Abstract
In this paper, partial transient liquid phase (PTLP) diffusion bonding between Si3N4 ceramics and Ht250 cast iron was carried out by using an Ti/Cu/Kovar/Cu/Ti interlayer. The effects of the heating temperature and holding time on the microstructure, formation mechanism, and mechanical properties of Si3N4/Ht250 cast iron joints were studied. The results show that the maximum shear strength of the joint is 112 MPa when the welding temperature is 1000 °C and the holding time is 1 h. In addition, the problems of Ti/Cu/Ti intermetallic compound formation and Cu/Si3N4 ceramic residual thermal stress in the joint can be effectively alleviated.
1. Introduction
Gray cast iron is characterized by the presence of flake graphite in the structure. It has good casting and cutting performance, and low casting cost. Therefore, it is the most widely used cast iron in the world []. It is widely used for hydraulic parts, cylinder blocks, pump bodies, compressors, and other pressure- and leakage-resistant parts [,,]. Si3N4 ceramics have many excellent properties, such as high hardness, high strength, low density, low coefficient of thermal expansion, high temperature resistance, and so on [,,,]. They are suitable for the heat insulation parts of diesel engines, but the high brittleness limits the possibility of processing them into an integral component. Through the bonding of Si3N4 ceramics/cast iron, problems with the quality of gray cast iron casting and difficult processing of Si3N4 ceramics due to poor plasticity can be solved [].
At present, AgCuTi active metal brazing is still the main method for joining ceramics and metals [], but the problem of stress concentration at the weld caused by this method is difficult to solve, and it is difficult to maintain high service performance under a high-temperature and high-wear environment for a long time. PTLP diffusion bonding combines the advantages of brazing bonding and diffusion bonding, and has broad application prospects in the field of ceramic matrix composites [,,]. Zou et al. [] successfully carried out secondary PTLP bonding of Si3N4 ceramics using a Ti/Cu/Ni interlayer. Zheng et al. [] used a Ti/Ni multilayer interlayer to conduct partial transient liquid phase connection of silicon nitride ceramics under the vacuum conditions of 1273–1423 k.
This paper aima at the problems of over-sized Ti/Cu intermetallics being formed when Ti/Cu/Ti is used as the intermediate. The residual thermal stress produced by Cu and Si3N4 ceramics in the joint is too large. A Kovar alloy with a thermal expansion coefficient that is more suitable for Si3N4 ceramics was selected as the interlayer to alleviate the aggregation of Ti–Cu intermetallics near the reaction layer of the Si3N4 interface, thus greatly reducing the stress near the ceramic interface. The bonding mechanism under different welding temperature and holding time is analyzed in detail. In addition, the fracture mechanism of the Si3N4/Ti/Cu/Kovar/Cu/Ti/Ht250 joint is studied.
2. Materials and Methods
The Si3N4 ceramic cube with a side length of 7 mm was provided by Shanghai Silicate Research Institute (Shanghai, China). The Ht250 cast iron with a side length of 7 mm was provided by the Engine Research Institute (Tianjin, China); the SEM micro-morphology distribution of graphite on its surface is shown in Figure 1, and the main chemical composition is shown in Table 1. The interlayer materials used include 10-μm-thick Ti foil, 20-μm-thick Cu foil, and a 250-μm-thick Kovar alloy sheet (brand 4J29) whose main chemical composition is as shown in Table 2. Before joining, the surface of Si3N4 to be connected was smoothed with W3.5 grinding paste, and the surfaces of the Ht250 cast iron, 250-μm-thick Kovar alloy sheet, 20-μm-thick Cu foil, and 10-μm-thick Ti foil were polished with metallographic sandpaper to remove the surface oxide film and impurities, followed by acetone ultrasonic cleaning for 10 min.
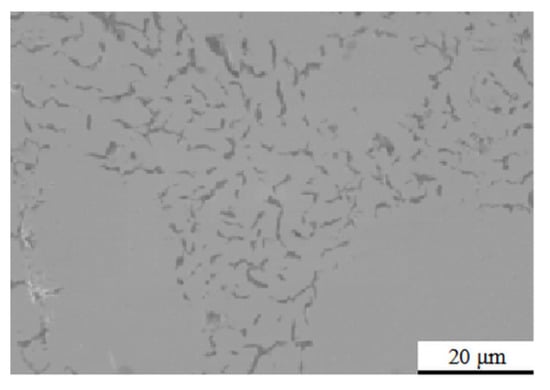
Figure 1.
Micromorphology distribution of graphite on the surface of Ht250 cast iron.

Table 1.
Main components of Ht250 cast iron.

Table 2.
Main components of 4J29 fungible alloy.
Before joining, the Si3N4 ceramics, interlayer materials, and Ht250 were assembled sequentially, as shown in Figure 2a. The assembled samples were then put into a ZM (Y) series vacuum hot pressing furnace (Figure 2b) (Shanghai Chenghua Electric Furnace Co., Ltd., Shanghai, China). The samples were heated to 940–1060 °C in a vacuum at 5 × 10−3 Pa for 1–3 h. The process temperature curve is shown in Figure 2c. The micromorphology of the interface was observed and analyzed by a FEIQuanta250F (Nanjing, China) scanning electron microscope (SEM) equipped with an energy-dispersive spectrometer (EDS) to analyze the element distribution. The hardness of the interlayer was measured by a Vickers hardness tester. The phase analysis at the interface of the joint was carried out by using the Raman spectrometer of model DXR2. The mechanical properties of the joint were tested by an electronic universal mechanical testing machine (Figure 2d) at a moving speed of 0.2 mm/min, and the shear strength of each parameter was the average of 3–4 samples.
