High-Lead Glazed Ceramic Production in Western Iberia (Gharb al-Andalus) between the 10th and Mid-13th Centuries: An Approach from the City of Évora (Portugal)
Abstract
1. Introduction
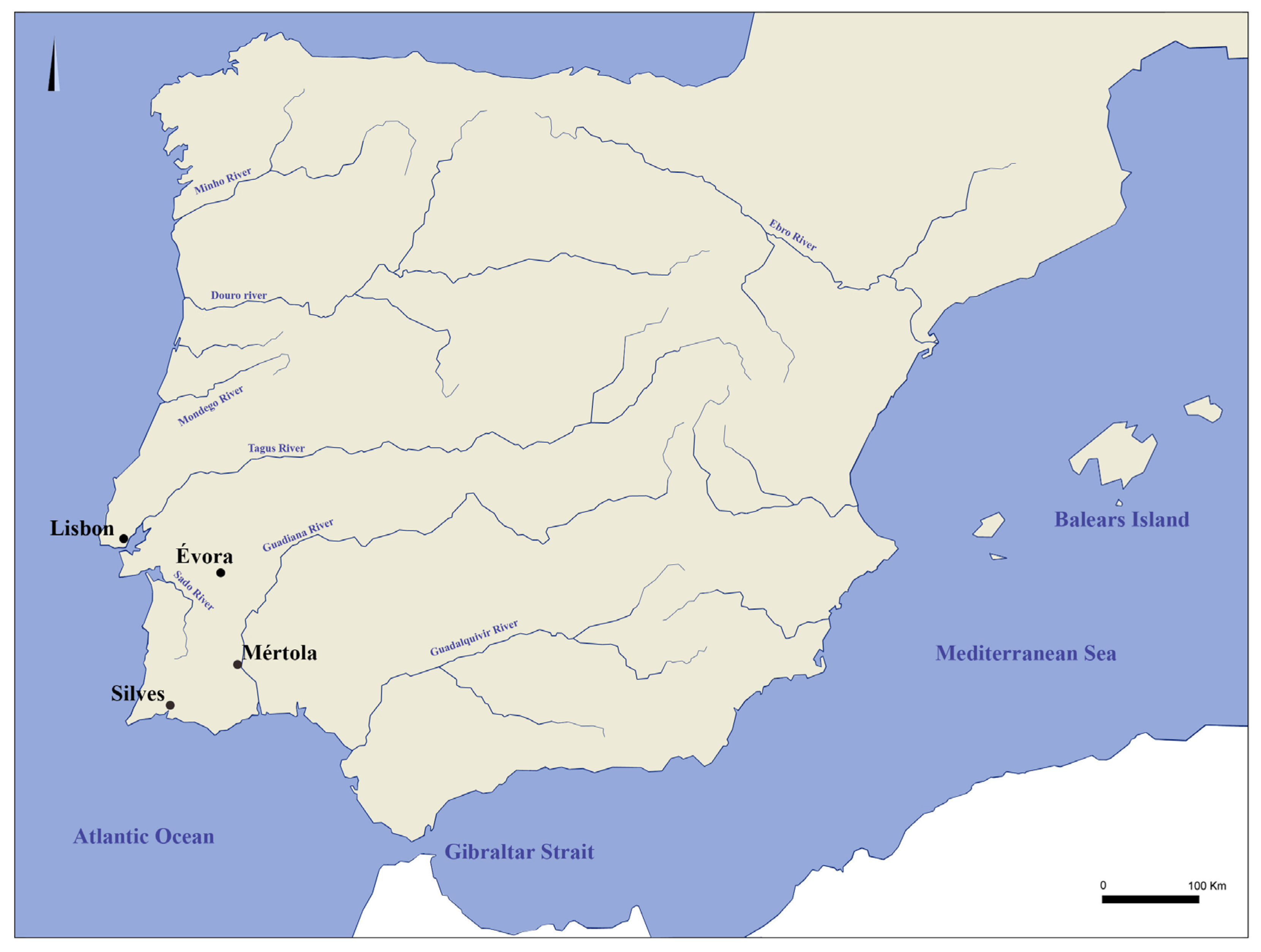
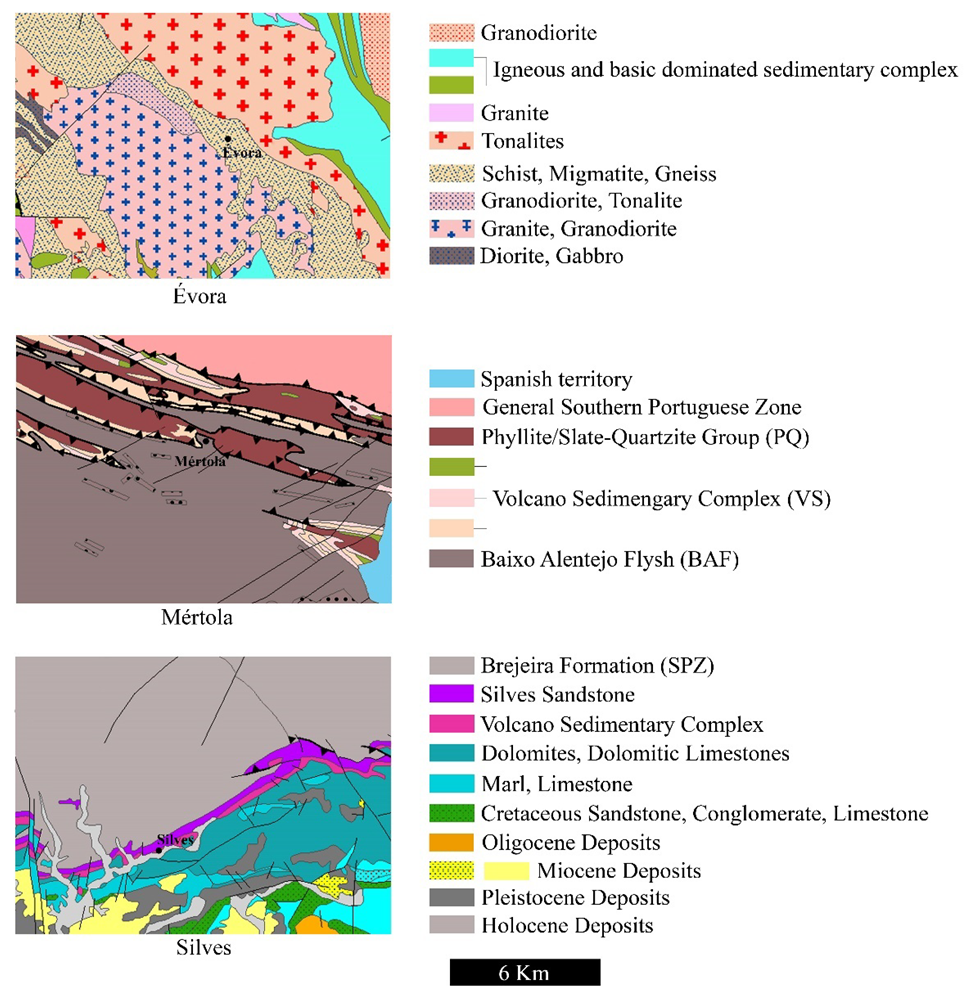
2. Geological Settings of the Cities of Évora, Mértola, and Silves
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Optical Microscopy (OM)
3.2.2. X-ray Diffraction (XRD)
3.2.3. X-ray Fluorescence (XRF)
3.2.4. Microanalysis by SEM-EDS
4. Results and Discussion
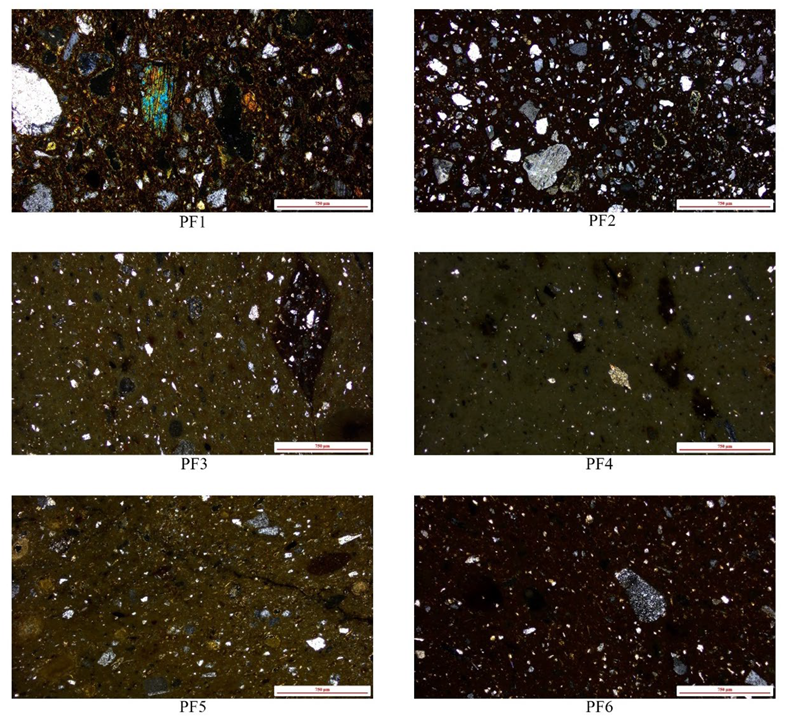
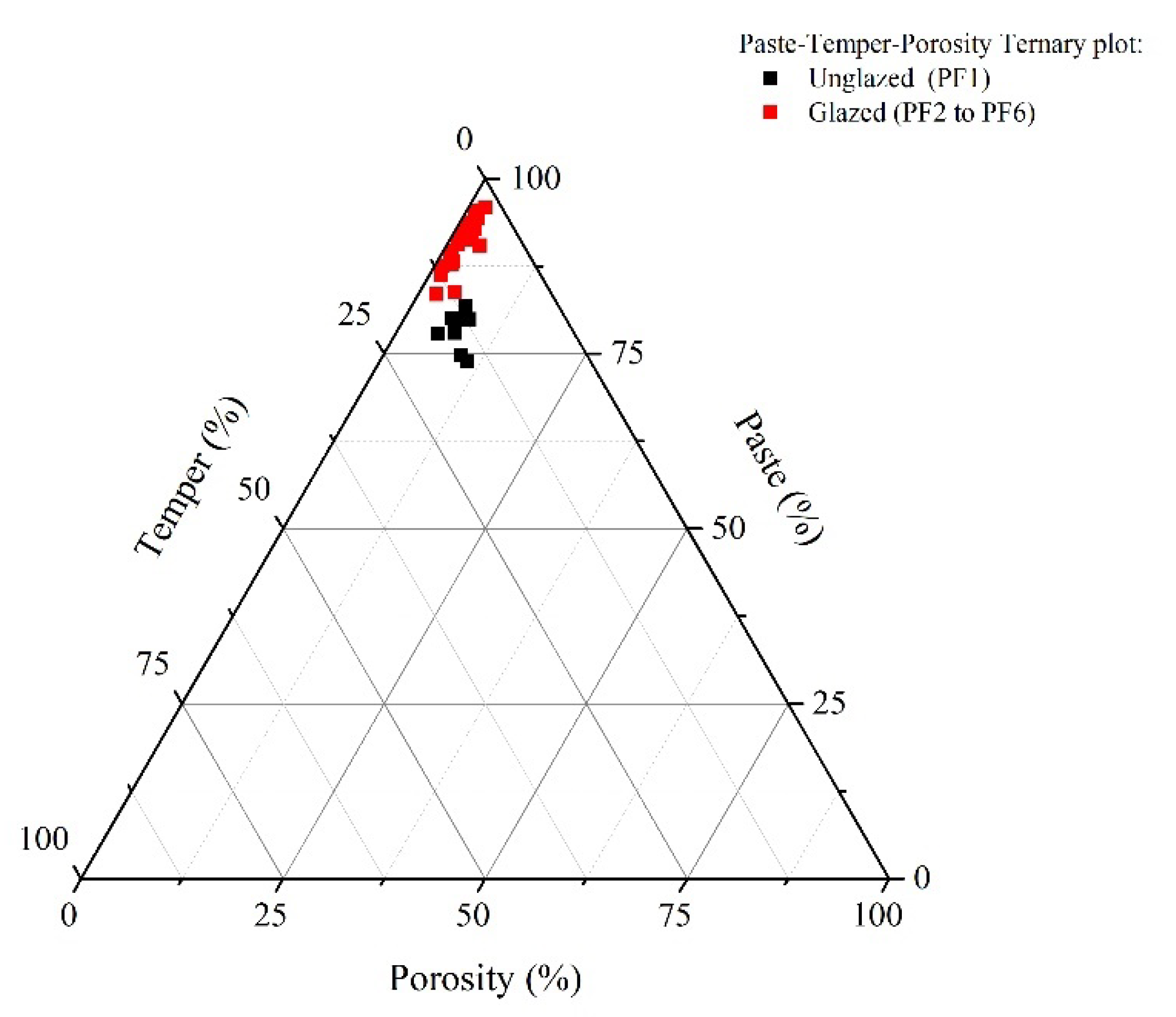
4.1. Optical Microscopy (OM)
4.1.1. PF 1
4.1.2. PF 2
4.1.3. PF 3
4.1.4. PF 4
4.1.5. PF 5
4.1.6. PF 6
4.2. X-ray Diffraction (XRD)
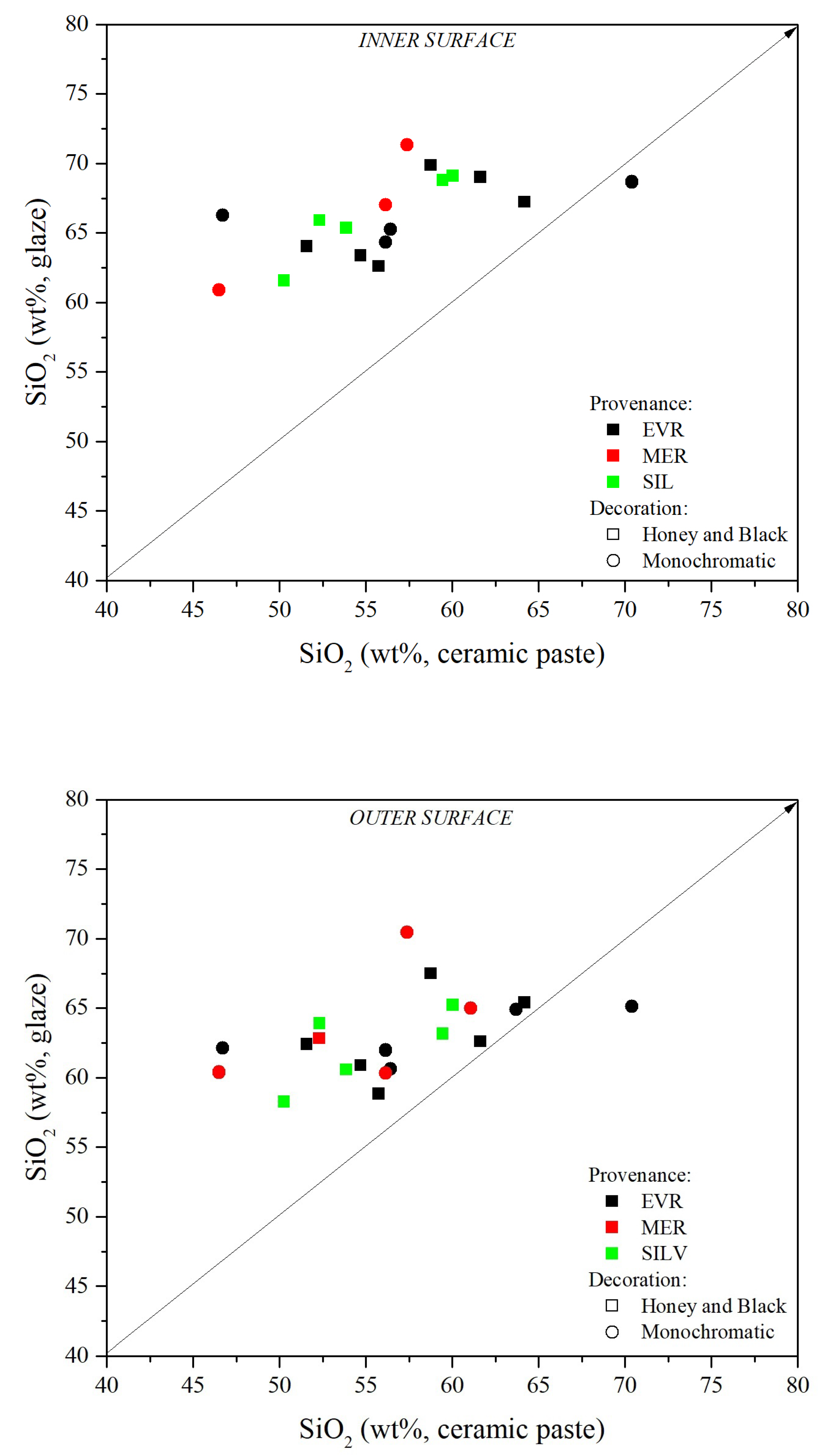
4.3. X-ray Fluorescence (XRF)
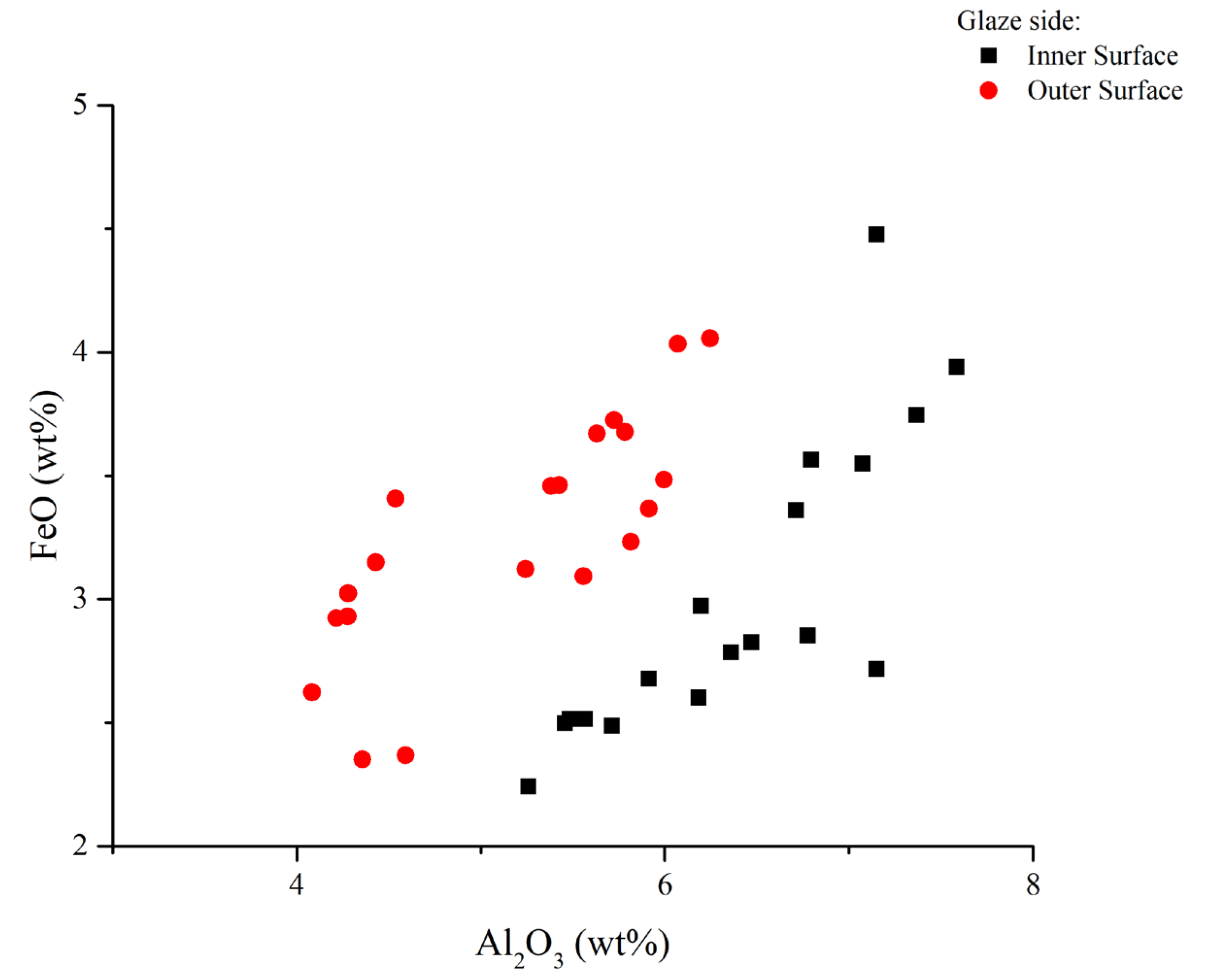
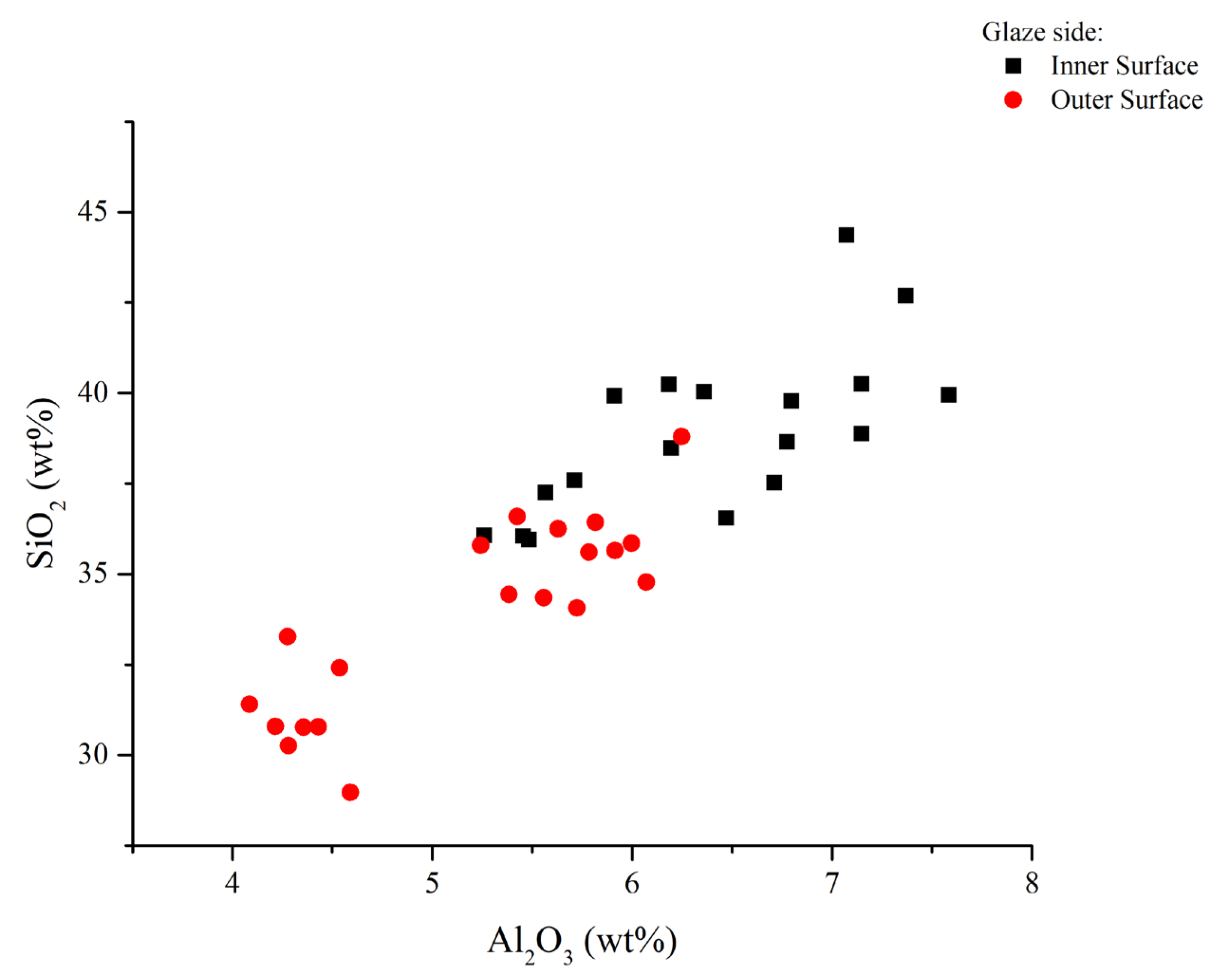
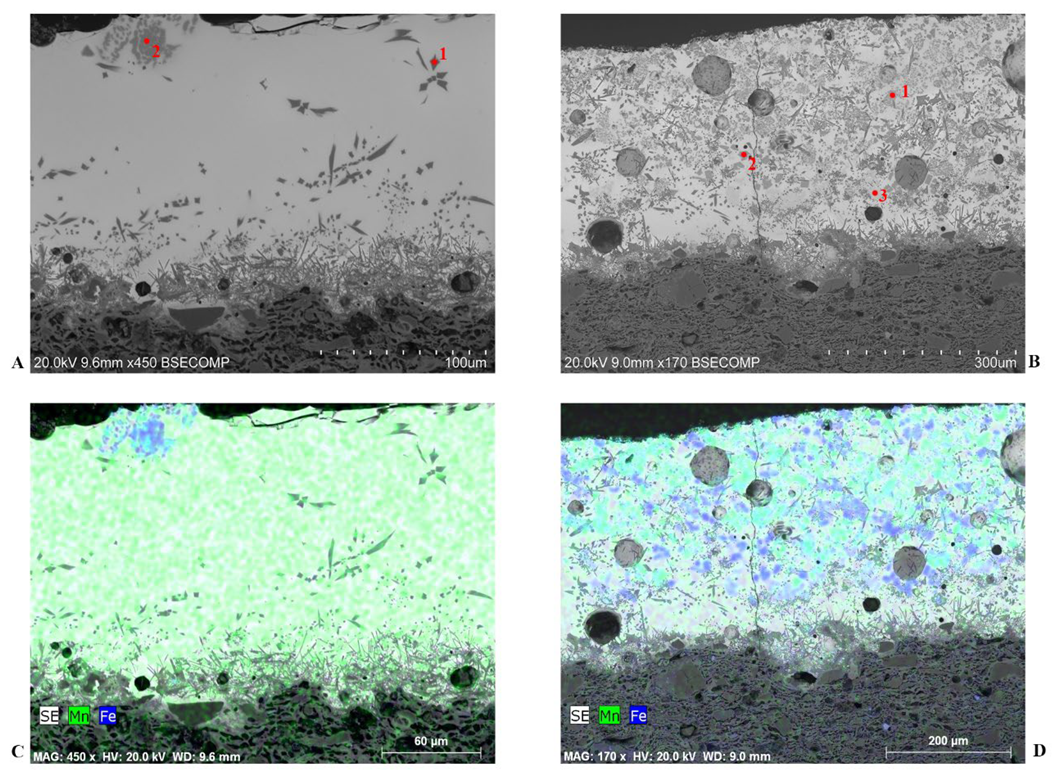
4.4. Micro-Structural and Chemical Analysis of Glazed Decorations by SEM-EDS
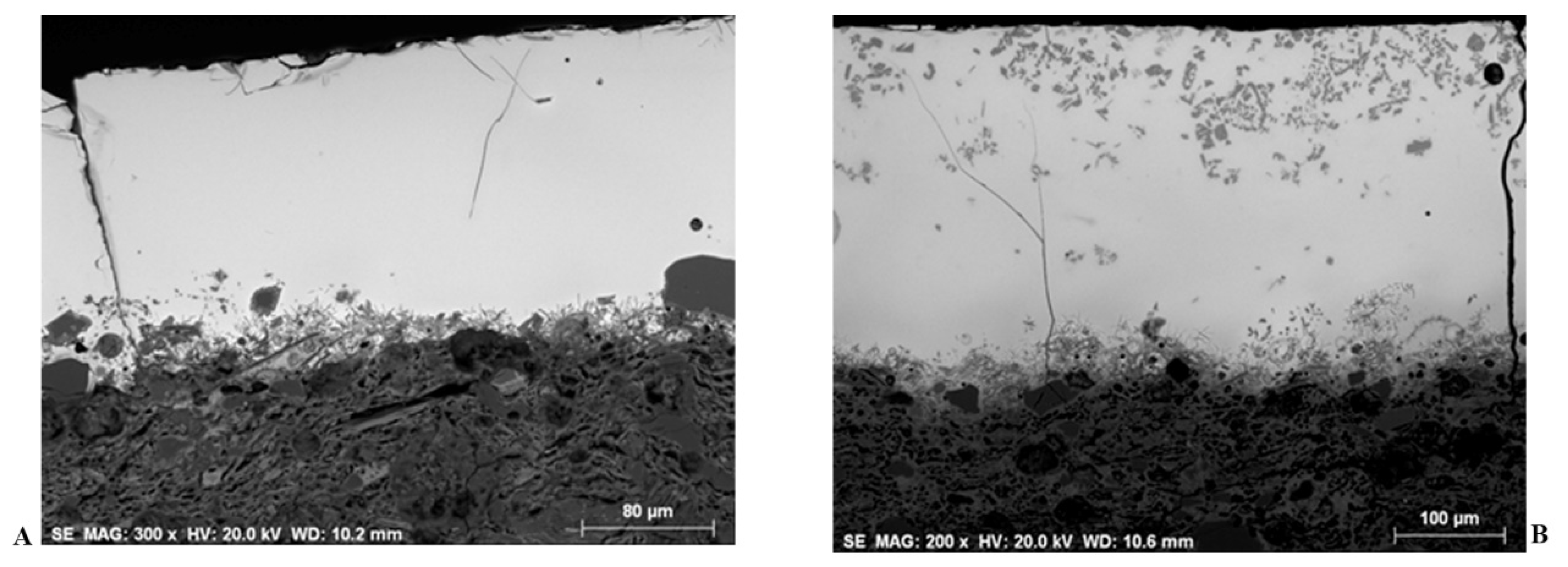
4.4.1. Micro-Structural and Chemical Characteristics of the Inner and Outer Glazes
4.4.2. Firing Technology (Single vs. Double Firing)
4.4.3. Glaze Application Technique
4.4.4. Micro-Structural and Chemical Characteristics of Black/Brown Glazed Decorations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Salinas, E.; Pradell, T.; Molera, J. Glaze production at early Islamic workshop in al-Andalus. Archaeol. Anthropol. Sci. 2018, 11, 2201–2213. [Google Scholar] [CrossRef]
- Salinas, E.; Pradell, T. The introduction of the glaze in al-Andalus: Technological waves and Oriental influences. Libyan Stud. 2020, 51, 87–98. [Google Scholar] [CrossRef]
- Salinas, E.; Pradell, T. The first glaze production centres in al-Andalus (late 9th early 10th centuries): Pechina, Cordoba and Malaga. In Proceedings of the Tecnología de los Vidriados en el Oeste Mediterráneo: Tradiciones Islámicas y Cristianas, Valencia, Spain, 25 January 2018; Coll Conesa, J., Salinas, E., Eds.; Ministerio de Cultura y Deporte: Madrid, Spain, 2021; pp. 49–60. [Google Scholar]
- Almodóvar, G.R.; Yesares, L.; Sáez, R.; Toscano, M.; González, F.; Pons, J.M. Massive sulfide ores in the Iberian Pyrite Belt: Mineralogical and textural evolution. Minerals 2019, 9, 653. [Google Scholar] [CrossRef]
- Tite, M.S.; Freestone, I.; Mason, R.; Molera, J.; Vendrell-Saz, M.; Wood, N. Lead glazes in antiquity. Methods Prod. Reason. Use. Archaeom. 1998, 40, 241–260. [Google Scholar] [CrossRef]
- Molera, J.; García-Vallés, M.; Pradell, T.; Vendrell-Saz, M. Hispano-Moresque pottery production of the fourteenth-century workshop of Testar del Molí (Paterna, Spain). Archaeometry 1996, 38, 67–80. [Google Scholar] [CrossRef]
- Quinn, P.S. Ceramic Petrography: The Interpretation of Archaeological Pottery & Related Artefacts in Thin Section, 1st ed.; Archaeopress: Oxford, UK, 2013; ISBN 978-1-905739-59-2. [Google Scholar]
- Pradell, T.; Molera, J. Ceramic technology. How to characterize ceramic glazes. Archaeol. Anthropol. Sci. 2020, 12, 189. [Google Scholar] [CrossRef]
