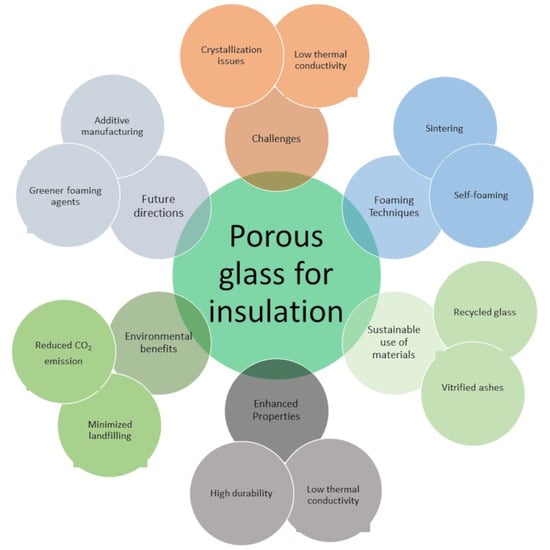
Porous Glass for Thermal Insulation in Buildings with a Focus on Sustainable Materials and Technologies: Overview and Challenges
Abstract
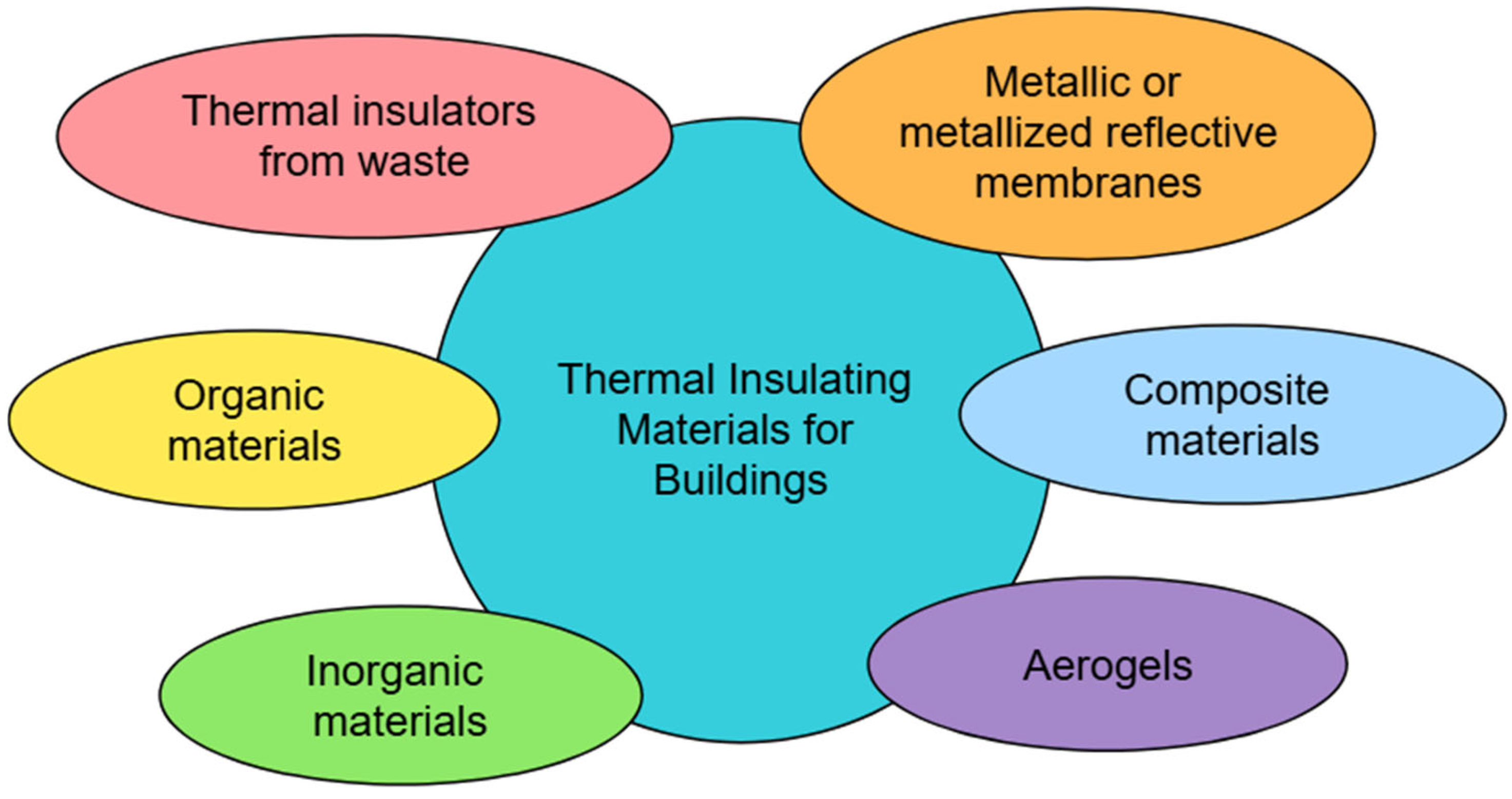
1. Introduction
- (1)
- Organic materials;
- (2)
- Inorganic materials;
- (3)
- Metallic or metallized reflective membranes;
- (4)
- Aerogels;
- (5)
- Thermal insulators from waste;
- (6)
- Composite materials.
2. Factors Affecting the Functional Properties
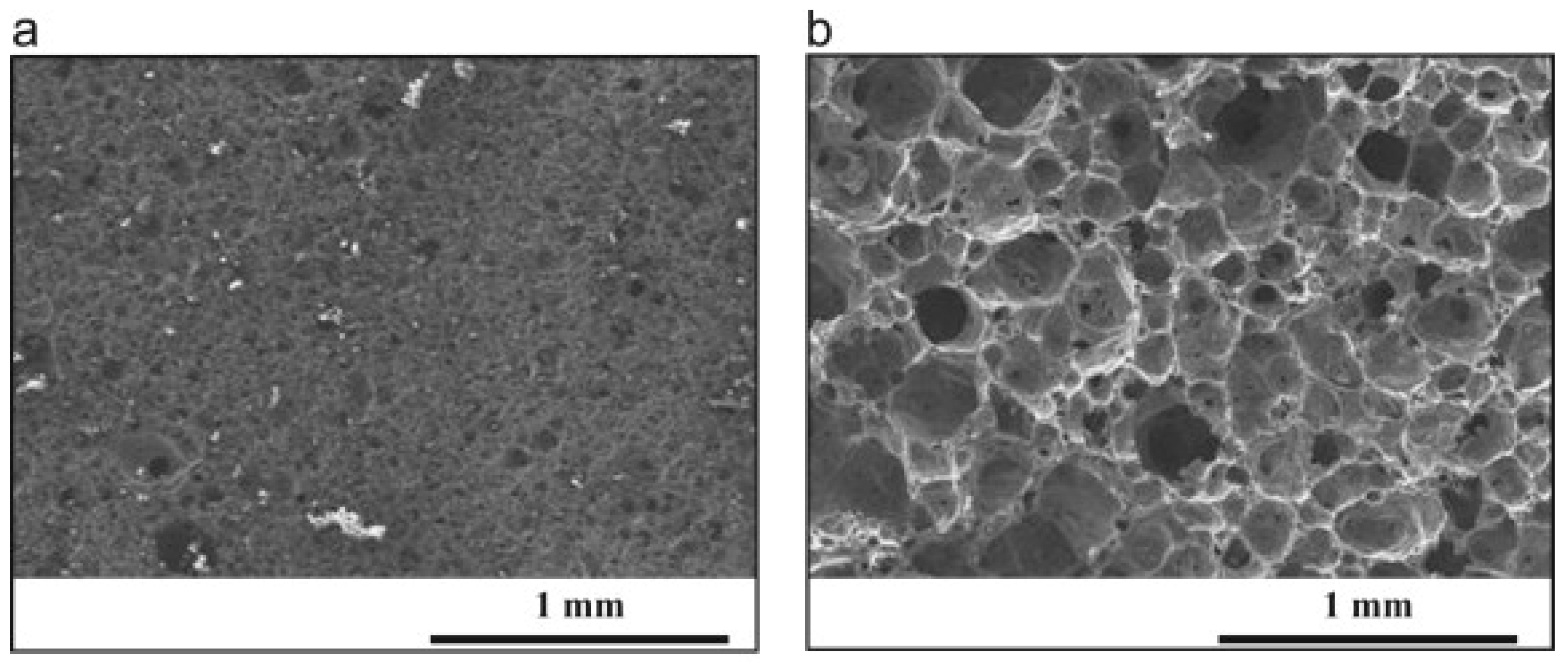
2.1. Porosity and Porous Microstructure
| Material | Thermal Conductivity (W/(m·K)) | Porosity (vol.%) | Reference |
|---|---|---|---|
| Porous silicon carbide (SiC) ceramics | 37.9–5.8 | 30–63 | [18] |
| Porous anorthite ceramics | 0.018–0.13 | 69–91 | [19] |
| Porous mullite ceramics | 0.09 | 73–86 | [20] |
| Porous yttria-stabilized zirconia (YSZ) ceramics | 0.06 | 52–76 | [22] |
| Highly porous fibrous ceramics | 0.18–0.06 | 73–90 | [23] |
| Glass wool | 0.03 | - | [38] |
| Vitrified bottom ash-based porous granules | 0.13 | - | [39] |
| 40 wt.% coal fly ash and 60 wt.% waste glass with 30 wt.% borax and 0.5 wt.% calcium carbonate | 0.36 | - | [40] |
| Aerogel | 0.017–0.04 | - | [9] |
| Al2O3 hollow glass sphere foam | 0.0244 | 94 | [28] |
| Glass-ceramic foams produced from zeolite-poor rock and eggshells | 0.07–0.4 | Only the density is reported: 0.54–1 g/cm3 | [29] |
| High-entropy ceramic foams | 0.0343–0.0592 | 90.13–96.13 | [30] |
2.2. Material Composition
3. Porous Glasses for Thermal Insulation in Buildings: Overview and Market Survey
- Impermeability to water (it does not absorb humidity and does not swell, remaining dimensionally/geometrically stable over time);
- Incombustibility (in case of fire, it does not spread flames, burn-off, or develop harmful gases);
- High thermal insulation (or low thermal conductivity);
- Resistance to parasites, rodents, microorganisms, and bacteria (this is due to its inorganic nature and can be an advantage over polymers);
- Constant compressive strength, even over a long period, and possible use also in load-bearing conditions (structural applications);
- Dimensional stability to variations in temperature (low coefficient of thermal expansion) and humidity, so that no cracks or shrinkage are generated;
- Resistance to acids and organic solvents;
- Ease of processing, cutting, and shaping;
- Recyclability (glass foams keep their properties unaltered for long periods of time, so they can be reused even after disposal as a filler or insulating granulate);
- Very low density (between 100 and 170 kg/m3).
4. Toward the Future: New Sources of Waste Materials, Technologies, and Approaches
4.1. The Potential of Vitrified Ashes from MSWIs
4.2. The Search for “Sustainable” Pore-Forming Agents
4.3. The Potential of Alkali-Activation Treatment
4.4. The Potential of Additive Manufacturing
5. Conclusions and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, L.; Li, T.; Wang, F. Heat insulation and thermal insulation method of passive low energy consumption residential building exterior envelope structure based on BIM. Results Eng. 2024, 23, 102734. [Google Scholar] [CrossRef]
- Ali, A.; Issa, A.; Elshaer, A. A Comprehensive Review and Recent Trends in Thermal Insulation Materials for Energy Conservation in Buildings. Sustainability 2024, 16, 8782. [Google Scholar] [CrossRef]
- Fragkos, P.; Tasios, N.; Paroussos, L.; Capros, P.; Tsani, S. Energy system impacts and policy implications of the European Intended Nationally Determined Contribution and low-carbon pathway to 2050. Energy Policy 2017, 100, 216–226. [Google Scholar] [CrossRef]
- Kearney, D. EIA’s Outlook Through 2035. In Annual Energy Outlook 2010; Energy Information Administration: Washington, DC, USA, 2010; pp. 1–15. [Google Scholar]
- Al-Homoud, M.S. Performance characteristics and practical applications of common building thermal insulation materials. Build. Environ. 2005, 40, 353–366. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Xu, H. Properties of vulcanized rubber nanocomposites filled with nanokaolin and precipitated silica. Appl. Clay Sci. 2008, 42, 232–237. [Google Scholar] [CrossRef]
- Villasmil, W.; Fischer, L.J.; Worlitschek, J. A review and evaluation of thermal insulation materials and methods for thermal energy storage systems. Renew. Sustain. Energy Rev. 2019, 103, 71–84. [Google Scholar] [CrossRef]
- Zhuang, J.; Ghaffar, S.H.; Fan, M.; Corker, J. Restructure of expanded cork with fumed silica as novel core materials for vacuum insulation panels. Compos. Part B Eng. 2017, 127, 215–221. [Google Scholar] [CrossRef]
