Tagetes erecta—Mediated Green Synthesis of ZnO–Ag Nanocomposites: Characterization and Dual Applications in Solar Photocatalytic Degradation and Antibacterial Activity
Abstract
1. Introduction
2. Materials and Methods
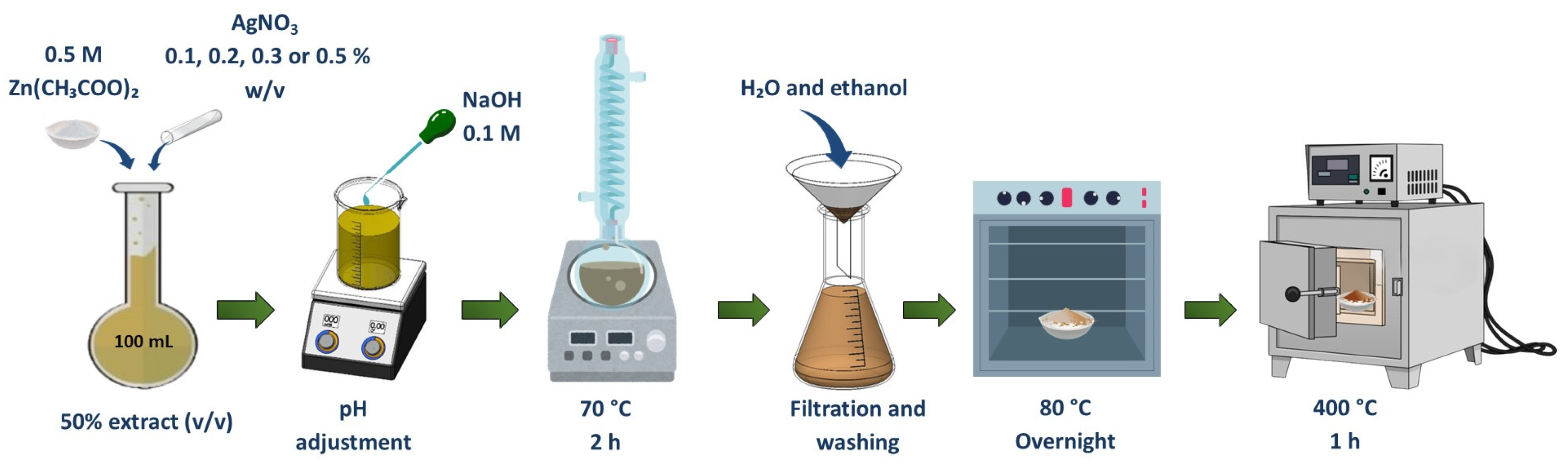
2.1. Synthesis of ZnO–Ag Nanocomposites
2.2. Characterization of ZnO–Ag Nanocomposites
2.2.1. UV-Vis Spectroscopy
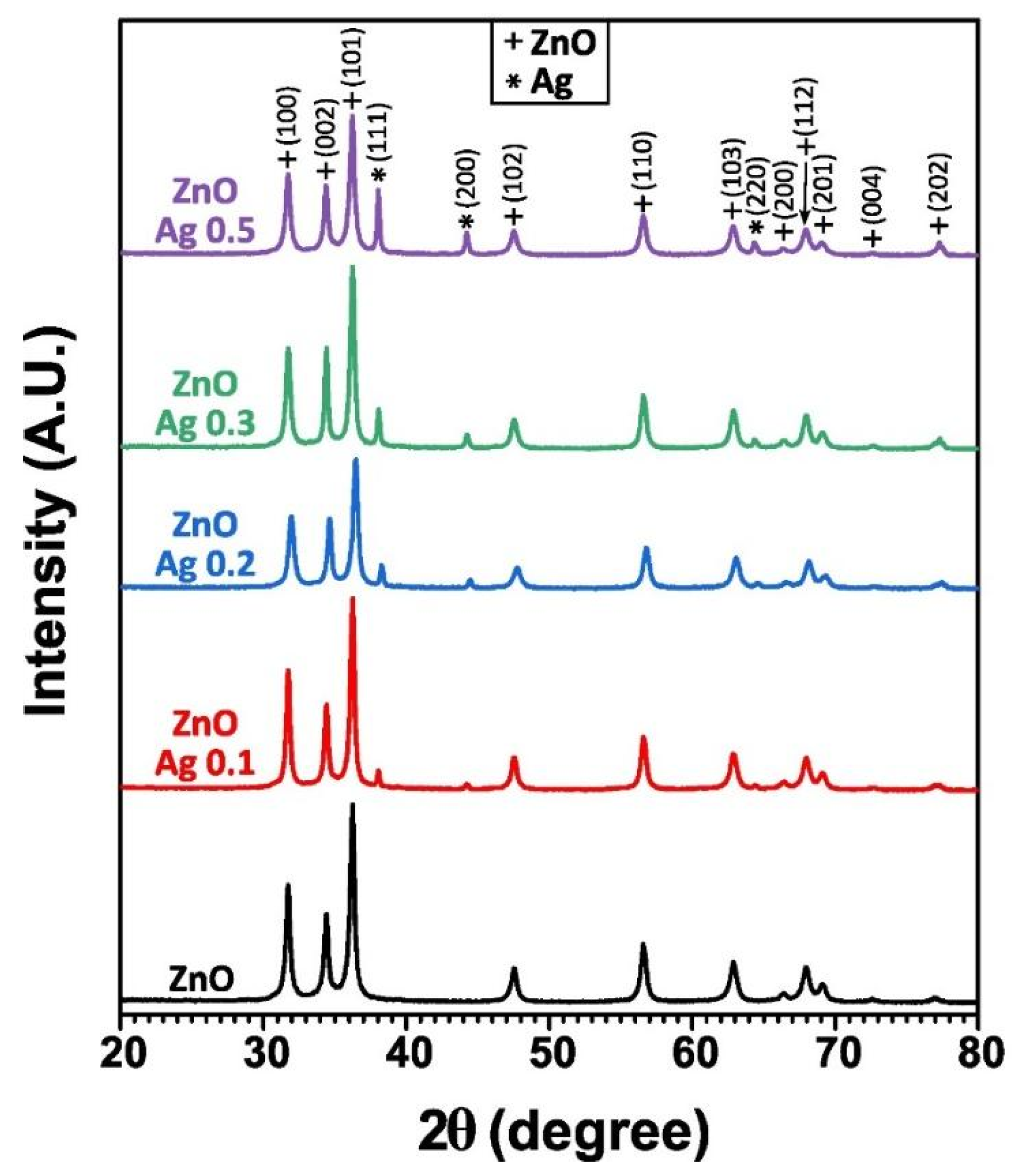
2.2.2. X-Ray Diffraction (XRD)
2.2.3. Transmission Electron Microscopy (TEM)
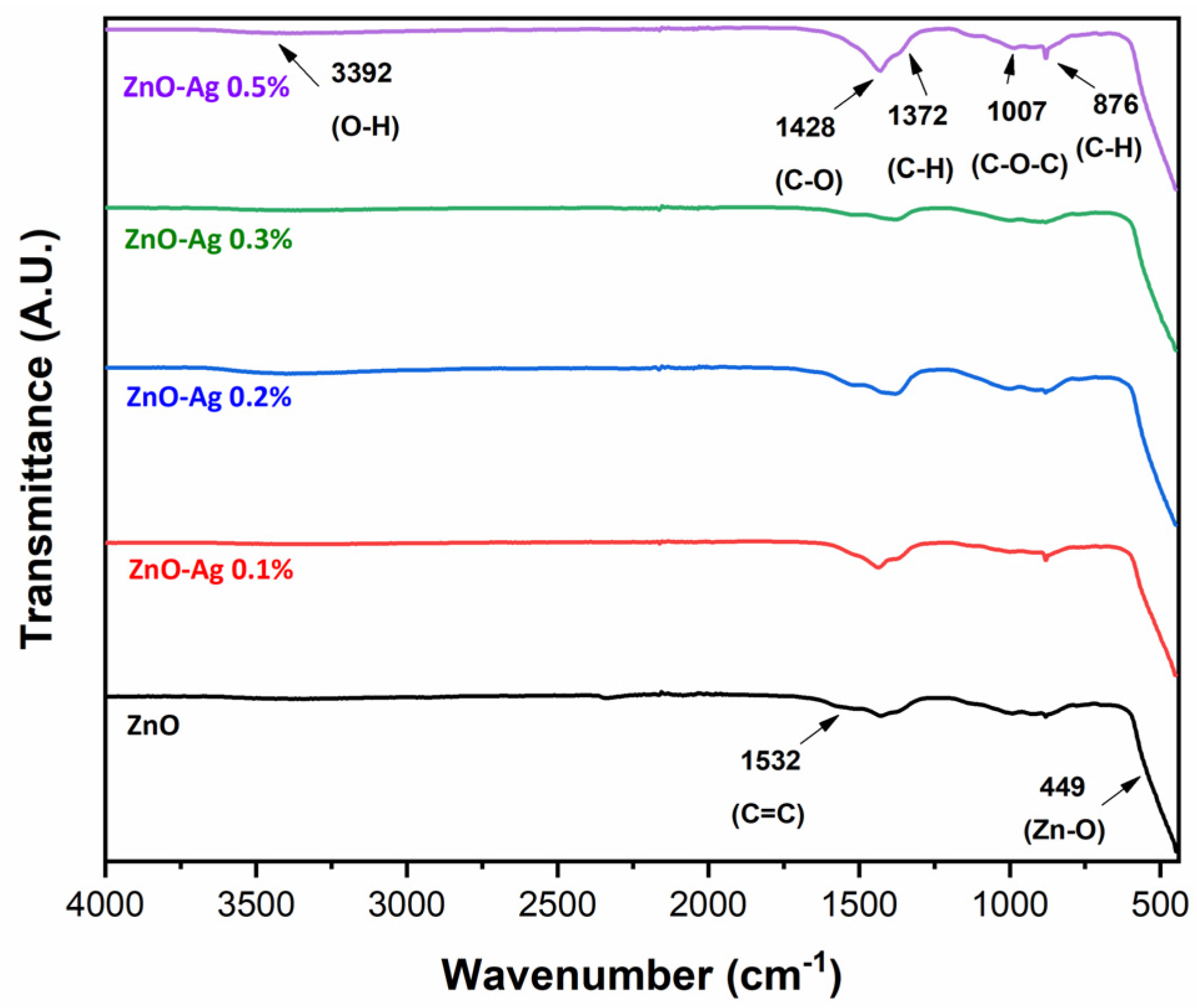
2.2.4. Fourier Transform Infrared (FTIR)