- Beltrame, M.; Sitzia, F.; Liberato, M.; Santos, H.; Themudo, F.; Columbu, S.; Mirão, J. Comparative pottery technology between the Middle Ages and Modern times (Santarém, Portugal). Archaeol. Anthropol. Sci. 2020, 12, 130. [Google Scholar] [CrossRef]
- Molera, J.; Pradell, T.; Merino, L.; García-Vallés, M.; García-Orellana, J.; Salvadó, N.; Vendrell-Saz, M. La tecnología de la cerámica Islámica y Mudéjar. Caesaraugusta 1997, 73, 15–41. [Google Scholar]
- Molera, J.; Vendrell-Saz, M.; García-Vallés, M. Technology and colour development of Hispano-Moresque lead-glazed pottery. Archaeometry 1997, 39, 23–39. [Google Scholar] [CrossRef]
- Molera, J.; Coll, J.; Labrador, A.; Pradell, T. Manganese brown decorations in 10th to 18th century Spanish tin glazed ceramics. Appl. Clay Sci. 2013, 82, 86–90. [Google Scholar] [CrossRef]
- Molera, J.; Carvajal, J.C.; Molina, G.; Pradell, T. Glazes, colourants and decorations in early Islamic glazed ceramics from the Vega of Granada (9th to 12th centuries CE). J. Archaeol. Sci. Rep. 2017, 21, 1141–1151. [Google Scholar] [CrossRef]
- Salinas, E.; Zozaya, J. Pechina: El antecedente de las cerámicas vidriadas Islámicas en el al-Andalus. In Proceedings of the X Congresso Internacional a Cerâmica Medieval no Mediterrâneo, Silves-Mértola, Portugal, 22–27 October 2012; Gonçalves, M.J., Gómez-Martínez, S., Eds.; Câmara Municipal de Silves & Campo Arqueológico de Mértola: Silves/Mértola, Portugal, 2015; pp. 573–576. [Google Scholar]
- Gómez, S. La cerámica en al-Andalus: Producción y comercio. In Economía y Trabajo: Las Bases Materiales de la Vida en al-Andalus; Delgado, M.M., Pérez-Aguilar, L., Eds.; Ediciones Alfar: Seville, Spain, 2019; pp. 199–234. ISBN 978-84-7898-839-6. [Google Scholar]
- Gómez, S.; Gonçalves, M.J.; Inácio, I.; dos Santos, C.; Coelho, C.; Liberato, M.; Gomes, A.S.; Bugalhão, J.; Catarino, H.; Cavaco, S.; et al. A cidade e o seu território no Gharb al-Andalus através da cerâmica. In Proceedings of the X Congresso Internacional a Cerâmica Medieval no Mediterrâneo, Silves-Mértola, Portugal, 22–27 October 2012; Gonçalves, M.J., Gómez-Martínez, S., Eds.; Câmara Municipal de Silves & Campo Arqueológico de Mértola: Silves/Mértola, Portugal, 2015; pp. 19–50. [Google Scholar]
- Gómez, S.; Cavaco, S.; Coelho, C.; Covanerio, J.; Fernandes, I.C.; Gomes, A.S.; Gonçalves, M.J.; Linácio, I.; Liberato, M.; Lopes, G.; et al. El uso del vidriado en el Garb al-Ándalus y su lenta difusión. In Proceedings of the Tecnología de los Vidriados en el Oeste Mediterráneo: Tradiciones Islámicas y Cristianas, Valencia, Spain, 25 January 2018; Coll Conesa, J., Salinas, E., Eds.; Ministerio de Cultura y Deporte: Madrid, Spain, 2021; pp. 129–152. [Google Scholar]
- Bugalhão, J.; Sousa, M.; Gomes, A. Vestígios de produção oleira islâmica no Mandarim Chinês, Lisboa. Rev. Port. Arqueol. 2004, 7, 575–643. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=1039244 (accessed on 27 February 2023).
