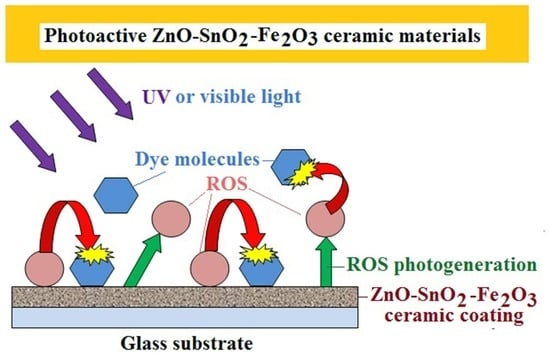
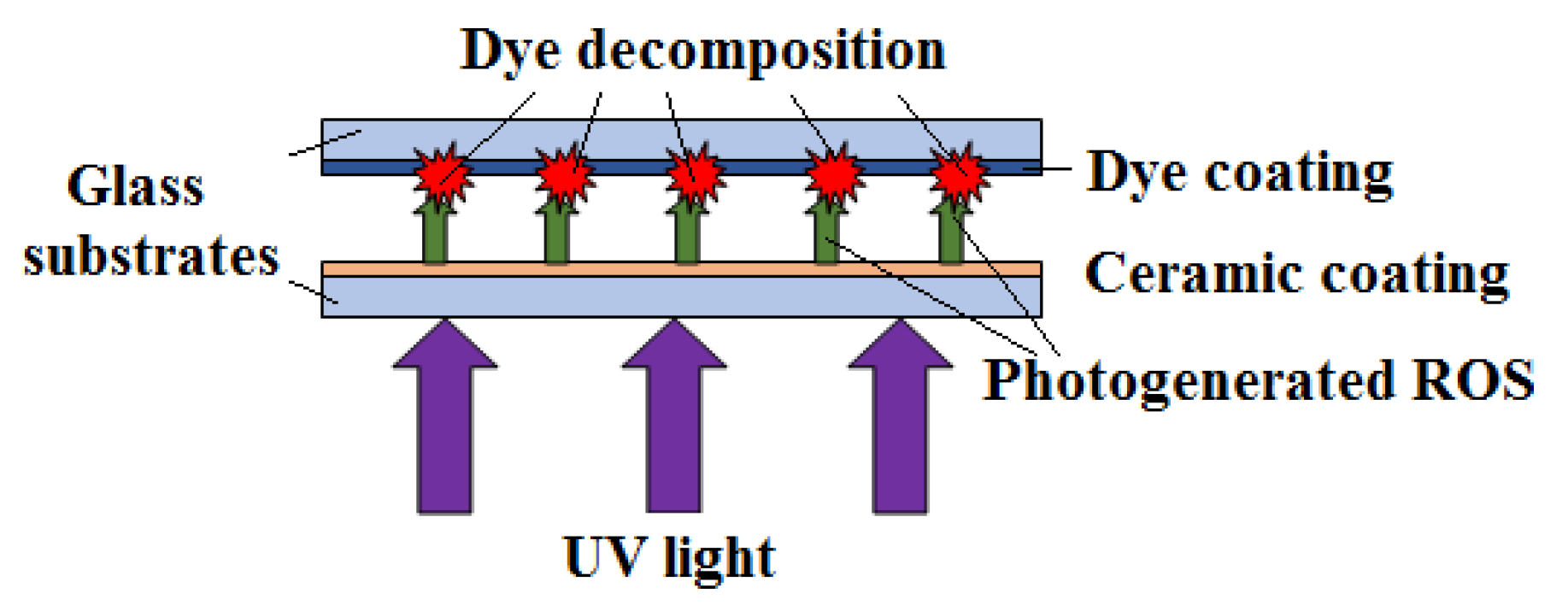
Ceramic ZnO-SnO2-Fe2O3 Powders and Coatings -Effective Photogenerators of Reactive Oxygen Species
Abstract
1. Introduction
2. Materials and Methods
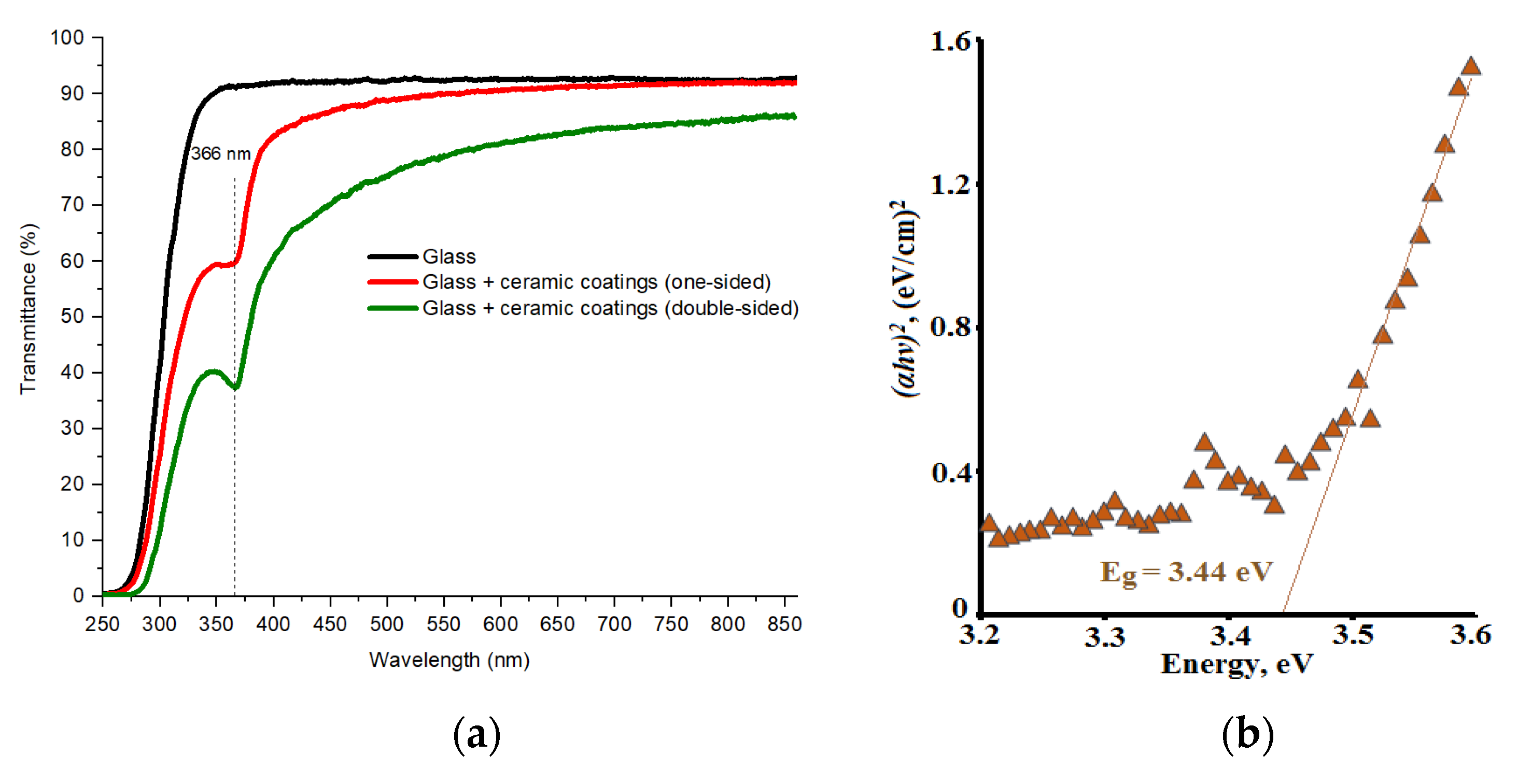
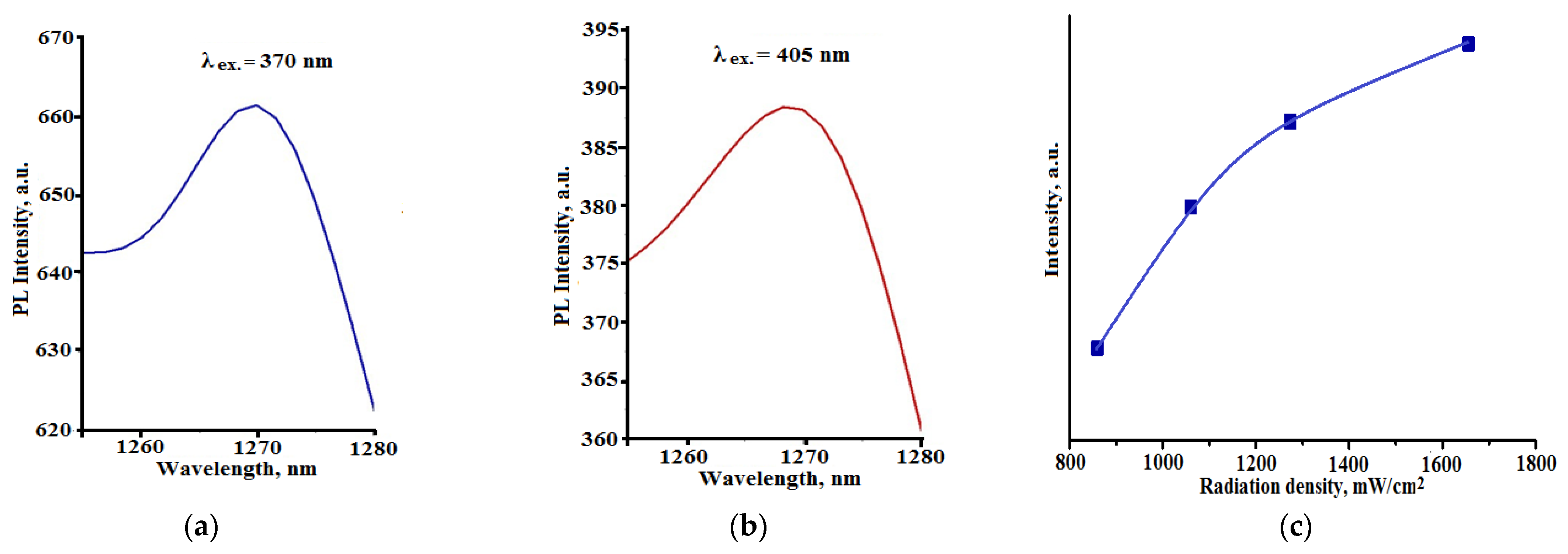
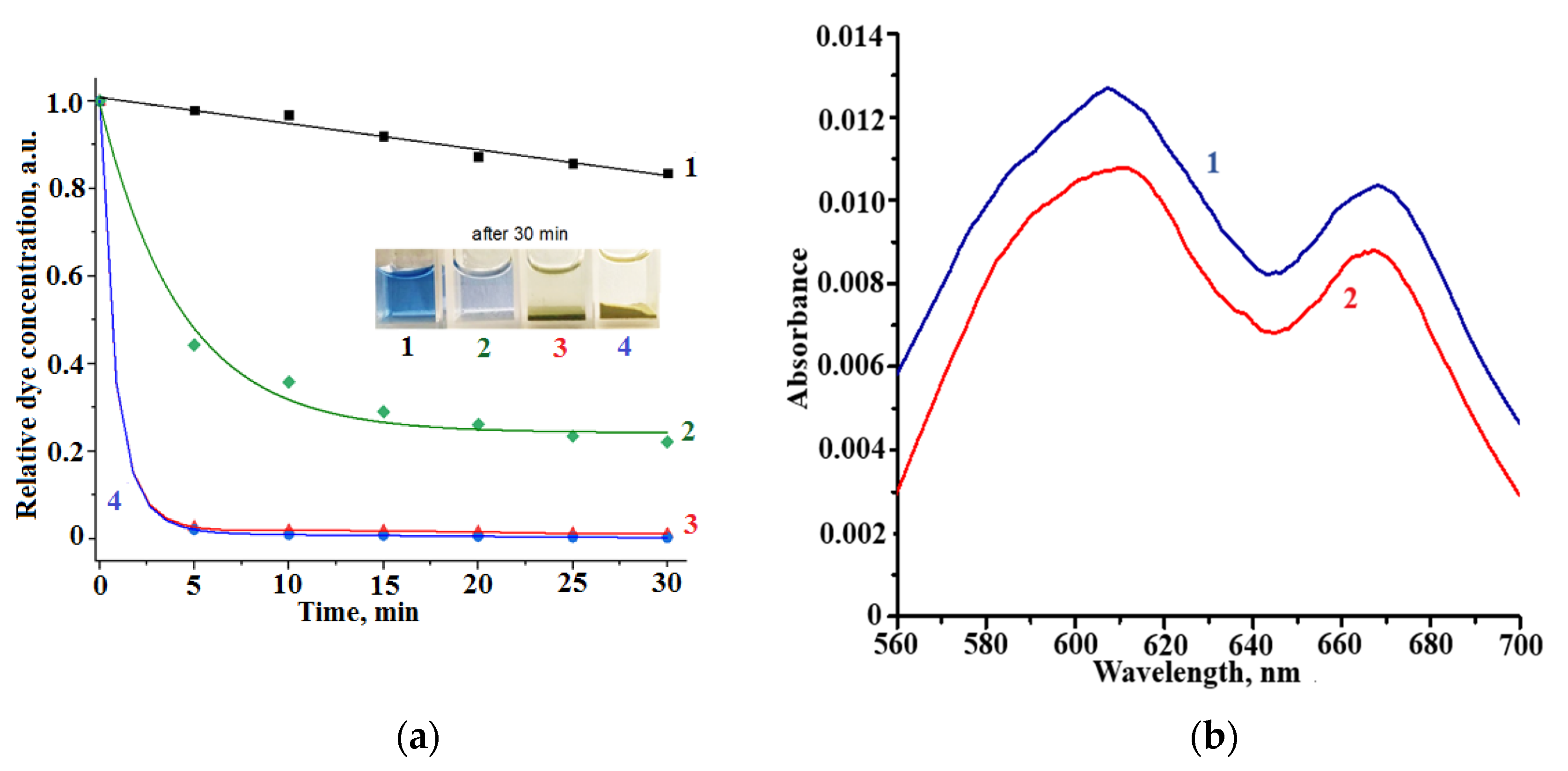
3. Results
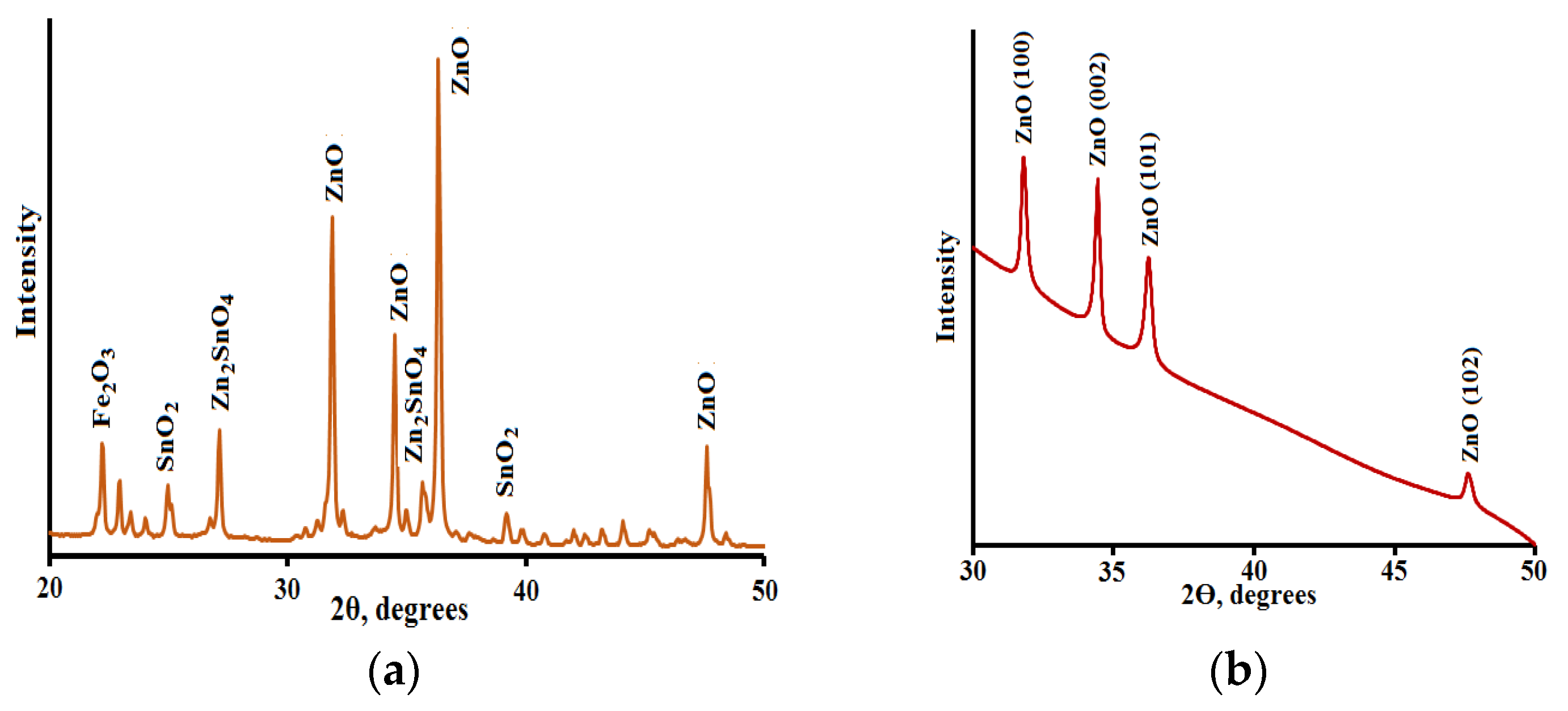
3.1. XRD Analysis
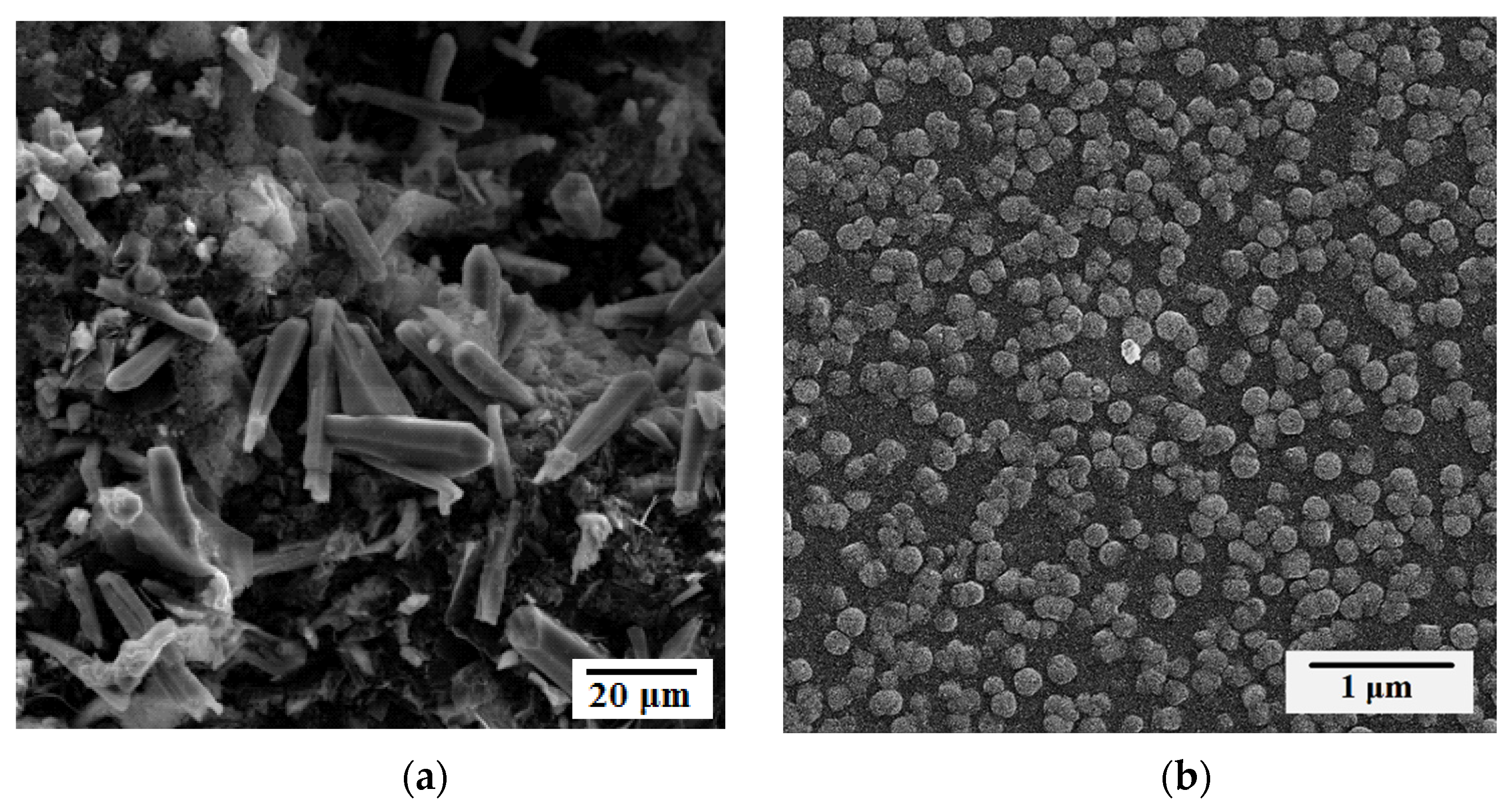
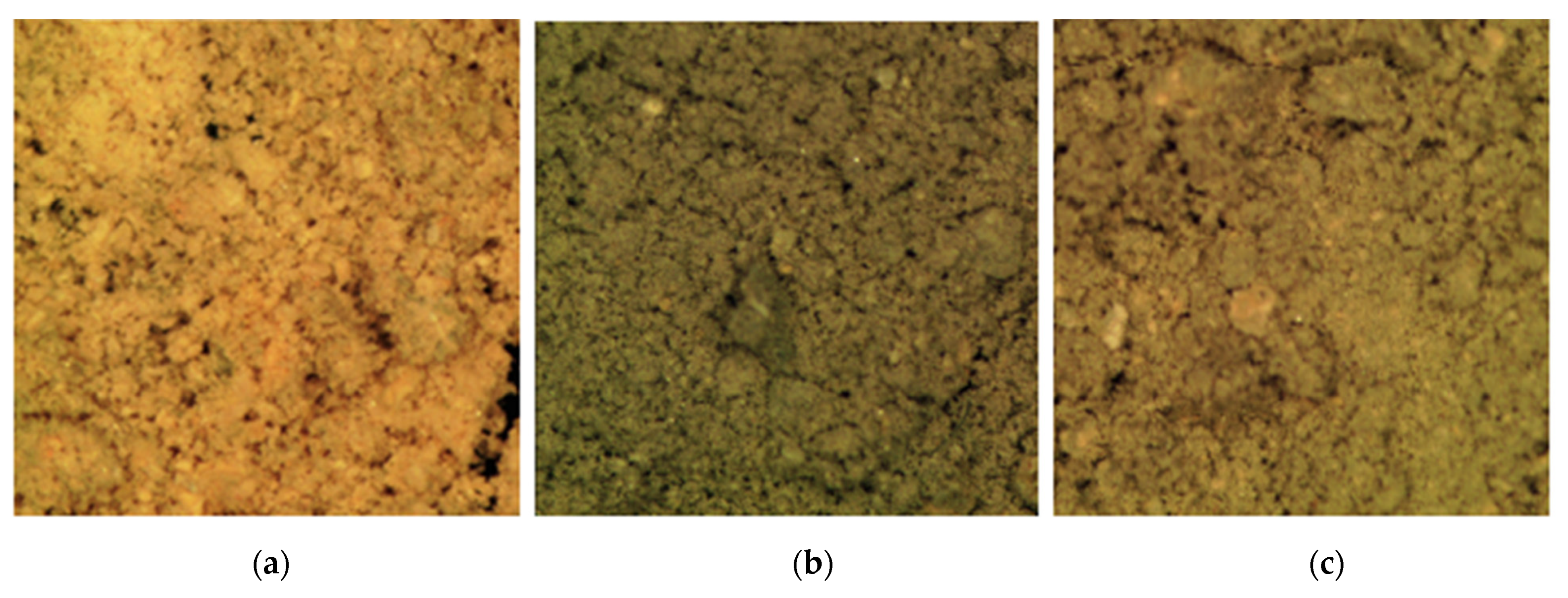
3.2. SEM Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vela, N.; Calín, M.; Yáñez-Gascón, M.J.; Garrido, I.; Pérez-Lucas, G.; Fenoll, J.; Navarro, S. Photocatalytic oxidation of six endocrine disruptor chemicals in wastewater using ZnO at pilot plant scale under natural sunlight. Environ. Sci. Pollut. Res. 2018, 25, 34995–35007. [Google Scholar] [CrossRef] [PubMed]
- Matos, R.S.; Attah-Baah, J.M.; Monteiro, M.D.S.; Costa, B.F.O.; Mâcedo, M.A.; Junior, R.S.S.; da Fonseca Filho, H.D.; Oliveira, R.M.P.B.; Ferreira, N.S. Effect of the amapá-latex chelating agent contens on the microstructure and photocatalytic properties of ZnO nanoparticles. J. Mater. Res. Technol. 2023, 22, 2673–2689. [Google Scholar] [CrossRef]
- Palai, A.; Panda, N.R.; Sahu, D. Novel ZnO blended SnO2 nanocatalysts exhibiting superior degradation of hazardous pollutants and enhanced visible photoemission properties. J. Mol. Struct. 2021, 1244, 131245. [Google Scholar] [CrossRef]
- Evstropiev, S.K.; Lesnykh, L.V.; Karavaeva, A.V.; Nikonorov, N.V.; Oreshkina, K.V.; Mironov, L.Y.; Maslennikov, S.Y.; Kolobkova, E.V.; Bagrov, I.V. Intensification of photodecomposition of organics contaminations by nanostructured ZnO-SnO2 coatings prepared by polymer-salt method. Chem. Eng. Process-Process Intens. 2019, 142, 107587. [Google Scholar] [CrossRef]
