Progress of Spark Plasma Sintering (SPS) Method, Systems, Ceramics Applications and Industrialization
Abstract
1. Introduction
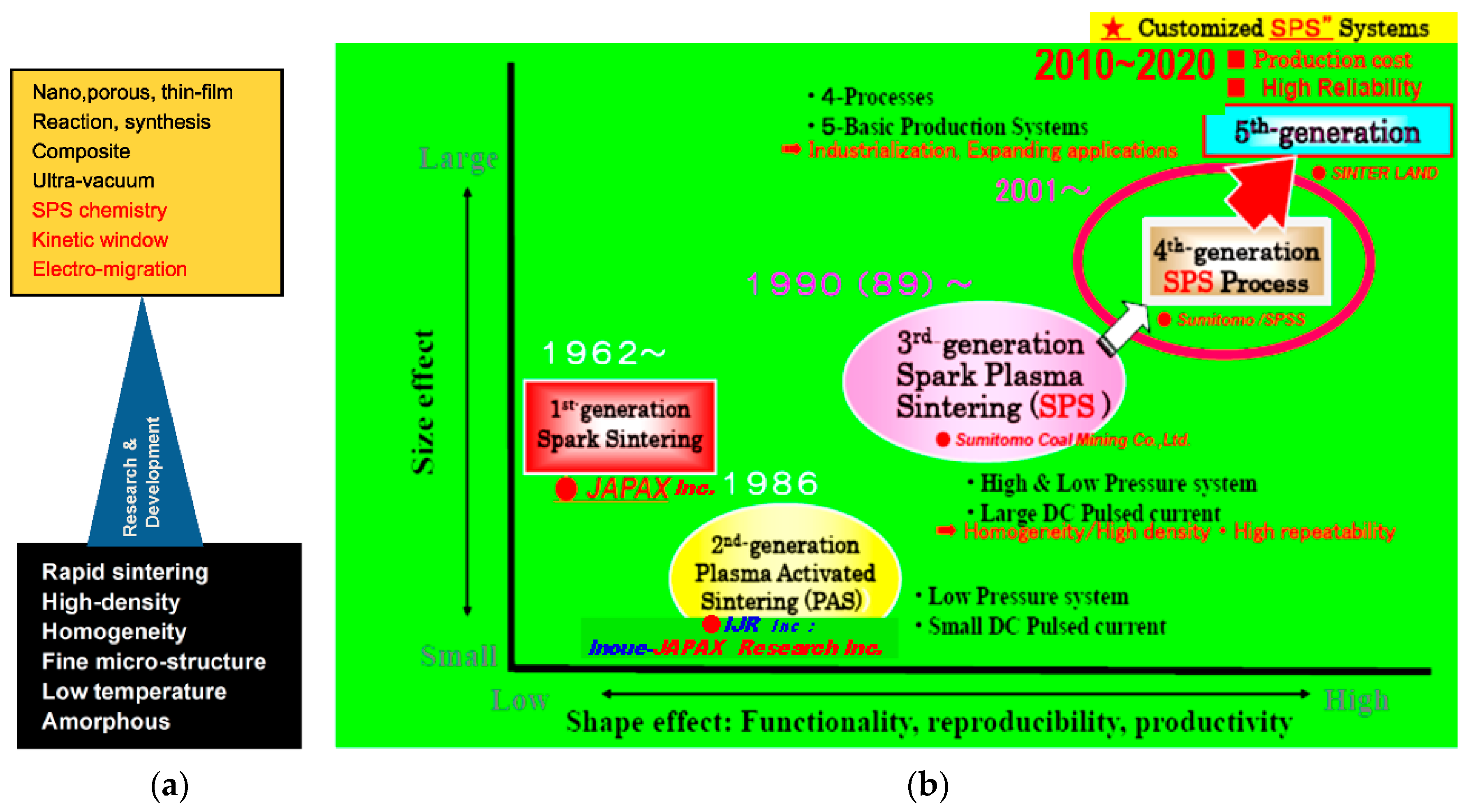
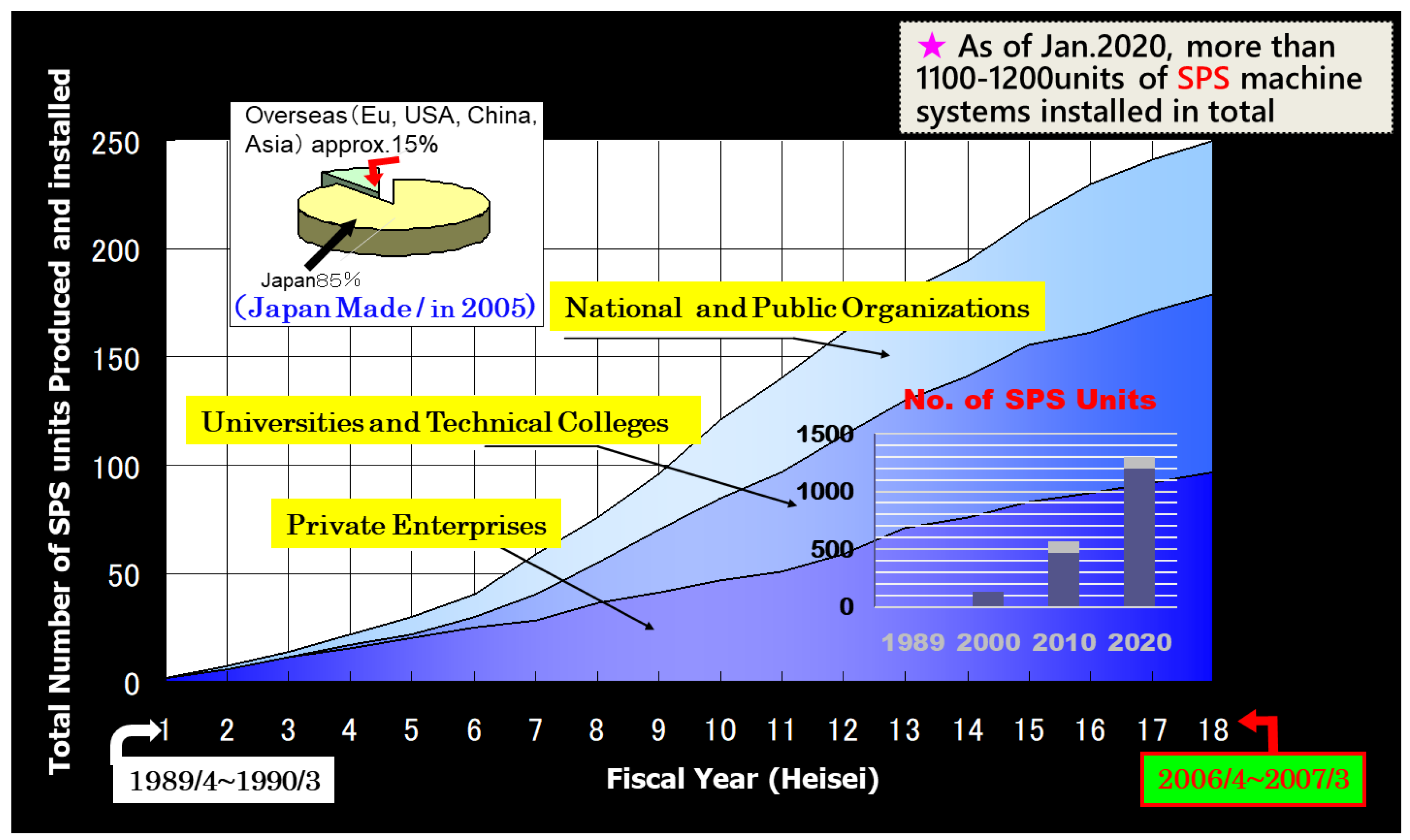
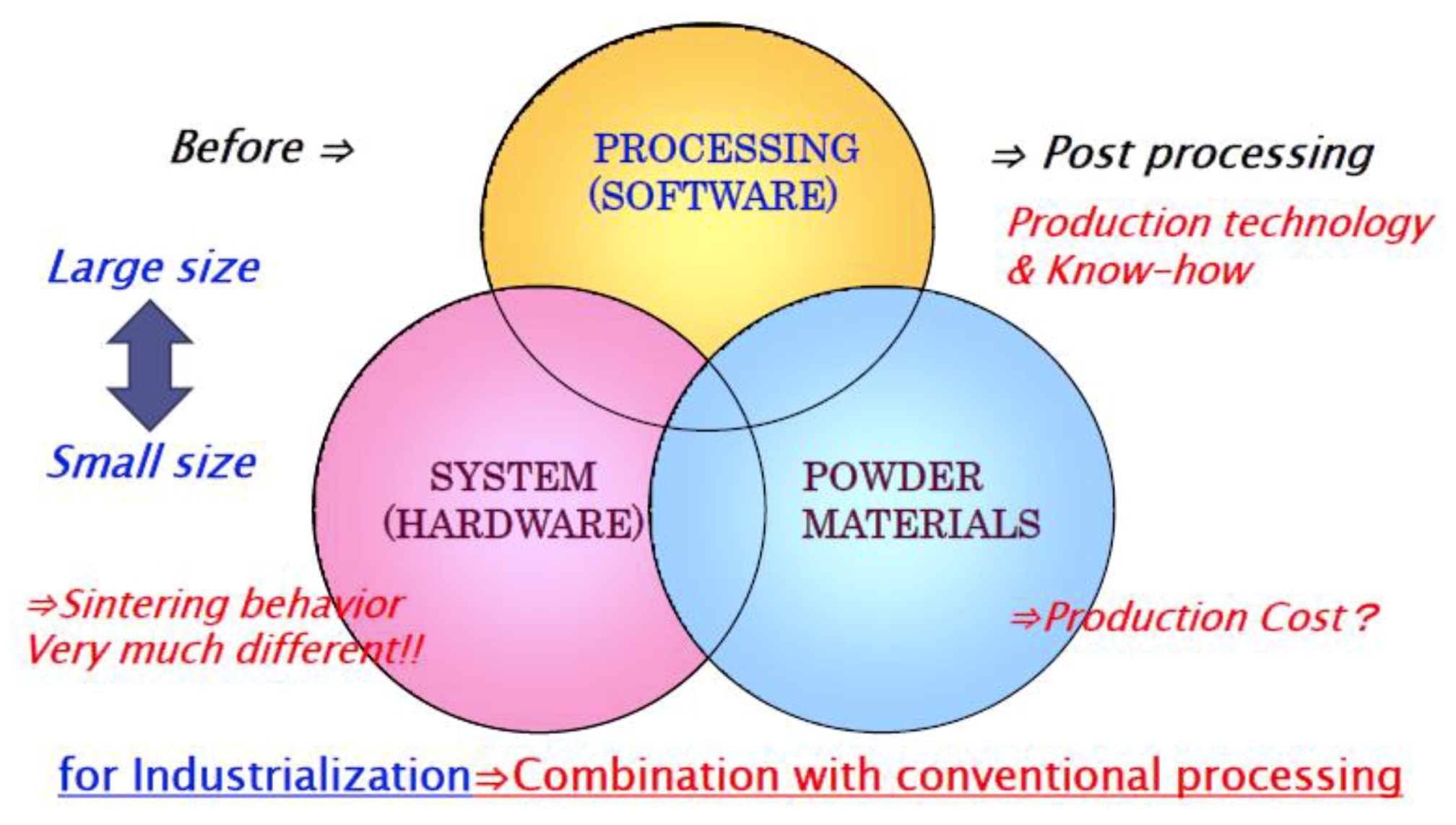
2. Historical Background and Increase of SPS Market
3. Spark Plasma Sintering (SPS)
3.1. Materials for SPS
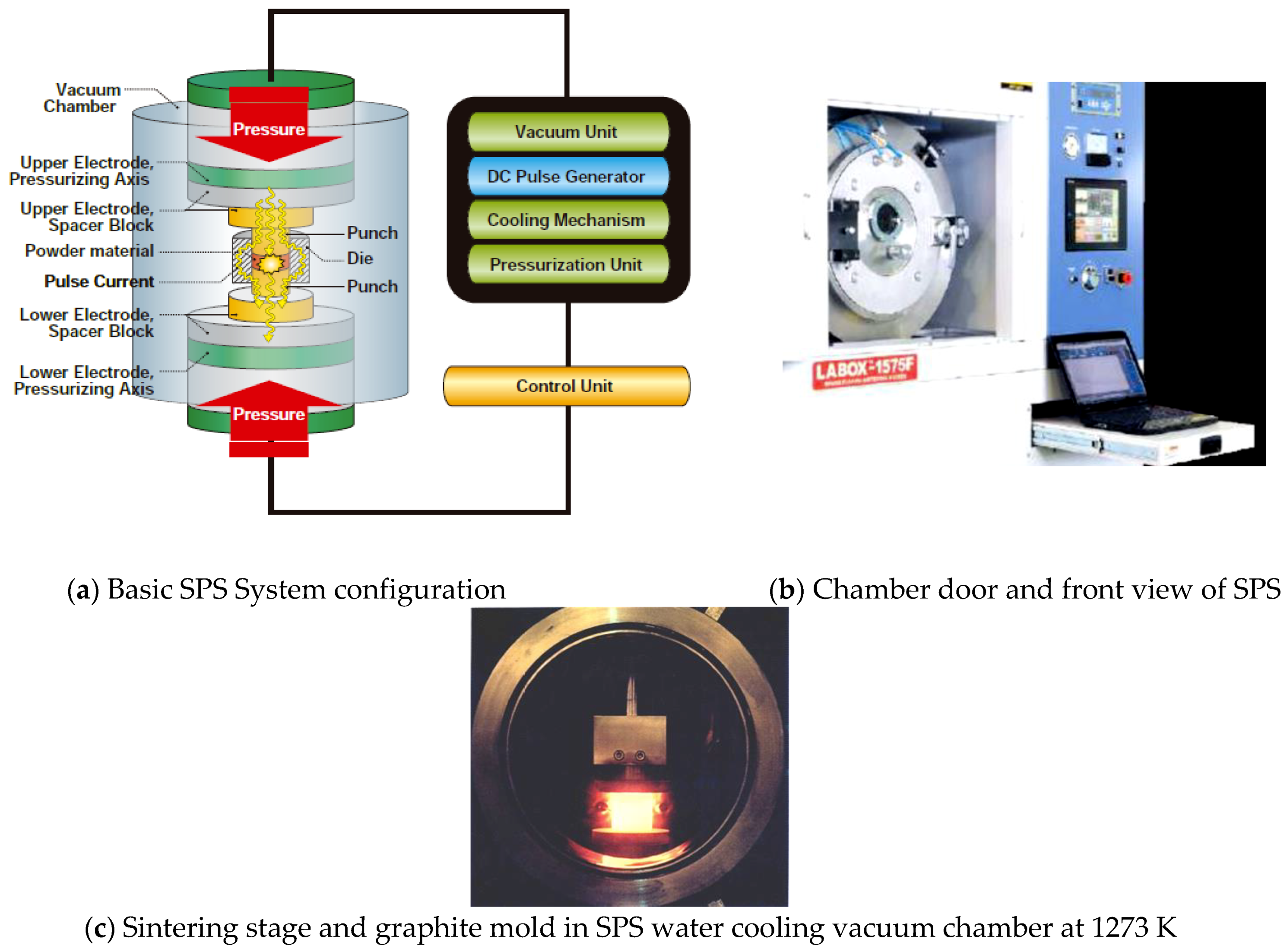
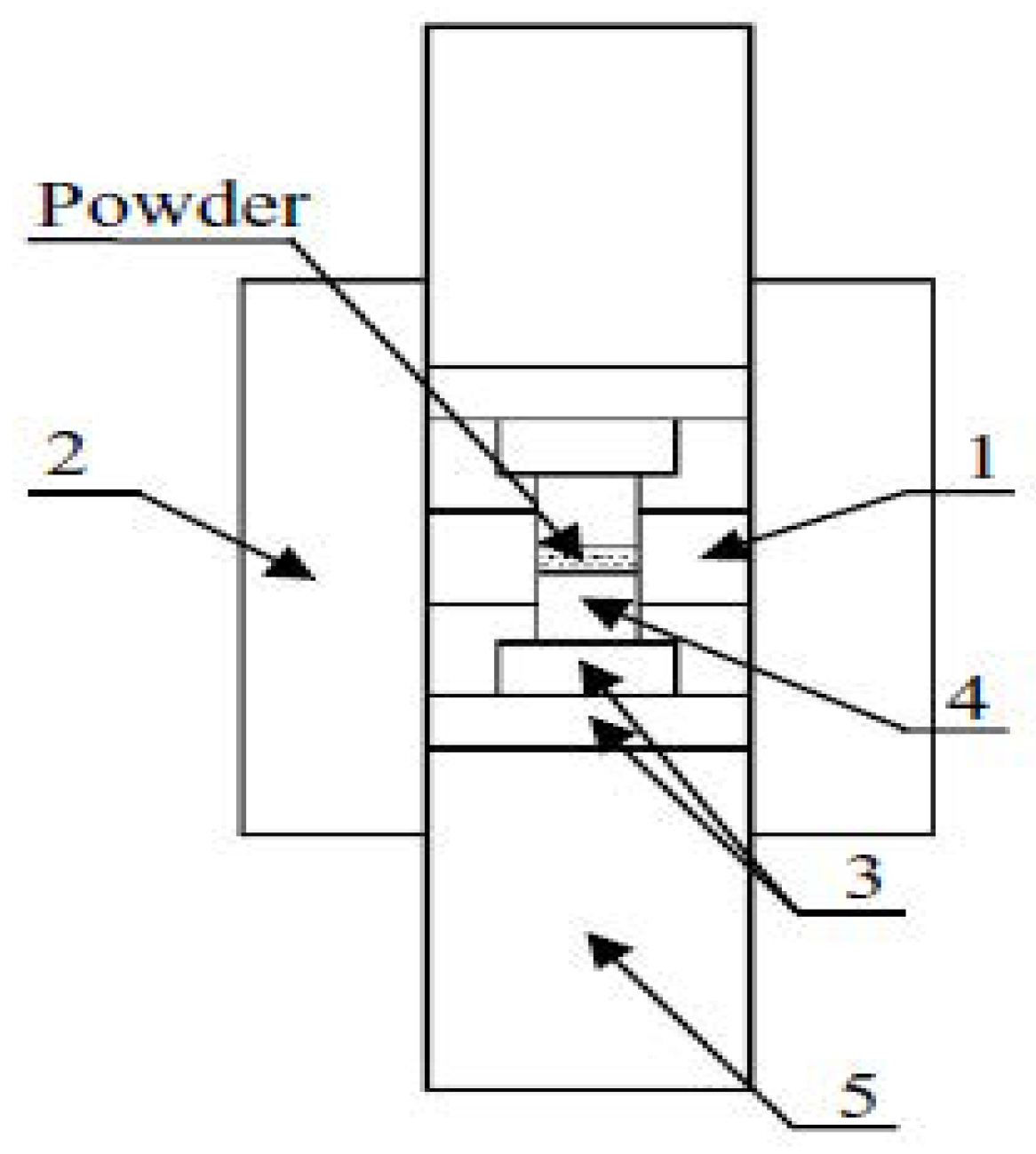
3.2. Basic Configuration of SPS System
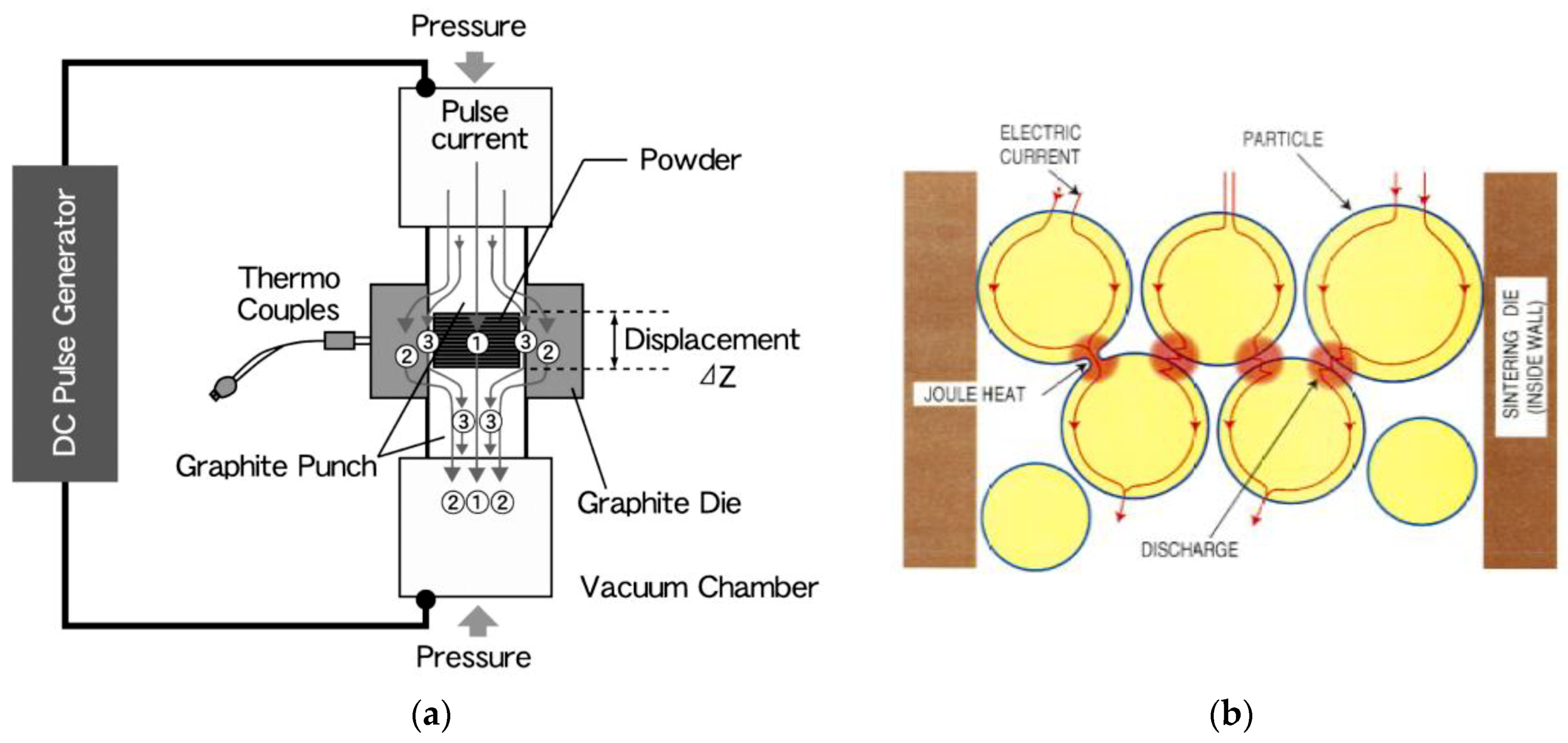
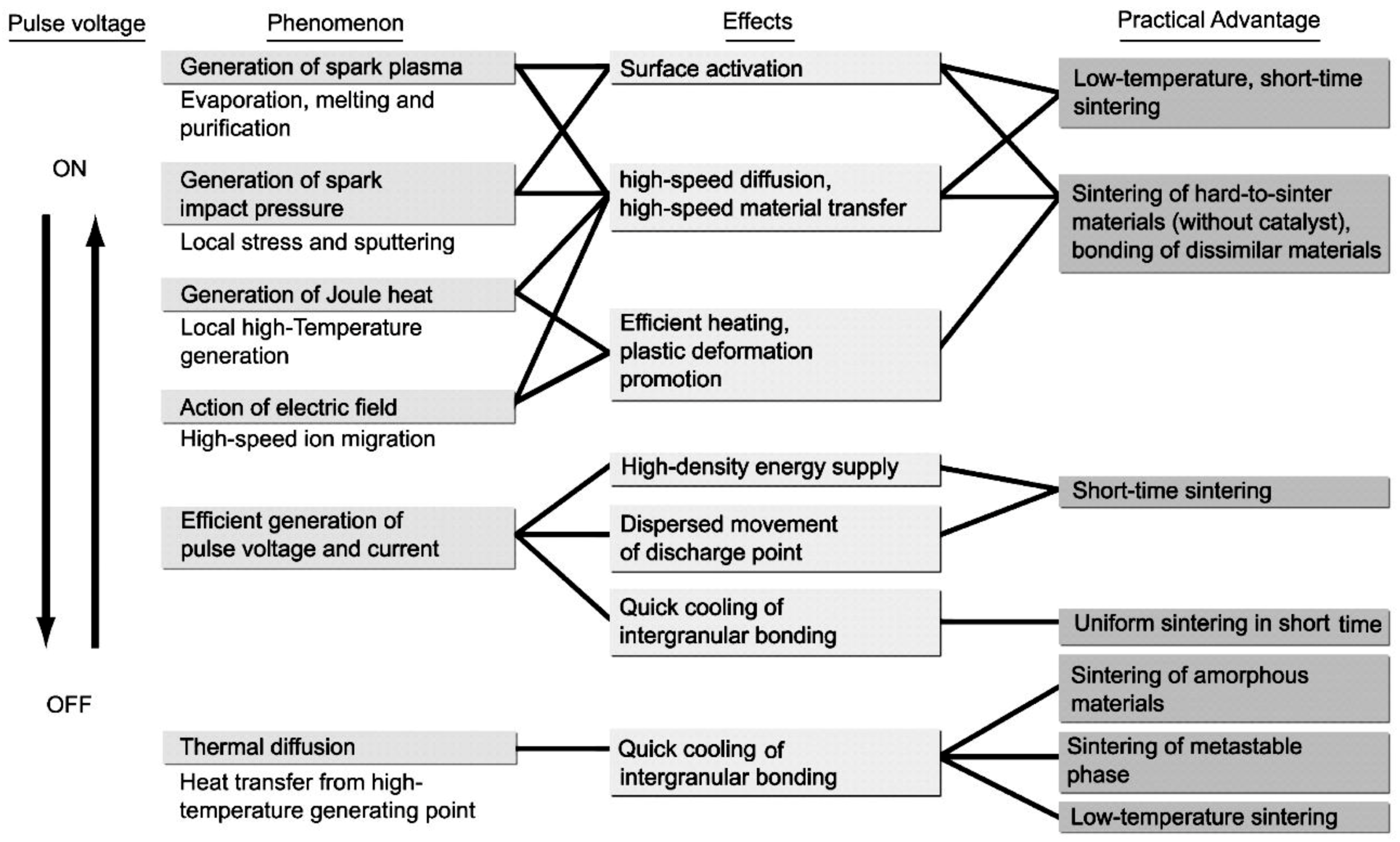
3.3. Basic Mechanism of SPS Process
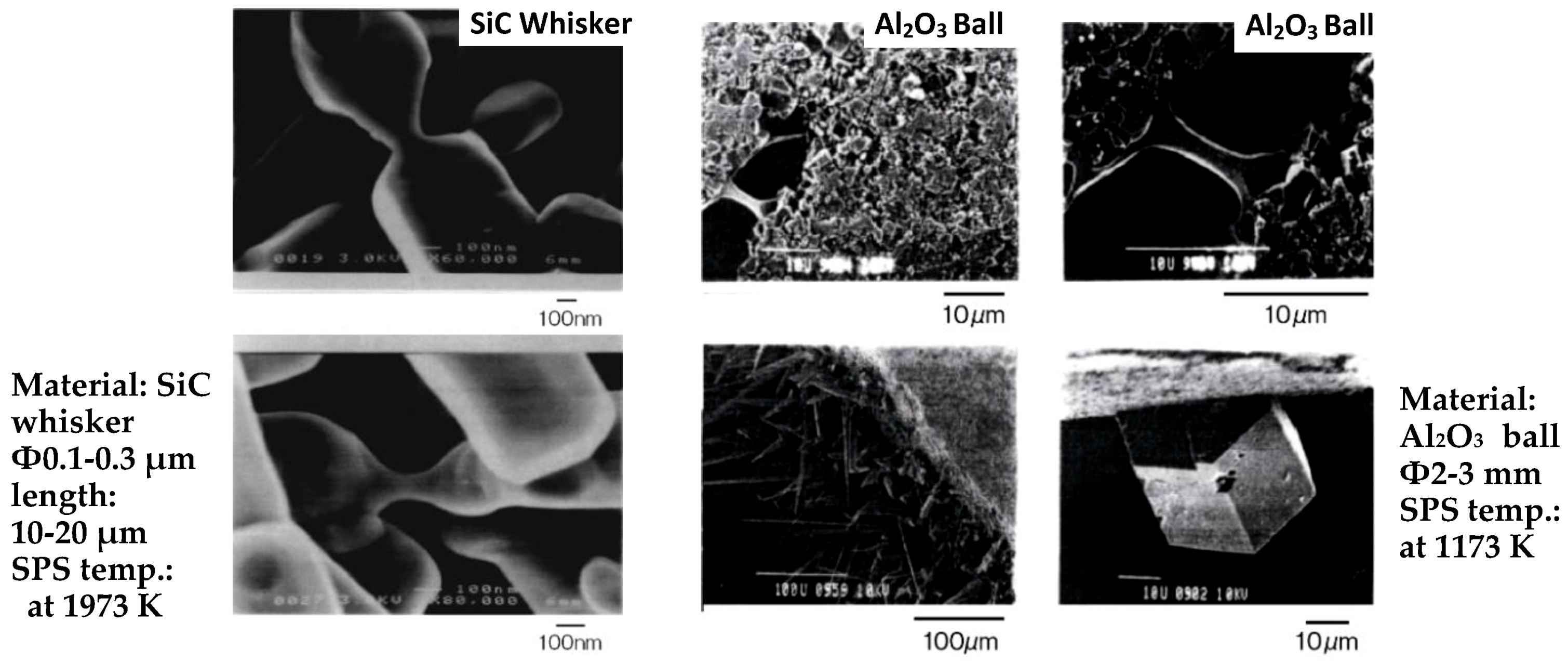
3.4. Neck Formation and Densification by SPS
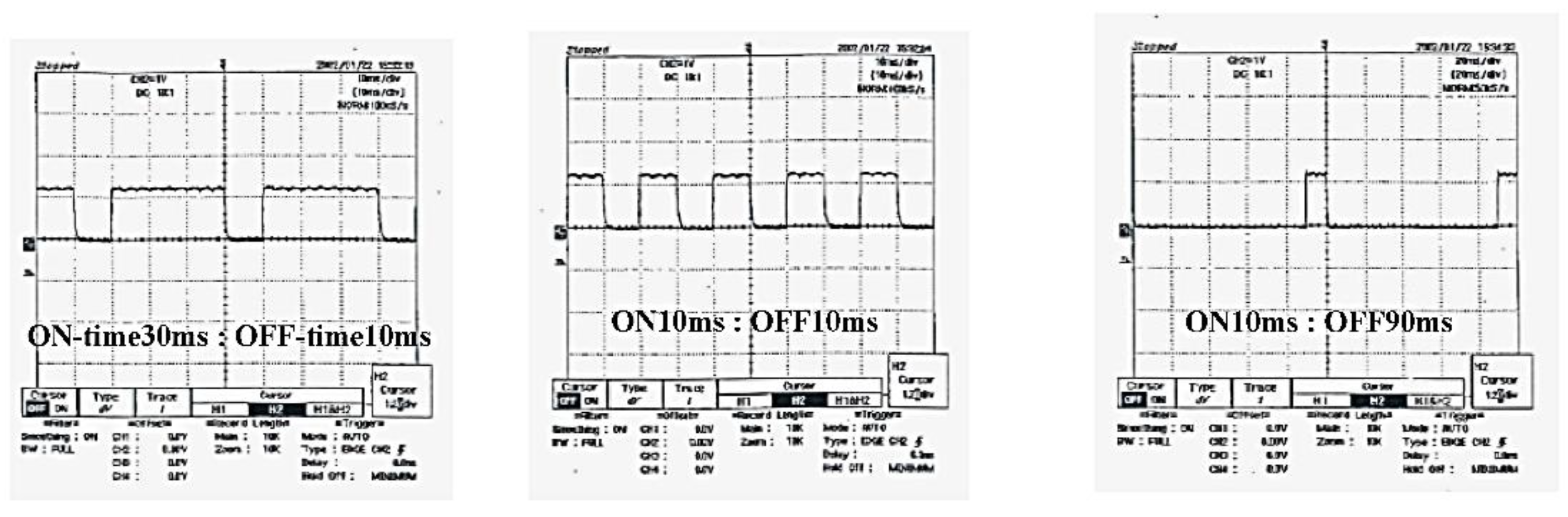
3.5. DC Pulse Generator for SPS System
