Inhibition Mechanism of Corrosion of Aluminium Alloy in Ordinary Portland Cement Paste by Polyaluminium Sulphate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
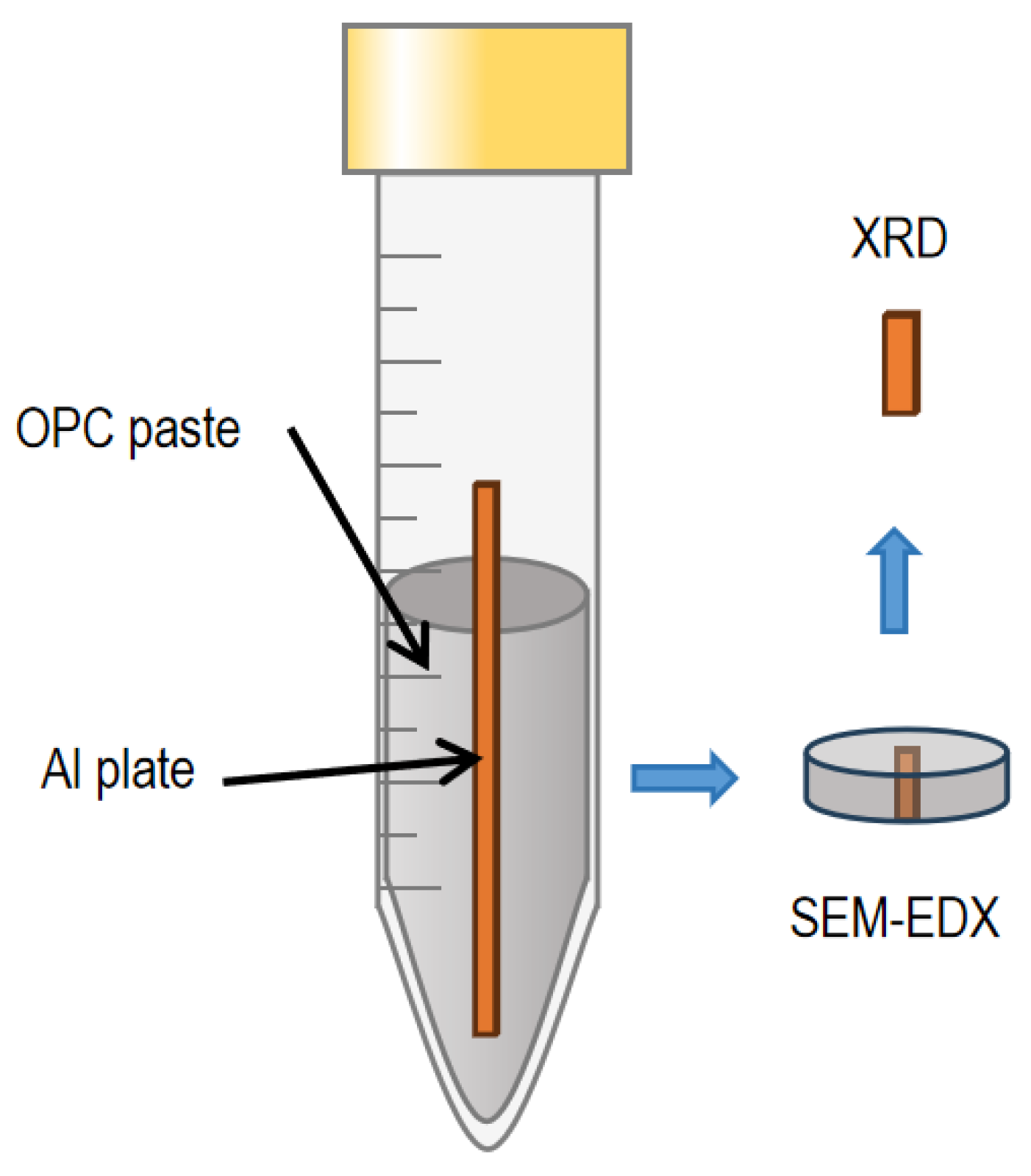
2.2.1. Sample Preparation for the Appearance of Hardened OPC Pastes Embedding Al Plates
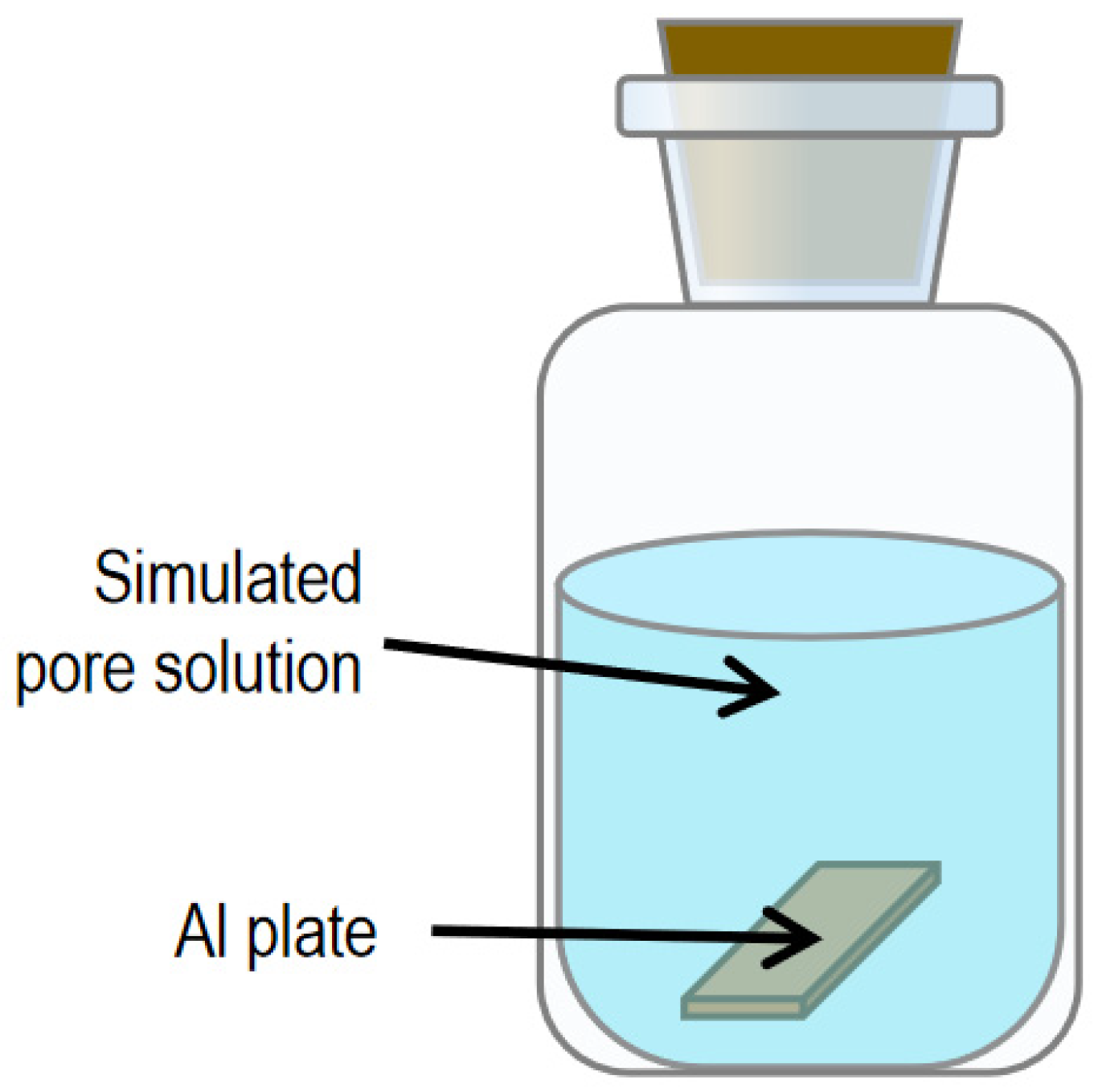
2.2.2. Sample Preparation for Al Plates’ Immersion in Simulated Pore Solutions
2.3. Test Methods
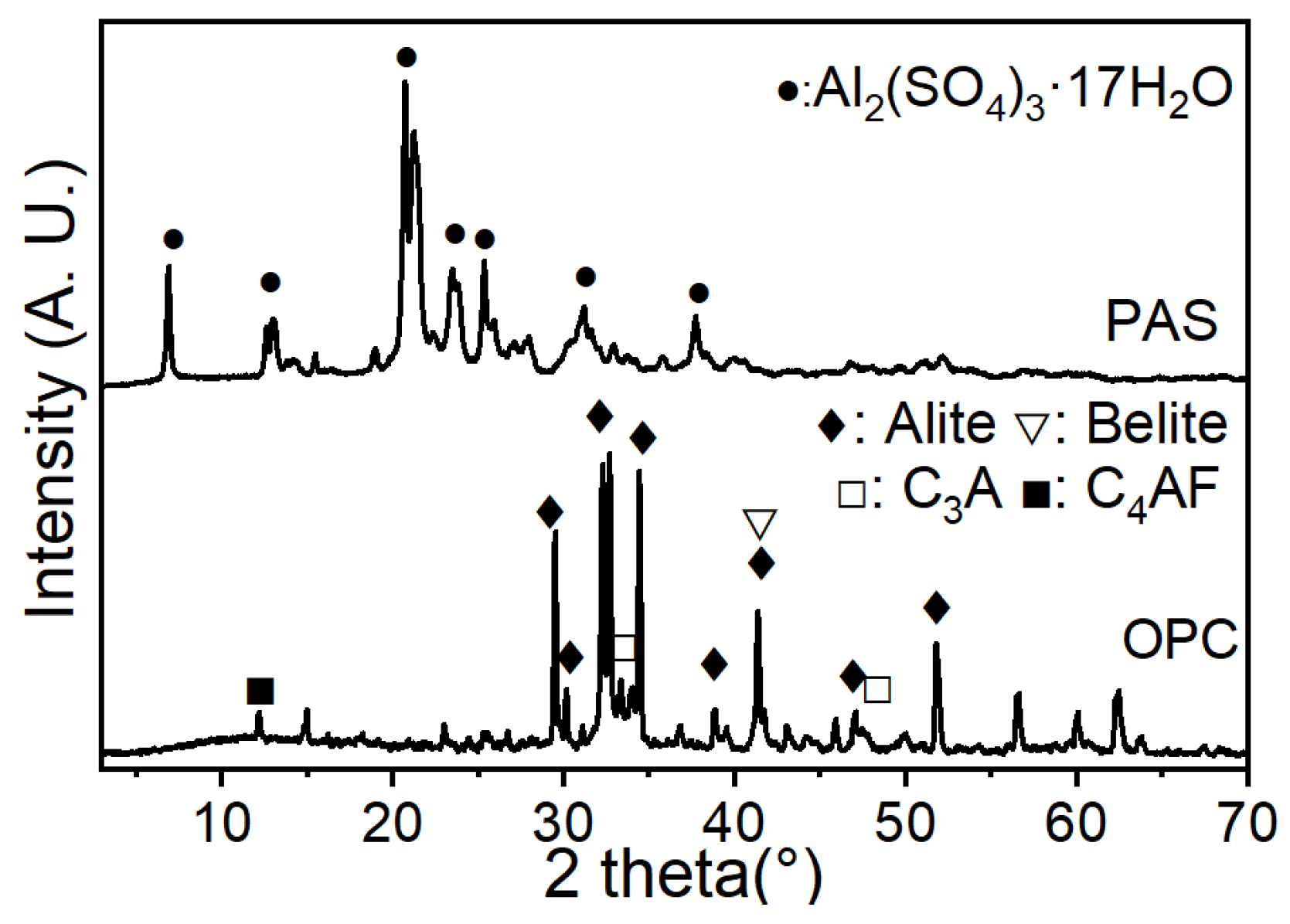
2.3.1. Microstructure and Chemical Composition
2.3.2. Phase Assemblage on the Surface of Al Plates
2.3.3. Polarisation Curve Analysis and Corrosion Rate of Al Plates in Simulated Pore Solution
3. Results and Discussions
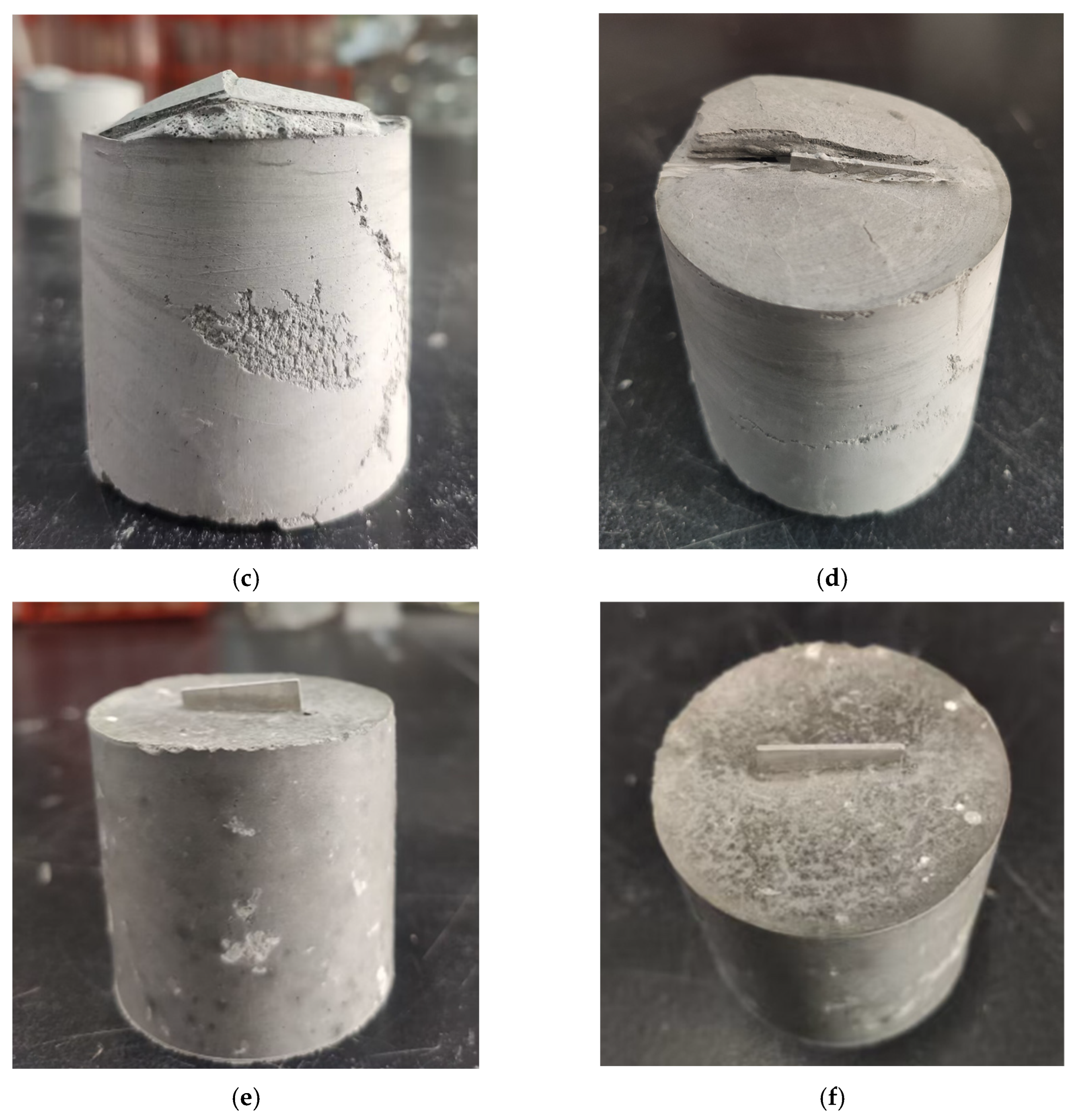
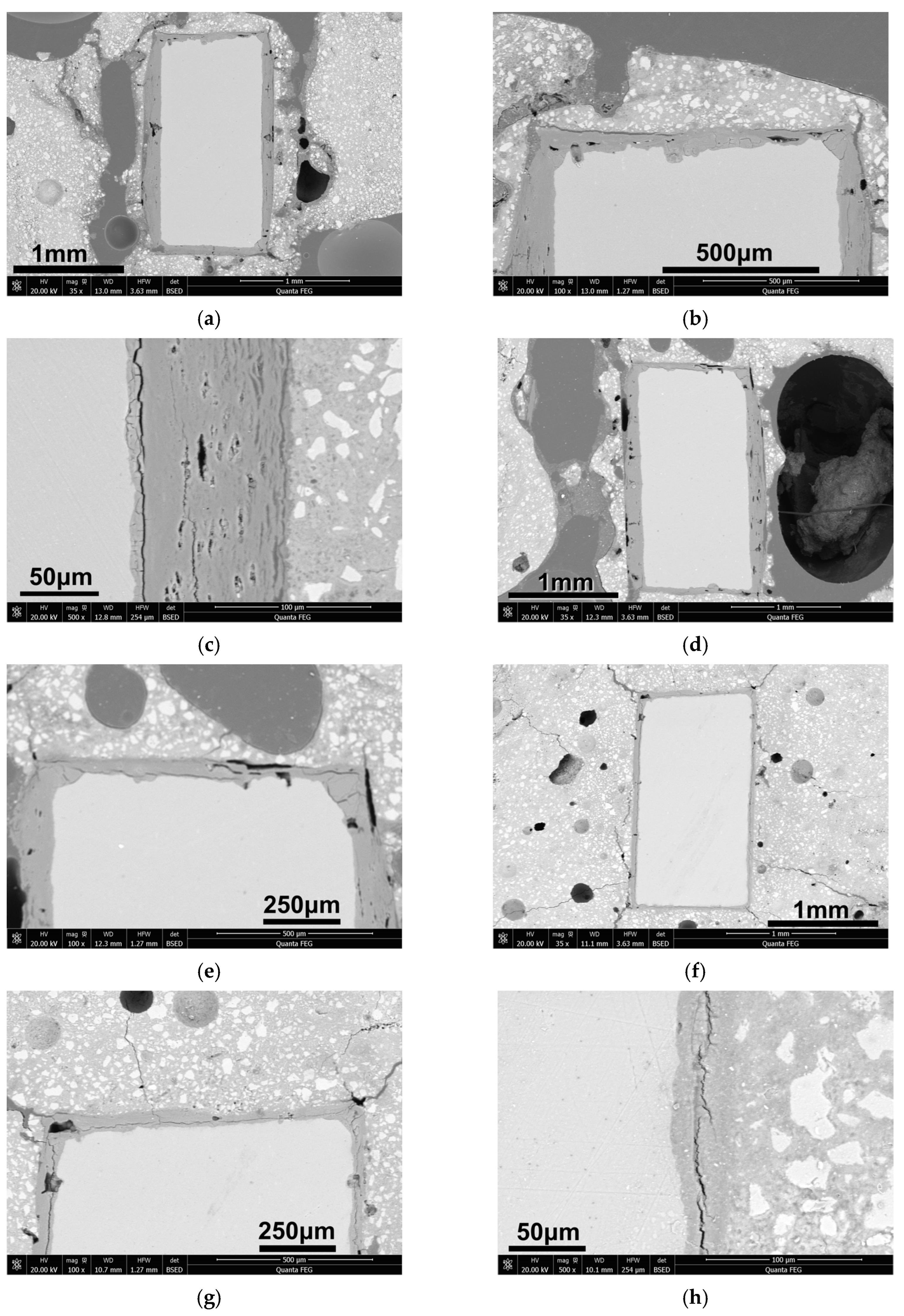
3.1. Appearance of Hardened OPC Paste Embedding Al Plate
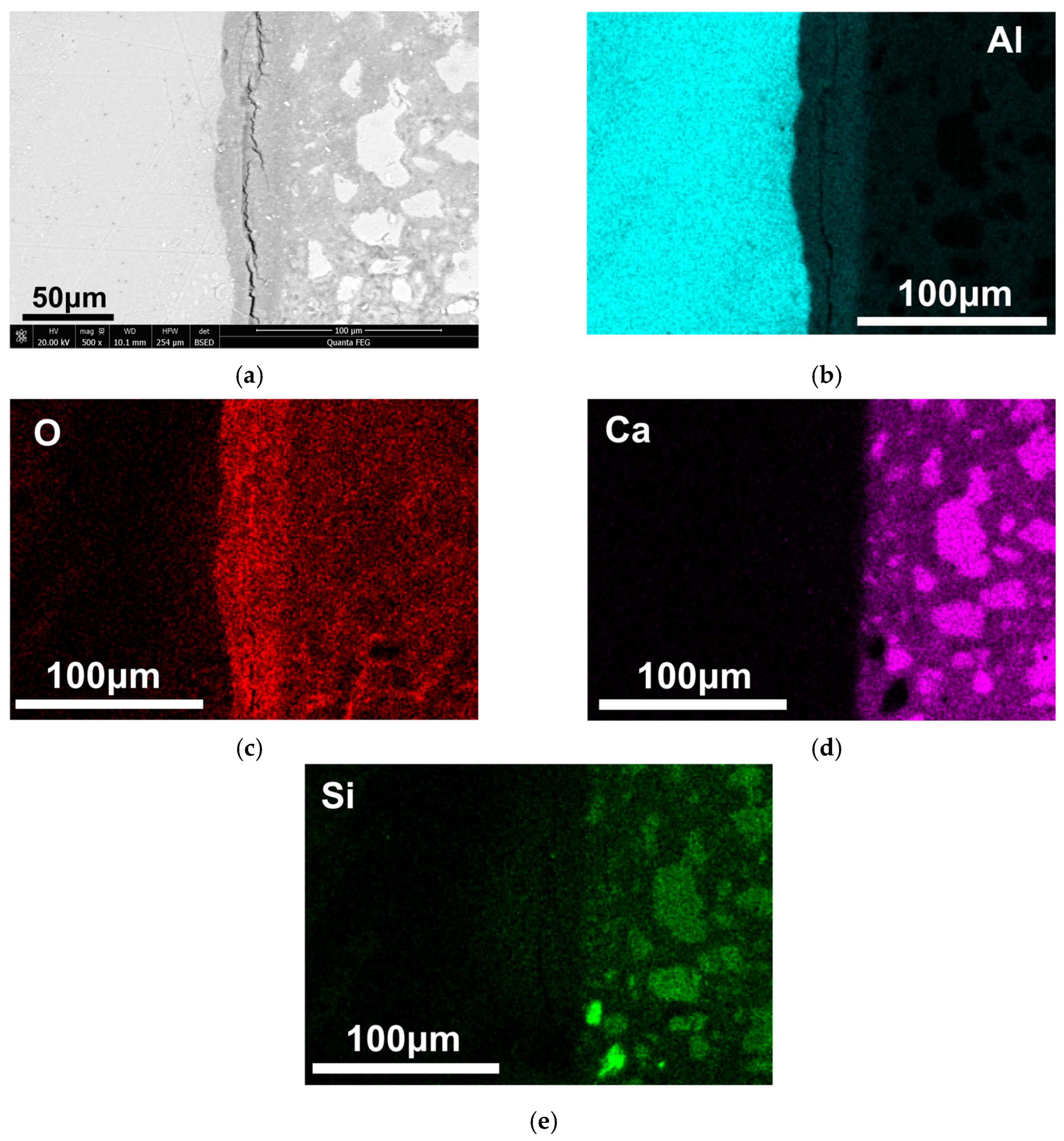
3.2. Corrosion of Al Plates in Hardened OPC Paste
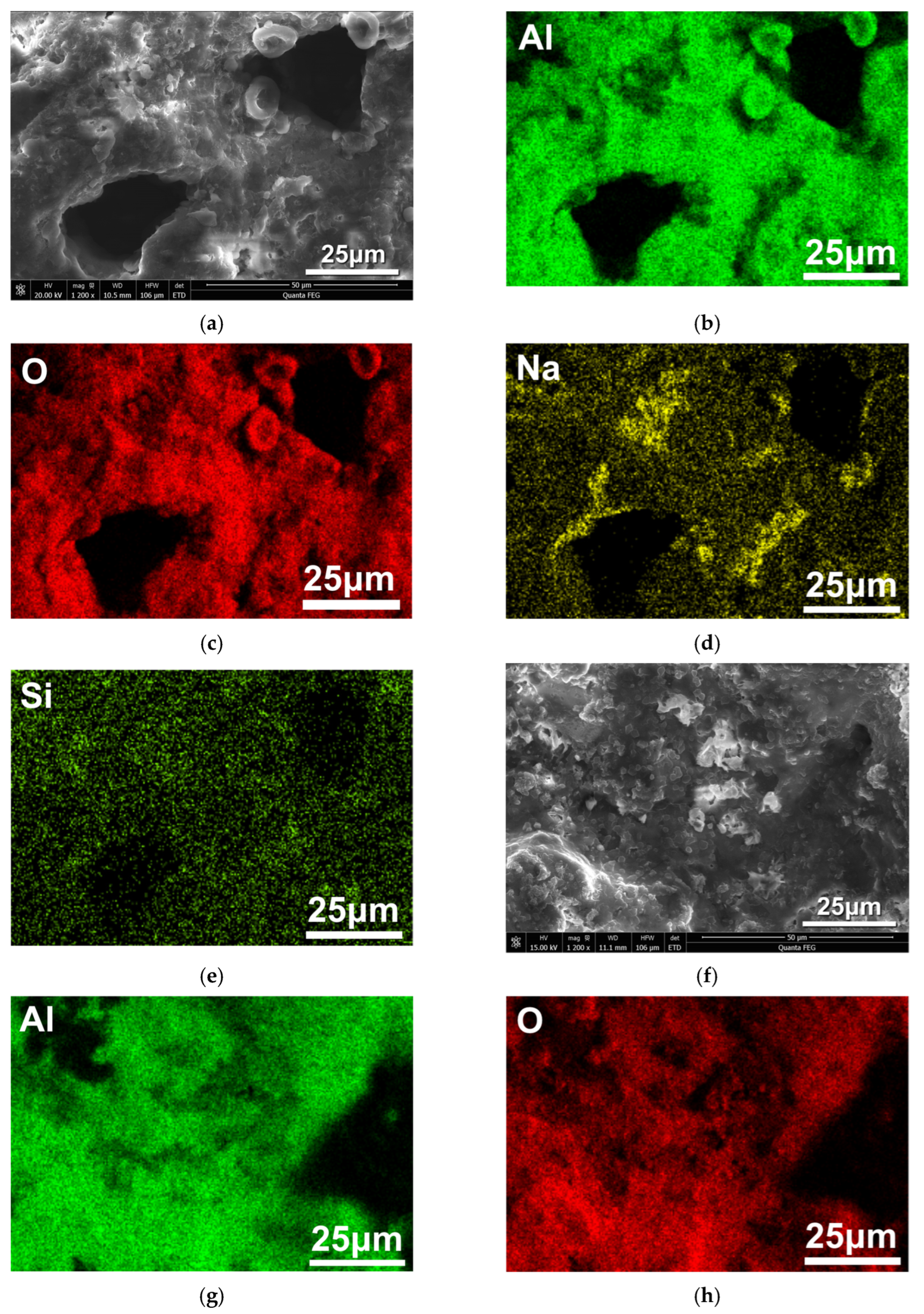
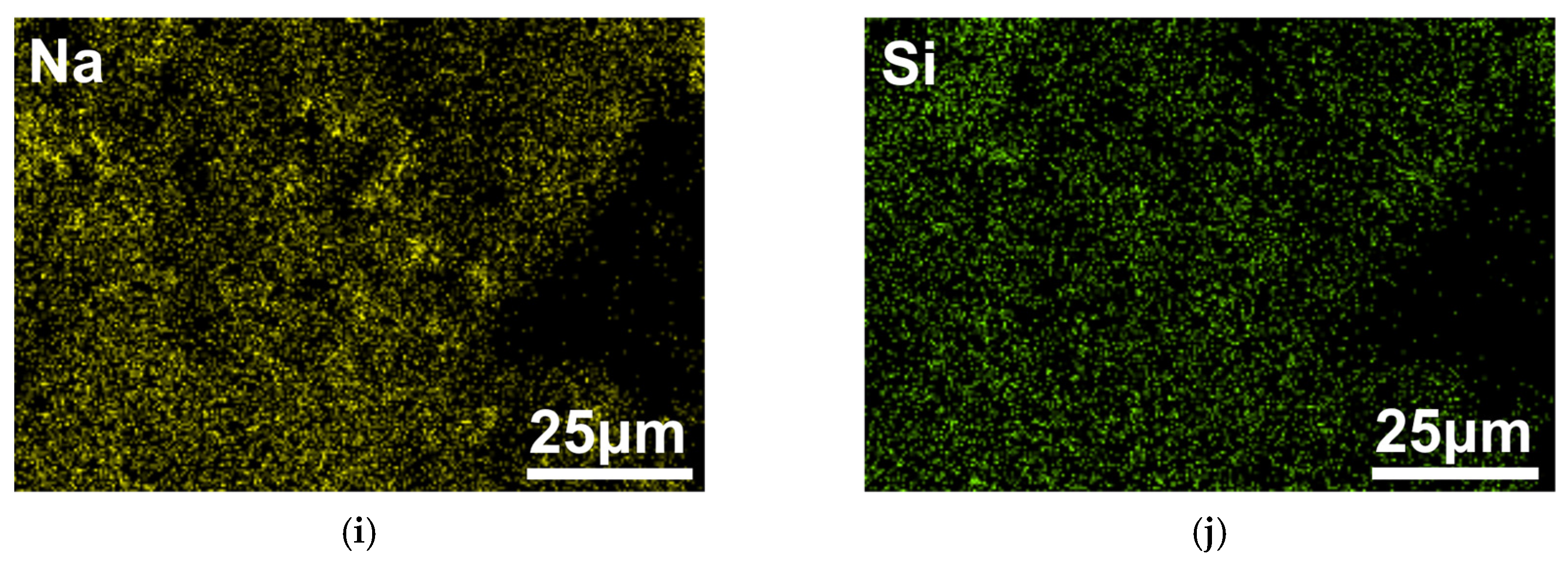
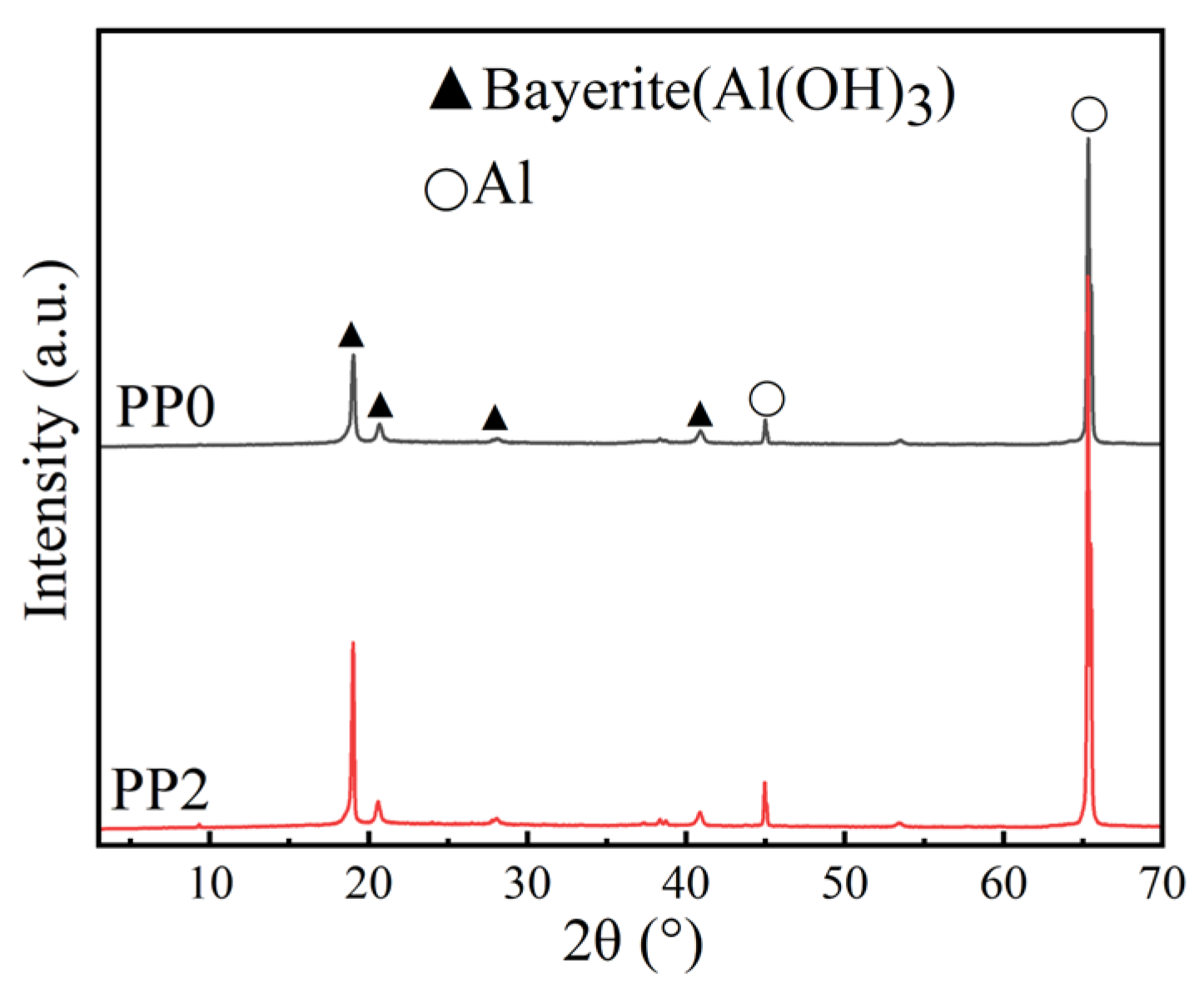
3.3. Mechanism of Corrosion Inhibition of Al Alloy in OPC Paste by PAS
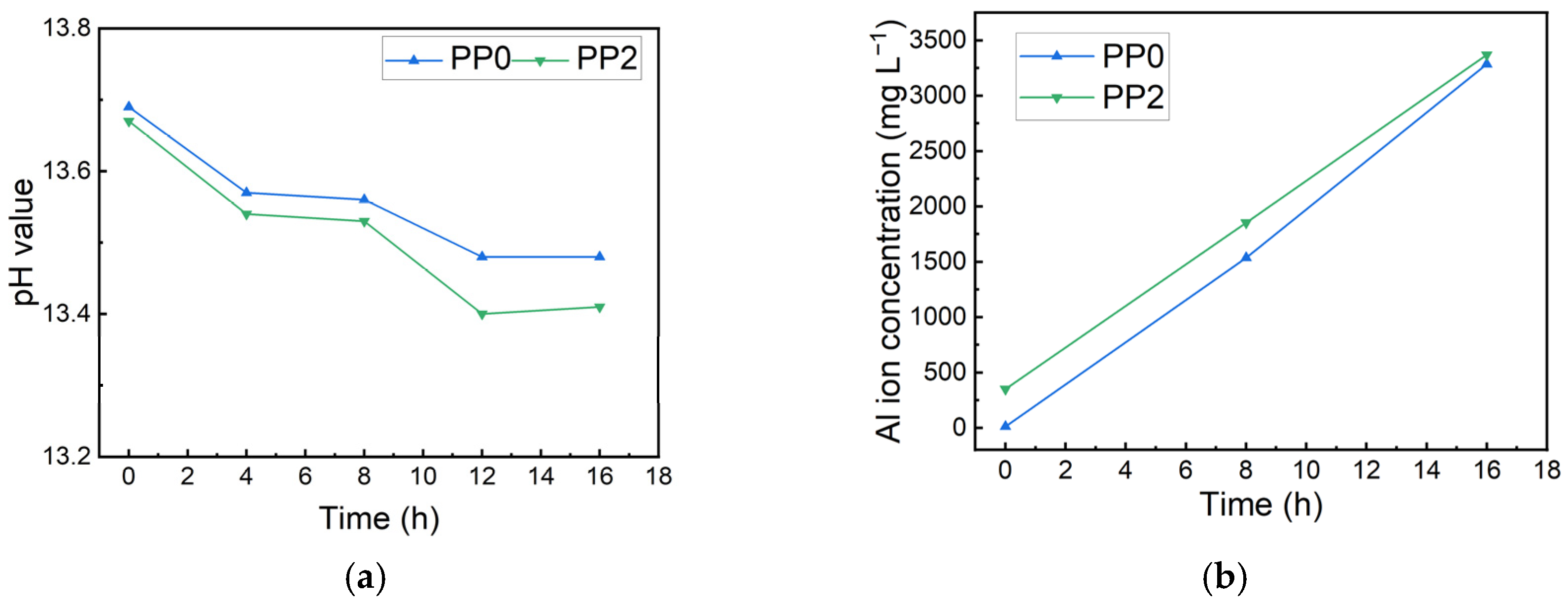
3.4. Corrosion Rate of Al Alloy in OPC Paste
3.5. Mechanism of Corrosion Inhibition of PAS on the Al Alloy in OPC Paste