- Matos, R.S.; Attah-Baah, J.M.; Monteiro, M.D.S.; Costa, B.F.O.; Mâcedo, M.A.; Da Paz, S.P.A.; Angélica, R.S.; de Souza, T.M.; Ţălu, S.; Oliveira, R.M.P.B.; et al. Evaluation of the photocatalytic activity of distinctive-shaped ZnO nanocrystals synthesized using latex of different plants native to the Amazon rainforest. Nanomaterials 2022, 12, 2889. [Google Scholar] [CrossRef]
- Zaman, F.; Xie, B.; Zhang, J.; Gong, T.; Cui, K.; Hou, L.; Xu, J.; Zhai, Z.; Yuan, C. MOFs derived hetero-ZnO/Fe2O3 nanoflowers with enhanced photocatalytic performance towards efficient degradation of organic dyes. Nanomaterials 2021, 11, 3239. [Google Scholar] [CrossRef]
- Khomutinnikova, L.L.; Evstropiev, S.K.; Danilovich, D.P.; Meshkovskii, I.K.; Bulyga, D.V. Structural engineering of photocatalytic ZnO-SnO2-Fe2O3 composites. J. Comp. Sci. 2022, 6, 331. [Google Scholar] [CrossRef]
- Ramos, P.G.; Sánchez, L.A.; Rodriguez, J.M. A review on improving the efficiency of photocatalytic water decontamination using ZnO nanorods. J. Sol-Gel Sci. Technol. 2022, 102, 105–124. [Google Scholar] [CrossRef]
- Istomina, O.V.; Evstropiev, S.K.; Kolobkova, E.V.; Trofimov, A.O. Photolysis of diazo dye in solutions and films containing zinc and silver oxides. Opt. Spectr. 2018, 124, 774–778. [Google Scholar] [CrossRef]
- Lu, Y.H.; Xu, M.; Xu, L.; Zhang, C.L.; Zhang, Q.P.; Xu, X.V.; Xu, S.; Ostrikov, K. Enhanced ultraviolet photocatalytic activity of Ag/ZnO nanoparticles synthesized by modified polymer-network gel method. J. Nanopart. Res. 2015, 17, 350. [Google Scholar] [CrossRef]
- Dousthah, E.; Esmat, M.; Fukata, N.; Ide, Y.; Hanaor, D.A.H.; Assadi, M.H.N. MOF-derived nanocrystalline ZnO with controlled orientation and photocatalytic activity. Chemosphere 2022, 303, 134932. [Google Scholar] [CrossRef]