- Dias, M.I.; Prudêncio, M.I.; Bugalhão, J.; Gomes, S.; Sousa, M.J.; Folgado, D. A produção de cerâmicas no arrabalde occidental de Lisboa islâmica. Primeros resultados arqueométricos. A Ocupação Islâmica da Península Ibérica. In Proceedings of the IV Congresso de Arqueologia Peninsular, Faro, Portugal,, 14–19 September 2004; Ferreira, N., Ed.; University of Algarve: Algarve, Portugal, 2008; pp. 157–167. [Google Scholar]
- Beltrame, M.; Liberato, M.; Mirão, J.; Santos, H.; Barrulas, P.; Branco, F.; Gonçalves, L.; Candeias, A.; Schiavon, N. Islamic and post Islamic ceramics from the town of Santarém (Portugal): The continuity of ceramic technology in a transforming society. J. Archaeol. Sci. Rep. 2019, 23, 910–928. [Google Scholar] [CrossRef]
- Beltrame, M.; Sitzia, F.; Arruda, A.M.; Barrulas, P.; Barata, F.T.; Mirão, J. The Islamic ceramic of the Santarém Alcaçova: Raw materials, technology, and trade. Archaeometry 2021, 63, 1157–1177. [Google Scholar] [CrossRef]
- Gómez, S. La Cerámica Islámica de Mértola: Producción y Comercio. Ph.D. Thesis, Complutense University of Madrid, Madrid, Spain, 2004. Available online: eprints.ucm.es/id/eprint/7087/ (accessed on 11 June 2021).
- Bridgman, R. Re-examining Almohad economies in south-western al-Andalus through petrological analysis of archaeological ceramics. In Revisiting Al-Andalus. Perspectives on the Material Culture of Islamic Iberia and Beyond, 1st ed.; Anderson, G.D., Rosser-Owen, M., Eds.; Brill: Leiden, The Netherlands, 2007; Volume 34, pp. 143–165. ISBN 978-90-04-16227-3. [Google Scholar]
- Coll Conesa, J.; Garcia Porras, A. Tipologia, cronologia e produzione dei forni per ceramica in al-Andalus. Fornaci. Tecnologie e produzione della ceramica in Età Medievale e Moderna. In Proceedings of the XLII Convegno Internazionale della Ceramica, Savona, Italy, 29–30 May 2009; Centro Ligure per la Storia della Ceramica: Firenze, Italia, 2010; pp. 25–44. [Google Scholar]
- Karagiannopoulou, M.; Mirão, J.A.P.; Beltrame, M.; Gonçalves, M.J. Islamic Almohad pottery from Silves, Portugal. In Proceedings of the 12th Congress AIECM on Medieval and Modern Period Mediterranean Ceramic, Athens, Greece, 21–27 October 2018; Petridis, P., Yangaki, A.G., Liaros, N., Bia, E.-E., Eds.; National Hellenistic Research Foundation: Athens, Greece, 2021; pp. 255–262. [Google Scholar]
- Ma, B.; Liu, L.; Feng, S.; Xu, Q.; Feng, X. Analyisis of the elemental composition of Tang Sancai from the four major kilns in China using EDXRF. Nucl. Instrum. Methods Phys. Res. Sect. B 2014, 319, 95–99. [Google Scholar] [CrossRef]
- Buravlev, I.Y.; Gelman, E.I.; Lapo, E.G.; Pimenov, V.A.; Martynenko, A.V. Three-colored Sancai glazed ceramics excavated from Bohai sites in Primorye (Russia). J. Archaeol. Sci. Rep. 2022, 41, 103346. [Google Scholar] [CrossRef]
- Moita, P.; Santo, J.F.; Francisco, M. Layered granitoids: Interaction between continental crust recycling processes and mantle-derived magmatism. Examples from the Évora Massif (Ossa-Morena Zone, southwest Iberia, Portugal). Lithos 2009, 111, 125–141. [Google Scholar] [CrossRef]
