Formation and Photophysical Properties of Silver Clusters in Bulk of Photo-Thermo-Refractive Glass
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuznetsov, A.S.; Tikhomirov, V.K.; Shestakov, M.V.; Moshchalkov, V.V. Ag Nanocluster Functionalized Glasses for Efficient Photonic Conversion in Light Sources, Solar Cells and Flexible Screen Monitors. Nanoscale 2013, 5, 10065–10075. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Jiang, P.; Xu, B.; Qiu, J. Single-Pulse-Induced Ultrafast Spatial Clustering of Metal in Glass: Fine Tunability and Application. Adv. Photonics Res. 2021, 2, 2000121. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, H.; Li, R.; Lin, S.; Wu, C.; Huang, Q.; Xu, J.; Cheng, Y.; Wang, Y. Laser-Direct-Writing of Molecule-like Agmx+ nanoclusters in Transparent Tellurite Glass for 3D Volumetric Optical Storage. Nanoscale 2021, 13, 19663–19670. [Google Scholar] [CrossRef] [PubMed]
- De Cremer, G.; Sels, B.F.; Hotta, J.I.; Roeffaers, M.B.J.; Bartholomeeusen, E.; Coutiño-Gonzalez, E.; Valtchev, V.; De Vos, D.E.; Vosch, T.; Hofkens, J. Optical Encoding of Silver Zeolite Microcarriers. Adv. Mater. 2010, 22, 957–960. [Google Scholar] [CrossRef]
- Dong, X.Y.; Si, Y.; Yang, J.S.; Zhang, C.; Han, Z.; Luo, P.; Wang, Z.Y.; Zang, S.Q.; Mak, T.C.W. Ligand Engineering to Achieve Enhanced Ratiometric Oxygen Sensing in a Silver Cluster-Based Metal-Organic Framework. Nat. Commun. 2020, 11, 3678. [Google Scholar] [CrossRef]
- Qian, S.; Wang, Z.; Zuo, Z.; Wang, X.; Wang, Q.; Yuan, X. Engineering Luminescent Metal Nanoclusters for Sensing Applications. Coord. Chem. Rev. 2022, 451, 214268. [Google Scholar] [CrossRef]
