Powders Synthesized from Solutions of Calcium Chloride, Sodium Hydrogen Phosphate, and Sodium Sulfate for Bioceramics Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Powders and Ceramic Samples Preparation
2.2. Characterization of Samples
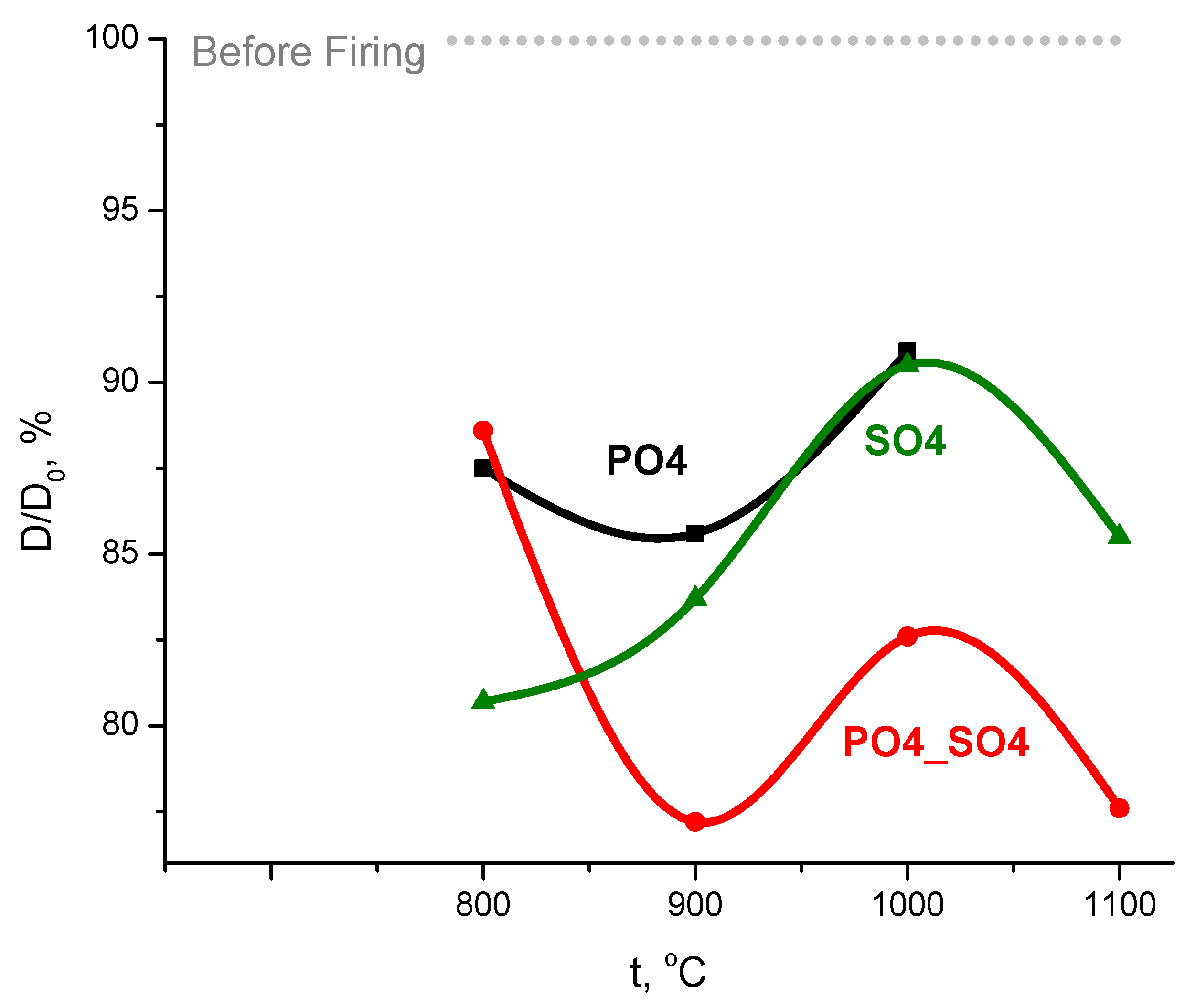
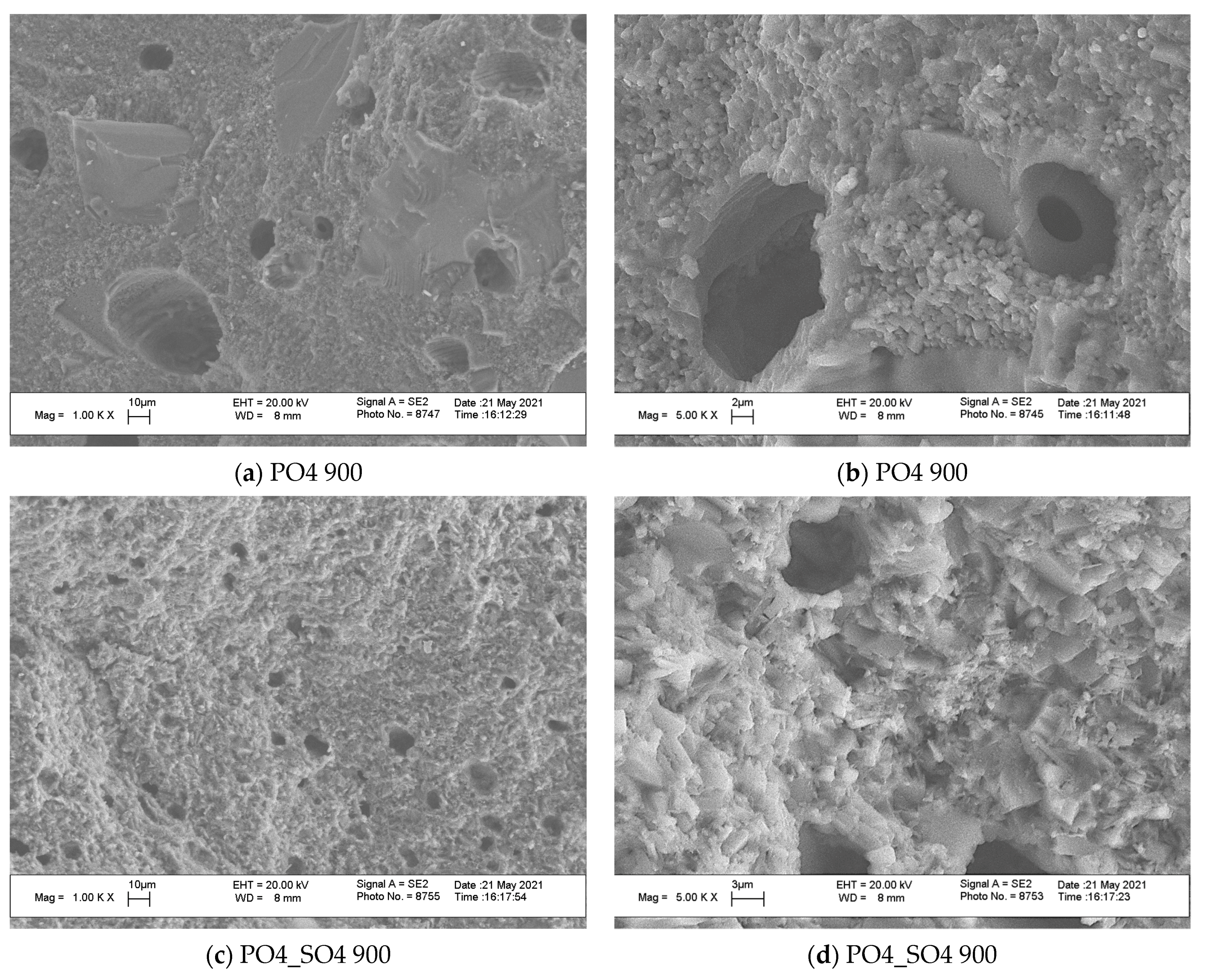
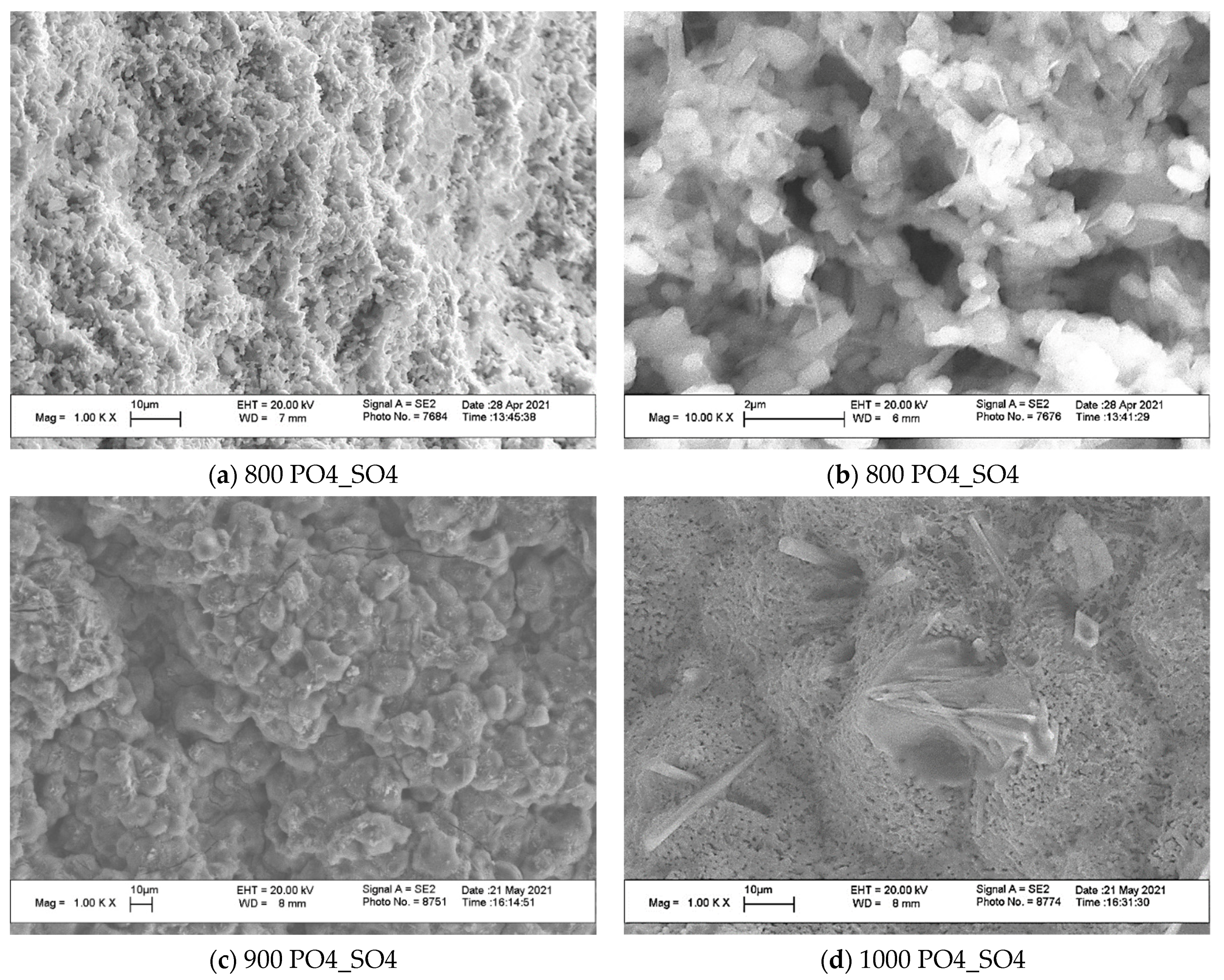
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Navarro, M.; Michiardi, A.; Castan, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef]
- Safronova, T.V. Inorganic materials for regenerative medicine. Inorg. Mater. 2021, 57, 443–474. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef]
- Niu, Y.Q.; Liu, J.H.; Aymonier, C.; Fermani, S.; Kralj, D.; Falini, G.; Zhou, C.H. Calcium carbonate: Controlled synthesis, surface functionalization, and nanostructured materials. Chem. Soc. Rev. 2022, 51, 7883–7943. [Google Scholar] [CrossRef]
- Combes, C.; Bareille, R.; Rey, C. Calcium carbonate–calcium phosphate mixed cement compositions for bone reconstruction. J. Biomed. Mater. Res. A 2006, 79A, 318–328. [Google Scholar] [CrossRef]
- Yukna, R.A. Clinical evaluation of coralline calcium carbonate as a bone replacement graft material in human periodontal osseous defects. J. Periodontol. 1994, 65, 177–185. [Google Scholar] [CrossRef]
- Youness, R.A.; Tag El-deen DMTaha, M.A. A Review on Calcium Silicate Ceramics: Properties, Limitations, and Solutions for Their Use in Biomedical Applications. Silicon 2022. [Google Scholar] [CrossRef]
- Almulhim, K.S.; Syed, M.R.; Alqahtani, N.; Alamoudi, M.; Khan, M.; Ahmed, S.Z.; Khan, A.S. Bioactive Inorganic Materials for Dental Applications: A Narrative Review. Materials 2022, 15, 6864. [Google Scholar] [CrossRef]
- Ros-Tárraga, P.; Mazón, P.; Meseguer-Olmo, L.; De Aza, P.N. Revising the subsystem Nurse’s a-phase-silicocarnotite within the system Ca3(PO4)2–Ca2SiO4. Materials 2016, 9, 322. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. A review of bioactive silicate ceramics. Biomed. Mater. 2013, 8, 032001. [Google Scholar] [CrossRef]
- Kuo, S.-T.; Wu, H.-W.; Tuan, W.-H.; Tsai, Y.-Y.; Wang, S.-F.; Sakka, Y. Porous calcium sulfate ceramics with tunable degradation rate. J. Mater. Sci. Mater. Med. 2012, 23, 2437–2443. [Google Scholar] [CrossRef]
- Ene, R.; Nica, M.; Ene, D.; Cursaru, A.; Cirstoiu, C. Review of calcium-sulphate-based ceramics and synthetic bone substitutes used for antibiotic delivery in PJI and osteomyelitis treatment. EFORT Open Rev. 2021, 6, 297–304. [Google Scholar] [CrossRef]
- Yahav, A.; Kurtzman, G.M.; Katzap, M.; Dudek, D.; Baranes, D. Bone regeneration: Properties and clinical applications of biphasic calcium sulfate. Dent. Clin. 2020, 64, 453–472. [Google Scholar] [CrossRef]