- Ferreira, N.S.; Sasaki, J.M.; Silva JR, R.S.; Attah-Baah, J.M.; Macêdo, M.A. Visible light-responsive photocatalytic activity significantly enhanced by active (VZn + Vo+) defects in self-assembled ZnO nanoparticles. Inorg. Chem. 2021, 69, 4475–4496. [Google Scholar] [CrossRef]
- Gatou, M.-A.; Lagopati, N.; Vagena, I.-A.; Gazouli, M.; Pavlatou, E.A. ZnO Nanoparticles from Different Precursors and Their Photocatalytic Potential for Biomedical Use. Nanomaterials 2023, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Gonullu, M.P.; Cergel, M.S.; Efkere, H.I.; Ates, H. Investigations of some physical properties of ALD growth ZnO films: Effect of crystal orientation on photocatalytic activity. J. Mater. Sci. Mater. Electron. 2021, 32, 12059–12074. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef] [PubMed]
- Shelemanov, A.A.; Evstropiev, S.K.; Karavaeva, A.V.; Nikonorov, N.V.; Vasilyev, V.N.; Podruhin, Y.F.; Kiselev, V.M. Enhanced Singlet Oxygen Photogeneration by Bactericidal ZnO–MgO–Ag Nanocomposites. Mater. Chem. Phys. 2022, 276, 125204. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, S.; Wang, S.; Zhang, Y.; Long, C.; Xie, J.; Fan, X.; Zhao, W.; Xu, P.; Fan, Y.; et al. Enabling specific photocatalytic methane oxidation by controlling free radical type. J. Am. Chem. Soc. 2023, 145, 2698–2707. [Google Scholar] [CrossRef]
- Turchi, C.S.; Ollis, D.F. Photocatalytic degradation of organic water contaminants: Mechanisms involving hydroxyl radical attack. J. Catal. 1990, 122, 178–192. [Google Scholar] [CrossRef]
- Deng, Y. Developing a Langmuir-type excitation equilibrium equation to describe the effect of light intensity on the kinetics of the photocatalytic oxidation. Chem. Eng. J. 2018, 337, 220–237. [Google Scholar] [CrossRef]
- Puma, G.L.; Salvadό-Estivill, I.; Obee, T.N.; Hay, S.O. Kinetics rate model of the photocatalytic oxidation of trichloroethylene in air over TiO2 thin films. Sep. Purif. Technol. 2009, 67, 226–232. [Google Scholar] [CrossRef]
