Abstract
The energy efficiency of buildings can be greatly improved by decreasing the energy embodied in installed materials. In this contribution, we investigated the possibility of foaming waste bottle glass in the air atmosphere with the addition of water glass, which would reduce the energy used in the production of foamed glass boards. The results show that with the increased addition of water glass, the crystallinity and the thermal conductivity decrease, however, the remaining crystal content prevents the formation of closed-porous foams. The added water glass only partly protects the carbon from premature oxidation, and the foaming mechanism in the air is different than in the argon atmosphere. The lowest obtained foam density in the air atmosphere is 123 kg m−3, while the lowest thermal conductivity is 53 mW m−1 K−1, with an open porosity of 50% for the sample obtained in the air, containing 12 wt% of water glass, 2 wt% of B2O3, 2 wt% AlPO4 and 2 wt% K3PO4.
1. Introduction
One of the main focuses of energy savings in the European Union (EU) is energy use related to buildings, making it crucial to improve construction materials by lowering the embodied energy and further improving buildings’ energy efficiency. These actions include the development of greener thermal insulation materials, which are one of the key materials used in energy-efficient buildings. Foamed glass is considered a sustainable insulation material, as it can be made from waste glass and is stable on a long timescale. However, producing high-quality foamed glass with superior insulation properties is an energy-intensive process that could be improved by eliminating the step of remelting waste glass (remelting represents approx. 20% of energy use) [1]. In comparison to conventional thermal insulation materials used in the building sector, i.e., mineral wool and organic foams (EPS, XPS), the best foamed glass reaches similar thermal conductivity values (36 mW m−1 K−1 vs. 30–35 mW m−1 K−1), while having much better mechanical properties and long-term stability [2,3].
To avoid the remelting step, foamed glass is often produced through direct foaming of a mixture of finely milled waste glass and foaming agents, such as carbon or carbonate [1,2,3,4,5,6]. Carbon-based foaming agents react with chemically bonded oxygen (present as polyvalent ions in higher oxidation states) in the glass and release gases. To reduce the oxidation dependency on the glass composition, foaming mixtures can be supplemented with oxidizing agents, such as Fe2O3, manganese oxide in various oxidation states, and sulfates [7,8,9]. During the foaming process, the metal oxides incorporate, fully or partly, into the glass structure, which can trigger undesired crystallization [9]. Since the foaming temperatures are low, typically 750–850 °C, the glass is not completely homogenized, which results in local fluctuations in glass composition and glass instabilities. Glasses with lower glass stability are more susceptible to crystallization, which can hinder expansion and result in an open-porous foamed glass. Moreover, the finely milled glass is itself prone to crystallization [10].
In our previous research [10], we have shown that container waste glass exhibits low glass stability in a mixture with carbon and manganese oxide (Mn3O4) under foaming conditions. Glass with a common soda-lime-silica (SLS) composition manifested complex crystallization with the formation of several crystalline phases. It was evidenced that when re-melted, the glass stability against crystallization improved, and fewer crystalline phases formed. However, the improvement was minor. Thereafter, we showed that crystallization can be more effectively suppressed by the addition of selected additives (borax, B2O3, Al2O3). Furthermore, we introduced phosphates in the foamed glass mixture, which improved the homogeneity of the foams and increased the content of closed pores [11]. The addition of the phosphates also decreased the densities of the foams below 150 kg m−3, and the thermal conductivity of these foams was in the range of 57–66 mW m−1 K−1. These samples were prepared under an oxygen-free atmosphere, which is used in the industry but is related to a higher energy consumption due to under-stoichiometric gas burning [5].
This study aims to investigate the influence of water glass (WG) addition on the foaming process and the possibility of transferring the foaming process from an oxygen-free atmosphere to an air atmosphere. Water glass is a known additive used in industrial processes. It was shown that WG could be used as a single foaming agent or in combination with carbonaceous foaming agents [12,13,14]. The proposed mechanism of the foaming with WG is due to the decomposition of carbonates, which are formed when wet water-glass-coated glass powder is in contact with the air atmosphere [15]. This study focuses on the foaming process of container glass waste using a foaming/oxidizing agent couple, with the addition of various crystallization inhibitors and water glass, the latter possibly enabling the foaming process in the air atmosphere. The effects of the different additives and foaming atmosphere on the properties of the foamed samples, such as density, porosity, crystallinity, and thermal conductivity, were investigated and compared. The underlying reactions and their influence on the properties of the foams are discussed.