- Zheng, W.; Li, P.; Wang, C.; Qiao, X.; Qian, G.; Fan, X. Tuning Ag Quantum Clusters in Glass as an Efficient Spectral Converter: From Fundamental to Applicable. J. Non. Cryst. Solids 2023, 599, 121910. [Google Scholar] [CrossRef]
- Fares, H.; Santos, S.N.C.; Santos, M.V.; Franco, D.F.; Souza, A.E.; Manzani, D.; Mendonça, C.R.; Nalin, M. Highly Luminescent Silver Nanocluster-Doped Fluorophosphate Glasses for Microfabrication of 3D Waveguides. RSC Adv. 2017, 7, 55935–55944. [Google Scholar] [CrossRef]
- Aslani, M.; Talebi, R.; Vashaee, D. Coupling Light in Ion-Exchanged Waveguides by Silver Nanoparticle-Based Nanogratings: Manipulating the Refractive Index of Waveguides. ACS Appl. Nano Mater. 2022, 5, 5439–5447. [Google Scholar] [CrossRef]
- de Castro, T.; Fares, H.; Khalil, A.A.; Laberdesque, R.; Petit, Y.; Strutinski, C.; Danto, S.; Jubera, V.; Ribeiro, S.J.L.; Nalin, M.; et al. Femtosecond Laser Micro-Patterning of Optical Properties and Functionalities in Novel Photosensitive Silver-Containing Fluorophosphate Glasses. J. Non. Cryst. Solids 2019, 517, 51–56. [Google Scholar] [CrossRef]
- Sholom, S.; McKeever, S.W.S. Silver Molecular Clusters and the Properties of Radiophotoluminescence of Alkali-Phosphate Glasses at High Dose. Radiat. Meas. 2023, 163, 106924. [Google Scholar] [CrossRef]
- McKeever, S.W.S.; Sholom, S.; Shrestha, N.; Klein, D.M. Build-up of Radiophotoluminescence (RPL) in Ag-Doped Phosphate Glass in Real-Time Both during and after Exposure to Ionizing Radiation: A Proposed Model. Radiat. Meas. 2020, 132, 106246. [Google Scholar] [CrossRef]
- Han, Z.; Dong, X.Y.; Luo, P.; Li, S.; Wang, Z.Y.; Zang, S.Q.; Mak, T.C.W. Ultrastable Atomically Precise Chiral Silver Clusters with More than 95% Quantum Efficiency. Sci. Adv. 2020, 6, eaay0107. [Google Scholar] [CrossRef]
