Synthesis and Sintering of Novel High-Entropy Barium Cerates Designed Through the Cluster-Plus-Glue Atom Model
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Design via the Cluster-Plus-Glue Atom Model
- [TVO6]Ba2TV;
- [REO6]RE3;
- [REO6]Ba3RE.
- where TV stands for a tetravalent cation (i.e., Ce, Zr in our systems) and RE stands for a generic trivalent rare-earth (i.e., Yb, Sm, La, Gd, Nd in our systems).
3.2. Geometric Descriptors
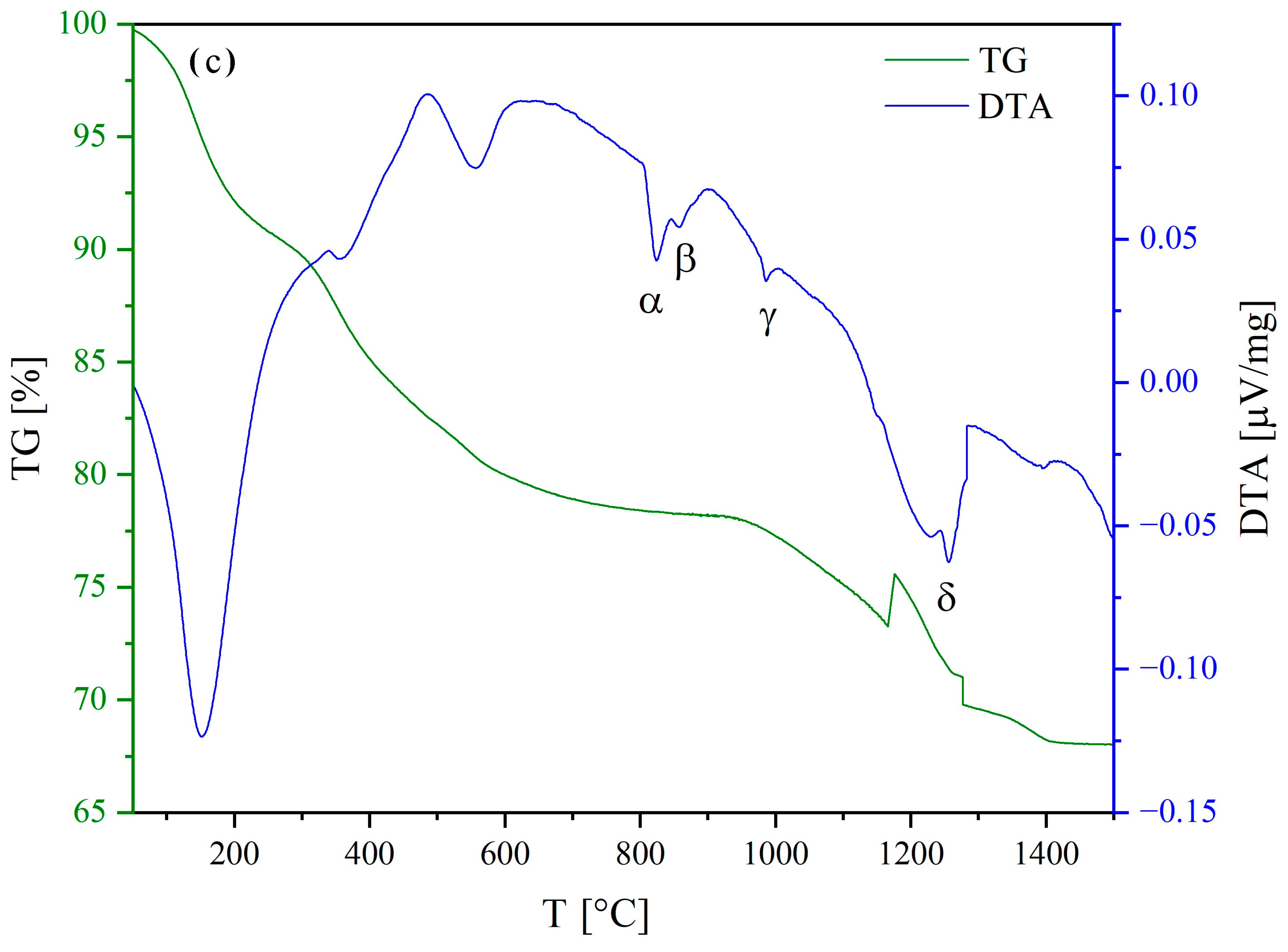
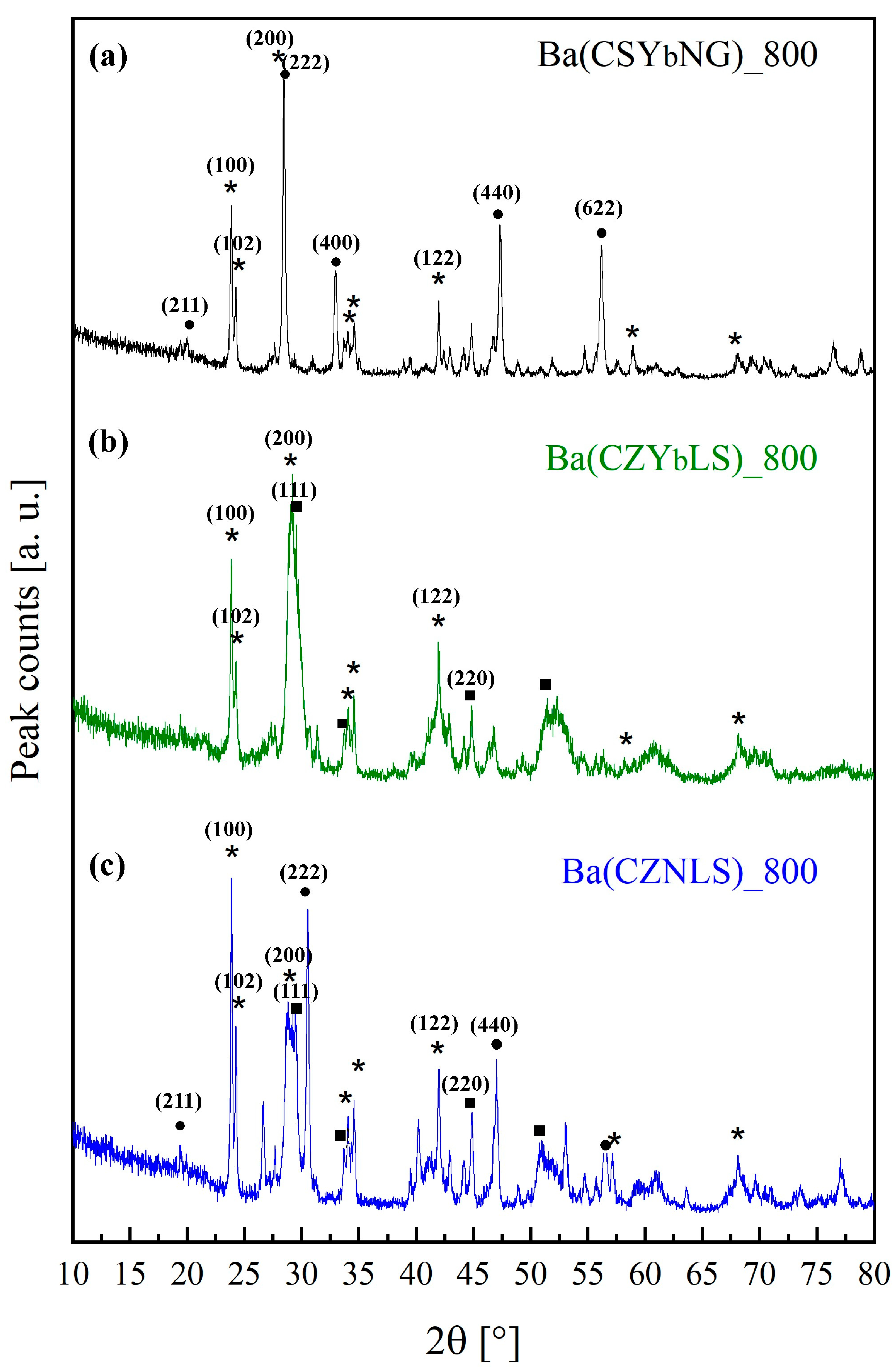
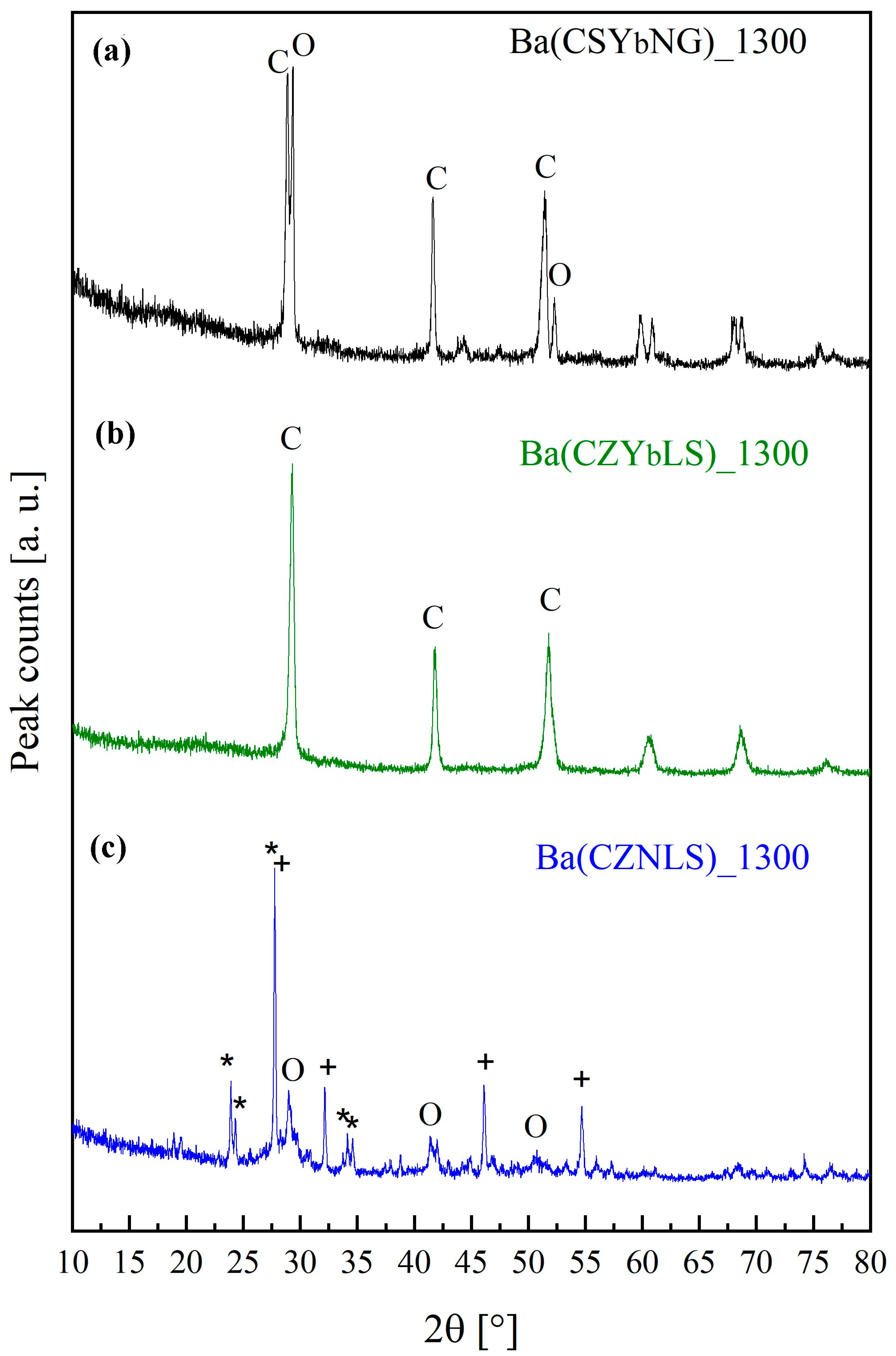
3.3. Structural and Thermal Characterization
BaCO3 + 0.2 CeO2 + 0.3 RE2O3 + 0.2 ZrO2 + (0.1x + 0.3y + 0.2z) H2O↑ + 1.2 CO2↑
- Endothermic orthorhombic-to-hexagonal polymorphic transformation (around 800 °C) of barium carbonate [57];
- Endothermic hexagonal-to-cubic polymorphic transformation (around 850 °C) of barium carbonate [57];
- Possible formation of the entropy-stabilized fluorite/bixbyite single phase involving the non-Ba cations (around 950 °C);
- Possible formation of the entropy-stabilized perovskite single phase upon completion of barium carbonate decomposition at around 1250 °C (according to reaction (9)).