- Belovolova, L.V. Reactive oxygen species in aqueous media (a review). Opt. Spectr. 2020, 128, 932–951. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide ion: Generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef]
- Abebe, B.; Ananda Murthy, H.C.; Amare, E. Summary on adsorption and photocatalysis for pollutant remediation: Mini review. J. Encapsulation Adsorpt. Sci. 2018, 8, 225–255. [Google Scholar] [CrossRef]
- Sun, X.; Xu, K.; Chatzitakis, A.; Norby, T. Photocatalytic generation of gas phase reactive oxygen species from adsorbed water: Remote action and electrochemical detection. J. Environ. Chem. Eng. 2021, 9, 104809. [Google Scholar] [CrossRef]
- Saratovskii, A.S.; Bulyga, D.V.; Evstrop’ev, S.K.; Antropova, T.V. Adsorption and photocatalytic activity of the porous glass-ZnO-Ag composite and ZnO-Ag nanopowder. Glass Phys. Chem. 2022, 48, 10–17. [Google Scholar] [CrossRef]
- Evstropiev, S.K.; Soshnikov, I.P.; Kolobkova, E.V.; Evstropyev, K.S.; Nikonorov, N.V.; Khrebtov, A.I.; Dukelskii, K.V.; Kotlyar, K.P.; Oreshkina, K.V.; Nashekin, A.V. Polymer-salt synthesis and characterization of MgO-ZnO ceramic coatings with the high transparency in UV spectral range. Opt. Mater. 2018, 82, 81–87. [Google Scholar] [CrossRef]
- Sokolov, I.S.; Maslennikov, S.Y.; Evstropiev, S.K.; Mironov, L.Y.; Nikonorov, N.V.; Oreshkina, K.V. YAG:Ce3+ phosphor nanopowders and thin textured coatings prepared by polymer-salt method. Opt. Eng. 2019, 58, 027103. [Google Scholar] [CrossRef]
- Evstropiev, S.K.; Karavaeva, A.V.; Petrova, M.A.; Nikonorov, N.V.; Vasilyev, V.N.; Lesnykh, L.L.; Dukelskii, K.V. Antibacterial effect of nanostructured ZnO-SnO2 coatings: The role of microstructure. Mater. Today Commun. 2019, 21, 100628. [Google Scholar] [CrossRef]