- Hoseini, A.; McCague, C.; Andisheh-Tadbir, M.; Bahrami, M. Aerogel blankets: From mathematical modeling to material characterization and experimental analysis. Int. J. Heat Mass Transf. 2016, 93, 1124–1131. [Google Scholar] [CrossRef]
- Nadir, N.; Bouguettaia, H.; Boughali, S.; Bechki, D. Use of a new agricultural product as thermal insulation for solar collector. Renew. Energy 2019, 134, 569–578. [Google Scholar] [CrossRef]
- Apostolopoulou-Kalkavoura, V.; Munier, P.; Bergström, L. Thermally insulating nanocellulose-based materials. Adv. Mater. 2021, 33, 2001839. [Google Scholar] [CrossRef]
- Chao, C.-W.; Liao, C.-J. Approaches to eliminate waste and reduce cost for recycling glass. Waste Manag. 2011, 31, 2414–2421. [Google Scholar] [CrossRef] [PubMed]
- Nodehi, M.; Mohamad Taghvaee, V. Sustainable concrete for circular economy: A review on use of waste glass. Glass Struct. Eng. 2022, 7, 3–22. [Google Scholar] [CrossRef]
- Pavlenko, A.; Cheilytko, A.; Ilin, S.I.; Koshlak, H. Porous structures and their effect on thermophysical properties of thermal protection elements. Solid State Phenom. 2019, 291, 20–27. [Google Scholar] [CrossRef]
- Guo, T.; Liu, Z.; Yu, C.; Ding, J.; Yu, P.; Deng, C. Effect of pore structure evolution on mechanical properties and thermal conductivity of porous SiC-Mullite ceramics. Ceram. Int. 2023, 49, 33618–33627. [Google Scholar] [CrossRef]
- Neumann, M.; Gräfensteiner, P.; Santos de Oliveira, C.; Martins de Souza e Silva, J.; Koppka, S.; Enke, D.; Huber, P.; Schmidt, V. Morphology of nanoporous glass: Stochastic 3D modeling, stereology and the influence of pore width. Phys. Rev. Mater. 2024, 8, 045605. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, X.-P. Investigating effects of pore-scale variables and pore geometries on the thermal behaviors of porous concretes. J. Build. Eng. 2023, 75, 106895. [Google Scholar] [CrossRef]
- Kultayeva, S.; Ha, J.-H.; Malik, R.; Kim, Y.-W.; Kim, K.J. Effects of porosity on electrical and thermal conductivities of porous SiC ceramics. J. Eur. Ceram. Soc. 2020, 40, 996–1004. [Google Scholar] [CrossRef]
- Han, Y.; Li, C.; Bian, C.; Li, S.; Wang, C.-A. Porous anorthite ceramics with ultra-low thermal conductivity. J. Eur. Ceram. Soc. 2013, 33, 2573–2578. [Google Scholar] [CrossRef]
- Gong, L.; Wang, Y.; Cheng, X.; Zhang, R.; Zhang, H. Porous mullite ceramics with low thermal conductivity prepared by foaming and starch consolidation. J. Porous Mater. 2014, 21, 15–21. [Google Scholar] [CrossRef]
- Živcová, Z.; Gregorová, E.; Pabst, W.; Smith, D.S.; Michot, A.; Poulier, C. Thermal conductivity of porous alumina ceramics prepared using starch as a pore-forming agent. J. Eur. Ceram. Soc. 2009, 29, 347–353. [Google Scholar] [CrossRef]
- Hu, L.; Wang, C.-A.; Huang, Y. Porous yttria-stabilized zirconia ceramics with ultra-low thermal conductivity. J. Mater. Sci. 2010, 45, 3242–3246. [Google Scholar] [CrossRef]
- Sun, J.; Hu, Z.; Zhuo, J.; Wang, X.; Sun, C. Thermal properties of highly porous fibrous ceramics. In Proceedings of the 5th International Conference on Porous Media and Their Applications in Science, Engineering and Industry, Kona, HI, USA, 22–27 June 2014. [Google Scholar]
- Құлтаева, Ш.М.; Құрманбекoва, Э.Б. Tuning the Thermal Conductivity of Porous Silicon Carbide Ceramics: A Review. Вестн. КазГАСА 2024, 3, 160–171. [Google Scholar]
- Smith, D.S.; Alzina, A.; Bourret, J.; Nait-Ali, B.; Pennec, F.; Tessier-Doyen, N.; Otsu, K.; Matsubara, H.; Elser, P.; Gonzenbach, U.T. Thermal conductivity of porous materials. J. Mater. Res. 2013, 28, 2260–2272. [Google Scholar] [CrossRef]
- Gregorová, E.; Pabst, W.; Sofer, Z.; Jankovský, O.; Matějíček, J. Porous alumina and zirconia ceramics with tailored thermal conductivity. J. Phys. Conf. Ser. 2012, 395, 012022. [Google Scholar] [CrossRef]
- Li, X.; Pan, M.; Tao, M.; Liu, W.; Gao, Z.; Ma, C. Preparation of high closed porosity foamed ceramics from coal gangue waste for thermal insulation applications. Ceram. Int. 2022, 48, 37055–37063. [Google Scholar] [CrossRef]
- Wang, C.; Rong, Y.; Zhang, B.; Yang, J. Facile Method for Preparing Hierarchical Al2O3–Glass Foam Ceramics with Superior Thermal Insulating Property. Langmuir 2022, 38, 1141–1150. [Google Scholar] [CrossRef]
- Ibrahim, J.E.F.; Gömze, L.A.; Koncz-Horvath, D.; Filep, Á.; Kocserha, I. Preparation, characterization, and physicomechanical properties of glass-ceramic foams based on alkali-activation and sintering of zeolite-poor rock and eggshell. Ceram. Int. 2022, 48, 25905–25917. [Google Scholar] [CrossRef]