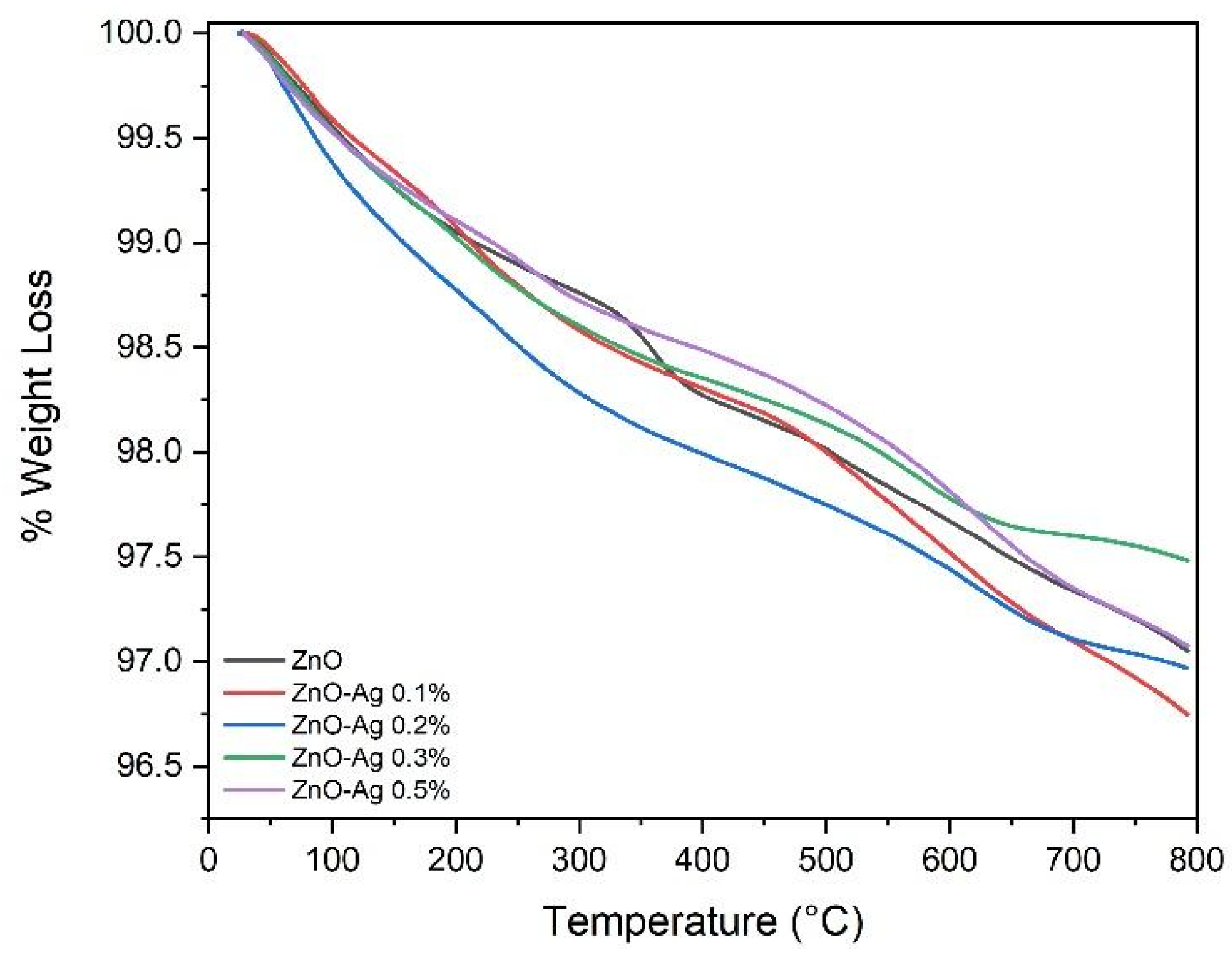
2.2.5. Thermogravimetric Analysis (TGA)
2.3. Photocatalytic Performance of ZnO–Ag Nanocomposites
2.4. Antibacterial Activity of ZnO–Ag Nanocomposites
2.4.1. Strains and Bacterial Culture Media
2.4.2. ZnO–Ag Nanocomposites Evaluated in the Antibacterial Assays
2.4.3. Inhibition of Bacterial Growth in the Presence of Nanoparticles
3. Results and Discussion
3.1. Characterization of ZnO NPs
3.1.1. UV-Vis Spectroscopy
3.1.2. X-Ray Diffraction (XRD)
3.1.3. Transmission Electron Microscopy (TEM)
