Franklinite-Zincochromite-Gahnite Solid Solutions for Cool Red Ceramic Pigments with Visible Light Photocatalysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
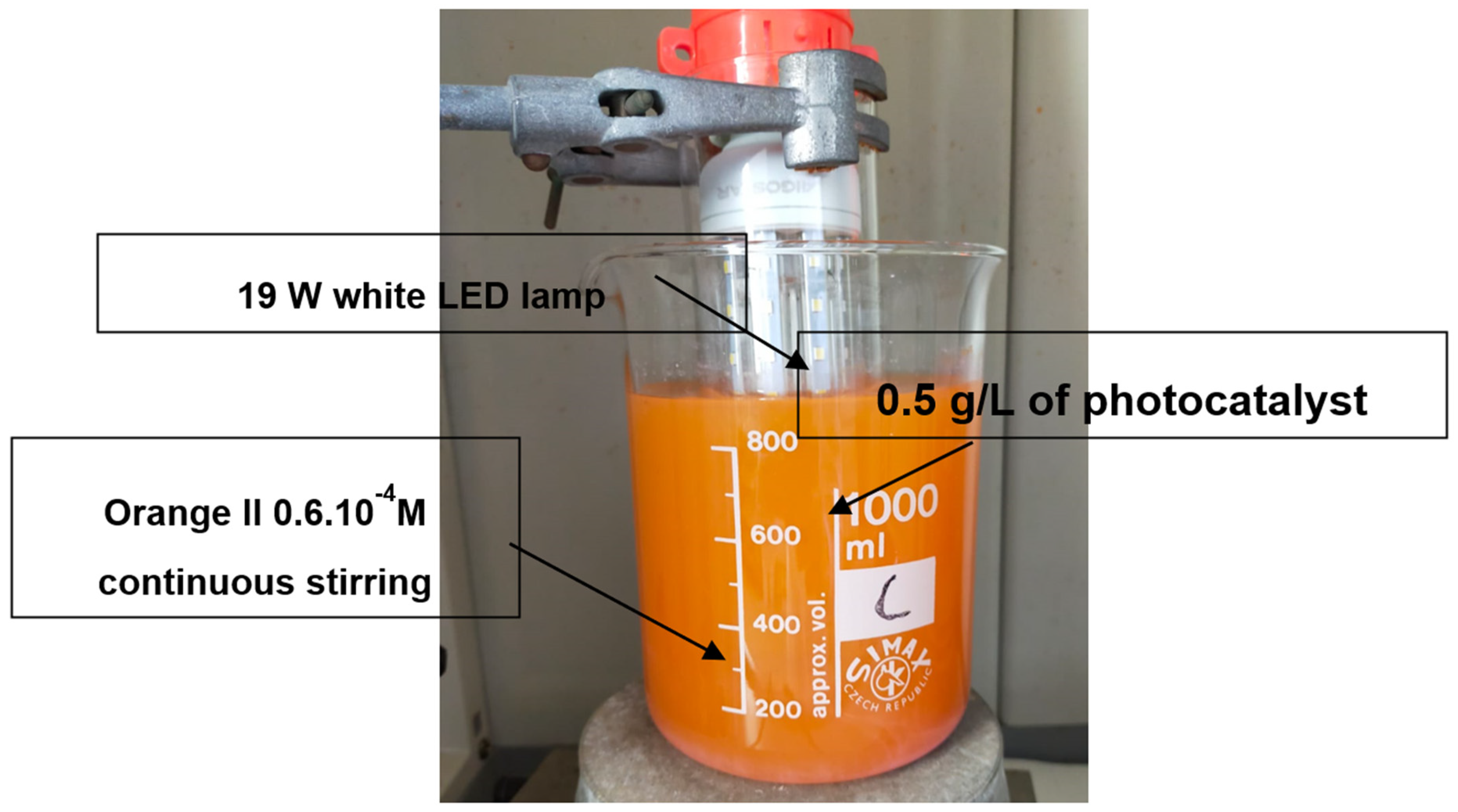
2.2. Samples Characterisation
2.3. Application of Pigments
3. Results and Discussion
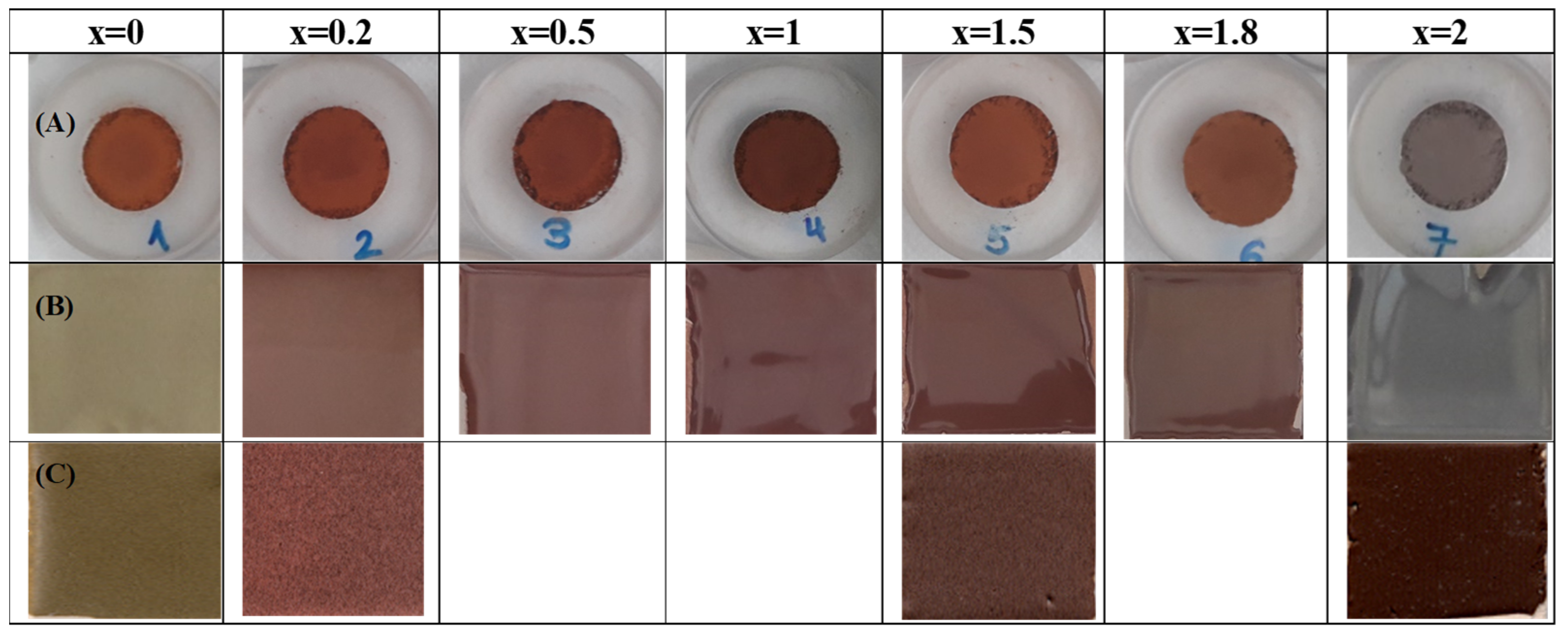
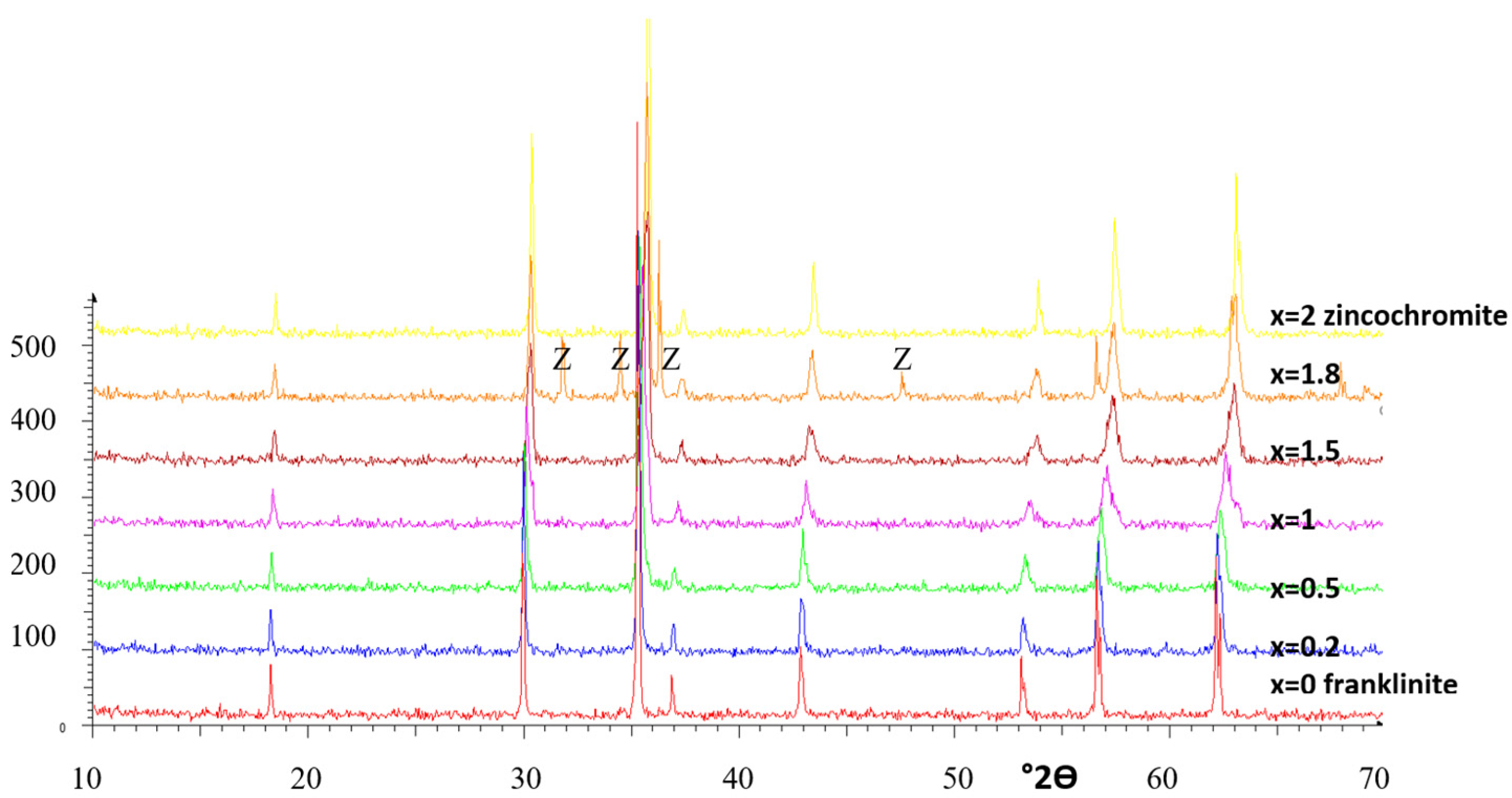
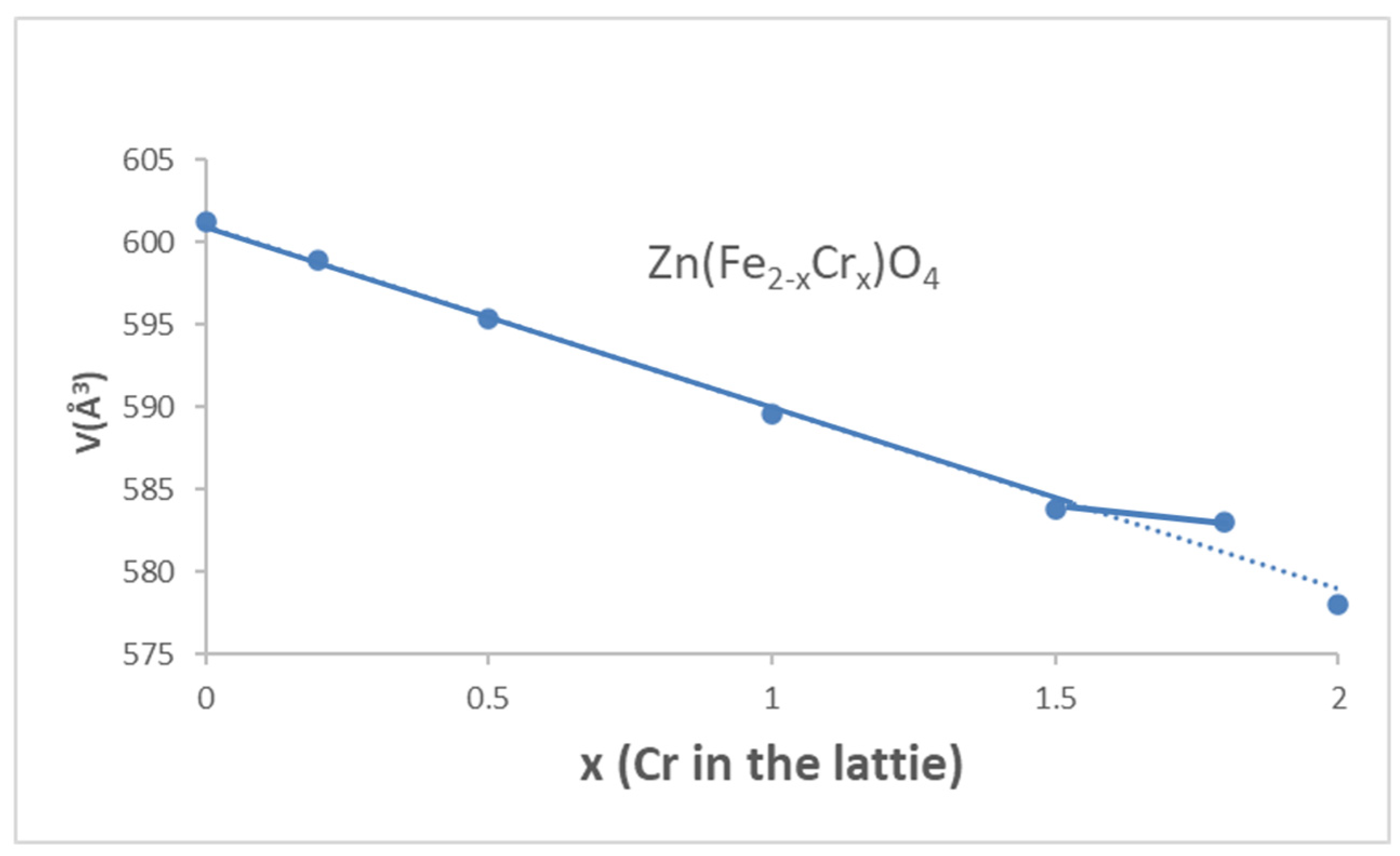
3.1. Franklinite-Zincochromite Zn(Fe2−xCrx)O4 Solid Solutions
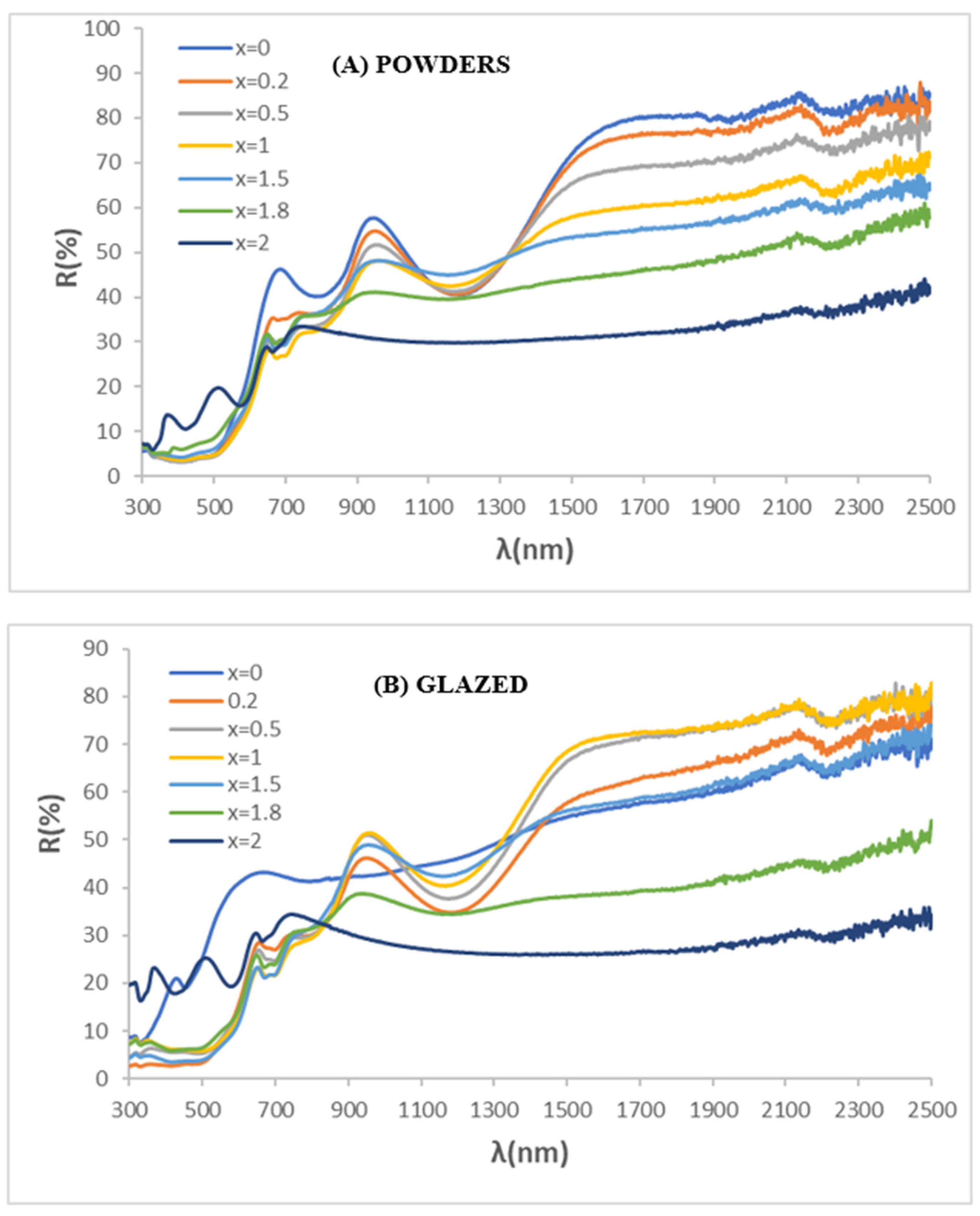
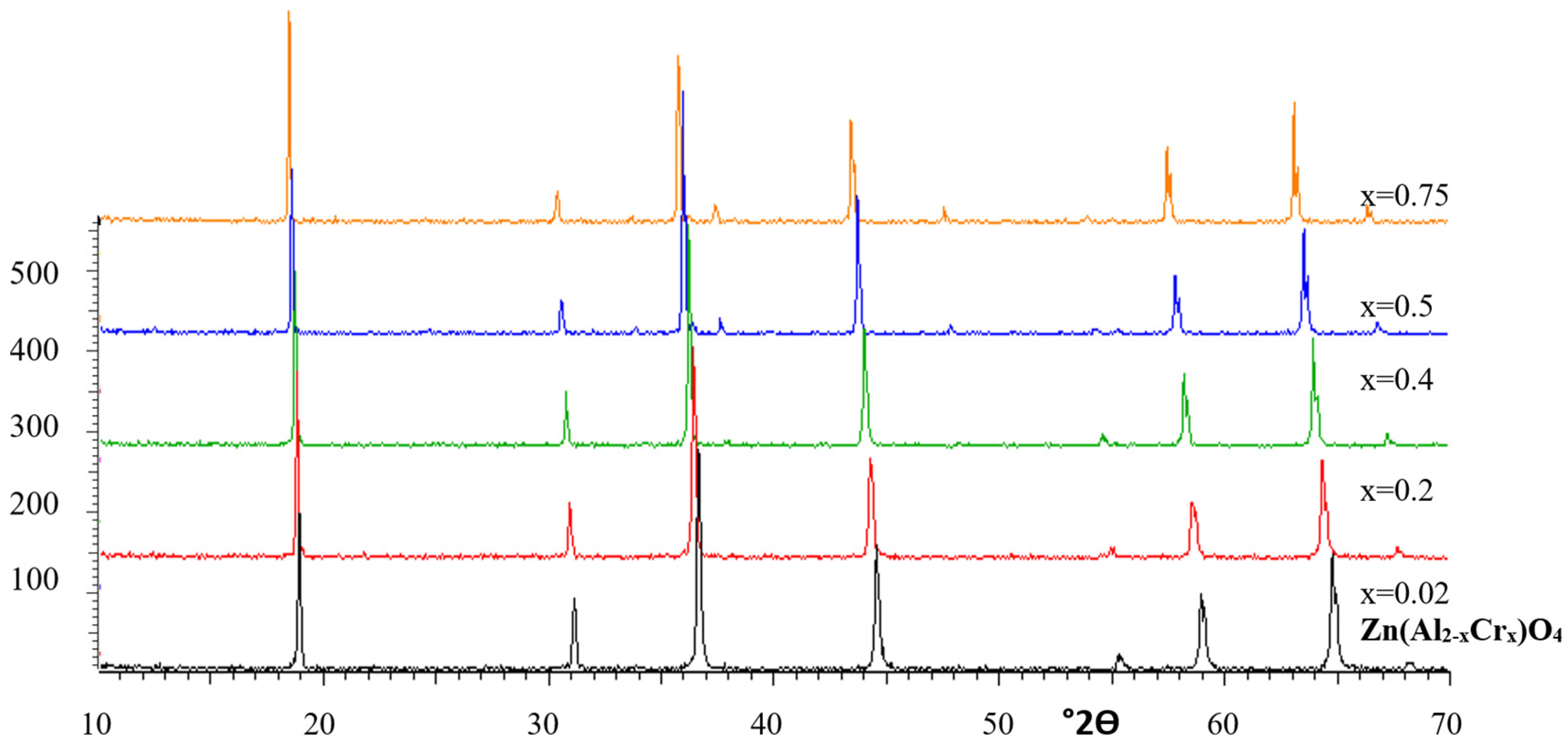
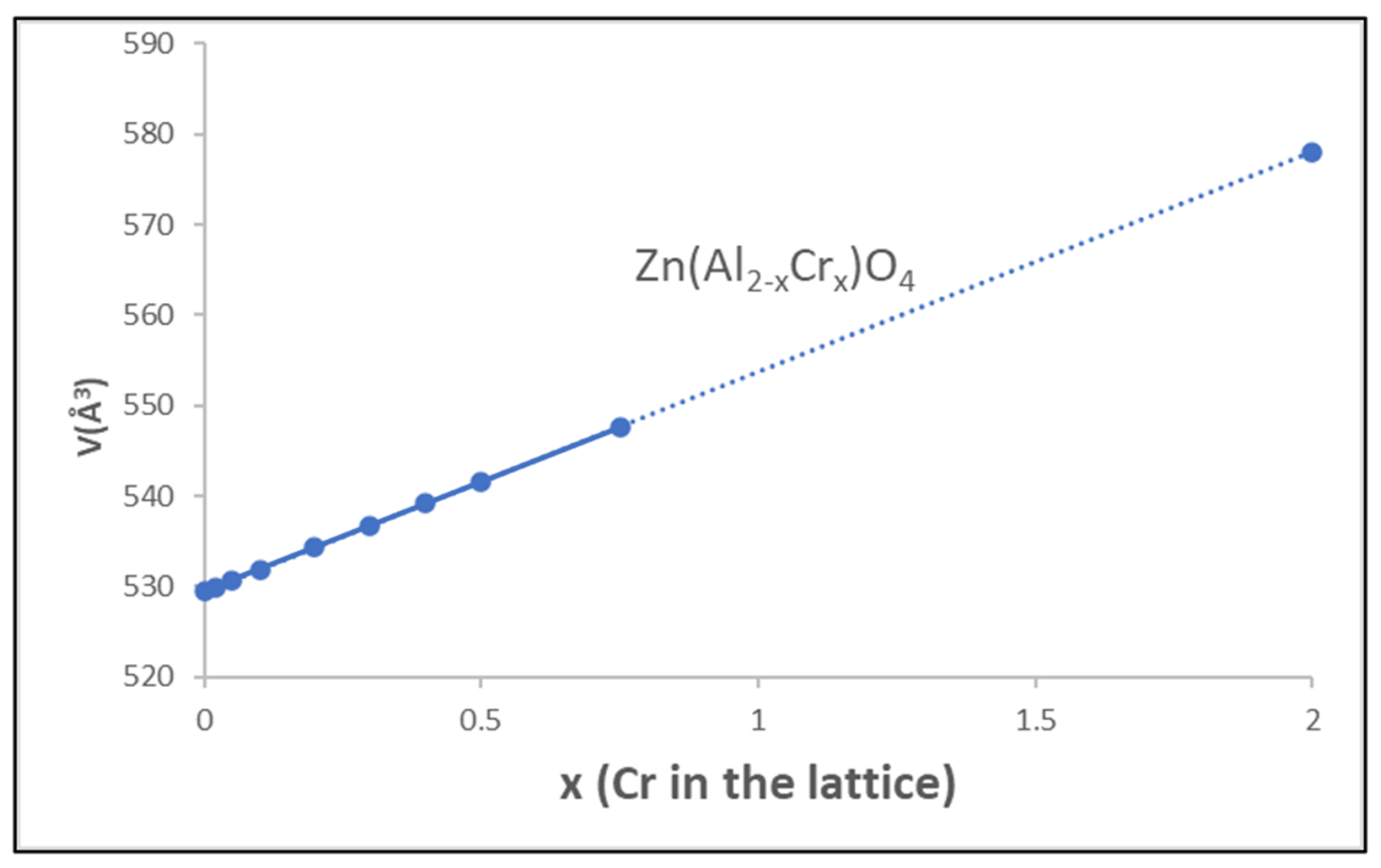
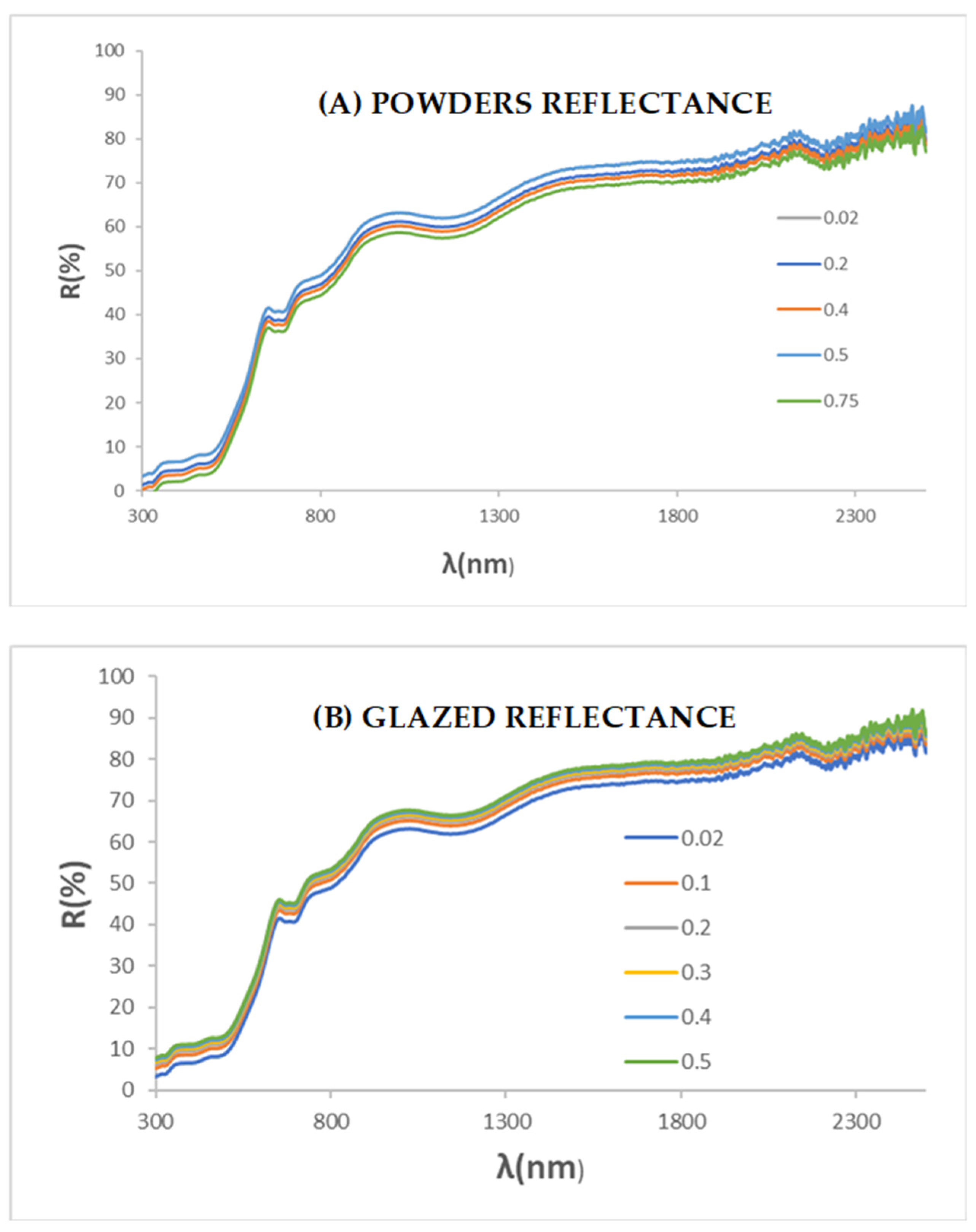
3.2. Gahnite-Zincochromite Zn(Al2−xCrx)O4 Solid Solutions
3.3. Gahnite-Zincochromite-Franklinite Zn(Al1.5−xCr0.5Fex)O4 Solid Solutions
3.4. Summary of Optimised Samples Compared with a Reference
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sickafus, K.E.; Wills, J.M.; Grimes, N.W. Structure of Spinel. J. Am. Ceram. Soc. 1999, 82, 3279–3292. [Google Scholar] [CrossRef]
- Burdett, J.K.; Price, G.L.; Price, S.L. Role of the crystal-field theory in determining the structures of spinels. J. Am. Chem. Soc. 1982, 104, 92–95. [Google Scholar] [CrossRef]
- O’Neil, H.S.T.; Navrotsky, A. Simple spinels: Crystallographic parameters, cation radii, lattice energies, and cation distribution. Am. Mineral. 1983, 68, 181–194. [Google Scholar]