- Yang, R.; Liang, Y.; Xu, J.; Meng, X.; Zhu, J.; Cao, S.; Wei, M.; Zhang, R.; Yang, J.; Gao, F. Rare-earth-niobate high-entropy ceramic foams with enhanced thermal insulation performance. J. Mater. Sci. Technol. 2022, 116, 94–102. [Google Scholar] [CrossRef]
- Sun, J.; Liu, P. Optimization of structural parameters for the sound absorption performance of a cellular ceramic foam. Multidiscip. Model. Mater. Struct. 2021, 17, 1108–1118. [Google Scholar] [CrossRef]
- Chen, J.; Liu, P.; Sun, J. Sound absorption performance of a lightweight ceramic foam. Ceram. Int. 2020, 46, 22699–22708. [Google Scholar] [CrossRef]
- Lucio, M.D.S.; Kultayeva, S.; Kim, Y.-W. Improved mechanical strength and thermal resistance of porous SiC ceramics with gradient pore sizes. J. Eur. Ceram. Soc. 2022, 42, 6785–6794. [Google Scholar] [CrossRef]
- Niyogi, S.; Gupta, B.S. Mechanical properties and pore size distribution in athermal porous glasses. Soft Matter 2021, 17, 9716–9724. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, N.; Omoto, Y.; Hoshide, T. Numerical simulation on effects of pore characteristics on strength in porous ceramics. Mech. Eng. J. 2019, 6, 19-00234. [Google Scholar] [CrossRef]
- Pereira, C.; Rice, R.; Skalny, J. Pore structure and its relationship to properties of materials. MRS Online Proc. Libr. 1988, 137, 3. [Google Scholar] [CrossRef]
- Jung, D.; Kwon, Y. Investigation of Structure-Property Relations in Porous Metals Using Finite Element Simulation. Korean J. Met. Mater. 2019, 57, 747–754. [Google Scholar] [CrossRef]
- Jeon, C.-K.; Lee, J.-S.; Chung, H.; Kim, J.-H.; Park, J.-P. A study on insulation characteristics of glass wool and mineral wool coated with a polysiloxane agent. Adv. Mater. Sci. Eng. 2017, 2017, 3938965. [Google Scholar] [CrossRef]
- Baino, F.; Ferraris, M. Production and characterization of ceramic foams derived from vitrified bottom ashes. Mater. Lett. 2019, 236, 281–284. [Google Scholar] [CrossRef]
- Dele-Afolabi, T.; Hanim, M.A.; Norkhairunnisa, M.; Sobri, S.; Calin, R. Research trend in the development of macroporous ceramic components by pore forming additives from natural organic matters: A short review. Ceram. Int. 2017, 43, 1633–1649. [Google Scholar] [CrossRef]
- Park, S.; Kwon, Y.-P.; Kwon, H.-C.; Lee, H.-W.; Lee, J.C. Effect of composition on thermal conductivity of silica insulation media. J. Nanosci. Nanotechnol. 2008, 8, 5052–5056. [Google Scholar] [CrossRef]
- Rodin, A.; Ermakov, A.; Erofeeva, I.; Erofeev, V. Effect of Chlorides Content on the Structure and Properties of Porous Glass Ceramics Obtained from Siliceous Rock. Materials 2022, 15, 3268. [Google Scholar] [CrossRef]
- Jiang, K.; Xia, M.; Tang, Y.; Xu, Y.; Deng, T.; Li, B.; Chen, W. Formation of closed pore structure in CaO-MgO-Al2O3-SiO2 (CMAS) porous glass-ceramics via Fe2O3 modified foaming for thermal insulation. J. Eur. Ceram. Soc. 2023, 43, 1689–1697. [Google Scholar] [CrossRef]
- Apkaryan, A.; Kulkov, S. Formation of structure and closed porosity under high-temperature firing of granules of porous glass-ceramic material. Inorg. Mater. Appl. Res. 2018, 9, 286–290. [Google Scholar] [CrossRef]
- Kim, H.D.; Baek, C.R.; Jang, Y.C. Advancing glass recycling and environmental applications with porous glass: A mini-review. J. Mater. Cycles Waste Manag. 2024, 26, 2620–2633. [Google Scholar] [CrossRef]
- He, R.; Qu, Z.; Cheng, X. Effects of starch addition amount on microstructure, mechanical properties and room temperature thermal conductivity of porous Y2SiO5 ceramics. Ceram. Int. 2016, 42, 2257–2262. [Google Scholar] [CrossRef]
- Müller, A.; Leydolph, B.; Stanelle, K. Recycling mineral wool waste: Technologies for the conversion of the fiber structure, Part 1. Interceram 2009, 58, 378–381. [Google Scholar]
- Jagadeeswaran, I.; Sriram, H. EU 1272/2008—Classification, Labelling and Packaging of Substances and Mixtures. In Medical Device Guidelines and Regulations Handbook; Springer: Berlin/Heidelberg, Germany, 2022; pp. 261–295. [Google Scholar]
- Mesa Sanchez, M.; Giro Guasch, E.; Pasalaigua Huguet, J. Biosoluble Composition of Glass Fibres for the Production of Glass Wools and Similar Patent ES2212962T3, 16 August 2004.
- Tulyaganov, D.; Fernandes, H.; Agathopoulos, S.; Ferreira, J. Preparation and characterization of high compressive strength foams from sheet glass. J. Porous Mater. 2006, 13, 133–139. [Google Scholar] [CrossRef]
- Bernardo, E.; Scarinci, G.; Bertuzzi, P.; Ercole, P.; Ramon, L. Recycling of waste glasses into partially crystallized glass foams. J. Porous Mater. 2010, 17, 359–365. [Google Scholar] [CrossRef]
- Owens Corning FOAMGLAS® Thermal Insulation Made of Cellular Glass. Available online: https://www.foamglas.com/it-ch (accessed on 10 December 2024).