3.1.4. Fourier Transform Infrared (FTIR) Spectroscopy
3.1.5. Thermogravimetric Analysis
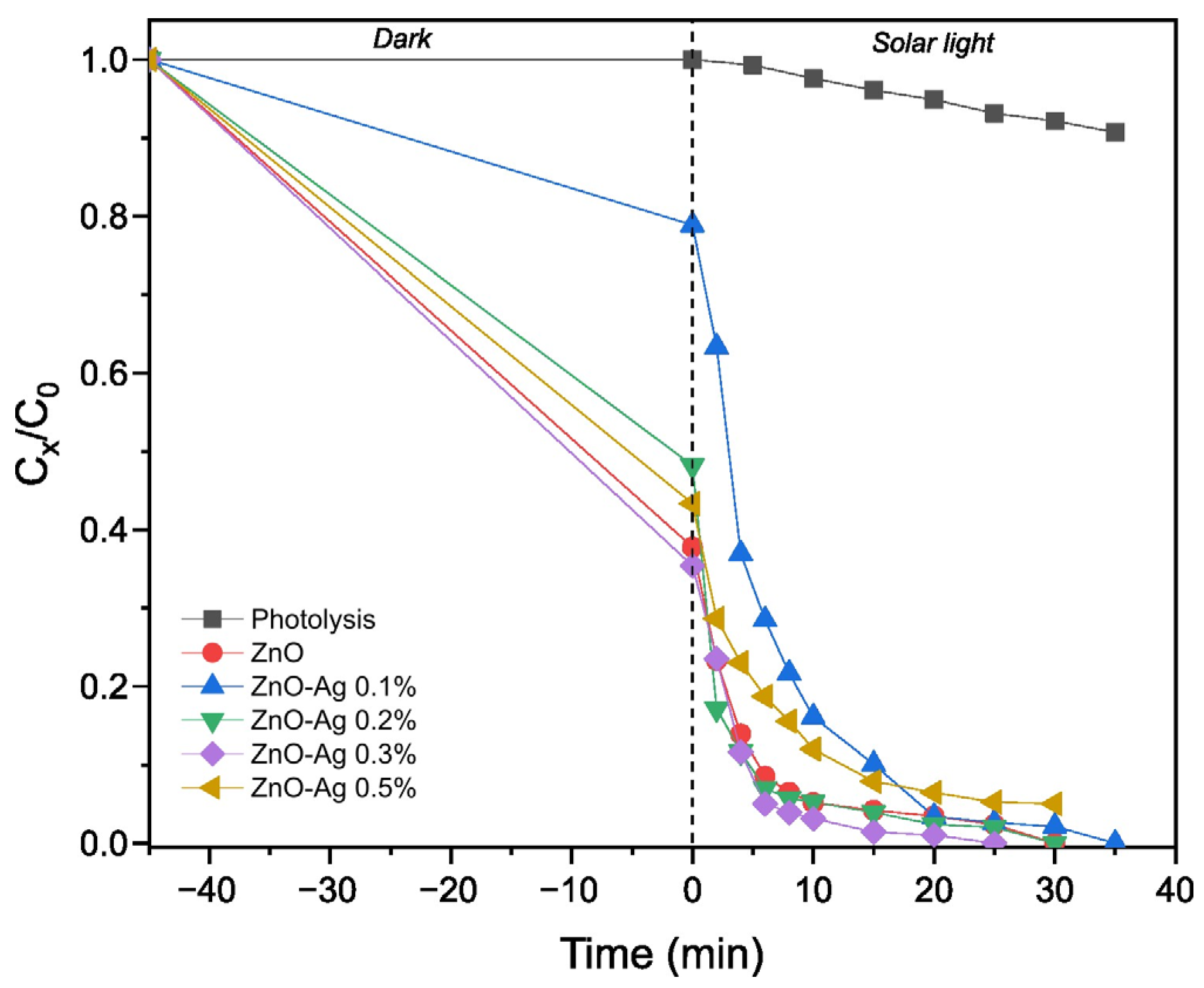
3.2. Photocatalytic Performance
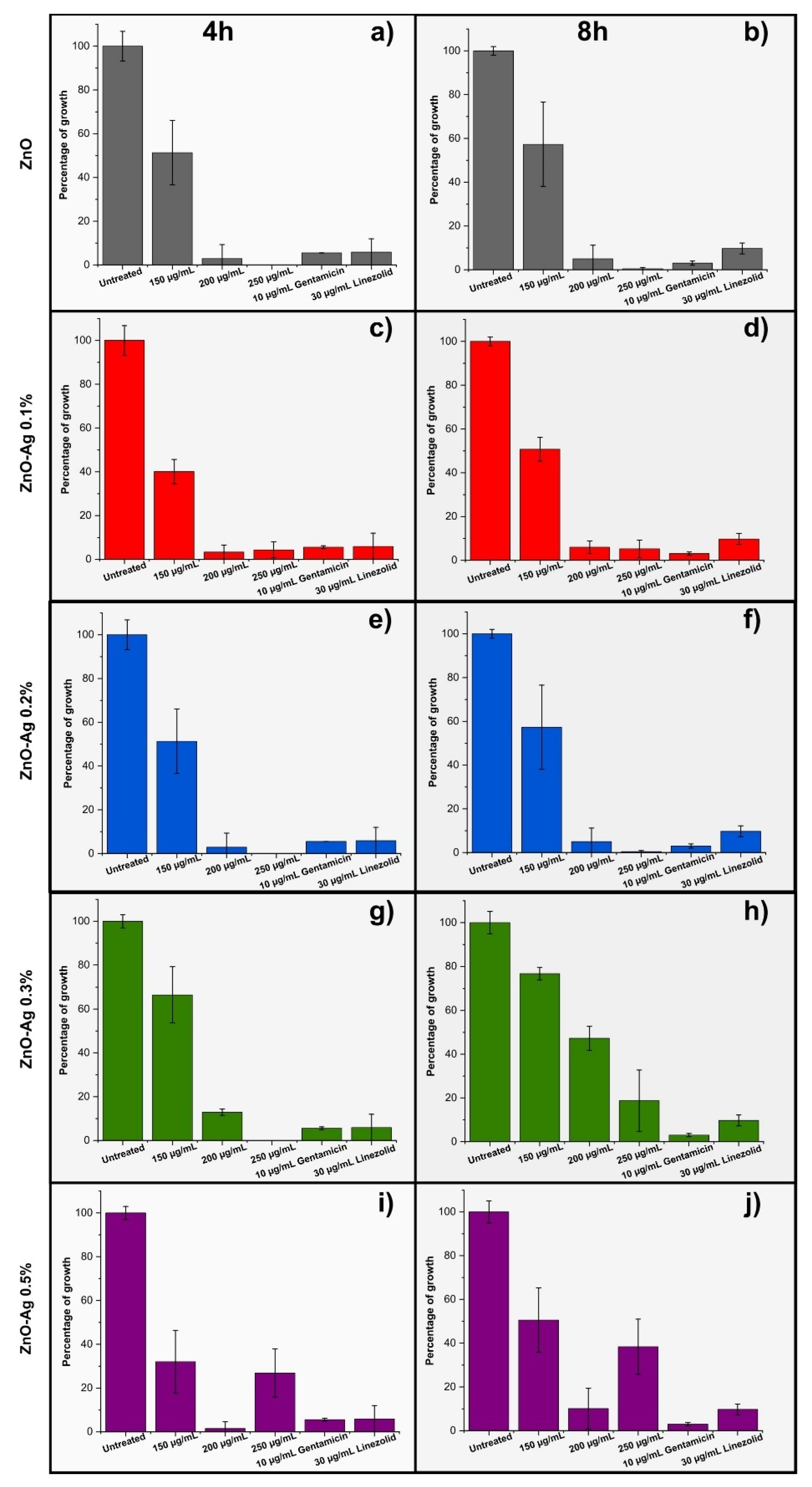
3.3. Antibacterial Activity
3.3.1. Antibacterial Activity of ZnO and Ag with Marigold Flower Extract Against S. aureus ATCC 14923
3.3.2. Antibacterial Activity of ZnO and Ag with Marigold Flower Extract Against E. coli ATCC 25922
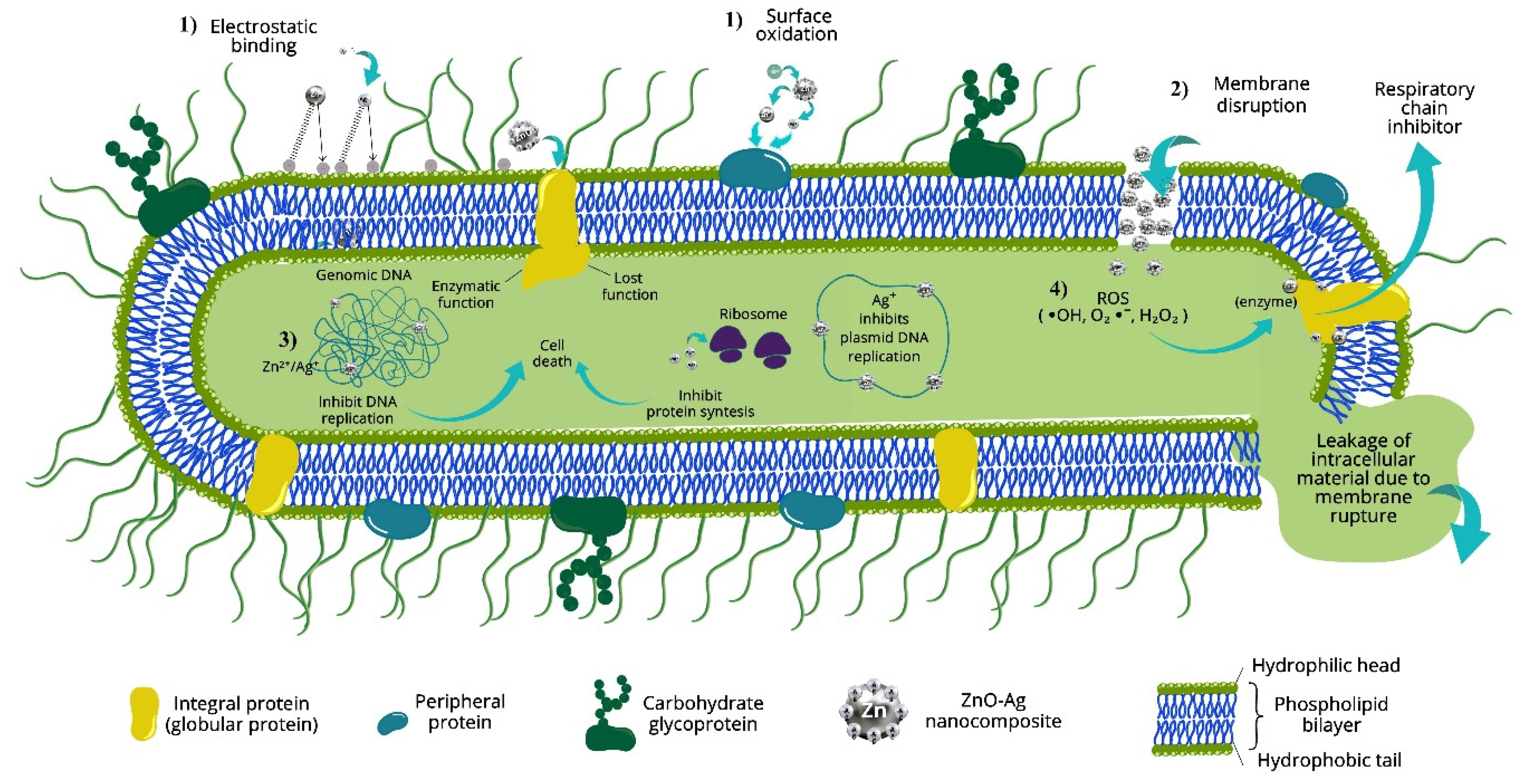
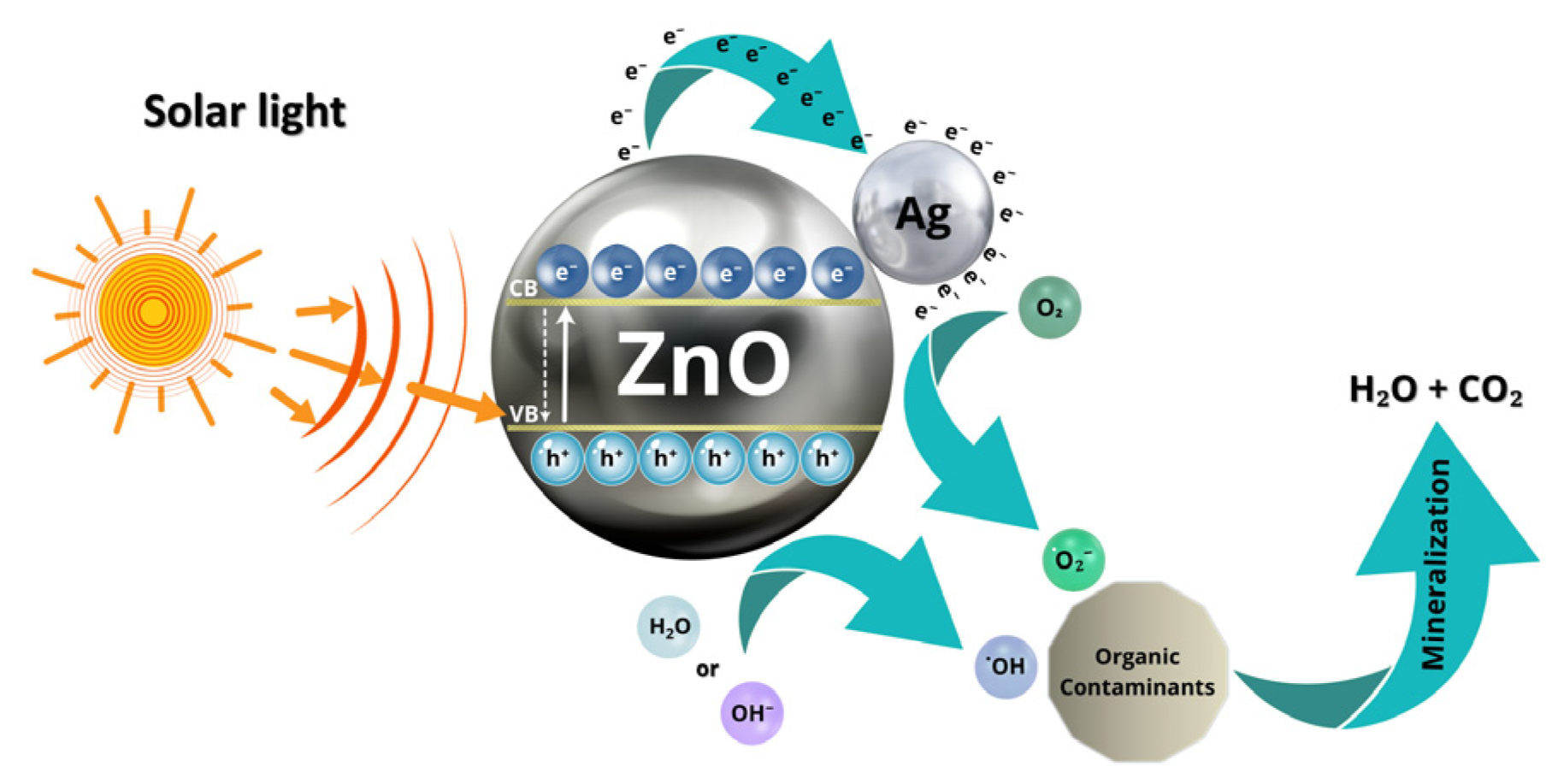
3.4. Proposed Mechanism of Photocatalytic and Antibacterial Activity of Prepared Nanocomposites
- Physical damage to the cell membrane. ZnO–Ag nanoparticles adhere to the surface of bacteria through electrostatic interactions between the released Zn2+ and Ag+ ions and the negatively charged bacterial cell wall [58]. This interaction can cause physical damage to the cell membrane, such as deformation, loss of integrity, and even cell lysis. Electron microscopic studies have demonstrated morphological alterations in bacteria such as E. coli, including cell elongation and membrane rupture [59,60].