- Walsh, W.R.; Morberg, P.; Yu, Y.; Yang, J.L.; Haggard, W.; Sheath, P.C.; Bruce, W.J.M. Response of a calcium sulfate bone graft substitute in a confined cancellous defect. Clin. Orthop. Relat. Res. 2003, 406, 228–236. [Google Scholar] [CrossRef]
- Medvecky, L.; Giretova, M.; Stulajterova, R.; Luptakova, L.; Sopcak, T. Tetracalcium Phosphate/Monetite/Calcium Sulfate Hemihdrate Biocement Powder Mixtures Prepared by the One-Step Synthesis for Preparation of Nanocrystalline Hydroxyapatite Biocement-Properties and In Vitro Evaluation. Materials 2021, 14, 2137. [Google Scholar] [CrossRef]
- Yang, D.A.; Yang, Z.; Li, X.; Di, L.Z.; Zhao, H. A study of hydroxyapatite/calcium sulphate bioceramics. Ceram. Int. 2005, 31, 1021–1023. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, D.A.; Zhao, H. Degradation behavior of calcium sulfate/β-tricalcium phosphate composites in Tris. Key Eng. Mater. 2007, 336, 1635–1637. [Google Scholar] [CrossRef]
- Urban, R.M.; Turner, T.M.; Hall, D.J.; Inoue, N.; Gitelis, S. Increased bone formation using calcium sulfate-calcium phosphate composite graft. Clin. Orthop. Relat. Res. 2007, 459, 110–117. [Google Scholar] [CrossRef]
- Guo, H.; Wei, J.; Liu, C.S. Development of a degradable cement of calcium phosphate and calcium sulfate composite for bone reconstruction. Biomed. Mater. 2006, 1, 193–197. [Google Scholar] [CrossRef]
- Laskus, A.; Kolmas, J. Ionic Substitutions in Non-Apatitic Calcium Phosphates. Int. J. Mol. Sci. 2017, 18, 2542. [Google Scholar] [CrossRef]
- Safronova, T.; Kiselev, A.; Selezneva, I.; Shatalova, T.; Lukina, Y.; Filippov, Y.; Toshev, O.; Tikhonova, S.; Antonova, O.; Knotko, A. Bioceramics Based on β-Calcium Pyrophosphate. Materials 2022, 15, 3105. [Google Scholar] [CrossRef]
- Gbureck, U.; Hölzel, T.; Biermann, I.; Barralet, J.E.; Grover, L.M. Preparation of tricalcium phosphate/calcium pyrophosphate structures via rapid prototyping. J. Mater. Sci. Mater. Med. 2008, 19, 1559–1563. [Google Scholar] [CrossRef]
- Safronova, T.V.; Belokozenko, M.A.; Yahyoev, S.O.; Shatalova, T.B.; Kazakova, G.K.; Peranidze, K.K.; Toshev, O.U.; Khasanova, S.S. Ceramics Based on CaSO4⋅2H2O Powder Synthesized from Ca(NO3)2 and (NH4)2SO4. Inorg. Mater. 2021, 57, 867–873. [Google Scholar] [CrossRef]
- Hsu, P.Y.; Chang, M.P.; Tuan, W.H.; Lai, P.L. Effect of physical and chemical characteristics on the washout resistance of calcium sulfate pellets. Ceram. Int. 2018, 44, 8934–8939. [Google Scholar] [CrossRef]
- Chang, M.P.; Hsu, H.C.; Tuan, W.H.; Lai, P.L. A Feasibility Study Regarding the Potential Use of Silica-Doped Calcium Sulfate Anhydrite as a Bone Void Filler. J. Med. Biol. Eng. 2017, 37, 879–886. [Google Scholar] [CrossRef]
