Investigation of the Effect of Milling Duration on a Ce-Gd Doped Zirconolite Phase Assemblage Synthesised by Hot Isostatic Pressing
Abstract
1. Introduction
2. Experimental Method
2.1. Sample Preparation
2.2. Sample Characterisation
3. Results and Discussion
3.1. Phase Assemblage
3.2. Microstructure Analysis
3.3. Oxidation State and Local Environment of Ce
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nuclear Decommissioning Authority. Progress on Plutonium Consolidation, Storage and Disposition. 2019. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/791046/Progress_on_Plutonium.pdf (accessed on 15 January 2023).
- Hyatt, N.C. Plutonium management policy in the United Kingdom: The need for a dual track strategy. Energy Policy 2017, 101, 303–309. [Google Scholar] [CrossRef]
- Hyatt, N.C. Safe management of the UK separated plutonium inventory: A challenge of materials degradation. NPJ Mater. Degrad. 2020, 4, 28. [Google Scholar] [CrossRef]
- Ewing, R.C.; Weber, W.J.; Lian, J. Nuclear waste disposal—Pyrochlore (A2B2O7): Nuclear waste form for the immobilization of plutonium and “minor” actinides. J. Appl. Phys. 2004, 95, 5949–5971. [Google Scholar] [CrossRef]
- Weber, W.J.; Ewing, R.C.; Catlow, C.R.A.; de la Rubia, T.D.; Hobbs, L.W.; Kinoshita, C.; Matzke, H.; Motta, A.T.; Nastasi, M.; Salje, E.K.H.; et al. Radiation effects in crystalline ceramics for the immobilization of high-level nuclear waste and plutonium. J. Mater. Res. 1998, 13, 1434–1484. [Google Scholar] [CrossRef]