2. Experimental Section
Waste glass with a typical SLS composition [10] was mixed with foaming/oxidizing agent couple 0.5 wt% carbon black (CB, acetylene black, Alfa Aesar, Kandel, Germany) and 6.356 wt% Mn3O4 and with different amounts of water glass (12 and 24 wt%), Table 1. Mn3O4 was prepared from MnO2 (99%, Bie & Berntsen, Rødovre, Denmark) by thermal treatment at 1250 °C for 4 h. The mixture was homogenized in yttria-stabilized zirconia (YSZ) jar with 10 mm YSZ balls at 250 rpm in a planetary ball mill for 35 min. As crystallization inhibitors, we used fluxing agents: B2O3, and phosphates: K3PO4 and AlPO4, which were also previously used as supporting materials for foaming waste glass [9,16]. WG was then mixed with the homogenized mixture in an agate mortar. The mixture with WG was finally dried at 200 °C for 1 h.

Table 1.
Composition of the mixtures of the glass, 0.5 wt% CB, 6.356 wt% Mn3O4, 12 wt% WG or 24 wt% WG, foamed in air and Ar atmosphere.
Small samples were prepared from these mixtures by uniaxial pressing (ϕ 12) of ~1 g of the batch. The samples were heat-treated at 865 °C for 10 min with a heating rate of 10 K min−1 in an Ar and air atmosphere in a laboratory electrical tube furnace (Protherm ASP11/150/500, Ankara, Turkey).
The thermal behavior of foaming mixtures during heat treatment was investigated using a thermo-gravimetric analyzer (TGA) coupled with a mass spectrometer (MS). Specifically, the instrument used was the NETZSCH STA 449 C/6/G Jupiter TGA coupled with an Aëoloss QMS 403 mass spectrometer. In the study, approximately 10 mg of a powder mixture was analyzed. The samples were subjected to heating at a rate of 10 K min−1 and reached a maximum temperature of 800 °C. The analysis was carried out under two different gas atmospheres: synthetic air and Ar (flow 50 mL min−1).
Powder X-ray diffraction (XRD; Malvern PANalytical Empyrean diffractometer, Malvern, United Kingdom) using a Cu-Kα radiation source was employed to identify the crystalline phases present in the foamed samples. The XRD data were collected within the 2θ range of 10–70°, with a step size of 0.0263° and a time per step of 500 s. The obtained diffraction patterns were compared with the patterns in the Joint Committee on Powder Diffraction Standards (JCPDS) database using Highscore Plus software for phase identification.
The apparent density (ρapp) of the resulting foams was determined using the Archimedes principle in water for small samples, with a measurement error of ±1%. The pycnometric volume of the small samples was determined by the Archimedes method in absolute ethanol, with a measurement error of ±2%. To eliminate air from the open pores, the small samples were submerged in boiling ethanol under reduced pressure. The submerged samples’ volume was then measured in absolute ethanol using the Archimedes principle (Vpyc).
The porosity of the foamed samples was determined based on the apparent density, pycnometer density, and powder density (measured as 2.50 g cm−3). The following equations were used to calculate the total porosity φ (measurement error ±1%), open porosity (OP), and closed porosity (CP) (measurement error ±2%):
where the volume (Vfoam) of foam is:
Vfoam = VCP + VOP + Vglass.
For the large samples, the apparent density was calculated using the sample’s dimensions (geometric volume from the sample’s dimensions) and weight. The total porosity of the samples was calculated based on the apparent density using Equation (1). The closed porosity was determined using an Ultrapyc 5000 Foam instrument (Anton Paar GmbH, Graz, Austria).
The thermal conductivity of the large foam samples, which were cut into dimensions of 8 cm × 8 cm × 2 cm, was measured using a heat-flow meter (HFM 446 Lambda Small, Stirolab, Sežana, Slovenia) following the DIN EN 12667 standard. The instrument was calibrated using a NIST Standard Reference Material® 1450 d. The typical accuracy of the HFM is ± 1%. The mean temperature of the sample during the measurement was 10 °C, with the upper and lower plate temperatures set at 5 °C and 15 °C, respectively.
The pore size distribution was analyzed based on a magnified cross-section image of the sample. A transparent foil was then placed over the image, and the pores were manually outlined. The resulting image was scanned and processed using ImageJ 1.53t software.