- Cho, J.; Park, J.H.; Kim, J.K.; Schubert, E.F. White Light-Emitting Diodes: History, Progress, and Future. Laser Photonics Rev. 2017, 11, 1600147. [Google Scholar] [CrossRef]
- Grandjean, D.; Coutiño-Gonzalez, E.; Cuong, N.T.; Fron, E.; Baekelant, W.; Aghakhani, S.; Schlexer, P.; D’Acapito, F.; Banerjee, D.; Roeffaers, M.B.J.; et al. Origin of the Bright Photoluminescence of Few-Atom Silver Clusters Confined in LTA Zeolites. Science 2018, 361, 686–690. [Google Scholar] [CrossRef]
- Coutino-Gonzalez, E.; Baekelant, W.; Grandjean, D.; Roeffaers, M.B.J.; Fron, E.; Aghakhani, M.S.; Bovet, N.; Van Der Auweraer, M.; Lievens, P.; Vosch, T.; et al. Thermally Activated LTA(Li)-Ag Zeolites with Water-Responsive Photoluminescence Properties. J. Mater. Chem. C 2015, 3, 11857–11867. [Google Scholar] [CrossRef]
- Baekelant, W.; Aghakhani, S.; Fron, E.; Martin, C.; Woong-Kim, C.; Steele, J.A.; De Baerdemaeker, T.; D’Acapito, F.; Chernysov, D.; Van Der Auweraer, M.; et al. Luminescent Silver-Lithium-Zeolite Phosphors for near-Ultraviolet LED Applications. J. Mater. Chem. C 2019, 7, 14366–14374. [Google Scholar] [CrossRef]
- Fenwick, O.; Coutiño-Gonzalez, E.; Grandjean, D.; Baekelant, W.; Richard, F.; Bonacchi, S.; De Vos, D.; Lievens, P.; Roeffaers, M.; Hofkens, J.; et al. Tuning the Energetics and Tailoring the Optical Properties of Silver Clusters Confined in Zeolites. Nat. Mater. 2016, 15, 1017–1022. [Google Scholar] [CrossRef]
- Coutino-Gonzalez, E.; Roeffaers, M.B.J.; Dieu, B.; De Cremer, G.; Leyre, S.; Hanselaer, P.; Fyen, W.; Sels, B.; Hofkens, J. Determination and Optimization of the Luminescence External Quantum Efficiency of Silver-Clusters Zeolite Composites. J. Phys. Chem. C 2013, 117, 6998–7004. [Google Scholar] [CrossRef]