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adhikari, S.; Hebert, K.R. Participation of Aluminum Hydride in the Anodic Dissolution of Aluminum in Alkaline Solutions. J. Electrochem. Soc. 2008, 155, C189. [Google Scholar] [CrossRef]
- Mercier, D.; Barthés-Labrousse, M.G. The role of chelating agents on the corrosion mechanisms of aluminium in alkaline aqueous solutions. Corros. Sci. 2009, 51, 339–348. [Google Scholar] [CrossRef]
- Muller, U.; Rubner, K. The microstructure of concrete made with municipal waste incinerator bottom ash as an aggregate component. Cem. Concr. Res. 2006, 36, 1434–1443. [Google Scholar] [CrossRef]
- Bertolini, L.; Carsana, M.; Cassago, D.; Quadrio Curzio, A.; Collepardi, M. MSWI ashes as mineral additions in concrete. Cem. Concr. Res. 2004, 34, 1899–1906. [Google Scholar] [CrossRef]
- Xuan, D.; Tang, P.; Poon, C.S. Limitations and quality upgrading techniques for utilization of MSW incineration bottom ash in engineering applications—A review. Constr. Build. Mater. 2018, 190, 1091–1102. [Google Scholar] [CrossRef]
- Grosso, M.; Biganzoli, L.; Rigamonti, L. A quantitative estimate of potential aluminium recovery from incineration bottom ashes. Resour. Conserv. Recycl. 2011, 55, 1178–1184. [Google Scholar] [CrossRef]
- Biganzoli, L.; Grosso, M. Aluminium recovery from waste incineration bottom ash, andits oxidation level. Waste Manag. Res. 2013, 31, 954–959. [Google Scholar] [CrossRef]