- Chang, M.P.; Tsung, Y.C.; Hsu, H.C.; Tuan, W.H.; Lai, P.L. Addition of a small amount of glass to improve the degradation behavior of calcium sulfate bioceramic. Ceram. Int. 2015, 41, 1155–1162. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, C.; Feng, P.; Tao Xiao, T.; Shuai, C.; Peng, S. Calcium sulfate bone scaffolds with controllable porous structure by selective laser sintering. J. Porous. Mater. 2015, 22, 1171–1178. [Google Scholar] [CrossRef]
- Zhou, J.; Yuan, F.; Peng, S.; Xie, H.; Wu, P.; Feng, P.; Gao, C.; Yang, Y.; Guo, W.; Lai, D.; et al. Tunable Degradation Rate and Favorable Bioactivity of Porous Calcium Sulfate Scaffolds by Introducing Nano-Hydroxyapatite. Appl. Sci. 2016, 6, 411. [Google Scholar] [CrossRef]
- Toshima, T.; Hamai, R.; Tafu, M.; Takemura, Y.; Fujita, S.; Chohji, T.S.; Tanda, S.; Li Qin, G.W. Morphology control of brushite prepared by aqueous solution synthesis. J. Asian Ceram. Soc. 2014, 2, 52–56. [Google Scholar] [CrossRef]
- Boanini, E.; Pagani, S.; Tschon, M.; Rubini, K.; Fini, M.; Bigi, A. Monetite vs. Brushite: Different Influences on Bone Cell Response Modulated by Strontium Functionalization. J. Funct. Biomater. 2022, 13, 65. [Google Scholar] [CrossRef]
- Safronova, T.V.; Shatalova, T.B.; Tikhonova, S.A.; Filippov, Y.Y.; Krut’ko, V.K.; Musskaya, O.N.; Kononenko, N.E. Synthesis of Calcium Pyrophosphate Powders from Phosphoric Acid and Calcium Carbonate. Inorg. Mater. Appl. Res. 2021, 12, 986–992. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; Gbureck, U.; Bhaduri, S.B.; Sikder, P. Monetite, an important calcium phosphate compound–Its synthesis, properties and applications in orthopedics. Acta Biomater. 2021, 127, 41–55. [Google Scholar] [CrossRef]
- Safronova, T.V.; Sadilov, I.S.; Chaikun, K.V.; Shatalova, T.B.; Filippov, Y.Y. Synthesis of Monetite from Calcium Hydroxyapatite and Monocalcium Phosphate Monohydrate under Mechanical Activation Conditions. Russ. J. Inorg. Chem. 2019, 64, 1088–1094. [Google Scholar] [CrossRef]
- Safronova, T.V.; Putlyaev, V.I.; Kurbatova, S.A.; Shatalova, T.B.; Larionov, D.S.; Kozlov, D.A.; Evdokimov, P.V. Properties of amorphous calcium pyrophosphate powder synthesized via ion exchange for the preparation of bioceramics. Inorg. Mater. 2015, 51, 1177–1184. [Google Scholar] [CrossRef]
- Safronova, T.V.; Putlyaev, V.I.; Ivanov, V.K.; Knot’ko, A.V.; Shatalova, T.B. Powders mixtures based on ammonium pyrophosphate and calcium carbonate for preparation of biocompatible porous ceramic in the CaO–P2O5 system. Refract. Ind. Ceram. 2016, 56, 502–509. [Google Scholar] [CrossRef]