- Kuznetsov, A.S.; Tikhomirov, V.K.; Moshchalkov, V.V. UV-Driven Efficient White Light Generation by Ag Nanoclusters Dispersed in Glass Host. Mater. Lett. 2013, 92, 4–6. [Google Scholar] [CrossRef]
- Hu, T.; Zheng, W.; Liu, Z.; Jia, J.; Xu, X.; Xu, Q.; Qiao, X.; Fan, X. Strategies to Host Silver Quantum Clusters in Borosilicate Glass: How to Mutually Fulfill PL Efficiency and Chemical Stability? J. Non-Crystalline Solids X 2022, 16, 100132. [Google Scholar] [CrossRef]
- Sgibnev, Y.M.; Nikonorov, N.V.; Ignatiev, A.I. High Efficient Luminescence of Silver Clusters in Ion-Exchanged Antimony-Doped Photo-Thermo-Refractive Glasses: Influence of Antimony Content and Heat Treatment Parameters. J. Lumin. 2017, 188, 172–179. [Google Scholar] [CrossRef]
- Dubrovin, V.D.; Ignatiev, A.I.; Nikonorov, N.V.; Sidorov, A.I.; Shakhverdov, T.A.; Agafonova, D.S. Luminescence of Silver Molecular Clusters in Photo-Thermo-Refractive Glasses. Opt. Mater. 2014, 36, 753–759. [Google Scholar] [CrossRef]
- Mironov, L.Y.; Marasanov, D.V.; Ulshina, M.D.; Sgibnev, Y.M.; Kolesnikov, I.E.; Nikonorov, N.V. The Role of Thermally Activated Quenching and Energy Migration in Luminescence of Silver Clusters in Glasses. J. Phys. Chem. C 2022, 126, 13863–13869. [Google Scholar] [CrossRef]
- West, B.R. Ion-Exchanged Glass Waveguide Technology: A Review. Opt. Eng. 2011, 50, 071107. [Google Scholar] [CrossRef]
- Stepanov, A.L.; Hole, D.E.; Townsend, P.D. Formation of Silver Nanoparticles in Soda-Lime Silicate Glass by Ion Implantation near Room Temperature. J. Non. Cryst. Solids 1999, 260, 65–74. [Google Scholar] [CrossRef]