- Schermerhorn, L.J.G. An outline stratigraphy of the Iberian Pyrite Belt. Boletín Geológico E Min. España 1971, 82, 304–308. [Google Scholar]
- Oliveira, J.T.; Silva, J.B. Notícia Explicativa de Folha 46-D, Mértola; Instituto Nacional de Engenharia, Tecnologia e Inovação: Lisbon, Portugal, 2007. [Google Scholar]
- Fletcher, W.J. Holocene Landscape History of Southern Portugal. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2005. [Google Scholar] [CrossRef]
- Trindade, M.J.; Dias, M.I.; Coroado, J.; Rocha, F. Mineralogical transformation of calcareous rich clays with firing: A comparative study between calcite and dolomite rich clays from Algarve, Portugal. Appl. Clay Sci. 2009, 42, 345–355. [Google Scholar] [CrossRef]
- Trindade, M.J.; Rocha, F.; Dias, M.I.; Prudêncio, M.I. Mineralogy and grain-size distribution of clay-rich rock units of the Algarve basin (South Portugal). Clay Miner. 2013, 48, 59–83. [Google Scholar] [CrossRef]
- Trindade, M.J.; Dias, M.I.; Rocha, F.; Prudêncio, M.I.; Marques, R. Geochemistry of mudrock units from the Meso-Cenozoic Algarve Basin, Portugal. Geosci. J. 2018, 22, 733–749. [Google Scholar] [CrossRef]
- Bugalhão, J.; Catarino, H.; Cavaco, S.; Covaneiro, J.; Fernandes, I.C.; Gomes, A.; Gómez, S.; Gonçalves, M.; Grangé, M.; Inácio, I.; et al. CIGA: Projecto de sistematização para a cerãmica islãmica do Gharb al-Ândalus. XELB 2010, 10, 455–476. Available online: https://hdl.handle.net/10400.26/6580 (accessed on 15 February 2021).
- Cavaco, S.; Covaneiro, J.; Fernandes, I.; Gómez, S.; Gonçalves, M.J.; Grangé, M.; Inácio, I.; Lopes, G.; Santos, C.; Bugalhão, J.; et al. Contextos sócio-territoriais de distribuição. In O Arqueólogo Português, 1st ed.; Museu Nacional de Arqueologia, Ed.; Imprensa Nacional-Casa da Moeda: Lisbon, Portugal, 2013; Volume 3(V), pp. 349–380. ISSN 0870-094X. [Google Scholar]
- Lopez, G.; Santos, J.R. Cerâmicas islâmicas da Natatio das Termas Romanas de Évora. In Proceedings of the X Congresso Internacional a Cerâmica Medieval no Mediterrâneo, Silves-Mértola, Portugal, 22–27 October 2012; Gonçalves, M.J., Gómez-Martínez, S., Eds.; Câmara Municipal de Silves & Campo Arqueológico de Mértola: Silves/Mértola, Portugal, 2015; pp. 346–352. [Google Scholar]
- Santos, J.R. Conjunto de cerâmica Omíada (sêculos X–XI) do Colégio Dos Meninos do Coro da Sé de Évora. Arqueol. Mediev. 2016, 13, 81–90. [Google Scholar]
- Acién, M.; Castillo, F.; Fernandez, M.I.; Martinez, R.; Peral, C.; Vallego, A. Evolución de los tipos cerámicos en el S.E. de Al-Andalus. In Proceedings of the 5ème Colloque sur la Céramique Médiévale en Méditerranée Occidentale, Rabat, Morocco, 11–17 November 1991; El Hraîki, R., Erbati, E., Eds.; Institut National des Sciences de l’Archéologie et du Patrimoine: Rabat, Morocco, 1995; pp. 125–139. [Google Scholar]
- Gonçalves, M.J. Silves Islâmica. A Muralha do Arravalde Oriental e a Dinâmica de Ocupacão do Espaço Adjacente. Master’s Thesis, University of Algarve, Algarve, Portugal, 2008. Available online: hdl.handle.net/10400.1/267 (accessed on 16 July 2021).