- Mary Joseph, A.; Snellings, R.; Nielsen, P.; Matthys, S.; De Belie, N. Pre-treatment and utilisation of municipal solid waste incineration bottom ashes towards a circular economy. Constr. Build. Mater. 2020, 260, 120485. [Google Scholar] [CrossRef]
- Xuan, D.; Poon, C.S. Removal of metallic Al and Al/Zn alloys in MSWI bottom ash by alkaline treatment. J. Hazard. Mater. 2018, 344, 73–80. [Google Scholar] [CrossRef]
- Pezzato, L.; Coelho, L.B.; Bertolini, R.; Settimi, A.G.; Brunelli, K.; Olivier, M.; Dabala, M. Corrosion and mechanical properties of plasma electrolytic oxidation-coated AZ80 magnesium alloy. Mater. Corros. 2019, 70, 2103–2112. [Google Scholar] [CrossRef]
- Yu, X.W.; Li, G.Q. XPS study of cerium conversion coating on the anodized 2024 aluminum alloy. J. Alloys Compd. 2004, 364, 193–198. [Google Scholar] [CrossRef]
- Chen, J.H.; He, Z.; Liu, J.M.; Wang, Y.X.; Hodgson, M.; Gao, W. Antibacterial anodic aluminium oxide-copper coatings on aluminium alloys: Preparation and long-term antibacterial performance. Chem. Eng. J. 2023, 461, 141873. [Google Scholar] [CrossRef]
- Usman, B.J.; Scenini, F.; Curioni, M. The effect of exposure conditions on performance evaluation of post-treated anodic oxides on an aerospace aluminium alloy: Comparison between salt spray and immersion testing. Surf. Coat. Technol. 2020, 399, 126157. [Google Scholar] [CrossRef]