3. Results
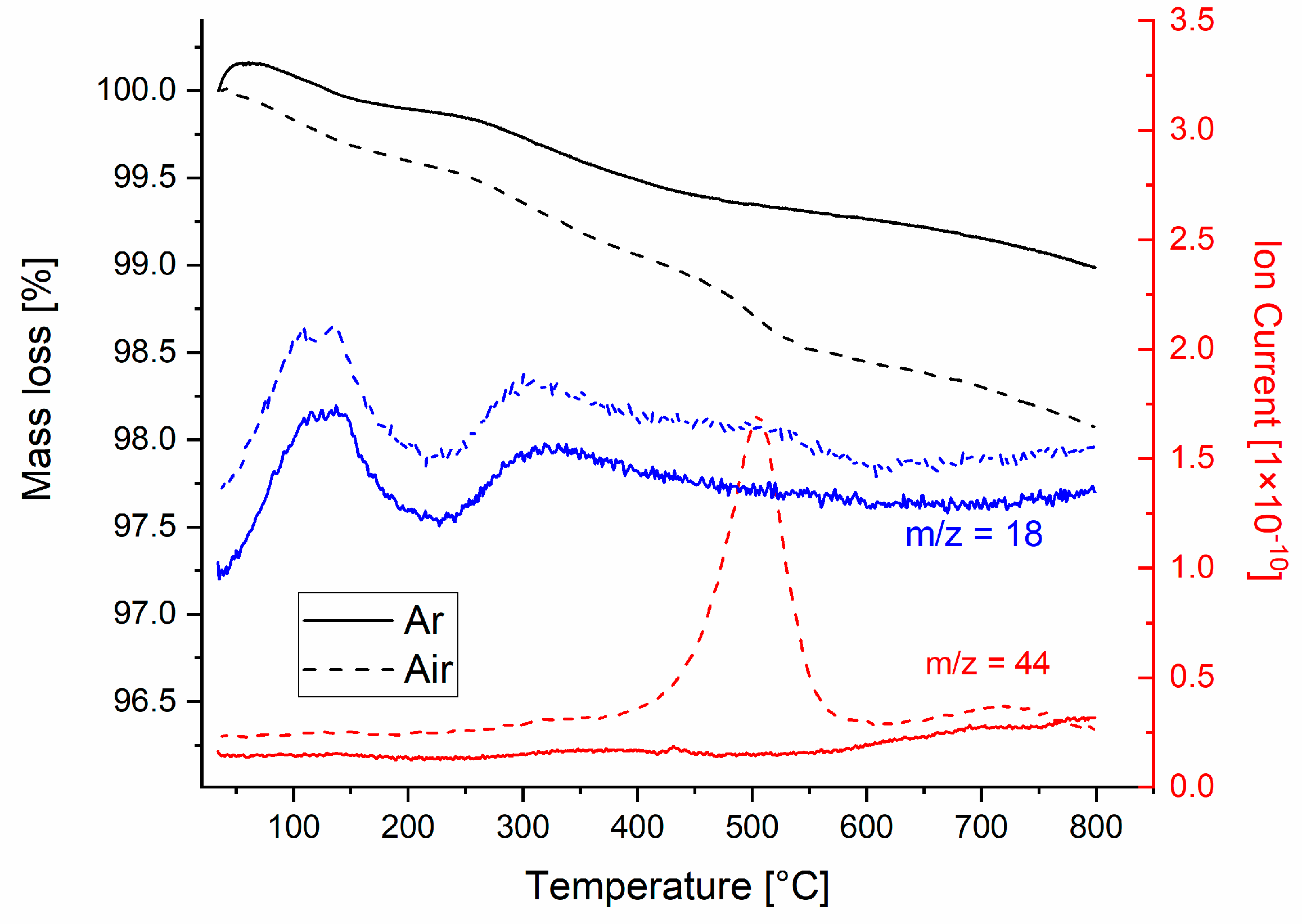
Thermal analysis coupled with evolved gas analysis of the sample 12WG-2B-2Al-2K with 12 wt% WG in Ar and air atmosphere revealed important differences (Figure 1). A mass loss (black curves, Figure 1) in both atmospheres occurs practically over the whole temperature range and is related to the release of H2O and CO2. A major part of the mass loss is related to a gradual release of water bounded in the water glass [14]. Two peaks in the release of water are located at 120 and 320 °C, while water vapor is present in the evolved gases up to 600 °C.
Figure 1.
Thermal analysis coupled with evolved gas analysis of the sample.
The main mass loss related to CO2 release is visible in the sample foamed in an air atmosphere peaking at 500 °C. This is related to the premature oxidation of the added carbon to oxygen present in the atmosphere, which is unwanted [17]. In the sample processed in the Ar atmosphere, a small, i.e., negligible, mass loss is observed at 440 °C. The samples analyzed were in powder form, so sharp peaks of gas release above the sintering temperature are not present. Despite that, in both samples, the signal of CO2 is observed at temperatures above 600 °C. This is related to (i) the presence of carbon, which is protected from the atmosphere by the added WG and reacts with oxygen from the glass, and (ii) the decomposition of carbonates, which are formed when a wet mixture with WG is exposed to the air atmosphere [15]. Both sources of CO2 are present in the sample foamed in the Ar atmosphere, while the second one is predominantly present in the sample processed in the air atmosphere. From the mass loss occurring at 500 °C in the air atmosphere, accompanied by the large CO2 peak, we calculate that around 80% of carbon is burned out. For the sample tested in the Ar atmosphere, the CO2 signal increases from 600 to 800 °C, while in the sample tested in the air atmosphere, the CO2 signal peaks at 720 °C and then decreases gradually, indicating that the source of CO2 is diminishing.