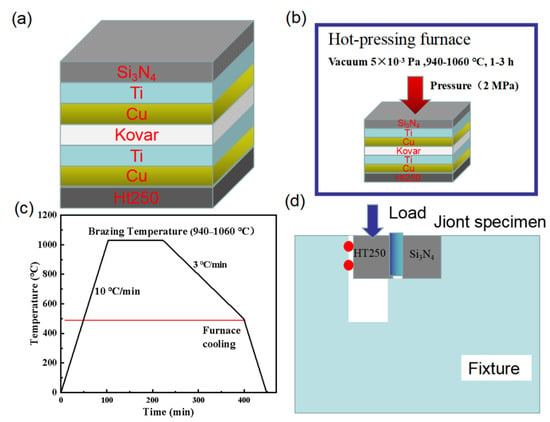
Figure 2.
Schematic diagram of (a) workpiece assembly, (b) experimental process, (c) thermal cycle curve, and (d) shear test fixture.
3. Results and Discussion
3.1. Microstructure of the Si3N4/Ti/Cu/Kovar/Cu/Ti/Ht250 Joints
Figure 3 shows SEM images of the cross sections of the Si3N4/Ht250 joint joined by the Ti/Cu/Kovar/Cu/Ti interlayer; the interface was free of defects such as holes and cracks. There was a certain width of diffusion reaction zone between Si3N4 ceramics, Ht250, and the Kovar alloy, which allows for diffusion bonding. As shown in Figure 3c, there was a dense reaction layer of about 7 μm at the interface of Si3N4 ceramics, and the right side of the dense reaction layer was about 10 μm wide for reactants with dispersion distribution; the distribution changes from dense to dispersed. A light colored empty band of about 15 μm existed to the right of the dispersion reactants, which should be a Cu-rich zone. As seen in Figure 3d, there was a diffuse reaction zone of about 25 μm on the Ht250 side, where there was a dense reaction layer of about 2 μm at the Ht250 interface, and a light colored empty band of about 20 μm to the left of the dense reaction layer, which was also a Cu-rich zone.
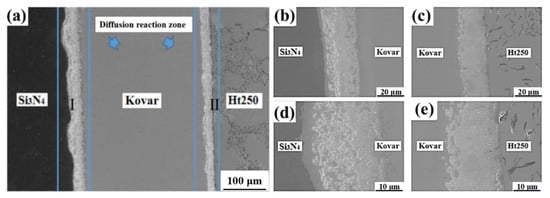
Figure 3.
The morphology of the Si3N4/Ti/Cu/Kovar/Ti/Cu/Ht250 integral joint maintained at 1000 °C for 1 h: (a,b,d) microscopic magnification diagrams of diffusion reaction zone I; (c,e) microscopic magnification diagram of diffusion reaction zone II.
As shown in Figure 4, the C element in Ht250 cast iron existed not only in the form of Fe3C, but also in the form of flake graphite, which covered the whole surface of Ht250—one of the main reasons for its poor weldability. The purpose of this experimental design was to use Ti/Cu/Kovar/Cu/Ti as the intermediate layer in the hope that the active element Ti would react with the graphite on the surface of Ht250 to form a TiC phase to increase its wettability and cooperate with part of the transient liquid phase formed by Cu–Ti to form a compact and high-performing joint. Therefore, EDS line scanning of graphite near the Ht250/Ti/Cu interface was carried out, and it was found that, when the line scanning path runs through the graphite, the cps curve (the diffusion curve of the element, which is defined as the cps curve below) of the C element shows a peak shape, while the cps curve of the Ti element shows a less obvious peak at the edge of the graphite, indicating that the Ti element may have gathered at the edge of the graphite. In order to further confirm the distribution of Ti around graphite, the line scanning path was converted to be tangential to graphite and scanned again.
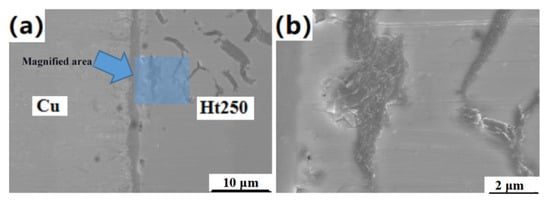
Figure 4.
Enlarged view of graphite near the Ht250/Ti/Cu interface: (a) Schematic diagram of the location of the area to be swept; (b) enlarged view of the area to be swept.
As shown in Figure 5b, the cps curve of C element showed a peak at the tangent point, while the cps value of the Ti element also showed a peak value, which is highly consistent with the trend of the C element, and proves that there was obvious aggregation of the Ti element here, combined with the results of two line scans. It can be inferred that Ti reacted with graphite in Ht250 cast iron to form a TiC phase. Similarly, graphite on the surface of PTLP diffusion bonding of Ht250 cast iron more easily made contact with the Ti element, and the reaction between them was stronger, which greatly increased the wettability of Ti–Cu liquid to the surface of Ht250 cast iron. The Raman spectrum scanning test was carried out at the interface of the joint, and the existence of TiC phase was detected near 800 cm−1 (Figure 5c), which proved that the active element Ti could reacte with graphite in Ht250 to form a TiC phase, which was consistent with the previous analysis.