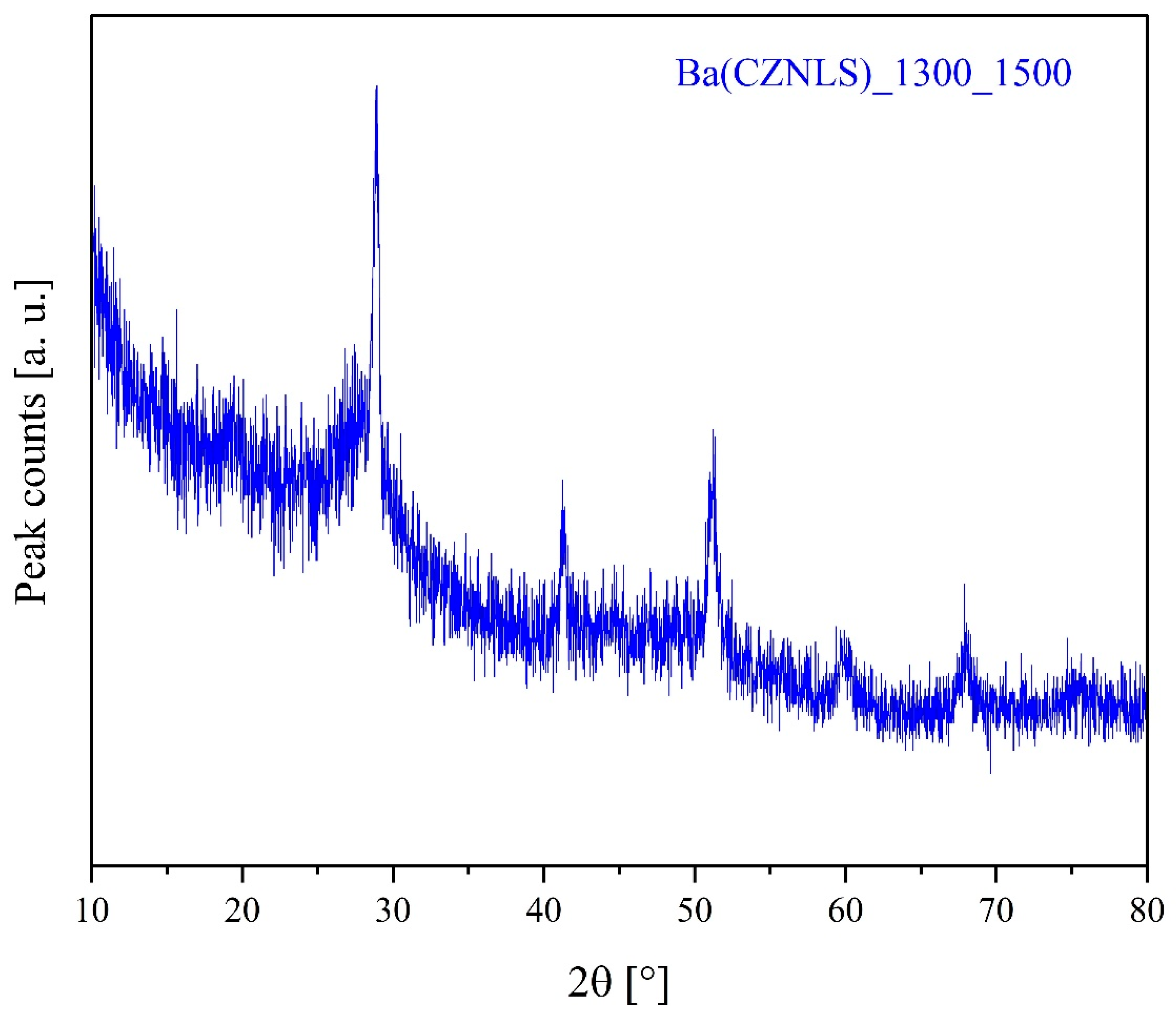
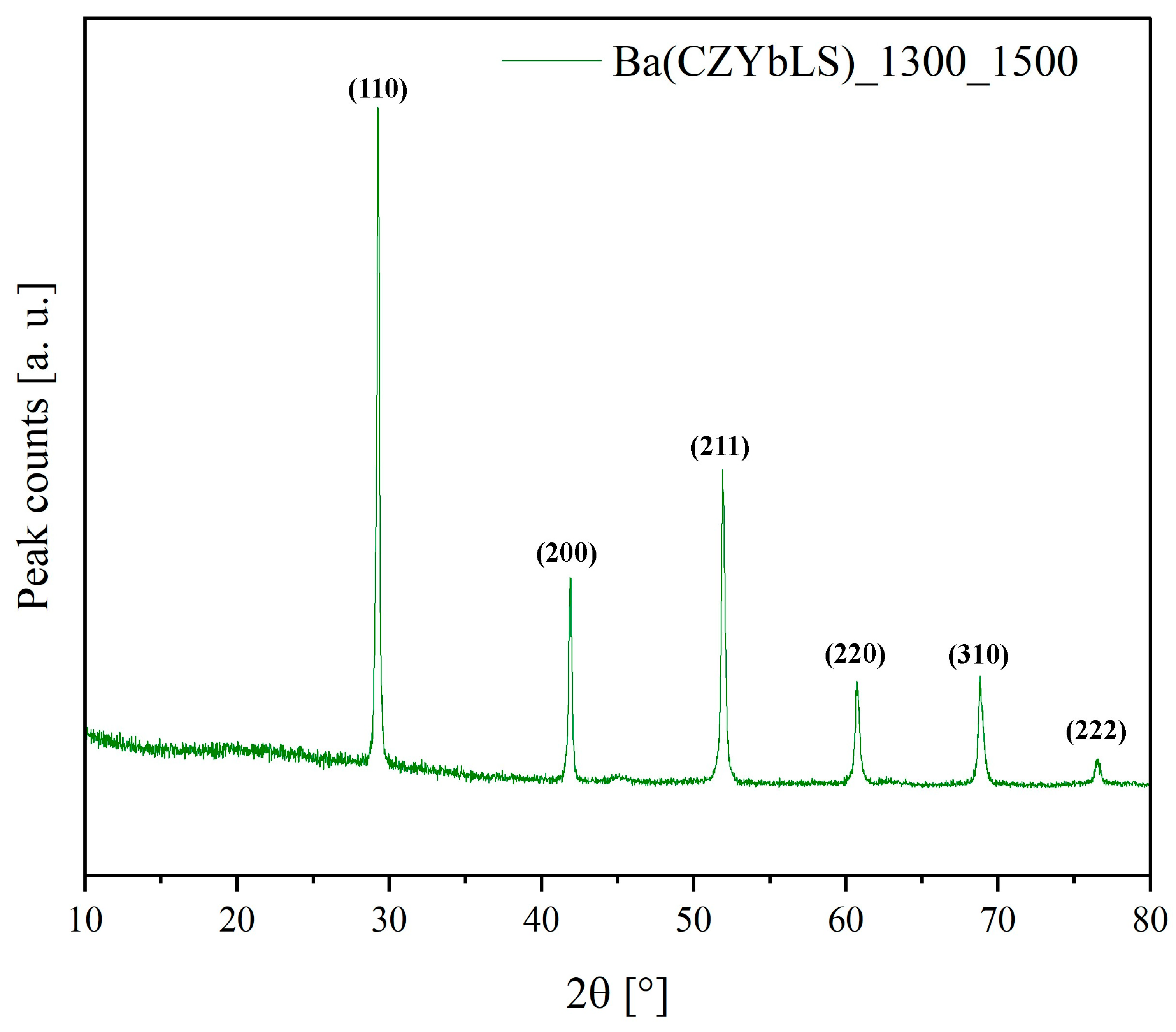
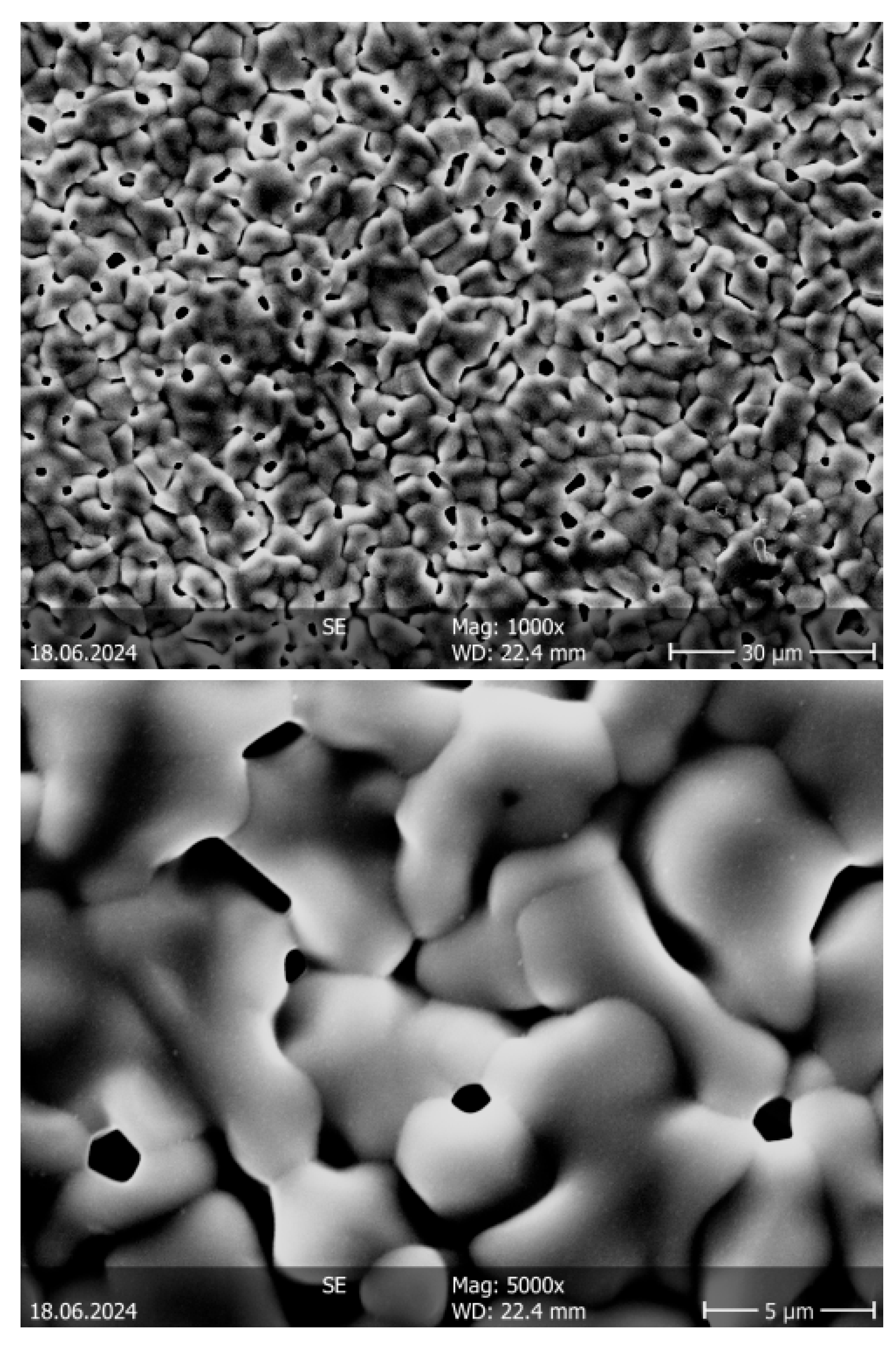
3.4. Sintering Behavior of System Ba(CZYbLS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar]
- Murty, B.S.; Yeh, J.W.; Ranganathan, S.; Bhattacharjee, P.P. High-Entropy Alloys; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–12. [Google Scholar]
- Otto, F.; Yang, Y.; Bei, H.; George, E.P. Relative effects of enthalpy and entropy on the phase stability of equiatomic high-entropy alloys. Acta Mater. 2013, 61, 2628–2638. [Google Scholar]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.P. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar]
- Dąbrowa, J.; Stygar, M.; Mikuła, A.; Knapik, A.; Mroczka, K.; Tejchman, W.; Danielewski, M.; Martin, M. Synthesis and microstructure of the (Co, Cr, Fe, Mn, Ni) 3O4 high entropy oxide characterized by spinel structure. Mater. Lett. 2018, 216, 32–36. [Google Scholar]
- Wang, J.; Cui, Y.; Wang, Q.; Wang, K.; Huang, X.; Stenzel, D.; Sarkar, A.; Azmi, R.; Bergfeldt, T.; Bhattacharya, S.S.; et al. Lithium containing layered high entropy oxide structures. Sci. Rep. 2020, 10, 18430. [Google Scholar]
- Gild, J.; Samiee, M.; Braun, J.L.; Harrington, T.; Vega, H.; Hopkins, P.E.; Vecchio, K.; Luo, J. High-entropy fluorite oxides. J. Eur. Ceram. Soc. 2018, 38, 3578–3584. [Google Scholar]
- Spiridigliozzi, L.; Dell’Agli, G. A novel high-entropy rare-earth hydroxycarbonate synthesized via facile hydrothermal synthesis with superior decomposition temperature. Mater. Lett. 2024, 372, 137090. [Google Scholar]
- Teng, Z.; Zhu, L.; Tan, Y.; Zeng, S.; Xia, Y.; Wang, Y.; Zhang, H. Synthesis and structures of high-entropy pyrochlore oxides. J. Eur. Ceram. Soc. 2020, 40, 1639–1643. [Google Scholar]
- Vinnik, D.A.; Trofimov, E.A.; Zhivulin, V.E.; Zaitseva, O.V.; Gudkova, S.A.; Starikov, A.Y.; Zherebtsov, D.A.; Kirsanova, A.A.; Häßner, M.; Niewa, R. High-entropy oxide phases with magnetoplumbite structure. Ceram. Int. 2019, 45, 12942–12948. [Google Scholar]
- Dippo, O.F.; Vecchio, K.S. A universal configurational entropy metric for high-entropy materials. Scr. Mater. 2021, 201, 113974. [Google Scholar]
- Aamlid, S.S.; Oudah, M.; Rottler, J.; Hallas, A.M. Understanding the role of entropy in high entropy oxides. J. Am. Chem. Soc. 2023, 145, 5991–6006. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.B.; Motola, M.; Rauf, S.; Li, C.J.; Li, C.X. Recent advancements, doping strategies and the future perspective of perovskite-based solid oxide fuel cells for energy conversion. Chem. Eng. J. 2022, 428, 132603. [Google Scholar] [CrossRef]
