meerbeek, B. Inhibition of enzymatic degradation of adhesive-dentin interfaces. J. Esthet. Restor. Dent. 2011, 23, 350–352. [Google Scholar] [CrossRef]
- Breschi, L.; Martin, P.; Mazzoni, A.; Nato, F.; Carrilho, M.; Tjäderhane, L.; Visintini, E.; Cadenaro, M.; Tay, F.R.; Dorigo, E.D.S. Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dent. Mater. 2010, 26, 571–578. [Google Scholar] [CrossRef]
- Yiu, C.K.; Hiraishi, N.; Tay, F.R.; King, N.M. Effect of chlorhexidine incorporation into dental adhesive resin on durability of resin-dentin bond. J. Adhes. Dent. 2012, 14, 355. [Google Scholar] [PubMed]
- Mazzoni, A.; Angeloni, V.; Apolonio, F.M.; Scotti, N.; Tjäderhane, L.; Tezvergil-Mutluay, A.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; Breschi, L. Effect of carbodiimide (EDC) on the bond stability of etch-and-rinse adhesive systems. Dent. Mater. 2013, 29, 1040–1047. [Google Scholar] [PubMed]
- Montagner, A.; Sarkis-Onofre, R.; Pereira-Cenci, T.; Cenci, M. MMP inhibitors on dentin stability: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 733–743. [Google Scholar] [PubMed]
- Abunawareg, M.; Abuelenain, D.; Elkassas, D.; Haimed, T.A.; Al-Dharrab, A.; Zidan, A.; Hassan, A.; Pashley, D. Role of dentin cross-linking agents in optimizing dentin bond durability. Int. J. Adhes. Adhes. 2017, 78, 83–88. [Google Scholar] [CrossRef]
- Mazzoni, A.; Angeloni, V.; Comba, A.; Maravic, T.; Cadenaro, M.; Tezvergil-Mutluay, A.; Pashley, D.H.; Tay, F.R.; Breschi, L. Cross-linking effect on dentin bond strength and MMPs activity. Dent. Mater. 2018, 34, 288–295. [Google Scholar]
- Zhou, J.; Chiba, A.; Scheffel, D.L.; Hebling, J.; Agee, K.; Tagami, J.; Tan, J.; Abuelenain, D.; Nawareg, M.A.; Hassan, A.H. Cross-linked dry bonding: A new etch-and-rinse technique. Dent. Mater. 2016, 32, 1124–1132. [Google Scholar] [CrossRef]
- Husain, S.; Al-Samadani, K.H.; Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Qasim, S.B. Chitosan biomaterials for current and potential dental applications. Materials. 2017, 10, 602. [Google Scholar]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2012, 10, 28–55. [Google Scholar]
- Abu-Nawareg, M.M.; Abouelseoud, H.K.; Zidan, A.Z. Effect of Salvadora persica on resin-dentin bond stability. BMC Oral Health 2024, 24, 505. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar]
- Tisler, C.E.; Chifor, R.; Badea, M.E.; Moldovan, M.; Prodan, D.; Carpa, R.; Cuc, S.; Chifor, I.; Badea, A.F. Photodynamic Therapy (PDT) in Prosthodontics: Disinfection of Human Teeth Exposed to Streptococcus mutans and the Effect on the Adhesion of Full Ceramic Veneers, Crowns, and Inlays: An In Vitro Study. Biomedicines 2022, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-P.; Douglas, W.H. Failure mechanisms at the human dentin-resin interface: A fracture mechanics approach. J. Biomech. 1994, 27, 1037–1047. [Google Scholar] [PubMed]
- Al-karadsheh, O.; Albalsheh, M.; Zabadi, S.; Alhaddad, A.; Hassona, Y.; Sawair, F. The Social Impact of Dental Implant Research: An Altmetric Analysis. J. Int. Dent. Med. Res. 2024, 17, 1371–1379. [Google Scholar]
- De Moraes, I.Q.S.; do Nascimento, T.G.; da Silva, A.T.; de Lira, L.M.S.S.; Parolia, A.; de Moraes Porto, I.C.C. Inhibition of matrix metalloproteinases: A troubleshooting for dentin adhesion. Restor. Dent. Endod. 2020, 45, e31. [Google Scholar]
