Oxidation Resistance of γ-TiAl Based Alloys Modified by C, Si and Y2O3 Microdopants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Initial Powders
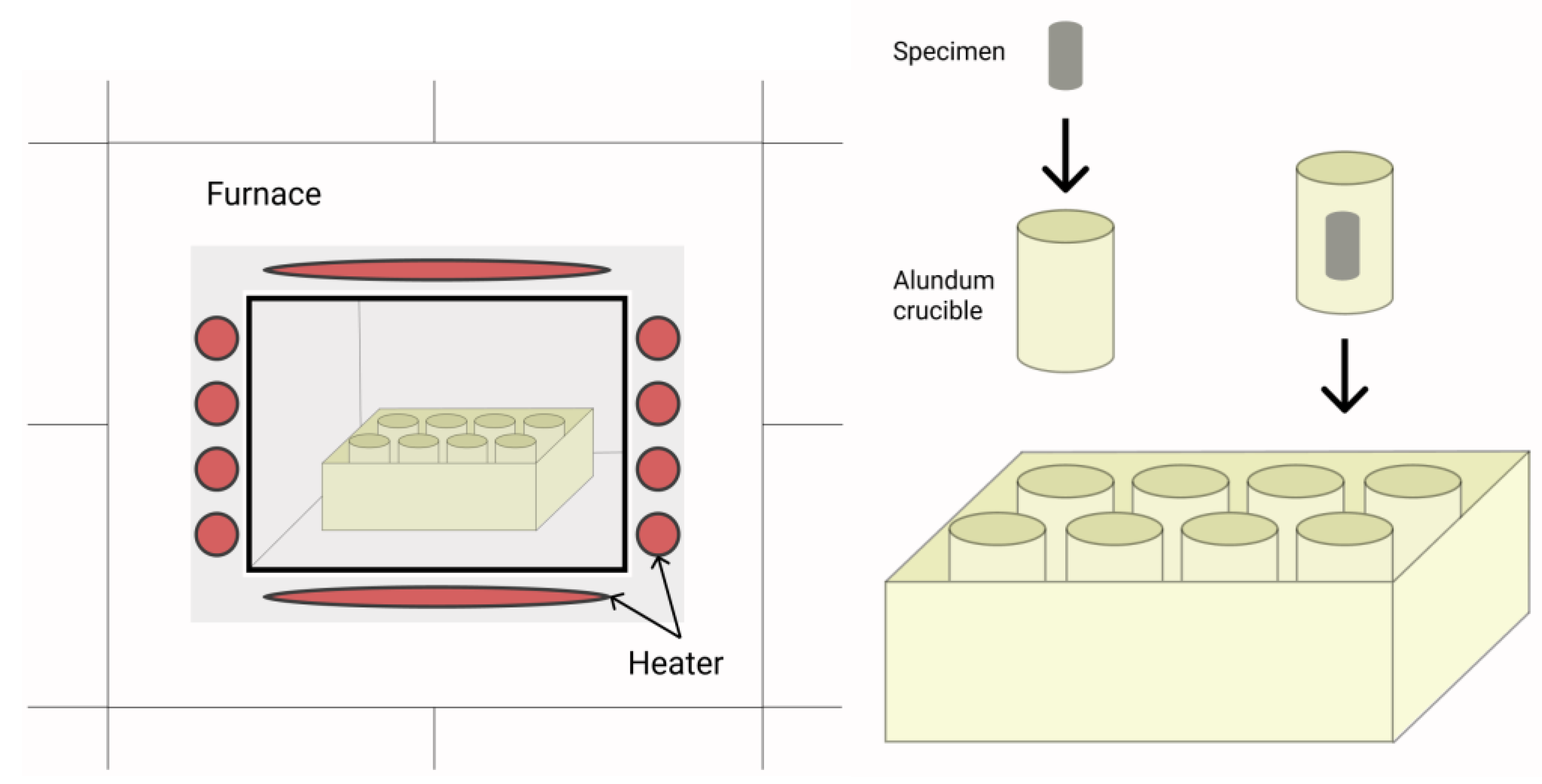
2.2. Manufacturing and Investigation Methods
3. Results and Discussion
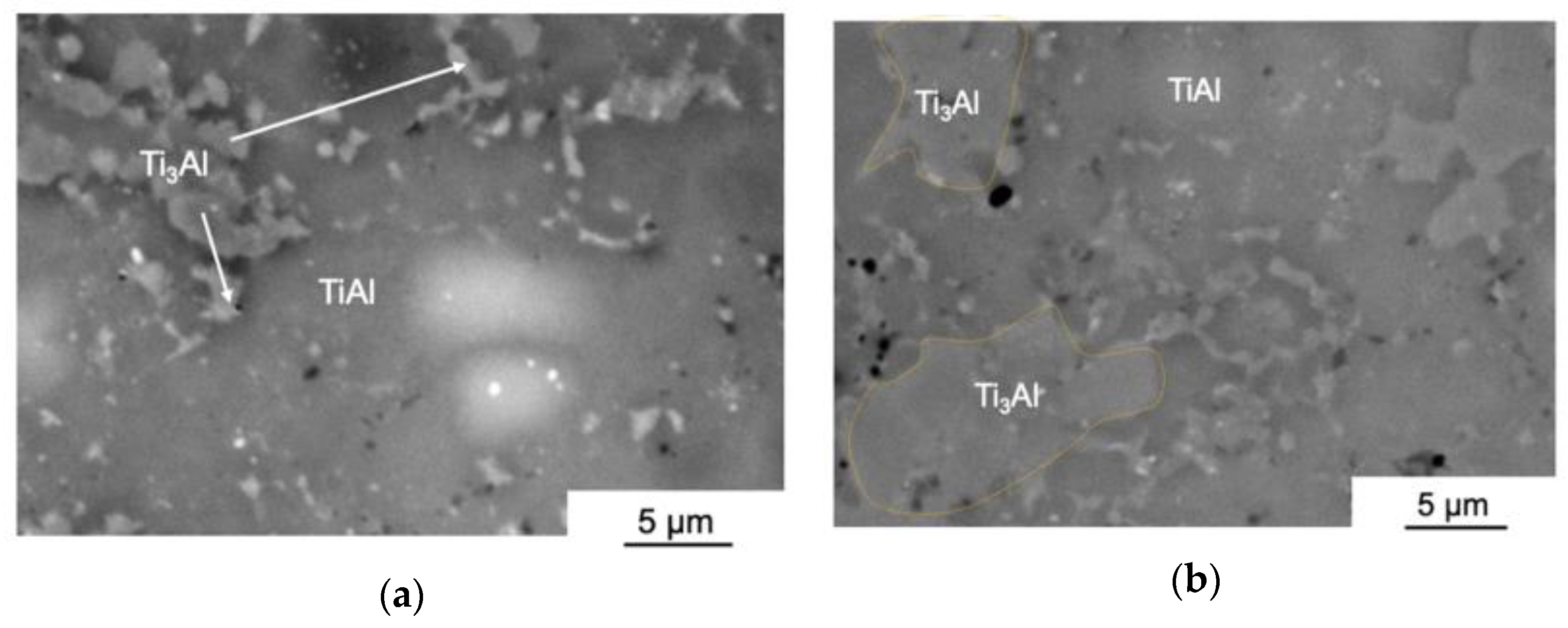
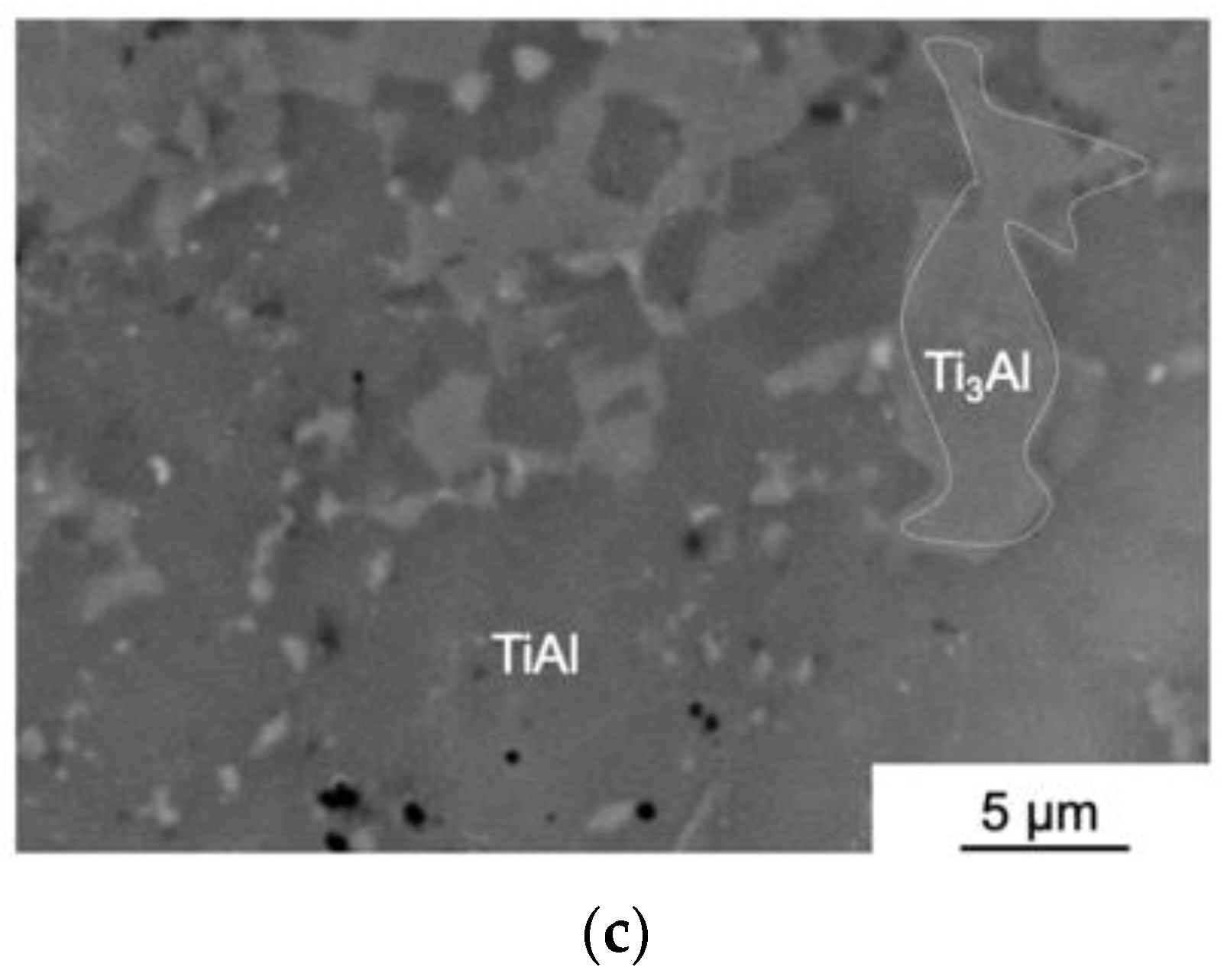
3.1. The Structure and Phase Composition of as-HIPed Samples
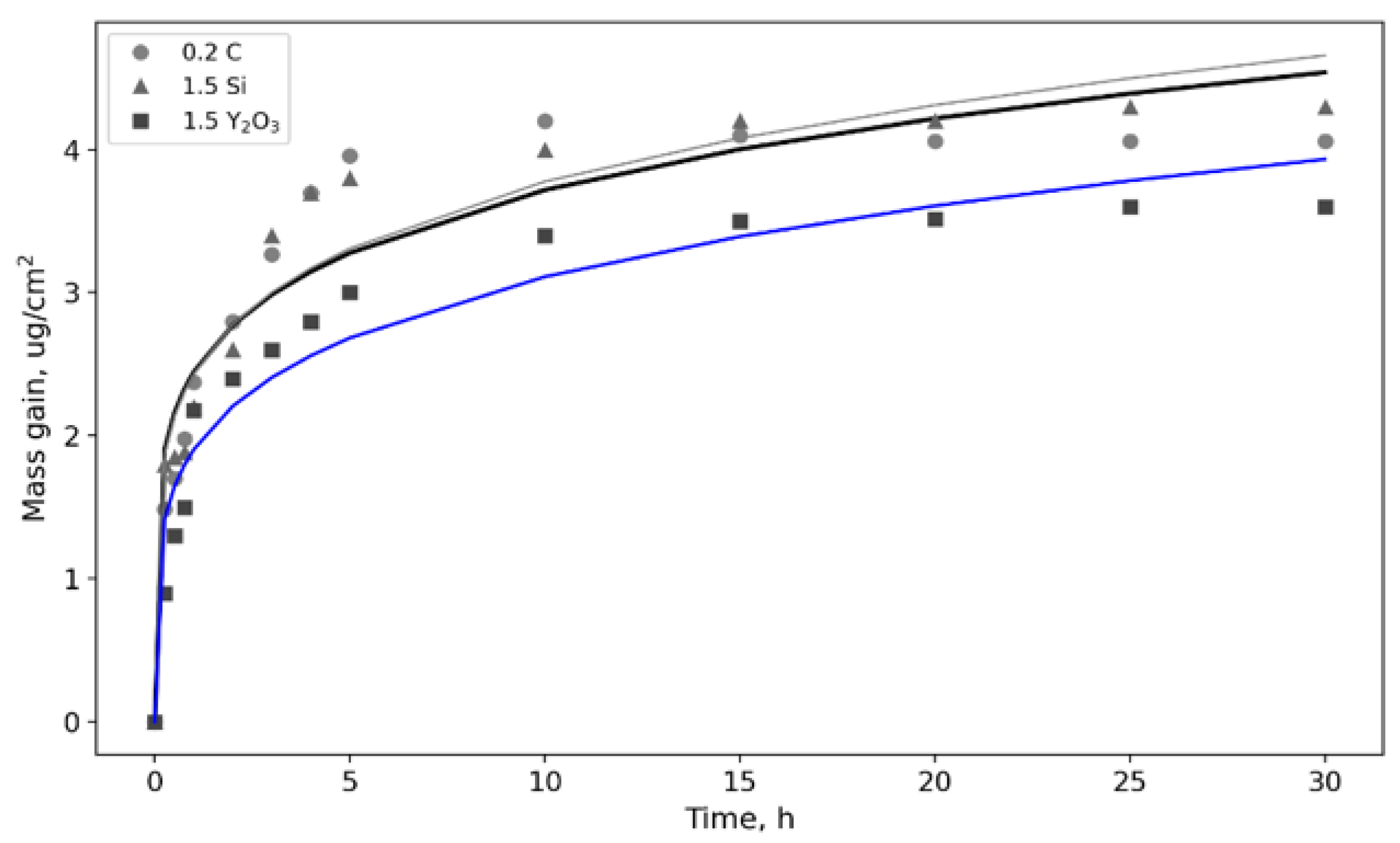
3.2. Oxidation Kinetics at 800 °C; of TiAl-Based Alloys
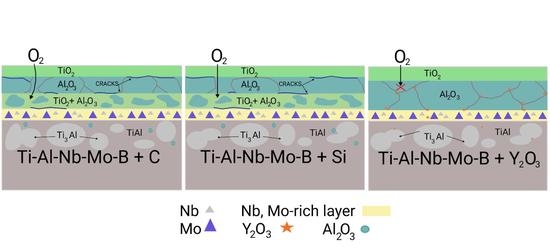
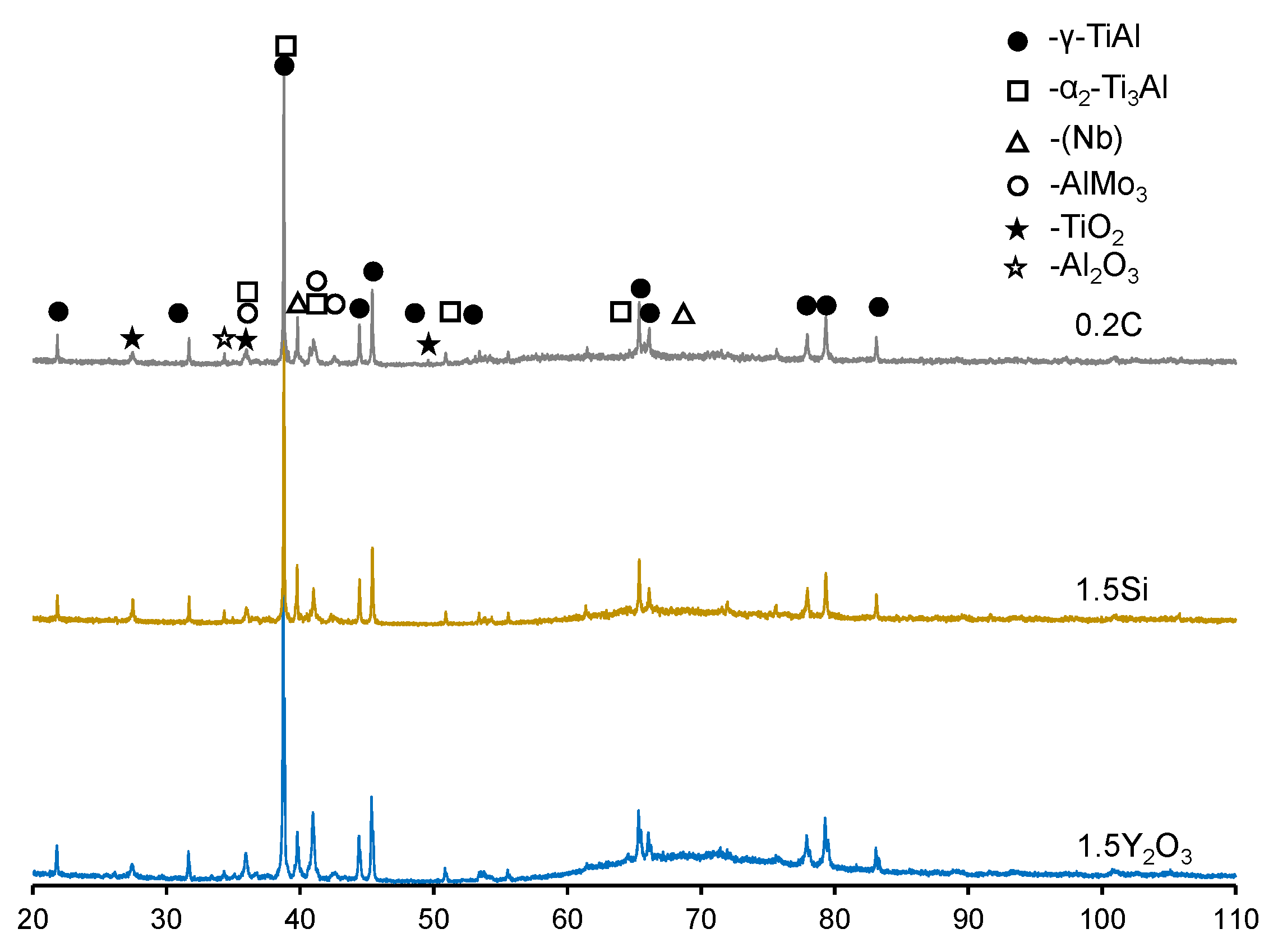
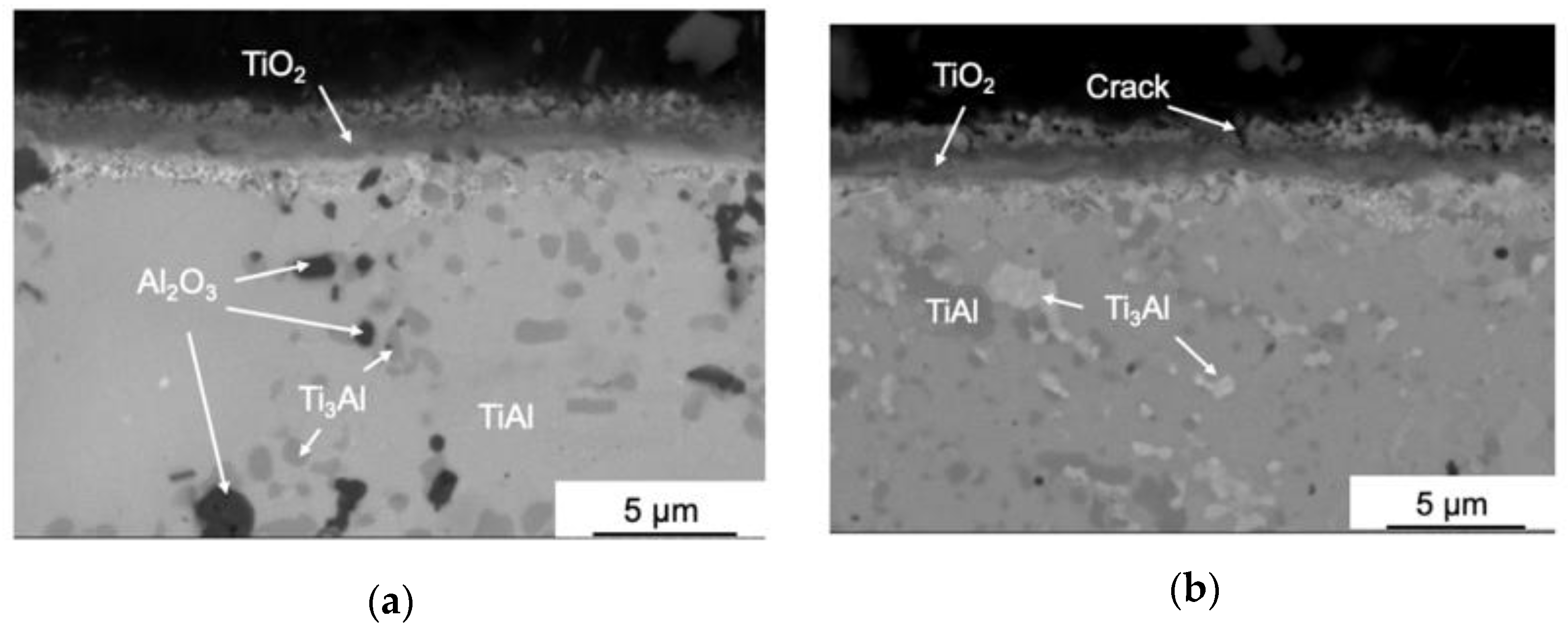
3.3. Oxidation Kinetics at 1100 °C of TiAl-Based Alloys
4. Discussion
5. Conclusions
- All of the alloys exhibited a high oxidation resistance at 800 °C. The oxidation kinetics can be described by a power–law with a power–law exponent of 5.461–5.580. A coating based on rutile TiO2 (~2 µm thick) was formed on the surface;
- Oxidation of the 0.2C and 1.5Si alloys at 1100 °C caused the formation of three-layer TiO2/Al2O3/(TiO2 + Al2O3) coatings with a porous structure and a large number of cracks, resulting in spallation;
- The alloy modified with 1.5Y2O3 had the best oxidation resistance at 1100 °C. During annealing, the oxide layer formed the Y2Ti2O7 phase, which has low oxygen permeability and high temperature stability. The formation of Y2Ti2O7 was accompanied by a decrease in the concentration of undesirable TiO2 oxide;
- An interlayer rich in niobium and molybdenum was formed at the oxide layer/matrix interface. This interlayer ensures thermal stability of the alloy by preventing oxygen diffusion into the material bulk as well as titanium and aluminum diffusion toward the surface.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, R.K.; Pant, B. Titanium aluminides. Intermet. Matrix Compos. 2018, 4, 71–93. [Google Scholar] [CrossRef]
