Ceramic Materials in Na2O-CaO-P2O5 System, Obtained via Heat Treatment of Cement-Salt Stone Based on Powder Mixture of Ca3(C6H5O7)2∙4H2O, Ca(H2PO4)2∙H2O and NaH2PO4
Abstract
1. Introduction
2. Materials and Methods
2.1. Initial Reagents and Synthesis
2.2. Preparation of the Calcium Pyrophosphate and Sodium Rhenanite Ceramics
+ 4H3C6H5O7 + 14H2O (CaNa)
2.3. Characterization
2.3.1. XRD
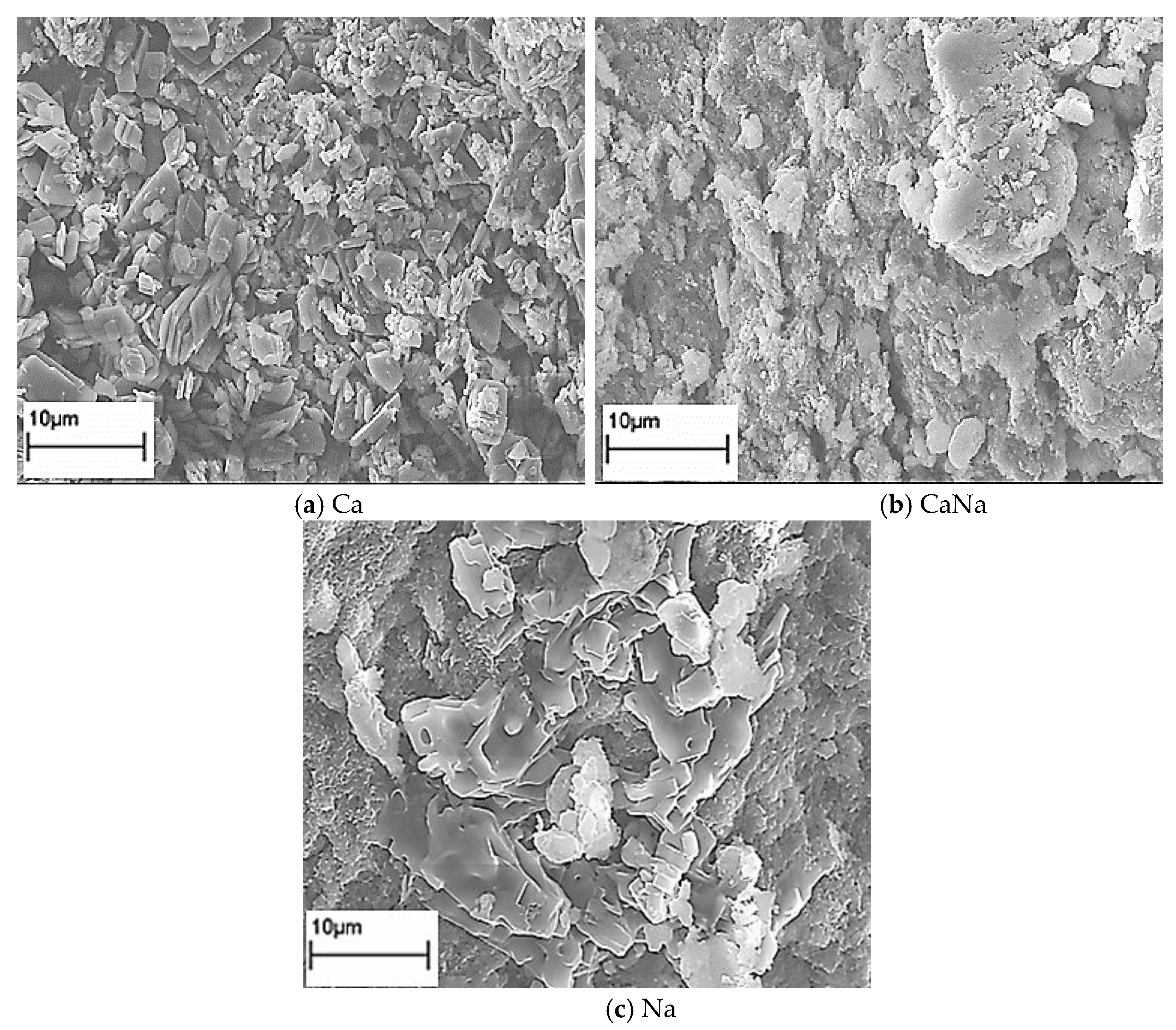