- Gregg, D.J.; Vance, E.R. Synroc tailored waste forms for actinide immobilization. Radiochim. Acta 2017, 105, 907–925. [Google Scholar] [CrossRef]
- Vance, E.R.; Stewart, M.W.A.; Moricca, S.A. Progress at ANSTO on SYNROC. J. Aust. Ceram. Soc. 2014, 50, 38–48. [Google Scholar]
- Jantzen, C.M.; Ojovan, M.I. On Selection of Matrix (Wasteform) Material for Higher Activity Nuclear Waste Immobilization (Review). Russ. J. Inorg. Chem. 2019, 64, 1611–1624. [Google Scholar] [CrossRef]
- Gregg, D.J.; Farzana, R.; Dayal, P.; Holmes, R.; Triani, G. Synroc technology: Perspectives and current status (Review). J. Am. Ceram. Soc. 2020, 103, 5424–5441. [Google Scholar] [CrossRef]
- Lumpkin, G.R. Ceramic Waste Forms for Actinides. Elements 2006, 2, 365–372. [Google Scholar] [CrossRef]
- Begg, B.D.; Vance, E.R.; Hunter, B.A.; Hanna, J.V. Zirconolite transformation under reducing conditions. J. Mater. Res. 1998, 13, 3181–3190. [Google Scholar] [CrossRef]
- Zhang, Y.; Gregg, D.J.; Kong, L.; Jovanovich, M.; Triani, G. Zirconolite glass-ceramics for plutonium immobilization: The effects of processing redox conditions on charge compensation and durability. J. Nucl. Mater. 2017, 490, 238–241. [Google Scholar] [CrossRef]
- Aldean, I.; Sun, S.-K.; Wilkins, M.C.D.; Gardner, L.J.; Mason, A.R.; Stennett, M.C.; Corkhill, C.L.; Hyatt, N.C.; Blackburn, L.R. Synthesis and characterisation of Ce-doped zirconolite Ca0.80Ce0.20ZrTi1.60M0.40O7 (M = Fe, Al) formed by reactive spark plasma sintering (RSPS). MRS Adv. 2022, 7, 75–80. [Google Scholar] [CrossRef]