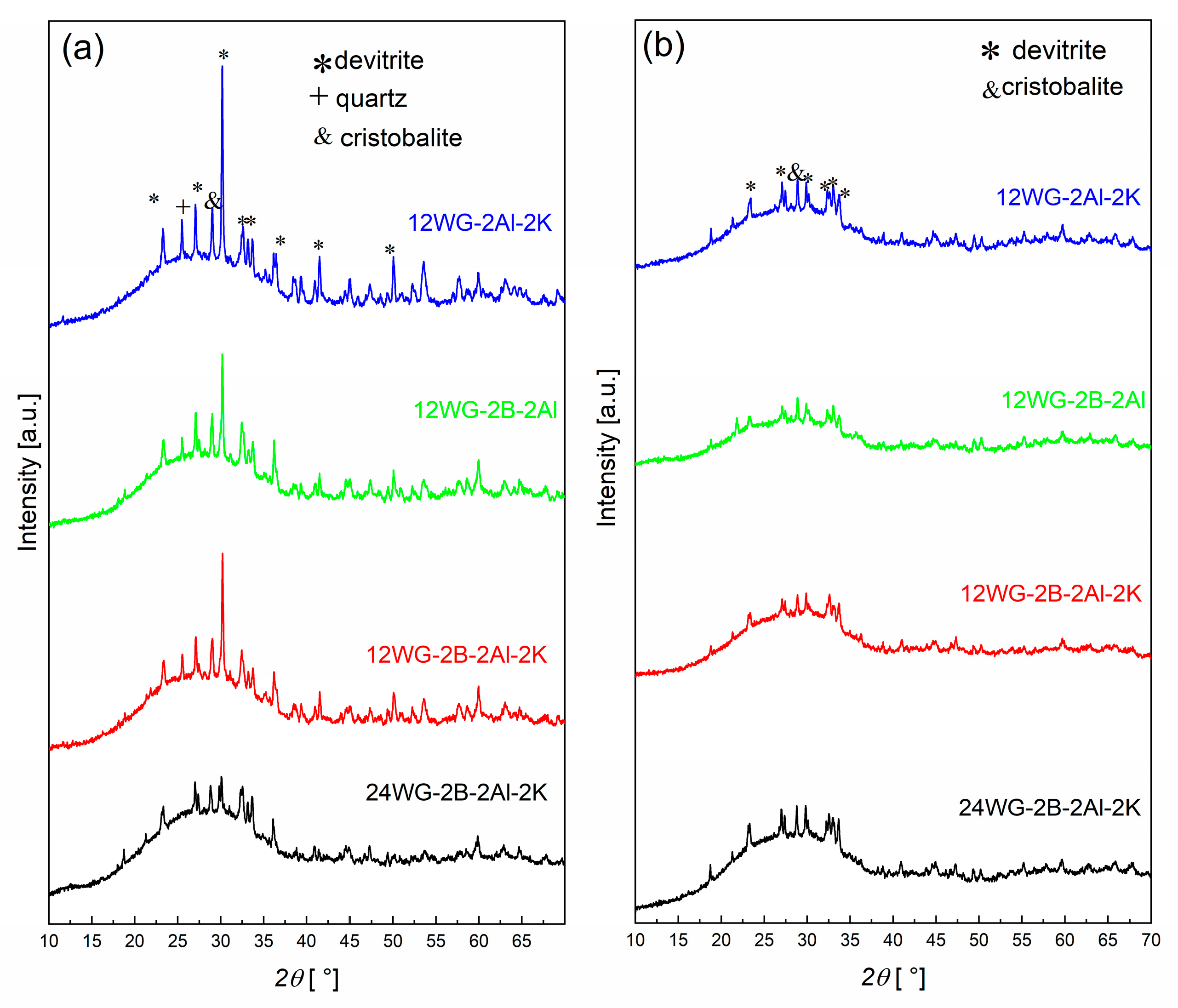
The XRD patterns of the samples foamed in Ar and air atmospheres are shown in Figure 2. The samples processed in the Ar atmosphere contain a higher amount of crystalline phase than the samples foamed in the air atmosphere. The exact mechanism behind this is not known; however, it is most likely related to the presence of carbon, which is higher in the sample foamed in the Ar atmosphere. The carbon binds the oxygen from the glass, thereby changing the oxidation state of the glass, which influences the crystallization processes. In comparison to the samples without added WG [11], the crystalline phase content is lower. In general, the samples with 24 wt% of WG have a significantly lower crystalline content and are very similar in both atmospheres (Figure 2; note: only the XRD pattern of the sample with all additives and 24 wt% WG is shown). For the samples with 24 wt% of WG, the samples processed in both atmospheres exhibit practically identical XRD patterns, and there are negligible differences, e.g., a peak at 36° 2θ foamed in Ar.