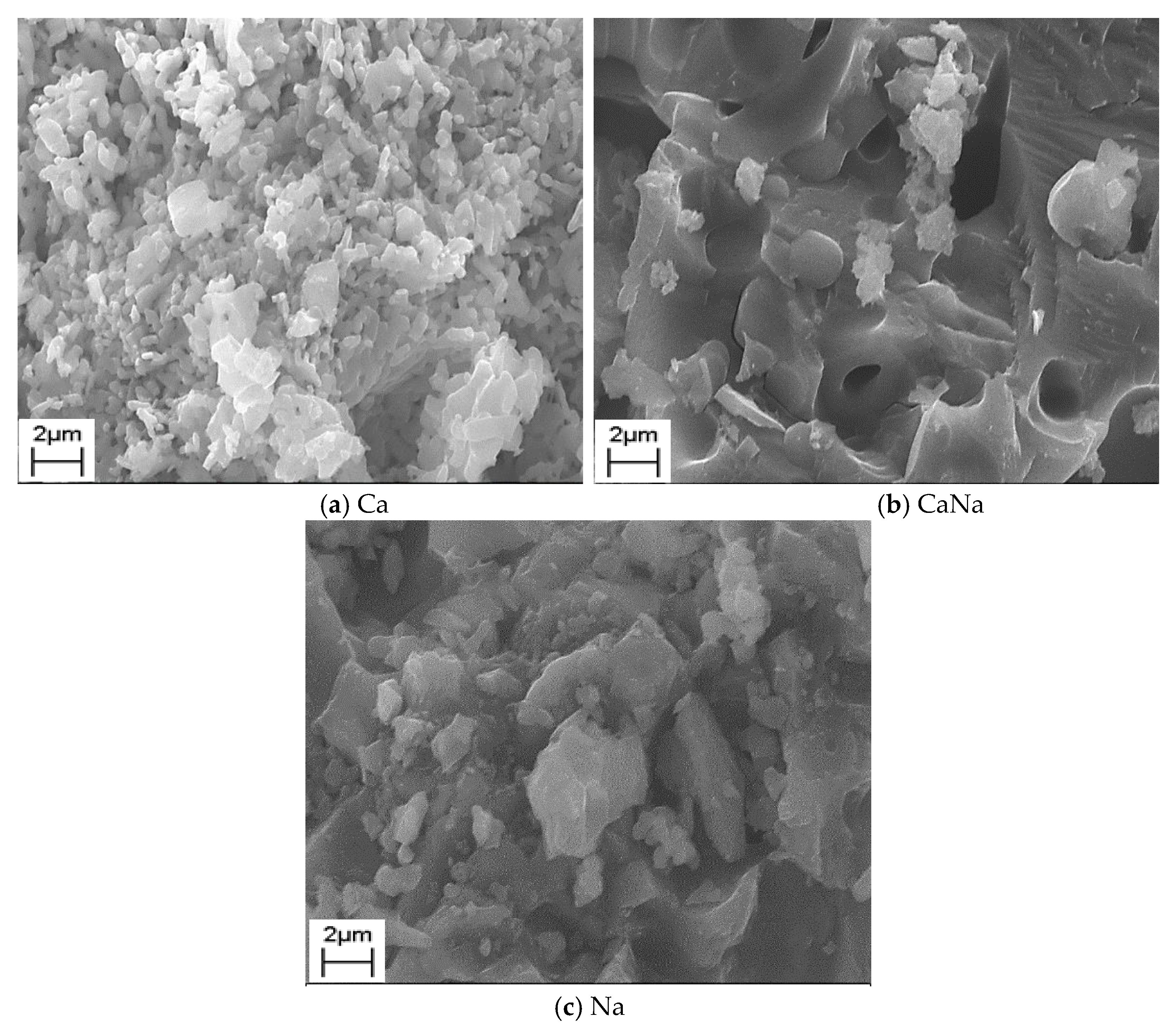
2.3.2. SEM
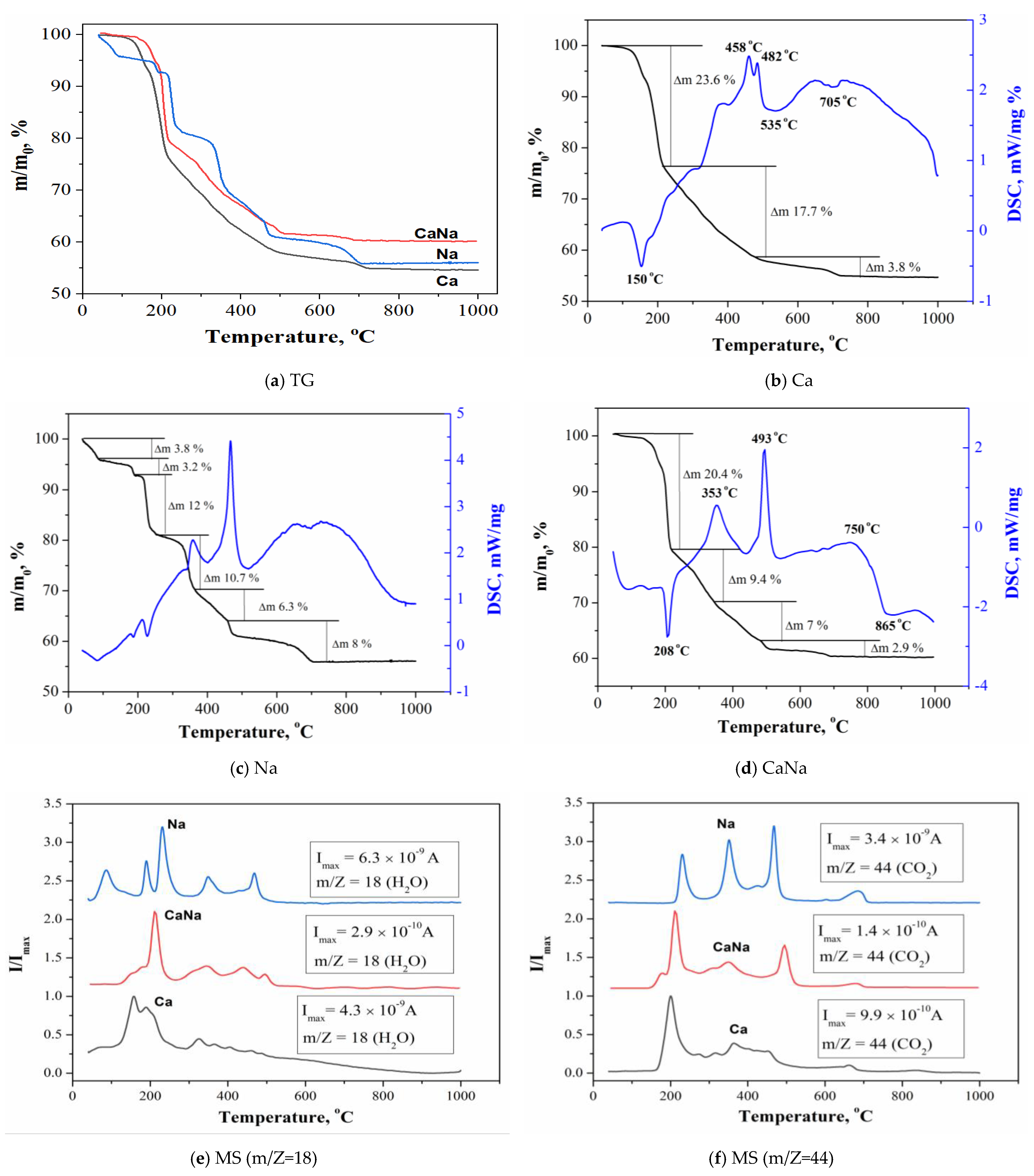
2.3.3. Thermal Analysis
2.3.4. Determination of Strength Properties of the Ceramic Samples
2.3.5. Determination of Shrinkage and Density of the Ceramic Samples
3. Results and Discussion
→ 9CaHPO4∙2H2O + 2H3C6H5O7 + Na2HC6H5O7 + NaH2C6H5O7
→ 9CaHPO4 + 2H3C6H5O7 + Na2HC6H5O7 + NaH2C6H5O7 + 11H2O
4. Conclusions
- 1.