- Toshev, O.; Safronova, T.; Kaimonov, M.; Shatalova, T.; Klimashina, E.; Lukina, Y.; Malyutin, K.; Sivkov, S. Biocompatibility of Ceramic Materials in Ca2P2O7–Ca(PO3)2 System Obtained via Heat Treatment of Cement-Salt Stone. Ceramics 2022, 5, 516–532. [Google Scholar] [CrossRef]
- Bolarinwa, A.; Gbureck, U.; Purnell, P.; Bold, M.; Grover, L.M. Cement casting of calcium pyrophosphate based bioceramics. Adv. Appl. Ceram. 2010, 109, 291–295. [Google Scholar] [CrossRef]
- Safronova, T.V.; Korneichuk, S.A.; Shatalova, T.B.; Lukina Yu, S.; Sivkov, S.P.; Filippov, Y.Y.; Krut’ko, V.K.; Musskaya, O.N. Ca2P2O7–Ca(PO3)2 Ceramic Obtained by Firing β-Tricalcium Phosphate and Monocalcium Phosphate Monohydrate Based Cement Stone. Glass Ceram. 2020, 77, 165–172. [Google Scholar] [CrossRef]
- Asadi-Eydivand, M.; Solati-Hashjin, M.; Shafiei, S.S.; Mohammadi, S.; Hafezi, M.; Abu Osman, N.A. Structure, properties, and in vitro behavior of heat-treated calcium sulfate scaffolds fabricated by 3D printing. PloS ONE 2016, 11, e0151216. [Google Scholar] [CrossRef]
- Rahman, F. Calcium sulfate precipitation studies with scale inhibitors for reverse osmosis desalination. Desalination 2013, 319, 79–84. [Google Scholar] [CrossRef]
- Combes, C.; Rey, C. Amorphous calcium phosphates: Synthesis, properties and uses in biomaterials. Acta biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef]
- Mosina, M.; Locs, J. Synthesis of Amorphous Calcium Phosphate: A Review. Key Eng. Mater. 2020, 850, 199–206. [Google Scholar] [CrossRef]
- Kukueva, E.V.; Putlyaev, V.I.; Tikhonov, A.A.; Safronova, T.V. Octacalcium phosphate as a precursor for the fabrication of composite bioceramics. Inorg. Mater. 2017, 53, 212–219. [Google Scholar] [CrossRef]
- Kukueva, E.V.; Putlyaev, V.I.; Safronova, T.V.; Tikhonov, A.A. Composite bioceramic based on octacalcium phosphate decomposition products. Glass Ceram. 2017, 74, 67–72. [Google Scholar] [CrossRef]
- Safronova, T.V.; Putlyaev, V.I.; Filippov, Y.; Knot’ko, A.V.; Klimashina, E.S.; Peranidze, K.K.; Vladimirova, S.A. Powders Synthesized from Calcium Acetate and Mixed-Anionic Solutions, Containing Orthophosphate and Carbonate Ions, for Obtaining Bioceramic. Glass Ceram. 2018, 75, 118–123. [Google Scholar] [CrossRef]
- Joksa, A.A.; Komarovska, L.; Ubele-Kalnina, D.; Viksna, A.; Gross, K.A. Role of carbonate on the crystallization and processing of amorphous calcium phosphates. Materialia 2023, 27, 101672. [Google Scholar] [CrossRef]
- Glazov, I.E.; Krut’ko, V.K.; Musskaya, O.N.; Kulak, A.I. Low-Temperature Formation and Identification of Biphasic Calcium Carbonate Phosphates. Russ. J. Inorg. Chem. 2022, 67, 1718–1730. [Google Scholar] [CrossRef]