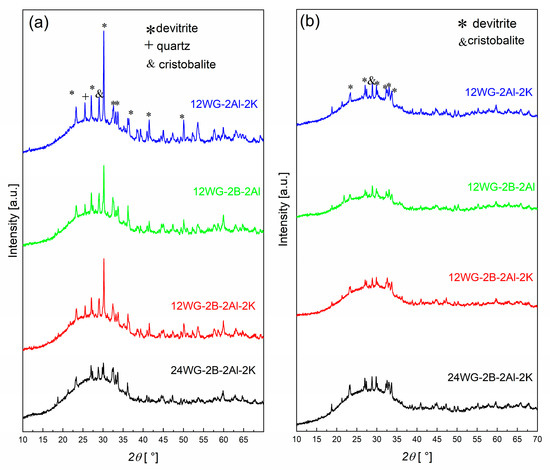
Figure 2.
XRD patterns of the samples foamed in (a) Ar and (b) air atmosphere at 865 °C.
The densities of the samples with 12 wt% of WG foamed in Ar atmosphere are in the range of 143–157 kg m−3, while the samples foamed in air exhibit higher densities in the range of 224–290 kg m−3. In general, the samples with both phosphate additions have a lower density. Closed porosity is the highest in the samples containing all additives. When the amount of added WG is increased to 24 wt%, the densities of the samples foamed in Ar remain at the same level, while for the samples foamed in air atmosphere, the densities decrease significantly. This decrease can be related to a higher amount of carbon being protected by the added WG. The open porosity, however, increases in almost all samples with a higher addition of WG. In Ar, the lowest density obtained is 130 kg m−3, while in the air atmosphere it is 156 kg m−3, in both cases with 24 wt% WG (Table 2).

Table 2.
The density, closed porosity, and total porosity of the samples foamed for 10 min at 865 °C in Ar and air atmosphere.
4. Large Samples
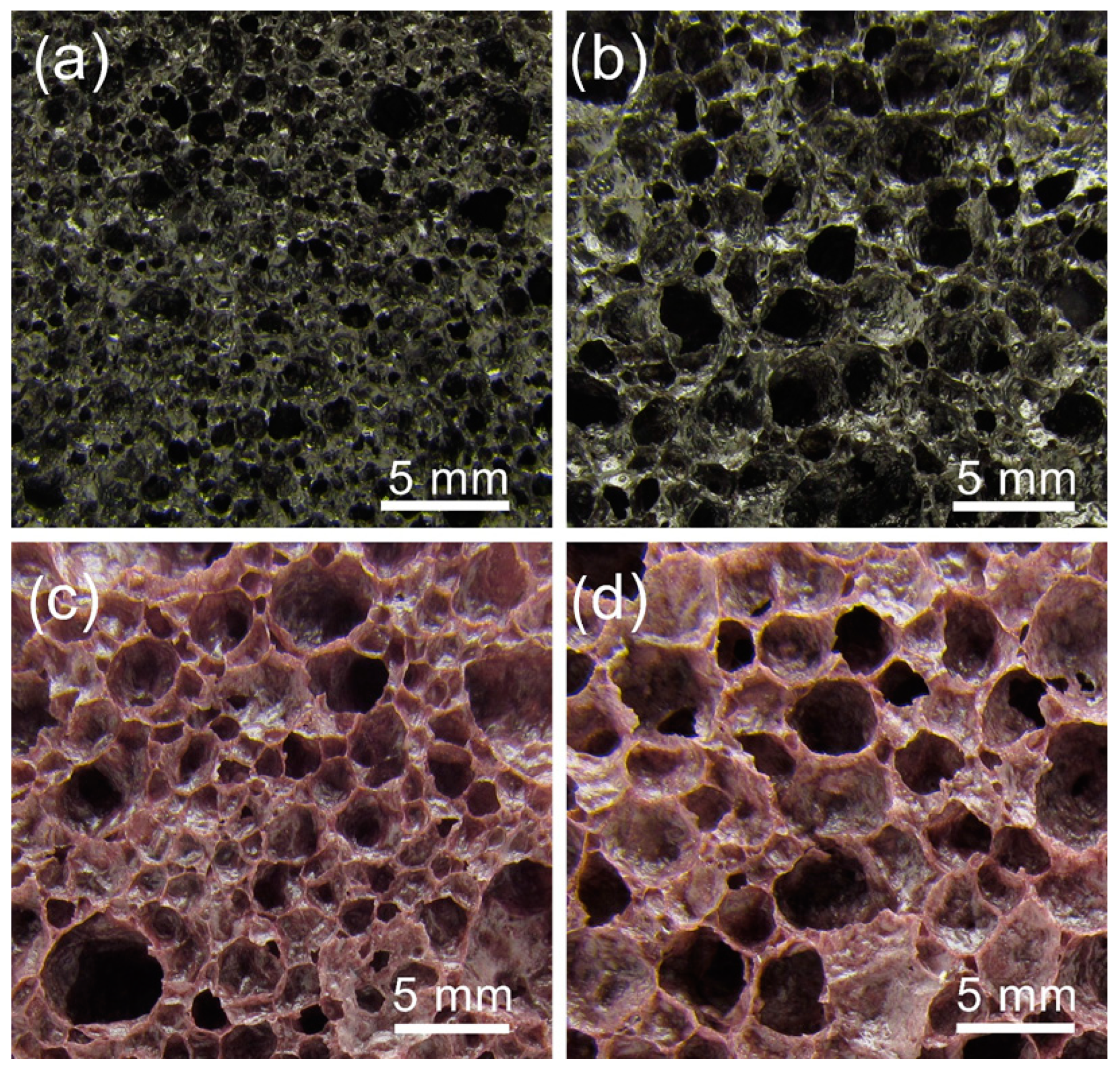
Large samples from the compositions containing all additives and 12 or 24 wt% WG were prepared to properly evaluate the achievable densities and thermal conductivities. The pore structure of the large samples is shown in Figure 3. The samples foamed in the Ar atmosphere exhibit a more homogeneous pore structure than the samples foamed in the air atmosphere, which also contain larger pores. These differences indicate that the foaming mechanism changes when the atmosphere changes. In the Ar atmosphere, carbon is fully protected and remains in the sintered sample. Oxidation of carbon, with the chemically bounded oxygen in the glass and manganese oxide [17], as well as the decomposition of carbonates formed on addition of WG [15], contribute to the foaming process. The color of the samples is gray (to better understand the color references mentioned, readers are directed to consult the online version of the article). In the air atmosphere, only a small part of carbon remains in the sample, although it is expected for the amount to increase with an increasing sample size and WG content. Thus, the foaming is in major part related to the release of oxygen from the manganese oxide and the decomposition of carbonates formed in the wet foaming mixture with WG. The color of the samples is more purple than gray, indicating that the majority of manganese in the foamed sample is present as Mn3+ [18]. The larger pore sizes of these samples foamed in the air indicate that the foaming time could be shortened in order to obtain a sample with smaller pores.