- Hanif, M.B.; Rauf, S.; Motola, M.; Babar ZU, D.; Li, C.J.; Li, C.X. Recent progress of perovskite-based electrolyte materials for solid oxide fuel cells and performance optimizing strategies for energy storage applications. Mater. Res. Bull. 2022, 146, 111612. [Google Scholar] [CrossRef]
- Zhang, M.; Du, Z.; Zhang, Y.; Zhao, H. Progress of perovskites as electrodes for symmetrical solid oxide fuel cells. ACS Appl. Energy Mater. 2022, 5, 13081–13095. [Google Scholar] [CrossRef]
- Li, H.; Yu, J.; Gong, Y.; Lin, N.; Yang, Q.; Zhang, X.; Wang, Y. Perovskite catalysts with different dimensionalities for environmental and energy applications: A review. Sep. Purif. Technol. 2023, 307, 122716. [Google Scholar] [CrossRef]
- Bian, Z.; Wang, Z.; Jiang, B.; Hongmanorom, P.; Zhong, W.; Kawi, S. A review on perovskite catalysts for reforming of methane to hydrogen production. Renew. Sustain. Energy Rev. 2020, 134, 110291. [Google Scholar] [CrossRef]
- Ali, S.A.; Ahmad, T. Treasure trove for efficient hydrogen evolution through water splitting using diverse perovskite photocatalysts. Mater. Today Chem. 2023, 29, 101387. [Google Scholar] [CrossRef]
- Zhu, H.; Teale, S.; Lintangpradipto, M.N.; Mahesh, S.; Chen, B.; McGehee, M.D.; Sargent, E.H.; Bakr, O.M. Long-term operating stability in perovskite photovoltaics. Nat. Rev. Mater. 2023, 8, 569–586. [Google Scholar] [CrossRef]
- Liu, X.; Luo, D.; Lu, Z.H.; Yun, J.S.; Saliba, M.; Seok, S.I.; Zhang, W. Stabilization of photoactive phases for perovskite photovoltaics. Nat. Rev. Chem. 2023, 7, 462–479. [Google Scholar] [CrossRef]
- Zhang, L.; Pu, Y.; Chen, M.; Peng, X.; Wang, B.; Shang, J. Design strategies of perovskite energy-storage dielectrics for next-generation capacitors. J. Eur. Ceram. Soc. 2023, 43, 5713–5747. [Google Scholar] [CrossRef]
- Sahoo, L.; Parida, B.N.; Nayak, N.C.; Parida, R.K. Revived BBFTO double perovskite with improved dielectric properties for some possible device applications. J. Mater.Sci. Mater. Electron. 2023, 34, 1019. [Google Scholar]
- Zhang, M.H.; Qi, J.L.; Liu, Y.Q.; Lan, S.; Luo, Z.X.; Pan, H.; Lin, Y.H. High energy storage capability of perovskite relaxor ferroelectrics via hierarchical optimization. Rare Met. 2022, 41, 730–744. [Google Scholar]
- Yang, Q.; Wang, G.; Wu, H.; Beshiwork, B.A.; Tian, D.; Zhu, S.; Yang, Y.; Lu, X.; Ding, Y.; Ling, Y.; et al. A high-entropy perovskite cathode for solid oxide fuel cells. J. Alloys Compd. 2021, 872, 159633. [Google Scholar]
- Zhang, D.; Wang, Y.; Peng, Y.; Luo, Y.; Liu, T.; He, W.; Chen, F.; Ding, M. Novel high-entropy perovskite-type symmetrical electrode for efficient and durable carbon dioxide reduction reaction. Adv. Powder Mater. 2023, 2, 100129. [Google Scholar]
- Guo, M.; Liu, Y.; Zhang, F.; Cheng, F.; Cheng, C.; Miao, Y.; Gao, F.; Yu, J. Inactive Al3+-doped La (CoCrFeMnNiAlx)1/(5+x) O3 high-entropy perovskite oxides as high performance supercapacitor electrodes. J. Adv. Ceram. 2022, 11, 742–753. [Google Scholar]