- Liu, H.; Zhao, X. Thermal conductivity analysis of high porosity structures with open and closed pores. Int. J. Heat Mass Transf. 2022, 183, 122089. [Google Scholar] [CrossRef]
- Ye, L.; Hong, J.; Ma, X.; Qi, C.; Yang, D. Life cycle environmental and economic assessment of ceramic tile production: A case study in China. J. Clean. Prod. 2018, 189, 432–441. [Google Scholar] [CrossRef]
- König, J.; Petersen, R.R.; Yue, Y.; Suvorov, D. Gas-releasing reactions in foam-glass formation using carbon and MnxOy as the foaming agents. Ceram. Int. 2017, 43, 4638–4646. [Google Scholar] [CrossRef]
- Sakai, S.-i.; Hiraoka, M. Municipal solid waste incinerator residue recycling by thermal processes. Waste Manag. 2000, 20, 249–258. [Google Scholar] [CrossRef]
- Ferraris, M.; Salvo, M.; Smeacetto, F.; Augier, L.; Barbieri, L.; Corradi, A.; Lancellotti, I. Glass matrix composites from solid waste materials. J. Eur. Ceram. Soc. 2001, 21, 453–460. [Google Scholar] [CrossRef]
- Scarinci, G.; Brusatin, G.; Barbieri, L.; Corradi, A.; Lancellotti, I.; Colombo, P.; Hreglich, S.; Dall’Igna, R. Vitrification of industrial and natural wastes with production of glass fibres. J. Eur. Ceram. Soc. 2000, 20, 2485–2490. [Google Scholar] [CrossRef]
- Islam, G.S.; Rahman, M.; Kazi, N. Waste glass powder as partial replacement of cement for sustainable concrete practice. Int. J. Sustain. Built Environ. 2017, 6, 37–44. [Google Scholar] [CrossRef]
- Sharifikolouei, E.; Canonico, F.; Salvo, M.; Baino, F.; Ferraris, M. Vitrified and nonvitrified municipal solid wastes as ordinary Portland cement (OPC) and sand substitution in mortars. Int. J. Appl. Ceram. Technol. 2020, 17, 573–583. [Google Scholar] [CrossRef]
- Maddalena, R.; Roberts, J.J.; Hamilton, A. Can Portland cement be replaced by low-carbon alternative materials? A study on the thermal properties and carbon emissions of innovative cements. J. Clean. Prod. 2018, 186, 933–942. [Google Scholar] [CrossRef]
- Sabbrojjaman, M.; Liu, Y.; Tafsirojjaman, T. A comparative review on the utilisation of recycled waste glass, ceramic and rubber as fine aggregate on high performance concrete: Mechanical and durability properties. Dev. Built Environ. 2024, 17, 100371. [Google Scholar] [CrossRef]
- Hamada, H.; Alattar, A.; Tayeh, B.; Yahaya, F.; Thomas, B. Effect of recycled waste glass on the properties of high-performance concrete: A critical review. Case Stud. Constr. Mater. 2022, 17, e01149. [Google Scholar] [CrossRef]
- Appendino, P.; Ferraris, M.; Matekovits, I.; Salvo, M. Production of glass–ceramic bodies from the bottom ashes of municipal solid waste incinerators. J. Eur. Ceram. Soc. 2004, 24, 803–810. [Google Scholar] [CrossRef]
- Fernandes, H.R.; Gunduz, O.; Tulyaganov, D.U. Synthesis of Bulk-Nucleated Glass–Ceramics and Porous Glass–Ceramic Composites through Utilization of Fly Ashes. Ceramics 2024, 7, 1014–1029. [Google Scholar] [CrossRef]
- Fernandes, H.; Tulyaganov, D.; Ferreira, J. Preparation and characterization of foams from sheet glass and fly ash using carbonates as foaming agents. Ceram. Int. 2009, 35, 229–235. [Google Scholar] [CrossRef]
- Zhu, M.; Ji, R.; Li, Z.; Wang, H.; Liu, L.; Zhang, Z. Preparation of glass ceramic foams for thermal insulation applications from coal fly ash and waste glass. Constr. Build. Mater. 2016, 112, 398–405. [Google Scholar] [CrossRef]
- Zeng, L.; Sun, H.-j.; Peng, T.-j.; Zheng, W.-m. The sintering kinetics and properties of sintered glass-ceramics from coal fly ash of different particle size. Results Phys. 2019, 15, 102774. [Google Scholar] [CrossRef]
- Chen, R.; Li, Y.; Xiang, R.; Li, S. Effect of particle size of fly ash on the properties of lightweight insulation materials. Constr. Build. Mater. 2016, 123, 120–126. [Google Scholar] [CrossRef]
- Li, M.; Gao, Q.; He, Q.; Li, S.; Quan, Y.; Wu, M.; Li, C. Low-cost porous thermal insulation materials with tunable pore structures derived from fly ash by foam-gelcasting. Int. J. Appl. Ceram. Technol. 2024, 21, 267–277. [Google Scholar] [CrossRef]
- Sharifikolouei, E.; Baino, F.; Salvo, M.; Tommasi, T.; Pirone, R.; Fino, D.; Ferraris, M. Vitrification of municipal solid waste incineration fly ash: An approach to find the successful batch compositions. Ceram. Int. 2021, 47, 7738–7744. [Google Scholar] [CrossRef]
- Ding, L.; Ning, W.; Wang, Q.; Shi, D.; Luo, L. Preparation and characterization of glass–ceramic foams from blast furnace slag and waste glass. Mater. Lett. 2015, 141, 327–329. [Google Scholar] [CrossRef]
- Rincón, A.; Marangoni, M.; Cetin, S.; Bernardo, E. Recycling of inorganic waste in monolithic and cellular glass-based materials for structural and functional applications. J. Chem. Technol. Biotechnol. 2016, 91, 1946–1961. [Google Scholar] [CrossRef]
- Fernandes, H.R.; Andreola, F.; Barbieri, L.; Lancellotti, I.; Pascual, M.J.; Ferreira, J.M. The use of egg shells to produce Cathode Ray Tube (CRT) glass foams. Ceram. Int. 2013, 39, 9071–9078. [Google Scholar] [CrossRef]
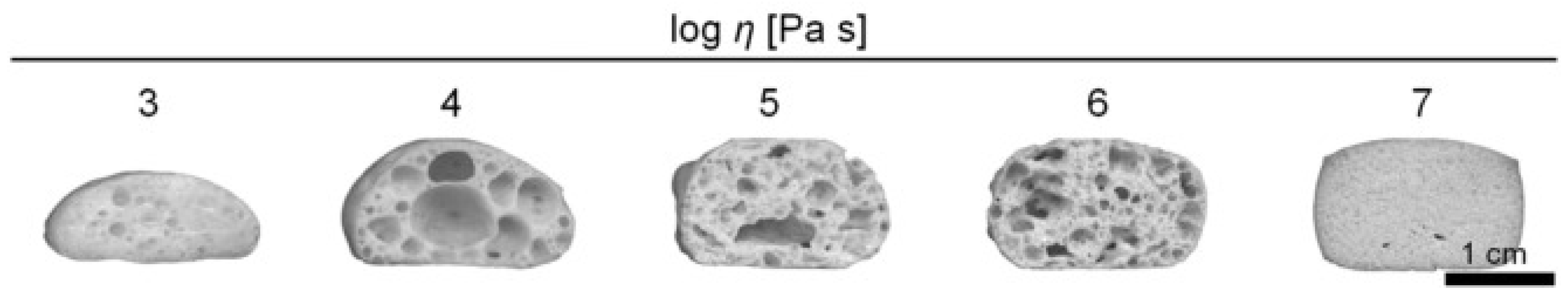
- Petersen, R.R.; König, J.; Yue, Y. The viscosity window of the silicate glass foam production. J. Non-Cryst. Solids 2017, 456, 49–54. [Google Scholar] [CrossRef]
- Fedosov, S.; Bakanov, M.; Grushko, I. Using anthropogenic raw materials in the process of synthesizing foam glass with heterogeneous microstructure. Vestn. MGSU 2024, 19, 258–269. [Google Scholar] [CrossRef]
- Cozzarini, L.; De Lorenzi, L.; Fortuna, L.; Bevilacqua, P. Recycling of glass waste and spent alkaline batteries cathodes into insulation materials. Sustain. Mater. Technol. 2023, 38, e00767. [Google Scholar] [CrossRef]
- Fernandes, F.A.d.S.; Costa, D.d.S.d.O.; Martin, C.A.G.; Rossignolo, J.A. Vitreous foam with thermal insulating property produced with the addition of waste glass powder and rice husk ash. Sustainability 2023, 15, 796. [Google Scholar] [CrossRef]
- Odewole, O.P.; Kashim, I.B.; Akinbogun, T.L. Investigation into the viability of the properties of porous glass-ceramics produced from granite dust and maize cob for use in thermal insulation of external walls of residential buildings. J. Mech. Eng. Sci. 2022, 16, 8943–8952. [Google Scholar] [CrossRef]
- Goltsman, B.M.; Yatsenko, E.A. Role of carbon phase in the formation of foam glass porous structure. Materials 2022, 15, 7913. [Google Scholar] [CrossRef]
- Paunescu, L.; Axinte, S.M.; Dragoescu, M.F.; Cosmulescu, F.; Paunescu, B.V. Simultaneous Use of Liquid and Solid Foaming Agents by a Nonconventional Technique to Obtain a High-Strength Glass Foam with Fine Porosity. Nonconv. Technol. Rev. 2021, 25, 3–9. [Google Scholar]
- Sooksaen, P.; Sudyod, N.; Thongtha, N.; Simsomboonphol, R. Fabrication of lightweight foam glasses for thermal insulation applications. Mater. Today Proc. 2019, 17, 1823–1830. [Google Scholar] [CrossRef]