- Rashid, K.H.; Khadom, A.A. Sulfosalicylic/oxalic acid anodizing process of 5854 aluminum-magnesium alloy: Influence of sealing time and corrosion tendency. Results Chem. 2022, 4, 100289. [Google Scholar] [CrossRef]
- Machado, C.d.S.C.; Klumpp, R.E.; Ayusso, V.H.; Donatus, U.; Milagre, M.X.; Araujo, J.V.d.S.; Machado, G.A.F.; Costa, I. Effect of surface treatments on the localized corrosion resistance of the AA2198-T8 aluminum lithium alloy welded by FSW process. Surf. Interface Anal. 2019, 51, 1231–1239. [Google Scholar] [CrossRef]
- Gad, S.M.; Jin, Z.; Emad, S.; Vergara, J.E.; Yawas, D.S.; Dagwa, I.M.; Momoh-Bello Omiogbemi, I. Potential of rare-earth compounds as anticorrosion pigment for protection of aerospace AA2198-T851 alloy. Heliyon 2023, 9, e14693. [Google Scholar] [CrossRef]
- Shahzad, M.; Chaussumier, M.; Chieragatti, R.; Mabru, C.; Rezai-Aria, F. Effect of sealed anodic film on fatigue performance of 2214-T6 aluminum alloy. Surf. Coat. Technol. 2012, 206, 2733–2739. [Google Scholar] [CrossRef]
- Hu, N.; Dong, X.; He, X.; Browning, J.F.; Schaefer, D.W. Effect of sealing on the morphology of anodized aluminum oxide. Corros. Sci. 2015, 97, 17–24. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Jang, H.; Chung, W. Cr2O3 sealing of anodized aluminum alloy by heat treatment. Surf. Coat. Technol. 2014, 243, 34–38. [Google Scholar] [CrossRef]