- Poongodi, G.; Ananan, P.; Mohan Kumar, R.; Jayavel, R. Studies on visible light photocatalytic and antibacterial activities of nanostructured cobalt doped ZnO thin films prepared by sol-gel coating method. Spectrochim. Acta A Mol. Biomol. Spectr. 2015, 148, 237–243. [Google Scholar] [CrossRef]
- Boltenkov, I.S.; Kolobkova, E.V.; Evstropiev, S.K. Synthesis and characterization of transparent photocatalytic ZnO-Sm2O3 and ZnO-Er2O3 coatings. J. Photochem. Photobiol. A Chem. 2018, 367, 458–464. [Google Scholar] [CrossRef]
- Talinungsang; Upadhaya, D.; Kumar, P.; Purkayastha, D.D. Superhydrophilicity of photocatalytic ZnO/SnO2 heterostructure for self-cleaning applications. J. Sol-Gel Sci. Technol. 2019, 92, 575–584. [Google Scholar] [CrossRef]
- Gayle, A.J.; Lenef, J.D.; Huff, P.A.; Wang, J.; Fu, F.; Dadheech, G.; Dasgupta, N.P. Visible-light-driven photocatalysts for self-cleaning transparent surfaces. Langmuir 2022, 38, 11641–11649. [Google Scholar] [CrossRef]
- Hamrouni, A.; Moussa, N.; Parrino, F.; Di Paola, A.; Houas, A.; Palmisano, L. Sol-gel synthesis and photocatalytic activity of ZnO-SnO2 nanocomposites. J. Molec. Catal. A Chem. 2014, 390, 133–141. [Google Scholar] [CrossRef]
- Sabry, R.S.; Rahmah, M.I.; Aziz, W.J. A systematic study to evaluate effects of stearic acid on superhydrophobicity and photocatalytic properties of Ag –doped ZnO nanostructures. J. Mat. Sci. Mater. Electron. 2020, 31, 13382–13391. [Google Scholar] [CrossRef]
- Zarei, S.; Hasheminiasari, M.; Masoudpanah, S.M.; Javadpour, J. Photocatalytic properties of ZnO/SnO2 nanocomposite films: Role of morphology. J. Mater. Res. Technol. 2022, 17, 2305–2312. [Google Scholar] [CrossRef]
- Xu, L.; Xian, F.; Zhang, Y.; Wang, W.; Qiu, K.; Xu, J. Synthesis of ZnO-decorated SnO2 nanopowder with enhanced photocatalytic performance. Optik 2019, 194, 162965. [Google Scholar] [CrossRef]