- Ślósarczyk, A.; Paszkiewicz, Z.; Paluszkiewicz, C. FTIR and XRD evaluation of carbonated hydroxyapatite powders synthesized by wet methods. J. Mol. Struct. 2005, 744, 657–661. [Google Scholar] [CrossRef]
- Peranidze, K.; Safronova, T.V.; Filippov, Y.; Kazakova, G.; Shatalova, T.; Rau, J.V. Powders Based on Ca2P2O7-CaCO3-H2O System as Model Objects for the Development of Bioceramics. Ceramics 2022, 5, 423–434. [Google Scholar] [CrossRef]
- Safronova, T.V.; Knot’ko, A.V.; Shatalova, T.B.; Evdokimov, P.V.; Putlyaev, V.I.; Kostin, M.S. Calcium phosphate ceramic based on powder synthesized from a mixed-anionic solution. Glass Ceram. 2016, 73, 25–31. [Google Scholar] [CrossRef]
- Golubchikov, D.; Safronova, T.V.; Nemygina, E.; Shatalova, T.B.; Tikhomirova, I.N.; Roslyakov, I.V.; Khayrutdinova, D.; Platonov, V.; Boytsova, O.; Kaimonov, M.; et al. Powder Synthesized from Aqueous Solution of Calcium Nitrate and Mixed-Anionic Solution of Orthophosphate and Silicate Anions for Bioceramics Production. Coatings 2023, 13, 374. [Google Scholar] [CrossRef]
- Zarga, Y.; Boubaker, H.B.; Ghaffour, N.; Elfil, H. Study of calcium carbonate and sulfate co-precipitation. Chem. Eng. Sci. 2013, 96, 33–41. [Google Scholar] [CrossRef]
- Khayrutdinova, D.R.; Goldberg, M.A.; Antonova, O.S.; Krokhicheva, P.A.; Fomin, A.S.; Obolkina, T.O.; Konovalov, A.A.; Akhmedova, S.A.; Sviridova, I.K.; Kirsanova, V.A.; et al. Effects of Heat Treatment on Phase Formation in Cytocompatible Sulphate-Containing Tricalcium Phosphate Materials. Minerals 2023, 13, 147. [Google Scholar] [CrossRef]
- Safronova, T.V.; Putlyaev, V.I.; Filippov, Y.; Shatalova, T.B.; Fatin, D.S. Ceramics based on brushite powder synthesized from calcium nitrate and disodium and dipotassium hydrogen phosphates. Inorg. Mater. 2018, 54, 195–207. [Google Scholar] [CrossRef]
- Safronova, T.V. Phase composition of ceramic based on calcium hydroxyapatite powders containing byproducts of the synthesis reaction. Glass Ceram. 2009, 66, 136–139. [Google Scholar] [CrossRef]
- Safronova, T.V.; Secheiko, P.A.; Putlyaev, V.I. Multiphase ceramics based on powders synthesized from sodium pyrophosphate and soluble calcium salts using mechanical activation. Glass Ceram. 2012, 69, 276–282. [Google Scholar] [CrossRef]
- Safronova, T.V.; Putlyaev, V.I.; Kuznetsov, A.V.; Ketov, N.A.; Veresov, A.G. Properties of calcium phosphate powder synthesized from calcium acetate and sodium hydrophosphate. Glass Ceram. 2011, 68, 131–135. [Google Scholar] [CrossRef]
- Safronova, T.V.; Steklov, M.Y.; Putlyaev, V.I.; Shekhirev, M.A. Na-substituted Ca-deficient carbonate hydroxyapatite for the production of ceramic materials. Compos. Mater. Constr. (Konstr. Iz Kompoz. Mater.) 2006, 4, 34–39. (In Russian) [Google Scholar]