- Appel, F.; Brossmann, U.; Christoph, U.; Eggert, S.; Janschek, P.; Lorenz, U.; Mullauer, J.; Oehring, M.; Paul, J.D.H. Recent progress in the development of gamma titanium aluminide alloys. Adv. Eng. Mater. 2000, 2, 699–720. [Google Scholar] [CrossRef]
- Shi, J.; Zheng, A.; Lin, Z.; Chen, R.; Zheng, J.; Cao, Z. Effect of process control agent on alloying and mechanical behavior of L21 phase Ni–Ti–Al alloys. Mater. Sci. Eng. A 2019, 740–741, 130–136. [Google Scholar] [CrossRef]
- Nepapushev, A.A.; Moskovskikh, D.O.; Buinevich, V.S.; Vadchenko, S.G.; Rogachev, A.S. Production of Rounded Reactive Composite Ti/Al Powders for Selective Laser Melting by High-Energy Ball Milling. Met. Mater. Trans. B 2019, 50, 1241–1247. [Google Scholar] [CrossRef]
- Kenel, C.; Lis, A.; Dawson, K.; Stiefel, M.; Pecnik, C.; Barras, J.; Colella, A.; Hauser, C.; Tatlock, G.; Leinenbach, C.; et al. Mechanical performance and oxidation resistance of an ODS γ-TiAl alloy processed by spark plasma sintering and laser additive manufacturing. Intermetallics 2017, 91, 169–180. [Google Scholar] [CrossRef]
- Meng, K.; Guo, K.; Yu, Q.; Miao, D.; Yao, C.; Wang, Q.; Wang, T. Effect of annealing temperature on the microstructure and corrosion behavior of Ti-6Al-3Nb-2Zr-1Mo alloy in hydrochloric acid solution. Corros. Sci. 2021, 183, 109320. [Google Scholar] [CrossRef]
- Huang, Z.; Lin, J.; Sun, H. Microstructural changes and mechanical behaviour of a near lamellar γ-TiAl alloy during long-term exposure at 700 °C. Intermetallics 2017, 85, 59–68. [Google Scholar] [CrossRef]
- Galetz, M.; Ulrich, A.; Oskay, C.; Fähsing, D.; Laska, N.; Schulz, U.; Schütze, M. Oxidation-induced microstructural changes of the TiAl TNM-B1 alloy after exposure at 900 °C in air. Intermetallics 2020, 123, 106830. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Zhang, C.J.; Du, Z.X.; Hou, Z.; Lin, P.; Kong, F.T.; Chen, Y.Y. Deformation behavior of high Nb containing TiAl based alloy in α + γ two phase field region. Mater. Des. 2016, 90, 225–229. [Google Scholar] [CrossRef]