- Manoharan, C.; Pavithra, G.; Dhanapandian, S.; Dhamodaran, P.; Shanthi, B. Properties of spray pyrolised ZnO:Sn thin films and their antibacterial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 141, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Tahir, D.; Ilyas, S.; Rahmat, R.; Heryanto, H.; Fahri, A.N.; Rahmi, M.H.; Abdullah, B.; Hong, C.C.; Kang, H.J. Enhanced visible-light absorption of Fe2O3 covered by activated carbon for multifunctional purposes: Tuning the structural, electronic, optical, and magnetic properties. ACS Omega 2021, 6, 28334–28346. [Google Scholar] [CrossRef] [PubMed]
- Abbott, L.C.; Batchelor, S.N.; Oakes, J.; Lindsay Smith, J.R.; Moore, J.N. Spectroscopic studies of the intermolecular interactions of a Bis-Azo Dye, Direct Blue 1, on Di and trimerization in aqueous solution and in cellulose. J. Phys. Chem. B 2004, 108, 13726–13735. [Google Scholar] [CrossRef]
- El-Bahy, Z.M.; Ismail, A.A.; Mohamed, R.M. Enhancement of titania by doping rare earth for photodegradation of organic dye (Direct Blue). J. Hazard. Mater. 2009, 165, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Volkova, N.A.; Evstropiev, S.K.; Istomina, O.V.; Kolobkova, E.V. Photolysis of diazo dye in aqueous solutions of metal nitrates. Opt. Spectr. 2018, 124, 489–493. [Google Scholar] [CrossRef]
- Mohamed, G.R.M.; Mkhalid, I.A.; Al-Thabaiti, S.A.; Mohamed, M. Nano Cu metal doped on TiO2-SiO2 nanoparticle catalysts in photocatalytic degradation of direct blue dye. J. Nanosci. Nanotechnol. 2013, 13, 4975–4980. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, K.; Vasanthi, M.; Thirumurugan, K.; Sakthivel, B.; Karthika, K. Annealing induced reorientation of crystallites in Sn doped ZnO films. Opt. Mater. 2014, 37, 59–64. [Google Scholar] [CrossRef]