3.6. Vacuum Exhausting System
3.7. SPS Sintering Temperature Measurement Difference
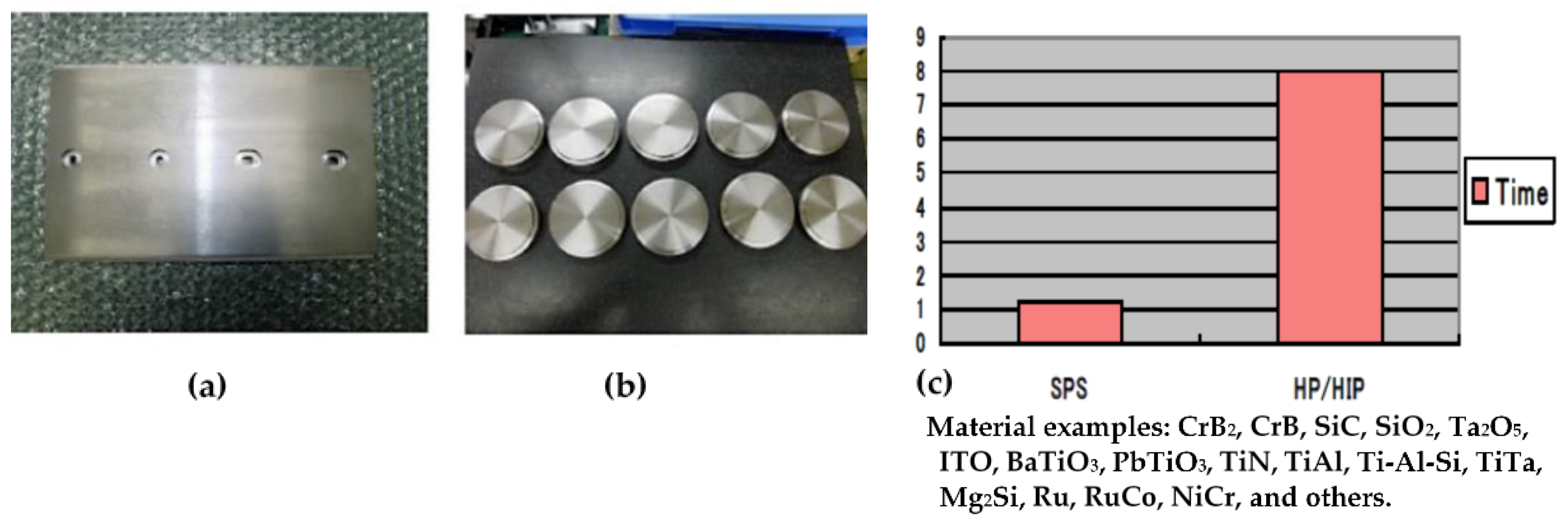
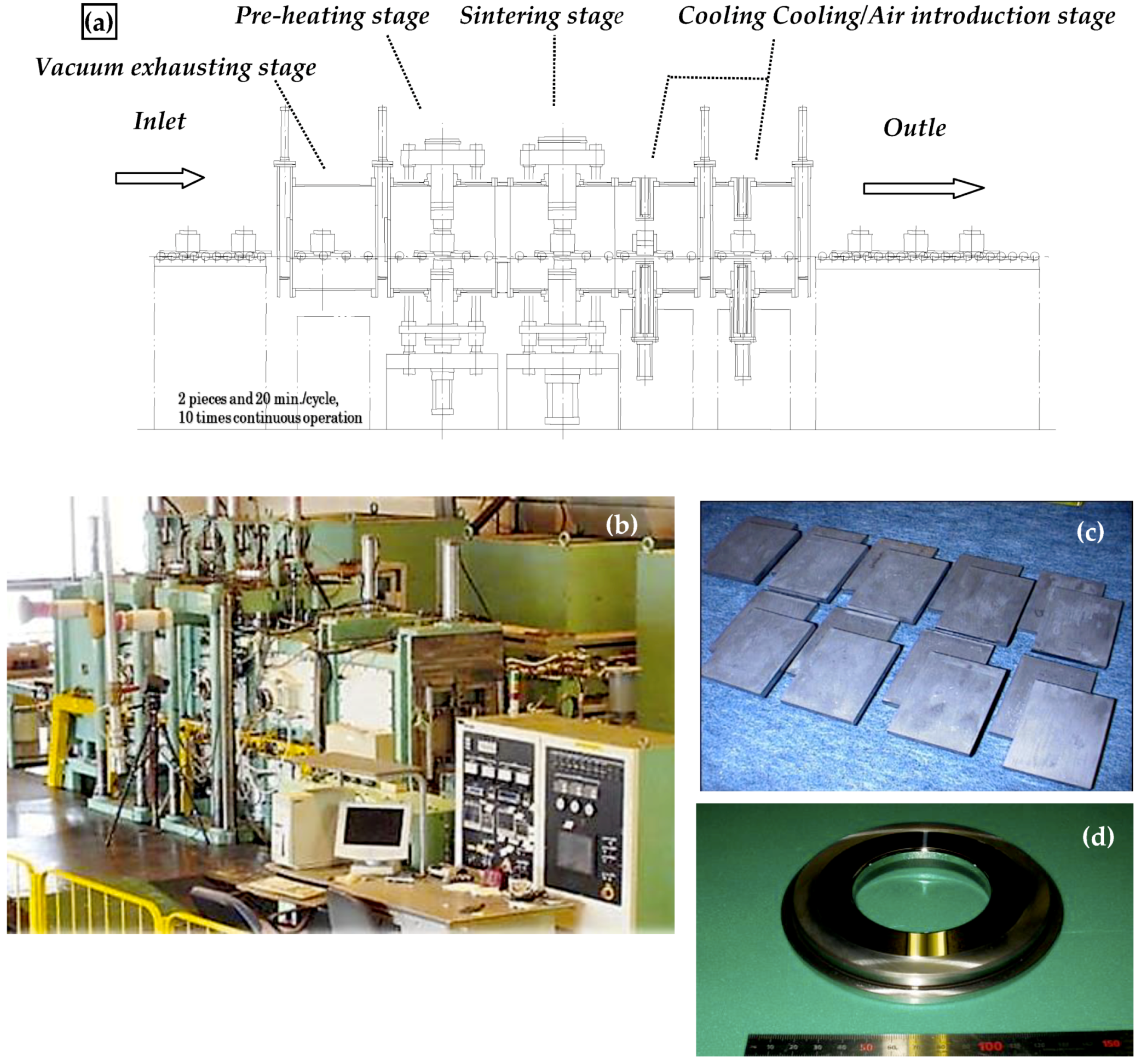
3.8. SPS Production Systems
4. Typical Example of SPS Phenomena on Ceramics Materials
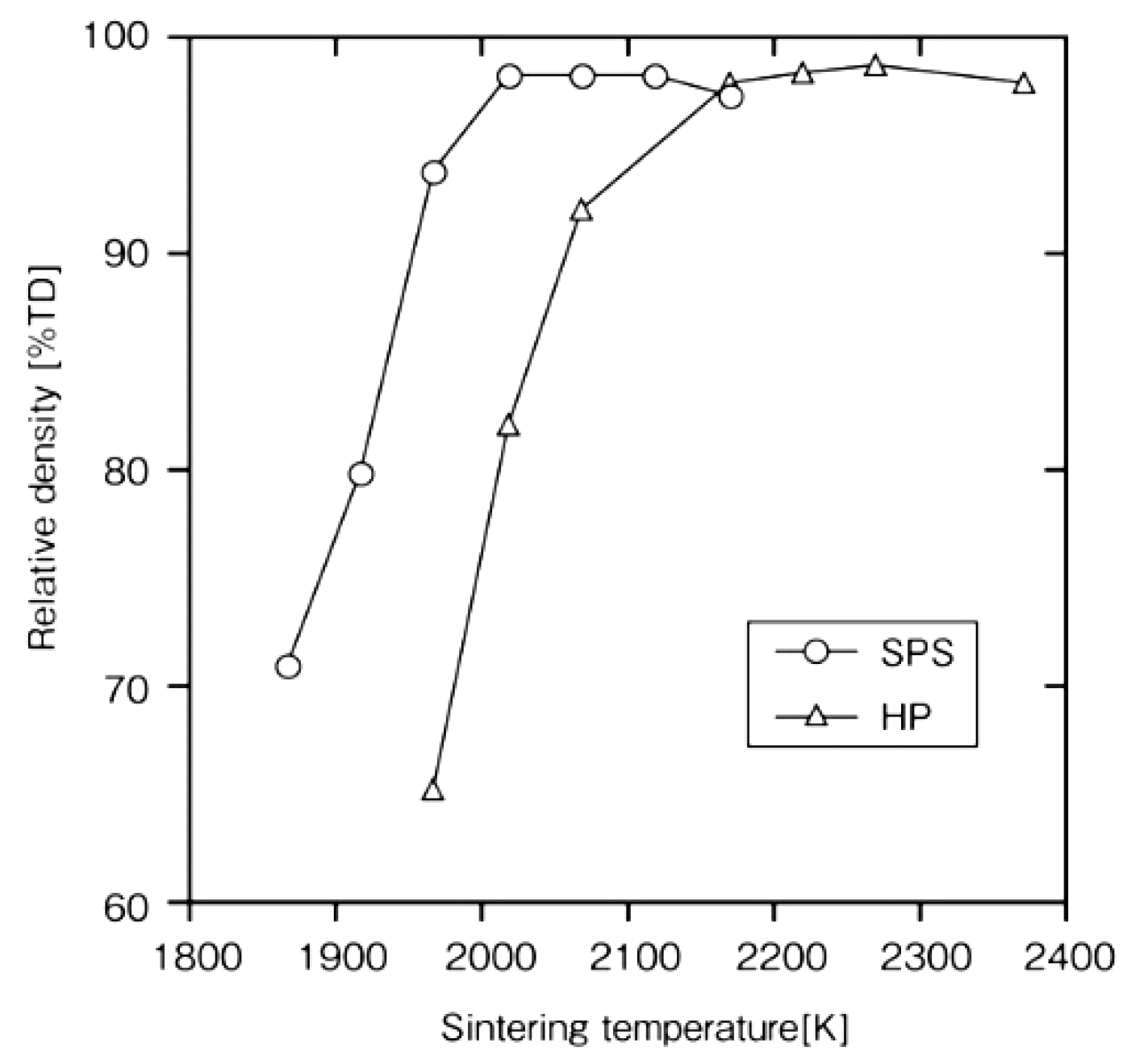
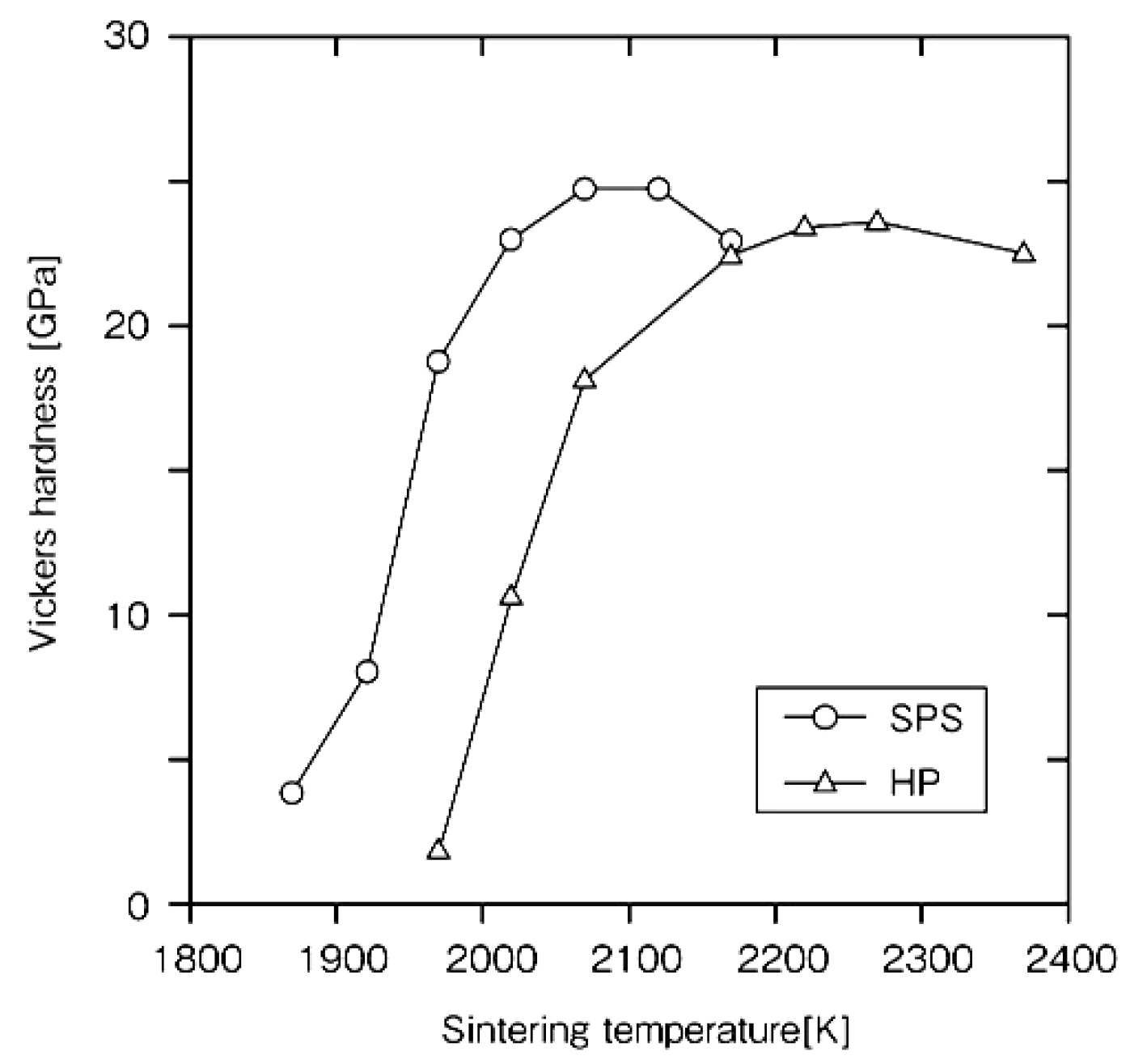
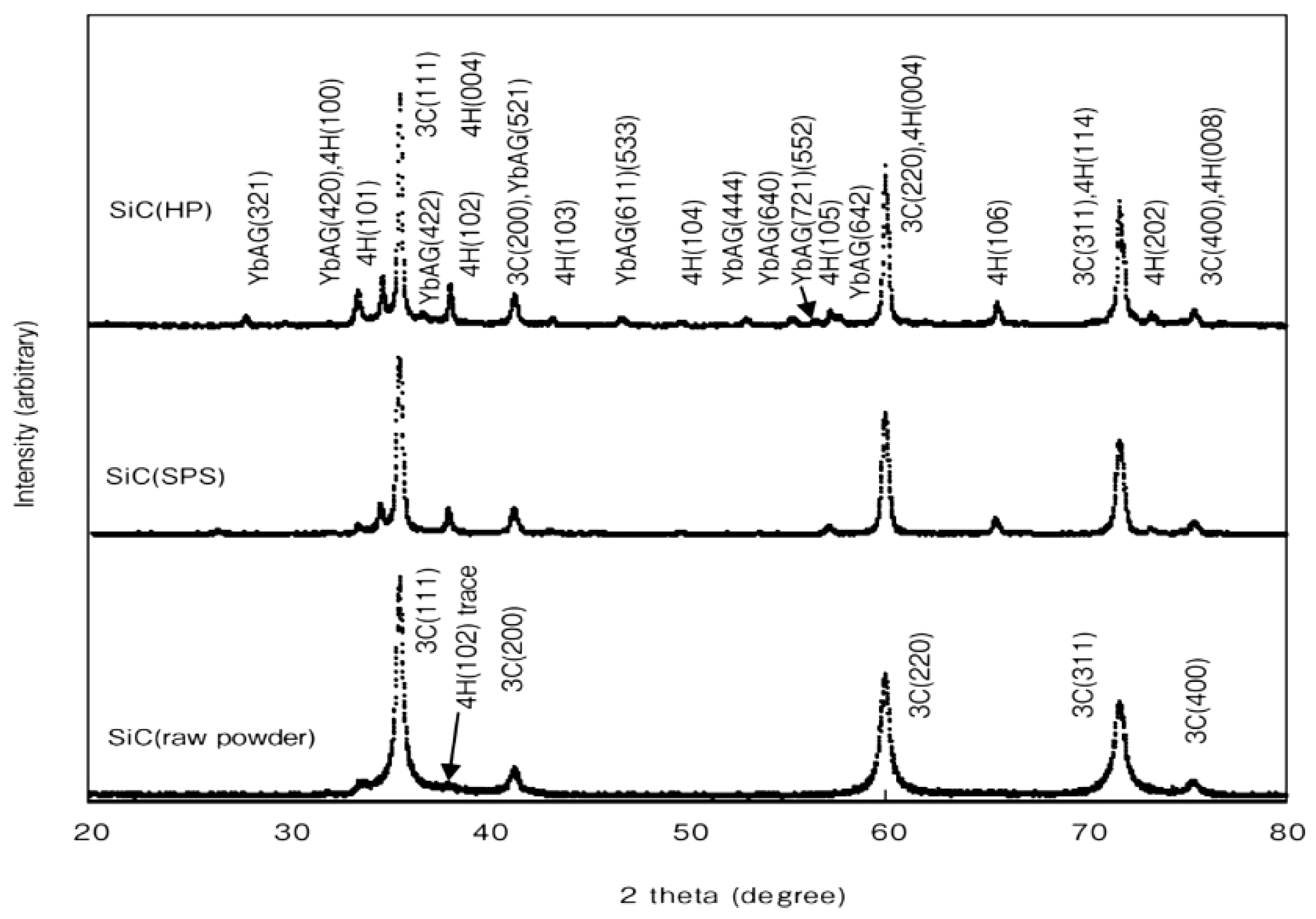
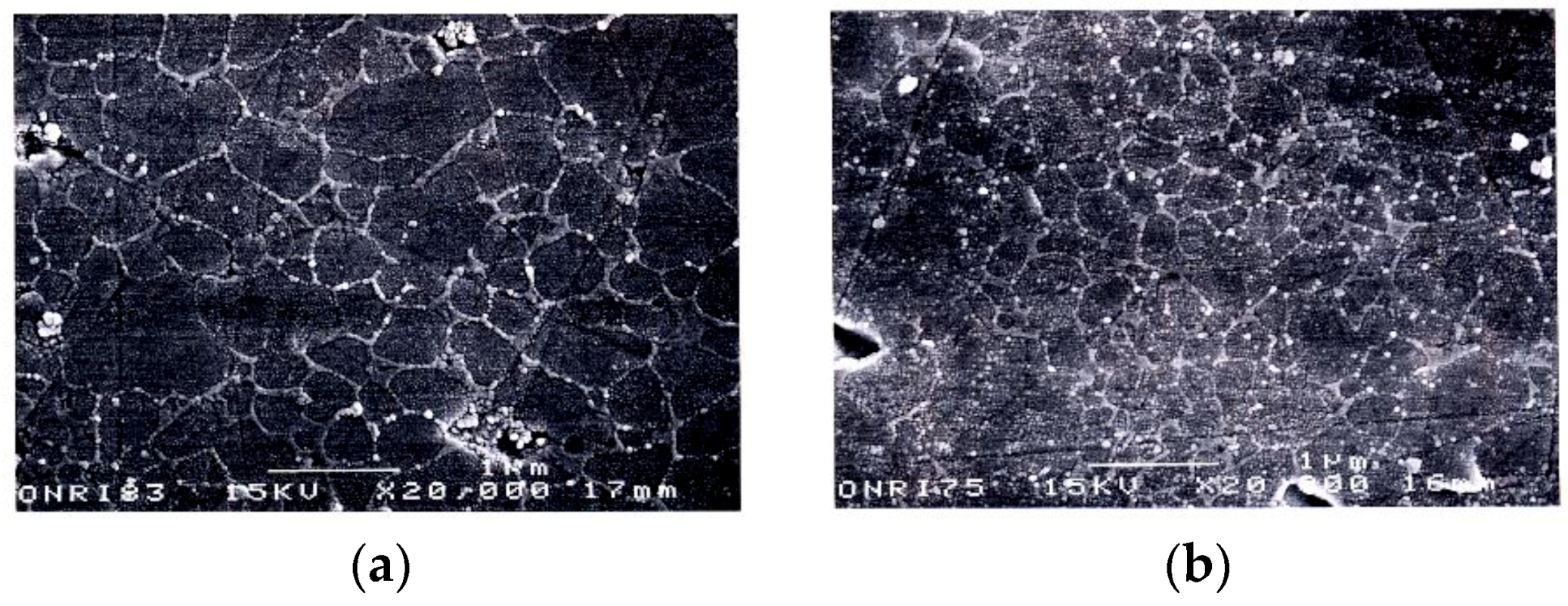
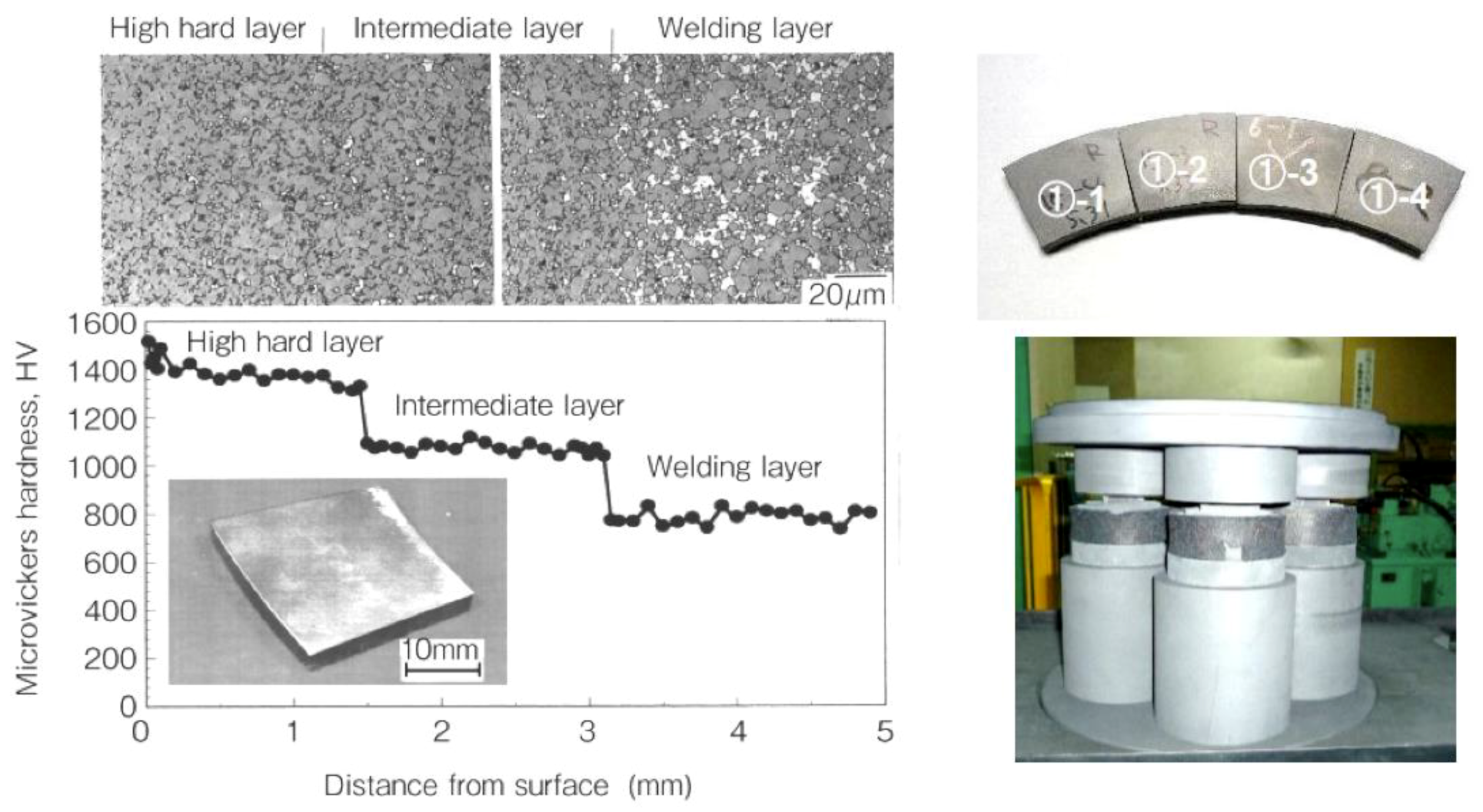
4.1. SiC with Al2O3/Yb2O3 Consolidated by SPS and Its Mechanical Properties
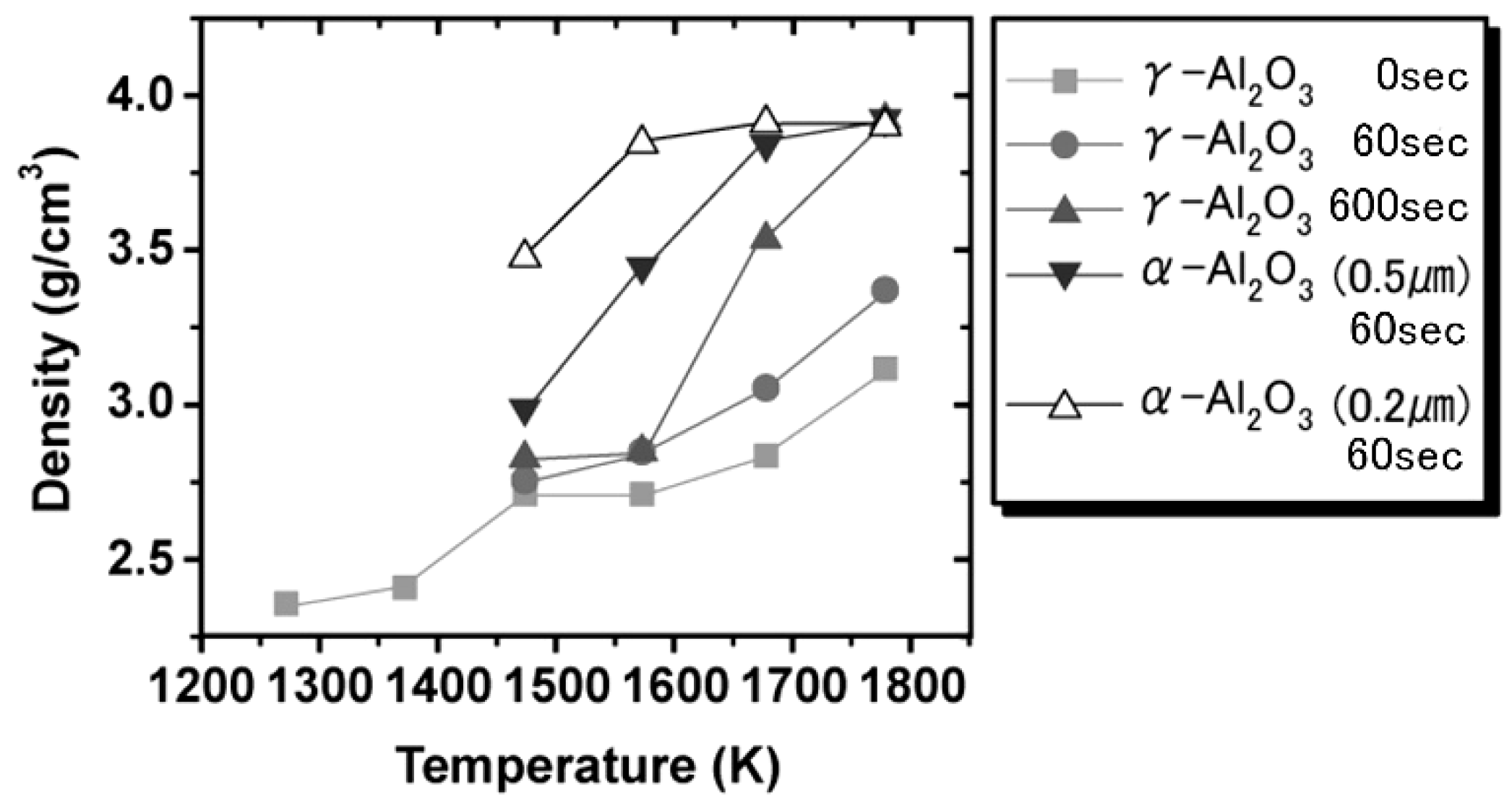
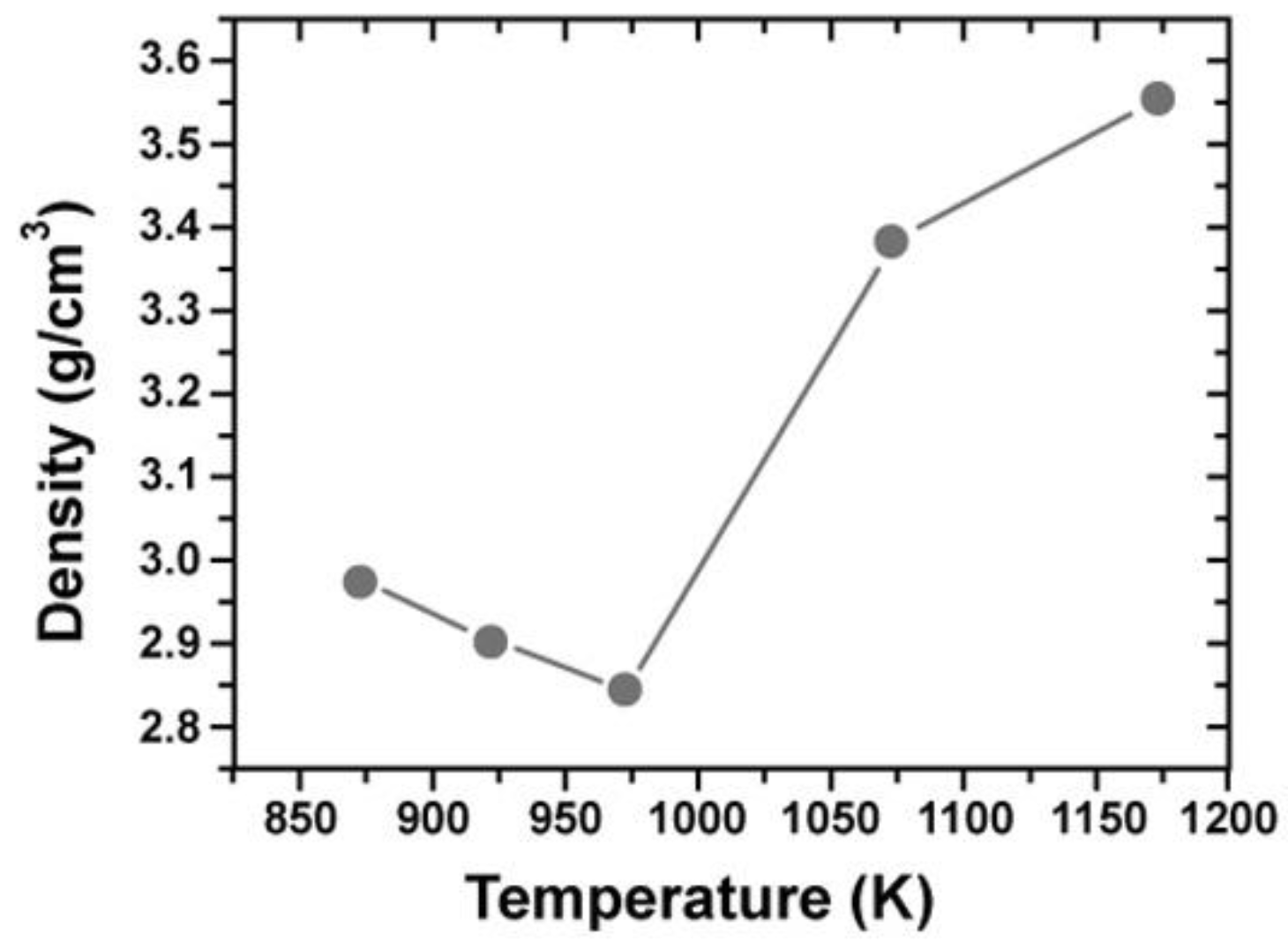
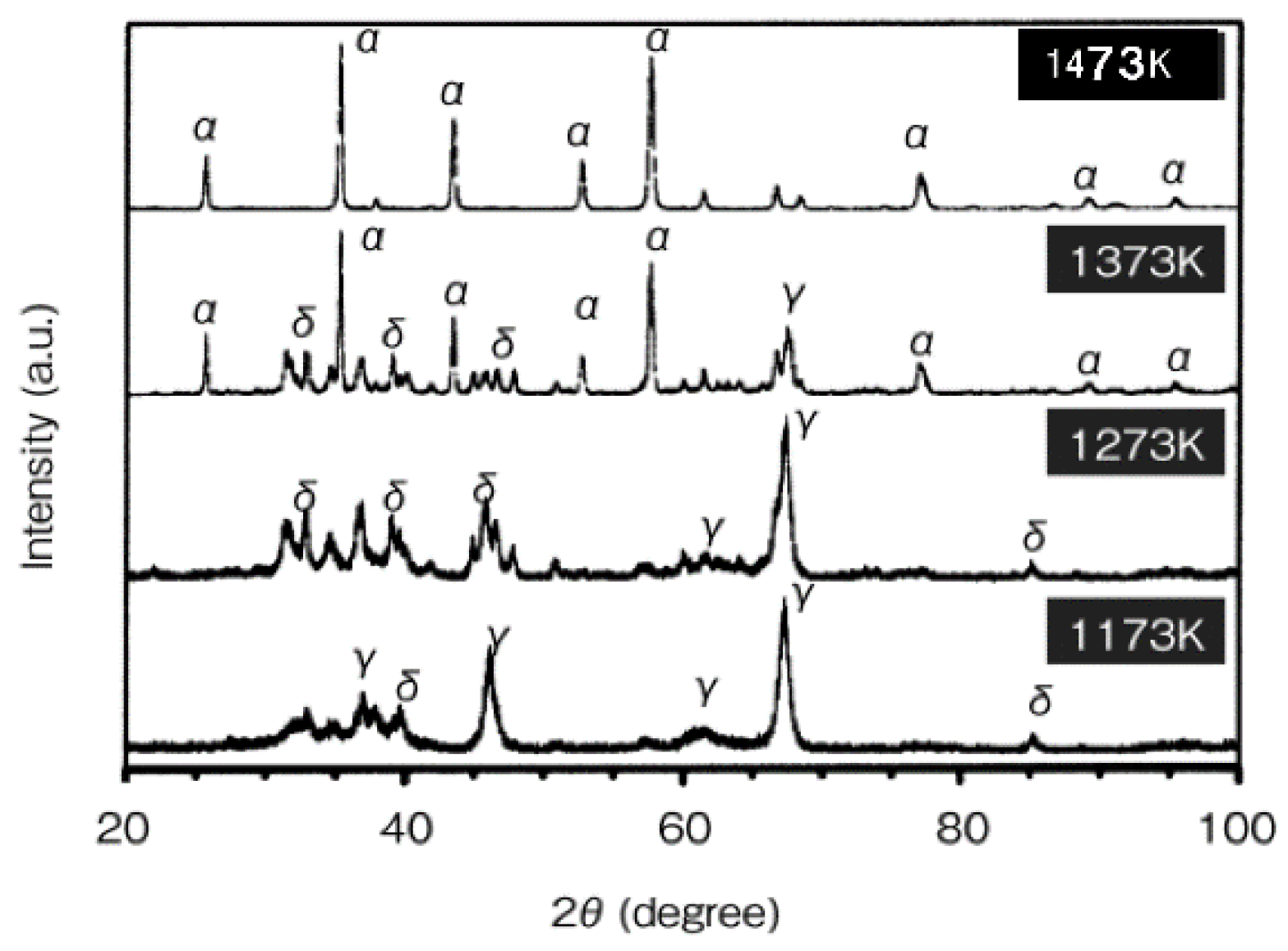
4.2. Consolidation of Nano-Al2O3, Phase Transformation, and Grain Growth
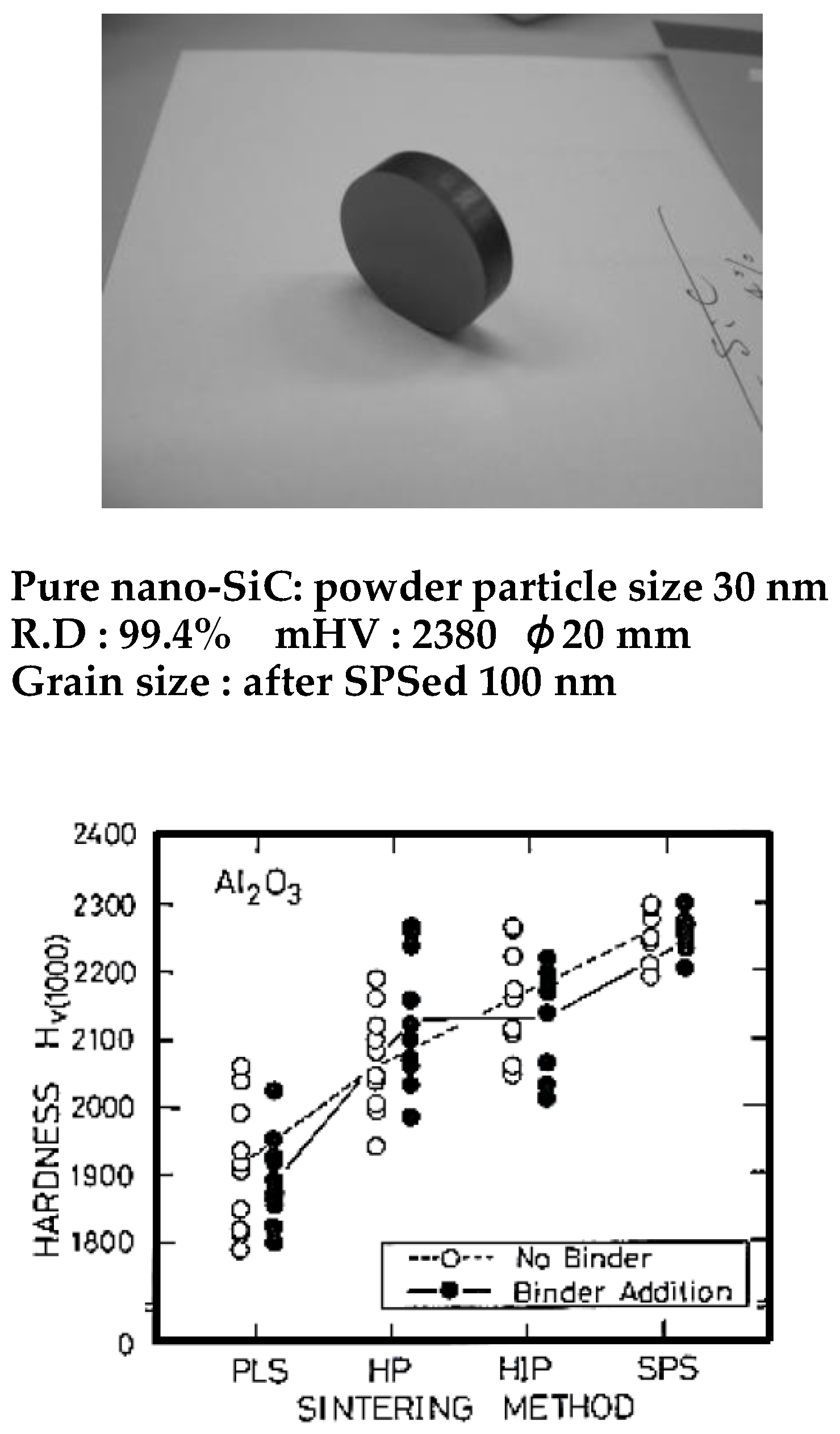