- In the present work, an approach to obtaining bioresorbable ceramic materials in Na2O-CaO-P2O5 system with a given phase composition, including β-CPP β-Ca2P2O7, sodium rhenanite CaNaPO4, double calcium-sodium pyrophosphate Na2CaP2O7, and Na–substituted tricalcium phosphate Сa10Na(PO4)7 was obtained by firing cement-salt stone from a powder mixture including calcium citrate tetrahydrate Ca3(C6H5O7)2∙4H2O, MCPM Ca(H2PO4)2∙H2O and sodium dihydrogen phosphate NaH2PO4. This approach involved the preparation of a powder mixtures with a given molar ratios of Na:Ca:P = 0:1:1(Ca), Na:Ca:P = 0.5:0.5:1(CaNa), Na:Ca:P = 1:1:1(Na), which were capable of entering into a chemical reaction; molding samples of cement-salt stone; and firing samples of cement-salt stone to obtain ceramics.
- 2.
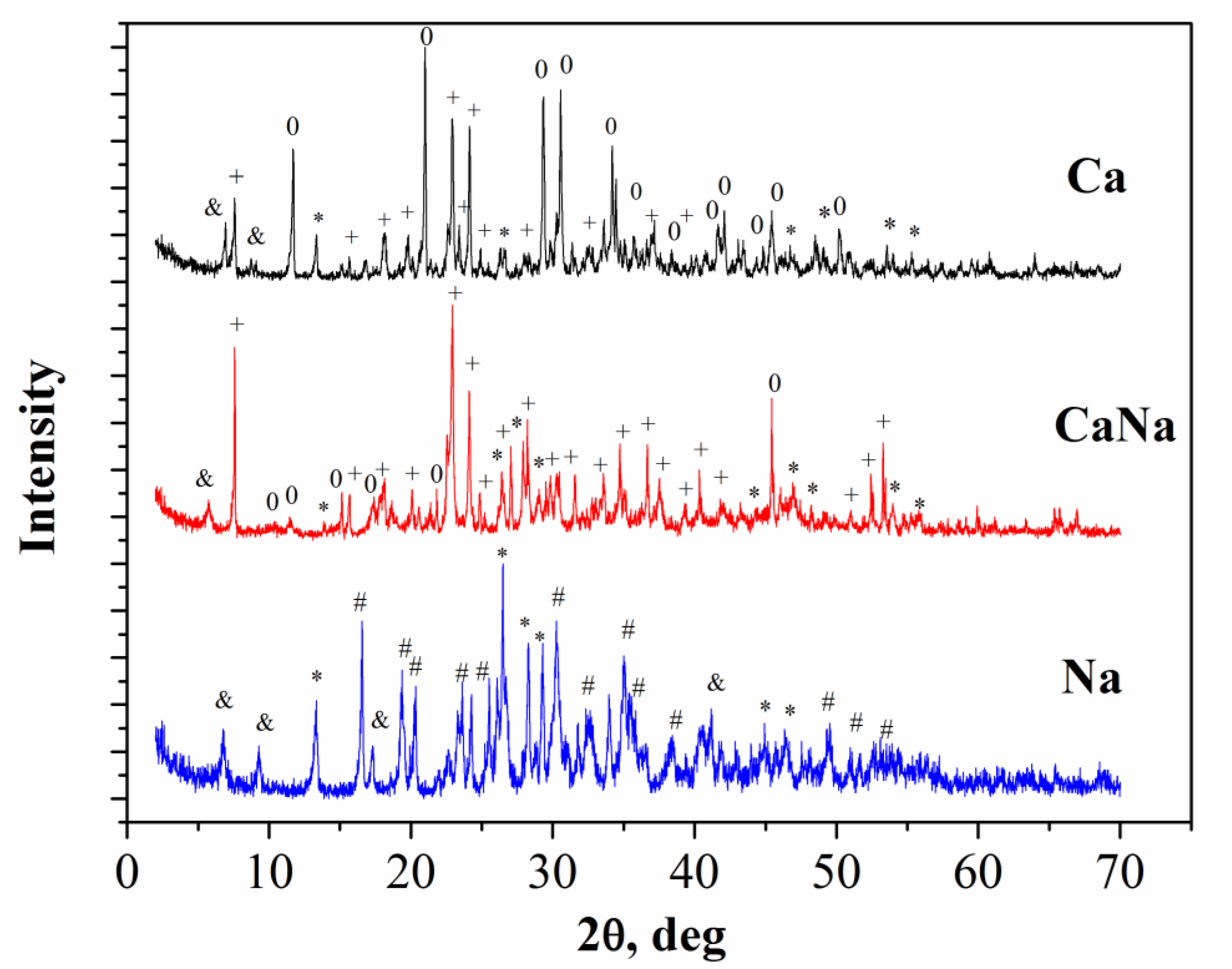
- The phase composition of Ca and Na cement-salt stone samples was represented by brushite (CaHPO4∙2H2O), monetite (CaHPO4) and unreacted Ca3(C6H5O7)2∙4H2O, Ca(H2PO4)2∙H2O and NaH2PO4 respectively. CaNa cement-salt stone samples were prepared from a powder mixture with a molar ratio of Na:Ca:P = 0.5:0.5:1, including calcium citrate tetrahydrate Ca3(C6H5O7)2∙4H2O, MCPM Ca(H2PO4)2∙H2O and sodium dihydrogen phosphate NaH2PO4. The phase composition of cement-salt stone samples based on Ca3(C6H5O7)2∙4H2O, Ca(H2PO4)2∙H2O and NaH2PO4 was represented mainly by brushite CaHPO4∙2H2O, monetite CaHPO4, as well as unreacted Ca(H2PO4)2∙H2O, NaH2PO4 and Ca3(C6H5O7)2∙4H2O.
- 3.