- Lumpkin, G.; Whittle, K.; Howard, C.; Zhang, Z.; Berry, F.; Oates, G.; Williams, C.; Zaitsev, A. Crystal Chemistry and Cation Ordering in Zirconolite 2M. MRS Online Proc. Libr. 2006, 932, 53. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, L.; Ionescu, M.; Gregg, D.J. Current advances on titanate glass-ceramic composite materials as waste forms for actinide immobilization: A technical review. J. Eur. Ceram. Soc. 2022, 42, 1852–1876. [Google Scholar] [CrossRef]
- Yudintsev, S.V.; Stefanovsky, S.V.; Nikonov, B.S.; Nikol’Skii, M.S.; Livshits, T.S. Potential matrices for immobilization of the rare earth-actinide fraction of high-level waste in the REE2Zr2O7-REE2Ti2O7 system. Radiochemistry 2015, 57, 187–199. [Google Scholar] [CrossRef]
- Stefanovsky, S.V.; Troole, A.Y.; Lapina, M.I.; Nikonov, B.S.; Sivtsov, A.V.; Yudintsev, S.V. XRD, SEM and TEM Study of the Gd-Doped Zirconolites. MRS Proc. 2002, 713, 345–350. [Google Scholar] [CrossRef]
- Zhang, K.; Yin, D.; He, Z.; Luo, B.; Zhang, H. Combustion synthesis of Hf-doped zirconolite-rich composite waste forms and the aqueous durability. J. Adv. Ceram. 2019, 8, 448–455. [Google Scholar] [CrossRef]
- Gardner, L.J.; A Walling, S.; Hyatt, N.C. Hot isostatic pressing: Thermal treatment trials of inactive and radioactive simulant UK intermediate level waste. IOP Conf. Ser. Mater. Sci. Eng. 2020, 818, 012009. [Google Scholar] [CrossRef]