- Kuznetsov, B.; Serdechnova, M.; Tedim, J.; Starykevich, M.; Kallip, S.; Oliveira, M.P.; Hack, T.; Nixon, S.; Ferreira, M.G.S.; Zheludkevich, M.L. Sealing of tartaric sulfuric (TSA) anodized AA2024 with nanostructured LDH layers. RSC Adv. 2016, 6, 13942–13952. [Google Scholar] [CrossRef]
- Liu, J.; Shi, H.; Yu, M.; Du, R.; Rong, G.; Li, S. Effect of divalent metal ions on durability and anticorrosion performance of layered double hydroxides on anodized 2A12 aluminum alloy. Surf. Coat. Technol. 2019, 373, 56–64. [Google Scholar] [CrossRef]
- Fang, X.; Zeng, Y.; Mu, S.; Du, J.; Guo, J.; Li, W.; Fan, Y. Study on the effect of acetate ions on the sealing treatment for anodic oxide film of 6063 aluminum alloy. Surf. Coat. Technol. 2023, 472, 129961. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, C.; Zhao, S.; Niu, A.; Ma, Y.; Liu, B. Enhanced long-term corrosion protection of 2A14 aluminum alloy: Hybrid effect of micro-arc oxidation coating and cerium based conversion treatment. Surf. Coat. Technol. 2023, 464, 129579. [Google Scholar] [CrossRef]
- Kinoshita, H.; Swift, P.; Utton, C.; Carro-Mateo, B.; Marchand, G.; Collier, N.; Milestone, N. Corrosion of aluminium metal in OPC- and CAC-based cement matrices. Cem. Concr. Res. 2013, 50, 11–18. [Google Scholar] [CrossRef]