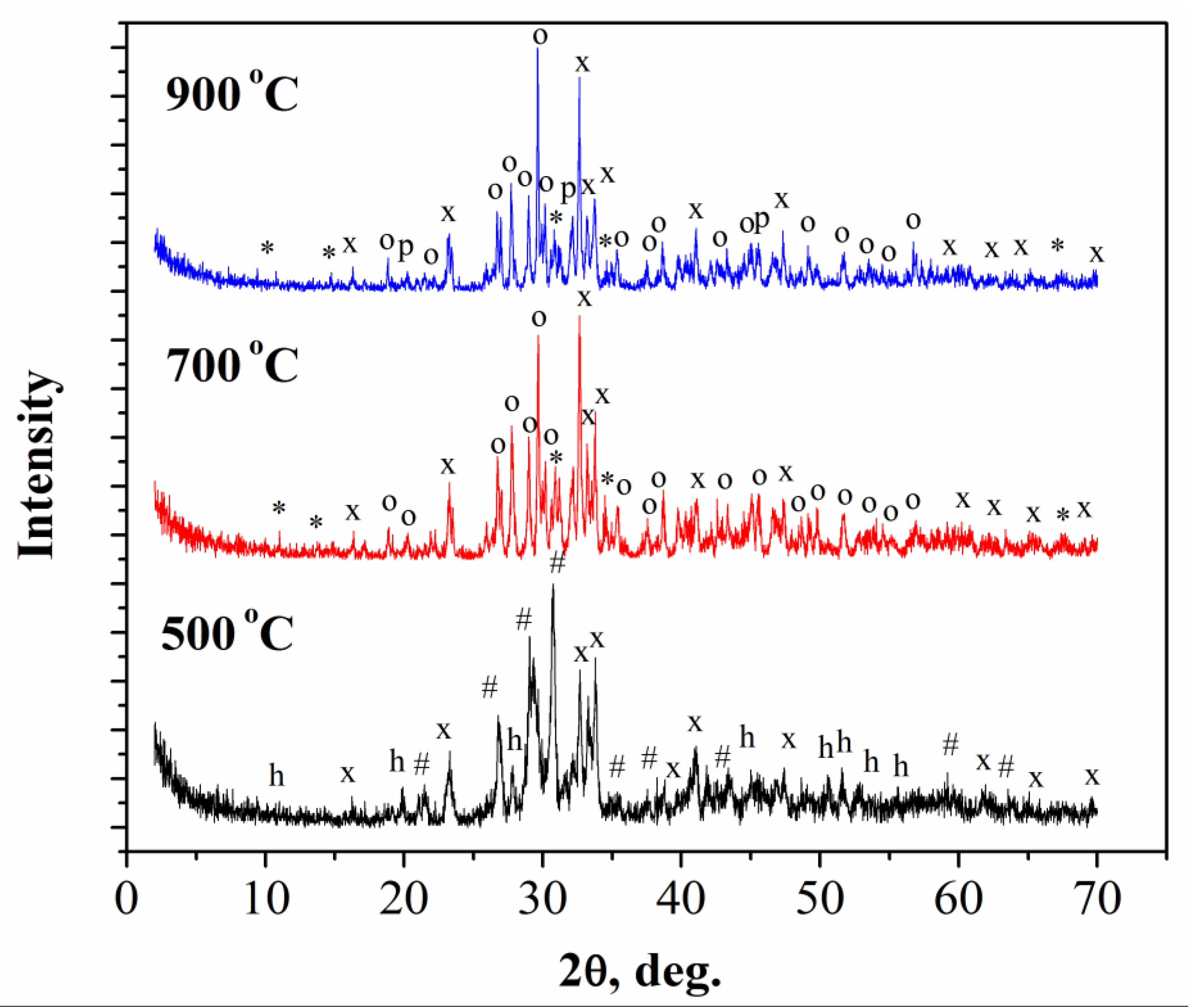
- After annealing of cement-salt stone Ca at 500 °C phase composition of the ceramic materials was represented by γ-Ca2P2O7 and γ-Ca(PO3)2. In the temperature range of 700–900 °C γ-Ca(PO3)2 and γ-Ca2P2O7 phases passed into a higher-temperature modifications (β-Ca(PO3)2 and β-Ca2P2O7). And after annealing at 1000 °C the phase composition of ceramics was presented only with β-Ca2P2O7.During the heat treatment of cement-salt stone Na at temperatures of 500 and 700 °C in addition to the target phase β-CaNaPO4, hydroxyapatite Ca10(PO4)6(OH)2 was formed. At 700 °C, in addition to β-CaNaPO4 and Ca10(PO4)6(OH)2, phases of double calcium-sodium pyrophosphate CaNa2P2O7 and β-Ca3(PO4)2 phases were formed. At 900 °C, only the target phase β-CaNaPO4 was found.Heat treatment of cement-salt stone CaNa at a temperature of 500 °C led to the formation of a phase composition, which included the β-CaNaPO4, Ca10(PO4)6(OH)2, and γ-Ca2P2O7 phases. At temperatures of 700 °C and 900 °C, in addition to β-CaNaPO4, phases of β-Ca2P2O7, double calcium-sodium pyrophosphate Na2CaP2O7 and Na–substituted tricalcium phosphate Сa10Na(PO4)7 phases were formed in minor quantities.
- 4.
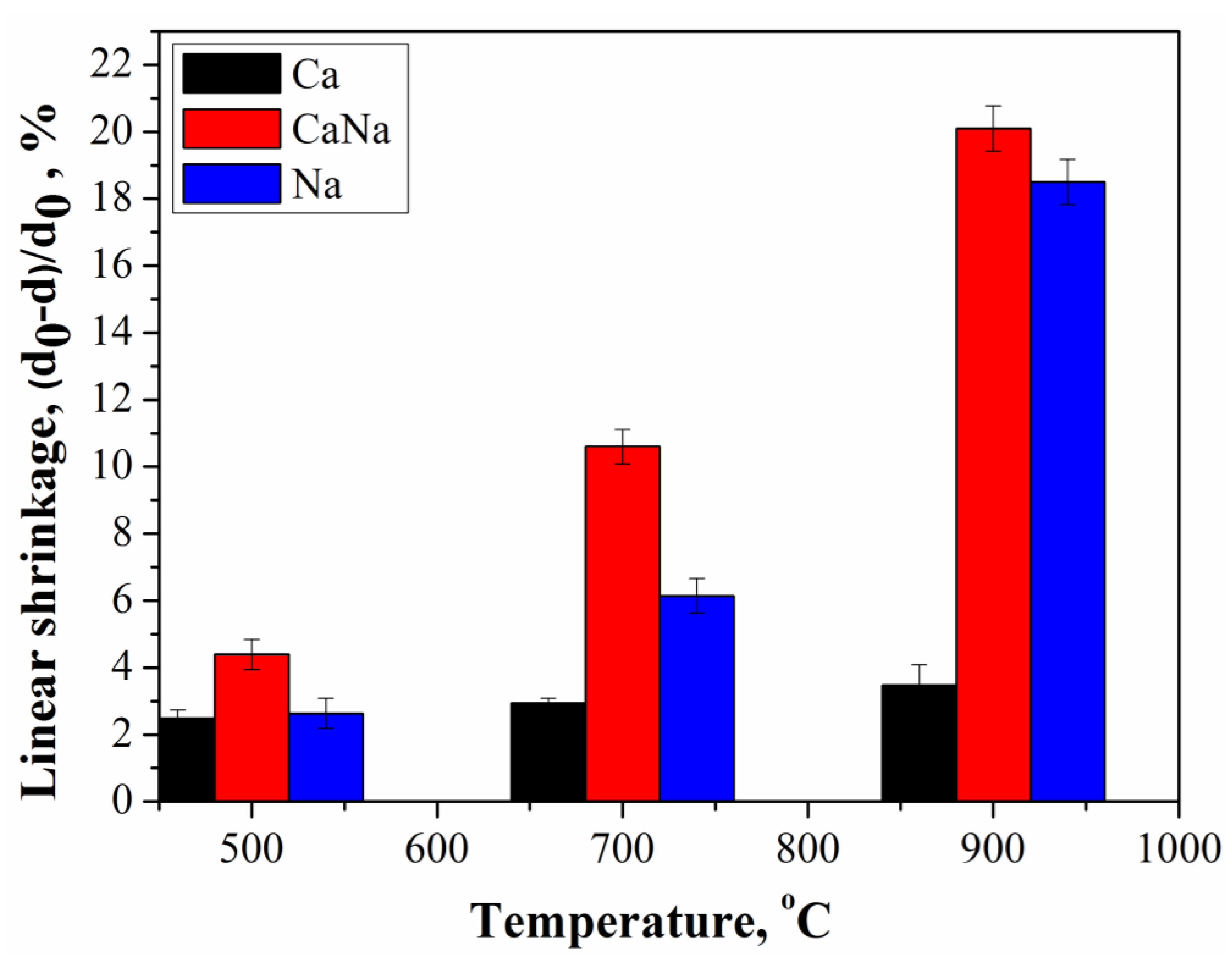
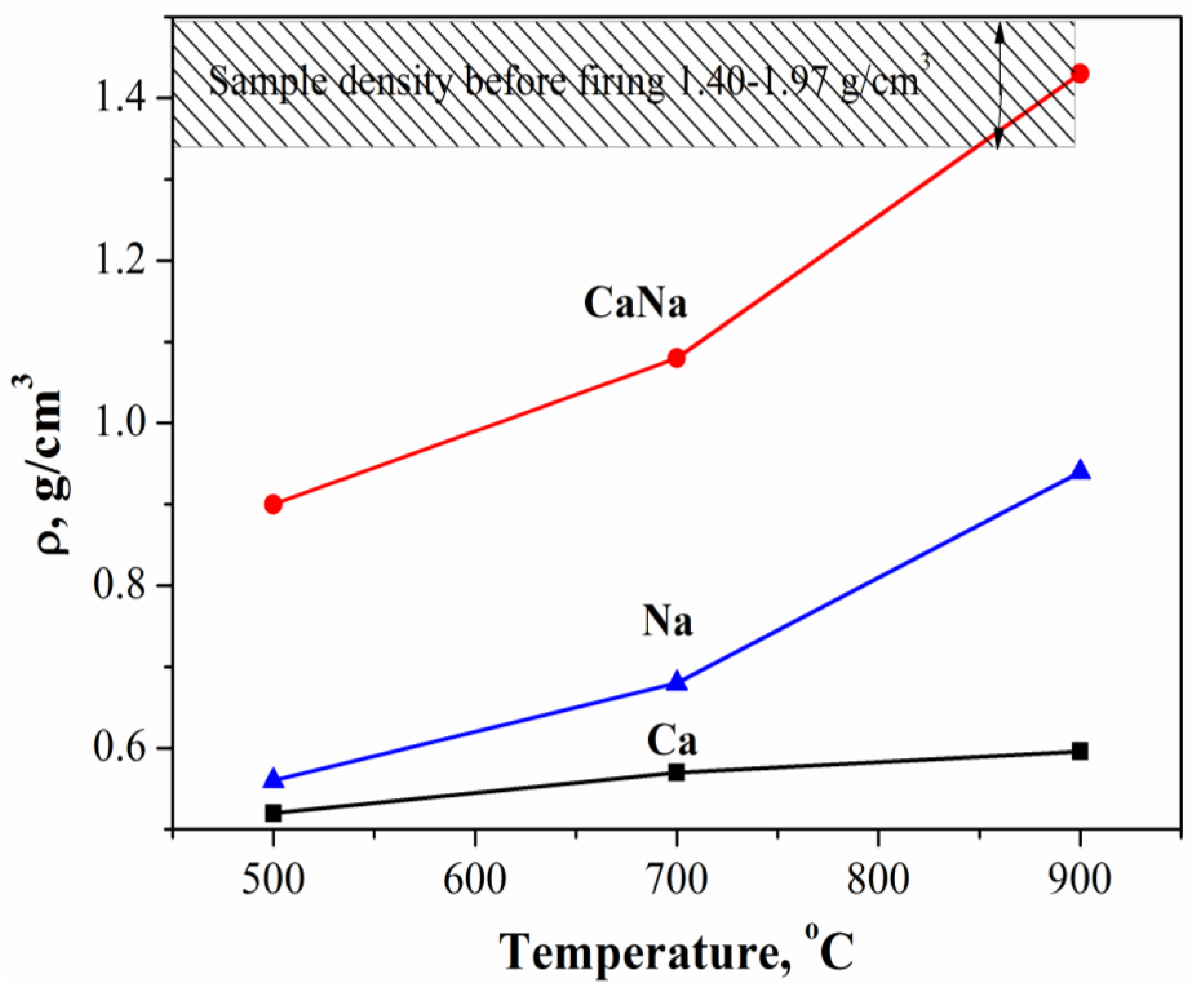
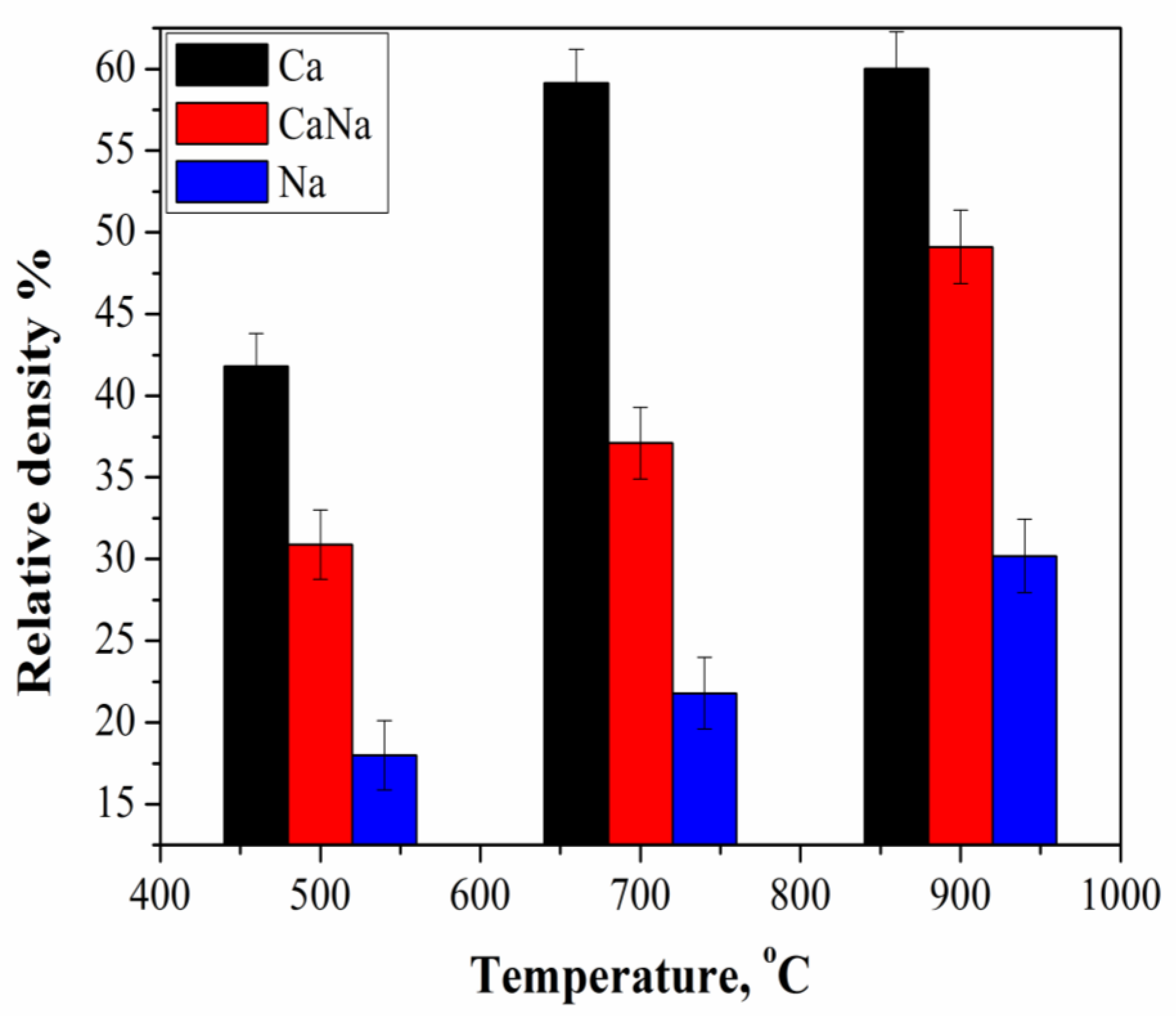
- The density of Ca samples increased from 0.52 g/cm3 to 0.59 g/cm3 with an increase in firing temperature from 500 °C to 900 °C or from 41.8% to 60% relatively to the density of β-Ca2P2O7 equal to 3.09 g/cm3. The shrinkage of the Ca samples was 2.5% and 3.5% at 500 °C and 900 °C, respectively.With an increase in firing temperature from 500 °C to 900 °C, the density of Na samples increased from 0.56 g/cm3 to 0.94 g/cm3 or from 18% to 30.2% relatively to the density of β-CaNaPO4 equal to 3.11 g/cm3. The shrinkage of the Na samples was 2.7% and 18.5% at 500 °C and 900 °C, respectively.The density of CaNa samples increased from 0.9 g/cm3 to 1.43 g/cm3 or from 28.9% to 45.9% relatively to the density of β-CaNaPO4 equal to 3.11 g/cm3. The shrinkage of the CaNa samples was 4.4% and 20.1% at 500 °C and 900 °C, respectively.
- 5.