- Thornber, S.M.; Heath, P.G.; Da Costa, G.P.; Stennett, M.C.; Hyatt, N.C. The effect of pre-treatment parameters on the quality of glass-ceramic wasteforms for plutonium immobilisation, consolidated by hot isostatic pressing. J. Nucl. Mater. 2017, 485, 253–261. [Google Scholar] [CrossRef]
- Stewart, M.W.A.; Moricca, S.A.; Eddowes, T.; Zhang, Y.; Vance, E.R.; Lumpkin, G.R.; Carter, M.L.; Dowson, M.; James, M. The Use of Hot-Isostatic Pressing to Process Nuclear Waste Forms. In Proceedings of the ASME 2009 12th International Conference on Environmental Remediation and Radioactive Waste Management, Liverpool, UK, 11–15 October 2009; pp. 611–616. [Google Scholar] [CrossRef]
- Hyatt, N.C.; Corkhill, C.L.; Stennett, M.C.; Hand, R.J.; Gardner, L.J.; Thorpe, C.L. The HADES Facility for High Activity Decommissioning Engineering & Science: Part of the UK National Nuclear User Facility. IOP Conf. Series: Mater. Sci. Eng. 2020, 818, 012022. [Google Scholar] [CrossRef]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- Blackburn, L.R.; Bailey, D.J.; Sun, S.-K.; Gardner, L.J.; Stennett, M.C.; Corkhill, C.L.; Hyatt, N.C. Review of zirconolite crystal chemistry and aqueous durability. Adv. Appl. Ceram. 2021, 120, 69–83. [Google Scholar] [CrossRef]