4.3. Examples of Nano Structural Ceramic Materials
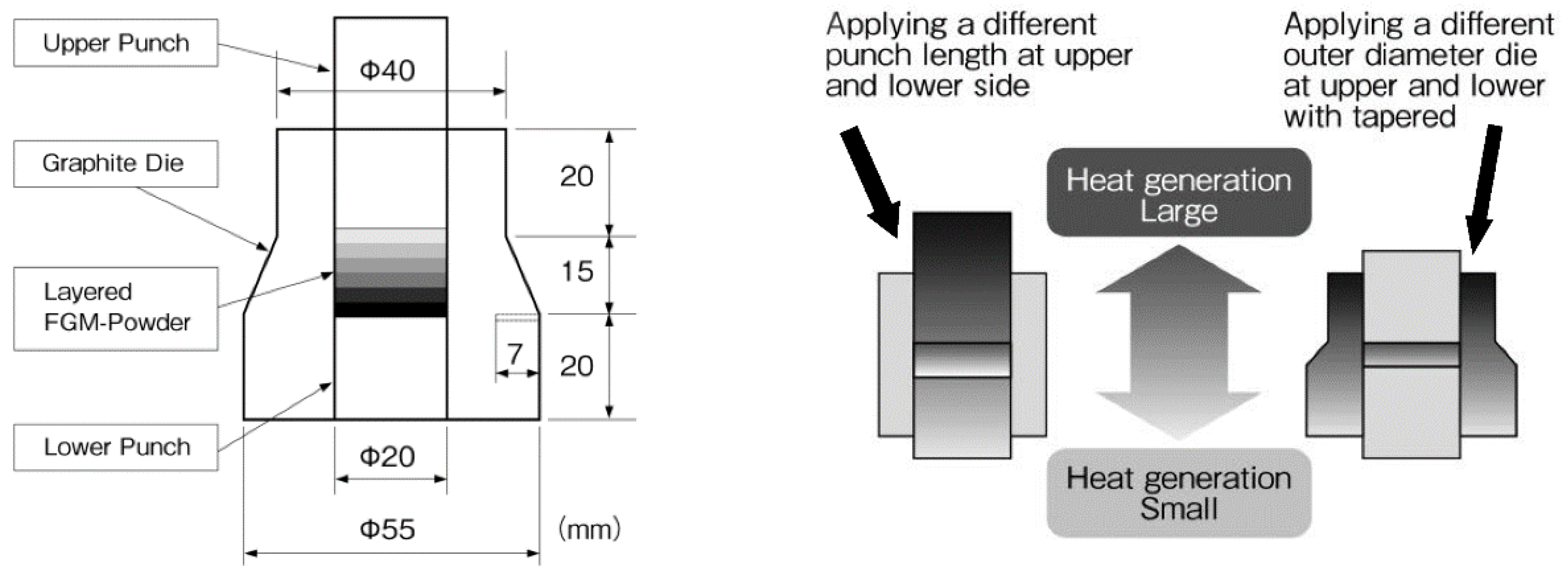
4.4. Examples of Synthesized Functionally Graded Materials (FGMs) by SPS
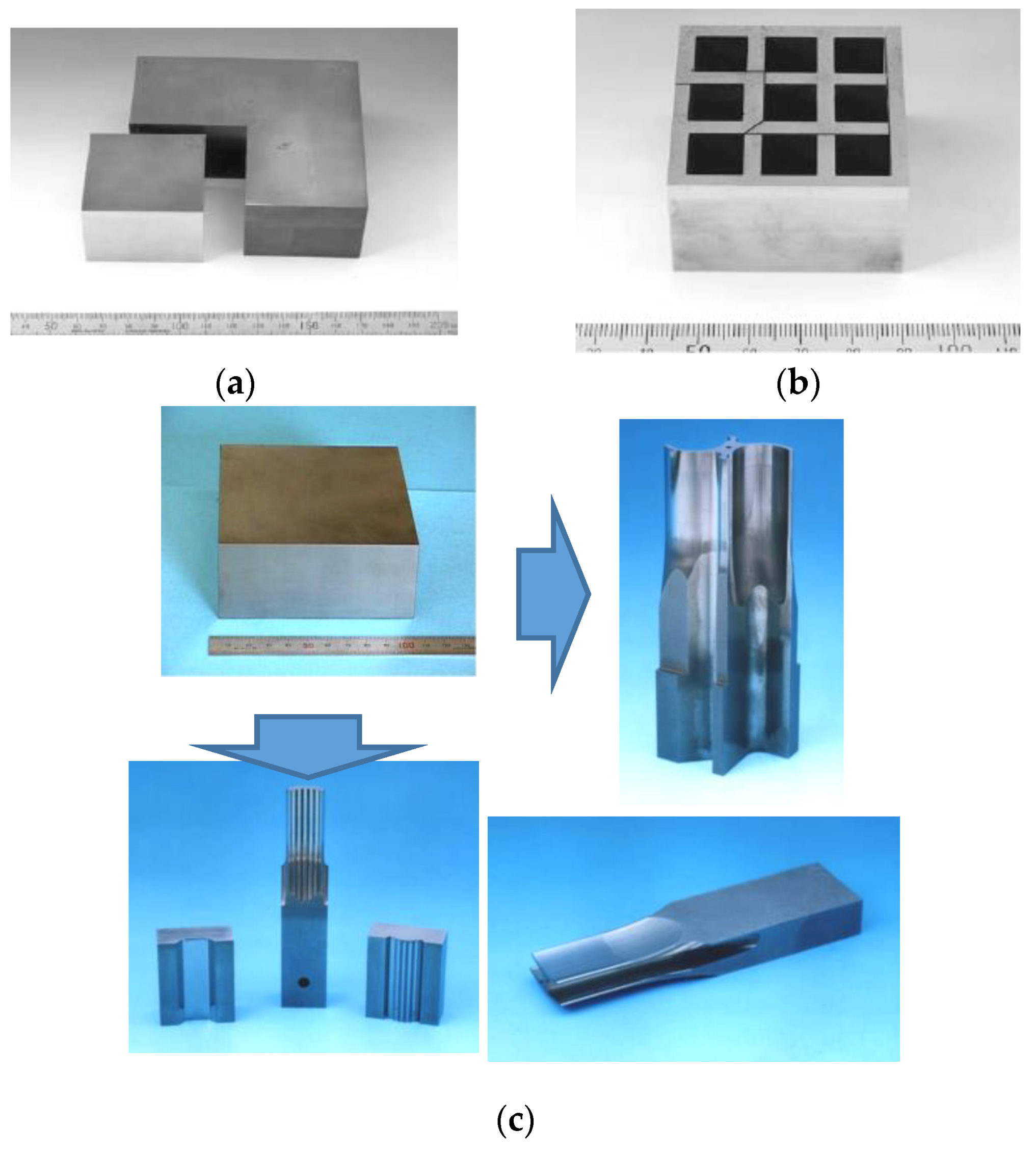
4.5. 3D Near-Net Shape/Net Shape Forming
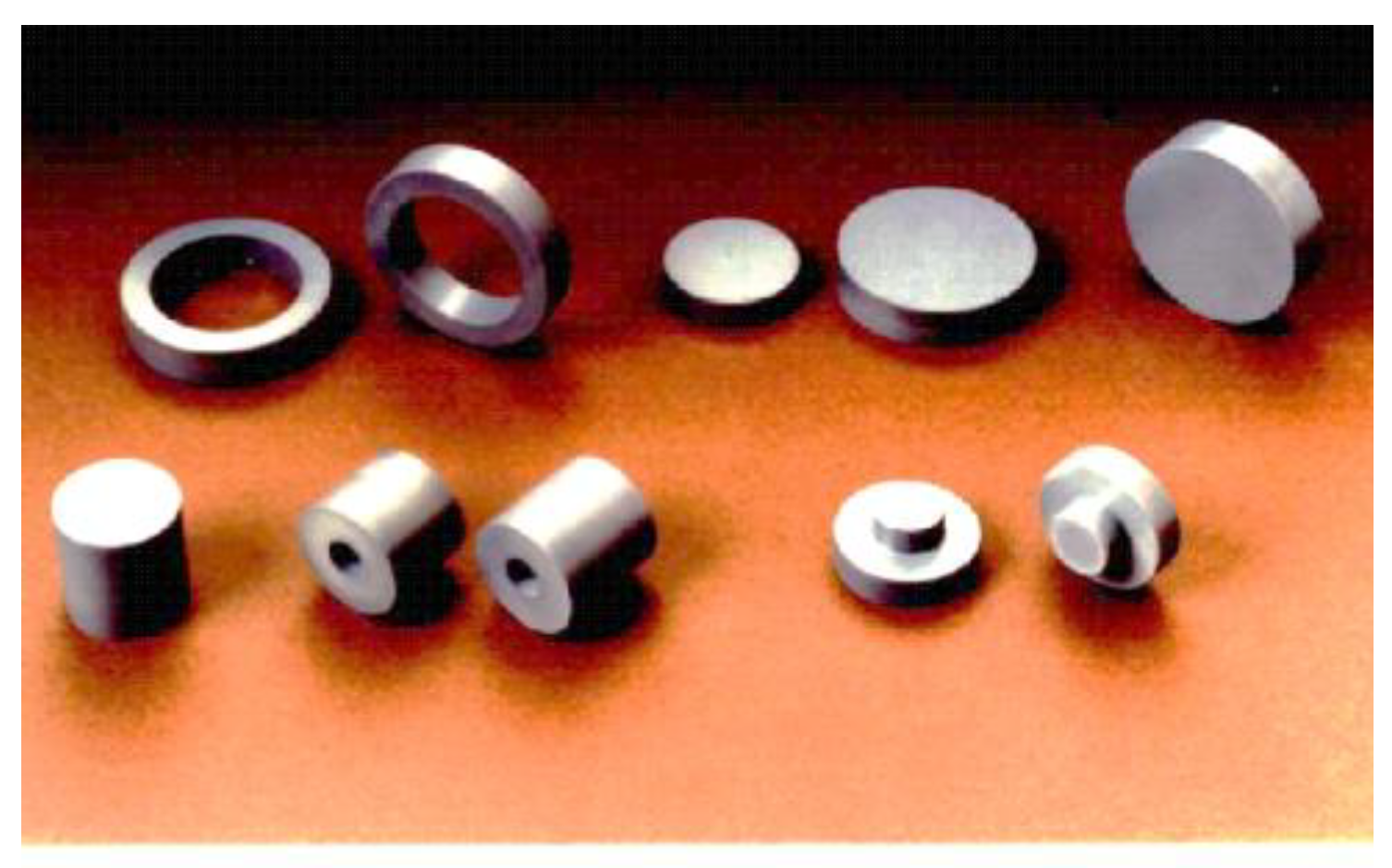
5. Ceramics Applications by SPS
5.1. Nanoparticle of Pure WC (Tungsten Carbide) Aspheric Glass Lens Mold
5.2. 3-Dimensional Complex Net- Shape Forming of Al2O3 Blasting Nozzle
5.3. Near-Net Shape Formed Si3N4/Al2O3 Composite Compacts for Homogenizer Component
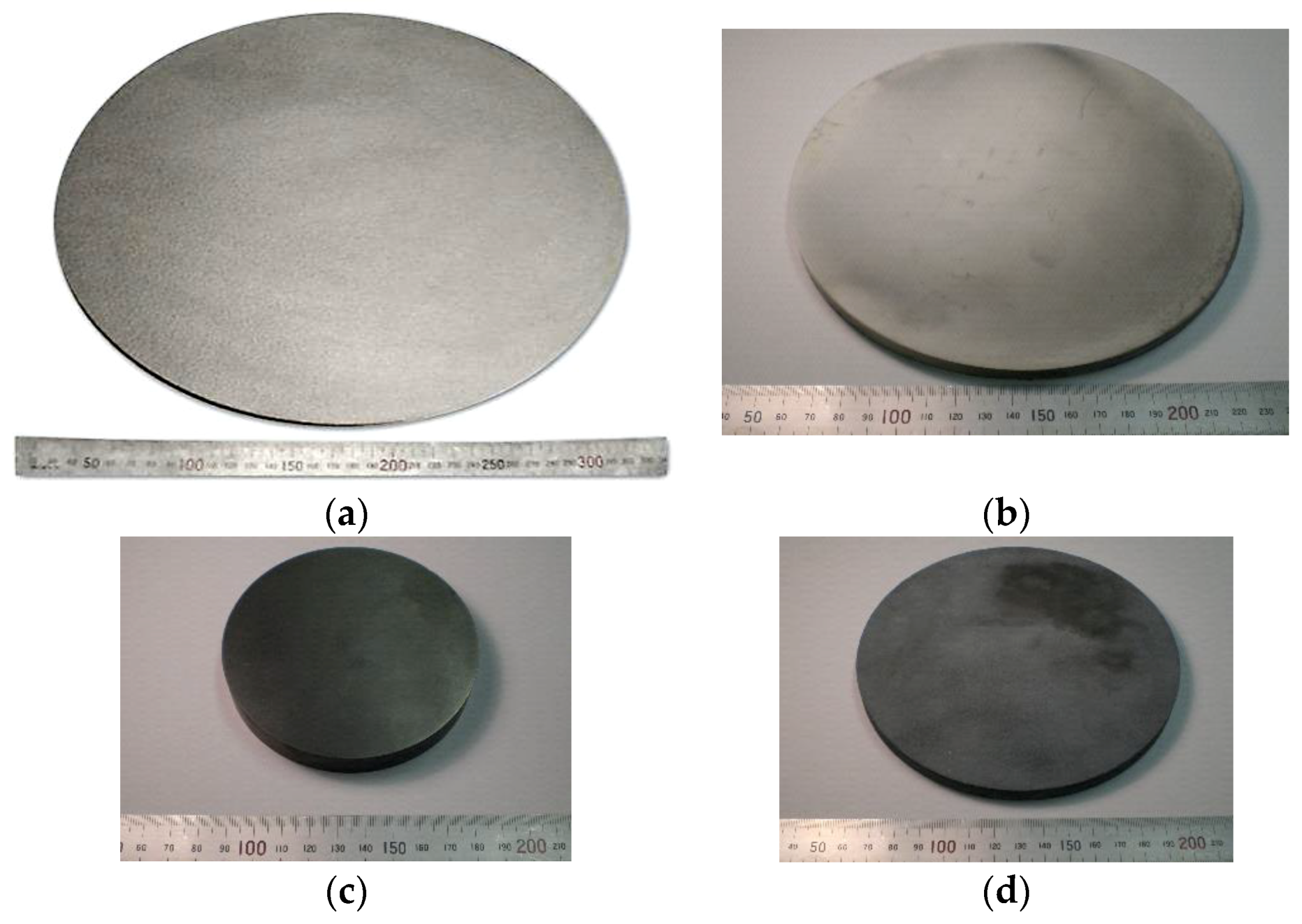
5.4. Sputtering Target Material and Fabrication of Large-Sized Ceramics and Metals Compact
5.5. Development of Al2O3/Ti System FGMs and ZrO2/Ti/Ti-6Al-4V Alloy FGMs Horn Tip Tool for Ultra-Sonic Homogenizer