- Guo, L.; Yang, S.; Yang, C.; Yu, P.; Wang, J.; Ge, W.; Wong, G.K.L. Highly monodisperse polymer-capped ZnO nanoparticles: Preparation and optical properties. Appl. Phys. Lett. 2000, 76, 2901–2903. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Mang, A.; Reimann, K.; Rübenacke, S. Band gap, crystal field splitting, spin-orbit coupling and exciton binding energies in ZnO under hydrostatic pressure. Solid State Commun. 1995, 94, 251–254. [Google Scholar] [CrossRef]
- Koike, K.; Hama, K.; Nakashima, I.; Takada, G.; Ogata, K.; Sasa, S.; Inoue, M.; Yano, M. Molecular beam epitaxial growth of wide bandgap ZnMgO alloy films on (111)-oriented Si substrate toward UV-detector applications. J. Cryst. Growth 2005, 278, 288–292. [Google Scholar] [CrossRef]
- Ohtomo, A.; Kawasaki, M.; Koida, T.; Masubuchi, K.; Koinuma, H.; Sakurai, Y.; Yoshida, Y.; Yasuda, T.; Segawa, Y. MgxZn1-xO as a II-VI wide gap semiconductor alloy. Appl. Phys. Lett. 1998, 72, 2466. [Google Scholar] [CrossRef]
- Toshihiro, D.; Yoshio, N. Formation and behavior of singlet molecular oxygen in TiO2 photocatalysis studied by detection of near-infrared phosphorescence. J. Phys. Chem. C 2007, 111, 4420–4424. [Google Scholar]
- Bell, S.; Will, G.; Bell, J. Light intensity effects on photocatalytic water splitting with a titania catalyst. Int. J. Hydrogen Energy 2013, 38, 6938–6947. [Google Scholar] [CrossRef]
| Compound | Supplier | Specification |
|---|---|---|
| Zn(NO3)2·6 H2O | Neva Reactive Co., (Neva Reactive, Saint-Petersburg, Russia) | UPCR-5106.F00250 CAS: 0196-18-6 |
| SnCl2·2 H2O | MERCK | MERCK-1078150250 CA S: 10025-69-1 ACS. ISO. Reag. PhEur. |
| FeCl3· 6 H2O | Neva Reactive Co., (Neva Reactive, Saint-Petersburg, Russia) | ACROS-217091000 CAS: 10025-77-1 |
| Polyvinylpyrrolidone | Sigma-Aldrich | K30; CAS: 9003-39-8 |
| Chicago Sky Blue 6 B | Sigma-Aldrich | CAS: 2610-05-1 |