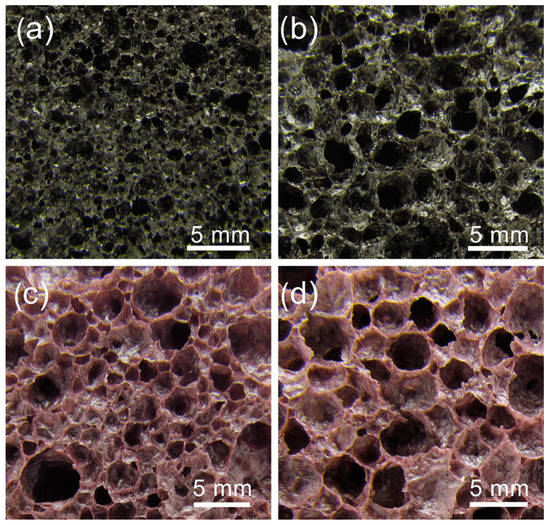
Figure 3.
Microstructure of the foamed large samples: (a) 12WG-2B-2Al-2K at 865 °C in Ar, (b) 24WG-2B-2Al-2K at 865 °C in Ar, (c) 12WG-2B-2Al-2K at 880 °C in Air and (d) 24WG-2B-2Al-2K at 855 °C in air.
The densities of the large samples with 12 wt% of WG are similar to those of the small samples; however, the densities of the large samples foamed with 24 wt% of WG are significantly lower, Table 3. This is related to the larger size of the sample, where more released water and carbon can stay in the sample, since the diffusion path of the gases in the compacted powder becomes longer. Similarly, the higher content of remaining hydroxyl groups and water in the large samples can contribute to pore coalescence through the decrease of viscosity. The 12 wt% addition is not adequate for triggering such differences. Additionally, the viscosity decreases with an increasing WG content due to the increase of sodium content in the glass [14].

Table 3.
Density, porosity and thermal conductivity of the samples foamed for 20 min in Ar and air atmosphere.
The thermal conductivities are in the range of 53–66 mW m−1 K−1, which is slightly lower than in the samples without WG addition [11] and in the range of commercial foamed glass produced in air atmosphere [19], Table 3. The samples prepared from the composition with 12 wt% WG foamed in Ar and air atmospheres have the same density, but the thermal conductivity of the sample foamed in air is much lower, despite the higher open porosity. Although not measured on this set of samples, based on our previous reports [19,20], we presume that CO2 is present in the closed pores (thermal conductivity 16 mW m−1 K−1), while air with a higher thermal conductivity (25 mW m−1 K−1) fills the open pores. From these results, we can conclude that the difference in the thermal conductivity is in major part related to the contribution of the solid (glassy) phase. The crystallinity of the samples with 12 wt% WG foamed in Ar is higher than of those foamed in air, which means that the contribution of the solid conductivity is higher. The sample with 24 wt% WG foamed in Ar has a similar crystalline content to the sample with 12 wt% WG foamed in air, and its thermal conductivity is lower, despite the higher open-porous content. This result again shows that the prevention of crystallization is an important parameter in the production of foamed glass with improved insulation properties [19,21].