- Whittle, K.R.; Hyatt, N.; Smith, K.L.; Margiolaki, I.; Berry, F.J.; Knight, K.S.; Lumpkin, G.R. Combined neutron and X-ray diffraction determination of disorder in doped zirconolite-2M. Am. Miner. 2012, 97, 291–298. [Google Scholar] [CrossRef]
- Blackburn, L.R.; Sun, S.; Gardner, L.J.; Maddrell, E.R.; Stennett, M.C.; Hyatt, N.C. A systematic investigation of the phase assemblage and microstructure of the zirconolite CaZr1-xCexTi2O7 system. J. Nucl. Mater. 2020, 535, 152137. [Google Scholar] [CrossRef]
- Blackburn, L.R.; Crawford, R.; Walling, S.A.; Gardner, L.J.; Cole, M.R.; Sun, S.-K.; Gausse, C.; Mason, A.R.; Stennett, M.C.; Maddrell, E.R.; et al. Influence of accessory phases and surrogate type on accelerated leaching of zirconolite waste forms. NPJ Mater. Degrad. 2021, 5, 1–11. [Google Scholar] [CrossRef]
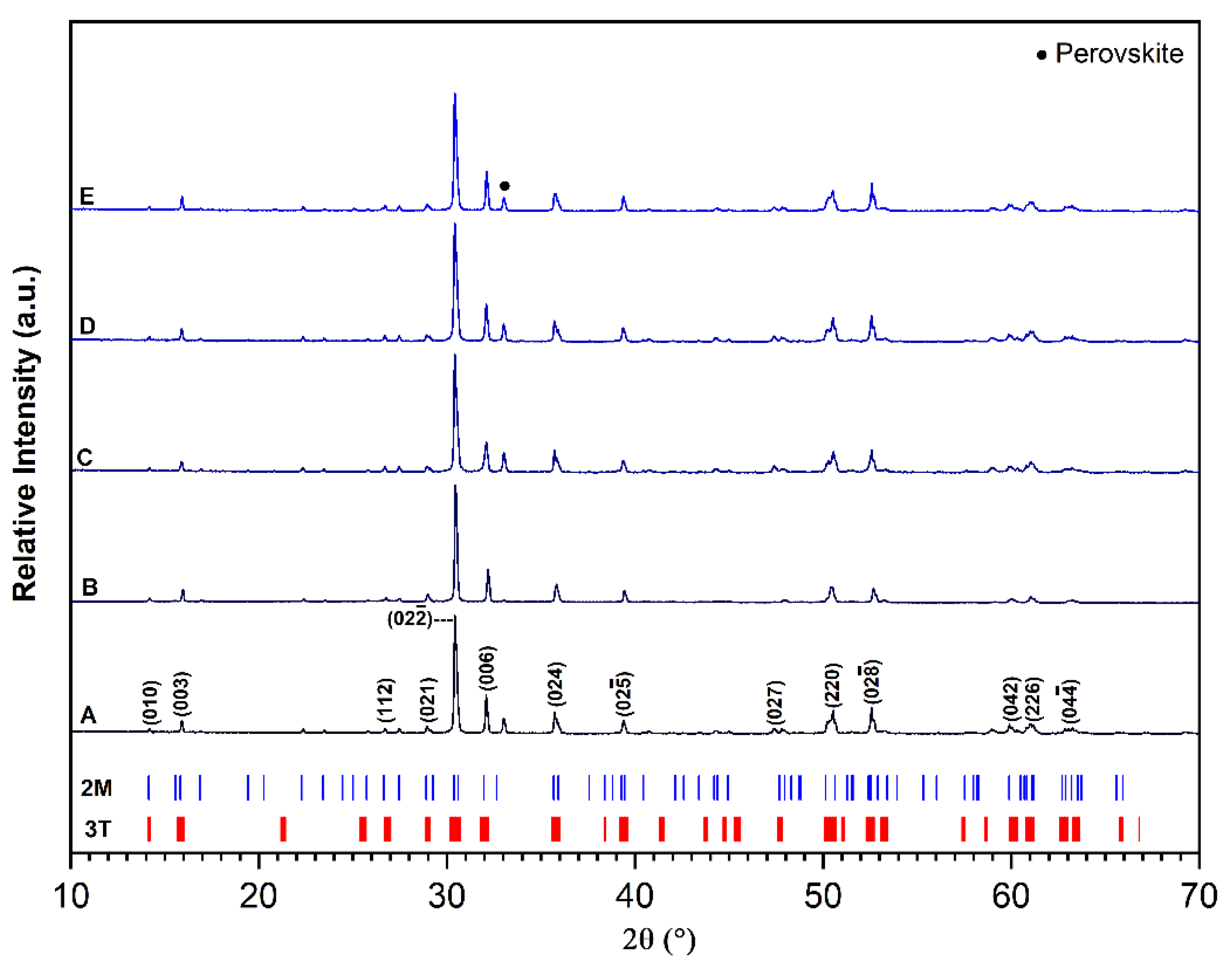
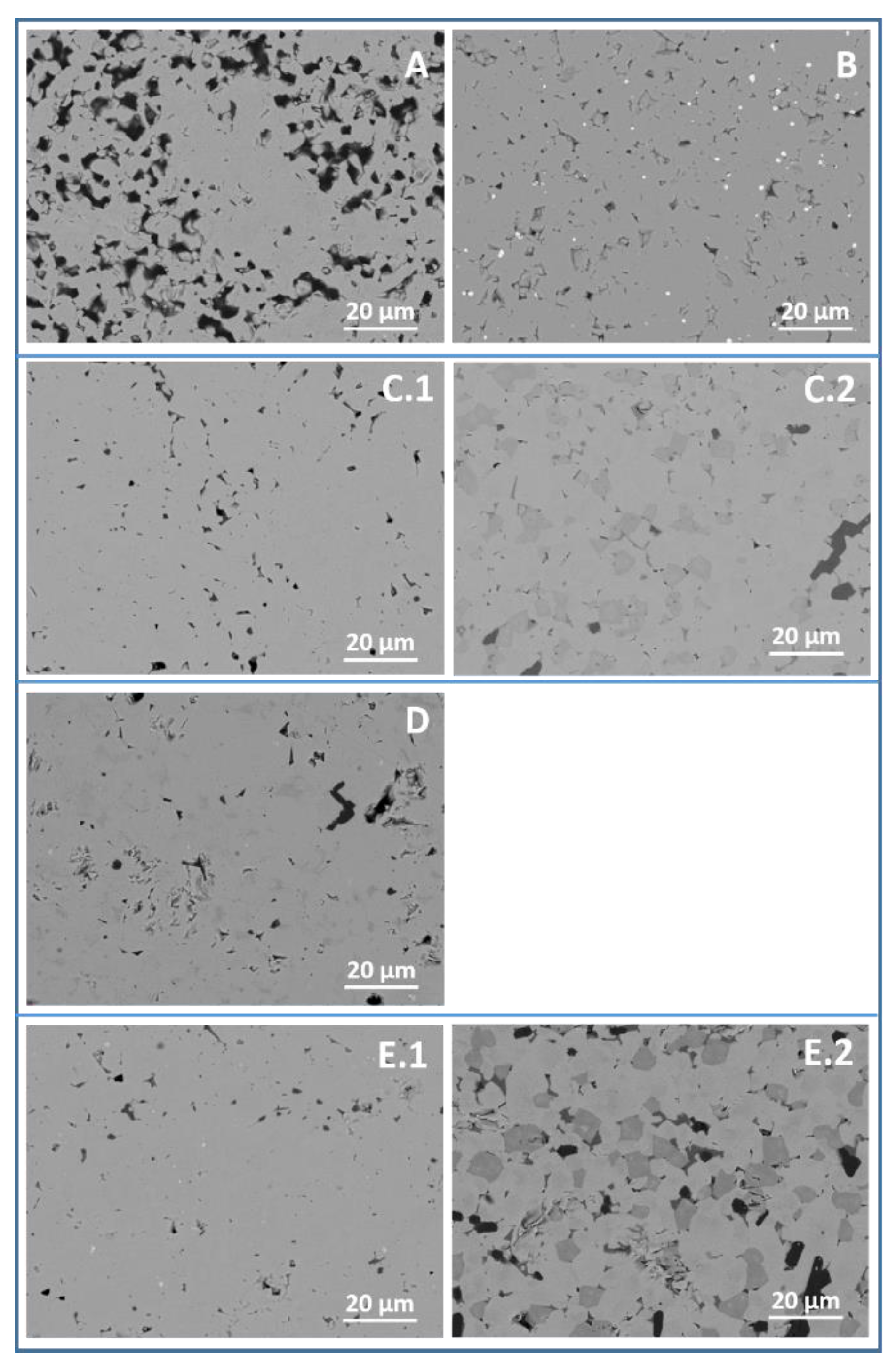
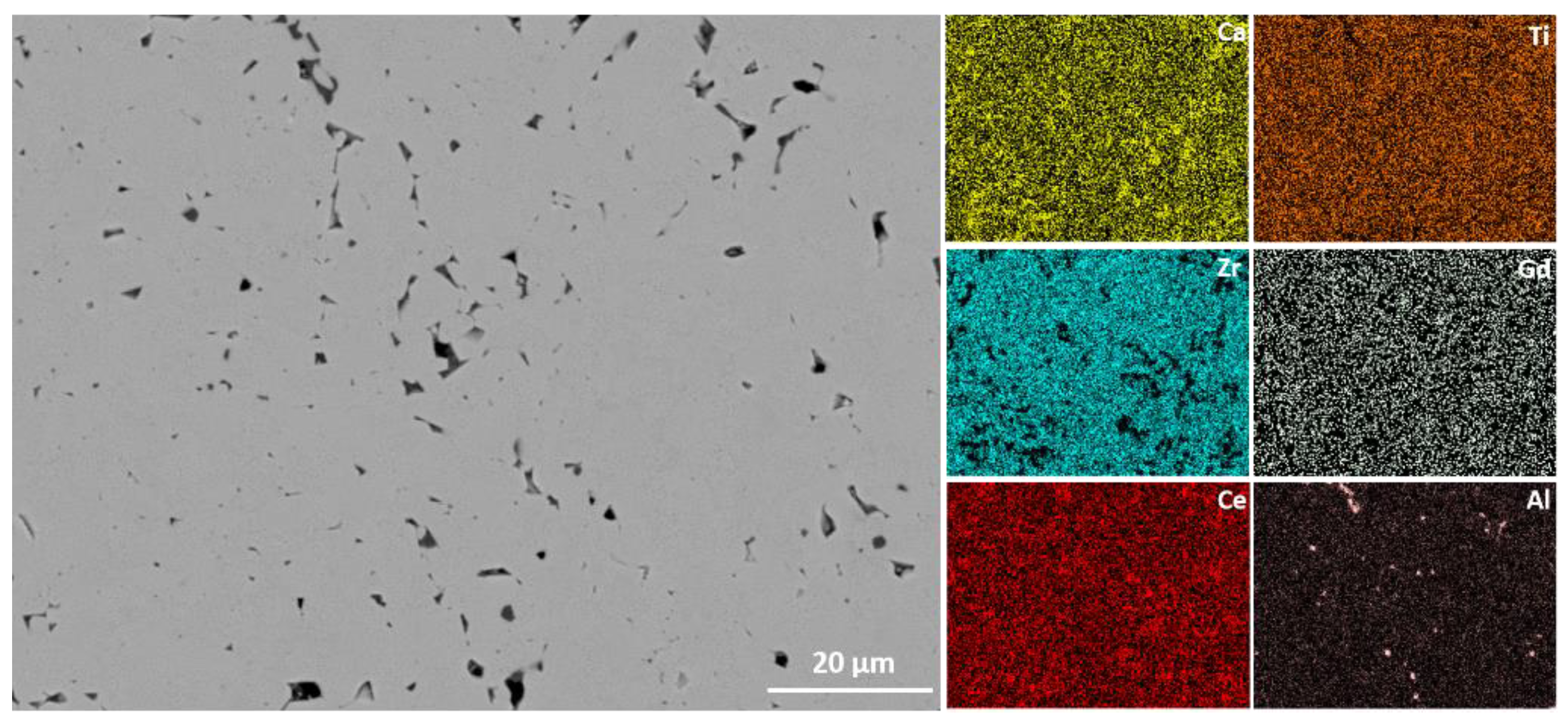
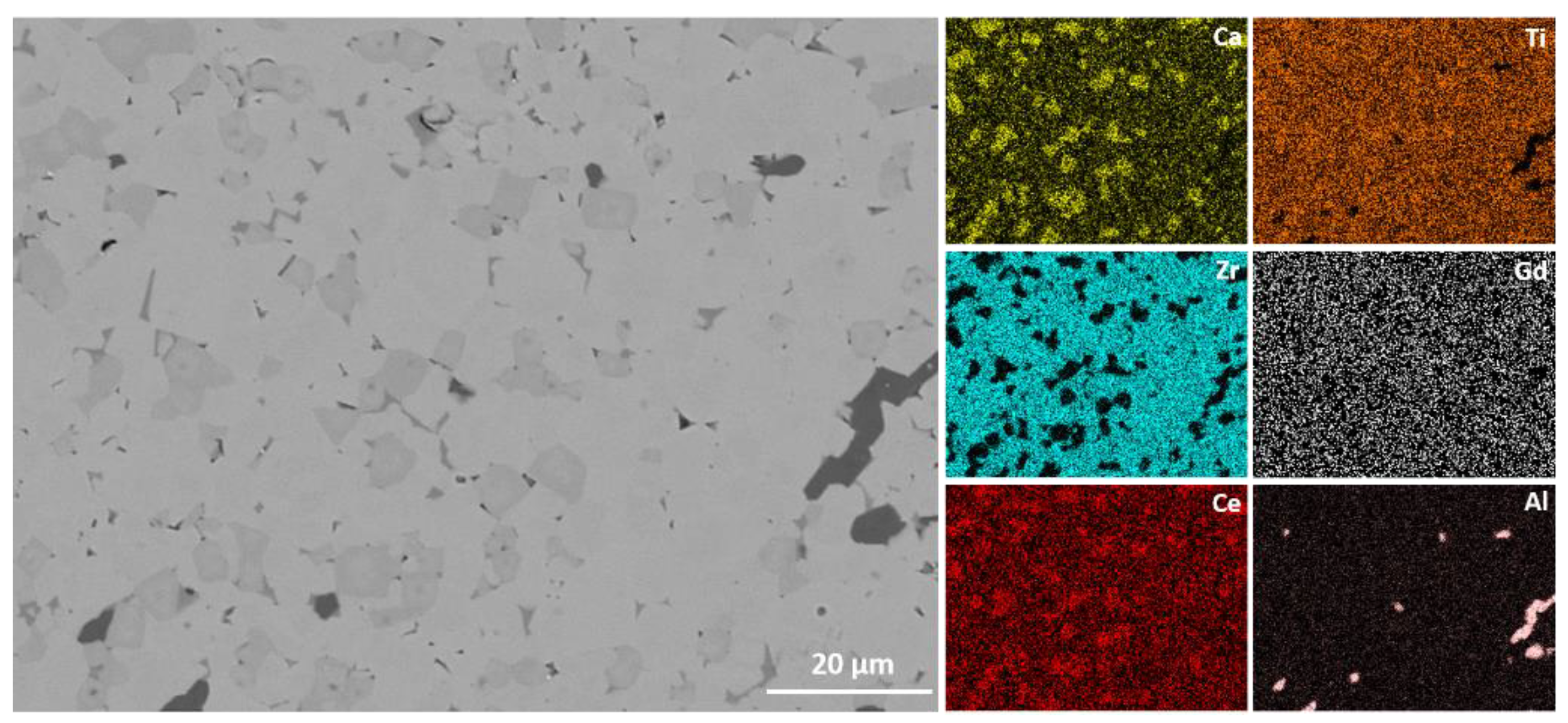

| Sample | Milling Parameters at 400 rpm |
|---|---|
| A | 40 min with the direction reversed at a 20 min interval |
| B | 60 min with the direction reversed at 20 min intervals |
| C | 120 min with the direction reversed at 20 min intervals (20 min break after 1 h) |
| D | Pre-milled CeO2 added; 20 min with the direction reversed at 10 min intervals, and then 60 min with the direction reversed at 20 min intervals |
| E | 60 min with the direction reversed at 20 min intervals, using CeO2 with a finer particle size (<5 µm) |
| Sample | Zirc-2M (%) | Zirc-3T (%) | Perovskite (%) | Rwp | R | χ2 |