| Planes (hkl) Planes | Relative Peaks Intensities | Peaks Positions 2Q, Degrees | ||||
|---|---|---|---|---|---|---|
| JCPDS Card No. 36-1451 | Ceramic Powder | Ceramic Coating | JCPDS Card No. 36-1451 | Ceramic Powder | Ceramic Coating | |
| (100) | 57 | 55 | 82 | 31.770 | 31.85 | 31.78 |
| (002) | 44 | 36 | 100 | 34.422 | 34.50 | 34.42 |
| (101) | 100 | 100 | 70 | 36.253 | 36.30 | 36.21 |
| Photocatalysts | Constant Rate, min−1 | References |
|---|---|---|
| ZnO 100% | 0.022 | [4] |
| ZnO 93.2 mol.% + SnO2 6.8 mol.% | 0.026 | [4] |
| ZnO 95.7 mol.% + Er2O3 4.3 mol.% | 0.012 | [26] |
| ZnO 95.3 mol.% + Er2O3 4.7 mol.% | 0.017 | [26] |
| ZnO 96.2 mol.% + Sm2O3 3.8 mol.% | 0.014 | [26] |
| ZnO 95 mol.% + SnO2 3 mol.% + Fe2O3 2 mol.% | 0.056 | present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khomutinnikova, L.; Evstropiev, S.; Meshkovskii, I.; Bagrov, I.; Kiselev, V. Ceramic ZnO-SnO2-Fe2O3 Powders and Coatings -Effective Photogenerators of Reactive Oxygen Species. Ceramics 2023, 6, 886-897. https://doi.org/10.3390/ceramics6020051
Khomutinnikova L, Evstropiev S, Meshkovskii I, Bagrov I, Kiselev V. Ceramic ZnO-SnO2-Fe2O3 Powders and Coatings -Effective Photogenerators of Reactive Oxygen Species. Ceramics. 2023; 6(2):886-897. https://doi.org/10.3390/ceramics6020051
Chicago/Turabian StyleKhomutinnikova, Larisa, Sergey Evstropiev, Igor Meshkovskii, Igor Bagrov, and Valery Kiselev. 2023. "Ceramic ZnO-SnO2-Fe2O3 Powders and Coatings -Effective Photogenerators of Reactive Oxygen Species" Ceramics 6, no. 2: 886-897. https://doi.org/10.3390/ceramics6020051
APA StyleKhomutinnikova, L., Evstropiev, S., Meshkovskii, I., Bagrov, I., & Kiselev, V. (2023). Ceramic ZnO-SnO2-Fe2O3 Powders and Coatings -Effective Photogenerators of Reactive Oxygen Species. Ceramics, 6(2), 886-897. https://doi.org/10.3390/ceramics6020051