- Thus, ceramic materials in Na2O-CaO-P2O5 system developed here, consisting of biocompatible and bioresorbable β-CPP β-Ca2P2O7, β-sodium rhenanite β-CaNaPO4, double calcium-sodium pyrophosphate Na2CaP2O7, and Na–substituted tricalcium phosphate Сa10Na(PO4)7 phases can be used in regenerative methods for the treatment of bone tissue defects.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tavoni, M.; Dapporto, M.; Tampieri, A.; Sprio, S. Bioactive calcium phosphate-based composites for bone regeneration. J. Compos. Sci. 2021, 5, 227. [Google Scholar] [CrossRef]
- Kanazawa, T. Inorganic Phosphate Materials; Elsevier Science Ltd.: Oxford, UK, 1989; 306p. [Google Scholar]
- Bohner, M.; Santoni, B.L.G.; Dobelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef]
- Toshev, O.; Safronova, T.; Kaimonov, M.; Shatalova, T.; Klimashina, E.; Lukina, Y.; Malyutin, K.; Sivkov, S. Biocompatibility of ceramic materials in Ca2P2O7–Ca(PO3)2 system obtained via heat treatment of cement-salt stone. Ceramics. 2022, 5, 516–532. [Google Scholar] [CrossRef]
- Safronova, T.; Kiselev, A.; Selezneva, I.; Shatalova, T.; Lukina, Y.; Filippov, Y.; Toshev, O.; Tihonova, S.; Antonova, O.; Knotko, A. Bioceramics based on β-calcium pyrophosphate. Materials 2022, 15, 3105. [Google Scholar] [CrossRef]
- Safronova, T.V.; Mukhin, E.A.; Putlyaev, V.I.; Knotko, A.V.; Evdokimov, P.V.; Shatalova, T.B.; Filippov, Y.Y.; Sidorov, E.A.; Karpushkin, E.A. Amorphous calcium phosphate powder synthesized from calcium acetate and polyphosphoric acid for bi-oceramics application. Ceram. Int. 2017, 43, 1310–1317. [Google Scholar] [CrossRef]
- Yuan, Y.; Yuan, Q.; Wu, C.; Ding, Z.; Wang, X.; Li, G.; Gu, Z.; Li, L.; Xie, H. Enhanced osteoconductivity and osseointegration in calcium polyphosphate bioceramic scaffold via lithium doping for bone regeneration. ACS Biomater. Sci. Eng. 2019, 5, 5872–5880. [Google Scholar] [CrossRef]
- Orlov, N.K.; Putlayev, V.I.; Evdokimov, P.V.; Safronova, T.V.; Garshev, A.V.; Milkin, P.A. Composite bioceramics engineering based on analysis of phase equilibria in the Ca3(PO4)2-CaNaPO4-CaKPO4 system. Inorg. Mater. 2019, 55, 516–523. [Google Scholar] [CrossRef]
- Orlov, N.; Kiseleva, A.; Milkin, P.; Evdokimov, P.; Putlayev, V.; Günster, J.; Biesuz, M.; Sglavod, V.M.; Tyablikov, A. Sintering of mixed Ca–K–Na phosphates: Spark plasma sintering vs flash-sintering. Open Ceram. 2021, 5, 100072. [Google Scholar] [CrossRef]
- Safronova, T.V.; Korneichuk, S.A.; Shatalova, T.B.; Lukina, Y.S.; Sivkov, S.P.; Filippov, Y.; Krut’ko, V.K.; Musskaya, O.N. Ca2P2O7–Ca(PO3)2 ceramic obtained by firing β-tricalcium phosphate and monocalcium phosphate monohydrate based cement stone. Glass Ceram. 2020, 77, 165–172. [Google Scholar] [CrossRef]