- Intracellular inclusion of nanoparticles. Once the nanoparticles penetrate the cell, leakage from the cytoplasm occurs, causing membrane shrinkage and loss of cellular functionality. This leads to bacterial cell death by structural collapse [18].
- Excitation of ZnO–Ag nanoparticles under light irradiation. When ZnO–Ag nanoparticles are irradiated with light (UV or visible), electronic excitation occurs in the ZnO semiconductor (with a bandgap of about 3.37 eV). Photons with sufficient energy excite electrons from the valence band (VB) to the conduction band (CB), generating free electrons (e−) in the CB and positive holes (h⁺) in the VB, as indicated in the following expression [64]:ZnO + hν → e− (BC) + h+ (BV)
- Role of silver (Ag) as an electron sink. The incorporation of silver nanoparticles into ZnO forms a heterojunction that significantly improves the photocatalytic performance since silver acts as an electron sink, capturing electrons from the conduction band of ZnO and reducing the e−/h+ recombination [16]:e− (BC, ZnO) → Ag
- Generation of reactive oxygen species (ROS). Electrons transferred to silver can reduce molecular oxygen adsorbed on the surface, forming superoxide radicals, while holes in the valence band of ZnO can oxidize water molecules or hydroxyl ions to generate hydroxyl radicals, as follows:Oxygen reduction:O2 + e− → ●O2−Oxidation of water or hydroxyl ions:H2O + h+ → ●OH + H+OH− + h+→ ●OH
- Degradation of organic pollutants. The generated radicals attack organic pollutant molecules, such as dyes or pharmaceutical compounds, oxidizing them to carbon dioxide, water, and other harmless products:Contaminant + ●OH → Intermediates → CO2 + H2O
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Drinking-Water. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 3 March 2025).
- Bharathi, D.; Thiruvengadam Nandagopal, J.G.; Rajamani, R.; Pandit, S.; Kumar, D.; Pant, B.; Pandey, S.; Kumar Gupta, P. Enhanced photocatalytic activity of St-ZnO nanorods for methylene blue dye degradation. Mater. Lett. 2022, 311, 131637. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Stathopoulos, V.N. Titanium dioxide (TiO2)-based photocatalyst materials activity enhancement for contaminants of emerging concern (CECs) degradation: In the light of modification strategies. Chem. Eng. J. Adv. 2022, 10, 100262. [Google Scholar] [CrossRef]
- Le, V.T.; Doan, V.D.; Le, T.T.N.; Dao, M.U.; Vo, T.-T.T.; Do, H.H.; Viet, D.Q.; Tran, V.A. Efficient photocatalytic degradation of crystal violet under natural sunlight using Fe3O4/ZnO nanoparticles embedded carboxylate-rich carbon. Mater. Lett. 2021, 283, 128749. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Dong, X.-X.; Lv, Y.-K. Functionalized metal-organic frameworks for photocatalytic degradation of organic pollutants in the environment. Chemosphere 2020, 242, 125144. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yu, D.; Eom, S.H.; Kim, S.H.; Oh, J.; Jung, W.K.; Kim, Y.M. Synergistic Antibacterial Effects of Chitosan-Caffeic Acid Conjugate against Antibiotic-Resistant Acne-Related Bacteria. Mar. Drugs 2017, 15, 167. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Pradhan, D.; Halder, J.; Biswasroy, P.; Rai, V.K.; Dubey, D.; Kar, B.; Ghosh, G.; Rath, G. Metal nanoparticles against multi-drug-resistance bacteria. J. Inorg. Biochem. 2022, 237, 111938. [Google Scholar] [CrossRef]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fievet, F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef]
- Ghosh, T.; Das, A.B.; Jena, B.; Pradhan, C. Antimicrobial effect of silver zinc oxide (Ag-ZnO) nanocomposite particles. Front. Life Sci. 2015, 8, 47–54. [Google Scholar] [CrossRef]
- Alharthi, F.A.; Alghamdi, A.A.; Al-Zaqri, N.; Alanazi, H.S.; Alsyahi, A.A.; Marghany, A.E.; Ahmad, N. Facile one-pot green synthesis of Ag–ZnO Nanocomposites using potato peeland their Ag concentration dependent photocatalytic properties. Sci. Rep. 2020, 10, 20229. [Google Scholar] [CrossRef]
- Adhikari, S.; Banerjee, A.; Eswar, N.K.; Sarkar, D.; Madras, G. Photocatalytic inactivation of E. coli by ZnO–Ag nanoparticles under solar radiation. RSC Adv. 2015, 5, 51067–51077. [Google Scholar] [CrossRef]
- Yeganeh-Faal, A.; Bordbar, M.; Negahdar, N.; Nasrollahzadeh, M. Green synthesis of the Ag/ZnO nanocomposite using Valeriana officinalis L. root extract: Application as a reusable catalyst for the reduction of organic dyes in a very short time. IET Nanobiotechnol. 2017, 11, 669–676. [Google Scholar] [CrossRef]
- Zare, M.; Namratha, K.; Alghamdi, S.; Mohammad, Y.H.E.; Hezam, A.; Zare, M.; Drmosh, Q.A.; Byrappa, K.; Chandrashekar, B.N.; Ramakrishna, S.; et al. Novel Green Biomimetic Approach for Synthesis of ZnO-Ag Nanocomposite; Antimicrobial Activity against Food-borne Pathogen, Biocompatibility and Solar Photocatalysis. Sci. Rep. 2019, 9, 8303. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, P.; Shinde, S.; Suryawanshi, S.S.; Teli, S.B.; Patil, P.S.; Ramteke, A.A.; Hiremath, N.G.; Prasad, N.R. Green AgNPs Decorated ZnO Nanocomposites for Dye Degradation and Antimicrobial Applications. Eng. Sci. 2020, 12, 79–94. [Google Scholar] [CrossRef]
- Noohpisheh, Z.; Amiri, H.; Farhadi, S.; Mohammadi-Gholami, A. Green synthesis of Ag-ZnO nanocomposites using Trigonella foenum-graecum leaf extract and their antibacterial, antifungal, antioxidant and photocatalytic properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 240, 118595. [Google Scholar] [CrossRef]
- Chauhan, A.; Verma, R.; Kumari, S.; Sharma, A.; Shandilya, P.; Li, X.; Batoo, K.M.; Imran, A.; Kulshrestha, S.; Kumar, R. Photocatalytic dye degradation and antimicrobial activities of Pure and Ag-doped ZnO using Cannabis sativa leaf extract. Sci. Rep. 2020, 10, 7881. [Google Scholar] [CrossRef] [PubMed]