- Reedy, C.L.; Anderson, J.; Reedy, T.J.; Liu, Y. Image analysis in quantitative particle studies of archaeological ceramic thin sections. Adv. Archaeol. Pract. 2014, 2, 252–268. [Google Scholar] [CrossRef][Green Version]
- Maritan, L. Archaeo-ceramic 2.0: Investigating ancient ceramics using modern technological approaches. Archaeol. Anthropol. Sci. 2019, 11, 5085–5093. [Google Scholar] [CrossRef]
- Sitzia, F.; Beltrame, M.; Lisci, C.; Mirão, J. Micro destructive analysis for the characterization of ancient mortars: A case study from the Little Roman Bath of Nora (Sardinia, Italy). Heritage 2021, 4, 144. [Google Scholar] [CrossRef]
- Sitzia, F.; Beltrame, M.; Mirão, J. The particle-size distribution of concrete and mortar aggregates by image analysis. J. Build. Rehabil. 2022, 7, 74. [Google Scholar] [CrossRef]
- Adams, A.E.; MacKenzie, W.S.; Guilford, C. Atlas of Sedimentary Rocks under the Microscope, 1st ed.; Longman Group: London, UK, 1984; ISBN 0-582-02701-2. [Google Scholar]
- Duminuco, P.; Messiga, B.; Riccardi, M.P. Firing process of natural clays. Some microtextures and related phase compositions. Thermochim. Acta 1998, 321, 185–190. [Google Scholar] [CrossRef]
- Riccardi, M.P.; Messiga, B.; Duminuco, P. An approach to the dynamics of clay firing. Appl. Clay Sci. 1999, 15, 393–409. [Google Scholar] [CrossRef]
- Cultrone, G.; Rodriguez-Navarro, C.; Sebastian, E.; Cazalla, O.; De La Torre, M.J. Carbonate and silicate phase reactions during ceramic firing. Eur. J. Mineral. 2001, 13, 621–634. [Google Scholar] [CrossRef]
- El Ouahabi, M.; Daoudi, L.; Hatert, F.; Fagel, N. Modified mineral phases during clay ceramic firing. Clays Clay Miner. 2015, 63, 404–413. [Google Scholar] [CrossRef]
- Heimann, R.B.; Maggetti, M. The struggle between thermodynamics and kinetics: Phase evolution of ancient and historical ceramics. Eur. Mineral. Union Notes Mineral. 2019, 20, 233–281. [Google Scholar] [CrossRef]
- Heiri, O.; Lotter, A.F.; Lemcke, G. Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results. J. Paleolimnol. 2001, 25, 101–110. [Google Scholar] [CrossRef]
- Walton, M.S.; Tite, M.S. Production technology of Roman lead-glazed pottery and its continuance into Late Antiquity. Archaeometry 2010, 52, 733–759. [Google Scholar] [CrossRef]
- Molera, J.; Pradell, T.; Salvadó, N.; Vendrell-Saz, M. Interactions between clay bodies and lead glazes. J. Am. Ceram. Soc. 2001, 84, 1120–1128. [Google Scholar] [CrossRef]
- Nodari, L.; Marcuz, E.; Maritan, L.; Mazzoli, C.; Russo, U. Hematite nucleation and growth in the firing of carbonate-rich clay for pottery production. J. Eur. Ceram. Soc. 2007, 27, 4665–4673. [Google Scholar] [CrossRef]
- Malek, Z.; Balek, V.; Garfinkel-Shweky, D.; Yariv, S. The study of the dehydration and dehydroxylation of smectites by emanation thermal analysis. J. Therm Anal. 1997, 48, 83–92. [Google Scholar] [CrossRef]
- Fabbri, B.; Gualtieri, S.; Shoval, S. The presence of calcite in archaeological ceramics. J. Eur. Ceram. Soc. 2014, 34, 1899–1911. [Google Scholar] [CrossRef]
- Molera, J.; Pradell, T.; Salvadó, N.; Vendrell-Saz, M. Lead frits in Islamic and Hispano-moresque glazed productions. In From Mine to Microscope: Advances in the Study of Ancient Technology, 1st ed.; Shortland, A.J., Freestone, I.C., Rehren, T., Eds.; Oxbow Books: Oxford, UK, 2007; pp. 1–10. ISBN 978-184217-259-9. [Google Scholar]
- Di Febo, R.; Molera, J.; Pradell, T.; Vallcorba, O.; Capelli, C. Technological implications of neo-formed hematite crystals in ceramic lead glazes. Sci. Technol. Archaeol. Res. 2017, 3, 366–375. [Google Scholar] [CrossRef]