- Arnold, G.W.; Borders, J.A. Aggregation and Migration of Ion-implanted Silver in Lithia-alumina-silica Glass. J. Appl. Phys. 1977, 48, 1488–1496. [Google Scholar] [CrossRef]
- Lumeau, J.; Zanotto, E.D. A Review of the Photo-Thermal Mechanism and Crystallization of Photo-Thermo-Refractive (PTR) Glass. Int. Mater. Rev. 2017, 62, 348–366. [Google Scholar] [CrossRef]
- Ivanov, S.; Dubrovin, V.; Nikonorov, N.; Stolyarchuk, M.; Ignatiev, A. Origin of Refractive Index Change in Photo-Thermo-Refractive Glass. J. Non. Cryst. Solids 2019, 521, 119496. [Google Scholar] [CrossRef]
- Efimov, A.M.; Ignatiev, A.I.; Nikonorov, N.V.; Postnikov, E.S. Quantitative UV–VIS Spectroscopic Studies of Photo-Thermo-Refractive Glasses. II. Manifestations of Ce3+ and Ce(IV) Valence States in the UV Absorption Spectrum of Cerium-Doped Photo-Thermo-Refractive Matrix Glasses. J. Non. Cryst. Solids 2013, 361, 26–37. [Google Scholar] [CrossRef]
- Paul, A.; Mulholland, M.; Zaman, M.S. Ultraviolet Absorption of Cerium(III) and Cerium(IV) in Some Simple Glasses. J. Mater. Sci. 1976, 11, 2082–2086. [Google Scholar] [CrossRef]
- Hövel, H.; Fritz, S.; Hilger, A.; Kreibig, U.; Vollmer, M. Width of Cluster Plasmon Resonances: Bulk Dielectric Functions and Chemical Interface Damping. Phys. Rev. B 1993, 48, 18178–18188. [Google Scholar] [CrossRef]
- Arnold, G.W. Near-surface Nucleation and Crystallization of an Ion-implanted Lithia-alumina-silica Glass. J. Appl. Phys. 1975, 46, 4466–4473. [Google Scholar] [CrossRef]
- Jiménez, J.A.; Sendova, M.; Liu, H. Evolution of the Optical Properties of a Silver-Doped Phosphate Glass during Thermal Treatment. J. Lumin. 2011, 131, 535–538. [Google Scholar] [CrossRef]
- Sontakke, A.D.; Ueda, J.; Tanabe, S. Effect of Synthesis Conditions on Ce3+ Luminescence in Borate Glasses. J. Non. Cryst. Solids 2016, 431, 150–153. [Google Scholar] [CrossRef]