- Niu, H.Z.; Chen, X.J.; Chen, Y.F.; Zhao, S.; Liu, G.H.; Zhang, D.L. Microstructural stability, phase transformation and mechanical properties of a fully-lamellar microstructure of a Mo-modified high-Nb γ-TiAl alloy. Mater. Sci. Eng. A 2020, 784, 139313. [Google Scholar] [CrossRef]
- Bizot, Q.; Politano, O.; Nepapushev, A.A.; Vadchenko, S.G.; Rogachev, A.S.; Baras, F. Reactivity of the Ti–Al system: Experimental study and molecular dynamics simulations. J. Appl. Phys. 2020, 127, 145304. [Google Scholar] [CrossRef]
- Ismaeel, A.; Wang, C.-S. Effect of Nb additions on microstructure and properties of γ-TiAl based alloys fabricated by selective laser melting. Trans. Nonferrous Met. Soc. China 2019, 29, 1007–1016. [Google Scholar] [CrossRef]
- Zhang, S.; Song, Z.; Han, J.; Zhang, C.; Lin, P.; Zhu, D.; Kong, F.; Chen, Y. Effect of 2–6 at.% Mo addition on microstructural evolution of Ti-44Al alloy. J. Mater. Sci. Technol. 2018, 34, 1196–1204. [Google Scholar] [CrossRef]
- Dahar, M.S.; Tamirisakandala, S.A.; Lewandowski, J.J. Fatigue crack growth and fracture behavior of as-cast Ti-43.5Al-4Nb-1Mo-0.1B (TNM) compared to Ti-48Al-2Nb-2Cr (4822). Intermetallics 2017, 91, 158–168. [Google Scholar] [CrossRef]
- Bernal, D.; Chamorro, X.; Hurtado, I.; Madariaga, I. Evolution of lamellar microstructures in a cast TNM alloy modified with boron through single-step heat treatments. Intermetallics 2020, 124, 106842. [Google Scholar] [CrossRef]
- Imayev, V.; Gaisin, R.; Rudskoy, A.; Nazarova, T.; Shaimardanov, R.; Imayev, R. Extraordinary superplastic properties of hot worked Ti–45Al–8Nb–0.2C alloy. J. Alloy Compd. 2016, 663, 217–224. [Google Scholar] [CrossRef]
- Huang, Z.W. Thermal stability of Ti–44Al–4Nb–4Zr–0.2Si–1B alloy. Intermetallics 2013, 42, 170–179. [Google Scholar] [CrossRef]
- Dahar, M.S.; Tamirisakandala, S.A.; Lewandowski, J.J. Evolution of fatigue crack growth and fracture behavior in gamma titanium aluminide Ti-43.5Al-4Nb-1Mo-0.1B (TNM) forgings. Int. J. Fatigue 2018, 111, 54–69. [Google Scholar] [CrossRef]
