Preparation and Characterization of Freeze-Dried β-Tricalcium Phosphate/Barium Titanate/Collagen Composite Scaffolds for Bone Tissue Engineering in Orthopedic Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of β-TCP Powder
2.2. Fabrication of Composite Collagen Scaffolds
2.3. Characterization
2.4. Mechanical Properties
2.5. Porosity Measurement
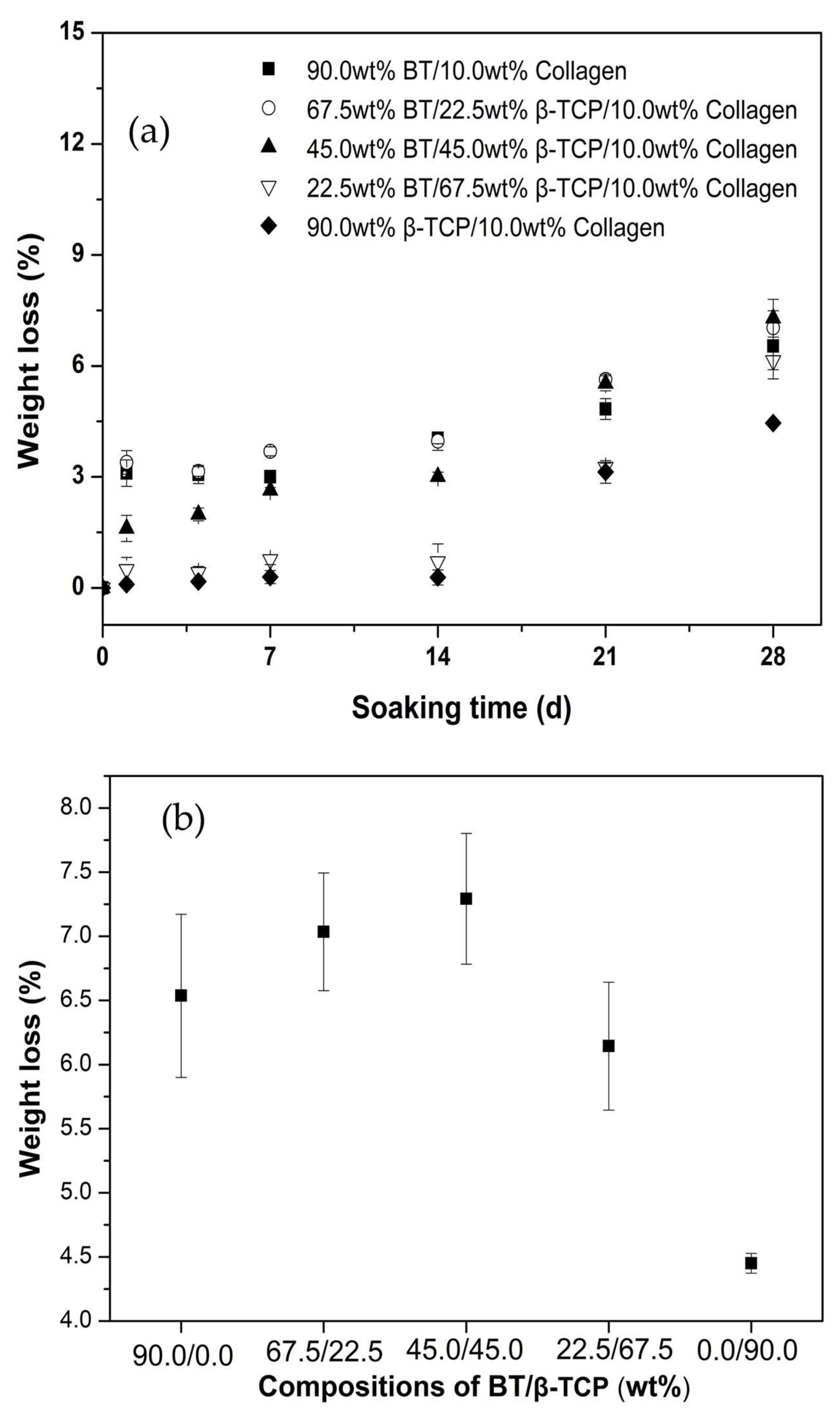
2.6. In Vitro Biodegradation
2.7. In Vitro Cytotoxicity
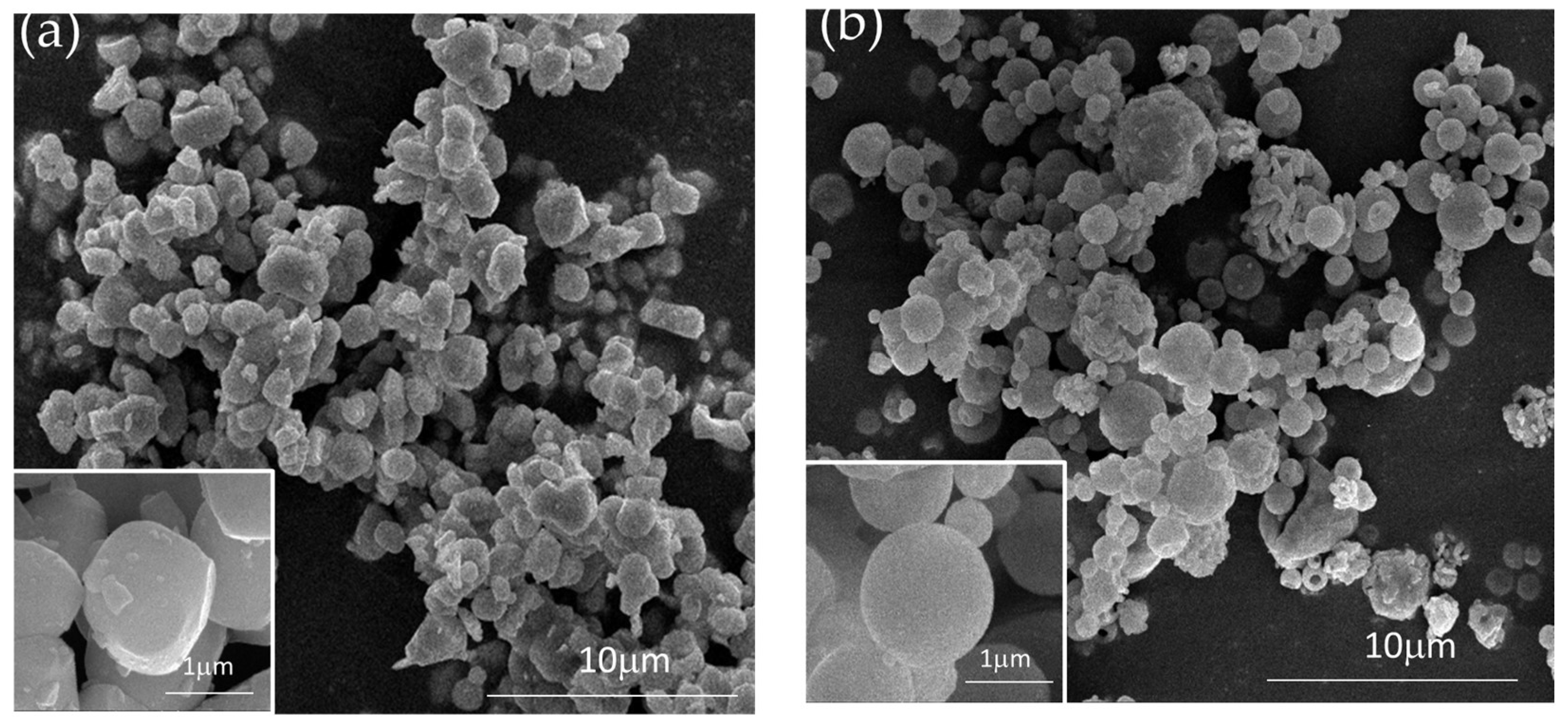
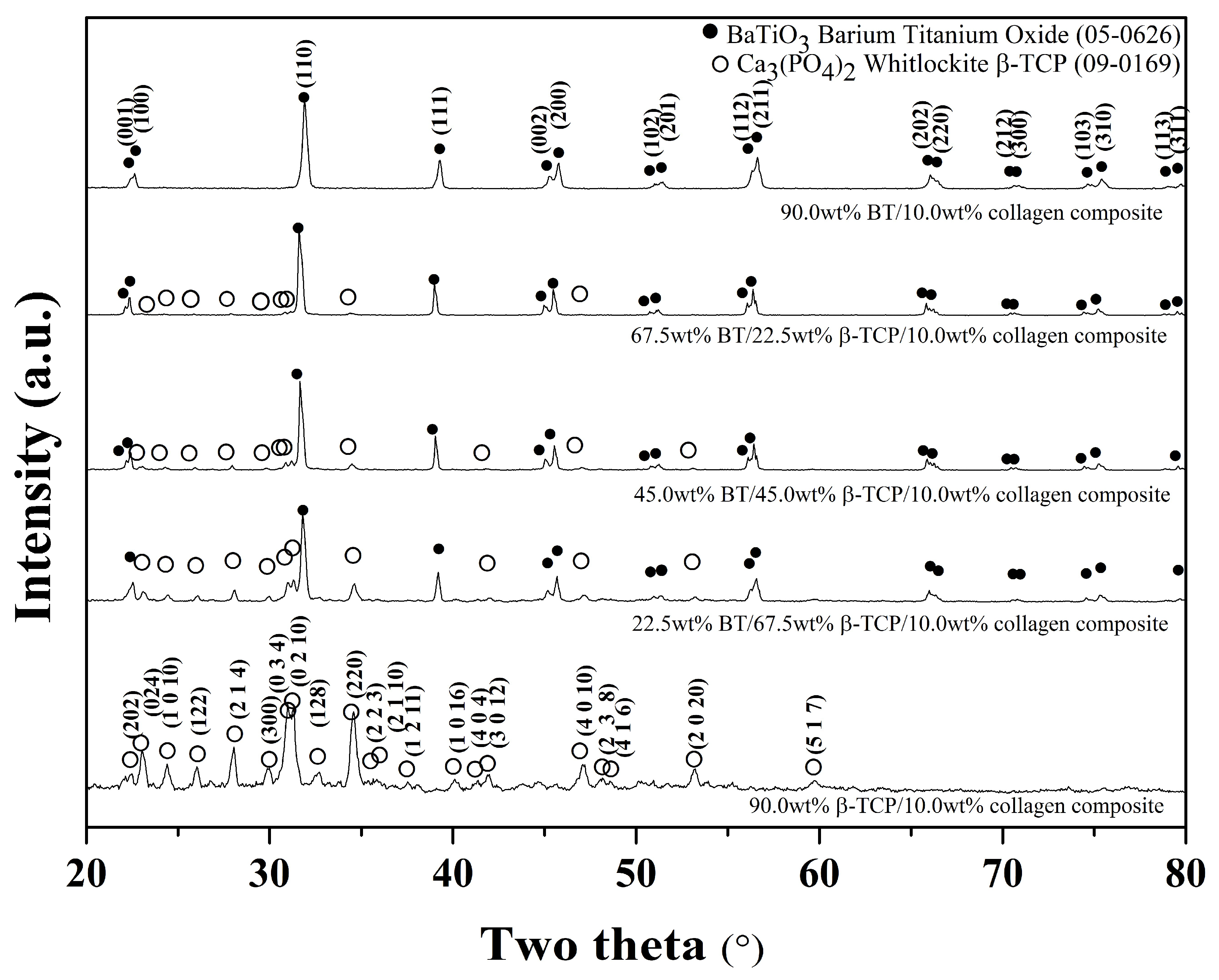
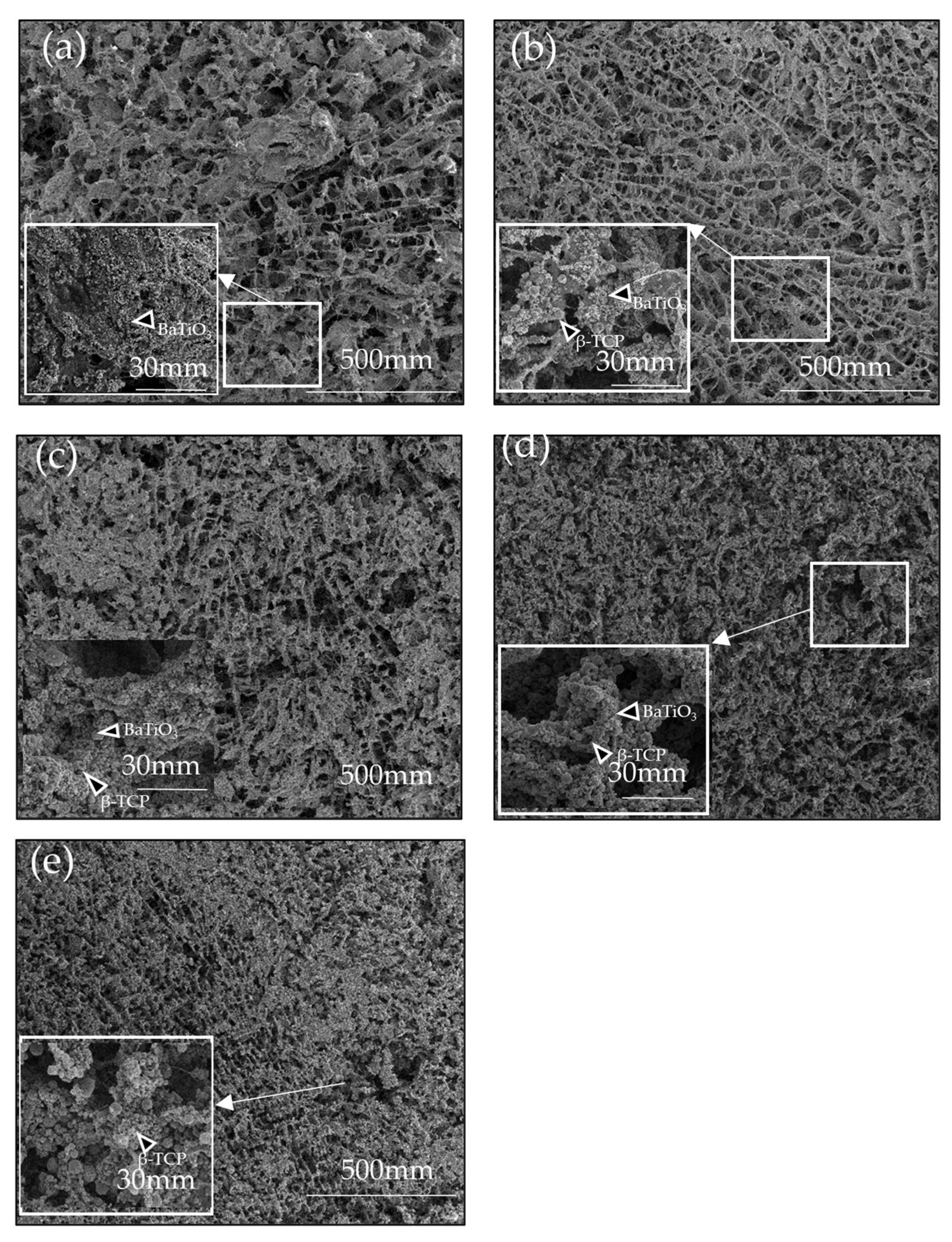
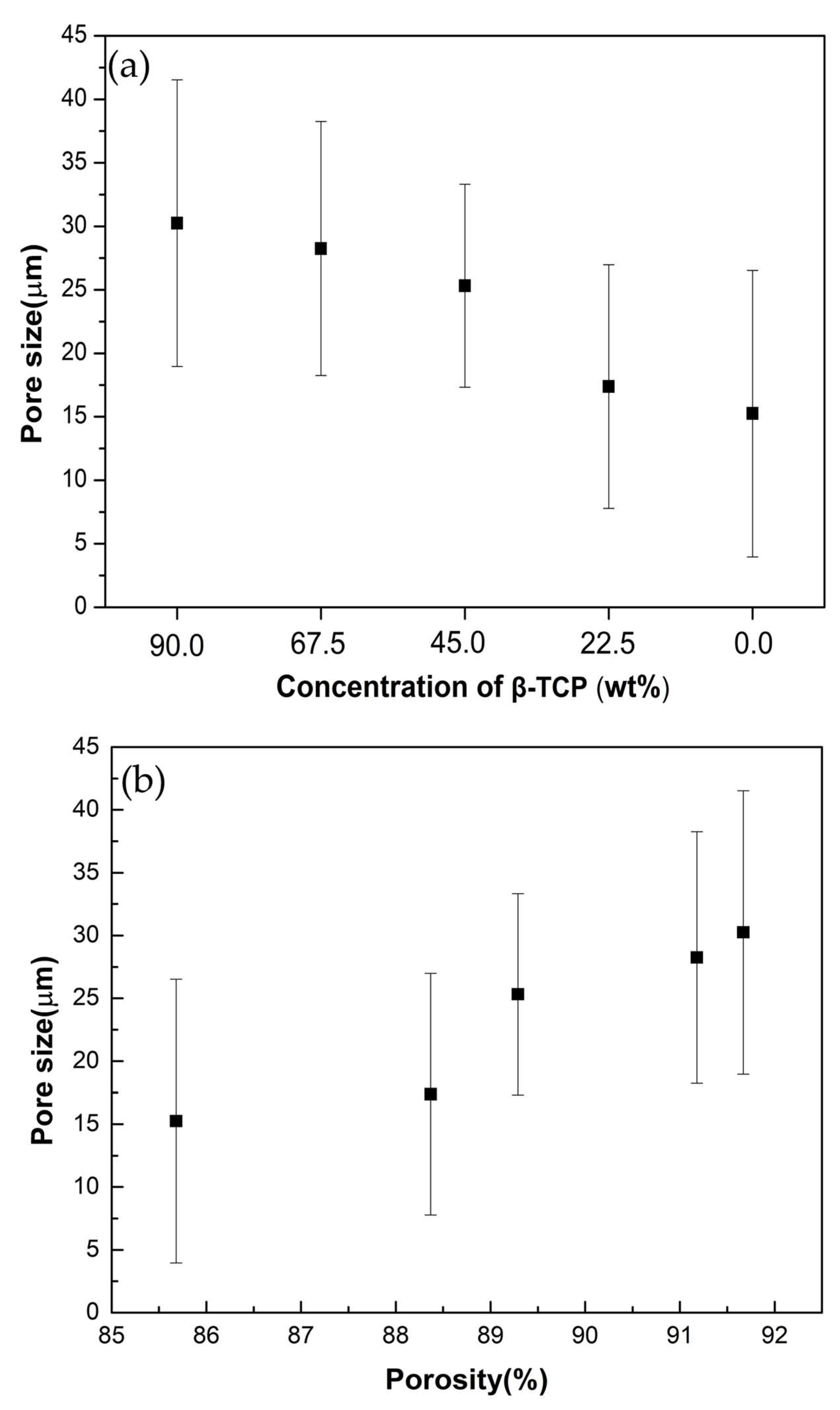
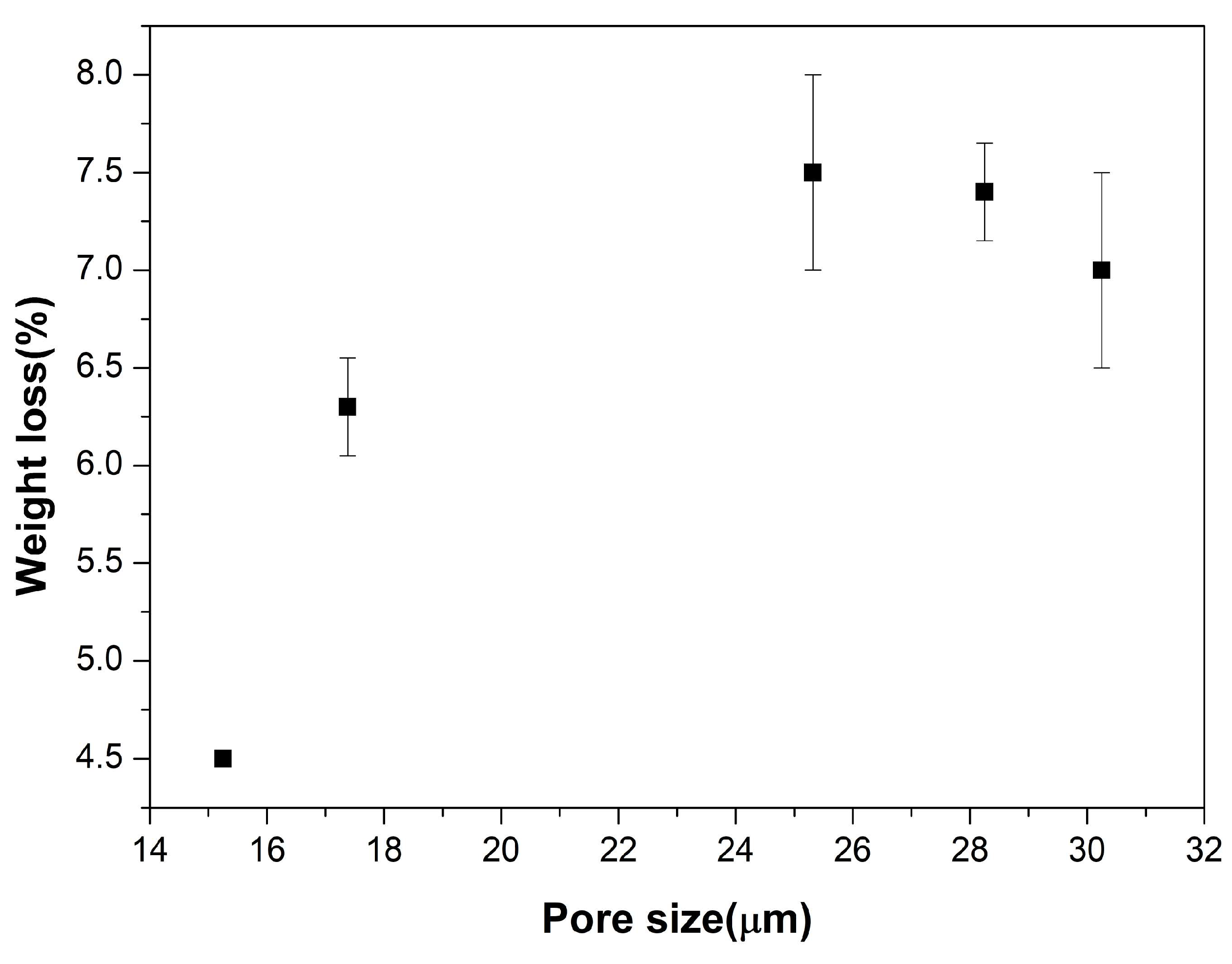
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boyce, S.T.; Lalley, A.L. Tissue engineering of skin and regenerative medicine for wound care. Burn. Trauma 2018, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Visser, R.; Rico-Llanos, G.A.; Pulkkinen, H.; Becerra, J. Peptides