- Oh, S.; Kim, D.; Ryu, H.J.; Lee, K.T. A Novel High-Entropy Perovskite Electrolyte with Improved Proton Conductivity and Stability for Reversible Protonic Ceramic Electrochemical Cells. Adv. Funct. Mater. 2024, 34, 2311426. [Google Scholar] [CrossRef]
- Ning, Y.; Pu, Y.; Wu, C.; Zhou, S.; Zhang, L.; Zhang, J.; Zhang, X.; Shang, Y. Enhanced capacitive energy storage and dielectric temperature stability of A-site disordered high-entropy perovskite oxides. J. Mater. Sci. Technol. 2023, 145, 66–73. [Google Scholar]
- Zhai, Y.; Ren, X.; Wang, B.; Liu, S. High-Entropy Catalyst—A Novel Platform for Electrochemical Water Splitting. Adv. Funct. Mater. 2022, 32, 2207536. [Google Scholar] [CrossRef]
- Wang, Q.; Xuan, Y.; Gao, K.; Sun, C.; Gao, Y.; Liu, J.; Chang, S.; Liu, X. High-entropy perovskite oxides for direct solar-driven thermochemical CO2 splitting. Ceram. Int. 2024, 50, 1564–1573. [Google Scholar]
- Guo, R.; He, T. High-entropy perovskite electrolyte for protonic ceramic fuel cells operating below 600 °C. ACS Mater. Lett. 2022, 4, 1646–1652. [Google Scholar] [CrossRef]
- Zhou, G.; Li, Y.; Luo, Y.; Wang, X.; Ding, Y. The structure and electrical properties of novel BaSn0.15Ce0.35Hf0.25Y0.1Yb0.1Ho0.05O3-δ high-entropy proton-conducting electrolyte. J. Alloys Compd. 2024, 971, 172668. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. Die gesetze der krystallochemie. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Tilley, R.J. Perovskites: Structure-Property Relationships; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 1–40. [Google Scholar]
- Wang, Y.; Liu, J.; Song, Y.; Yu, J.; Tian, Y.; Robson, M.J.; Wang, J.; Zhang, Z.; Lin, X.; Zhou, G.; et al. High-entropy perovskites for energy conversion and storage: Design, synthesis, and potential applications. Small Methods 2023, 7, 2201138. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Y.; Li, Z.; Wang, H.; Liu, S.; Yao, T.; Jiang, W.; Wang, N.; Liu, C.; Ding, W.; et al. Composition design, thermodynamic calculation and synthesis of rocksalt structure Mg–Co–Ni–Cu–Zn–O HEOs based on cluster-plus-glue-atom model. Ceram. Int. 2024, 50, 15240–15244. [Google Scholar] [CrossRef]
- Spiridigliozzi, L.; Ferone, C.; Cioffi, R.; Dell’Agli, G. Compositional design of single-phase rare-earth based high-entropy oxides (HEOs) by using the cluster-plus-glue atom model. Ceram. Int. 2023, 49, 7662–7669. [Google Scholar] [CrossRef]
- Spiridigliozzi, L.; Biesuz, M.; Sglavo, V.M.; Dell’Agli, G. Design, synthesis and formation mechanism of a novel entropy-stabilized perovskite oxide derived from barium cerate/zirconate. J. Eur. Ceram. Soc. 2024, 44, 2223–2232. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Q.; Qiang, J.B.; Wang, Y.M.; Jiang, N.; Han, G.; Li, Y.H.; Wu, J.; Xia, J.H. From clusters to phase diagrams: Composition rules of quasicrystals and bulk metallic glasses. J. Phys. D Appl. Phys. 2007, 40, R273. [Google Scholar] [CrossRef]