- Bulidon, N.; Pelissier, K.; Boissy, C.; Mendibide, C.; Maillot, V.; Bourbon, X.; Crusset, D. Hydrogen production through aluminium corrosion in a cement-based matrix. Mater. Corros. 2023, 74, 1765–1776. [Google Scholar] [CrossRef]
- Delpech, S.; Cannes, C.; Barré, N.; Tran, Q.T.; Sanchez, C.; Lahalle, H.; Lambertin, D.; Gauffinet, S.; Coumes, C.C.D. Kinetic Model of Aluminum Behavior in Cement-Based Matrices Analyzed by Impedance Spectroscopy. J. Electrochem. Soc. 2017, 164, C717–C727. [Google Scholar] [CrossRef]
- Liu, S.H.; Chang, S.; Tu, Y.J.; Luo, S.Q. Immobilisation mechanism for nuclear waste containing aluminium by supersulfated cement containing phosphogypsum. Cem. Concr. Compos. 2023, 139, 104991. [Google Scholar] [CrossRef]
- Setiadi, A.; Milestone, N.B.; Hill, J.; Hayes, M. Corrosion of aluminium and magnesium in BFS composite cements. Adv. Appl. Ceram. 2006, 105, 191–196. [Google Scholar] [CrossRef]
- Ghali, E. Fundamentals of Electrochemical Corrosion. In Corrosion Resistance of Aluminum and Magnesium Alloys; Revie, R.W., Ghali, E., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 1–48. [Google Scholar]
- Chen, Z.Y.; Luan, Z.K.; Jia, Z.P.; Li, X. Study on the hydrolysis/precipitation behavior of Keggin Al13 and Al30 polymers in polyaluminum solutions. J. Environ. Manag. 2009, 90, 2831–2840. [Google Scholar] [CrossRef]