- Hannas, A.R.; Pereira, J.C.; Granjeiro, J.M.; Tjäderhane, L. The role of matrix metalloproteinases in the oral environment. Acta Odontol. Scand. 2007, 65, 1–13. [Google Scholar]
- Vasei, F. Effect of chitosan treatment on shear bond strength of composite to deep dentin using self-etch and total-etch adhesive systems. Braz. Dent. Sci. 2021, 24, 2440. [Google Scholar]
- Milani, S.; Seraj, B.; Khoshlafz, Z.; Abazarian, N. Effect of dentin pretreatment with chlorhexidine on push-out bond strength of composite restorations in severely damaged primary anterior teeth. Front. Dent. 2020, 17, 1–6. [Google Scholar]
- Zaghloul, S.A.; Gabal, Z.A.; Abdul-Rahman, F.A.-Z.M. Shear Bond Strength of Ceramics to Chlorhexidine versus Pomegranate Peel Extract–Pretreated Dentin: A Randomized Control Study. J. Nat. Sci. Med. 2023, 6, 109–113. [Google Scholar]
- Borompiyasawat, P.; Putraphan, B.; Luangworakhun, S.; Sukarawan, W.; Techatharatip, O. Chlorhexidine gluconate enhances the remineralization effect of high viscosity glass ionomer cement on dentin carious lesions in vitro. BMC Oral Health 2022, 22, 60. [Google Scholar]
- Uctasli, M.; Mutluay, M.M.; Tezvergil-Mutluay, A. Effect of High Intensity Light Curing, Cavity Depth and Aging on Bond Strength. Dent. Mater. 2023, 39, e77. [Google Scholar]
- Burrer, P.; Dang, H.; Par, M.; Attin, T.; Tauböck, T.T. Effect of over-etching and prolonged application time of a universal adhesive on dentin bond strength. Polymers 2020, 12, 2902. [Google Scholar] [CrossRef] [PubMed]
- Elkaffas, A.A.; Hamama, H.H.; Mahmoud, S.H.; Fawzy, A.S. Effect of acid etching on dentin bond strength of ultra-mild self-etch adhesives. Int. J. Adhes. Adhes. 2020, 99, 102567. [Google Scholar]
- Campos, E.A.d.; Correr, G.M.; Leonardi, D.P.; Pizzatto, E.; Morais, E.C. Influence of chlorhexidine concentration on microtensile bond strength of contemporary adhesive systems. Braz. Oral. Res. 2009, 23, 340–345. [Google Scholar] [PubMed]
- Collares, F.M.; Rodrigues, S.B.; Branco Leitune, V.C.; Celeste, R.K.; De Araújo, F.B.; Werner Samuel, S.M. Chlorhexidine application in adhesive procedures: A meta-regression analysis. J. Adhes. Dent. 2013, 15, 11–18. [Google Scholar]
- Meiers, J.; Kresin, J. Cavity disinfectants and dentin bonding. Oper. Dent. 1996, 21, 153–159. [Google Scholar]
- Tulunoglu, O.; Ayhan, H.; Olmez, A.; Bodur, H. The effect of cavity disinfectants on microleakage in dentin bonding systems. J. Clin. Pediatr. Dent. 1998, 22, 299–305. [Google Scholar]


| Materials | Description | Composition | Batch Number | Manufacturer |
|---|---|---|---|---|
| Salvadora persica Extract | 20% aqauos solution of Salvadora persica extract | - | - | - |
| Chlorhexidine | 2% chlorhexidine gluconate | - | 6776544 | Consepsis™, Ultradent, Inc., South Jordan, Utah, USA |
| IPS e.max CAD | Lithium disilicate | SiO2, Li2O, K2O, MgO, ZnO, Al2O3, P2O5, and other oxides. | M415688 | Ivoclar Vivadent, Amherst, NY, USA |
| Variolink Esthetic DC | Photopolymerized luting cement | Urethane dimethacrylate, decamethylene dimethacrylate, inorganic fillers, ytterbium trifluoride, initiators, stabilizers, and pigments | Z0025D | Ivoclar Vivadent, Amherst, NY, USA |