- Bohra, M.; Prasad, S.; Kumar, N.; Misra, D.S.; Sahoo, S.C.; Venkataramani, N.; Krishnan, R. Large room temperature magnetization in nanocrystalline zinc ferrite thin films. Appl. Phys. Lett. 2006, 488, 262506. [Google Scholar] [CrossRef]
- Tanaka, K.; Katsuta, M.; Nakashima, S.; Fujita, K.J. Hydrothermal Synthesis and Magnetic Properties of Zinc Ferrite Nanocrystals. Jpn. Soc. Powder Powder Metall. 2004, 52, 221–227. [Google Scholar] [CrossRef][Green Version]
- Monrós, G.; Badenes, J.A.; Llusar, M. Ecofriendly High NIR Reflectance Ceramic Pigments Based on Rare Earths Compared with Classical Chromophores Prepared by DPC Method. Ceramics 2022, 5, 614–641. [Google Scholar] [CrossRef]
- Ayana, Y.M.A.; El-Sawy, S.M.; Salah, S.H. Zinc-ferrite pigment for corrosion protection. Anti-Corros. Methods Mater. 1997, 44, 381–388. [Google Scholar] [CrossRef]
- Calbo, J.; Sorlí, S.; Llusar, M.; Tena, M.A.; Monrós, G. Minimisation of toxicity in nickel ferrite black pigment. Br. Ceram. Trans. 2004, 103, 3–9. [Google Scholar] [CrossRef]
- Schwarz, M.; Veverka, M.; Michalková, E.; Lalík, V.; Veverková, D. Utilisation of industrial waste for ferrite pigments production. Chem. Pap. 2012, 66, 248–258. [Google Scholar] [CrossRef]