|---|---|---|---|---|---|---|
| A | 77.35(4) | 12.75(1) | 9.90(1) | 9.16 | 7.04 | 3.92 |
| B | 77.23(4) | 20.12(1) | 2.65(1) | 11.62 | 8.93 | 4.84 |
| C | 85.61(2) | 4.75(1) | 9.64(4) | 8.69 | 6.74 | 3.85 |
| D | 78.16(4) | 10.12(1) | 11.72(4) | 8.38 | 6.49 | 3.96 |
| E | 80.61(3) | 11.50(1) | 7.89(4) | 9.16 | 7.05 | 4.17 |
| Sample | a (Å) | b (Å) | c (Å) | β (°) | Vol (Å3) |
|---|---|---|---|---|---|
| CaZrTi2O7 [26] | 12.4430(1) | 7.2729(1) | 11.3809(1) | 100.56(1) | 1012.48(2) |
| A | 12.490(1) | 7.264(6) | 11.3561(9) | 100.71(1) | 1012.5(1) |
| B | 12.521(2) | 7.252(1) | 11.329(2) | 100.57(2) | 1010.6(2) |
| C | 12.492(1) | 7.2671(6) | 11.3575(8) | 100.70(1) | 1013.1(1) |
| D | 12.484(2) | 7.2652(8) | 11.359(1) | 100.68(1) | 1012.4(2) |
| E | 12.496(1) | 7.2660(8) | 11.351(1) | 100.72(1) | 1012.7(2) |
| Sample | Experimental Formulation |
|---|---|
| A | (Ca0.83(4)Gd0.013(9)Ce0.20(2))(Zr0.76(5)Gd0.013(9)Ce0.10(1))(Ti1.72(4)Al0.33(4))O7 |
| B | (Ca0.78(4)Gd0.007(3)Ce0.14(2))(Zr0.95(7)Gd0.007(3)Ce0.07(1))(Ti1.62(6)Al0.40(4))O7 |
| C | (Ca0.81(7)Gd0.011(6)Ce0.14(2))(Zr0.81(9)Gd0.011(6)Ce0.07(1))(Ti1.54(5)Al0.32(3))O7 |
| D | (Ca0.84(8)Gd0.01(3)Ce0.14(2))(Zr0.94(10)Gd0.01(3)Ce0.07(1))(Ti1.60(9)Al0.37(4))O7 |
| E | (Ca0.80(5)Gd0.013(6)Ce0.17(2))(Zr0.86(8)Gd0.013(6)Ce0.08(1))(Ti1.67(5)Al0.37(3))O7 |
| Sample | Density (g cm−3) |
|---|---|
| A | 4.18(2) |
| B | 4.64(3) |
| C | 4.64(2) |
| D | 4.68(2) |
| E | 4.67(2) |