Open porosity is another critical parameter. In this set of samples, only the sample with 12 wt% of WG foamed in Ar at 865 °C has a closed porosity of 80%, while the other samples have much lower values of closed porosity. Open porosity also contributes to a higher thermal conductivity [19]. If the samples foamed in the air atmosphere would be fully closed-porous and the pores were filled with CO2, the thermal conductivity would decrease by 4–6 mW m−1 K−1. The achieved values would then be below the commercial reference of 52 mW m−1 K−1 [22]. In relation to open porosity, one could also expect that the thermal conduction through the solid phase would decrease, since all the material would be placed in the struts (no walls), which decreases the solid conduction [17]. However, the distribution of the solid mass between struts and walls in foamed glass is not so extreme as to trigger such an effect. Moreover, foamed glass with open porosity is not appropriate for thermal insulation in conventional applications on the outer surface of a wall [23] due to the danger of water penetration. Such foamed glass can only be used as acoustic or thermal insulation in the interior of buildings.
The presented results show that bottle glass composition has a lower potential for use in foamed glass boards production. The main issue is related to crystallization, which occurs during foaming and triggers an increase of open porosity and thermal conductivity of solid phase. A new approach is needed in order to be able to use waste bottle glass in the production of foamed glass boards, preferably in the air atmosphere, for thermal insulation applications. One possibility is to change the foaming agent(s), but also to prepare a mixture with flat glass [9]. Until then, this source of waste glass can effectively be used in the production of foamed glass gravel, which typically uses SiC as the foaming agent [24].
5. Conclusions
We investigated the use of water glass in the foaming of waste bottle glass with the carbon–manganese-oxide foaming couple in Ar and air atmosphere. The results show that with an increased addition of WG, the crystallinity and the thermal conductivity decrease in comparison to the samples without WG addition. However, the remaining crystallization greatly influences the properties of the prepared foams, resulting in open porosity and higher thermal conductivities in comparison to amorphous foams in the literature. The DTA analysis revealed that 12 wt% of added WG partly protects the carbon from premature oxidation by the air from the atmosphere at around 500 °C. However, as indicated by the differences in the large samples processed in Ar and air atmospheres, the foaming mechanism differs greatly, and it was not possible to obtain a closed porous sample in air atmosphere. With the WG addition, it was not possible to obtain a foam of proper quality from the waste bottle glass in the air atmosphere. The lowest obtained foam density in the air atmosphere was 123 kg m−3, while the lowest thermal conductivity was 53 mW m−1 K−1.
Author Contributions
Conceptualization, J.K. and S.S.; methodology, J.K. and S.S.; formal analysis, U.H.; investigation, S.S.; writing—original draft preparation, S.S. and J.K.; writing—review and editing, S.S., J.K. and U.H.; supervision, J.K.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
Slovenian Ministry of Education, Science and Sport, and the European Regional Development Fund (grant number C3330-19-952051) and Slovenian Research Agency (grant number L2-9221).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No further data available.
Acknowledgments
The authors want to acknowledge the support from the Slovenian Ministry of Education, Science and Sport, and the European Regional Development Fund (grant number C3330-19-952051), as well as the Slovenian Research Agency (grant number L2-9221).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rodrigues, C.; König, J.; Freire, F. Prospective life cycle assessment of a novel building system with improved foam glass incorporating high recycled content. Sustain. Prod. Consum. 2023, 36, 161–170. [Google Scholar] [CrossRef]
- Hill, C.; Norton, A.; Dibdiakova, J. A comparison of the environmental impacts of different categories of insulation materials. Energy Build. 2018, 162, 12–20. [Google Scholar] [CrossRef]
- Pittsburgh Corning Europe. Foamglas Industrial Insulation Handbook; Pittsburgh Corning Europe: Waterlo, Belgium, 1992. [Google Scholar]