- Sali, R.K.; Pujar, M.S.; Patil, S.; Sidarai, A.H. Green Synthesis of ZnO and Ag-ZnO Nanoparticles using Macrotyloma Uniflorum: Evaluation of Antibacterial Activity. Adv. Mater. Letters. 2021, 12, 1–7. [Google Scholar] [CrossRef]
- Mtavangu, S.G.; Machunda, R.L.; van der Bruggen, B.; Njau, K.N. In situ facile green synthesis of Ag–ZnO nanocomposites using Tetradenia riperia leaf extract and its antimicrobial efficacy on water disinfection. Sci. Rep. 2022, 12, 15359. [Google Scholar] [CrossRef]
- Primo, J.d.O.; Horsth, D.F.; Correa, J.d.S.; Das, A.; Bittencourt, C.; Umek, P.; Buzanich, A.G.; Radtke, M.; Yusenko, K.V.; Zanette, C.; et al. Synthesis and Characterization of Ag/ZnO Nanoparticles for Bacteria Disinfection in Water. Nanomaterials 2022, 12, 1764. [Google Scholar] [CrossRef]
- Chitrakar, B.; Zhang, M.; Bhandari, B. Edible flowers with the common name “marigold”: Their therapeutic values and processing. Trends Food Sci. Technol. 2019, 89, 76–87. [Google Scholar] [CrossRef]
- Rivas-Garcia, L.; Crespo-Antolin, L.; Forbes-Hernandez, T.Y.; Romero-Marquez, J.M.; Navarro-Hortal, M.D.; Arredondo, M.; Llopis, J.; Quiles, J.L.; Sanchez-Gonzalez, C. Bioactive Properties of Tagetes erecta Edible Flowers: Polyphenol and Antioxidant Characterization and Therapeutic Activity against Ovarian Tumoral Cells and Caenorhabditis elegans Tauopathy. Int. J. Mol. Sci. 2024, 25, 280. [Google Scholar] [CrossRef]
- Burlec, A.F.; Pecio, L.; Kozachok, S.; Mircea, C.; Corciova, A.; Verestiuc, L.; Cioanca, O.; Oleszek, W.; Hancianu, M. Phytochemical Profile, Antioxidant Activity, and Cytotoxicity Assessment of Tagetes erecta L. Flowers. Molecules 2021, 26, 1201. [Google Scholar] [CrossRef] [PubMed]
- López-López, J.; Tejeda-Ochoa, A.; López-Beltrán, A.; Herrera-Ramírez, J.; Méndez-Herrera, P. Sunlight Photocatalytic Performance of ZnO Nanoparticles Synthesized by Green Chemistry Using Different Botanical Extracts and Zinc Acetate as a Precursor. Molecules 2021, 27, 6. [Google Scholar] [CrossRef] [PubMed]
- López-López, J.R.; Tejeda-Ochoa, A.; Cervantes-Gaxiola, M.E.; Herrera-Ramirez, J.M.; Méndez-Herrera, P.F. Photocatalytic activity of ZnO nanoparticles synthesized from zinc nitrate and botanical extracts of neem, chrysanthemum, Mexican marigold and shiitake mushroom. J. Chem. Technol. Biotechnol. 2023, 98, 1810–1818. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-Ray Diffraction Procedures: For Polycrystalline and Amorphous Materials; Wiley: Hoboken, NJ, USA, 1974. [Google Scholar]
- Klinger, M.; Jäger, A.J.A.C. Crystallographic Tool Box (CrysTBox): Automated tools for transmission electron microscopists and crystallographers. J. Appl. Crystallogr. 2015, 48, 2012–2018. [Google Scholar] [CrossRef]
- Mohammadzadeh Kakhki, R.; Tayebee, R.; Ahsani, F. New and highly efficient Ag doped ZnO visible nano photocatalyst for removing of methylene blue. J. Mater. Sci.: Mater. Electron. 2017, 28, 5941–5952. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Xu, M.; Yuan, H.; Chen, Y.; Zhang, J.; Luo, K.; Zhang, J.; You, B. Novel and efficient synthesis of Ag-ZnO nanoparticles for the sunlight-induced photocatalytic degradation. Appl. Surf. Sci. 2019, 476, 632–640. [Google Scholar] [CrossRef]
- Balogun, S.W.; James, O.O.; Sanusi, Y.K.; Olayinka, O.H. Green synthesis and characterization of zinc oxide nanoparticles using bashful (Mimosa pudica), leaf extract: A precursor for organic electronics applications. SN Appl. Sci. 2020, 2, 504. [Google Scholar] [CrossRef]
- Moezzi, A.; McDonagh, A.M.; Cortie, M.B. Zinc oxide particles: Synthesis, properties and applications. Chem. Eng. J. 2012, 185-186, 1–22. [Google Scholar] [CrossRef]
- Jayachandran, A.; Aswathy, T.R.; Nair, A.S. Green synthesis and characterization of zinc oxide nanoparticles using Cayratia pedata leaf extract. Biochem. Biophys. Rep. 2021, 26, 100995. [Google Scholar] [CrossRef]
- Sharma, V.K.; Filip, J.; Zboril, R.; Varma, R.S. Natural inorganic nanoparticles–formation, fate, and toxicity in the environment. Chem. Soc. Rev. 2015, 44, 8410–8423. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. Hydrothermal growth of ZnO nanostructures. Sci. Technol. Adv. Mater. 2009, 10, 013001. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Liu, M.; Lin, L.; Cheng, Y.; Fang, C. A Study on Doping and Compound of Zinc Oxide Photocatalysts. Polymers 2022, 14, 4484. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Tharmalingam, N.; Varaprasad, K.; Mylonakis, E.; Yallapu, M.M. Biocidal and biocompatible hybrid nanomaterials from biomolecule chitosan, alginate and ZnO. Carbohydr. Polym. 2021, 274, 118646. [Google Scholar] [CrossRef] [PubMed]
- Maji, A.; Beg, M.; Das, S.; Aktara, M.N.; Nayim, S.; Patra, A.; Islam, M.M.; Hossain, M. Study on the antibacterial activity and interaction with human serum albumin of Tagetes erecta inspired biogenic silver nanoparticles. Process Biochem. 2020, 97, 191–200. [Google Scholar] [CrossRef]
- Camacho-Campos, C.; Pérez-Hernández, Y.; Valdivia-Ávila, A.; Ramírez-Pérez, H.L.; Gómez-Brisuela, L. Propiedades fitoquímicas y antibacterianas de extractos de Tagetes erecta L. (Asteraceae). Rev. Cuba. De Química 2019, 31, 53–64. [Google Scholar]
- Karthikeyan, C.; Varaprasad, K.; Akbari-Fakhrabadi, A.; Hameed, A.S.H.; Sadiku, R. Biomolecule chitosan, curcumin and ZnO-based antibacterial nanomaterial, via a one-pot process. Carbohydr. Polym. 2020, 249, 116825. [Google Scholar] [CrossRef]