- Di Febo, R.; Molera, J.; Pradell, T.; Vallcorba, O.; Melgarejo, J.C.; Capelli, C. Thin-section petrography and SR-µXRD for the identification of micro-crystallites in the brown decorations of ceramic lead glazes. Eur. J. Mineral. 2017, 29, 861–870. [Google Scholar] [CrossRef]
- Galiza, V. Contributo para o Conhecimento da Presença Islâmica em Yābura. Master’s Thesis, Nova University of Lisbon, Lisbon, Portugal, 2012. Available online: hdl.handle.net/10362/8109 (accessed on 2 October 2021).
- Santos, J.R. Um Olhar sobre o Quotidiano de Évora no Período Medieval-Islâmico. Séculos VIII-XI. Master’s Thesis, University of Évora, Évora, Portugal, 2015. Available online: https://hdl.handle.net/10174/18256 (accessed on 27 March 2021).


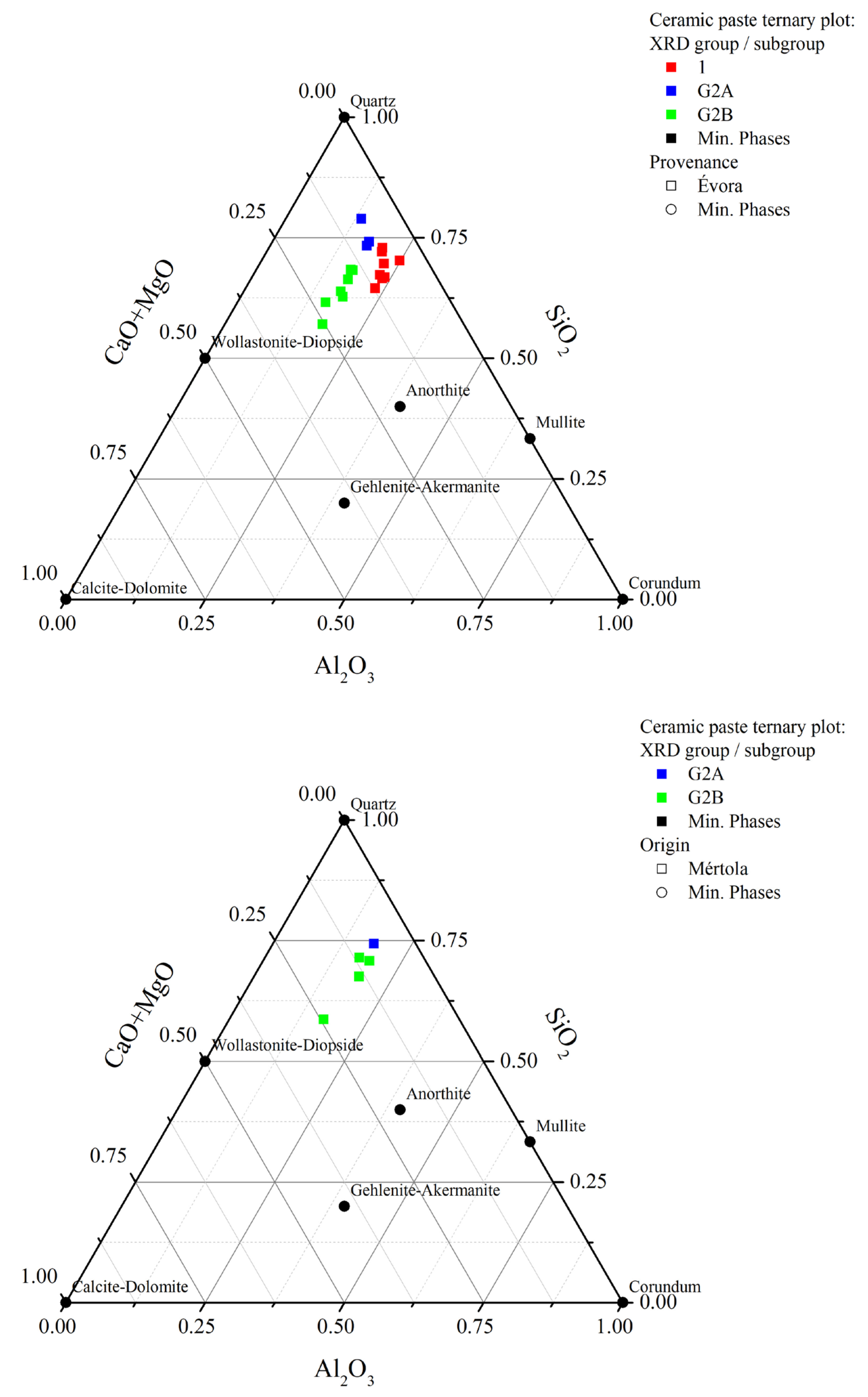
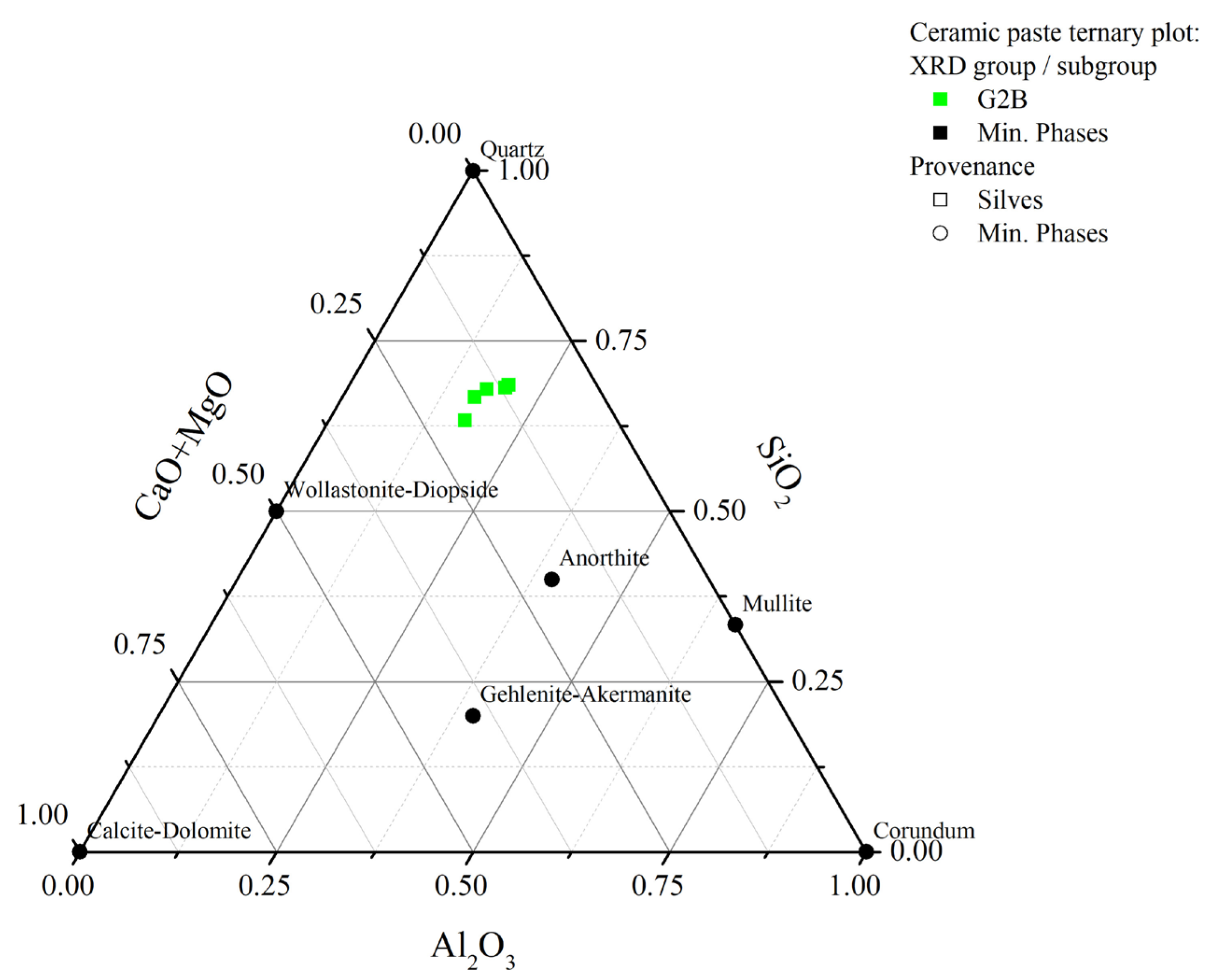
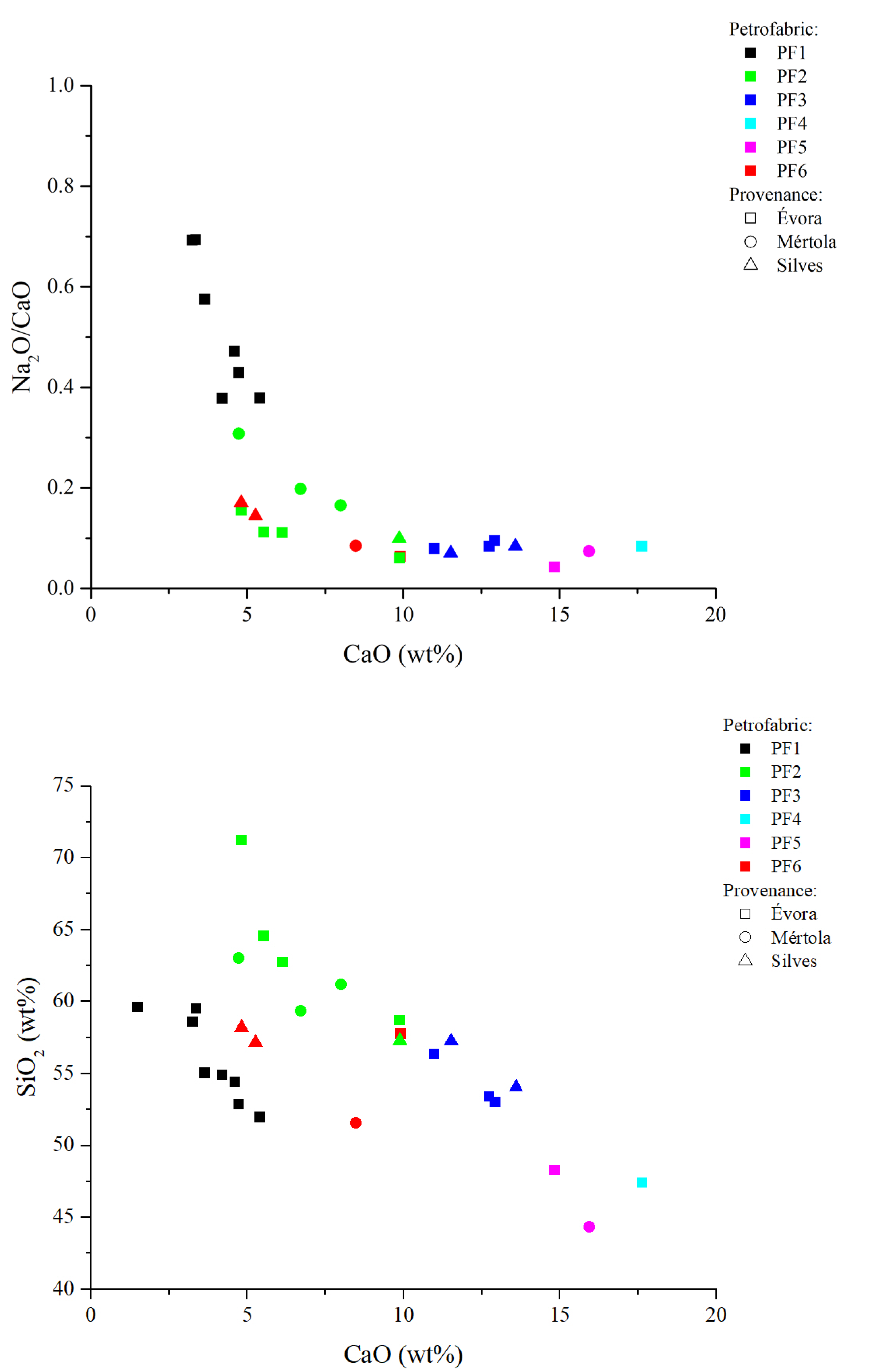
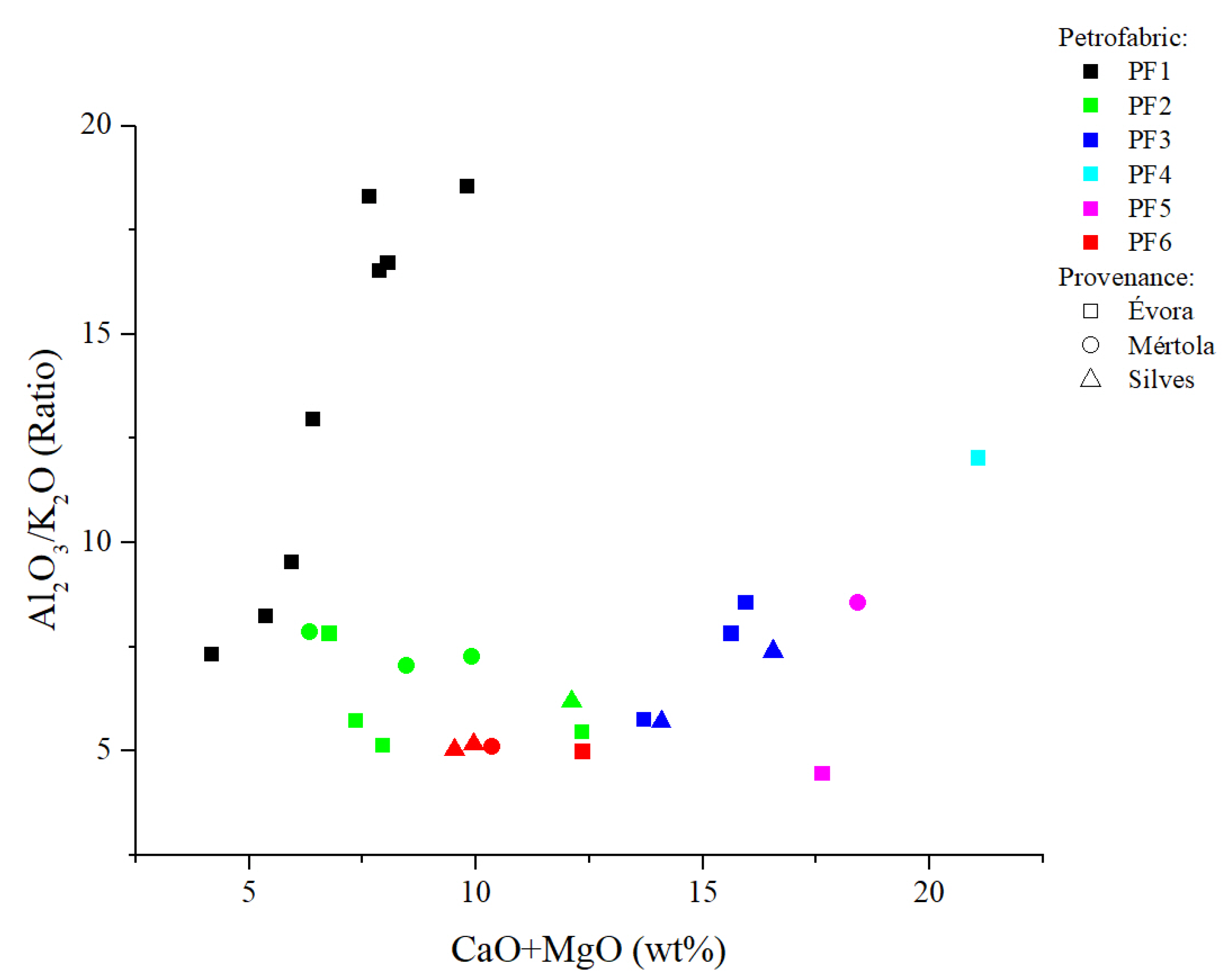



| Sample | Excavation Reference | Glaze Type | Glaze Colour (Inner/Outer) | Glazed Decoration Color | Typology | Function | Chronology | Period | Archaeological Site | Location |
|---|---|---|---|---|---|---|---|---|---|---|
| EVR-1 | CMCS.48/53 | Unglazed | Cookware | Cooking pot | X–XI | Caliphal | Colégio dos Meninos do Coro | Évora | ||
| EVR-2 | EVR.LOIOS.23 | Bichrome | Honey | Black/brown | Tableware | Medium jug | X–XI | Caliphal | Pousada dos Loios | Évora |
| EVR-3 | EVR.LOG.243/XII/90 | Bichrome | Honey | Black/brown | Tableware | Bowl | XI–XII | Taifa | Da Natatio das Termas Romanas | Évora |
| EVR-4 | CMCS.5/44 | Unglazed | Cookware | Cooking pot | X–XI | Caliphal | Colégio dos Meninos do Coro | Évora | ||
| EVR-5 | CMCS.25/6 | Unglazed | Cookware | Cooking pot | X–XI | Caliphal | Colégio dos Meninos do Coro | Évora | ||
| EVR-6 | CMCS.455 | Unglazed | Cookware | Casserole | X–XI | Caliphal | Colégio dos Meninos do Coro | Évora | ||
| EVR-7 | CMCS.830 | Unglazed | Tableware | Small jug | X–XI | Caliphal | Colégio dos Meninos do Coro | Évora | ||
| EVR-8 | CMCS.49 | Unglazed | Tableware | Small jug | X–XI | Caliphal | Colégio dos Meninos do Coro | Évora | ||
| EVR-9 | EVR-GOU.142 | Glaze drop | Green | Kiln tool | Tripod stand | X–XI | Caliphal | Casa de Burgos | Évora | |
| EVR-10 | EVT-92-12 | Unglazed | Cookware | Casserole | XI–XII | Taifa | Roman Temple | Évora | ||
| EVR-11 | PLG.S2.Si8 (2) 1136 | Bichrome | Honey | Black/brown | Tableware | Bowl | XI–XII | Taifa | Paço dos Lobo da Gama | Évora |
| EVR-12 | EVR.LOIOS.149 | Bichrome | Honey | Black/brown | Tableware | Small jug | XI–XII | Taifa | Pousada dos Loios | Évora |
| EVR-13 | PLG.S2.Si8 (2) 1119 | Monochrome | Honey | Tableware | Bowl | XI–XII | Taifa | Paço dos Lobo da Gama | Évora | |
| EVR-14 | EVR3-IV-F-1 | Bichrome | Honey | Black/brown | Tableware | Bowl | X–XI | Caliphal | Casa de Burgos | Évora |
| EVR-15 | PLG.S2.Si2 (1) 785 | Monochrome | Honey | Lighting | Oil lamp | XI–XII | Taifa | Paço dos Lobo da Gama | Évora | |
| EVR-16 | SEM.REF.PLG | Monochrome | Honey | Lighting | Oil lamp | XI–XII | Taifa | Paço dos Lobo da Gama | Évora | |
| EVR-17 | PLG.S2.Si8 (2) 1129 | Bichrome | Honey | Black/brown | Tableware | Bowl | XI–XII | Taifa | Paço dos Lobo da Gama | Évora |
| EVR-18 | PLG.S2.Si8 (2) 1118 | Monochrome | Honey | Tableware | Bowl | XI–XII | Taifa | Paço dos Lobo da Gama | Évora | |
| MER-19 | M (20.110) 24 | Bichrome | Honey | Black/brown | Tableware | Bowl | mid-XII/mid-XIII | Almohad | Encosta do Castelo | Mértola |
| MER-21 | M (20.110) 59 | Monochrome | Honey | Tableware | Bowl | mid-XII/mid-XIII | Almohad | Encosta do Castelo | Mértola | |
| MER-22 | M (20.110) 67 | Monochrome | Honey | Tableware | Bowl | mid-XII/mid-XIII | Almohad | Encosta do Castelo | Mértola | |
| MER-23 | M (20.110) 68 | Monochrome | Honey | Tableware | Small jug | mid-XII/mid-XIII | Almohad | Encosta do Castelo | Mértola | |