- Llaudis, A.S.; Tari, M.J.O.; Ten, F.J.G.; Bernardo, E.; Colombo, P. Foaming of flat glass cullet using Si3N4 and MnO2 powders. Ceram. Int. 2009, 35, 1953–1959. [Google Scholar] [CrossRef]
- Steiner, A.C. Foam Glass Production from Vitrified Municipal Waste Fly Ashes Door; Eindhoven University Press: Eindhoven, The Netherlands, 2006. [Google Scholar]
- García-Ten, J.; Saburit, A.; Orts, M.J.; Bernardo, E.; Colombo, P. Glass foams from oxidation/reduction reactions using SiC, Si3N4 and AlN powders. Glass Technol. Eur. J. Glass Sci. Technol. Part A 2011, 52, 103–110. [Google Scholar]
- Bayer, G. Foaming of borosilicate glasses by chemical reactions in the temperature range 950–1150 °C. J. Non-Cryst. Solids 1980, 38–39, 855–860. [Google Scholar] [CrossRef]
- Scarinci, G.; Brusatin, G.; Bernardo, E. Glass Foams. Cell. Ceram. Struct. Manuf. Prop. Appl. 2005, 2, 158–176. [Google Scholar] [CrossRef]
- König, J.; Petersen, R.R.; Iversen, N.; Yue, Y. Suppressing the effect of cullet composition on the formation and properties of foamed glass. Ceram. Int. 2018, 44, 11143–11150. [Google Scholar] [CrossRef]
- Smiljanić, S.; Hribar, U.; Spreitzer, M.; König, J. Influence of additives on the crystallization and thermal conductivity of container glass cullet for foamed glass preparation. Ceram. Int. 2021, 47, 32867–32873. [Google Scholar] [CrossRef]
- Smiljanić, S.; Spreitzer, M.; König, J. Application of the container waste glass in foamed glass production. Open Ceram. 2023, 14, 100339. [Google Scholar] [CrossRef]
- Hesky, D.; Aneziris, C.G.; Groß, U.; Horn, A. Water and waterglass mixtures for foam glass production. Ceram. Int. 2015, 41, 12604–12613. [Google Scholar] [CrossRef]
- Méar, F.O.; Podor, R.; Lautru, J.; Genty, S.; Lebullenger, R. Effect of the process atmosphere on glass foam synthesis: A high-temperature environmental scanning electron microscopy (HT-ESEM) study. Ceram. Int. 2021, 47, 26042–26049. [Google Scholar] [CrossRef]
- Hribar, U.; Østergaard, M.B.; Iversen, N.; Spreitzer, M.; König, J. The mechanism of glass foaming with water glass. J. Non-Cryst. Solids 2023, 600, 122025. [Google Scholar] [CrossRef]
- Hribar, U.; Spreitzer, M.; König, J. Applicability of water glass for the transfer of the glass-foaming process from controlled to air atmosphere. J. Clean. Prod. 2020, 282, 125428. [Google Scholar] [CrossRef]
- Østergaard, M.B.; Petersen, R.R.; König, J.; Yue, Y. Effect of alkali phosphate content on foaming of CRT panel glass using Mn3O4 and carbon as foaming agents. J. Non-Cryst. Solids 2018, 482, 217–222. [Google Scholar] [CrossRef]
- König, J.; Petersen, R.R.; Yue, Y.; Suvorov, D. Gas-releasing reactions in foam-glass formation using carbon and MnxOy as the foaming agents. Ceram. Int. 2017, 43, 4638–4646. [Google Scholar] [CrossRef]
- Iyel, A.; Oktem, D.; Akmaz, F. Parameters Affecting the Color Mechanism of Manganese Containing Colored Glasses. J. Chem. Chem. Eng. 2014, 9, 849–858. [Google Scholar]
- König, J.; Lopez-Gil, A.; Cimavilla-Roman, P.; Rodriguez-Perez, M.A.; Petersen, R.R.; Østergaard, M.B.; Iversen, N.; Yue, Y.; Spreitzer, M. Synthesis and properties of open- and closed-porous foamed glass with a low density. Constr. Build. Mater. 2020, 247, 118574. [Google Scholar] [CrossRef]
- PCimavilla-Román, P.; Villafañe-Calvo, J.; López-Gil, A.; König, J.; Rodríguez-Perez, M. Modelling of the mechanisms of heat transfer in recycled glass foams. Constr. Build. Mater. 2021, 274, 122000. [Google Scholar] [CrossRef]
- Østergaard, M.B.; Petersen, R.R.; König, J.; Johra, H.; Yue, Y. Influence of foaming agents on solid thermal conductivity of foam glasses prepared from CRT panel glass. J. Non-Cryst. Solids 2017, 465, 59–64. [Google Scholar] [CrossRef]
- Programme, D.; Declaration, P. GLAPOR cellular glass GLAPOR Werk Mitterteich GmbH. Mitterteich Ger. 2017, 1–8. Available online: https://www.glapor.de/ (accessed on 30 May 2023).
- EN 13167:2012+A1:2015; Thermal Insulation Products for Buildings—Factory Made Cellular Glass (CG). European Committee for Standardization: Brussels, Belgium, 2015. Available online: https://standards.iteh.ai/catalog/standards/cen/e2f42873-03de-457f-9fc8-6541f8541f64/en-13167-2012a1-2015 (accessed on 28 July 2022).
- Hibbert, M. Understanding the Production and Use of Foam Glass Gravel across Europe and Opportunities in the UK; Chartered Institution of Wastes Management: Northampton, UK, 2016. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).