- Singh, V.; Mondal, C.; Sarkar, R.; Bhattacharjee, P.P.; Ghosal, P. Dynamic recrystallization of a β(B2)-Stabilized γ-TiAl based Ti–45Al–8Nb–2Cr-0.2B alloy: The contributions of constituent phases and Zener-Hollomon parameter modulated recrystallization mechanisms. J. Alloys Compd. 2020, 828, 154386. [Google Scholar] [CrossRef]
- Narayana, P.L.; Kim, J.H.; Yun, D.W.; Kim, S.E.; Reddy, N.S.; Yeom, J.T.; Seo, D.; Hong, J.K. High temperature isothermal oxidation behavior of electron beam melted multi-phase γ-TiAl alloy. Intermetallics 2022, 141, 107424. [Google Scholar] [CrossRef]
- Gong, X.; Chen, R.R.; Fang, H.Z.; Ding, H.S.; Guo, J.J.; Su, Y.; Fu, H.Z. Synergistic effect of B and Y on the isothermal oxidation behavior of TiAl-Nb-Cr-V alloy. Corros. Sci. 2017, 131, 376–385. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, R.; Yang, Y.; Guo, J.; Su, Y.; Ding, H.; Fu, H. Effects of V and B, Y additions on the microstructure and creep behaviour of high-Nb TiAl alloys. J. Alloy Compd. 2018, 747, 640–647. [Google Scholar] [CrossRef]
- Shida, Y.; Anada, H. The influence of ternary element addition on the oxidation behaviour of TiAl intermetallic compound in high temperature air. Corros. Sci. 1993, 35, 945–953. [Google Scholar] [CrossRef]
- Yan, K.; Guo, H.B.; Gong, S.K. High-temperature oxidation behavior of β-NiAl with various reactive element dopants in dry and humid atmospheres. Corros. Sci. 2014, 83, 335–342. [Google Scholar] [CrossRef]
- Klein, L.; Shen, Y.; Killian, M.S.; Virtanen, S. Effect of B and Cr on the high temperature oxidation behaviour of novel γ/γ′-strengthened Co-base superalloys. Corros. Sci. 2011, 53, 2713–2720. [Google Scholar] [CrossRef]
- Yao, T.; Liu, Y.; Liu, B.; Song, M.; Zhao, K.; Zhang, W.; He, Y. Influence of carburization on oxidation behavior of High Nb contained TiAl alloy. Surf. Coat. Technol. 2015, 277, 210–215. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Han, W.; Ma, T.; Ma, D.; Zhang, Y. Achieving Superior High-Temperature Strength and Oxidation Resistance of TiAl Nanocomposite through In Situ Semicoherent MAX Phase Precipitation. ACS Appl. Mater. Interfaces 2022, 14, 8394–8403. [Google Scholar] [CrossRef]