for bone tissue engineering. J. Control. Release 2016, 244, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Yamamoto, K.; Ikeda, T.; Yanagiguchi, K.; Hayashi, Y. Potency of fish collagen as a scaffold for regenerative medicine. BioMed Res. Int. 2014, 2014, 302932. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.; Graves, S.; Wong, A.; Triffitt, J.; Francis, M.; Czernuszka, J. Investigation into the formation and mechanical properties of a bioactive material based on collagen and calcium phosphate. J. Mater. Sci. Mater. Med. 1993, 4, 107–110. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of collagen scaffold in tissue engineering: Recent advances and new perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef]
- Aichelmann-Reidy, M.E.; Yukna, R.A. Bone replacement grafts: The bone substitutes. Dent. Clin. N. Am. 1998, 42, 491–503. [Google Scholar] [CrossRef]
- Bissada, N.F.; Hangorsky, U. Alveolar bone induction: Alloplasts. Dent. Clin. N. Am. 1980, 24, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Fukada, E.; Yasuda, I. On the piezoelectric effect of bone. J. Phys. Soc. Jpn. 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Liang, D.; Hsiao, B.S.; Chu, B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Deliv. Rev. 2007, 59, 1392–1412. [Google Scholar] [CrossRef]
- Keshaw, H.; Thapar, N.; Burns, A.J.; Mordan, N.; Knowles, J.C.; Forbes, A.; Day, R.M. Microporous collagen spheres produced via thermally induced phase separation for tissue regeneration. Acta Biomater. 2010, 6, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Lee, K.; Wang, X.; Yoshitomi, T.; Kawazoe, N.; Yang, Y.; Chen, G. Interconnected collagen porous scaffolds prepared with sacrificial PLGA sponge templates for cartilage tissue engineering. J. Mater. Chem. B 2021, 9, 8491–8500. [Google Scholar] [CrossRef]