- Lahmer, M.A. The effect of degree of inversion on the electronic and optical properties of ZnAl2O4: A first-principles study. Comput. Condens. Matter 2023, 37, e00857. [Google Scholar] [CrossRef]
- Granone, L.I.; Dillert, R.; Heitjans, P.; Bahnemann, D.W. Effect of the degree of inversion on the electrical conductivity of spinel ZnFe2O4. ChemistrySelect 2019, 4, 1232–1239. [Google Scholar] [CrossRef]
- Quintero, J.J.M.; Rodríguez, K.L.S.; Torres, C.E.R.; Errico, L.A. Ab initio study of the role of defects on the magnetic response and the structural, electronic and hyperfine properties of ZnFe2O4. J. Alloy Compd. 2019, 775, 1117. [Google Scholar] [CrossRef]
- Peng, C.; Gao, L. Optical and Photocatalytic Properties of Spinel ZnCr2O4 Nanoparticles Synthesized by a Hydrothermal Route. J. Am. Ceram. Soc. 2008, 9, 2388–2390. [Google Scholar] [CrossRef]
- Mousavi, Z.; Soofivand, F.; Mahdiyeh Esmaeili-Zare, M.; Salavati-Niasari, M.; Bagheri, S. ZnCr2O4 Nanoparticles: Facile Synthesis, Characterization and Photocatalytic Properties. Sci. Rep. 2016, 6, 20071. [Google Scholar] [CrossRef] [PubMed]
- Bendiganavale, A.K.; Malshe, V.C. Infrared Reflective Inorganic Pigments. Recent Pat. Chem. Eng. 2008, 1, 67–79. [Google Scholar]