5.6. WC Matrix Diamond Dicing Blade for Cutting Tool Industry
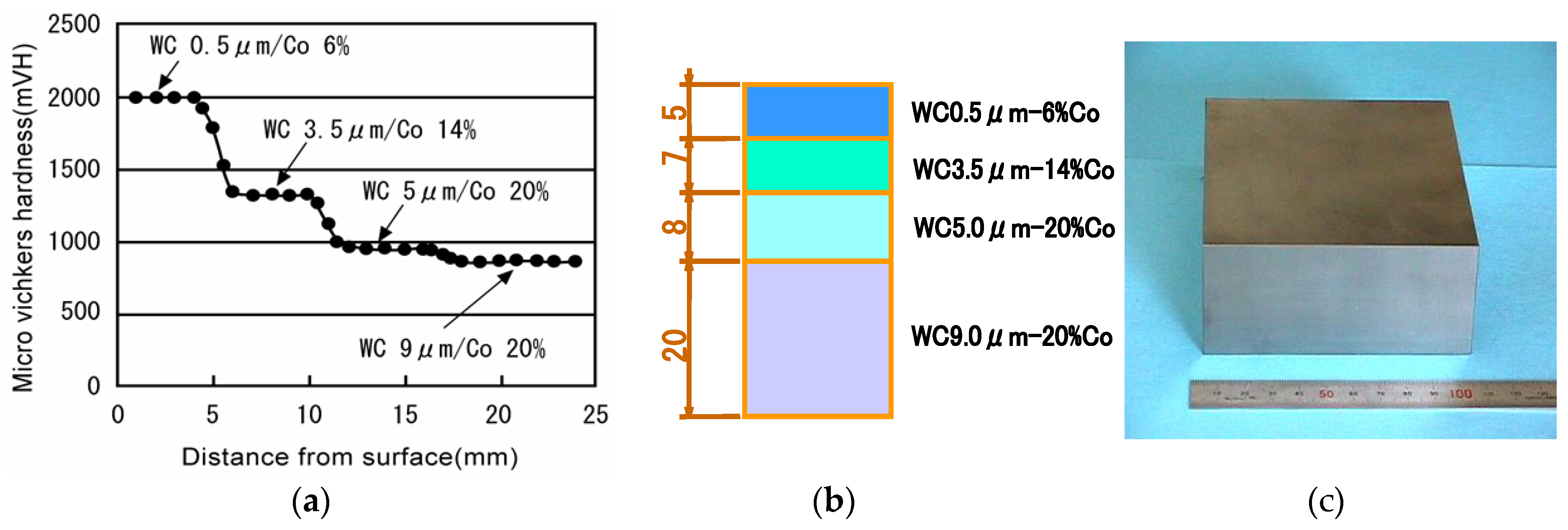
5.7. Fine WC/Co Hard Alloy FGMs for Die & Mold and Wear Resistant Materials Industries
5.8. Examples of WC/Co and WC/Co/Ni FGMs for Industrial Applications
5.9. Other Industrial Applications by SPS and Recent Demands from Industries
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tokita, M. Development of Advanced Spark Plasma Sintering (SPS) Systems and Its Industrial Applications. Ceram. Trans. Am. Ceram. Soc. 2006, 194, 51–60. [Google Scholar]
- Munir, Z.A.; Anselmi-Tamburini, U.; Ohyanagi, M. The effect of electric field and pressure on the synthesis and consolidation of materials: A review of the spark plasma sintering method. J. Mater. Sci. 2006, 41, 763–777. [Google Scholar] [CrossRef]