- Ma, Y.; Dong, D.; Wu, A.; Dong, C. Composition formulas of inorganic compounds in terms of cluster plus glue atom model. Inorg. Chem. 2018, 57, 710–717. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, R.; Yu, C.; He, L.; Ren, H.; Jiang, L. Flexural behavior of simply supported beams consisting of gradient concrete and GFRP bars. Front. Mater. 2021, 8, 693905. [Google Scholar] [CrossRef]
- Spiridigliozzi, L.; Ferone, C.; Cioffi, R.; Dell’Agli, G. A simple and effective predictor to design novel fluorite-structured High Entropy Oxides (HEOs). Acta Mater. 2021, 202, 181–189. [Google Scholar] [CrossRef]
- Reddy, G.S.; Bauri, R. Y and In-doped BaCeO3-BaZrO3 solid solutions: Chemically stable and easily sinterable proton conducting oxides. J. Alloys Compd. 2016, 688, 1039–1046. [Google Scholar]
- Luo, Y.; Li, Y.; Zhang, N.; Ding, Y.; Li, H.; Chen, G. Electrical properties and chemical stability of Br addition in BaCe0.8Gd0.2O3-α proton-conducting electrolyte. Ceram. Int. 2020, 46, 26027–26034. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Cai, X.; Wang, X. Y-Gd-Zn tri-doped BaCeO3-BaZrO3 proton conducting electrolytes with improved electrical and transport properties. J. Mater. Sci. 2024, 59, 20944–20963. [Google Scholar]
- Accardo, G.; Frattini, D.; Yoon, S.P. Enhanced proton conductivity of Gd–Co bi-doped barium cerate perovskites based on structural and microstructural investigations. J. Alloys Compd. 2020, 834, 155114. [Google Scholar] [CrossRef]
- Danilov, N.A.; Starostina, I.A.; Starostin, G.N.; Kasyanova, A.V.; Medvedev, D.A.; Shao, Z. Fundamental understanding and applications of protonic Y-and Yb-coped Ba (Ce, Zr) O3 perovskites: State-of-the-art and perspectives. Adv. Energy Mater. 2023, 13, 2302175. [Google Scholar]
- Spiridigliozzi, L.; Accardo, G.; Audasso, E.; Yoon, S.P.; Dell’Agli, G. On the role of copper as a sintering aid in proton conducting Gd-doped barium cerate (BCGO). J. Alloys Compd. 2023, 960, 170762. [Google Scholar]
- Sonu, B.K.; Sinha, E. Structural, thermal stability and electrical conductivity of zirconium substituted barium cerate ceramics. J. Alloys Compd. 2021, 860, 158471. [Google Scholar]
- Yan, N.; Zeng, Y.; Shalchi, B.; Wang, W.; Gao, T.; Rothenberg, G.; Luo, J.L. Discovery and understanding of the ambient-condition degradation of doped barium cerate proton-conducting perovskite oxide in solid oxide fuel cells. J. Electrochem. Soc. 2015, 162, F1408. [Google Scholar]