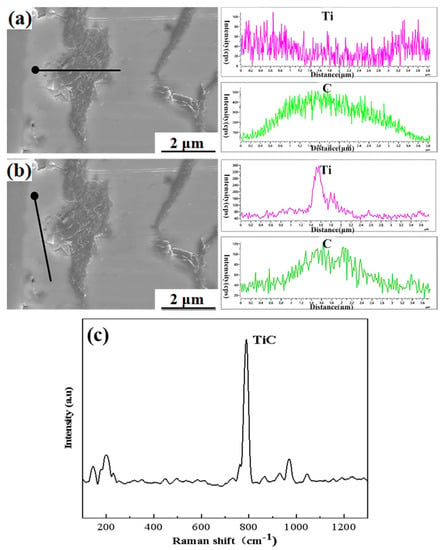
Figure 5.
Graphite line scan path and analysis near the Ht250/Ti/Cu joint: (a) Line scan path through graphite; (b) line scan path tangential to graphite; (c) the TiC Raman spectrum analysis.
3.2. The Influence of Process Parameters on the PTLP Diffusion Joints at the Si3N4/Ti/Cu/Kovar/Cu/Ti/Ht250 Interface
3.2.1. The Effect of Welding Temperature on PTLP Diffusion Bonding
When the welding pressure was 2 MPa and the holding time was 1 h, the Si3N4/Ti/Cu/Kovar/Cu/Ti/Ht250 was welded at 940 °C, 970 °C, 1000 °C, 1030 °C and 1060 °C. Because the whole joint was asymmetrical, the joints on both sides were analyzed separately.
It can be seen from Figure 6 that, in the temperature range of 940–1060 °C, tightly connected joints were obtained, but the thickness and distribution of the interface reaction layer were significantly different. At 940 °C, there were some tiny pores at the interface because the activation energy of interatomic diffusion is smaller at a lower temperature, which also made the diffusion of Ti atoms in the Cu–Ti liquid phase into the Kovar alloy lower. As a result, a large number of Ti–Fe and Ti–Ni compounds were segregated at the interface of the Kovar alloy, resulting in defects such as holes. When the temperature increased to 970 °C, the pores on the interface decreased. When the temperature further increased to 1000 °C, an obvious reaction layer formed at the Si3N4 ceramic interface, the distribution width of the dispersed reactants became wider, the joint transition was more natural, and the interface had no defects such as holes. When the welding temperature reached 1060 °C, the Si3N4 ceramics were closely combined with the Ti/Cu/Kovar intermediate layer. At this time, the dense reaction layer at the Si3N4 ceramic interface was integrated with the surrounding dispersed reactants, and the transition was natural. However, at 1060 °C, due to the high temperature, the Ti and Si atoms fully reacted, and finally a variety of Ti–Cu and Ti–Si intermetallic compounds were formed and grown, which was detrimental to the mechanical properties of the joint. Therefore, we hypothesize that the Si3N4/Ti/Cu/Kovar joints at different welding temperatures are all closely connected, but with an increase in temperature, the longer the Ti–Cu eutectic liquid phase lasts, the more interatomic diffusion there will be, and the transition of the reaction layer will be more natural. For further verification, EDS line scanning and point scanning analysis were performed on the interface under different welding temperatures.
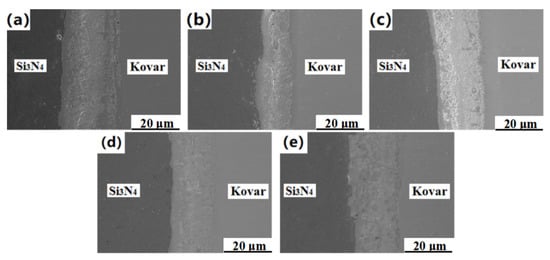
Figure 6.
The microstructure of Si3N4/Ti/Cu/Kovar joints at (a) 940 °C, (b) 970 °C, (c) 1000 °C, (d) 1030 °C and (e) 1060 °C.
At three different welding temperatures, the N, Si, Ti, and Cu elements were obviously diffused in the diffusion reaction layer, and no fault was seen. It can be seen from Figure 7b that, at a welding temperature of 1000 °C, the width of the Si3N4 ceramic side diffusion reaction layer was about 13 μm, and that of the Kovar alloy side diffusion reaction layer was about 14 μm. There was sufficient time to diffuse to both sides, so the final diffusion reaction layer was more concentrated, and the new phase was more stable; the interdiffusion of Ti and Fe elements was more obvious on the Kovar side. In order to further study the effect of different soldering temperatures on the tight connection of the Si3N4/Ti/Cu/Kovar interface, a point analysis was carried out on some of the joints. The EDS results of chemical compositions and possible phases for these spots are shown in Table 3.
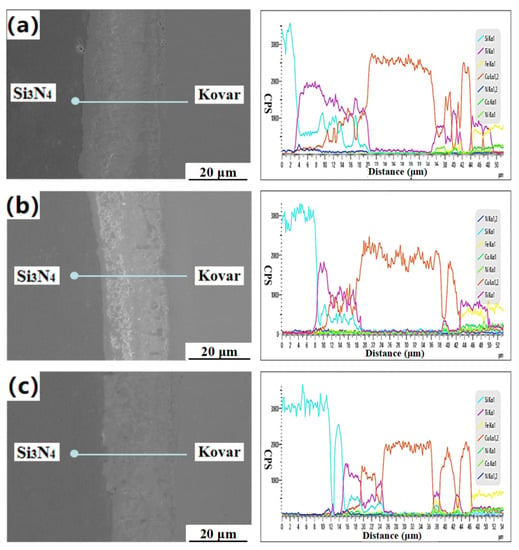
Figure 7.