- Ma, T.; Li, Q.; Wang, Y.; Wang, X.; Dong, D.; Zhu, D. Microstructure and mechanical properties of micro-nano Ti2AlC-reinforced TiAl composites. Intermetallics 2022, 146, 107563. [Google Scholar] [CrossRef]
- Lim, H.P.; Liew, W.Y.; Melvin, G.J.H.; Jiang, Z.-T. A Short Review on the Phase Structures, Oxidation Kinetics, and Mechanical Properties of Complex Ti-Al Alloys. Materials 2021, 14, 1677. [Google Scholar] [CrossRef]
- Crespo-Villegas, J.; Cavarroc, M.; Knittel, S.; Martinu, L.; Klemberg-Sapieha, J.E. Protective TixSiy coatings for enhanced oxidation resistance of the ɣ-TiAl alloy at 900 °C. Surf. Coat. Technol. 2021, 430, 127963. [Google Scholar] [CrossRef]
- Bauer, P.-P.; Swadźba, R.; Klamann, L.; Laska, N. Aluminum diffusion inhibiting properties of Ti5Si3 at 900 °C and its beneficial properties on Al-rich oxidation protective coatings on γ-TiAl. Corros. Sci. 2022, 201, 110265. [Google Scholar] [CrossRef]
- Zhang, K.; Xin, L.; Ma, T.; Chang, H.; Lu, Y.; Feng, C.; Zhu, S.; Wang, F. Investigation of the role of silicon in TiAlSiN coating deposited on TiAl alloys during long-term oxidation. Corros. Sci. 2022, 204, 110394. [Google Scholar] [CrossRef]
- Zhang, K.; Xin, L.; Lu, Y.; Cheng, Y.; Wang, X.; Zhu, S.; Wang, F. Improving oxidation resistance of γ-TiAl based alloy by depositing TiAlSiN coating: Effects of silicon. Corros. Sci. 2021, 179, 109151. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, W.-Y.; Jiang, M.-Y.; Cao, F.-H.; Wu, H.-X.; Sun, D.-B.; Yu, H.-Y.; Wu, L.-K. Improved oxidation performance of TiAl alloy by a novel Al–Si composite coating. Surf. Coat. Technol. 2020, 381, 125126. [Google Scholar] [CrossRef]
- Bobzin, K.; Brögelmann, T.; Kalscheuer, C.; Liang, T. Al-Si and Al-Si-Y coatings deposited by HS-PVD for the oxidation protection of γ-TiAl. Surf. Coat. Technol. 2018, 350, 587–595. [Google Scholar] [CrossRef]