- Chaima, S.; Andrea, S.; Peter, B. Investigation of foamed glass using natural waste materials as foaming agent. Multidiszcip. Tudományok 2022, 12, 280–291. [Google Scholar]
- Da Silva, L.L.; Ribeiro, L.C.N.; Santacruz, G.; Arcaro, S.; Alves, A.K.; Bergmann, C.P. Glass Foams Produced from Glass and Yerba Mate (Ilex paraguarinensis) Waste. FME Trans. 2018, 46, 70–79. [Google Scholar]
- Yatsenko, E.; Gol’tsman, B.; Smolii, V.; Gol’tsman, N.; Yatsenko, L. Study on the possibility of applying organic compounds as pore-forming agents for the synthesis of foam glass. Glass Phys. Chem. 2019, 45, 138–142. [Google Scholar] [CrossRef]
- Stochero, N.; de Souza Chami, J.; Souza, M.; de Moraes, E.; de Oliveira, A.N. Green glass foams from wastes designed for thermal insulation. Waste Biomass Valorization 2021, 12, 1609–1620. [Google Scholar] [CrossRef]
- Da Silva, R.C.; Kubaski, E.T.; Tenório-Neto, E.T.; Lima-Tenório, M.K.; Tebcherani, S.M. Foam glass using sodium hydroxide as foaming agent: Study on the reaction mechanism in soda-lime glass matrix. J. Non-Cryst. Solids 2019, 511, 177–182. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, B.; Zhao, S.; Shen, H.; Liu, J.; Zhang, S. Preparation and characterization of glass ceramic foams based on municipal solid waste incineration ashes using secondary aluminum ash as foaming agent. Constr. Build. Mater. 2020, 262, 120781. [Google Scholar] [CrossRef]
- Mahmoud, M.; Kraxner, J.; Kaňková, H.; Hujová, M.; Chen, S.; Galusek, D.; Bernardo, E. Porous glass microspheres from alkali-activated fiber glass waste. Materials 2022, 15, 1043. [Google Scholar] [CrossRef]
- Rincón, A.; Giacomello, G.; Pasetto, M.; Bernardo, E. Novel ‘inorganic gel casting’process for the manufacturing of glass foams. J. Eur. Ceram. Soc. 2017, 37, 2227–2234. [Google Scholar] [CrossRef]
- Elsayed, H.; Rincón Romero, A.; Ferroni, L.; Gardin, C.; Zavan, B.; Bernardo, E. Bioactive glass-ceramic scaffolds from novel ‘inorganic gel casting’ and sinter-crystallization. Materials 2017, 10, 171. [Google Scholar] [CrossRef]
- Rincón, A.; Desideri, D.; Bernardo, E. Functional glass-ceramic foams from ‘inorganic gel casting’and sintering of glass/slag mixtures. J. Clean. Prod. 2018, 187, 250–256. [Google Scholar] [CrossRef]
- Monich, P.R.; Romero, A.R.; Höllen, D.; Bernardo, E. Porous glass-ceramics from alkali activation and sinter-crystallization of mixtures of waste glass and residues from plasma processing of municipal solid waste. J. Clean. Prod. 2018, 188, 871–878. [Google Scholar] [CrossRef]
- Romero, A.R.; Salvo, M.; Bernardo, E. Up-cycling of vitrified bottom ash from MSWI into glass-ceramic foams by means of ‘inorganic gel casting’and sinter-crystallization. Constr. Build. Mater. 2018, 192, 133–140. [Google Scholar] [CrossRef]
- Monich, P.R.; Romero, A.R.; Rambaldi, E.; Bernardo, E. Case studies of up-cycling of partially crystallized ceramic waste in highly porous glass-ceramics. Constr. Build. Mater. 2020, 261, 119971. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, B.; Zhang, X.; Shen, H.; Liu, J.; Zhang, S. Co-vitrification of municipal solid waste incinerator fly ash and bottom slag: Glass detoxifying characteristics and porous reformation. Ecotoxicol. Environ. Saf. 2022, 243, 113995. [Google Scholar] [CrossRef] [PubMed]
- UNI EN 12457-2:2004; Caratterizzazione dei Rifiuti–Lisciviazione–Prova di Conformità per la Lisciviazione di Rifiuti Granulari e di Fanghi. In Parte 2: Prova a Singolo Stadio, con un Rapporto Liquido/Solido di 10 L/kg, per Materiali con Particelle di Dimensioni Minori di 4 mm (con o Senza Riduzione Delle Dimensioni). UNI: Rome, Italy, 2004.
- Souza, M.T.; Onghero, L.; Passos, A.B.; Simão, L.; Piva, R.H.; Repette, W.L.; de Oliveira, A.P.N. Sustainable glass foams produced with stone waste as a pore-forming agent: Assessing the role of heating rate in foamability and glass foams recyclability. J. Clean. Prod. 2022, 338, 130596. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D printing of ceramics: A review. J. Eur. Ceram. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Villa, A.; Gianchandani, P.K.; Baino, F. Sustainable Approaches for the Additive Manufacturing of Ceramic Materials. Ceramics 2024, 7, 291–309. [Google Scholar] [CrossRef]
- Simorgh, S.; Alasvand, N.; Khodadadi, M.; Ghobadi, F.; Kebria, M.M.; Milan, P.B.; Kargozar, S.; Baino, F.; Mobasheri, A.; Mozafari, M. Additive manufacturing of bioactive glass biomaterials. Methods 2022, 208, 75–91. [Google Scholar] [CrossRef]
- Cuevas, K.; Chougan, M.; Martin, F.; Ghaffar, S.H.; Stephan, D.; Sikora, P. 3D printable lightweight cementitious composites with incorporated waste glass aggregates and expanded microspheres–Rheological, thermal and mechanical properties. J. Build. Eng. 2021, 44, 102718. [Google Scholar] [CrossRef]
- Deng, Q.; Zou, S.; Xi, Y.; Singh, A. Development and Characteristic of 3D-Printable Mortar with Waste Glass Powder. Buildings 2023, 13, 1476. [Google Scholar] [CrossRef]
- Sheng, Z.; Zhu, B.; Cai, J.; Han, J.; Zhang, Y.; Pan, J. Influence of waste glass powder on printability and mechanical properties of 3D printing geopolymer concrete. Dev. Built Environ. 2024, 20, 100541. [Google Scholar] [CrossRef]
- Derevianko, O.; Derevianko, O.; Zakiev, V.; Zgalat-Lozynskyy, O. 3D printing of porous glass products using the robocasting technique. Powder Met. Metal Ceram. 2022, 60, 546–555. [Google Scholar] [CrossRef]
- Nan, B.; Gołębiewski, P.; Buczyński, R.; Galindo-Rosales, F.J.; Ferreira, J.M. Direct ink writing glass: A preliminary step for optical application. Materials 2020, 13, 1636. [Google Scholar] [CrossRef]
- De Moraes, E.G.; Ferreira, I.M.; Teixeira, L.B.; Cartapati, L.H.; Souza, M.T.; de Oliveira, A.P.N. Additive manufacturing of cellular structures from recycled soda-lime glass printing inks by robocasting. Ceram. Int. 2023, 49, 6554–6562. [Google Scholar] [CrossRef]
- Smolii, V.; Kosarev, A.; Yatsenko, E. Cellular Heat Insulation Building Glass Materials Based on Wastes from Thermal Power Plants and Ferrous Metallurgy. Glass Ceram. 2017, 74, 52–54. [Google Scholar] [CrossRef]
- Omerašević, M.; Pavkov, V.; Rosić, M.; Egerić, M.; Nenadović, S.; Bučevac, D.; Potkonjak, N. Fabrication of Porous Anorthite Ceramic Insulation Using Solid Wastes. Materials 2024, 17, 1478. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.; Yan, S.; Wu, J.M.; Liu, J.; Chen, Y.; Qu, Y.; Tang, X.; Yang, J. A novel fabrication method for glass foams with small pore size and controllable pore structure. J. Am. Ceram. Soc. 2017, 100, 5502–5511. [Google Scholar] [CrossRef]
- Mann, C.J.; Hee, K.D.; Gyu, K.S. Porous Ultra Light Ceramic Insulator for Building External Insulation System Using Waste Glass and Coal Ash and Manufacturing Method Thereof Patent KR20160137716A, 1 December 2016.