Line scanning spectrogram of Si3N4/Ti/Cu/Kovar interface at (a) 940 °C, (b) 1000 °C and (c) 1060 °C.

Table 3.
Component content of each point in Figure 7 (at.%).
It can be seen from Table 3 that the Ti content at points A and E was higher than at point C. At point A, because the Ti element was not fully diffused, the liquid phase region had begun to solidify, resulting in a large number of Ti atoms remaining in the Kovar alloy. Near the interface, point E was because a large amount of Fe, Ni, and other elements in the Kovar alloy diffused to this point and reacted with the Ti element to form a Ti-containing compound. Points B, D, and F were all located inside the Kovar alloy. With the increase in temperature, the atomic diffusion ability increased, and the content of Ti and Cu elements in the area gradually increased; the Fe, Ni, and Co elements diffused to the Kovar alloy interface and liquid zone, and the content gradually decreased.
As shown in Figure 8, at a temperature range of 940–1060 °C, tight joints were obtained, but the thickness and morphology of the diffusion reaction layer were significantly different. It can be seen from Figure 8a that, at 940 °C, there was an obvious interface reaction layer at the Ht250 side interface, of about 3–4 μm, but only a few point-like reactants near the Kovar alloy interface; there were very few point-like reactants near the interface of the Kovar alloy, and obvious delamination at the joint, accompanied by cracks and hole defects. Defects such as joint delamination and voids were significantly improved when joined at higher temperatures. The dispersed reactants began to appear near the Ht250 interface reaction layer and gradually increased, extending to the Kovar alloy side; the reactants near the Kovar alloy interface also increased, and the transition to the two sides became more natural. Therefore, we deduce that the diffusion interface of Kovar/Cu/Ti/Ht250 at is more tightly welded a higher temperature and the diffusion between atoms is more sufficient. In order to verify this hypothesis, the bonding interface at different welding temperatures was analyzed by EDS line scanning and point scanning, as shown in Figure 9.
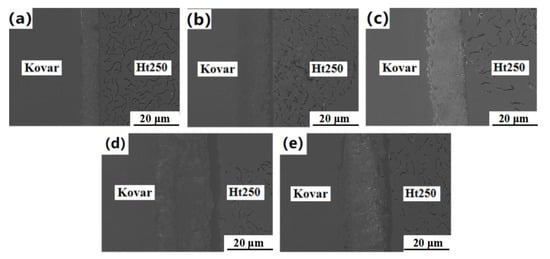
Figure 8.
The microstructure of Kovar/Cu/Ti/Ht250 joints at (a) 940 °C, (b) 970 °C, (c) 1000 °C, (d) 1030 °C and (e) 1060 °C.
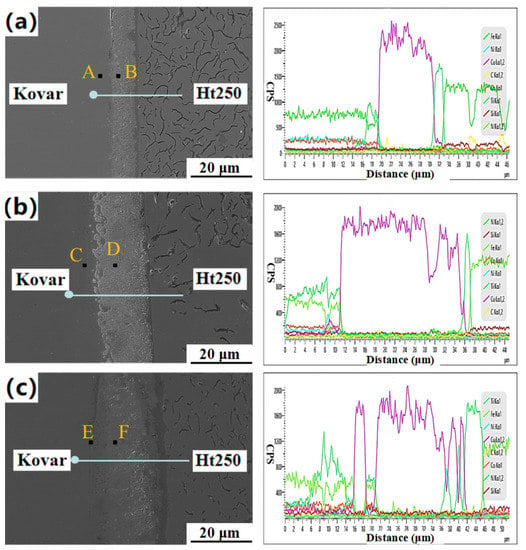
Figure 9.
Microstructure and line scan analysis spectrum of the Kovar/Cu/Ti/Ht250 interface at different welding temperatures: (a) 940 °C; (b) 1000 °C; (c) 1060 °C.
At three different temperatures, the cps value of each element continuously changed at the interface, and there was no gap or fracture, indicating that Ht250 was closely combined with the Ti/Cu/Kovar/Ti/Cu interlayer. With the increase in temperature, the diffusion of Ti element to the Kovar alloy side became more and more obvious. The diffusion distance of Ti element to the Kovar alloy was 4 μm at 940 °C, about 10 μm at 1000 °C, and about 19 μm at 1060 °C. Figure 9c shows that the Fe, Ni, and Co elements in the Kovar alloy diffused from the interface of 16 μm to 20 μm, and formed a reaction layer with Ti, which did not occur at other temperatures. On the Ht250 side, with the increase in temperature, the interdiffusion of Ti and Cu was more obvious, and it was easier to form Ti–Cu compounds at the joint. These reactants corresponded to the diffused reactants near the Ht250 interface reaction layer in the figure.
A point analysis of some characteristic points of the joint at different temperatures was carried out. According to Table 4, the content of Ti and Cu elements at point A was only 7.36 at.% and 0.15 at.%, respectively. It can be seen that the ability of Ti and Cu to diffuse into the Kovar alloy was weaker at a lower temperature. As can be seen from Figure 9a, the amount of Ti-containing reactants at point A was relatively small, and the Ti content at points C and E was 24.85 at.% and 25.19 at.%, respectively. Points B, D, and F were located on the right side of the Kovar alloy interface. Point B also contained a large amount of Cu (50.21 at.%), while the Cu content at the other two points was very low.