- Chewpraditkul, W.; Shen, Y.; Chen, D.; Yu, B.; Prusa, P.; Nikl, M.; Beitlerova, A.; Wanarak, C. Luminescence and Scintillation of Ce3+-Doped High Silica Glass. Opt. Mater. 2012, 34, 1762–1766. [Google Scholar] [CrossRef]
- Mironov, L.Y.; Marasanov, D.V.; Sgibnev, Y.M.; Sannikova, M.D.; Kulpina, E. V Influence of Reducing Agent Concentration on the Luminescence and Photophysical Processes Constant Rates of Silver Clusters in Silica-Based Glass. J. Lumin. 2023, 261, 119918. [Google Scholar] [CrossRef]
- He, Y.; Pei, G.; Liu, J.; Fang, Z.; Wang, L.; Jiang, S.; Yu, B.; Gou, J.; Liu, S.F. Utilizing the Energy Transfer of Ce4+- and Ce3+-Tb3+ to Boost the Luminescence Quantum Efficiency up to 100% in Borate Glass. J. Phys. Chem. C 2022, 126, 5838–5846. [Google Scholar] [CrossRef]
- Efimov, A.M.; Ignatiev, A.I.; Nikonorov, N.V.; Postnikov, E.S. Quantitative UV-VIS Spectroscopic Studies of Photo-Thermo-Refractive Glasses. I. Intrinsic, Bromine-Related, and Impurity-Related UV Absorption in Photo-Thermo-Refractive Glass Matrices. J. Non. Cryst. Solids 2011, 357, 3500–3512. [Google Scholar] [CrossRef]
- Marasanov, D.V.; Mironov, L.Y.; Sgibnev, Y.M.; Kolesnikov, I.E.; Nikonorov, N.V. Luminescence and Energy Transfer Mechanisms in Photo-Thermo-Refractive Glasses Co-Doped with Silver Molecular Clusters and Eu3+. Phys. Chem. Chem. Phys. 2020, 22, 23342–23350. [Google Scholar] [CrossRef]