- Lin, X.L.; Babar ZU, D.; Gao, Y.; Gao, J.T.; Li, C.X. Influence of triple sintering additives (BaO-CuO-B2O3) on the sintering behavior and conductivity of the proton-conducting BaZr0.1Ce0.7Y0.2O3−δ electrolyte sintered at 1150 °C. ACS Appl. Energy Mater. 2023, 6, 4833–4843. [Google Scholar] [CrossRef]
- Nasani, N.; Shakel, Z.; Loureiro, F.J.; Panigrahi, B.B.; Kale, B.B.; Fagg, D.P. Exploring the impact of sintering additives on the densification and conductivity of BaCe0.3Zr0.55Y0.15O3-δ electrolyte for protonic ceramic fuel cells. J. Alloys Compd. 2021, 862, 158640. [Google Scholar]
- Babar ZU, D.; Hanif, M.B.; Butt, M.K.; Motola, M.; Li, C.X. Towards highly dense electrolytes at lower sintering temperature (∼1200 °C): Optimization strategies for BaCe0.7Zr0.1CuxY0.2-xO3-δ in SOFCs. Ceram. Int. 2024, 50, 40261–40270. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Found. Crystallogr. 1976, 32, 751–767. [Google Scholar]
- Accardo, G.; Dell’ Agli, G.; Frattini, D.; Spiridigliozzi, L.; Nam, S.W.; Yoon, S.P. Electrical behaviour and microstructural characterization of magnesia co-doped ScSZ nanopowders synthesized by urea co-precipitation. Chem. Eng. Trans. 2017, 57, 1345–1350. [Google Scholar]
- Turco, R.; Bonelli, B.; Armandi, M.; Spiridigliozzi, L.; Dell’Agli, G.; Deorsola, F.A.; Esposito, S.; Di Serio, M. Active and stable ceria-zirconia supported molybdenum oxide catalysts for cyclooctene epoxidation: Effect of the preparation procedure. Catal. Today 2020, 345, 201–212. [Google Scholar]
- Arvanitidis, I.; Siche, D.; Seetharaman, S. A study of the thermal decomposition of BaCO3. Metall. Mater. Trans. B 1996, 27, 409–416. [Google Scholar]
- Earnest, C.M.; Miller, E.T. An assessment of barium and strontium carbonates as temperature and enthalpy standards. J. Therm. Anal. Calor. 2017, 130, 2277–2282. [Google Scholar]
- Tutuianu, M.; Inderwildi, O.R.; Bessler, W.G.; Warnatz, J. Competitive adsorption of NO, NO2, CO2, and H2O on BaO(100): A quantum chemical study. J. Phys. Chem. B 2006, 110, 17484–17492. [Google Scholar]
- Rahaman, M.N. Ceramic Processing and Sintering; Taylor & Francis: Boca Raton, FL, USA, 2003; pp. 540–619. [Google Scholar]
- Bassano, A.; Buscaglia, V.; Viviani, M.; Bassoli, M.; Buscaglia, M.T.; Sennour, M.; Thorel, A.; Nanni, P. Synthesis of Y-doped BaCeO3 nanopowders by a modified solid-state process and conductivity of dense fine-grained ceramics. Solid State Ion. 2009, 180, 168–174. [Google Scholar]
- Ricote, S.; Bonanos, N.; De Lucas, M.M.; Caboche, G. Structural and conductivity study of the proton conductor BaCe (0.9− x) ZrxY0. 1O (3− δ) at intermediate temperatures. J. Power Sources 2009, 193, 189–193. [Google Scholar]


| Chemical Composition | Thermal Treatments | Labeling |
|---|---|---|
| Ba(Ce0.2Zr0.2Yb0.2La0.2Sm0.2)O2.7 | As-precipitated | Ba(CZYbLS) |