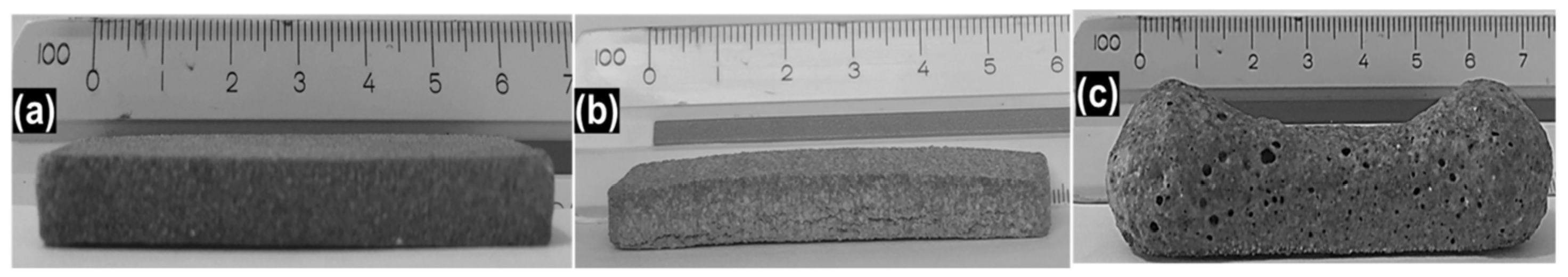
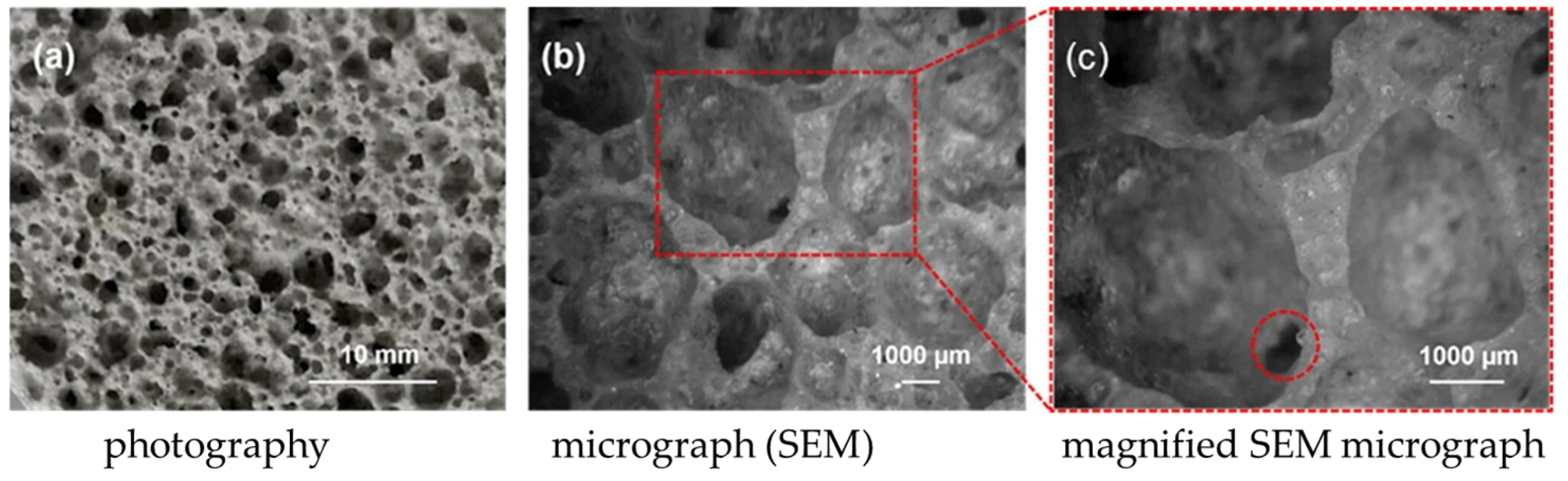


| Product | Applications |
|---|---|
| Foamglas® (without mechanical loads) | - Facades - Internal insulation |
| Foamglas® (with mechanical loads) | - Flat and sloping roofs - Facades - Insulation of floors and perimeters - Metal roofs and special roofs - Internal insulation for walls and ceilings |
| Misapor® | - Coupling with concrete - Perimeter insulation and foundation slabs - Roof insulation - Vertical insulation of walls in contact with the ground |
| Reapor® | - Fire insulation - Insulation of railway tunnels and ventilation shafts - Insulation of industrial plants and machines (thermal and acoustic) - Indoor insulation (thermal and acoustic) - Highway barriers (thermal and acoustic) |
| Type of Waste | Composition of Waste | Resulting Vitrified Product | Composition of the Vitrified Product and Crystalline Phases (If Glass-Ceramic) | Reference |
|---|---|---|---|---|
| Glass powder from obsolete cathode ray tubes | 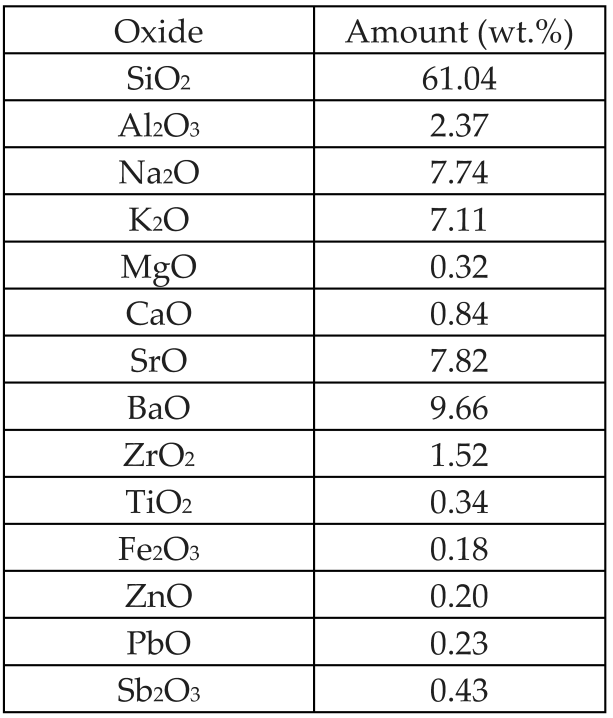 | Glass | - | [55] |
| Different dry bottom ashes (BA) from MSWIs and wastes from an aluminum alloy industry (AW) | 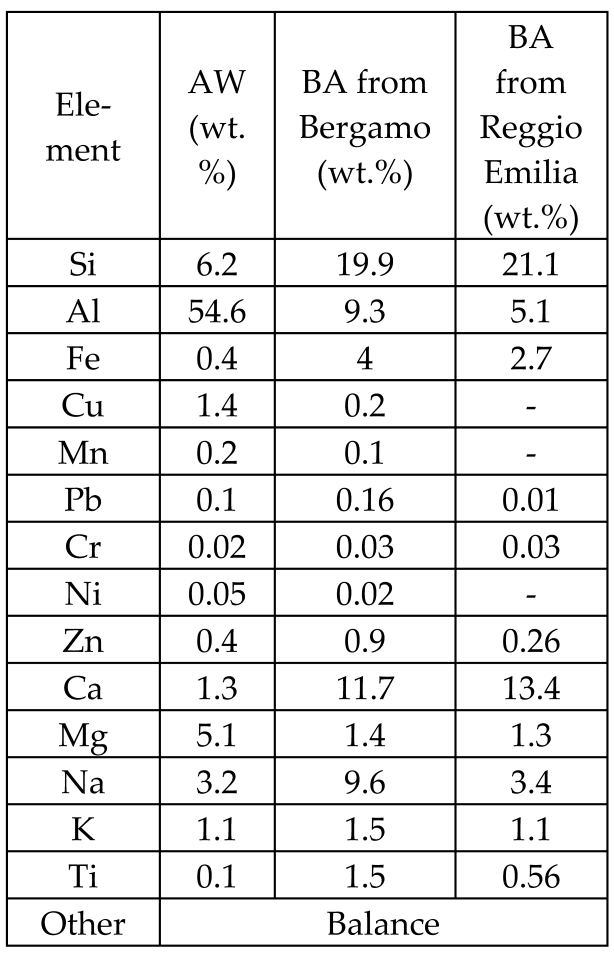 | Glass, glass-matrix composites | 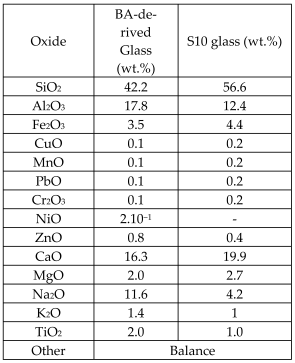 | [57] |
| Bottom ash (BA) from MSWIs, sludge, cullet glass | 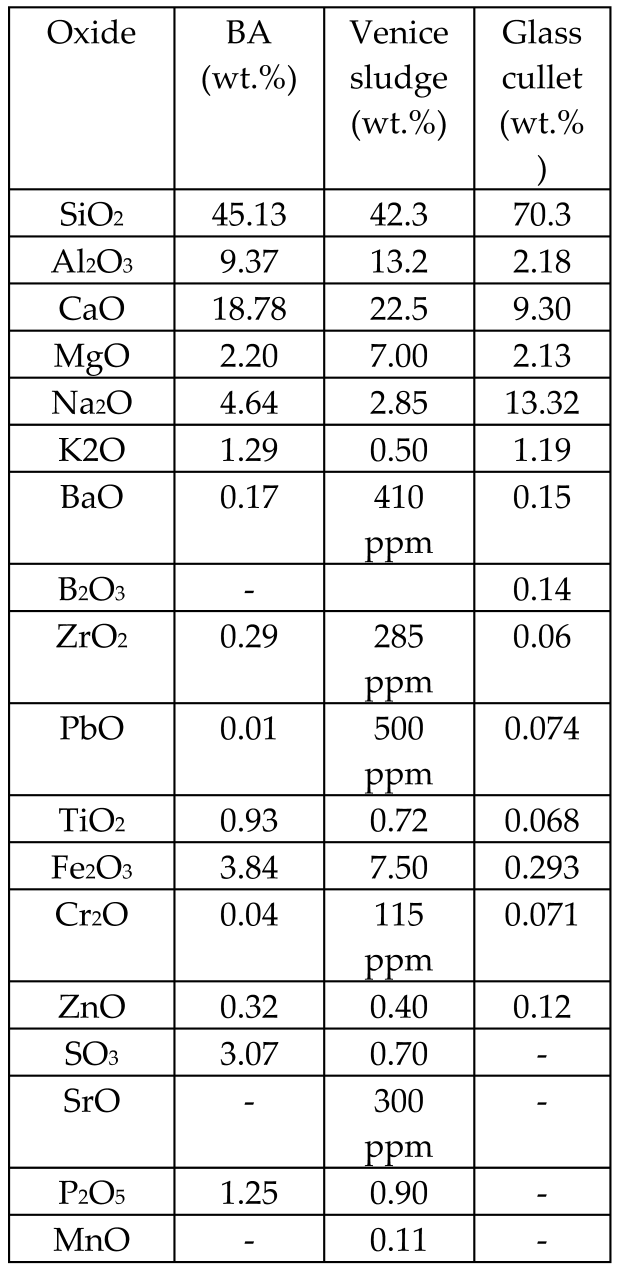 | Glass, glass fibers | - | [58] |
| Waste glass (clear and colored) |  | Glass, also combined with mortar | - | [59] |