- Cao, R.; Lu, Y.; Tian, Y.; Huang, F.; Guo, Y.; Xu, S.; Zhang, J. 2 Μm Emission Properties and Nonresonant Energy Transfer of Er3+ and Ho3+ Codoped Silicate Glasses. Sci. Rep. 2016, 6, 37873. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhou, B.; Ren, Z.; Xu, X.; Yang, G.; Qiao, X.; Yan, D.; Qian, G.; Fan, X. Fluorescence–Phosphorescence Manipulation and Atom Probe Observation of Fully Inorganic Silver Quantum Clusters: Imitating from and Behaving beyond Organic Hosts. Adv. Opt. Mater. 2022, 10, 2101632. [Google Scholar] [CrossRef]
- Li, L.; Yang, Y.; Zhou, D.; Yang, Z.; Xu, X.; Qiu, J. Influence of the Eu2+ on the Silver Aggregates Formation in Ag+-Na+ Ion-Exchanged Eu3+-Doped Sodium-Aluminosilicate Glasses. J. Am. Ceram. Soc. 2014, 97, 1110–1114. [Google Scholar] [CrossRef]
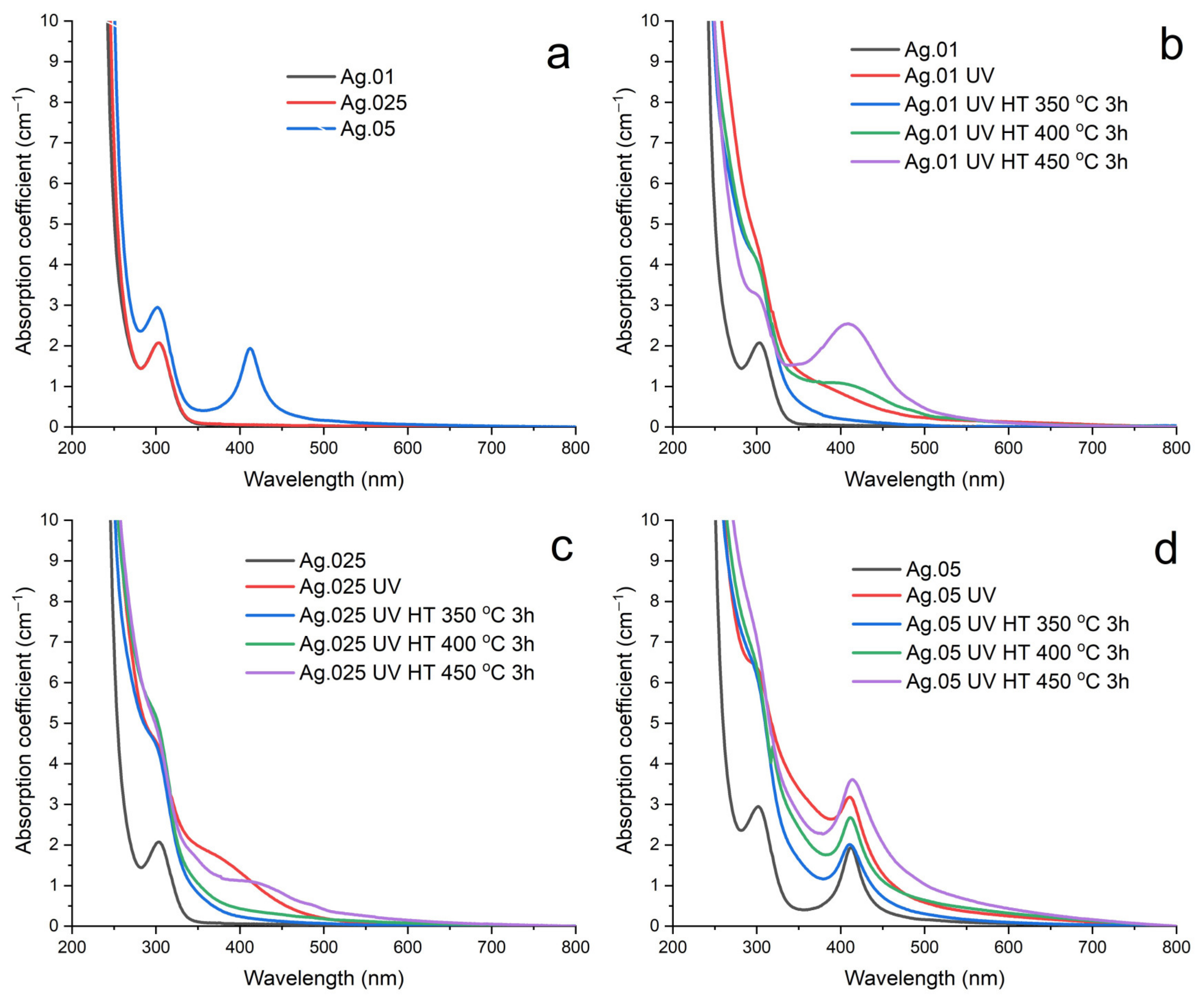

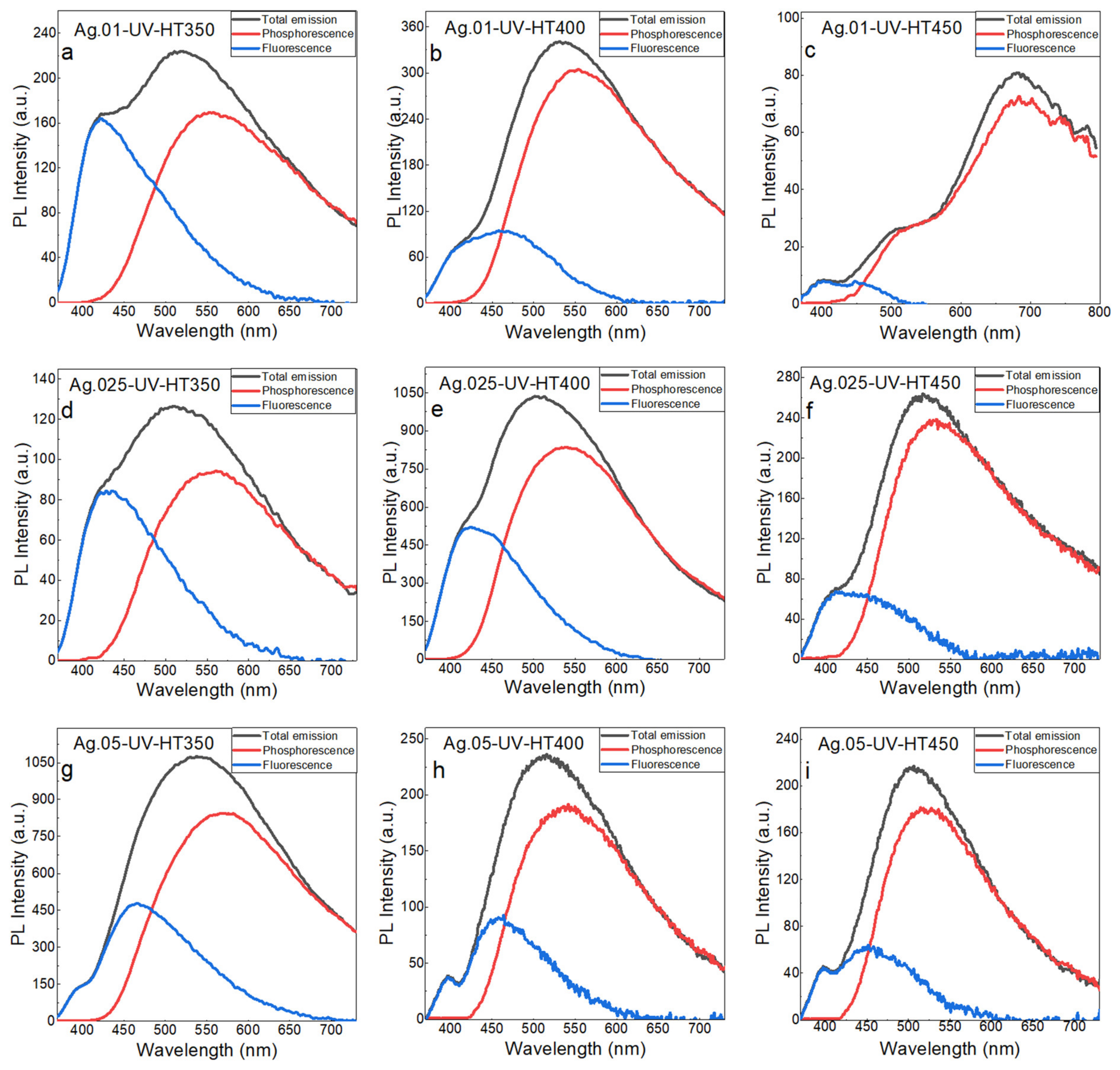
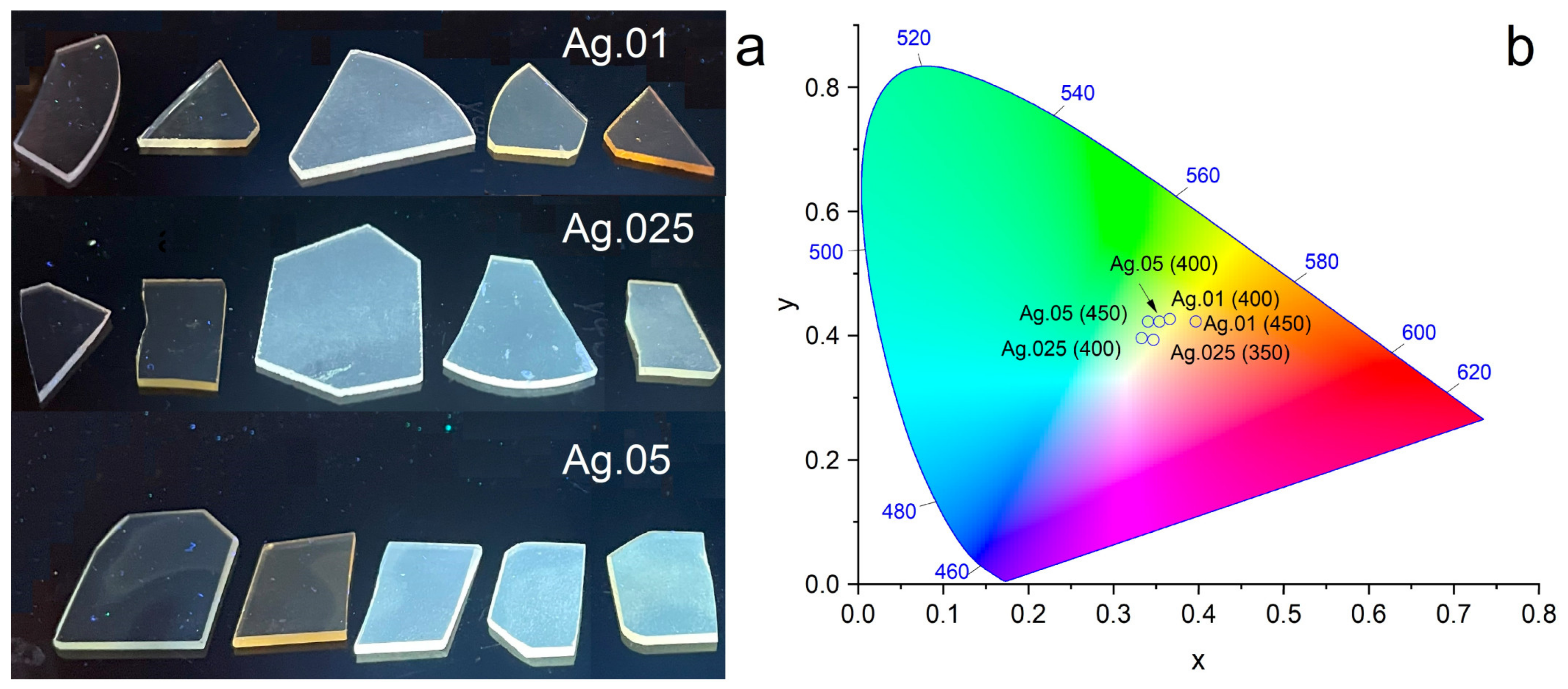
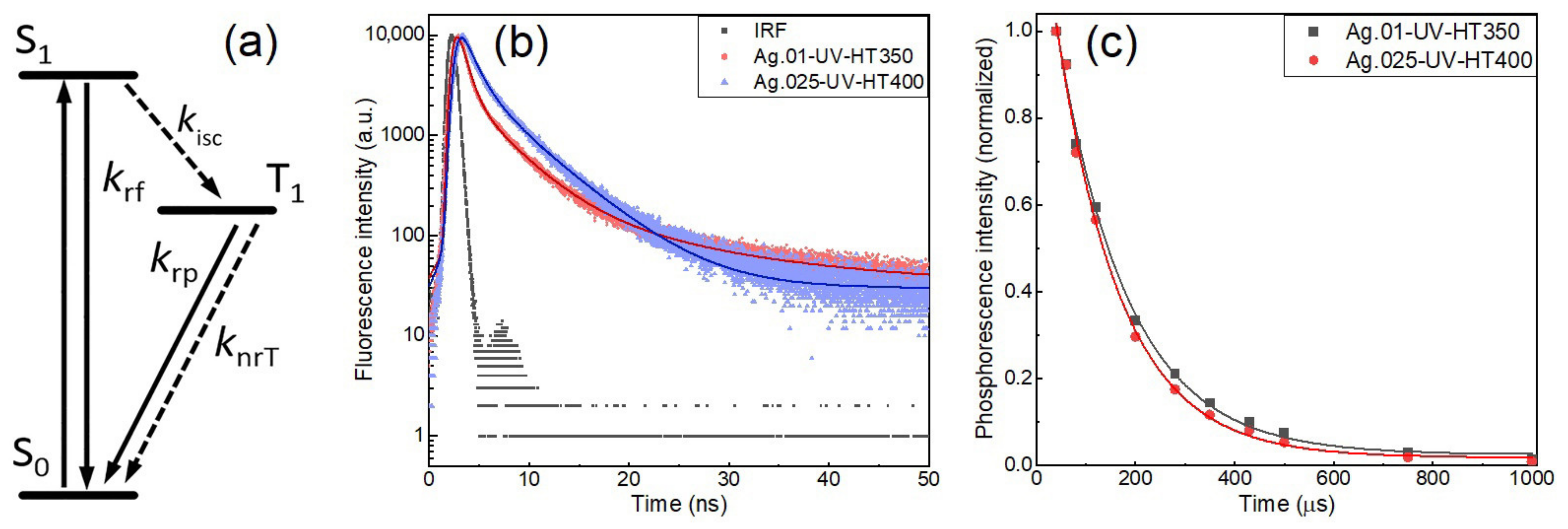
| Heat Treatment Temperature, °C | Ag.01 | Ag.025 | Ag.05 |
|---|---|---|---|
| Initial glass | - | - | 6 nm |
| 350 | - | - | 6 nm |
| 400 | 1 nm | - | 6 nm |
| 450 | 2 nm | 4 nm | 6 nm |
| Heat Treatment Temperature, °C | Ag.01 | Ag.025 | Ag.05 |
|---|---|---|---|
| Initial glass | 0.1 | 0.06 | 0.09 |
| UV-irradiated initial glass | 0.02 | 0.03 | 0.05 |
| 350 | 0.25 | 0.35 | 0.34 |
| 400 | 0.16 | 0.42 | 0.43 |
| 450 | 0.07 | 0.32 | 0.3 |
| Heat Treatment Temperature, °C | Ag.01 | Ag.025 | Ag.05 | |||
|---|---|---|---|---|---|---|
| τf_avg (ns) | τp (μs) | τf_avg (ns) | τp (μs) | τf_avg (ns) | τp (μs) | |
| Initial glass | 23.7 | - | 28.3 | - | 16.9 | 100 |
| 350 | 3.7 | 141 | 3.2 | 132 | 3.3 | 135 |
| 400 | 3.6 | 136 | 3.4 | 136 | 3.4 | 133 |
| 450 | 4.5 | 105 | 3.2 | 123 | 3.4 | 120 |
| Sample | τ1, ns | τ2, ns | τ3, ns | τf_avg, ns | τp, μs | Φlum | Reference |
|---|---|---|---|---|---|---|---|
| Ag.01-UV- HT350 | 0.8 | 3.8 | 14.5 | 3.7 | 141 | 0.25 | this work |