| Monobond plus | Universal primer | Silane methacrylate, phosphoric acid methacrylaye, and sulfide methacrylate | Z30WZW | Ivoclar Vivadent, Amherst, NY, USA |
| Ceramic etching gel | Hydrofluoric acid | <5% hydrofluoric acid | 100001664 | Ivoclar Vivadent, Amherst, NY, USA |
| N-Etch | 37% phosphoric acid gel | 85% phosphoric acid, distilled water, pigments, and thickiners | K17720 | Ivoclar Vivadent, Amherst, NY, USA |
| Try-in Paste | Glycerin | Glycerin, mineral fillers, and dyes | 7405123 | Ivoclar Vivadent, Amherst, NY, USA |
| Adhese Universal | Light curing, single bottle universal dental adhesive | HEMA, Bis-GMA, MDP, D3MA, etanol, water, methacrylate modified polyacylic acid, silicon dioxide, camphorquinone, ethyl-p-dimethyl aminobenzoate, and 2-di-methyl aminoethyl methacrylate | Z02R65 | Ivoclar Vivadent, Amherst, NY, USA |
| Control Group n = 10 | Chlorhexidine n = 10 | Salvadora persica n = 10 | ||
|---|---|---|---|---|
| Microtensile bond strength (μTBS) | 20.39 MPa ± 3.7 a | 27.80 MPa ± 4.1 b | 18.80 MPa ± 2.9 c | F = 17.78 p < 0.001 * |
| Control Group n = 10 | Chlorhexidine n = 10 | Salvadora persica n = 10 | ||
|---|---|---|---|---|
| Nanoleakage | 47.39 ± 8.6 a | 39.62 ± 7.9 b | 49.27 ± 9.1 c | F = 3.58 p = 0.04 * |
| Control/Experimental Group | Failure Mode | |||
|---|---|---|---|---|
| Cohesive Failures in Dentin | Adhesive Junction Failures | Cohesive Failures in Ceramics | Mixed Failures | |
| Control | 13/150 | 30/150 | 3/150 | 104/150 |
| Chlorhexidine | 11/150 | 20/150 | 3/150 | 116/150 |
| Salvadora persica | 12/150 | 27/150 | 4/150 | 107/150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almudahi, A.; Alshehri, A.; Alqahtani, A.R.; Almutairi, B.; Elkaffas, A.A.; Albaijan, R.S.; Abuelqomsan, M.A. A Novel Approach to Strengthening the Microtensile Bond Between Lithium Disilicate Ceramics Manufactured by CAD/CAM and Dentin Using Coatings of Natural and Synthetic Bio-Modifiers. Ceramics 2025, 8, 34. https://doi.org/10.3390/ceramics8020034
Almudahi A, Alshehri A, Alqahtani AR, Almutairi B, Elkaffas AA, Albaijan RS, Abuelqomsan MA. A Novel Approach to Strengthening the Microtensile Bond Between Lithium Disilicate Ceramics Manufactured by CAD/CAM and Dentin Using Coatings of Natural and Synthetic Bio-Modifiers. Ceramics. 2025; 8(2):34. https://doi.org/10.3390/ceramics8020034
Chicago/Turabian StyleAlmudahi, Abdulellah, Abdullah Alshehri, Ali R. Alqahtani, Basil Almutairi, Ali A. Elkaffas, Refal Saad Albaijan, and Mohammed Ali Abuelqomsan. 2025. "A Novel Approach to Strengthening the Microtensile Bond Between Lithium Disilicate Ceramics Manufactured by CAD/CAM and Dentin Using Coatings of Natural and Synthetic Bio-Modifiers" Ceramics 8, no. 2: 34. https://doi.org/10.3390/ceramics8020034
APA StyleAlmudahi, A., Alshehri, A., Alqahtani, A. R., Almutairi, B., Elkaffas, A. A., Albaijan, R. S., & Abuelqomsan, M. A. (2025). A Novel Approach to Strengthening the Microtensile Bond Between Lithium Disilicate Ceramics Manufactured by CAD/CAM and Dentin Using Coatings of Natural and Synthetic Bio-Modifiers. Ceramics, 8(2), 34. https://doi.org/10.3390/ceramics8020034