| Sample | Ce4+O2 (%) | Ce3+PO4 (%) | R-Factor |
|---|---|---|---|
| A | 79(1) | 21(3) | 0.02 |
| B | 60(2) | 40(4) | 0.04 |
| C | 31(3) | 69(4) | 0.10 |
| D | 30(3) | 70(4) | 0.10 |
| E | 32(3) | 68(4) | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuman, M.; Gardner, L.J.; Blackburn, L.R.; Stennett, M.C.; Hyatt, N.C.; Corkhill, C.L. Investigation of the Effect of Milling Duration on a Ce-Gd Doped Zirconolite Phase Assemblage Synthesised by Hot Isostatic Pressing. Ceramics 2023, 6, 707-716. https://doi.org/10.3390/ceramics6010043
Kuman M, Gardner LJ, Blackburn LR, Stennett MC, Hyatt NC, Corkhill CL. Investigation of the Effect of Milling Duration on a Ce-Gd Doped Zirconolite Phase Assemblage Synthesised by Hot Isostatic Pressing. Ceramics. 2023; 6(1):707-716. https://doi.org/10.3390/ceramics6010043
Chicago/Turabian StyleKuman, Merve, Laura J. Gardner, Lewis R. Blackburn, Martin C. Stennett, Neil C. Hyatt, and Claire L. Corkhill. 2023. "Investigation of the Effect of Milling Duration on a Ce-Gd Doped Zirconolite Phase Assemblage Synthesised by Hot Isostatic Pressing" Ceramics 6, no. 1: 707-716. https://doi.org/10.3390/ceramics6010043
APA StyleKuman, M., Gardner, L. J., Blackburn, L. R., Stennett, M. C., Hyatt, N. C., & Corkhill, C. L. (2023). Investigation of the Effect of Milling Duration on a Ce-Gd Doped Zirconolite Phase Assemblage Synthesised by Hot Isostatic Pressing. Ceramics, 6(1), 707-716. https://doi.org/10.3390/ceramics6010043