- Swadźba, R.; Mendala, B.; Bauer, P.-P.; Laska, N.; Schulz, U. Microstructure and cyclic oxidation resistance of Si-aluminide coatings on γ-TiAl at 850 °C. Surf. Coat. Technol. 2020, 403, 126361. [Google Scholar] [CrossRef]
- Wu, Y.; Hagihara, K.; Umakoshi, Y. Improvement of cyclic oxidation resistance of Y-containing TiAl-based alloys with equiaxial gamma microstructures. Intermetallics 2005, 13, 879–884. [Google Scholar] [CrossRef]
- Xiang, L.; Zhao, L.; Wang, Y.; Zhang, L.; Lin, J. Synergistic effect of Y and Nb on the high temperature oxidation resistance of high Nb containing TiAl alloys. Intermetallics 2012, 27, 6–13. [Google Scholar] [CrossRef]
- Loginov, P.A.; Kaplanskii, Y.Y.; Markov, G.M.; Patsera, E.I.; Vorotilo, K.V.; Korotitskiy, A.V.; Shvyndina, N.V.; Levashov, E.A. Structural and mechanical properties of Ti–Al–Nb–Mo–B alloy produced from the SHS powder subjected to high-energy ball milling. Mater. Sci. Eng. A 2021, 814, 141153. [Google Scholar] [CrossRef]
- Lu, W.; Chen, C.L.; He, L.L.; Wang, F.H.; Lin, J.P.; Chen, G.L. (S)TEM study of different stages of Ti–45Al–8Nb–0.2W–0.2B–0.02Y alloy oxidation at 900 °C. Corros. Sci. 2008, 50, 978–988. [Google Scholar] [CrossRef]
- Lin, J.P.; Zhao, L.L.; Li, G.Y.; Zhang, L.Q.; Song, X.P.; Ye, F.; Chen, G.L. Effect of Nb on oxidation behavior of high Nb containing TiAl alloys. Intermetallics 2011, 19, 131–136. [Google Scholar] [CrossRef]
- Maurice, V.; Despert, G.; Zanna, S.; Josso, P.; Bacos, M.-P.; Marcus, P. XPS study of the initial stages of oxidation of α2-Ti3Al and γ-TiAl intermetallic alloys. Acta Mater. 2007, 55, 3315–3325. [Google Scholar] [CrossRef]
- Li, X.T.; Huang, L.J.; Jiang, S.; Gao, Y.N.; An, Q.; Wang, S.; Zhang, R.; Geng, L. Microstructure and super oxidation resistance of the network structured Ti-Al-Si coating. J. Alloy Compd. 2019, 807, 151679. [Google Scholar] [CrossRef]
- Tanaka, M.; Matsudaira, T.; Kawai, E.; Kawashima, N.; Matsumoto, U.; Ogawa, T.; Takeuchi, M.; Kitaoka, S. Effect of chemical composition on mass transfer in Y2Ti2O7 under oxygen potential gradient at high temperatures. J. Ceram. Soc. Jpn. 2021, 129, 22–31. [Google Scholar] [CrossRef]