- Animut, T.Y.; Ningsih, H.S.; Shih, H.-H.; Wu, M.-H.; Shih, S.-J. Effect of Calcium Silicate and β-Tricalcium Phosphate Reinforcement on the Mechanical–Biological Properties of Freeze-Dried Collagen Composite Scaffolds for Bone Tissue Engineering Applications. Ceramics 2023, 6, 548–560. [Google Scholar] [CrossRef]
- Mabrouk, M.; Beherei, H.H.; Das, D.B. Recent progress in the fabrication techniques of 3D scaffolds for tissue engineering. Mater. Sci. Eng. C 2020, 110, 110716. [Google Scholar] [CrossRef]
- Finoli, A.; Ostrowski, N.; Schmelzer, E.; Nettleship, I.; Gerlach, J. Multiscale porous ceramic scaffolds for in vitro culturing of primary human cells. Adv. Appl. Ceram. 2012, 111, 262–268. [Google Scholar] [CrossRef]
- Wallin, R.F.; Arscott, E. A practical guide to ISO 10993-5: Cytotoxicity. Med. Device Diagn. Ind. 1998, 20, 96–98. [Google Scholar]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. Simulated body fluid (SBF) as a standard tool to test the bioactivity of implants. In Handbook of Biomineralization: Biological Aspects and Structure Formation; Wiley: Hoboken, NJ, USA, 2007; pp. 97–109. [Google Scholar]
- Liapis, A.I.; Bruttini, R. Freeze drying. In Handbook of Industrial Drying; CRC Press: Boca Raton, FL, USA, 2020; pp. 309–343. [Google Scholar]
- Spaniol, K.; Caldas, S.; Peres, A.; Dos Santos, E.; Acchar, W. β-TCP/PVA sheets crosslinked with citric acid produced via aqueous tape casting for bone regeneration. Ceram. Int. 2019, 45, 12417–12422. [Google Scholar] [CrossRef]
- Vázquez Lasa, B.; Pau Ginebra, M.; Gil, X.; Antón Planell, J.; San Román, J. Acrylic bone cements modified with b-TCP particles encapsulated with poly (ethylene glycol). Biomaterials 2005, 26, 4309–4316. [Google Scholar] [CrossRef]