- Safronova, T.V.; Shatalova, T.B.; Filippov, Y.; Krut’ko, V.K.; Musskaya, O.N.; Safronov, A.S.; Toshev, O.U. Ceramics in the Ca2P2O7–Ca (PO3)2 system obtained by annealing of the samples made from hardening mixtures based on calcium citrate tetrahydrate and monocalcium phosphate monohydrate. Inorg. Mater. Appl. Res. 2020, 11, 777–786. [Google Scholar] [CrossRef]
- Safronova, T.V.; Lukina, Y.S.; Sivkov, S.P.; Toshev, O.U.; Kazakova, G.K.; Shatalova, T.B.; Filippov, Y.Y.; Malyutin, K.V.; Aziziyan-Kalandarag, Y. Ceramics based on calcium pyrophosphate, obtained by annealing cement stone. Tehknika Tehnol. Silikatov. 2020, 27, 17–20. [Google Scholar]
- Zhou, H.; Yang, L.; Gbureck, U.; Bhaduri, S.B.; Sikder, P. Monetite, an important calcium phosphate compound–Its synthesis, properties and applications in orthopedics. Acta Biomaterialia 2021, 127, 41–55. [Google Scholar] [CrossRef]
- Toshima, T.; Hamai, R.; Tafu, M.; Takemura, Y.; Fujita, S.; Chohji, T.; Tanda, S.; Li, S.; Qin, G.W. Morphology control of brushite prepared by aqueous solution synthesis. J. Asian Ceram. Soc. 2014, 2, 52–56. [Google Scholar] [CrossRef]
- Safronova, T.V.; Sadilov, I.S.; Chaikun, K.V.; Shatalova, T.B.; Filippov, Y.Y. Synthesis of Monetite from Calcium Hydroxyapatite and Monocalcium Phosphate Monohydrate under Mechanical Activation Conditions. Russ. J. Inorg. Chem. 2019, 64, 1088–1094. [Google Scholar] [CrossRef]
- Webb, N.C. The crystal structure of β-Ca2P2O7. Acta Cryst. 1966, 21, 942–948. [Google Scholar] [CrossRef]
- Ding, L.; Wang, H.; Li, J.; Liu, D.; Bai, J.; Yuan, Z.; Yang, J.; Bian, L.; Zhao, X.; Li, B.; et al. Preparation and characterizations of an injectable and biodegradable high-strength iron-bearing brushite cement for bone repair and vertebral augmentation applications. Biomater. Sci. 2023, 11, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Michiardi, A.; Castano, O.; Planell, J.A. Biomaterials in orthopaedics. Biomater. Implants Tissue Eng. 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [PubMed]
- Holland, W.; Rheinberger, V.; Wegner, S.; Frank, M. Needle-like apatite-leucite glass-seramic as a base material for the veneering of metal restorations in dentistry. J. Mater. Sci. Mater. Med. 2000, 11, 11–17. [Google Scholar] [CrossRef]
- Apel, E.; Holland, W.; Rheinberger, V. Bioactive Rhenanite Glass. Ceramic. U.S. Patent 7,074,730, 11 July 2006. [Google Scholar]