- Bittner Fialová, S.; Rendeková, K.; Mučaji, P.; Nagy, M.; Slobodníková, L. Antibacterial Activity of Medicinal Plants and Their Constituents in the Context of Skin and Wound Infections, Considering European Legislation and Folk Medicine—A Review. Int. J. Mol. Sci. 2021, 22, 10746. [Google Scholar] [CrossRef]
- Zavaleta Espejo, G.; Saldaña Jiménez, J.; Jáuregui Rosas, S.R.; Pacherrez Gallardo, D.; Rivera Burgos, M.; Samanamud Moreno, F.V.; Perales Pérez, O.J. Efecto antibacteriano de nanopartículas de ZnO sobre Staphylococcus aureus y Salmonella typhi. Arnaldoa 2019, 26, 421–430. [Google Scholar] [CrossRef]
- Kaushik, M.; Niranjan, R.; Thangam, R.; Madhan, B.; Pandiyarasan, V.; Ramachandran, C.; Oh, D.-H.; Venkatasubbu, G.D. Investigations on the antimicrobial activity and wound healing potential of ZnO nanoparticles. Appl. Surf. Sci. 2019, 479, 1169–1177. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A Review of Microwave Synthesis of Zinc Oxide Nanomaterials: Reactants, Process Parameters and Morphoslogies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- Ji, H.; Zhou, S.; Fu, Y.; Wang, Y.; Mi, J.; Lu, T.; Wang, X.; Lü, C. Size-controllable preparation and antibacterial mechanism of thermo-responsive copolymer-stabilized silver nanoparticles with high antimicrobial activity. Mater. Sci. Eng. C 2020, 110, 110735. [Google Scholar] [CrossRef] [PubMed]
- More, P.R.; Pandit, S.; Filippis, A.D.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.d.M.R.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Wu, Y.; Liang, L.; Wei, Y.; Zheng, X.; Chen, Y. Altering sliver nanoparticles-induced inhibition to bacterial denitrification via visible light by regulating silver transformation and adaptive mechanism under anaerobic conditions. Chem. Eng. J. 2023, 452, 139268. [Google Scholar] [CrossRef]
- Nandi, A.; Giram, H.S.; Patel, V.P.; Mehera, R.; Das, S.; Choudhary, D.K.; Rahman, A.; Saha, D.; Chandra, P.; Singh, M.; et al. Single-step synthesis of ZnO nanoparticles using a phytosynthesis route and its characterization. Z. Fur Naturforschung A 2024, 79, 141–155. [Google Scholar] [CrossRef]
- Imparato, C.; Bifulco, A.; Silvestri, B.; Vitiello, G. Recent Advances in Endocrine Disrupting Compounds Degradation through Metal Oxide-Based Nanomaterials. Catalysts 2022, 12, 289. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Abu-Elghait, M.; Ahmed, N.E.; Salem, S.S. Eco-friendly Mycogenic Synthesis of ZnO and CuO Nanoparticles for In Vitro Antibacterial, Antibiofilm, and Antifungal Applications. Biol. Trace Elem. Res. 2021, 199, 2788–2799. [Google Scholar] [CrossRef]
- Dey, S.; Mohanty, D.l.; Divya, N.; Bakshi, V.; Mohanty, A.; Rath, D.; Das, S.; Mondal, A.; Roy, S.; Sabui, R. A critical review on zinc oxide nanoparticles: Synthesis, properties and biomedical applications. Intell. Pharm. 2025, 3, 53–70. [Google Scholar] [CrossRef]
- Alzahrani, E.A.; Nabi, A.; Kamli, M.R.; Albukhari, S.M.; Althabaiti, S.A.; Al-Harbi, S.A.; Khan, I.; Malik, M.A. Facile Green Synthesis of ZnO NPs and Plasmonic Ag-Supported ZnO Nanocomposite for Photocatalytic Degradation of Methylene Blue. Water 2023, 15, 384. [Google Scholar] [CrossRef]
- Shelar, S.G.; Mahajan, V.K.; Patil, S.P.; Sonawane, G.H. Effect of doping parameters on photocatalytic degradation of methylene blue using Ag doped ZnO nanocatalyst. SN Appl. Sci. 2020, 2, 820. [Google Scholar] [CrossRef]
- Rabbani, M.; Shokraiyan, J.; Rahimi, R.; Amrollahi, R. Comparison of photocatalytic activity of ZnO, Ag-ZnO, Cu-ZnO, Ag, Cu-ZnO and TPPS/ZnO for the degradation of methylene blue under UV and visible light irradiation. Water Sci. Technol. 2021, 84, 1813–1825. [Google Scholar] [CrossRef] [PubMed]
- Momeni, A.; Meshkatalsadat, M.H.; Bakhtiari Shahin, B.; Mousavi, Y. Photodegradation of methylene blue by phytosynthesized Ag–ZnO nanocomposites. Hybrid Adv. 2023, 3, 100050. [Google Scholar] [CrossRef]
- Cuadra, J.G.; Scalschi, L.; Vicedo, B.; Guc, M.; Izquierdo-Roca, V.; Porcar, S.; Fraga, D.; Carda, J.B. ZnO/Ag Nanocomposites with Enhanced Antimicrobial Activity. Appl. Sci. 2022, 12, 5023. [Google Scholar] [CrossRef]
- Dutta, G.; Chinnaiyan, S.K.; Sugumaran, A.; Narayanasamy, D. Sustainable bioactivity enhancement of ZnO-Ag nanoparticles in antimicrobial, antibiofilm, lung cancer, and photocatalytic applications. RSC Adv. 2023, 13, 26663–26682. [Google Scholar] [CrossRef] [PubMed]
- Asamoah, R.B.; Annan, E.; Mensah, B.; Nbelayim, P.; Apalangya, V.; Onwona-Agyeman, B.; Yaya, A. A Comparative Study of Antibacterial Activity of CuO/Ag and ZnO/Ag Nanocomposites. Adv. Mater. Sci. Eng. 2020, 2020, 1–18. [Google Scholar] [CrossRef]
- Sinha, R.; Karan, R.; Sinha, A.; Khare, S.K. Interaction and nanotoxic effect of ZnO and Ag nanoparticles on mesophilic and halophilic bacterial cells. Bioresour. Technol. 2011, 102, 1516–1520. [Google Scholar] [CrossRef]
- Liu, Y.; He, L.; Mustapha, A.; Li, H.; Hu, Z.Q.; Lin, M. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. J. Appl. Microbiol. 2009, 107, 1193–1201. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Interaction of ZnO nanoparticles with microbes-a physio and biochemical assay. J. Biomed. Nanotechnol. 2011, 7, 813–822. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Matai, I.; Sachdev, A.; Dubey, P.; Kumar, S.U.; Bhushan, B.; Gopinath, P. Antibacterial activity and mechanism of Ag-ZnO nanocomposite on S. aureus and GFP-expressing antibiotic resistant E. coli. Colloids Surf. B Biointerfaces 2014, 115, 359–367. [Google Scholar] [CrossRef]
- Saha, R.; Subramani, K.; Petchi Muthu Raju, S.A.K.; Rangaraj, S.; Venkatachalam, R. Psidium guajava leaf extract-mediated synthesis of ZnO nanoparticles under different processing parameters for hydrophobic and antibacterial finishing over cotton fabrics. Prog. Org. Coat. 2018, 124, 80–91. [Google Scholar] [CrossRef]