| Bottom ashes from MSWIs W1: vitrified bottom ashes collected from a Japanese MSWI equipped with a direct melting system (DMS) W2: bottom ashes collected from Lomello MSWI (Alessandria, Italy) and placed outdoors for 3 months under ambient humidity and CO2 (this step (weathering) is recommended by a large body of research on the stabilization of heavy metals in bottom ashes) | - | Glass, glass-ceramics | 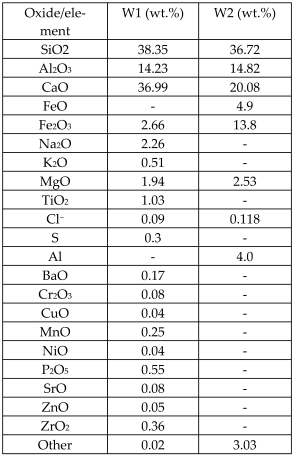 W1 contains gehlenite W1 contains gehleniteW2 contains quartz, gehlenite, anorthite, hematite, calcite | [60] |
| Dry bottom ash (BA) from MSWIs of Bergamo and Vercelli (Italy) were vitrified |  | Glass-ceramics | 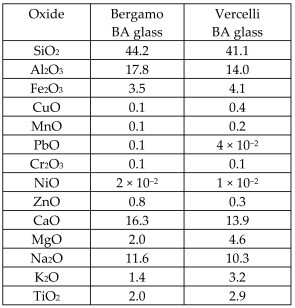 | [64] |
| Fly ash (FA) from thermal power plant + cullet of commercially produced sodium-calcium-silicate sheet glass Commercial dolomite and sludge from a marble cutting- polishing plant consisting of calcite were used as foaming gents | 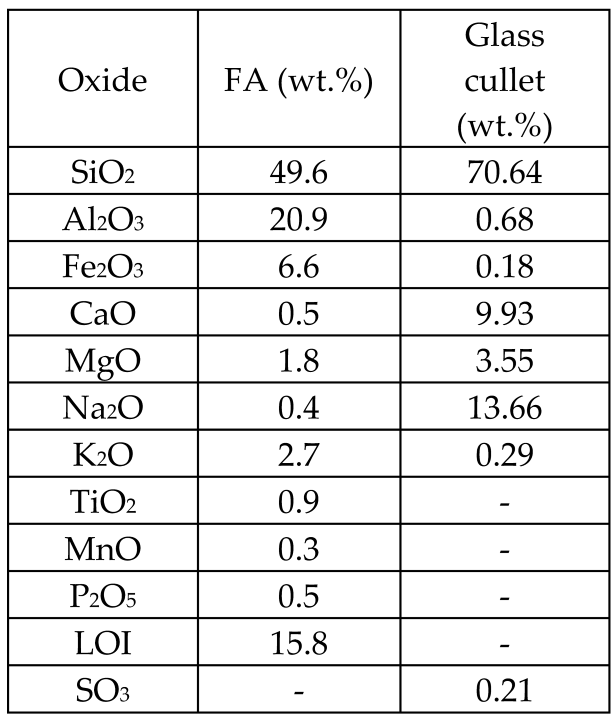 | Glass-ceramic | Crystalline phases: quartz, tridymite, pargasite, and augite | [66] |
| Coal fly ash (FA) from a thermal power plant + waste glass Borax added as fluxing agent, calcium carbonate chosen as foaming agent |  | Glass-ceramics | 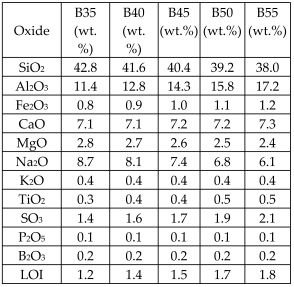 Crystalline phases: diopside, quartz Crystalline phases: diopside, quartz | [67] |
| Coal fly ash (FA) from a thermal power plant in three size fractions: CFA = 12.6 μm 20CFA = 7.5 μm 40CFA = 4.9 μm | 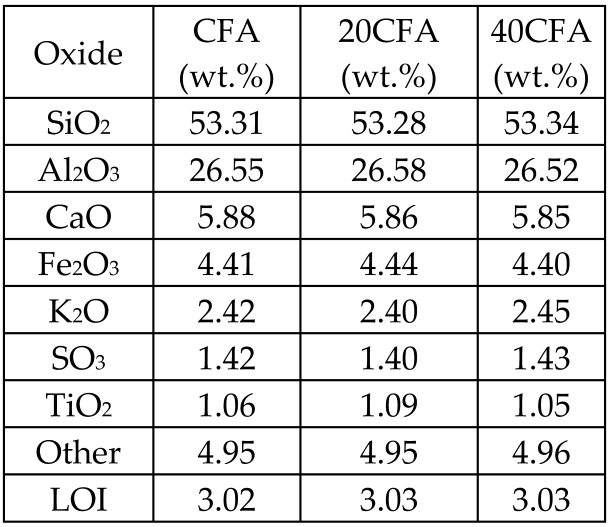 | Glass-ceramics |  Crystalline phases: quartz, mullite and hematite, with Crystalline phases: quartz, mullite and hematite, with amorphous metastable glassy phase forms | [68] |
| Fly ash, flint clay, kyanite, clay, saw dust + organic binder polyvinyl alcohol (2 wt.% solution) | 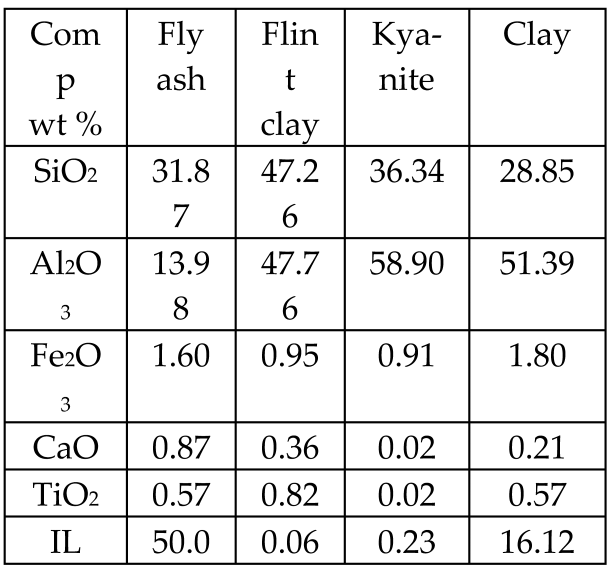 | Glass-ceramics | Crystalline phase: mullite | [69] |