- Rautaray, H.K.; Dash, R.N.; Mohanty, S.K. Phosphorus supplying power of some thermally promoted reaction products of phosphate rosks. Fertil. Res. 1995, 41, 67–75. [Google Scholar] [CrossRef]
- Suchanek, W.; Yashima, M.; Kakihana, M.; Yoshimura, M. β-rhenanite (β-NaCaPO4) as weak interphase for hydroxyapatite ceramics. J. Eur. Ceram. Soc. 1998, 18, 1923–1929. [Google Scholar] [CrossRef]
- Ramselaar, M.M.A.; Van Mullem, P.J.; Kalk, W.; Driessens, F.C.M.; Dewijn, J.R.; Stols, A.L.H. In vivo reactions to paniculate rhenanite and particulate hydroxyapatite after implantation in tooth sockets. J. Mater. Sci. Mater. Med. 1993, 4, 311–317. [Google Scholar] [CrossRef]
- Glasser, F.P.; Gunawardane, R.P. Fertilizer Material from Apatite. U.S. Patent 4,363,650, 14 December 1982. [Google Scholar]
- Toshev, O.U.; Safronova, T.V.; Kazakova, G.K.; Shatalova, T.B.; Boytsova, O.V.; Lukina, Y.S.; Sivkov, S.P. Ceramics based on sodium rhenanite CaNaPO4, obtained via firing of composite cement-salt stone. J. Compos. Sci. 2022, 6, 314. [Google Scholar] [CrossRef]
- Sventskaya, N.V.; Lukina, Y.S.; Larionov, D.S.; Andreev, D.V.; Sivkov, S.P. 3D-matrix based on bioactive glass and calcium phosphates with controllable resorption rate for bone tissue replacement. Glass Ceram. 2017, 73, 342–347. [Google Scholar] [CrossRef]
- Knotts, R.; Jalota, S.; Bhaduri, S.; Tas, A. Synthesis of Rhenanite (β-NaCaPO4)-Apatitic Calcium Phosphate Biphasics for Skeletal Repair. Adv. Bioceram. Porous Ceram. Ceram. Eng. Sci. Proc. 2009, 29, 151–164. [Google Scholar]
- Kaimonov, M.; Safronova, T.; Shatalova, T.; Filippov, Y.; Tikhomirova, I.; Lukina, Y. Composite Ceramics Based on Pastes Including Tricalcium Phosphate and an Aqueous Solution of Sodium Silicate. J. Compos. Sci. 2022, 6, 267. [Google Scholar] [CrossRef]
- Golubchikov, D.; Safronova, T.V.; Nemygina, E.; Shatalova, T.B.; Tikhomirova, I.N.; Roslyakov, I.V.; Khayrutdinova, D.; Platonov, V.; Boytsova, O.; Kaimonov, M.; et al. Powder Synthesized from Aqueous Solution of Calcium Nitrate and Mixed-Anionic Solution of Orthophosphate and Silicate Anions for Bioceramics Production. Coatings. 2023, 13, 374. [Google Scholar] [CrossRef]
- ICDD. International Centre for Diffraction Data; PDF-4+ 2010 (Database); Kabekkodu, S., Ed.; ICDD: Newtown Square, PA, USA, 2010; Available online: https://www.icdd.com/pdf-2/ (accessed on 20 February 2022).