Table 4.
Component content of each point in Figure 9 (at %).
3.2.2. Effect of Holding Time on PTLP Diffusion Bonding
In the 1–3 h holding time interval, tightly connected joints were obtained, but the thickness and distribution of the interface reaction layer were significantly different. As seen in Figure 10a, there was a 3–4-μm-thick reaction layer at the interface of Si3N4 ceramics, and dispersed reactants on the right side of the reaction layer. Dispersed reactants formed at the interface of the Kovar alloy. Notably, the width of the Si3N4 ceramic interface reaction layer and the nearby dispersed reactants both became thicker, the transition was more natural, and the reaction layer at the Kovar alloy interface became more obvious (Figure 10b). When the holding time reached 3 h, it can be seen from Figure 10c that the dispersed reactants on the Si3N4 ceramic side continued to extend to the Kovar side, almost connecting with the Kovar alloy interface reaction layer. Therefore, we suggest that the joints of Si3N4 ceramics/Ti/Cu/Kovar under different holding times are all tightly welded, but with the increase in holding time, the distribution of dispersed reactants in each reaction layer and the vicinity of the reaction layer changes. For further verification, EDS line scan and point scan analysis were performed on the connection interface under different incubation times.
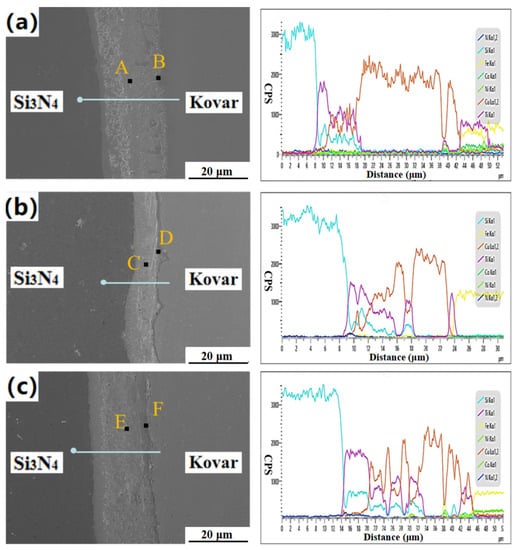
Figure 10.
Microstructure and line scan analysis of Si3N4/Ti/Cu/Kovar joint under different holding times: 1 h (a), 2 h (b), or 3 h (c).
The cps value of each element changed continuously at the interface, and there was no fault phenomenon, but the distribution of each element was very different. It can be seen from Figure 10b that, with the increase in the holding time, the range of the Cu elemental region in the joint was greatly reduced, and more Ti and Si elements diffused from the side of the Si3N4 ceramic, which corresponds to the one in Figure 10c. The dispersed reactants extended to the Kovar alloy side. At the same time, with the increase in holding time, the diffusion level of Ti into the Kovar alloy decreased, and Ti accumulated on the surface of the Kovar alloy; the corresponding area was the Kovar interface reaction layer.
The EDS results of chemical compositions and possible phase for these spots are shown in Table 5. The Cu element content at point A, located in the central area of the joint, was as high as 96.41 at.%, while the Cu content at points C and E was only 54.08 at.% and 22.97 at.%, respectively, and the content of Ti and Si increased significantly, which indicates that, with the increase in holding time, the diffusion distance of Ti, Si, and other elements at the interface of Si3N4 ceramics increased. In addition, point B was mainly composed of Ti, Cu, and Ni, and the content of Ni was 37.69 at.%. At this time, the diffusion ability of Ni was very strong. When the holding time was 2 h, the Kovar interface reaction layer was most obvious. The element composition at point D was Si 4.32 at.%, Ti 63.94 at.%, Cu 2.78 at.%, Fe 28.9 at.%, and Ni 0.06 at.%, indicating that the reaction layer was mainly TiFe and α-Ti phase, which hindered the diffusion of Ni and Co alloy elements to the Si3N4 side.

Table 5.
Component content of each point in Figure 10 (at.%).
As shown in Figure 11, the Ht250 and Ti/Cu/Kovar/Ti/Cu intermediate layers were closely connected within the holding time interval of 1–3 h, without holes, cracks, or other defects, but the morphology and transition of the interface reaction layer were significantly different. When the holding time was 1 h, there was obvious delamination at the Kovar interface, and with the increase in the holding time, the delamination improved until it was completely homogenized. When the holding time was 3 h, the thickness of the reaction layer at the Ht250 interface increased, and the dispersed reactants near the reaction layer turned into thin strips, extending all the way to the Kovar alloy side, with light-colored areas between the strips. The transition of the Kovar/Cu/Ti/Ht250 joint under a longer holding time was smoother and more natural. For further verification, EDS line scan and point scan analysis were performed on the joint connection interface under different incubation times. Similar to the Si3N4-side joint, with the increase in the holding time, the Cu elemental region in the Ht250 and Kovar joints decreased greatly, and the width of the Ti-rich reaction zone at the Ht250 interface increased. It can be seen from Figure 11c that, when the holding time was 3 h, the Fe element in the Kovar alloy diffused to the Ht250 side along the Cu–Ti liquid phase region, and the diffusion distance was about 10 μm. Figure 12 shows the concentration curves of Ti and C in Figure 11c. The peaks of Ti and C elements correspond to the band-shaped reaction layer near the Ht250 interface in Figure 11c. We speculate that the main phase is TiC. At this point, various brittle phases filled the entire joint structure.