- Feng, C.H.; Bi, Z.; Tang, H.X. Electrospray Ionization Time-of-Flight Mass Spectrum Analysis Method of Polyaluminum Chloride Flocculants. Environ. Sci. Technol. 2015, 49, 474–480. [Google Scholar] [CrossRef]
- Li, B.; Qin, J.F.; Li, Q.; Chen, W.; Cao, H.L.; Guo, Y. Promoting the dissolution of slag in blended cement via adding polyaluminum sulfate and controlling the inner spatial zonation. Constr. Build. Mater. 2023, 399, 132543. [Google Scholar] [CrossRef]
- Plusquellec, G.; Geiker, M.R.; Lindgård, J.; Duchesne, J.; Fournier, B.; De Weerdt, K. Determination of the pH and the free alkali metal content in the pore solution of concrete: Review and experimental comparison. Cem. Concr. Res. 2017, 96, 13–26. [Google Scholar] [CrossRef]





| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | Others | LOI * | |
|---|---|---|---|---|---|---|---|---|---|---|
| OPC | 19.76 | 4.74 | 3.30 | 64.14 | 0.99 | 4.45 | 0.19 | 0.97 | 1.27 | 0.19 |
| PAS | 0.1 | 8.72 | 0.01 | 0.08 | - | 15.95 | 0.07 | 0.003 | 0.047 | 75.02 |
| OPC | PAS | SP | Water | |
|---|---|---|---|---|
| PC-P0 | 100 | 0 | 0.5 | 35 |
| PC-P3 | 100 | 3 | 0.5 | 35 |
| PC-P6 | 100 | 6 | 0.5 | 35 |
| Ca(OH)2 | Na2SiO3·9H2O | KOH | NaOH | Na2SO4 | NaAlO2 | |
|---|---|---|---|---|---|---|