- Georgekutty, R.; Seery, M.K.; Pillai, S.C. A Highly Efficient Ag-ZnO Photocatalyst: Synthesis, Properties, and Mechanism. J. Phys. Chem. C 2008, 112, 13563–13570. [Google Scholar] [CrossRef]



| Sample | Particle Size (nm) ± SD 1 | Crystallite Size (nm) ± SD 1 |
|---|---|---|
| ZnO | 23.02 ± 11.61 | 17.40 ± 1.26 |
| ZnO–Ag 0.1% | 24.13 ± 9.52 | 19.45 ± 1.85 |
| ZnO–Ag0.2% | 17.39 ± 6.45 | 16.76 ± 1.84 |
| ZnO–Ag 0.3% | 20.64 ± 6.06 | 18.81 ± 1.26 |
| ZnO–Ag 0.5% | 18.48 ± 5.62 | 17.17 ± 0.69 |
| Material | kapp (min−1) | R2 | Degradation (%) | Degradation Time (min) |
|---|---|---|---|---|
| Without catalyst (photolysis) | 0.00273 ± 5.3342 × 10−5 | 0.99733 | 9.26 | 35 |
| ZnO | 0.13423 ± 0.01333 | 0.9269 | 100 | 30 |
| ZnO–Ag 0.1% | 0.13502 ± 0.00532 | 0.98622 | 100 | 35 |
| ZnO–Ag 0.2% | 0.15977 ± 0.01818 | 0.90618 | 100 | 30 |
| ZnO–Ag 0.3% | 0.20912 ± 0.0153 | 0.9639 | 100 | 25 |
| ZnO–Ag 0.5% | 0.0752 ± 0.00648 | 0.9308 | 97.01 | 45 |
| Summary of Methylene Blue Dye Photodegradation from Different Research Papers | |||
|---|---|---|---|
| Synthesis Conditions | Photocatalysis Conditions | Degradation Parameters | Reference |
| Green synthesis (Carthamus tinctorius L.) with 0.5 M zinc nitrate solution in 50 mL. g of as-prepared ZnO NPs in water + 50 mL (0.1 M) of silver nitrate solution | MB (10 mg/L); UV lamp (400 W); 0.6 g/L; Vol. = 50 mL; pH = 8 | 98% at 60 min | [51] |
| Modified Pechini co-precipitation method 0.5 M of zinc nitrate and 1 M of NaOH + silver nitrate | MB (10 mg/L); halogen lamp (500 W); 1 g/L; Vol. = 50 mL; pH = 8 | 95% was found for 10 mg/L for 4% Ag-doped ZnO at 100 min | [52] |
| Precipitation (molar ratio Ag to ZnO of 4:96): 0.038 M of zinc nitrate and 0.0016 M of silver nitrate | MB (10 mg/L); 400 W tungsten as a visible light source and 400 W high-pressure mercury lamps as a UV light;1 g/L; Vol. = 20 mL | 94.9% at 180 min at visible light | [53] |
| Green synthesis (Punica granatum), 1.347 g zinc nitrate, and 5 wt% siver nitrate, pH = 10 | MB (5 mg/L); sunlight; 2 g/L; Vol. = 30 mL, pH = 10 | 98% at 50 min | [54] |
| Green synthesis (Tagetes erecta), 0.5 M zinc acetate, and (0.1, 0.2, 0.3, and 0.4% w/v) silver nitrate. pH = 10 | MB (10 mg/L); sunlight; 1 g/L; Vol. = 300 mL, pH = 10 | 100% at 25 min | This work |
| Percentage growth of E. coli and S. aureus for different ZnO–Ag nanocomposites | |||
| Sol-gel method, 0.4 M of zinc acetate + ethanol + triethanolamine and 0-10% of silver acetate | S. aureus ATCC 29213 and the E. coli NCIMB 9484 strains; 0–10% Ag; 106 CFU/mL of S. aureus and 105 CFU/mL of E. coli; m/v of ZnO–Ag not indicated | 1% of Ag reduces the percentage of living cells by more than 90% | [55] |
| Green synthesis (Trichosanthes dioica), basic pH not specified. 0.2 M of zinc nitrate, and 0 or 0.1 M silver nitrate | 1 × 107 CFU/mL E. coli (ATCC 8739) S. aureus (ATCC 6538); 128 μg/mL for ZnO and 16 μg/mL for ZnO–Ag | E. coli and S. aureus biofilms were inhibited at 74.22% and 75.93% | [56] |
| Wet chemical precipitation method, 0.1 M zinc acetate, pH 11, 0.001 M silver nitrate, and 0.004 M sodium borohydride | 6 × 107 CFU/mL; 0, 0.05, 0.1, 0.25, 0.5, and 1 mg/mL. | 91.7% and 89.3% E. coli and S. aureus for 1 mg/mL | [57] |
| Green synthesis (Tagetes erecta), 0.5 M zinc acetate, and (0.1, 0.2, 0.3, and 0.4% w/v) silver nitrate. pH = 10 | 2 × 108 CFU/mL of each strain E. coli ATCC 25922 and S. aureus ATCC 25923; 150, 200, and 250 µg/mL | >95% for S. aureus and 100% for E. Coli with 250 μg/mL at 8 h | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-López, J.R.; Hernández-Chávez, M.A.; López-López, M.d.J.; Tejeda-Ochoa, A.; Cervantes-Gaxiola, M.E.; Parra-Unda, J.R.; Valenzuela-Ramírez, G.G.; Flores-Villaseñor, H.; León-Sicairos, N.; Canizalez-Roman, A.; et al. Tagetes erecta—Mediated Green Synthesis of ZnO–Ag Nanocomposites: Characterization and Dual Applications in Solar Photocatalytic Degradation and Antibacterial Activity. Ceramics 2025, 8, 45. https://doi.org/10.3390/ceramics8020045
López-López JR, Hernández-Chávez MA, López-López MdJ, Tejeda-Ochoa A, Cervantes-Gaxiola ME, Parra-Unda JR, Valenzuela-Ramírez GG, Flores-Villaseñor H, León-Sicairos N, Canizalez-Roman A, et al. Tagetes erecta—Mediated Green Synthesis of ZnO–Ag Nanocomposites: Characterization and Dual Applications in Solar Photocatalytic Degradation and Antibacterial Activity. Ceramics. 2025; 8(2):45. https://doi.org/10.3390/ceramics8020045
Chicago/Turabian StyleLópez-López, Juan R., Miguel A. Hernández-Chávez, María de J. López-López, Armando Tejeda-Ochoa, Maritza E. Cervantes-Gaxiola, Jesús R. Parra-Unda, Gladymar G. Valenzuela-Ramírez, Héctor Flores-Villaseñor, Nidia León-Sicairos, Adrián Canizalez-Roman, and et al. 2025. "Tagetes erecta—Mediated Green Synthesis of ZnO–Ag Nanocomposites: Characterization and Dual Applications in Solar Photocatalytic Degradation and Antibacterial Activity" Ceramics 8, no. 2: 45. https://doi.org/10.3390/ceramics8020045
APA StyleLópez-López, J. R., Hernández-Chávez, M. A., López-López, M. d. J., Tejeda-Ochoa, A., Cervantes-Gaxiola, M. E., Parra-Unda, J. R., Valenzuela-Ramírez, G. G., Flores-Villaseñor, H., León-Sicairos, N., Canizalez-Roman, A., Herrera-Ramírez, J. M., & Méndez-Herrera, P. F. (2025). Tagetes erecta—Mediated Green Synthesis of ZnO–Ag Nanocomposites: Characterization and Dual Applications in Solar Photocatalytic Degradation and Antibacterial Activity. Ceramics, 8(2), 45. https://doi.org/10.3390/ceramics8020045