- Safronova, T.V.; Putlyaev, V.I.; Knot’ko, A.V.; Shatalova, T.B.; Artemov, M.V.; Filippov, Y.Y. Properties of Calcium Phosphate Powder Synthesized from Calcium Chloride and Potassium Pyrophosphate. Inorg. Mater. Appl. Res. 2020, 11, 44–49. [Google Scholar] [CrossRef]
- Safronova, T.V.; Korneichuk, S.A.; Putlyaev, V.I.; Krut’ko, V.K. Ceramics based on calcium hydroxyapatite synthesized from calcium acetate, calcium hydroxide, and potassium hydrophosphate. Glass Ceram. 2012, 69, 30–36. [Google Scholar] [CrossRef]
- Shiryaev, M.; Safronova, T.; Putlyaev, V. Calcium phosphate powders synthesized from calcium chloride and potassium hydrophosphate. J. Therm. Anal. Calorim. 2010, 101, 707–713. [Google Scholar] [CrossRef]
- Safronova, T.V.; Shiryaev, M.A.; Putlyaev, V.I.; Murashov, V.A.; Protsenko, P.V. Ceramics based on hydroxyapatite synthesized from calcium chloride and potassium hydrophosphate. Glass Ceram. 2009, 66, 66–69. [Google Scholar] [CrossRef]
- Aslanian, S.; Stoilova, D. Étude de l’ardéalite, membre intermédiaire du système gypse˗brushite. Bull. De Minéralogie 1982, 105, 621–624. [Google Scholar] [CrossRef]
- Rinaudo, C.; Lanfranco, A.M.; Franchini-Angela, M. The system CaHPO4· 2H2O-CaSO4· 2H2O: Crystallizations from calcium phosphate solutions in the presence of SO2-4. J. Cryst. Growth 1994, 142, 184–192. [Google Scholar] [CrossRef]
- Sakae, T.; Nagata, H.; Sudo, T. The crystal structure of synthetic calcium phosphate-sulfate hydrate, Ca2HPO4 SO4·4H2O, and its relation to brushite and gypsum. Am. Mineral. 1978, 63, 520–527. [Google Scholar]
- Pinto, A.J.; Carneiro, J.; Katsikopoulos, D.; Jiménez, A.; Prieto, M. The link between brushite and gypsum: Miscibility, dehydration, and crystallochemical behavior in the CaHPO4·2H2O–CaSO4·2H2O system. Cryst. Growth Des. 2012, 12, 445–455. [Google Scholar] [CrossRef]
- Moussa, H.; El Hadad, A.; Sarrigiannidis, S.; Saad, A.; Wang, M.; Taqi, D.; Tamimi, F. High toughness resorbable brushite-gypsum fiber-reinforced cements. Mater. Sci. Eng. C 2021, 127, 112205. [Google Scholar] [CrossRef]
- Dumitraş, D.G. A re-investigation of ardealite from the type locality, the “dry” Cioclovina Cave (Şureanu Mountains, Romania). Eur. J. Mineral. 2017, 29, 1055–1066. [Google Scholar] [CrossRef]
- Dumitraş, D.G.; Marincea, Ş. Sequential dehydration of the phosphate–sulfate association from Gura Dobrogei Cave, Dobrogea, Romania. Eur. J. Mineral. 2021, 33, 329–340. [Google Scholar] [CrossRef]