- Kortüm, G. Reflectance Spectroscopy, Principles, Methods, Applications; Springer: Berlin/Heidelberg, Germany, 1969; ISBN 978-3-642-88071. [Google Scholar]
- Monrós, G. Scheelite and Zircon: Brightness, Color and NIR Reflectance in Ceramics; Nova Science Publishers: New York, NY, USA, 2021; ISBN 978-1-53619-332-9. [Google Scholar]
- May-Lozano, M.; Mendoza-Escamilla, V.; Rojas-García, E.; López-Medina, V.; Rivadeneyra-Romero, G.; Martinez-Delgadillo, S.A. Sonophotocatalytic degradation of Orange II dye using low cost photocatalyst. J. Clean. Prod. 2017, 148, 836–844. [Google Scholar] [CrossRef]
- Casbeer, E.; Sharma, V.K.; Li, X. Synthesis and photocatalytic activity of ferrites under visible light: A review. Sep. Purif. Technol. 2012, 87, 1–14. [Google Scholar] [CrossRef]
- CIE Comission International de l’Eclairage. Recommendations on Uniform Color Spaces, Colour Difference Equations, Psychometrics Colour Terms; Bureau Central de la CIE: Paris, France, 1978; Available online: https://cie.co.at/ (accessed on 14 September 2022).
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi 1996, 15, 627–637. [Google Scholar] [CrossRef]
- Standard G173-03; Standard Tables for Reference Solar Spectral Irradiances: Direct Normal and Hemispherical on 37° Tilted Surface. ASTM International: West Conshohocken, PA, USA, 2013.