- Perez, R.A.; Mestres, G. Role of pore size and morphology in musculo-skeletal tissue regeneration. Mater. Sci. Eng. C 2016, 61, 922–939. [Google Scholar] [CrossRef]
- Goodarzi, H.; Hashemi-Najafabadi, S.; Baheiraei, N.; Bagheri, F. Preparation and characterization of nanocomposite scaffolds (collagen/β-TCP/SrO) for bone tissue engineering. Tissue Eng. Regen. Med. 2019, 16, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Al Qaysi, M.; Owji, N.; Bayazit, M.; Xie, J.; Knowles, J.; Tang, J. Advanced biocomposites of poly (glycerol sebacate) and β-tricalcium phosphate by in situ microwave synthesis for bioapplication. Mater. Today Adv. 2020, 5, 100023. [Google Scholar] [CrossRef]
- Busuioc, C.; Voicu, G.; Jinga, S.-I.; Mitran, V.; Cimpean, A. The influence of barium titanate on the biological properties of collagen-hydroxiapatite composite scaffolds. Mater. Lett. 2019, 253, 317–322. [Google Scholar] [CrossRef]
- Lin, F.; Wang, X.; Wang, Y.; Yang, Y.; Li, Y. Preparation and biocompatibility of electrospinning PDLLA/β-TCP/collagen for peripheral nerve regeneration. RSC Adv. 2017, 7, 41593–41602. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putra, D.F.A.; Aji, B.B.; Ningsih, H.S.; Wu, T.-W.; Nakanishi, A.; Moriga, T.; Shih, S.-J. Preparation and Characterization of Freeze-Dried β-Tricalcium Phosphate/Barium Titanate/Collagen Composite Scaffolds for Bone Tissue Engineering in Orthopedic Applications. Ceramics 2023, 6, 2148-2161. https://doi.org/10.3390/ceramics6040132
Putra DFA, Aji BB, Ningsih HS, Wu T-W, Nakanishi A, Moriga T, Shih S-J. Preparation and Characterization of Freeze-Dried β-Tricalcium Phosphate/Barium Titanate/Collagen Composite Scaffolds for Bone Tissue Engineering in Orthopedic Applications. Ceramics. 2023; 6(4):2148-2161. https://doi.org/10.3390/ceramics6040132
Chicago/Turabian StylePutra, Dwi Fortuna Anjusa, Bramantyo Bayu Aji, Henni Setia Ningsih, Ting-Wei Wu, Akihiro Nakanishi, Toshihiro Moriga, and Shao-Ju Shih. 2023. "Preparation and Characterization of Freeze-Dried β-Tricalcium Phosphate/Barium Titanate/Collagen Composite Scaffolds for Bone Tissue Engineering in Orthopedic Applications" Ceramics 6, no. 4: 2148-2161. https://doi.org/10.3390/ceramics6040132
APA StylePutra, D. F. A., Aji, B. B., Ningsih, H. S., Wu, T.-W., Nakanishi, A., Moriga, T., & Shih, S.-J. (2023). Preparation and Characterization of Freeze-Dried β-Tricalcium Phosphate/Barium Titanate/Collagen Composite Scaffolds for Bone Tissue Engineering in Orthopedic Applications. Ceramics, 6(4), 2148-2161. https://doi.org/10.3390/ceramics6040132