- Grasso, S.; Sakka, Y.; Maizza, G. Electric current activated/assisted sintering (ECAS): A review of patents 1906–2008. Sci. Technol. Adv. Mater. 2009, 10, 1–24. [Google Scholar] [CrossRef]
- Hungria, T.; Galy, J.; Castro, A. Spark Plasma Sintering as a Useful Technique to the Nanostructuration of Piezo-Ferroelectric Materials. Adv. Eng. Mater. 2009, 11, 615–631. [Google Scholar] [CrossRef]
- Inoue, K. Electric Discharge Sintering. U.S. Patent No. 3,241,956, 22 March 1966. Ser.No.247,387 filed 29 October 1963. [Google Scholar]
- Inoue, K. Apparatus for Electrically Sintering Discrete Bodies. U.S. Patent No. 3,250,892, 10 May 1966. filed 29 December 1962. [Google Scholar]
- Taylor, G.F. Apparatus for Making Hard Metal Compositions. U.S. Patent No.1,896,854, 7 February 1933. [Google Scholar]
- Cremer, G.D. Sintering Together Powders Metals such as Bronze, Brass or Aluminum. U.S. Patent No. 2,355,954, 15 August 1944. [Google Scholar]
- Tokita, M. Trends in Advanced SPS Spark Plasma Sintering Systems and Technology. J. Soc. Powder Technol. Jpn. 1993, 30, 790–804. [Google Scholar] [CrossRef]
- Tokita, M. Mechanism of Spark Plasma Sintering (SPS). In Proceedings of the PM2000 Powder Metallurgy World Congress, Kyoto, Japan, 12–16 November 2001; Part-I. pp. 252–255. [Google Scholar]
- Tokita, M. Development of Automatic FGM Manufacturing Systems by the Spark Plasma Sintering (SPS) Method. Ceram. Trans. Am. Ceram. Soc. 2001, 114, 283–290. [Google Scholar]