| Ba(Ce0.2Zr0.2Yb0.2La0.2Sm0.2)O2.7 | Calcination at 800 °C | Ba(CZYbLS)_800 |
| Ba(Ce0.2Zr0.2Yb0.2La0.2Sm0.2)O2.7 | Calcination at 1300 °C | Ba(CZYbLS)_1300 |
| Ba(Ce0.2Zr0.2Yb0.2La0.2Sm0.2)O2.7 | Calcination at 1300 °C + Sintering at 1500 °C | Ba(CZYbLS)_1300_1500 |
| Ba(Ce0.2Sm0.2Yb0.2Nd0.2Gd0.2)O2.6 | As-precipitated | Ba(CSYbNG) |
| Ba(Ce0.2Sm0.2Yb0.2Nd0.2Gd0.2)O2.6 | Calcination at 800 °C | Ba(CSYbNG)_800 |
| Ba(Ce0.2Sm0.2Yb0.2Nd0.2Gd0.2)O2.6 | Calcination at 1300 °C | Ba(CSYbNG)_1300 |
| Ba(Ce0.2Sm0.2Yb0.2Nd0.2Gd0.2)O2.6 | Calcination at 1300 °C + Sintering at 1500 °C | Ba(CSYbNG)_1300_1500 |
| Ba(Ce0.2Zr0.2Nd0.2La0.2Sm0.2)O2.7 | As-precipitated | Ba(CZNLS) |
| Ba(Ce0.2Zr0.2Nd0.2La0.2Sm0.2)O2.7 | Calcination at 800 °C | Ba(CZNLS)_800 |
| Ba(Ce0.2Zr0.2Nd0.2La0.2Sm0.2)O2.7 | Calcination at 1300 °C | Ba(CZNLS)_1300 |
| Ba(Ce0.2Zr0.2Nd0.2La0.2Sm0.2)O2.7 | Calcination at 1300 °C + Sintering at 1500 °C | Ba(CZNLS)_1300_1500 |
| Sample Labelling | Standard Deviation of the B-Site Cationic Radii (sB) | Goldschmidt Tolerance Factor (t) | ΔCG Descriptor | |
|---|---|---|---|---|
| Ba(CSYbNG) | 0.9234 | 0.05216 | 0.914 | 0.278 |
| Ba(CZYbLS) | 0.8896 | 0.11680 | 0.928 | 0.333 |
| Ba(CZNLS) | 0.9126 | 0.12267 | 0.919 | 0.333 |
| Sample Labeling | Standard Deviation of the B-Site Cationic Radii (sB) | Goldschmidt Tolerance Factor (t) | ΔCG Descriptor |
|---|---|---|---|
| Ba(CZYbLS)—this work | 0.11680 | 0.928 | 0.333 |
| Ba(CZYLG)—[38] | 0.11385 | 0.927 | 0.333 |
| Sample Labeling | Apparent Density [g/cm3] | Relative Density [%] | Average Grain Size [mm] |
|---|---|---|---|
| Ba(CZYbLS)_1500 | 4.810 | 77.45 | 1.1 |
| Ba(CZYbLS)_1300_1500 | 5.891 | 94.86 | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiridigliozzi, L.; Marocco, A.; Monfreda, V.; Dell’Agli, G. Synthesis and Sintering of Novel High-Entropy Barium Cerates Designed Through the Cluster-Plus-Glue Atom Model. Ceramics 2025, 8, 32. https://doi.org/10.3390/ceramics8020032
Spiridigliozzi L, Marocco A, Monfreda V, Dell’Agli G. Synthesis and Sintering of Novel High-Entropy Barium Cerates Designed Through the Cluster-Plus-Glue Atom Model. Ceramics. 2025; 8(2):32. https://doi.org/10.3390/ceramics8020032
Chicago/Turabian StyleSpiridigliozzi, Luca, Antonello Marocco, Viviana Monfreda, and Gianfranco Dell’Agli. 2025. "Synthesis and Sintering of Novel High-Entropy Barium Cerates Designed Through the Cluster-Plus-Glue Atom Model" Ceramics 8, no. 2: 32. https://doi.org/10.3390/ceramics8020032
APA StyleSpiridigliozzi, L., Marocco, A., Monfreda, V., & Dell’Agli, G. (2025). Synthesis and Sintering of Novel High-Entropy Barium Cerates Designed Through the Cluster-Plus-Glue Atom Model. Ceramics, 8(2), 32. https://doi.org/10.3390/ceramics8020032