- ICDD. International Centre for Diffraction Data; PDF-4+ 2010 (Database); Kabekkodu, S., Ed.; ICDD: Newtown Square, PA, USA, 2010; Available online: https://www.icdd.com/pdf-2/ (accessed on 10 December 2022).
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 5th ed.; Wiley: New York, NY, USA, 1986; pp. 156–159. [Google Scholar]
- Taherdangkoo, R.; Tian, M.; Sadighi, A.; Meng, T.; Yang, H.; Butscher, C. Experimental Data on Solubility of the Two Calcium Sulfates Gypsum and Anhydrite in Aqueous Solutions. Data 2022, 7, 140. [Google Scholar] [CrossRef]
- Isa, K.; Okuno, H. Thermal decomposition of calcium sulfate dihydrate under self-generated atmosphere. Bull. Chem. Soc. Jpn. 1982, 55, 3733–3737. [Google Scholar] [CrossRef]
- Frost, R.L.; Palmer, S.J.; Pogson, R. Thermal stability of the ‘cave’ mineral ardealite Ca2(HPO4)(SO4)·4H2O. J. Therm. Anal. Calorim. 2012, 107, 549–553. [Google Scholar] [CrossRef]
- Ostroff, A.G.; Sanderson, R.T. Thermal stability of some metal sulphates. J. Inorg. Nucl. Chem. 1959, 9, 45–50. [Google Scholar] [CrossRef]
- Collier, N.C. Transition and decomposition temperatures of cement phases–a collection of thermal analysis data. Ceramics-Silikaty 2016, 60, 338–343. [Google Scholar] [CrossRef]
- Freyer, D.; Voigt, W. Crystallization and phase stability of CaSO4 and CaSO4–based salts. Mon. Für Chem./Chem. Mon. 2003, 134, 693–719. [Google Scholar] [CrossRef]
- Suchanek, W.; Yashima, M.; Kakihana, M.; Yoshimura, M. Hydroxyapatite ceramics with selected sintering additives. Biomaterials 1997, 18, 923–933. [Google Scholar] [CrossRef]
- Safronova, T.F.; Putlyaev, V.I.; Filippov, Y.Y.; Larionov, D.S.; Evdokimov, P.V.; Averina, A.E.; Klimashina, E.S.; Ivanov, V.K. Porous Ceramic Based on Calcium Pyrophosphate. Refract. Ind. Ceram. 2015, 56, 43–47. [Google Scholar] [CrossRef]
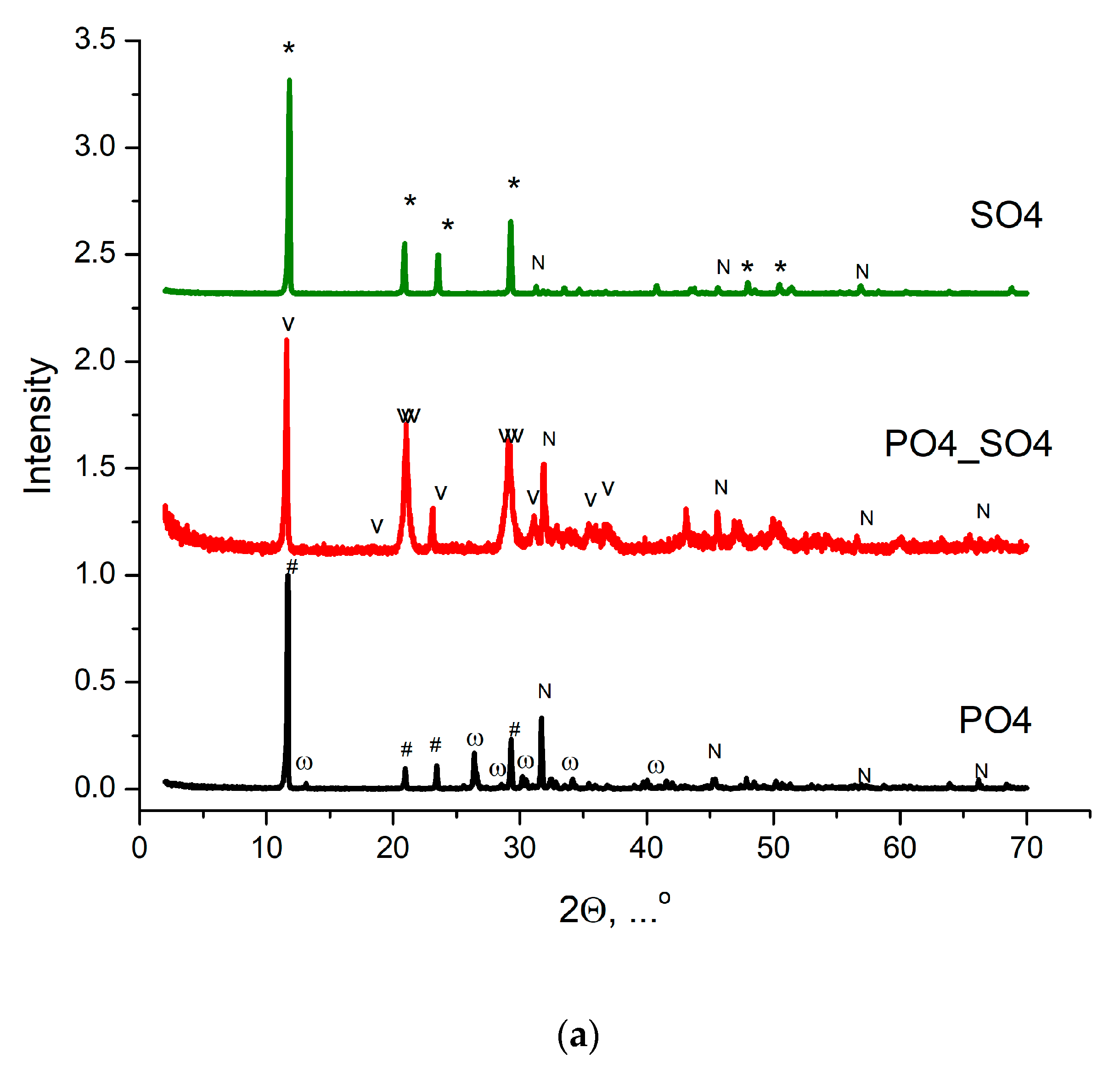
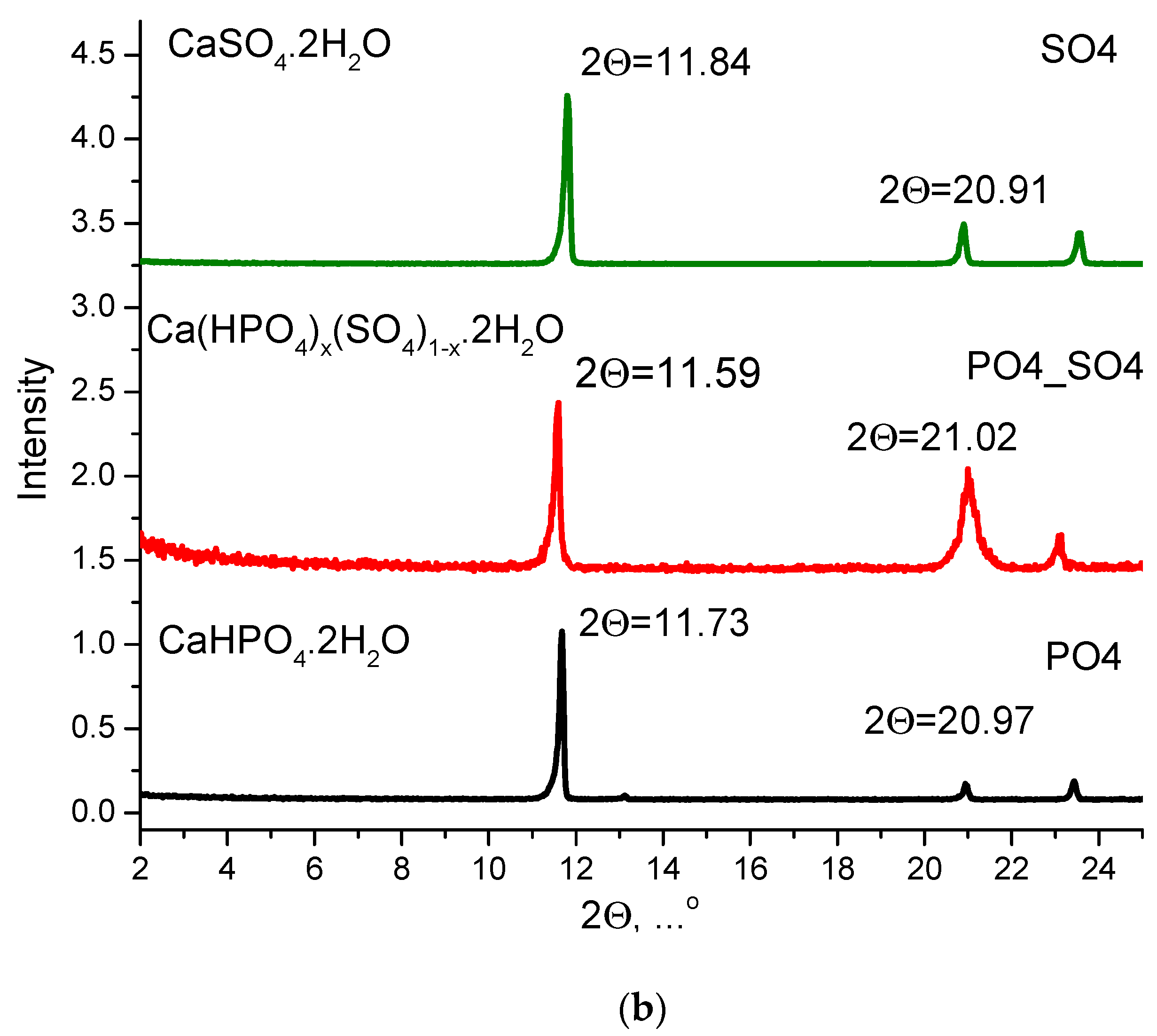
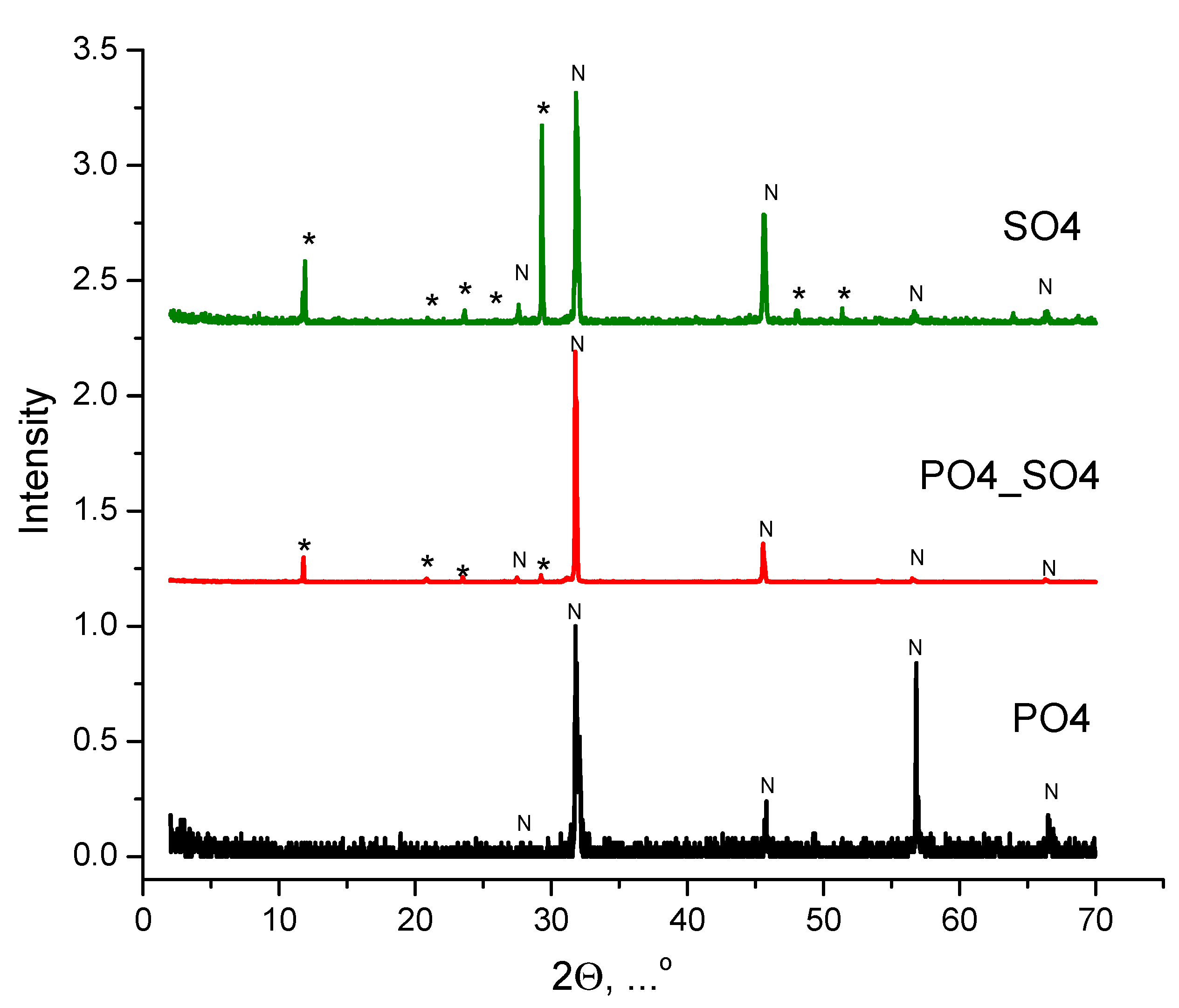
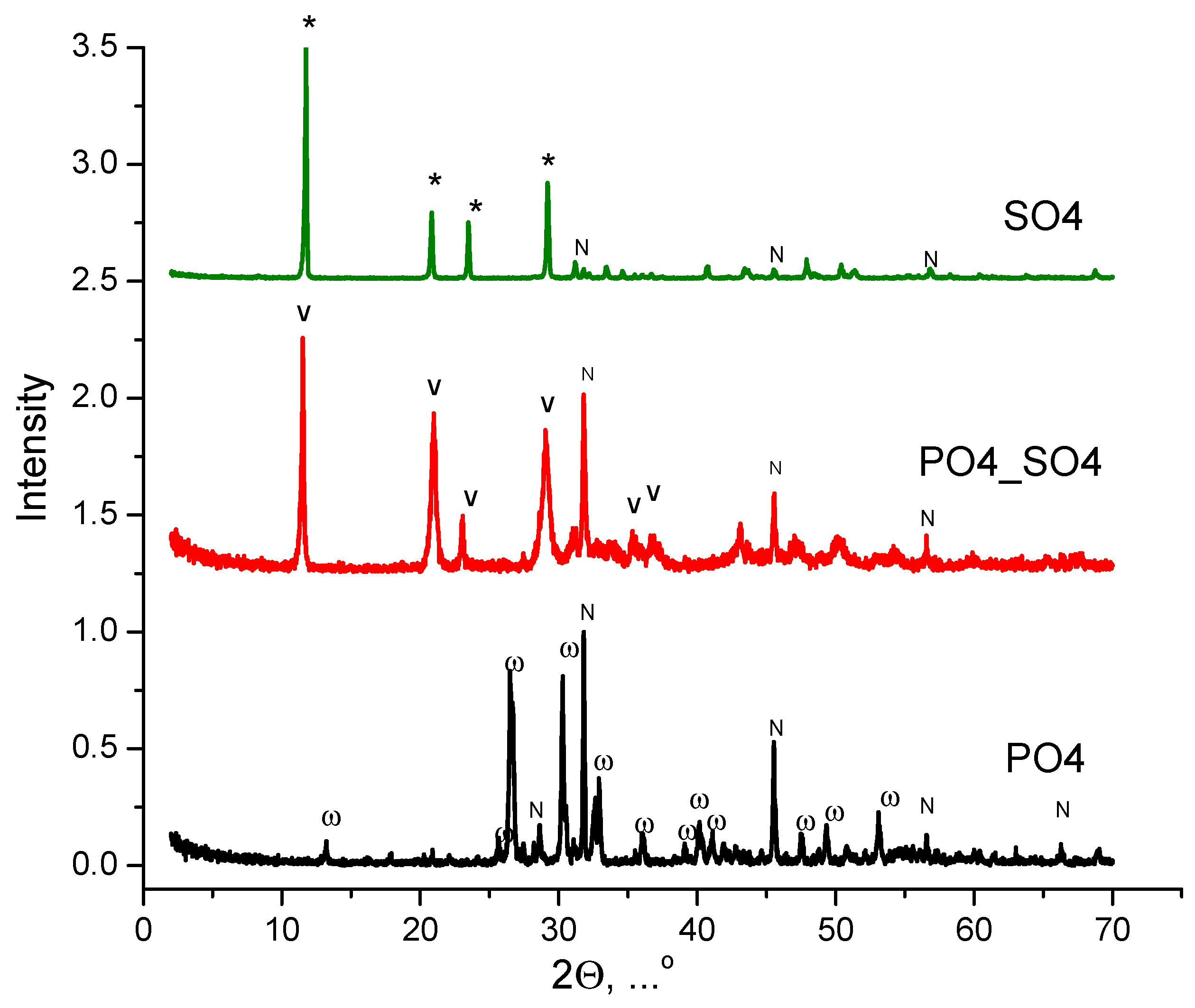

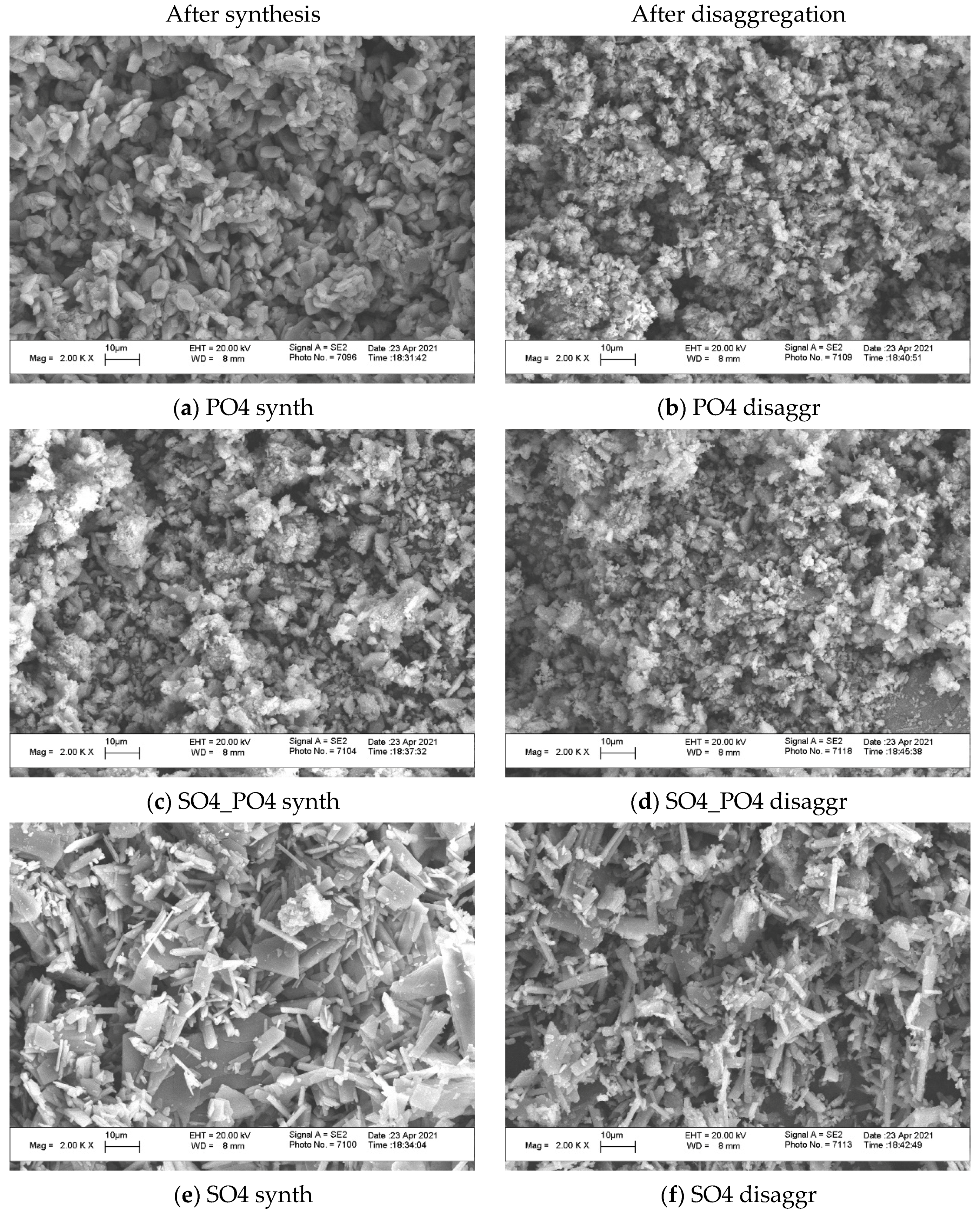
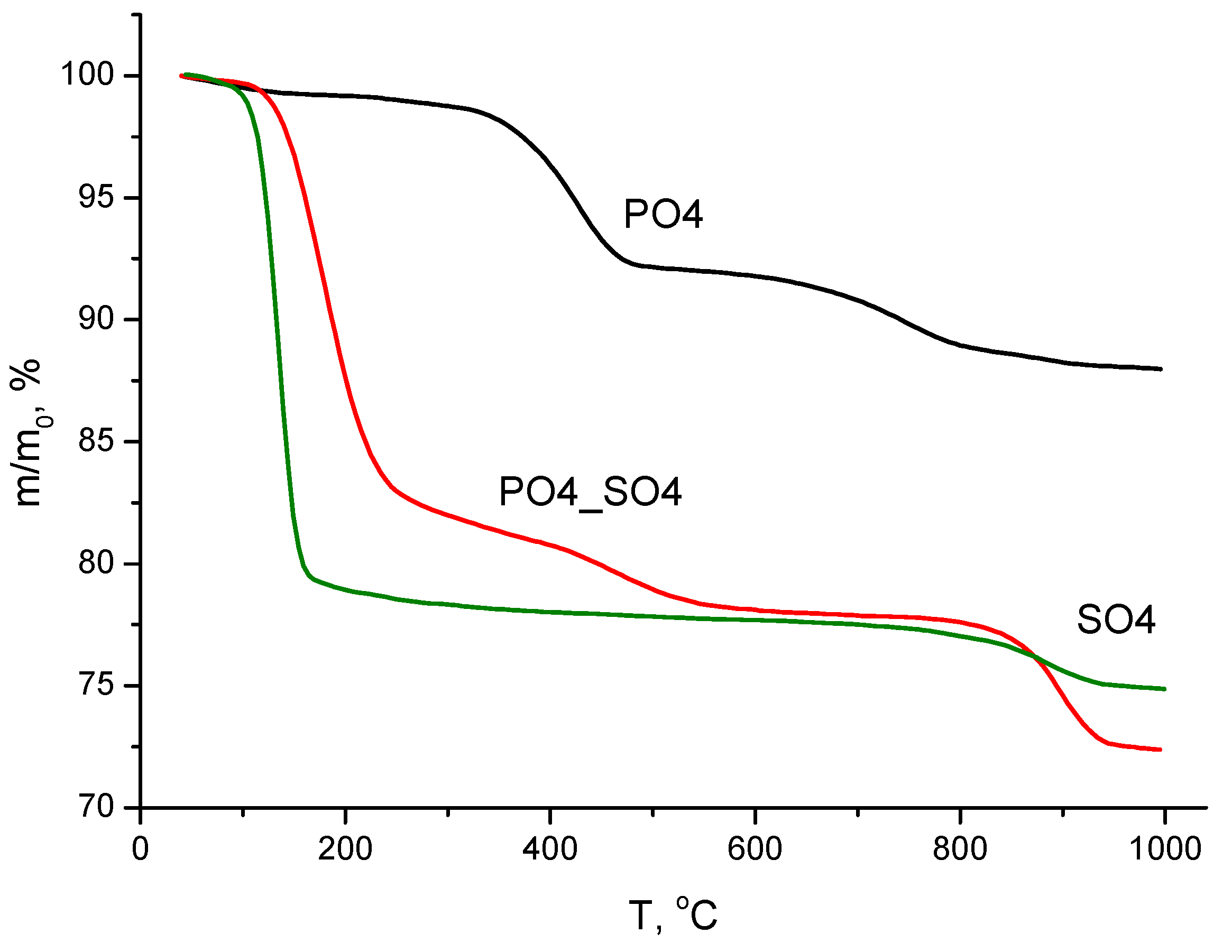
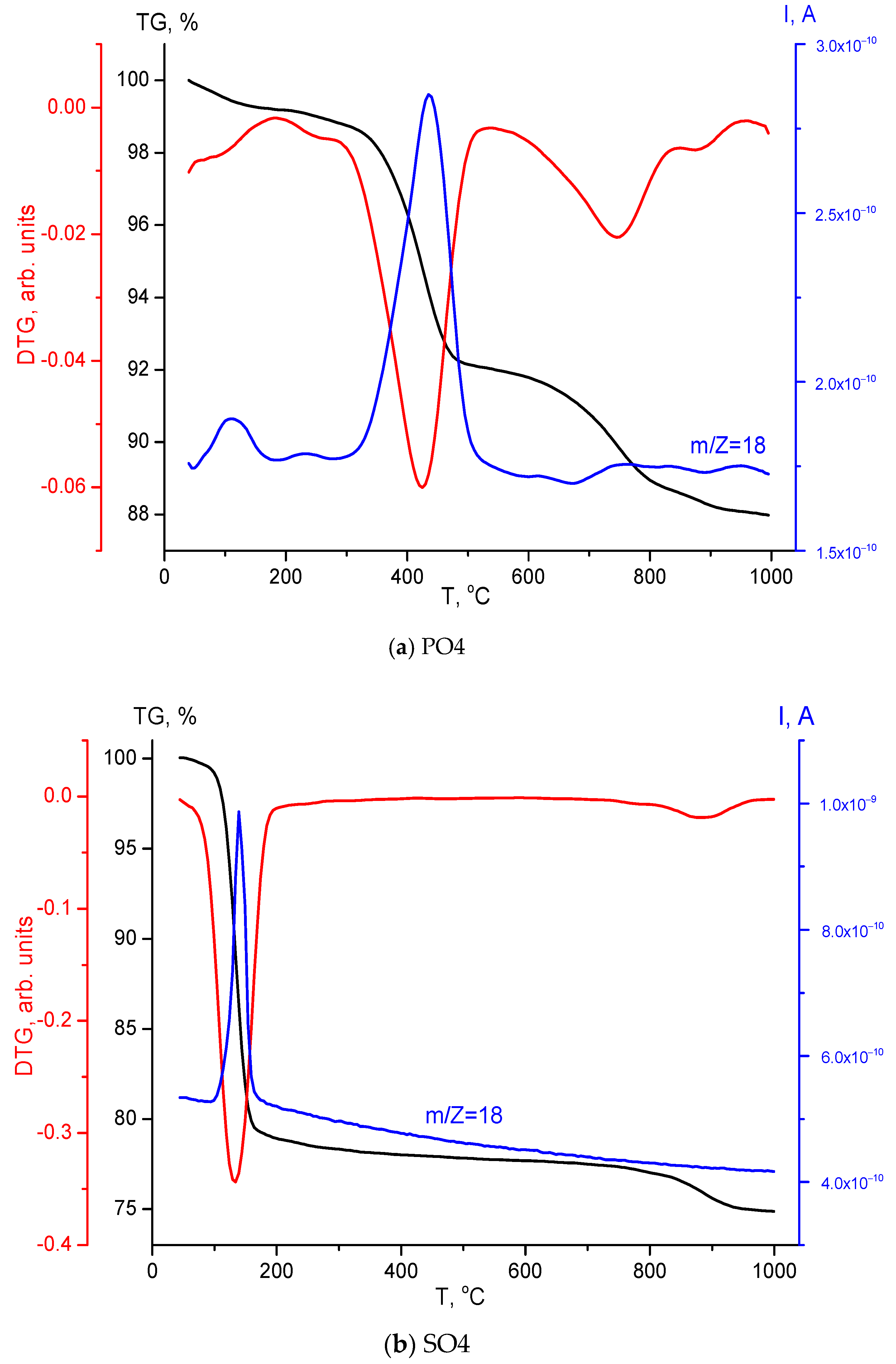


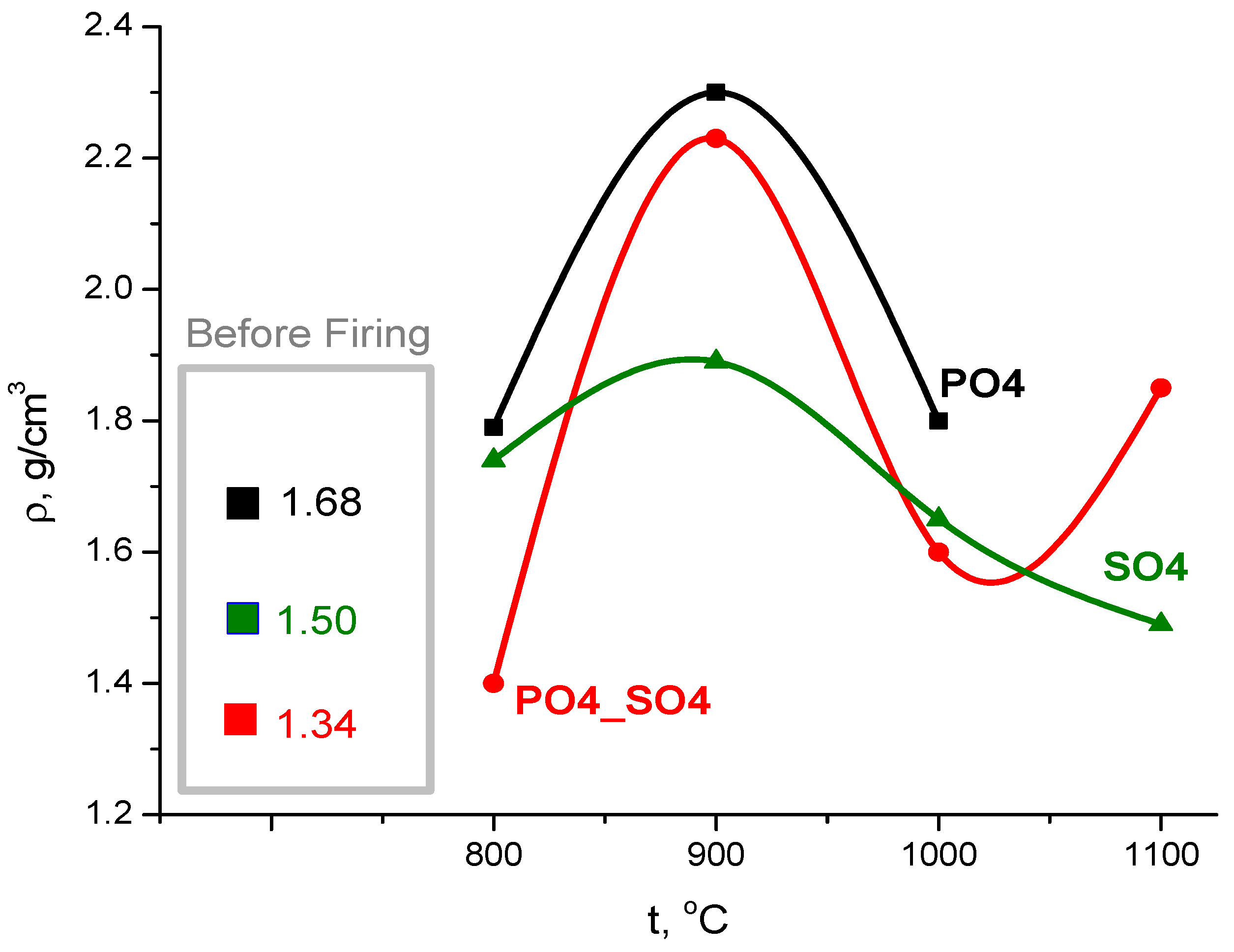

| Labeling of the Samples (Solutions, Powders, Ceramics) | Concentrations and Volumes of Aqueous Solutions | Molar Ratio of Salts in the Synthesis | ||||
|---|---|---|---|---|---|---|
| Ca-Containing Solution | Na-Containing Solution | |||||
| CaCl2 | Volume | Na2HPO4 | Na2SO4 | Volume | ||
| PO4 | 0.5 M | 300 mL | 0.5 M | 0 | 300 mL | [CaCl2]/[Na2HPO4] = 1:1 |
| PO4_SO4 | 0.5 M | 300 mL | 0.25 M | 0.25 M | 300 mL | [CaCl2]/[Na2HPO4]/[Na2SO4] = 1:0.5:0.5 |
| SO4 | 0.5 M | 300 mL | 0 | 0.5 M | 300 mL | [CaCl2]/[Na2SO4] = 1:1 |
| Sample | NaCl | CaSO4·2H2O |
|---|---|---|
| PO4 | 100.0 | - |
| PO4_SO4 | 89.9 | 11.1 |