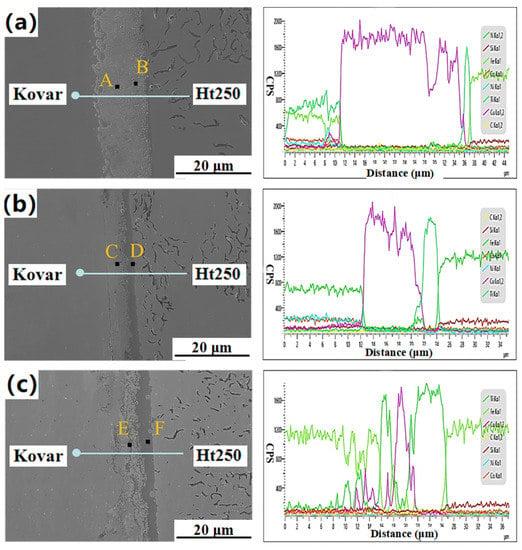
Figure 11.
Microstructure and line scan analysis of Kovar/Cu/Ti/Ht250 joint under different holding times: 1 h (a), 2 h (b), or 3 h (c).
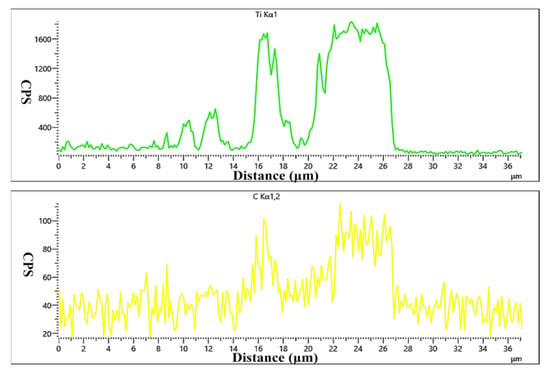
Figure 12.
Concentration curve of Ti and C.
It can be seen from Table 6 that the Cu content at points A and C was very high—72.5 at.% and 60.78 at.%, respectively—but the Cu element content at point E, which was also located in the middle area of the Kovar alloy and Ht250, was only 1.74 at.%. The contents of C and Ti were as high as 42.03 at.% and 52.72 at.%, respectively, which correspond to the thin ribbon-shaped reaction zone in Figure 11c, which was mainly composed of TiC. The element compositions and contents at points B, D, and F of the Ht250 interface reaction layer were similar, indicating that the increase in holding time only changed the thickness of the Ht250 interface reaction layer, but not its composition.

Table 6.
Component content of each point in Figure 11 (at.%).
3.3. The Influence of Process Parameters on the Mechanical Properties of Si3N4/Ti/Cu/Kovar/Cu/Ti/Ht250 Joints
3.3.1. The Effect of Welding Temperature on Mechanical Properties of Joints
Under a pressure of 2 MPa and holding time of 1 h, the influence on the strength of Si3N4/Ti/Cu/Kovar/Cu/Ti/Ht250 joints at different temperatures was studied, and a comparative test of Si3N4/Ti/Cu/Ti/Ht250 was designed. Shear strength and microhardness tests for the intermediate layer of the joint were carried out, and the results are shown in Figure 13.
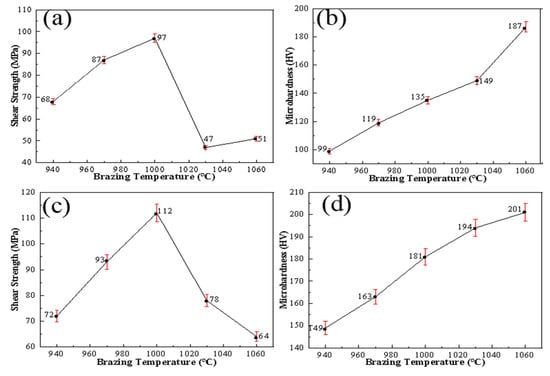
Figure 13.
Mechanical properties and microhardness of joints under different welding temperatures: (a,b) Ti/Cu/Ti shear strength and interlayer microhardness; (c,d) Ti/Cu/Kovar/Cu/Ti shear strength and interlayer microhardness.
As shown in Figure 13c, with the increase in welding temperature, the change trend of the shear strength of the joint was to increase first and then decrease, and it was 10–15% higher than that of Ti/Cu/Ti as the intermediate layer under the same process conditions, up to 112 MPa. The microhardness of the Kovar alloy was about 155 HV. It can be seen from Figure 13d that, as the temperature increased, the hardness of the Ti/Cu/Kovar/Cu/Ti interlayer in the joint changed without the Ti/Cu/Ti interlayer. The maximum hardness was 201 HV at 1060 °C, and the hardness increased by about 25%, which was less than the increase rate of the hardness of the Ti/Cu/Ti intermediate layer. This shows that, when the Kovar alloy is used as a buffer layer, the brittleness of intermetallic compounds has little effect on plasticity during welding, and can effectively relieve welding residual stress in ceramics and their reaction layers.
3.3.2. The Effect of Holding Time on the Mechanical Properties of Joints
To explore the effect of different holding times on the strength of Si3N4/Ti/Cu/Kovar/Cu/Ti/Ht250 joints under a pressure of 2 MPa and temperature of 1000 °C, a comparative test of Si3N4/Ti/Cu/Ti/Ht250 was designed. Shear strength and microhardness tests were carried out for the intermediate layer of the joint, and the results are shown in Figure 14.