- Liu, J.J.; Fu, X.L.; Chen, S.F.; Zhu, Y.F. Electronic structure and optical properties of Ag3PO4 photocatalyst calculated by hybrid density functional method. Appl. Phys. Lett. 2011, 99, 191903. [Google Scholar] [CrossRef]
- Kumar, K.V.; Porkodi, K.; Rocha, F. Langmuir–Hinshelwood kinetics—A theoretical study. Catal. Commun. 2008, 9, 82–84. [Google Scholar] [CrossRef]
- Verger, L.; Olivier, D.; Rousse, G.; Cotte, M.; Cormier, L. The Stability of Gahnite Doped with Chromium Pigments in Glazes from the French Manufacture of Sèvres. J. Am. Ceram. Soc. 2016, 100, 86–95. [Google Scholar] [CrossRef]
- Zen, E. Validity of Vegard’s law. Am. Mineral. 1956, 41, 523–524. [Google Scholar]
- Lambies, L.V.; Rincon, L.J.M. Mechanism of formation of a zircon-cadmium sulfoselenide pigment. Trans. J. Br. Ceram. Soc. 1981, 80, 105–108. [Google Scholar]
- Zhu, K.; Wang, J.; Wang, Y.; Jina, C.; Ganeshrajaa, A.S. Visible-light-induced photocatalysis and peroxymonosulfate activation over ZnFe2O4 fine nanoparticles for degradation of Orange II. Catal. Sci. Technol. 2016, 6, 2296–2304. [Google Scholar] [CrossRef]
- Kumbhar, S.S.; Mahadik, M.A.; Shinde, S.S.; Rajpure, K.Y.; Bhosale, C.H. Fabrication of ZnFe2O4 films and its application in photoelectrocatalytic degradation of salicylic acid. J. Photochem. Photobiol. B Biol. 2015, 142, 118–123. [Google Scholar] [CrossRef]

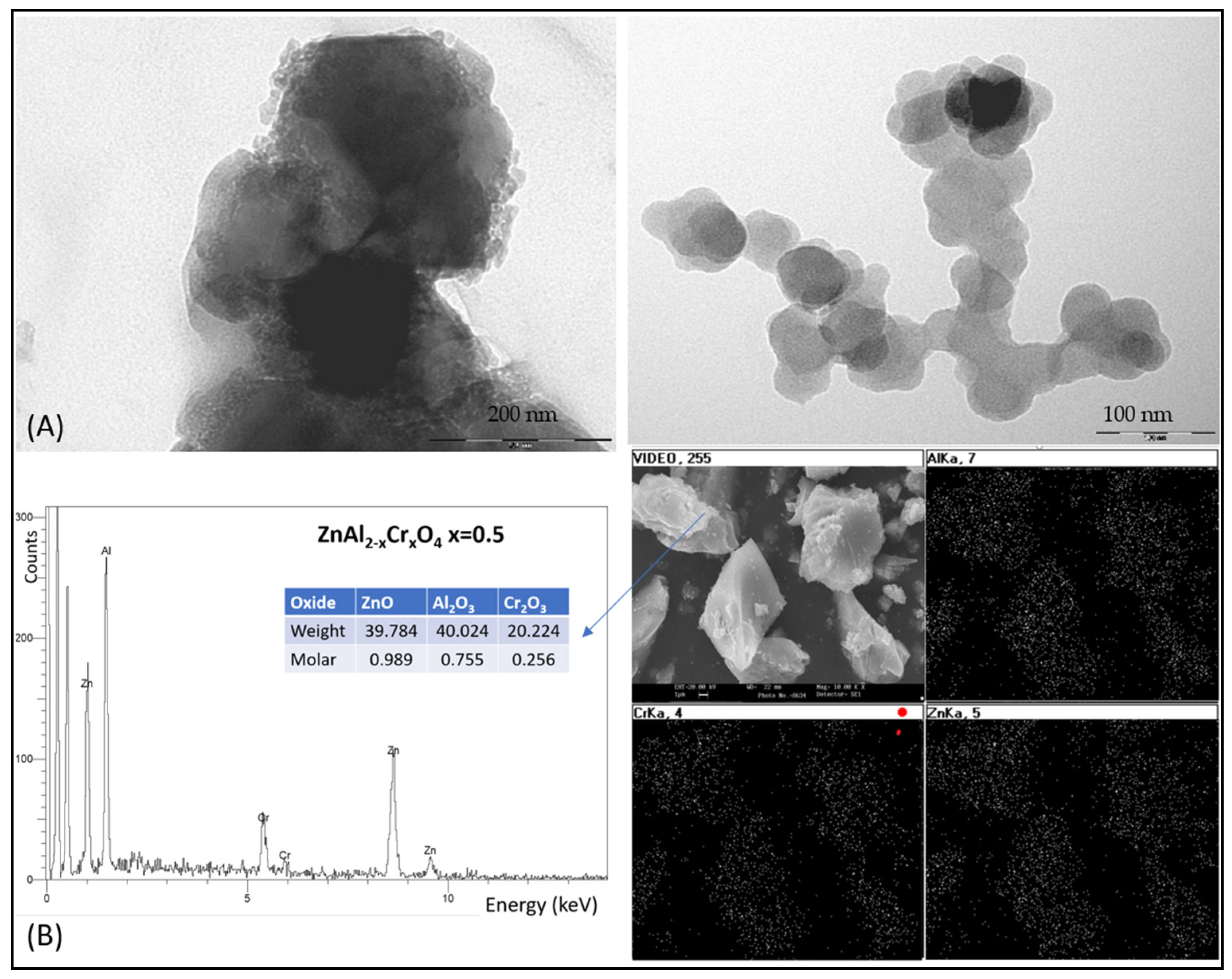
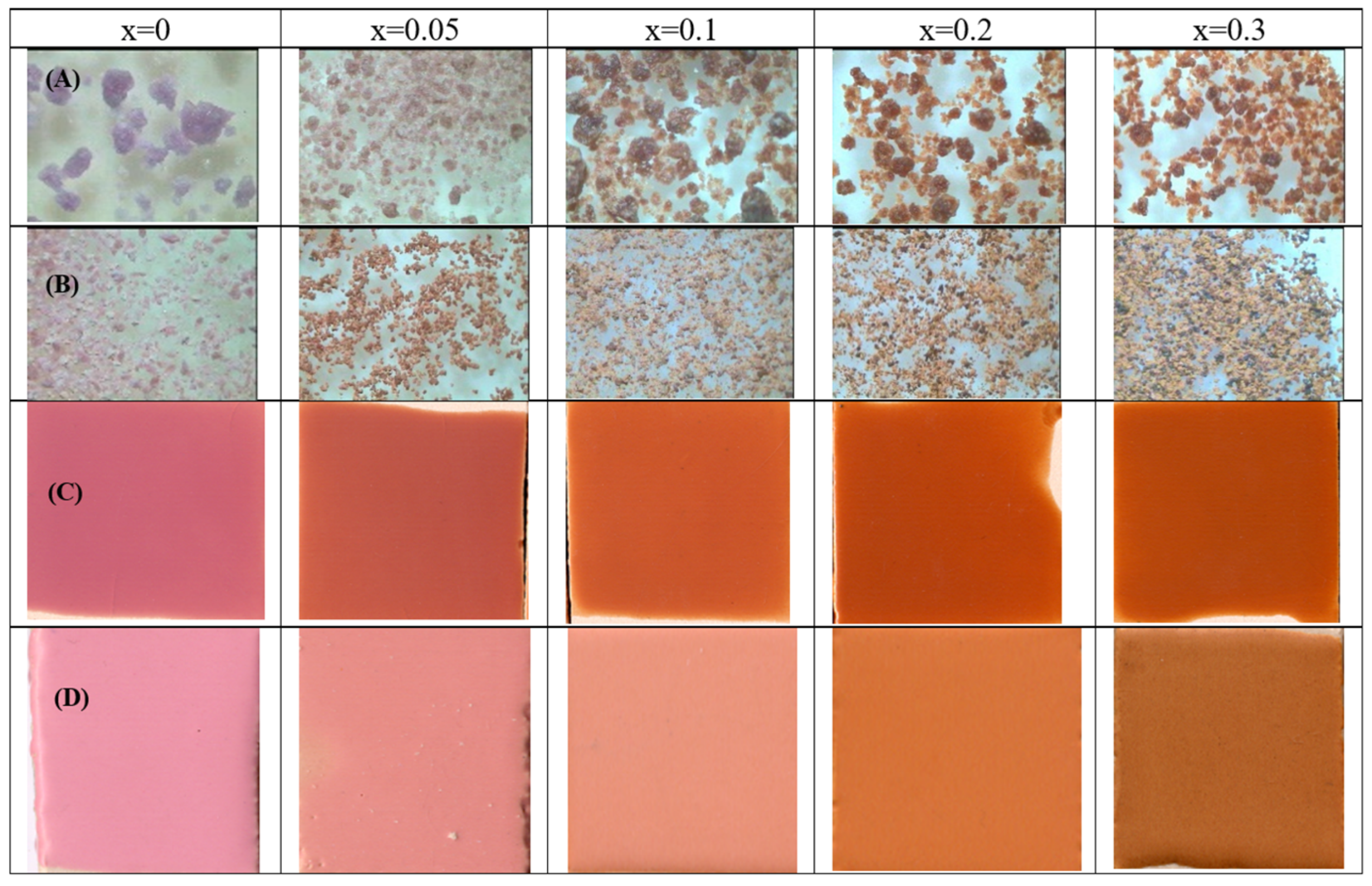
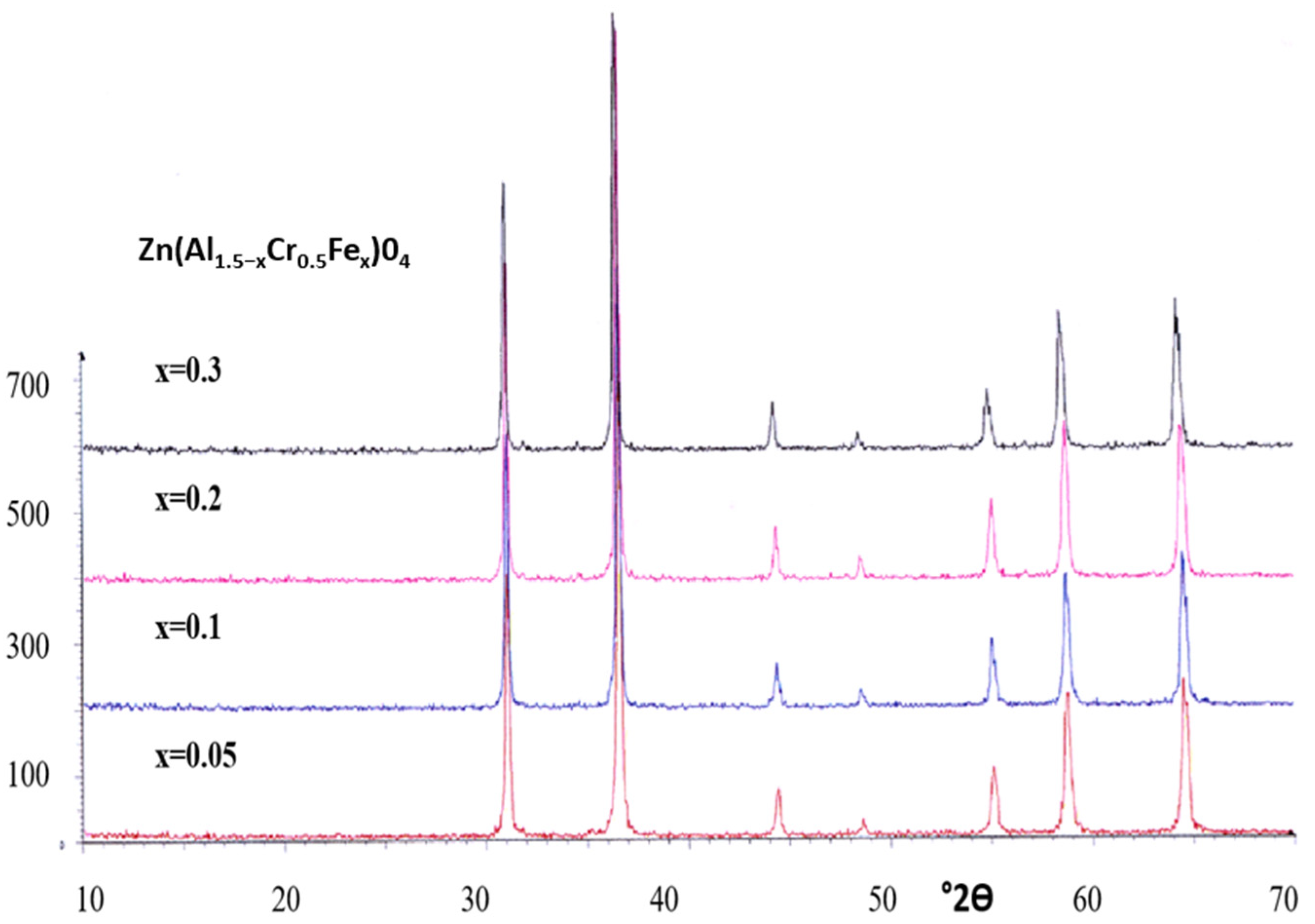
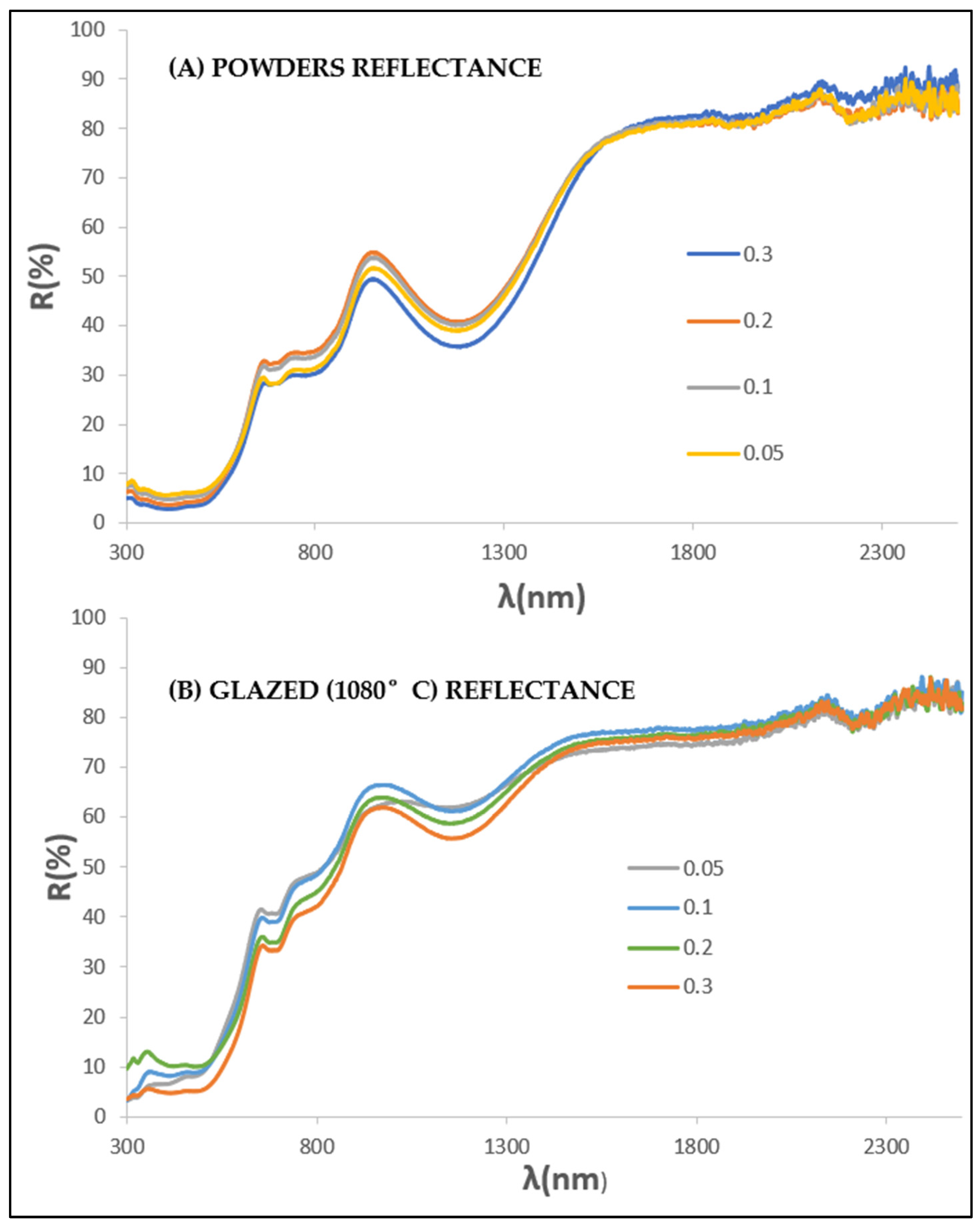
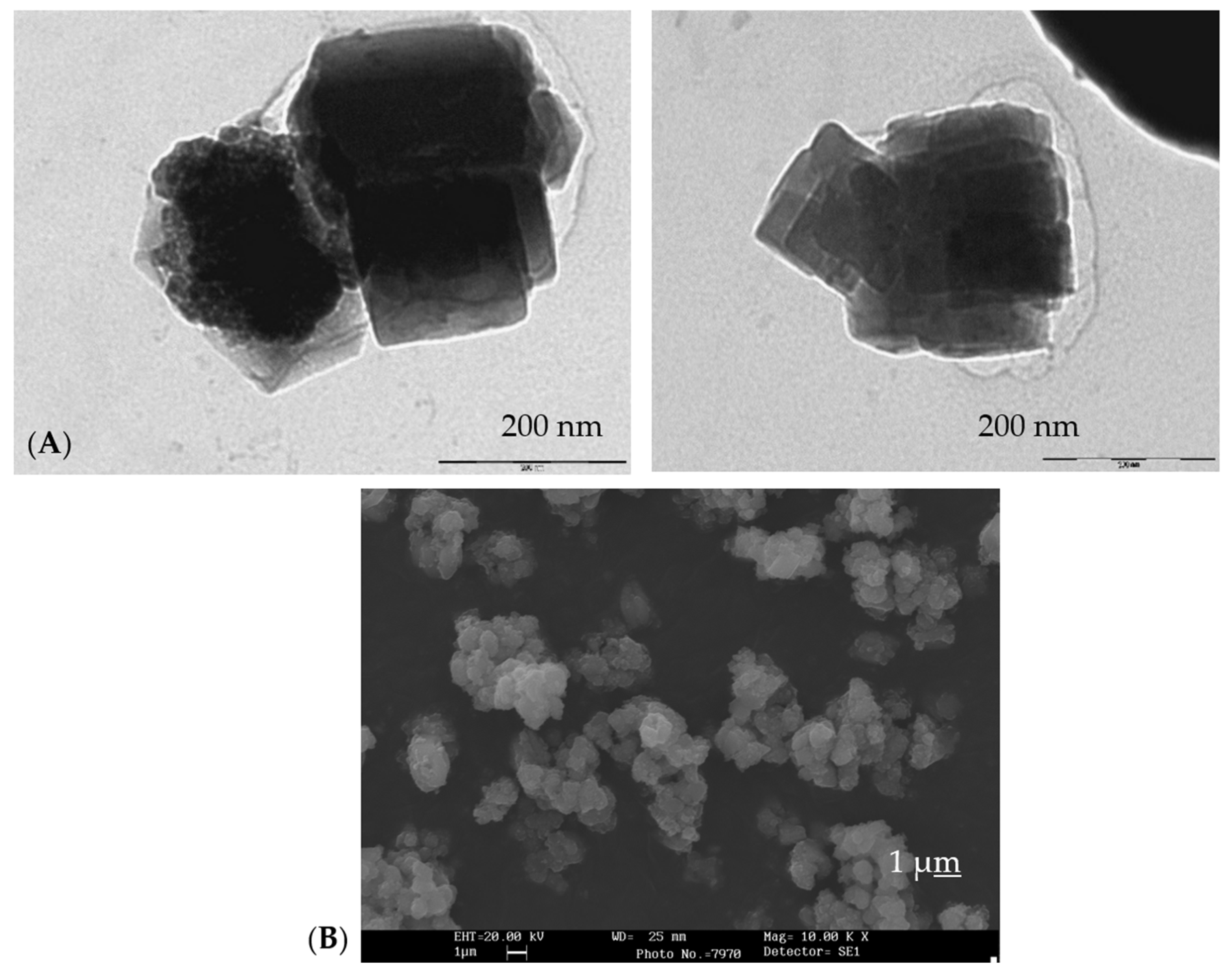
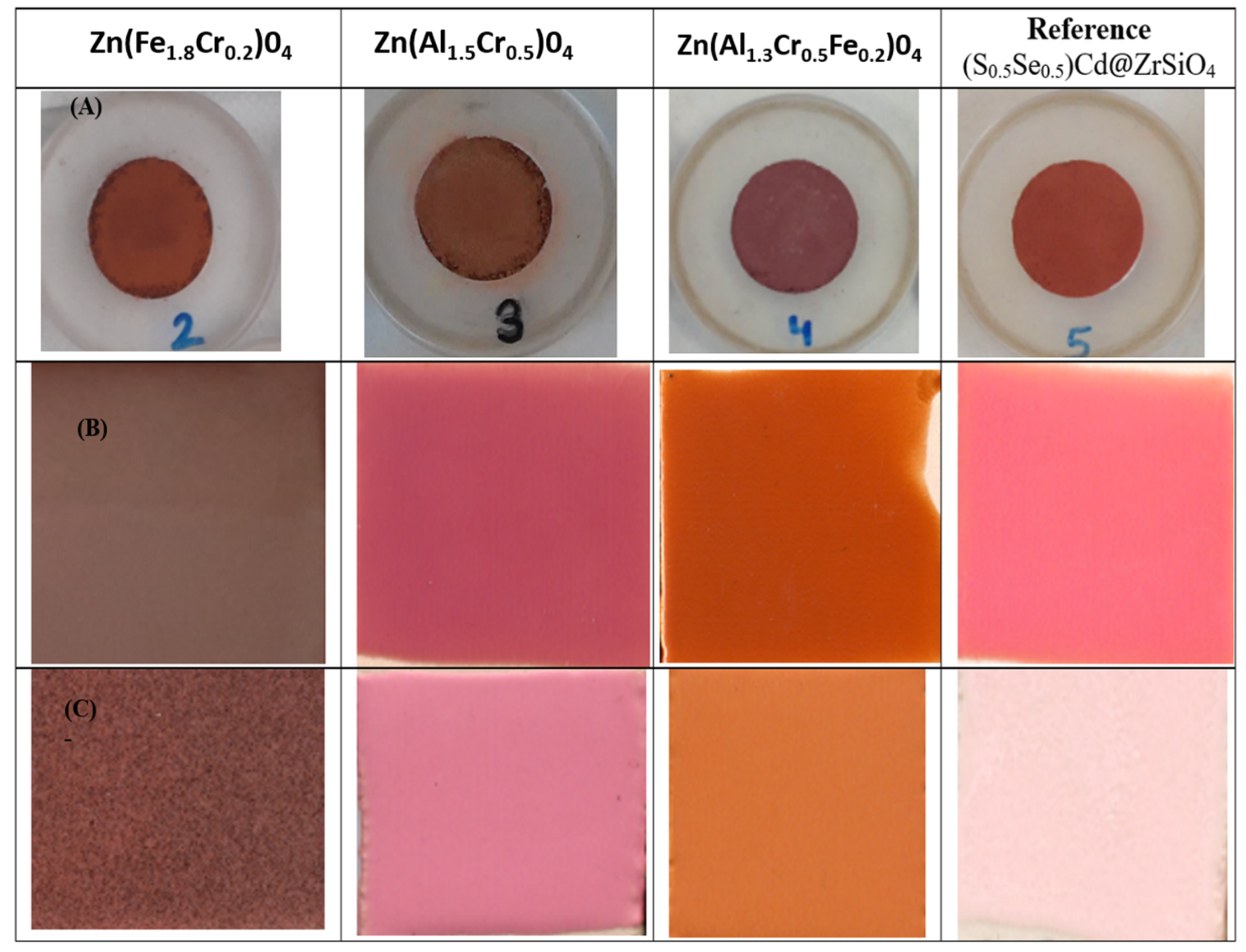