| Ag.025-UV- HT400 | 1.3 | 5.1 | - | 3.4 | 136 | 0.42 | this work |
| Ion-exchanged sample | 1.5 | 5.0 | - | 3.8 | 110 | 0.66 | [40] |
| Sample | krf (s−1) | kisc (s−1) | krp (s−1) | knrT (s−1) | Φf | Φisc | Φp | Reference |
|---|---|---|---|---|---|---|---|---|
| Ag.01-UV- HT350 | 1.6·107 | 2.5·108 | 1.4·103 | 5.6·103 | 0.06 | 0.94 | 0.20 | this work |
| Ag.025-UV- HT400 | 2.3·107 | 2.7·108 | 2.7·103 | 4.6·103 | 0.08 | 0.92 | 0.37 | this work |
| Ion-exchanged sample | 3.2·107 | 2.3·108 | 5.6·103 | 3.6·103 | 0.12 | 0.88 | 0.61 | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mironov, L.Y.; Marasanov, D.V.; Sannikova, M.D.; Zyryanova, K.S.; Slobozhaninov, A.A.; Kolesnikov, I.E. Formation and Photophysical Properties of Silver Clusters in Bulk of Photo-Thermo-Refractive Glass. Ceramics 2023, 6, 1546-1558. https://doi.org/10.3390/ceramics6030096
Mironov LY, Marasanov DV, Sannikova MD, Zyryanova KS, Slobozhaninov AA, Kolesnikov IE. Formation and Photophysical Properties of Silver Clusters in Bulk of Photo-Thermo-Refractive Glass. Ceramics. 2023; 6(3):1546-1558. https://doi.org/10.3390/ceramics6030096
Chicago/Turabian StyleMironov, Leonid Yu., Dmitriy V. Marasanov, Mariia D. Sannikova, Ksenia S. Zyryanova, Artem A. Slobozhaninov, and Ilya E. Kolesnikov. 2023. "Formation and Photophysical Properties of Silver Clusters in Bulk of Photo-Thermo-Refractive Glass" Ceramics 6, no. 3: 1546-1558. https://doi.org/10.3390/ceramics6030096
APA StyleMironov, L. Y., Marasanov, D. V., Sannikova, M. D., Zyryanova, K. S., Slobozhaninov, A. A., & Kolesnikov, I. E. (2023). Formation and Photophysical Properties of Silver Clusters in Bulk of Photo-Thermo-Refractive Glass. Ceramics, 6(3), 1546-1558. https://doi.org/10.3390/ceramics6030096