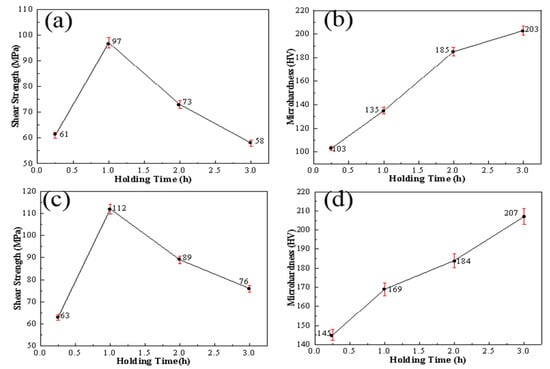
Figure 14.
Mechanical properties and microhardness of joints under different holding times: (a,b) Ti/Cu/Ti shear strength and interlayer microhardness; (c,d) Ti/Cu/Kovar/Cu/Ti shear strength and interlayer microhardness.
It can be seen from Figure 14c that, with the increase in holding time, the change trend of the shear strength of the joint was to increase first and then decrease. When the holding time was 1 h, the maximum shear strength reached 112 MPa. In Figure 14d, the hardness change of the Ti/Cu/Kovar/Cu/Ti interlayer in the joint was not as large as that of the Ti/Cu/Ti interlayer under the same process. The maximum hardness was 203 HV at 3 h. Compared with the normal Kovar, the increase was about 25%, which is less than the increase rate of the hardness of the Ti/Cu/Ti interlayer. This shows that, when a Kovar alloy is used as a buffer layer, the brittleness of intermetallic compounds has little effect on plasticity during welding, and can effectively relieve welding residual stress in ceramics and their reaction layers.
3.4. Fracture Analysis of Si3N4/Ti/Cu/Kovar/Cu/Ti/Ht250 Joints
Figure 15 shows the microstructure of the shear fracture of the Si3N4/Ti/Cu/Kovar/Cu/Ti/Ht250 joint under the two processes. The fractures were all on the Si3N4 ceramic side, but the specific fracture position was different, indicating that the bonding strength of the Ht250 side was higher. Table 7 and Table 8 give the EDS scanning results of each area in Figure 15. Table 7 shows that, under processing parameters of 2 MPa and 1000 °C for 15 min, the fracture mainly contained a small amount of TiN phase (region A) and Ti5Si3 phase (region B). Similar to the intermediate layer, this was at the reaction layer of Ti–N and Ti–Si. The reason for the fracture was that the holding time was too short, which made the reaction layer here too thin and the bonding force weak. It can be seen from Table 8 that, under processing parameters of 2 MPa and 1000 °C for 1 h, the fracture at the joint mainly contained Cu4Ti3, TiFe, TiFe2, and TiNi3 (region C) and a small amount of Cu4Ti3 (region D), corresponding to Figure 15b. The silvery white area contained a large amount of Ti–Ni compounds, and the brittle phase was the main reason for the fracture of the joint there.
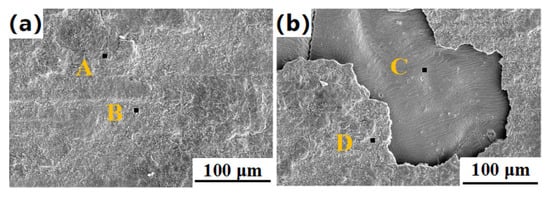
Figure 15.
Microstructure of the shear fracture of a Si3N4/Ti/Cu///Kovar/Cu/Ti/Ht250 joint: (a) 2 MPa, 1000 °C, 15 min; (b) 2 MPa, 1000 °C, 1 h.

Table 7.
Component content of each point in Figure 15a (at %).

Table 8.
Component content of each point in Figure 15b (at %).
As shown in Figure 16b, the position and mechanism of the fracture of the Si3N4/Ti/Cu/Kovar joint changed. The fracture was located in the Kovar interface reaction layer near the Kovar alloy interface on the Si3N4 ceramic side, which was mainly composed of Ti–Fe, Ti–Ni and a small amount of Ti–Cu, which was the weak point of the whole joint. This indicated that a large number of Ti elements diffused to the interface of Kovar alloy through the liquid zone of Ti–Cu, and an interface diffusion layer composed of Cu–Ti metal compounds formed on this interface. This greatly alleviated the aggregation of Ti–Cu intermetallic compounds near the Si3N4 interface reaction layer, thus greatly reducing the stress near the ceramic interface, so the fracture site changed to near the Kovar alloy interface layer. At the same time, the thermal expansion coefficient of the Kovar alloy itself was closer to that of Si3N4 ceramics, so the overall strength of the joint increased, and the maximum was 112 MPa.
4. Conclusions
In this paper, the PTLP diffusion bonding of Si3N4 ceramic and Ht250 cast iron with Ti/Cu/Kovar/Cu/Ti as an interlayer, which significantly improves the strength of the joint, was studied. The main conclusions are as follows:
- Si3N4/Ht250 PTLP diffusion bonding mainly depends on the eutectic liquid phase formed by Ti and Cu at high temperature. With the mutual diffusion of elements, the liquid phase advanced to both sides until Ti and Cu were completely dissolved, which alleviated the problem of the aggregation of a large number of Cu–Ti compounds near the ceramic interface. Finally, with the isothermal solidification process of the liquid region, a closely bonded joint formed.