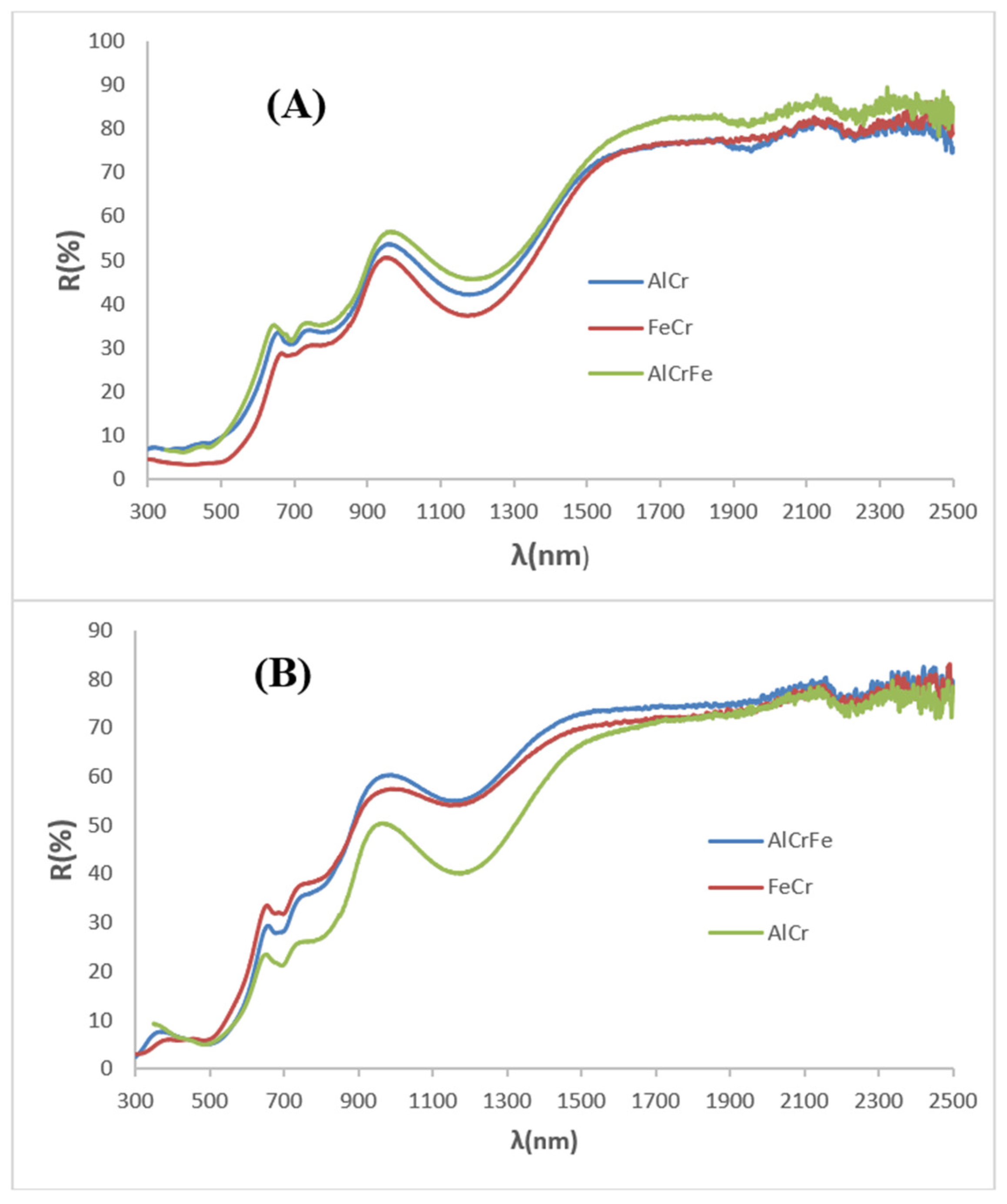
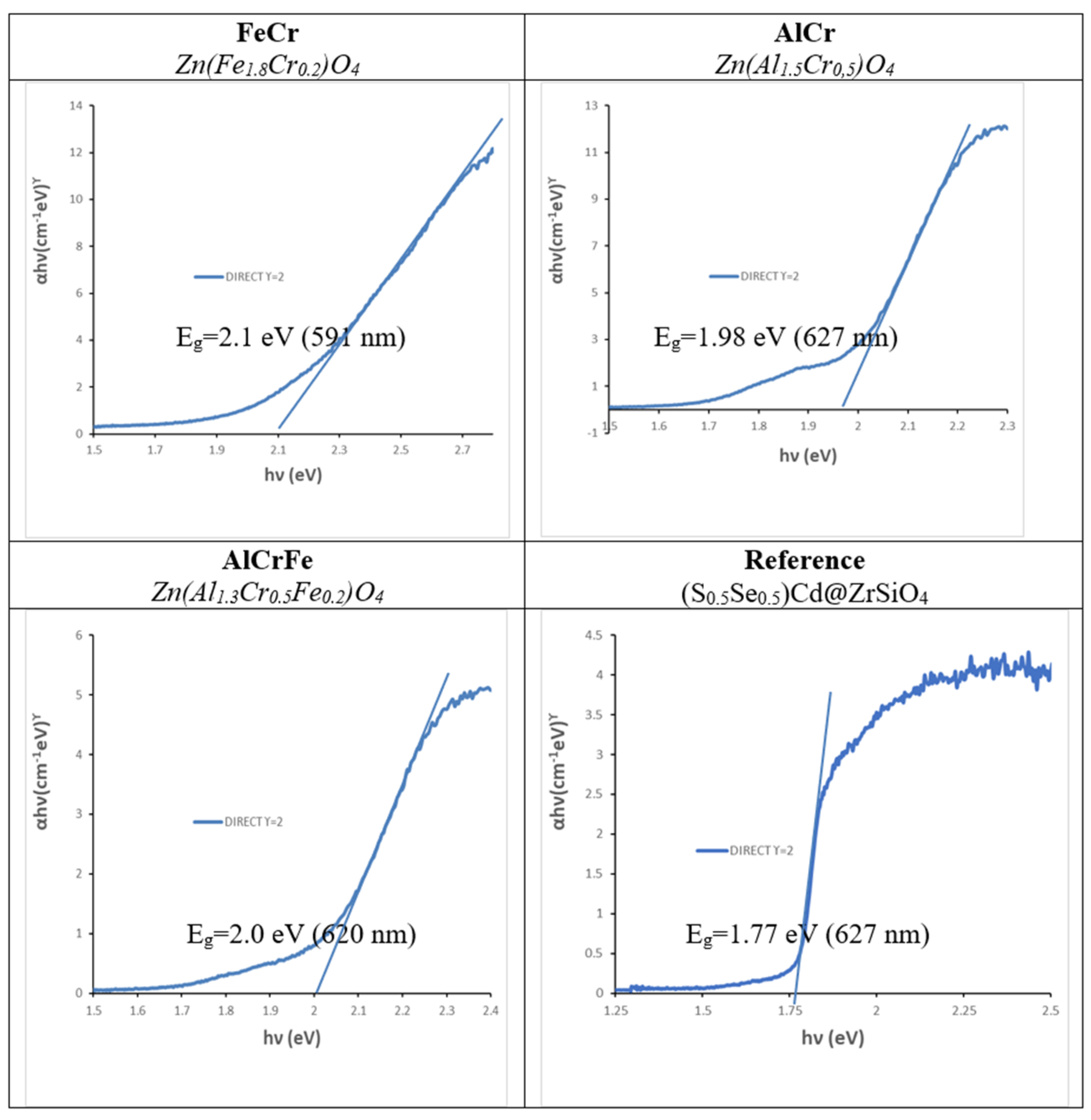

| OXIDE | (a) Double Firing with Pb, 1000 °C | (b) Single Firing, 1080 °C | (c) Single Firing Porcelain, 1200 °C |
|---|---|---|---|
| SiO2 | 75 | 65 | 67 |
| Na2O | 5 | 2 | - |
| K2O | 2 | 4 | 3 |
| CaO | 2 | 11 | 12.5 |
| MgO | - | 2 | 1.5 |
| ZnO | - | 7.5 | 6.5 |
| Al2O3 | 10 | 8.5 | 9 |
| PbO | 6 | - | - |
| x | L*a*b* | RVis/RNIR/R (%) |
|---|---|---|
| Powder | ||
| 0 | 46.6/25.3/34.8 | 22/54/36 |
| 0.2 | 44.6/20.4/22.6 | 18/52/33 |
| 0.5 | 42.5/20.1/22.5 | 16/50/31 |
| 1.0 | 42.9/18.3/19.8 | 15/47/29 |
| 1.5 | 47.5/16.6/19.3 | 17/47/30 |
| 1.8 | 45.9/15.3/23.1 | 19/41/29 |
| 2.0 | 53.6/0.4/10.9 | 21/32/26 |
| Glazed 1080 °C | ||
| 0 | 64.1/5.3/25.4 | 33/48/39 |
| 0.2 | 37.5/23.4/28.9 | 15/44/28 |
| 0.5 | 37.9/20.4/18.6 | 15/48/29 |
| 1.0 | 36.9/18.1/14.9 | 14/49/29 |
| 1.5 | 34.9/19.8/21.4 | 13/47/28 |
| 1.8 | 39.2/16.7/19.0 | 15/37/25 |
| 2.0 | 50.7/−2.0/8.0 | 25/29/27 |
| Glazed 1200 °C | ||
| 0 | 38.5/6.4/4.3 | |
| 0.2 | 48/8.9/15.2 | |
| 0.5 | - | |
| 1.0 | - | |
| 1.5 | 46.2/6.2/8.1 | |
| 1.8 | - | |
| 2.0 | 32.5/2.1/6.4 |
| x | L*a*b* | RVis/RNIR/R (%) |
|---|---|---|
| Gels | ||
| 0.02 | 68.2/1.3/2.2 | |
| 0.05 | 65.3/0.0/4.3 | |
| 0.1 | 60.5/−1.4/6.4 | |
| 0.2 | 54.1/−1.2/7.3 | |
| 0.3 | 48.1/−2.2/6.3 | |
| 0.4 | 44.2/−2.3/7.1 | |
| 0.5 | 40.2/−3.2/2.3 | |
| 0.75 | 31.1/−3.2/−1.2 | |
| Powder | ||
| 0.02 | 72.1/1/.2/2.1 | 36/45/41 |
| 0.05 | 70.4/3.2/3.1 | 25/45/35 |
| 0.1 | 63.4/6.2/4.2 | 22/45/33 |
| 0.2 | 57.3/8.2/5.3 | 20/44/32 |
| 0.3 | 51.2/8.3/4.3 | 19/43/31 |