| Fly ash floating beads with polycarboxylate (dispersant), dextrin (binder), sodium carboxymethyl cellulose (thickener), and sodium alkyl sulfate (foaming agent) |  | Glass-ceramics | Amount of crystalline phases of porous glass-ceramics prepared at different temperatures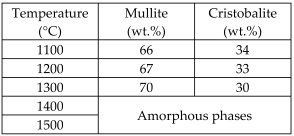 | [70] |
| Fly ashes from a waste-to-energy plant from two different sources (FA1, FA2) mixed in 50 wt.% combination with glass cullet and silica sand | 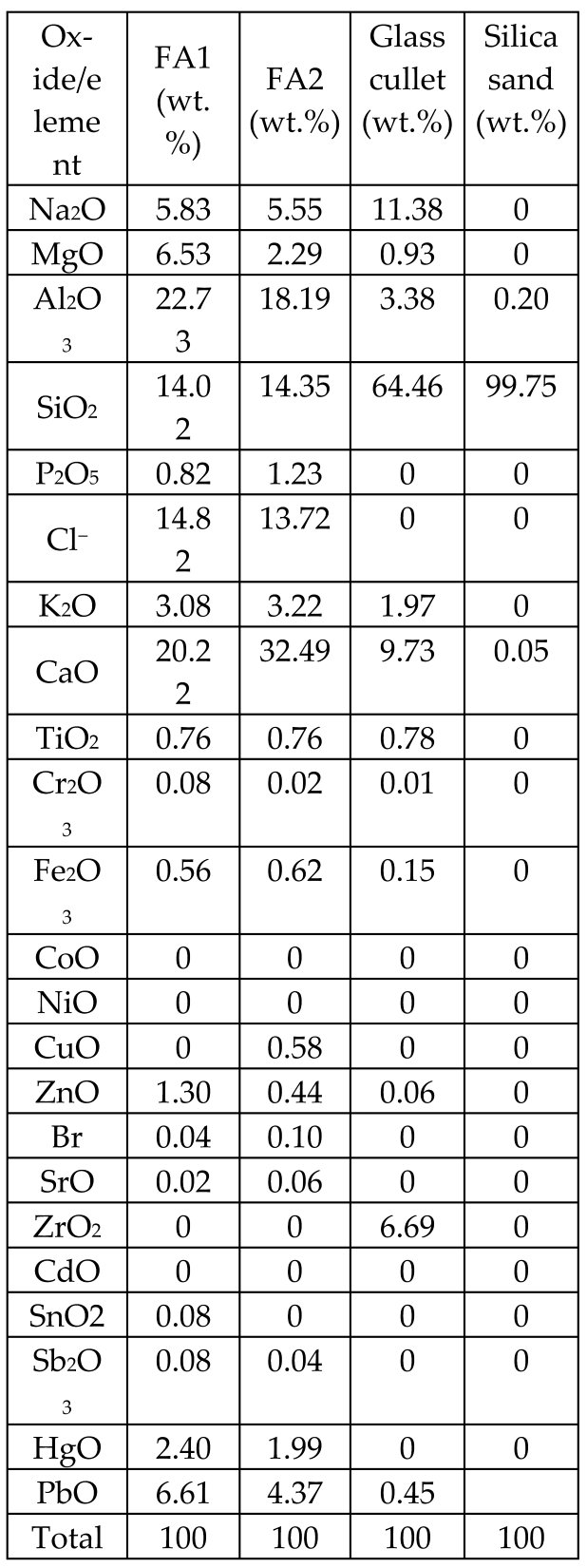 | Glass-ceramics | 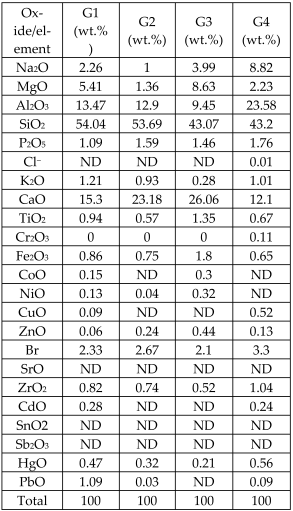 Crystalline phases: wollastonite (traces) Crystalline phases: wollastonite (traces) | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baino, F.; Kumar Gianchandani, P. Porous Glass for Thermal Insulation in Buildings with a Focus on Sustainable Materials and Technologies: Overview and Challenges. Ceramics 2025, 8, 28. https://doi.org/10.3390/ceramics8010028
Baino F, Kumar Gianchandani P. Porous Glass for Thermal Insulation in Buildings with a Focus on Sustainable Materials and Technologies: Overview and Challenges. Ceramics. 2025; 8(1):28. https://doi.org/10.3390/ceramics8010028
Chicago/Turabian StyleBaino, Francesco, and Pardeep Kumar Gianchandani. 2025. "Porous Glass for Thermal Insulation in Buildings with a Focus on Sustainable Materials and Technologies: Overview and Challenges" Ceramics 8, no. 1: 28. https://doi.org/10.3390/ceramics8010028
APA StyleBaino, F., & Kumar Gianchandani, P. (2025). Porous Glass for Thermal Insulation in Buildings with a Focus on Sustainable Materials and Technologies: Overview and Challenges. Ceramics, 8(1), 28. https://doi.org/10.3390/ceramics8010028