- Minh, D.P.; Lyczko, N.; Sebei, H.; Nzihou, A.; Sharrock, P. Synthesis of calcium hydroxyapatite from calcium carbonate and different orthophosphate sources: A comparative study. Mater. Sci. Eng. B 2012, 177, 1080–1089. [Google Scholar] [CrossRef]
- Ren, D.; Ruan, Q.; Tao, J.; Lo, J.; Nutt, S.; Moradian-Oldak, J. Amelogenin affects brushite crystal morphology and promotes its phase transformation to monetite. Cryst. Growth Desi. 2016, 16, 4981–4990. [Google Scholar] [CrossRef]
- Hurle, K.; Oliveira, J.M.; Reis, R.L.; Pina, S.; Goetz-Neunhoeffer, F. Ion-doped brushite cements for bone regeneration. Acta Biomater. 2021, 123, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Safronova, T.V.; Shatalova, T.B.; Tikhonova, S.A.; Filippov, Y.Y.; Krut’ko, V.K.; Musskaya, O.N.; Kononenko, N.E. Synthesis of calcium pyrophosphate powders from phosphoric acid and calcium carbonate. Inorg. Mater. Appl. Res. 2021, 12, 986–992. [Google Scholar] [CrossRef]
- Prihanto, A.; Fitriyana, D.F.; Muryanto, S.; Masykur, I.; Ismail, R.; Jamari, J.; Bayuseno, A.P. Aqueous crystallization of monocalcium phosphate monohydrate with green mussel shells (Verna piridis) for calcium sources. J. Environ. Chem. Eng. 2021, 9, 106913. [Google Scholar] [CrossRef]
- Jinawath, S.; Pongkao, D.; Suchanek, W.; Yoshimura, M. Hydrothermal synthesis of monetite and hydroxyapatite from monocalcium phosphate monohydrate. Int. J. Inorg. Mater. 2001, 3, 997–1001. [Google Scholar] [CrossRef]
- Sarda, S.; Fernández, E.; Nilsson, M.; Balcells, M.; Planell, J.A. Kinetic study of citric acid influence on calcium phosphate bone cements as water-reducing agent. J. Biomed. Mater. Res. 2002, 61, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.A.A. Thermal decomposition of calcium citrate tetrahydrate. Thermochim. Acta 1994, 233, 243–256. [Google Scholar] [CrossRef]
- Toshev, O.U.; Safronova, T.V.; Mironova, Y.S.; Matveeva, A.S.; Shatalova, T.B.; Filippov, Y.Y.; Knotko, A.V.; Akhmedov, M.R.; Kukueva, E.V.; Lukina, Y.S. Ultraporous Submicron-Grained β-Ca3(PO4)2 - Based Ceramics. Inorg. Mater. 2022, 58, 1208–1219. [Google Scholar] [CrossRef]




| Symbol at Graph | Labeling | Molar Ratio Na/Са/Р | The Composition of the Powder Mixture, g | Expected Phase Composition of Ceramics | ||
|---|---|---|---|---|---|---|
| Ca3(C6H5O7)2∙4H2O (g) | Ca(H2PO4)2∙H2O (g) | NaH2PO4 (g) | ||||
| a | Ca | 0/1/1 | 43 | 57 | 0 | β-Ca2P2O7 |
| b | CaNa | 0.5/0.5/1 | 50.6 | 33.5 | 15.9 | β-Ca2P2O7 + β-CaNaPO4 |
| c | Na | 1/1/1 | 61.3 | 0 | 38.7 | β-CaNaPO4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toshev, O.U.; Safronova, T.V.; Shatalova, T.B.; Lukina, Y.S. Ceramic Materials in Na2O-CaO-P2O5 System, Obtained via Heat Treatment of Cement-Salt Stone Based on Powder Mixture of Ca3(C6H5O7)2∙4H2O, Ca(H2PO4)2∙H2O and NaH2PO4. Ceramics 2023, 6, 600-618. https://doi.org/10.3390/ceramics6010036
Toshev OU, Safronova TV, Shatalova TB, Lukina YS. Ceramic Materials in Na2O-CaO-P2O5 System, Obtained via Heat Treatment of Cement-Salt Stone Based on Powder Mixture of Ca3(C6H5O7)2∙4H2O, Ca(H2PO4)2∙H2O and NaH2PO4. Ceramics. 2023; 6(1):600-618. https://doi.org/10.3390/ceramics6010036
Chicago/Turabian StyleToshev, Otabek U., Tatiana V. Safronova, Tatiana B. Shatalova, and Yulia S. Lukina. 2023. "Ceramic Materials in Na2O-CaO-P2O5 System, Obtained via Heat Treatment of Cement-Salt Stone Based on Powder Mixture of Ca3(C6H5O7)2∙4H2O, Ca(H2PO4)2∙H2O and NaH2PO4" Ceramics 6, no. 1: 600-618. https://doi.org/10.3390/ceramics6010036
APA StyleToshev, O. U., Safronova, T. V., Shatalova, T. B., & Lukina, Y. S. (2023). Ceramic Materials in Na2O-CaO-P2O5 System, Obtained via Heat Treatment of Cement-Salt Stone Based on Powder Mixture of Ca3(C6H5O7)2∙4H2O, Ca(H2PO4)2∙H2O and NaH2PO4. Ceramics, 6(1), 600-618. https://doi.org/10.3390/ceramics6010036