| MER-24 | M (20.110) 54 | Monochrome | Honey | Lighting | Oil lamp | mid-XII-mid-XIII | Almohad | Encosta do Castelo | Mértola | |
| SIL-25 | M (20.110) 43 | Bichrome | Honey | Black/brown | Tableware | Tureen | mid-XII/mid-XIII | Almohad | Arrabalde Islâmico | Silves |
| SIL-26 | BIB.03 M7 E10 1020 | Bichrome | Honey | Black/brown | Tableware | Small bowl | mid-XII/mid-XIII | Almohad | Arrabalde Islâmico | Silves |
| SIL-27 | BIB.03 K7 E6 | Bichrome | Honey | Black/brown | Tableware | Small bowl | mid-XII/mid-XIII | Almohad | Arrabalde Islâmico | Silves |
| SIL-28 | BIB.02 J2/E6 66 | Bichrome | Honey | Black/brown | Tableware | Small bowl | mid-XII/mid-XIII | Almohad | Arrabalde Islâmico | Silves |
| SIL-29 | BIB.04 J7 E2 4A 60 | Bichrome | Honey | Black/brown | Tableware | Small bowl | mid-XII/mid-XIII | Almohad | Arrabalde Islâmico | Silves |
| Sample Ref. | PF | XRD-Group | Quartz | Plagioclase Feldspar | K-Rich Feldspar | Pyroxene | Biotite | Illite/Muscovite | Amphibole | Calcite | Hematite | Analcime | Akermanite | Smectite |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EVR-1 | PF1 | G1 | xxx | xxxx | xx | x | x | xx | ||||||
| EVR-2 | PF6 | G2B | xxxx | xx | xx | xx | x | x | x | x | ||||
| EVR-3 | PF2 | G2A | xxxx | xx | xx | x | ||||||||
| EVR-4 | PF1 | G1 | xxx | xx | xx | xx | xxx | xx | x | |||||
| EVR-5 | PF1 | G1 | xxx | xxx | xx | tr | x | xx | xx | tr | x | |||
| EVR-6 | PF1 | G1 | xxxx | xx | xx | x | x | xx | tr | x | ||||
| EVR-7 | PF1 | G1 | xxxx | xxx | xxx | tr | x | xx | xx | x | ||||
| EVR-8 | PF1 | G1 | xxxx | xxx | xx | tr | xx | xx | xxx | x | x | |||
| EVR-9 | PF1 | G1 | xxxx | xx | xx | x | x | x | ||||||
| EVR-10 | PF1 | G1 | xxx | xxx | xxx | x | xx | x | ||||||
| EVR-11 | PF3 | G2B | xxxx | xx | xx | xxx | tr | xx | ||||||
| EVR-12 | PF4 | G2B | xxxx | xx | x | xxxx | xxx | xx | xxx | |||||
| EVR-13 | PF3 | G2B | xxxx | xxx | xx | xxx | tr | x | ||||||
| EVR-14 | PF2 | G2A | xxxx | x | x | x | tr | x | ||||||
| EVR-15 | PF2 | G2A | xxxx | x | x | x | ||||||||
| EVR-16 | PF5 | G2B | xxxx | x | x | xx | xx | xx | ||||||
| EVR-17 | PF3 | G2B | xxxx | xxx | xx | xxx | x | xx | ||||||
| EVR-18 | PF2 | G2B | xxxx | xxx | xx | xx | x | tr | ||||||
| MER-19 | PF6 | G2B | xxxx | x | xx | xx | x | x | xx | |||||
| MER-21 | PF2 | G2B | xxxx | xx | x | x | x | x | x | |||||
| MER-22 | PF2 | G2B | xxxx | xxx | xx | x | tr | x | tr | |||||
| MER-23 | PF2 | G2A | xxxx | xxx | x | x | ||||||||
| MER-24 | PF5 | G2B | xxxx | x | xx | xxx | x | x | xx | xxx | ||||
| SIL-25 | PF3 | G2B | xxxx | xx | x | x | x | x | x | |||||
| SIL-26 | PF3 | G2B | xxxx | xx | xx | xxx | xx | x | xx | |||||
| SIL-27 | PF2 | G2B | xxxx | xxx | xx | xx | x | x | ||||||
| SIL-28 | PF6 | G2B | xxxx | x | x | x | x | x | x | |||||
| SIL-29 | PF6 | G2B | xxxx | xx | xx | x | x | x | x | x |
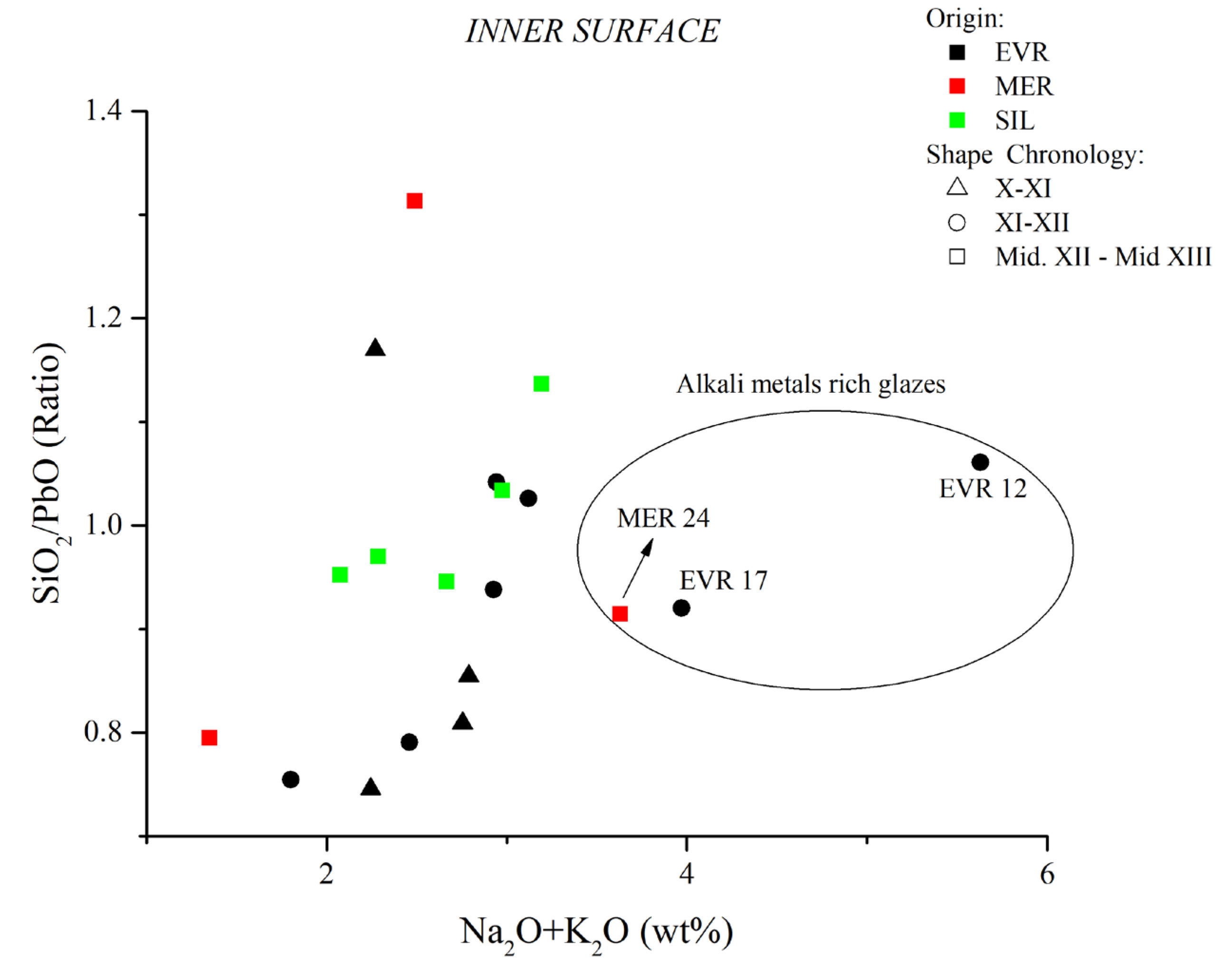
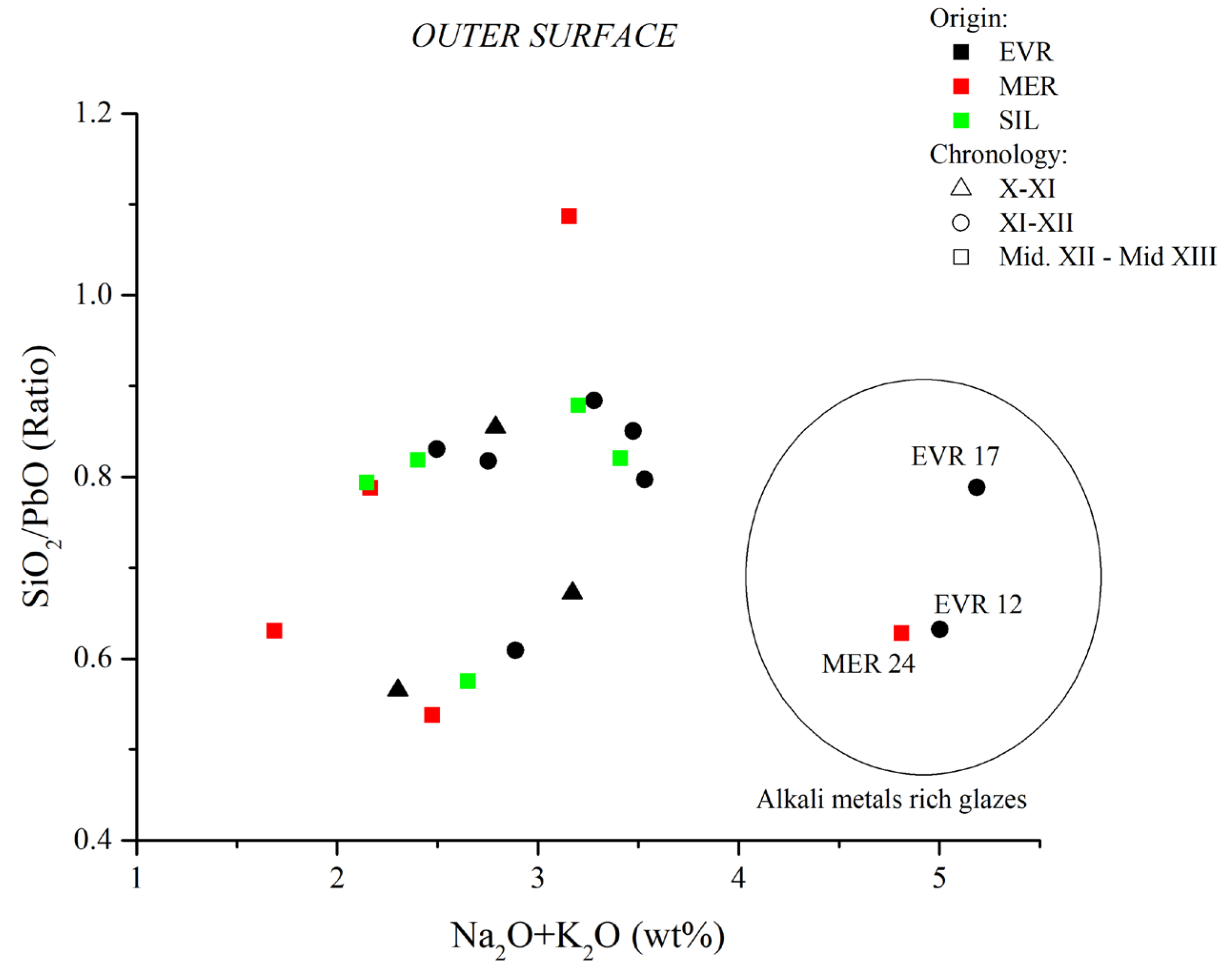
| Sample Ref. | PF | Glaze Type | Chronology | Inner | Outer | Inner | Outer | Inner | Outer | Inner | Outer |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PbO | SiO2 | SiO2/PbO | Na2O+K2O | ||||||||
| EVR-2 | 6 | Bichrome | X–XI | 48.36 | 54.40 | 36.07 | 30.77 | 0.75 | 0.57 | 2.24 | 2.30 |
| EVR-3 | 2 | Bichrome | XI–XII | 36.49 | 44.05 | 42.68 | 36.59 | 1.17 | 0.83 | 2.27 | 2.50 |
| EVR-9 | 1 | Glaze drops | X–XI | 42.66 | 36.46 | 0.85 | 2.79 | ||||
| EVR-11 | 3 | Bichrome | XI–XII | 37.90 | 40.88 | 38.88 | 34.77 | 1.03 | 0.85 | 3.12 | 3.47 |
| EVR-12 | 4 | Bichrome | XI–XII | 37.64 | 49.67 | 39.92 | 31.40 | 1.06 | 0.63 | 5.63 | 5.00 |
| EVR-13 | 3 | Monochrome | XI–XII | 41.03 | 43.20 | 38.49 | 34.44 | 0.94 | 0.8 | 2.93 | 3.53 |
| EVR-14 | 2 | Bichrome | X–XI | 46.03 | 48.22 | 37.25 | 32.42 | 0.81 | 0.67 | 2.75 | 3.17 |