| 0.4 | 47.1/9.2/4.2 | 20/43/31 |
| 0.5 | 45.2/11.2/3.2 | 21/45/32 |
| 0.75 | 36.4/2.1/2.0 | 18/42/28 |
| Glazed 1000 °C | ||
| 0.02 | 80.2/5.1/16.2 | 72/46/59 |
| 0.05 | 80.0/7.1/17.0 | 66/46/56 |
| 0.1 | 72.5/10.3/20.0 | 44/49/47 |
| 0.2 | 66.0/13.4/21.1 | 36/51/42 |
| 0.3 | 61.4/15.2/20.3 | 24/50/37 |
| 0.4 | 56.1/14.2/16.4 | 21/52/36 |
| 0.5 | 54.2/14.3/16.1 | 20/54/37 |
| 0.75 | 44.3/5.2/13.0 | 17/43/29 |
| x | L*a*b* | RVis/RNIR/R (%) |
|---|---|---|
| Gels | ||
| 0 | 54.2/−1.1/7.0 | |
| 0.05 | 52.3/2.2/5.4 | |
| 0.1 | 44.3/5.1/9.0 | |
| 0.2 | 44.2/5.2/8.3 | |
| 0.3 | 42.3/6.4/8.0 | |
| Powder | ||
| 0 | 57.0/8.1/5.2 | 21/45/33 |
| 0.05 | 52.1/10.0/12.1 | 22/46/34 |
| 0.1 | 53.2/12.2/17.2 | 20/50/37 |
| 0.2 | 50.2/11.1/17.2 | 19/51/34 |
| 0.3 | 49.2/12.1/18.2 | 19/50/33 |
| Glazed 1080 °C | ||
| 0 | 60.2/15.3/11.2 | 20/54/37 |
| 0.05 | 55.2/17.2/18.1 | 20/58/39 |
| 0.1 | 56.3/21.2/25.0 | 22/58/40 |
| 0.2 | 51.2/23.2/25.1 | 17/59/38 |
| 0.3 | 51.2/21.2/24.3 | 19/58/38 |
| Glazed 1200 °C | ||
| 0 | 69.1/13.0/9.2 | |
| 0.05 | 66.1/14.2/17.0 | |
| 0.1 | 66.4/16.0/22.2 | |
| 0.2 | 61.5/18.3/27.2 | |
| 0.3 | 57.5/15.2/24.3 |
| L*a*b* | RVis/RNIR/R (%) | |
|---|---|---|
| Powder | ||
| Zn(Fe1.8Cr0.2)O4 | 36.2/22.1/21.0 | 14/48/30 |
| Zn(Al1.5Cr0.5)O4 | 51.3/12.5/8.2 | 21/45/32 |
| Zn(Al1.3Cr0.5Fe0.2)O4 | 47.0/17.1/19.2 | 19/51/34 |
| Reference (S0.5Se0.5)Cd@ZrSiO4 | 53.2/23.1/12.2 | 21/58/39 |
| Glazed 1080 °C | ||
| Zn(Fe1.8Cr0.2)O4 | 38.3/23.2/29.3 | 18/56/36 |
| Zn(Al1.5Cr0.5)O4 | 60.2/15.0/11.0 | 20/54/37 |
| Zn(Al1.3Cr0.5Fe0.2)O4 | 51.5/23.4/25.3 | 17/59/38 |