- With the increase in the temperature and holding time, the transition of each interface reaction layer in the joint was more uniform, and the shear strength of the joint was the highest at 1000 °C for 1 h: 112 MPa. When the temperature or holding time increased further, the thermal residual stress at the joint became larger, and a brittle phase such as TiNi3 was produced, which led to a rapid decrease in the joint strength, to only 64–89 MPa.
- The fracture of the Si3N4/Ht250 joint was due to the interface reaction layer near the ceramic side of the Kovar alloy, which was mainly composed of Ti–Fe and TiNi3. The fracture type was brittle fracture, and was mainly due to the accumulation of brittle phase TiNi3.
Author Contributions
D.Z. and K.W. supervised the project and offered support and guidance with experiments and papers, X.L. put forward some suggestions for amendment. L.Z. and N.Z. carried out the main experimental work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We are grateful for the equipment and support provided by Nanjing University of Science and Technology. L.Z. and N.Z. also thank D.Z., X.L. and K.W. for their support and guidance.
Conflicts of Interest
No conflict of interest exists in the submission of this manuscript, and this manuscript was approved by all authors for publication.
References
- El-Sayed, M.S.; Hany, S.A.; Abdulhakim, A.A. Corrosion Behavior of Cast Iron in Freely Aerated Stagnant Arabian Gulf Seawater. Materials 2015, 8, 2127–2138. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Xu, X. Failure analysis of diesel engine cylinder head bolts. Eng. Fail. Anal. 2006, 13, 826–834. [Google Scholar] [CrossRef]
- Cueva, G.; Sinatora, A.; Guesser, W.; Tschiptschin, A. Wear resistance of cast irons used in brake disc rotors. Wear 2003, 255, 1256–1260. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.-H.; Lee, Y.-S.; Kim, D.-Y.; Youn, J.-G. Laser surface hardening of gray cast iron used for piston ring. J. Mater. Eng. Perform. 2002, 11, 294–300. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, Y.; Zhang, D.; Zhang, J.; Zhou, J.; Zhang, J.; Liu, X. Effect of surface nano-modification on the antioxidation properties of Si3N4 ceramics. J. Alloys Compd. 2018, 766, 678–685. [Google Scholar] [CrossRef]
- Song, W.; Wang, S.; Lu, Y.; Zhang, X.; Xia, Z. Friction behavior of PTFE-coated Si3N4/TiC ceramics fabricated by spray technique under dry friction-ScienceDirect. Ceram. Int. 2020, 47, 7487–7496. [Google Scholar] [CrossRef]
- Luo, S.-C.; Guo, W.-M.; Plucknett, K.; Lin, H.-T. Improved toughness of spark-plasma-sintered Si3N4 ceramics by adding HfB2. Ceram. Int. 2020, 47, 8717–8721. [Google Scholar] [CrossRef]
- Pazhouhanfar, Y.; Delbari, S.A.; Asl, M.S.; Shaddel, S.; Pazhouhanfar, M.; Van Le, Q.; Shokouhimehr, M.; Mohammadi, M.; Namini, A.S. Characterization of spark plasma sintered TiC–Si3N4 ceramics. Int. J. Refract. Met. Hard Mater. 2020, 95, 105444. [Google Scholar] [CrossRef]
- Ruigang, W.; Wei, P.; Mengning, J.; Jian, C.; Yongming, L. Investigation of the physical and mechanical properties of hot-pressed machinable Si3N4/h-BN composites and FGM. Mater. Sci. Eng. B 2002, 90, 261–268. [Google Scholar] [CrossRef]
- Ruigang, W.; Wei, P.; Mengning, J.; Jian, C.; Yongming, L. Residual stress induced failure of Ti-6Al4V/Si3N4 joints brazed with Ag-Cu-Ti filler: The effects of brazing zone’s elasto-plasticity and ceramics’ intrinsic properties. J. Eur. Ceram. Soc. 2021, 41, 6319–6329. [Google Scholar] [CrossRef]
- Lan, L.; Yu, J.; Yang, Z.; Li, C.; Ren, Z.; Wang, Q. Interfacial microstructure and mechanical characterization of silicon nitride/nickel-base superalloy joints by partial transient liquid phase bonding. Ceram. Int. 2015, 42, 1633–1639. [Google Scholar] [CrossRef]
- Cook, G.O., III; Sorensen, C.D. Overview of transient liquid phase and partial transient liquid phase bonding. J. Mater. Sci. 2011, 46, 5305–5323. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Jin, X.; Yan, N.; Yang, L. Influence of micro-oxidation on joints of C/C composites and GH3044 for large-size aerospace parts. Acta Astronaut. 2017, 140, 478–484. [Google Scholar] [CrossRef]
- Jia-sheng, Z.; Zhi-guo, J.; Ya-jie, C.; Zheng, C. Study on Double Partial Transient Liquid Phase Bonding with Si3N4 Ceramic Using Ti/Cu/Ni Interlayer. J. Aeronaut. Mater. 2005, 25, 29–33. [Google Scholar]
- Chen, Z.; Cao, M.; Zhao, Q.; Zou, J. Interfacial microstructure and strength of partial transient liquid-phase bonding of silicon nitride with Ti/Ni multi-interlayer. Mater. Sci. Eng. A 2004, 380, 394–401. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).