- D’Isanto, F.; Smeacetto, F.; Martin, H.-P.; Sedlák, R.; Lisnichuk, M.; Chrysanthou, A.; Salvo, M. Development and characterisation of a Y2Ti2O7-based glass-ceramic as a potential oxidation protective coating for titanium suboxide (TiOx). Ceram. Int. 2021, 47, 19774–19783. [Google Scholar] [CrossRef]
- Liu, T.; Wang, L.; Wang, C.; Shen, H. Effect of Al content on the oxidation behavior of Y2Ti2O7-dispersed Fe-14Cr ferritic alloys. Corros. Sci. 2016, 104, 17–25. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Nakayama, T.; Suematsu, H.; Suzuki, T.; Takeda, M.; Niihara, K. Low thermal conductivity Y2Ti2O7 as a candidate material for thermal/environmental barrier coatings. Ceram. Int. 2016, 42, 11314–11323. [Google Scholar] [CrossRef]
- Tanaka, M.; Kitaoka, S.; Yoshida, M.; Sakurada, O.; Hasegawa, M.; Nishioka, K.; Kagawa, Y. Structural stabilization of EBC with thermal energy reflection at high temperatures. J. Eur. Ceram. Soc. 2017, 37, 4155–4161. [Google Scholar] [CrossRef]
- Su, Y.J.; Trice, R.W.; Faber, K.T.; Wang, H.; Porter, W.D. Thermal Conductivity, Phase Stability, and Oxidation Resistance of Y3Al5O12 (YAG)/Y2O3–ZrO2 (YSZ) Thermal-Barrier Coatings. Oxid. Met. 2004, 61, 253–271. [Google Scholar] [CrossRef]
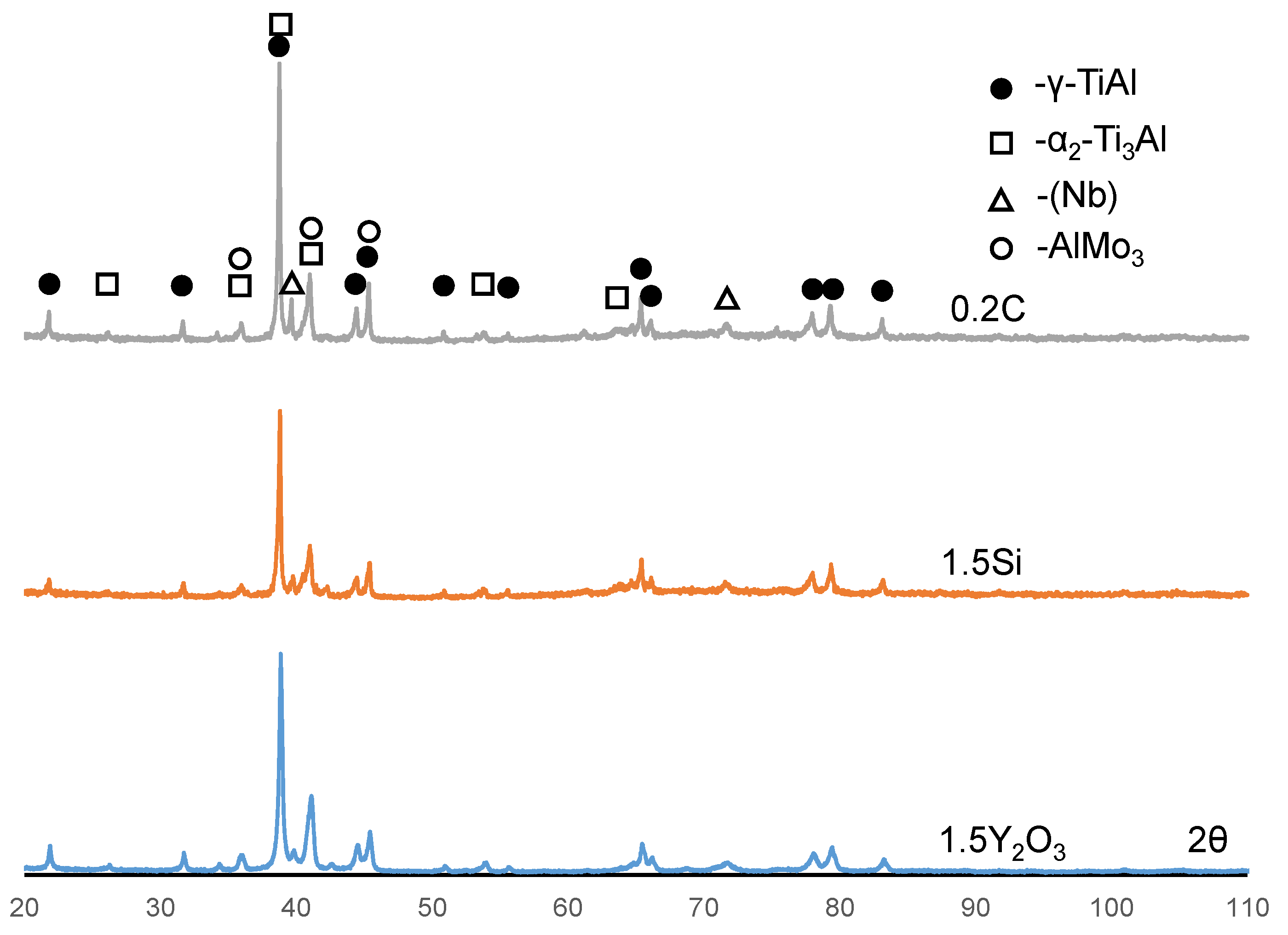
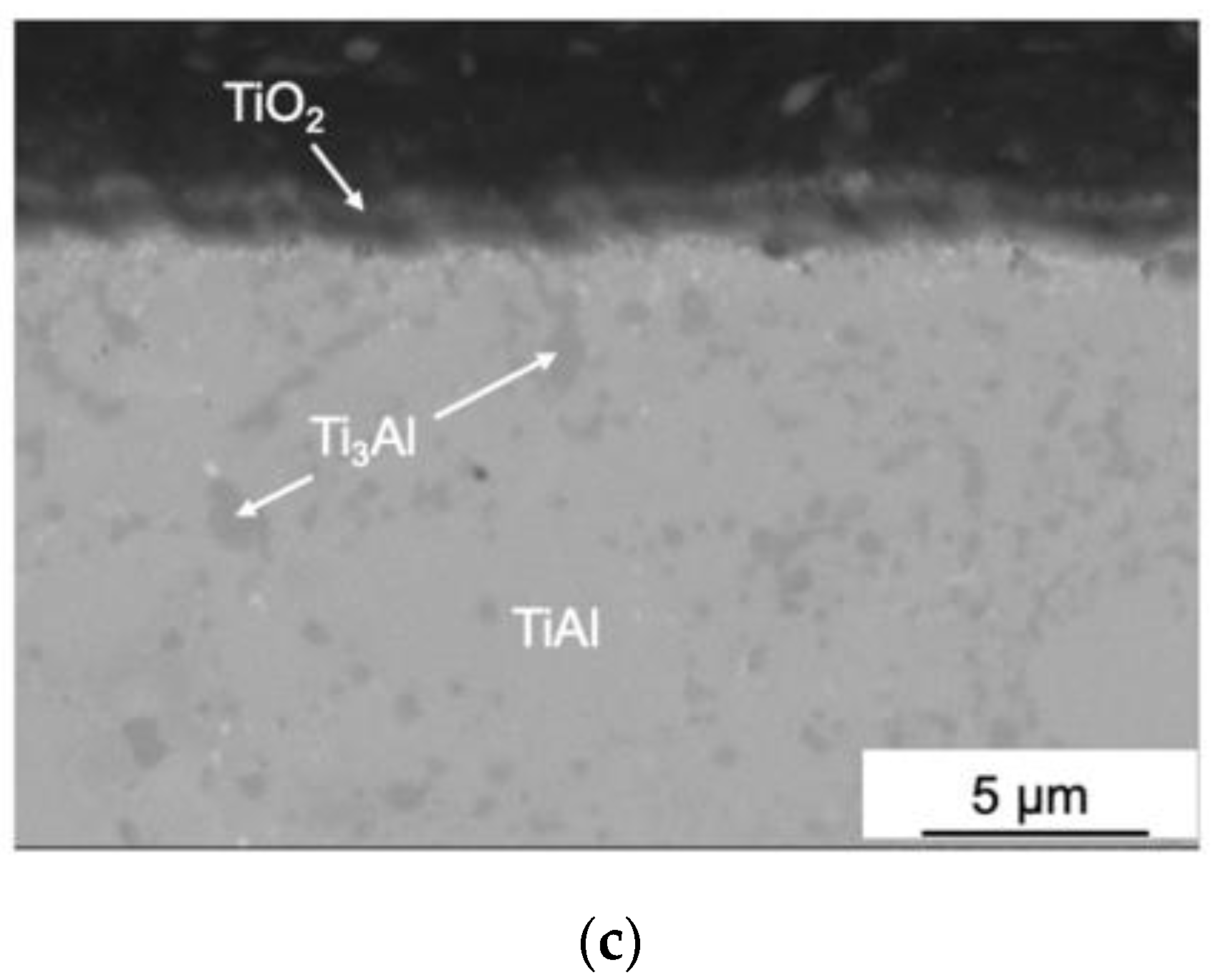

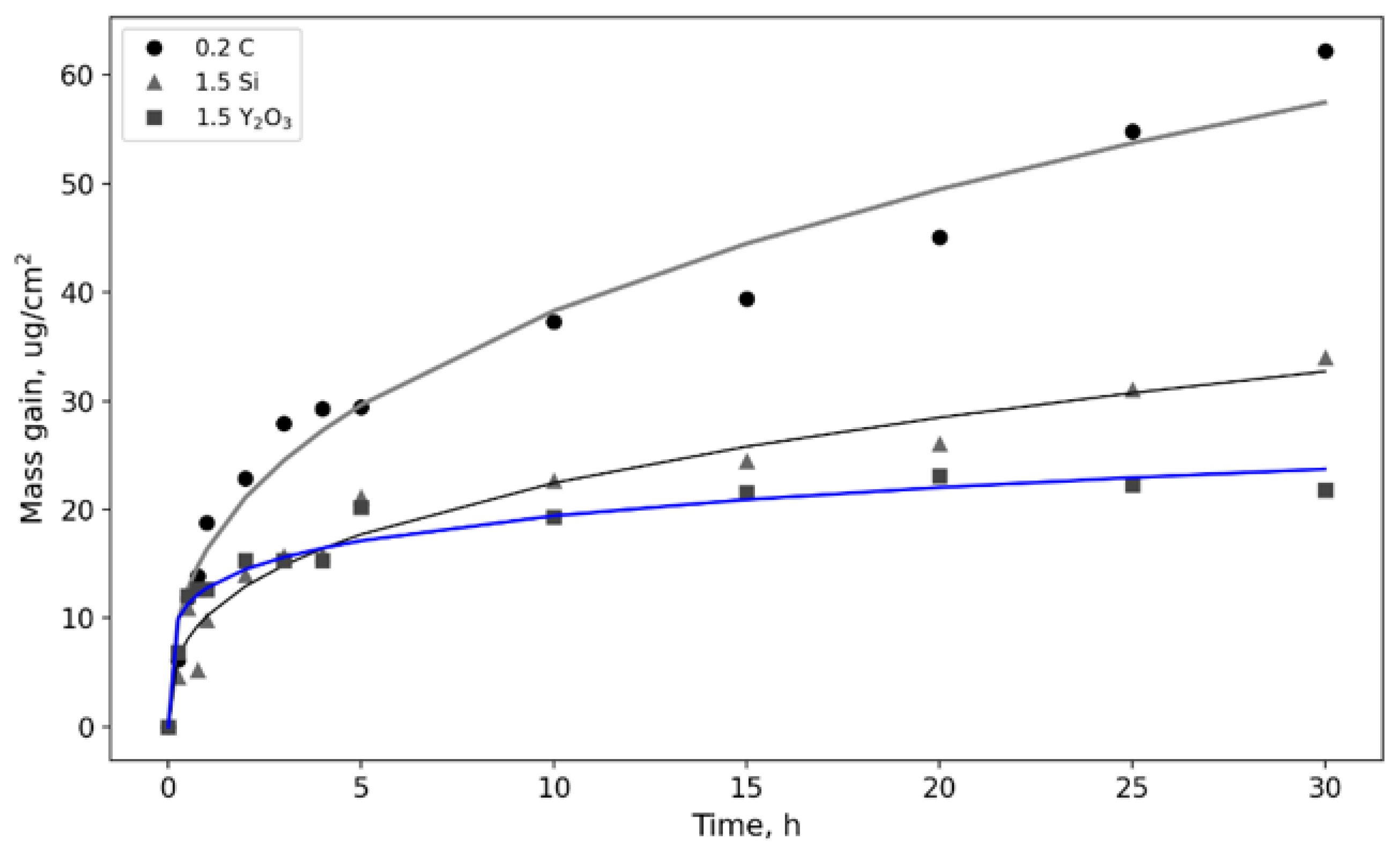

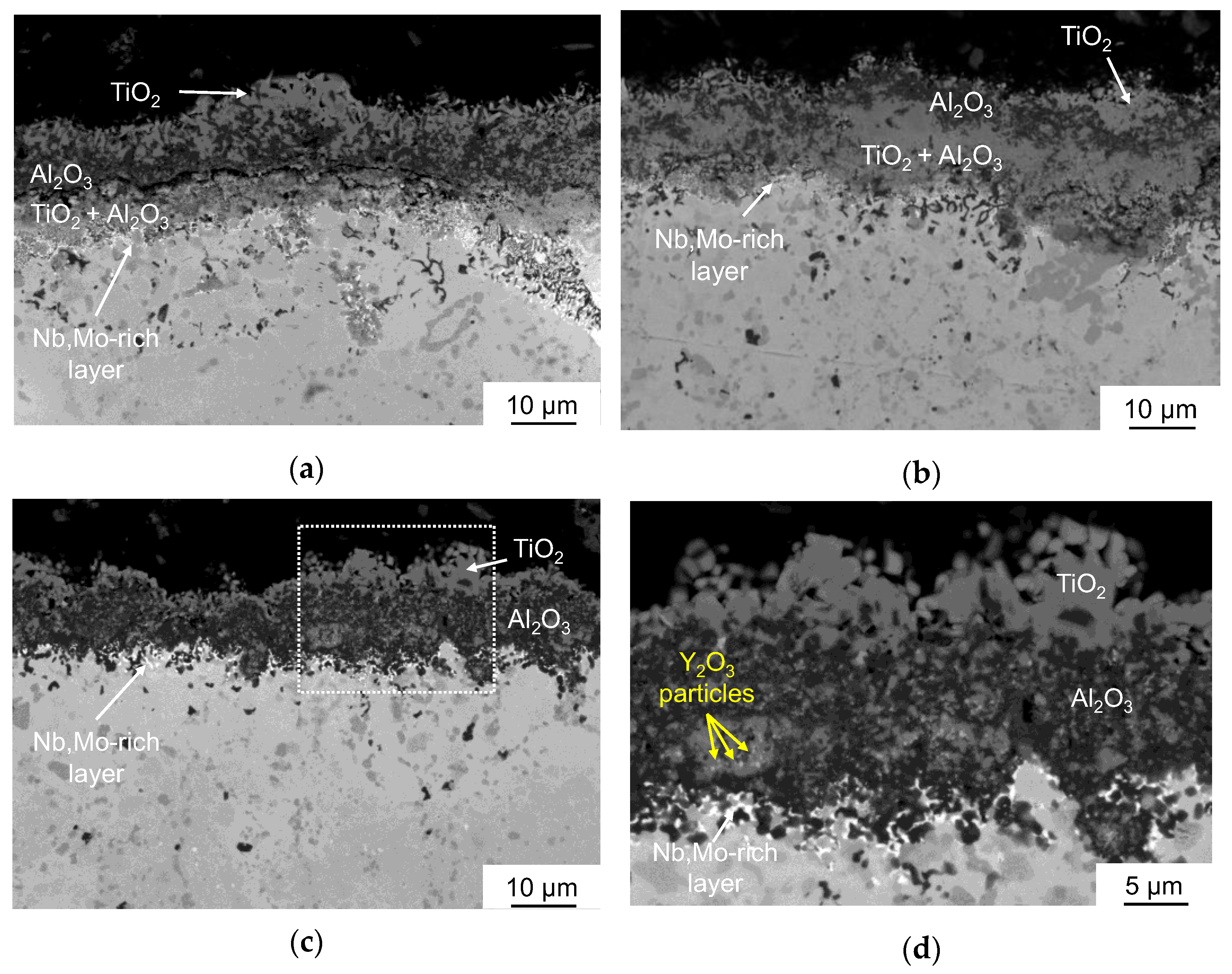

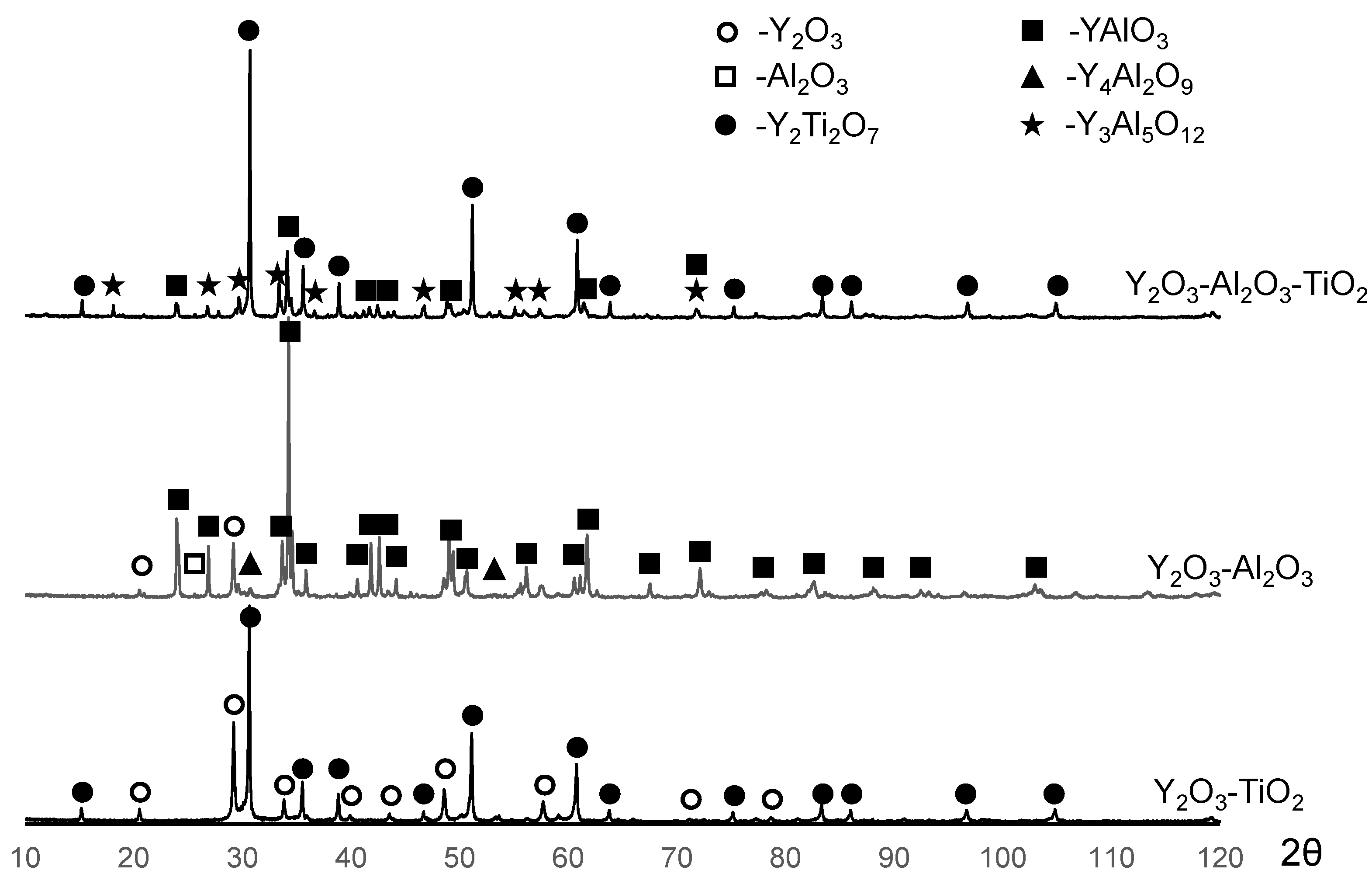
| Sample | Phase Concentration, wt. % | |||
|---|---|---|---|---|
| γ-TiAl P4/mmm | α2-Ti3Al P63/mmc | (Nb) Im-3m | AlMo3 Pm-3n | |
| 0.2C | 78 | 17 | 3 | 2 |
| 1.5Si | 80 | 15 | 3 | 2 |
| 1.5Y2O3 | 80 | 17 | 2 | 1 |
| Alloy | ΔM, µg/cm2 | k | n | h, µm |
|---|---|---|---|---|
| 0.2C | 3.9 | 1368 | 5498 | 2.06 |
| 1.5Si | 4.1 | 87.5 | 5580 | 2.28 |
| 1.5Y2O3 | 3.5 | 1446 | 5461 | 1.89 |
| Sample | Phase Concentration, wt. % | |||||