| Reference (S0.5Se0.5)Cd@ZrSiO4 | 62.2/33.4/16.2 | 26/64/45 |
| Glazed 1200 °C | ||
| Zn(Fe1.8Cr0.2)O4 | 48.0/8.9/15.2 | 15/43/29 |
| Zn(Al1.5Cr0.5)O4 | 69.3/13.2/9.1 | 26/57/42 |
| Zn(Al1.3Cr0.5Fe0.2)O4 | 61.0/18.2/27.3 | 20/62/41 |
| Reference (S0.5Se0.5)Cd@ZrSiO4 | colourless | - |
| Eg (eV) | t1/2 (min) | R2 | |
|---|---|---|---|
| Zn(Fe1.8Cr0.2) | 2.10 | 213 | 0.8341 |
| Zn(Al1.5Cr0.5) | 1.98 | 346 | 0.9679 |
| Zn(Al1.3Cr0.5Fe0.2)O4 | 2.00 | 433 | 0.8177 |
| ((S0.5Se0.5)Cd@ZrSiO4 | 1.77 | 267 | 0.9870 |
| Ag3PO4 | 2.45 | 230 | 0.9976 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monrós, G.; Badenes, J.A.; Llusar, M.; Delgado, C. Franklinite-Zincochromite-Gahnite Solid Solutions for Cool Red Ceramic Pigments with Visible Light Photocatalysis. Ceramics 2024, 7, 342-363. https://doi.org/10.3390/ceramics7010022
Monrós G, Badenes JA, Llusar M, Delgado C. Franklinite-Zincochromite-Gahnite Solid Solutions for Cool Red Ceramic Pigments with Visible Light Photocatalysis. Ceramics. 2024; 7(1):342-363. https://doi.org/10.3390/ceramics7010022
Chicago/Turabian StyleMonrós, Guillermo, José A. Badenes, Mario Llusar, and Carolina Delgado. 2024. "Franklinite-Zincochromite-Gahnite Solid Solutions for Cool Red Ceramic Pigments with Visible Light Photocatalysis" Ceramics 7, no. 1: 342-363. https://doi.org/10.3390/ceramics7010022
APA StyleMonrós, G., Badenes, J. A., Llusar, M., & Delgado, C. (2024). Franklinite-Zincochromite-Gahnite Solid Solutions for Cool Red Ceramic Pigments with Visible Light Photocatalysis. Ceramics, 7(1), 342-363. https://doi.org/10.3390/ceramics7010022