| EVR-15 | 2 | Monochrome | XI–XII | 47.64 | 44.34 | 35.95 | 36.25 | 0.75 | 0.82 | 1.80 | 2.75 |
| EVR-16 | 5 | Monochrome | XI–XII | 45.60 | 50.55 | 36.05 | 30.78 | 0.79 | 0.61 | 2.46 | 2.89 |
| EVR-17 | 3 | Bichrome | XI–XII | 40.78 | 43.56 | 37.52 | 34.35 | 0.92 | 0.79 | 3.97 | 5.19 |
| EVR-18 | 2 | Monochrome | XI–XII | 38.17 | 41.22 | 39.77 | 36.43 | 1.04 | 0.88 | 2.94 | 3.28 |
| MER-19 | 6 | Bichrome | mid-XII/mid-XIII | 53.88 | 28.97 | 0.54 | 2.47 | ||||
| MER-21 | 2 | Monochrome | mid-XII/mid-XIII | 47.30 | 52.77 | 37.59 | 33.27 | 0.79 | 0.63 | 1.35 | 1.69 |
| MER-22 | 2 | Monochrome | mid-XII/mid-XIII | 33.79 | 35.70 | 44.36 | 38.79 | 1.31 | 1.09 | 2.49 | 3.16 |
| MER-23 | 2 | Monochrome | mid-XII/mid-XIII | 45.21 | 35.61 | 0.79 | 2.17 | ||||
| MER-24 | 5 | Monochrome | mid-XII-mid-XIII | 39.97 | 49.02 | 36.55 | 30.79 | 0.91 | 0.63 | 3.63 | 4.81 |
| SIL-25 | 3 | Bichrome | mid-XII/mid-XIII | 40.86 | 40.79 | 38.65 | 35.85 | 0.95 | 0.88 | 2.67 | 3.20 |
| SIL-26 | 3 | Bichrome | mid-XII/mid-XIII | 35.13 | 41.52 | 39.94 | 34.07 | 1.14 | 0.82 | 3.19 | 3.41 |
| SIL-27 | 2 | Bichrome | mid-XII/mid-XIII | 38.93 | 52.64 | 40.25 | 30.26 | 1.03 | 0.57 | 2.97 | 2.65 |
| SIL-28 | 6 | Bichrome | mid-XII/mid-XIII | 42.05 | 45.10 | 40.03 | 35.79 | 0.95 | 0.79 | 2.08 | 2.15 |
| SIL-29 | 6 | Bichrome | mid-XII/mid-XIII | 41.49 | 43.55 | 40.24 | 35.65 | 0.97 | 0.82 | 2.29 | 2.40 |
| Oxides Wt%—Black/Brown Decoration—Spot Analyses | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | Spot | Na2O | MgO | Al2O3 | SiO2 | P2O5 | K2O | CaO | TiO2 | MnO | FeO | BaO | PbO |
| EVR-2 | 1 | 0.52 | 0.71 | 4.75 | 20.63 | 1.01 | 3.22 | 0.82 | 56.21 | 0.44 | 11.69 | ||
| 2 | 0.41 | 0.36 | 2.55 | 16.04 | 0.23 | 1.17 | 1.04 | 68.31 | 1.43 | 8.45 | |||
| 3 | 0.20 | 0.34 | 2.72 | 14.40 | 0.41 | 2.03 | 1.27 | 67.96 | 1.20 | 9.46 | |||
| EVR-11 | 1 | 0.53 | 0.55 | 2.90 | 11.86 | 0.43 | 1.73 | 1.61 | 68.87 | 11.52 | |||
| SIL-25 | 1 | 0.71 | 4.90 | 5.64 | 42.47 | 0.33 | 1.57 | 19.86 | 1.71 | 11.88 | 1.27 | 9.66 | |
| 2 | 0.43 | 0.12 | 2.76 | 6.76 | 0.14 | 0.33 | 0.91 | 0.02 | 1.98 | 80.02 | 1.59 | 4.93 | |
| SIL-26 | 1 | 0.70 | 3.70 | 3.08 | 12.77 | 0.82 | 2.53 | 1.04 | 15.63 | 44.68 | 15.06 | ||
| 2 | 0.00 | 0.43 | 2.08 | 6.62 | 0.31 | 1.28 | 0.81 | 3.54 | 80.04 | 4.88 | |||
| 3 | 0.73 | 0.55 | 3.58 | 13.00 | 0.53 | 2.53 | 1.60 | 5.01 | 47.80 | 24.67 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camara, C.A.; Gonçalves, M.J.; Mirão, J.A.P.; Martínez, S.G.; Beltrame, M. High-Lead Glazed Ceramic Production in Western Iberia (Gharb al-Andalus) between the 10th and Mid-13th Centuries: An Approach from the City of Évora (Portugal). Ceramics 2023, 6, 2213-2242. https://doi.org/10.3390/ceramics6040135
Camara CA, Gonçalves MJ, Mirão JAP, Martínez SG, Beltrame M. High-Lead Glazed Ceramic Production in Western Iberia (Gharb al-Andalus) between the 10th and Mid-13th Centuries: An Approach from the City of Évora (Portugal). Ceramics. 2023; 6(4):2213-2242. https://doi.org/10.3390/ceramics6040135
Chicago/Turabian StyleCamara, Carlos Andrés, María José Gonçalves, José Antonio Paulo Mirão, Susana Gómez Martínez, and Massimo Beltrame. 2023. "High-Lead Glazed Ceramic Production in Western Iberia (Gharb al-Andalus) between the 10th and Mid-13th Centuries: An Approach from the City of Évora (Portugal)" Ceramics 6, no. 4: 2213-2242. https://doi.org/10.3390/ceramics6040135
APA StyleCamara, C. A., Gonçalves, M. J., Mirão, J. A. P., Martínez, S. G., & Beltrame, M. (2023). High-Lead Glazed Ceramic Production in Western Iberia (Gharb al-Andalus) between the 10th and Mid-13th Centuries: An Approach from the City of Évora (Portugal). Ceramics, 6(4), 2213-2242. https://doi.org/10.3390/ceramics6040135