| Concentration (g L−1) | 0.047 | 0.232 | 26.46 | 2.22 | 1.611 | 0.022 |
| Group | PAS | Simulated Pore Solution of PC-P0 |
|---|---|---|
| PP0 | 0 | 500 |
| PP2 | 2 | 500 |
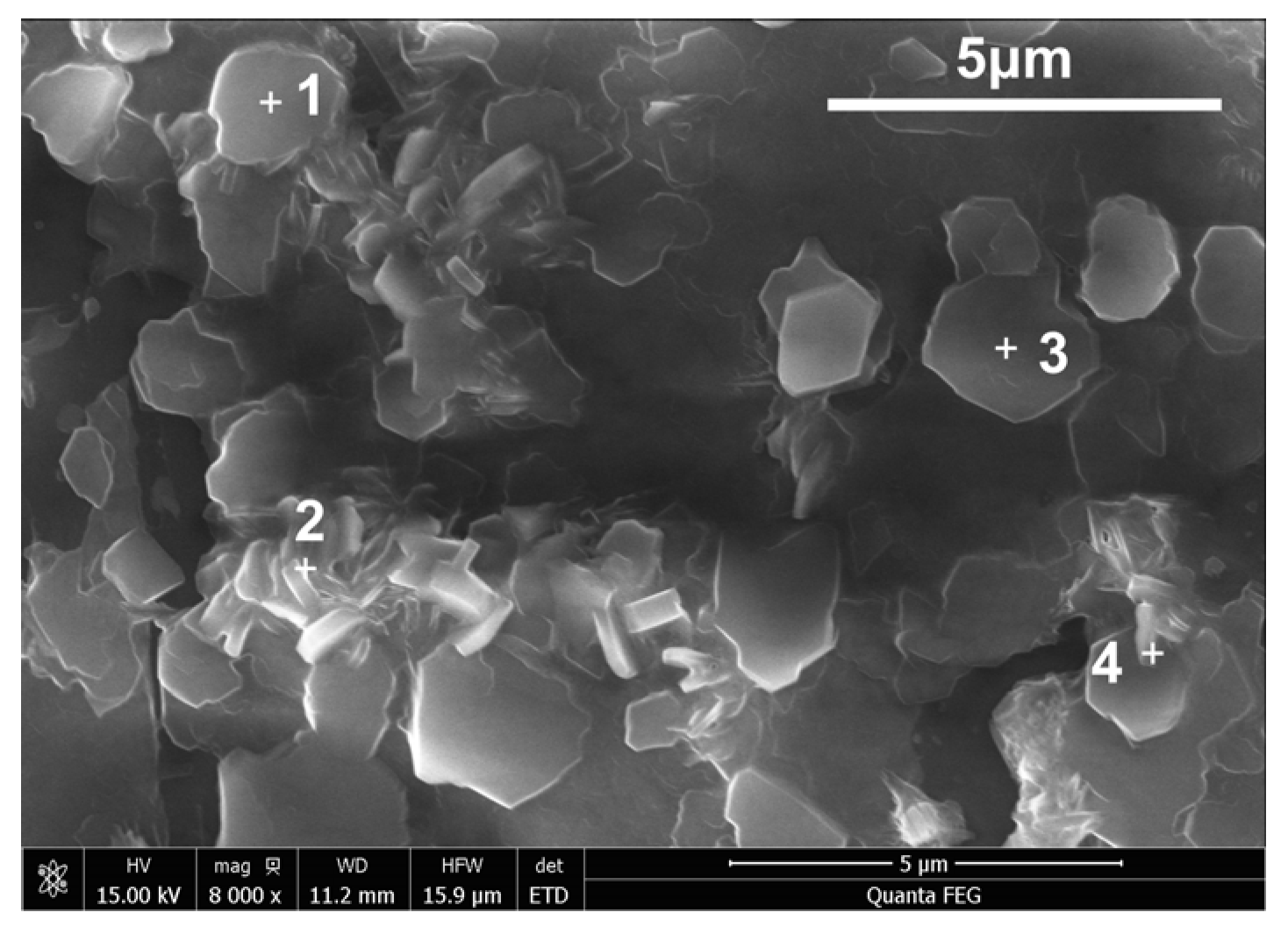
| Point | Al | O | Na | K |
|---|---|---|---|---|
| 1 | 21.00 | 77.08 | 0.34 | 1.59 |
| 2 | 20.61 | 77.42 | 0.41 | 1.56 |
| 3 | 21.02 | 76.56 | 0.41 | 2.01 |
| 4 | 23.91 | 73.15 | 0.52 | 2.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, H.; Wei, Q.; Ma, H.; Li, Q. Inhibition Mechanism of Corrosion of Aluminium Alloy in Ordinary Portland Cement Paste by Polyaluminium Sulphate. Ceramics 2025, 8, 27. https://doi.org/10.3390/ceramics8010027
Geng H, Wei Q, Ma H, Li Q. Inhibition Mechanism of Corrosion of Aluminium Alloy in Ordinary Portland Cement Paste by Polyaluminium Sulphate. Ceramics. 2025; 8(1):27. https://doi.org/10.3390/ceramics8010027
Chicago/Turabian StyleGeng, Haining, Qi Wei, Haosen Ma, and Qiu Li. 2025. "Inhibition Mechanism of Corrosion of Aluminium Alloy in Ordinary Portland Cement Paste by Polyaluminium Sulphate" Ceramics 8, no. 1: 27. https://doi.org/10.3390/ceramics8010027
APA StyleGeng, H., Wei, Q., Ma, H., & Li, Q. (2025). Inhibition Mechanism of Corrosion of Aluminium Alloy in Ordinary Portland Cement Paste by Polyaluminium Sulphate. Ceramics, 8(1), 27. https://doi.org/10.3390/ceramics8010027