| SO4 | 54.8 | 45.2 |
| Sample | Powder Composition | |||
|---|---|---|---|---|
| CaHPO4 | CaSO4·2H2O | Ca(HPO4)0.5(SO4) 0.5·2H2O | NaCl | |
| PO4 | 83.4 | - | - | 16.6 |
| PO4_SO4 | - | 72.5 | 27.5 | |
| SO4 | - | 98.1 | - | 1.9 |
| Sample | Powder after Synthesis | Powder after Disaggregation | Pre-Ceramic Sample after Pressing at 100 MPa | Densification, Degree |
|---|---|---|---|---|
| PO4 | 0.78 | 0.34 | 1.68 | 1.68/0.34 = 4.9 |
| PO4_SO4 | 0.55 | 0.44 | 1.34 | 1.34/0.44 = 3.0 |
| SO4 | 0.42 | 0.37 | 1.50 | 1.50/0.37 = 4.1 |
| Stage | Samples | ||
|---|---|---|---|
| PO4 | PO4_SO4 | SO4 | |
| Powders | |||
| Synthesis | CaHPO4·2H2O CaHPO4 NaCl | Ca(HPO4)x(SO4)1−x·2H2O (Ca(HPO4)0.5(SO4)0.5·2H2O) NaCl | CaSO4·2H2O NaCl |
| Disaggregation | CaHPO4 NaCl | Ca(HPO4)x(SO4)1−x·2H2O (Ca(HPO4)0.5(SO4)0.5·2H2O) NaCl | CaSO4·2H2O NaCl |
| Ceramics | |||
| Firing, 800 °C | β-Ca2P2O7 Ca5(PO4)3Cl amorphous phase | β-Ca2P2O7 CaSO4 Ca5(PO4)3Cl | CaSO4 |
| Firing, 900 °C | β-Ca2P2O7 Ca5(PO4)3Cl amorphous phase | β-Ca2P2O7 CaSO4 Ca5(PO4)3Cl amorphous phase | CaSO4 (Na0.8Ca0.1)2SO4 |
| Firing, 1000 °C | β-Ca2P2O7 Ca5(PO4)3Cl amorphous phase | CaSO4 Ca5(PO4)3Cl Ca3(PO4)2/Ca10Na(PO4)7 amorphous phase | CaSO4 (Na0.8Ca0.1)2SO4 |
| Firing, 1100 °C | Ca5(PO4)3Cl, amorphous phase | CaSO4 Ca5(PO4)3Cl amorphous phase | CaSO4 (Na0.8Ca0.1)2SO4 amorphous phase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safronova, T.V.; Khantimirov, A.S.; Shatalova, T.B.; Filippov, Y.Y.; Kolesnik, I.V.; Knotko, A.V. Powders Synthesized from Solutions of Calcium Chloride, Sodium Hydrogen Phosphate, and Sodium Sulfate for Bioceramics Production. Ceramics 2023, 6, 561-583. https://doi.org/10.3390/ceramics6010034
Safronova TV, Khantimirov AS, Shatalova TB, Filippov YY, Kolesnik IV, Knotko AV. Powders Synthesized from Solutions of Calcium Chloride, Sodium Hydrogen Phosphate, and Sodium Sulfate for Bioceramics Production. Ceramics. 2023; 6(1):561-583. https://doi.org/10.3390/ceramics6010034
Chicago/Turabian StyleSafronova, Tatiana V., Alexander S. Khantimirov, Tatiana B. Shatalova, Yaroslav Y. Filippov, Irina V. Kolesnik, and Alexander V. Knotko. 2023. "Powders Synthesized from Solutions of Calcium Chloride, Sodium Hydrogen Phosphate, and Sodium Sulfate for Bioceramics Production" Ceramics 6, no. 1: 561-583. https://doi.org/10.3390/ceramics6010034
APA StyleSafronova, T. V., Khantimirov, A. S., Shatalova, T. B., Filippov, Y. Y., Kolesnik, I. V., & Knotko, A. V. (2023). Powders Synthesized from Solutions of Calcium Chloride, Sodium Hydrogen Phosphate, and Sodium Sulfate for Bioceramics Production. Ceramics, 6(1), 561-583. https://doi.org/10.3390/ceramics6010034