|---|---|---|---|---|---|---|
| γ-TiAl P4/mmm | α2-Ti3Al P63/mmc | (Nb) Im-3m | AlMo3 Pm-3n | TiO2 P42/mnm | Al2O3 P63mc | |
| 0.2C | 74 | 8 | 3 | 2 | 9 | 4 |
| 1.5Si | 76 | 8 | 3 | 2 | 7 | 4 |
| 1.5Y2O3 | 77 | 6 | 3 | 1 | 10 | 3 |
| Alloy | ΔM, ug/cm2 | k | n | h, µm |
|---|---|---|---|---|
| 0.2C | 61 | 1911 | 2704 | 2086 |
| 1.5Si | 37 | 8862 | 2921 | 1744 |
| 1.5Y2O3 | 17 | 5641 | 1342 |
| Sample | Phase Concentration, wt. % | |||||
|---|---|---|---|---|---|---|
| TiO2 P42/mnm | (Ti,Nb)O2 P42/mnm | Al2O3 P63mc | (Nb) Im-3m | α2-Ti3Al P63/mmc | NbO Pm-3n | |
| 0.2C | 25 | 17 | 52 | 2 | 6 | 2 |
| 1.5Si | 27 | 19 | 42 | 3 | 7 | 2 |
| 1.5Y2O3 | 38 | - | 51 | 1 | 10 | - |
| Sample | Phase Concentration, wt. % | |||||
|---|---|---|---|---|---|---|
| Y2O3 Ia-3 | Al2O3 R-3c | Y2Ti2O7 Fd-3m | YAlO3 Pbnm | Y4Al2O9 P21/c | Y3Al5O12 Ia-3d | |
| Y2O3–TiO2 | 25 | - | 75 | - | - | - |
| Y2O3–Al2O3 | 6 | 5 | - | 80 | 11 | - |
| Y2O3–Al2O3–TiO2 | - | - | 70 | 10 | - | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loginov, P.A.; Markov, G.M.; Shvyndina, N.V.; Smirnov, G.V.; Levashov, E.A. Oxidation Resistance of γ-TiAl Based Alloys Modified by C, Si and Y2O3 Microdopants. Ceramics 2022, 5, 389-403. https://doi.org/10.3390/ceramics5030030
Loginov PA, Markov GM, Shvyndina NV, Smirnov GV, Levashov EA. Oxidation Resistance of γ-TiAl Based Alloys Modified by C, Si and Y2O3 Microdopants. Ceramics. 2022; 5(3):389-403. https://doi.org/10.3390/ceramics5030030
Chicago/Turabian StyleLoginov, Pavel A., Georgy M. Markov, Nataliya V. Shvyndina, Gleb V. Smirnov, and Evgeny A. Levashov. 2022. "Oxidation Resistance of γ-TiAl Based Alloys Modified by C, Si and Y2O3 Microdopants" Ceramics 5, no. 3: 389-403. https://doi.org/10.3390/ceramics5030030
APA StyleLoginov, P. A., Markov, G. M., Shvyndina, N. V., Smirnov, G. V., & Levashov, E. A. (2022). Oxidation Resistance of γ-TiAl Based Alloys Modified by C, Si and Y2O3 Microdopants. Ceramics, 5(3), 389-403. https://doi.org/10.3390/ceramics5030030