- Tokita, M. Method and System for Automatic Electrical Sintering. U.S. Patent No. 6,383,446 B1, 7 May 2002. [Google Scholar]
- Miyasaka, Y.; Tokita, M.; Karasawa, H.; Nishiyama, F. Electric Joining Methods and Apparatus and a Joined Unit of Members. U.S. Patent No. 6,515,250, 4 February 2003. [Google Scholar]
- Kawahara, M.; Tokita, M. New Application Fields of Spark Plasma Sintering (SPS) Technology Application examples of SPS for Surface Modification. In Corrosion Engineering; Allerton Press: Tokyo, Japan, 2001; Volume 50, pp. 63–71. [Google Scholar]
- Prawara, B.; Yara, H.; Miyagi, Y.; Fukushima, T. Spark plasma sintering as a post-spray treatment for thermally-sprayed coatings. Surf. Coat. Technol. 2003, 162, 234–241. [Google Scholar] [CrossRef]
- Khor, K.A.; Chen, X.J.; Chan, S.H.; Yu, L.G. Microstructure-property modifications in plasma sprayed 20 wt.% yttria stabilized zirconia electrolyte by spark plasma sintering (SPS) technique. Mater. Sci. Eng. 2004, A366, 120–126. [Google Scholar] [CrossRef]
- Makino, Y. Crystallographic behaviors of nano-powder anatase consolidated by SPS method. Pulse Electr. Curr. Synth. Process. Mater. 2006, 301–312. [Google Scholar] [CrossRef]
- Yu, M.; Grasso, S.; Mckinnon, R.; Saunder, T.; Reece, M. Review of flash sintering: Materials, mechanisms, and modelling. Adv. Appl. Ceram. 2016, 116, 24–26. [Google Scholar] [CrossRef]
- Olevsky, E.A.; Rolfing, S.M.; Maximenko, A.L. Flash (Ultra-Rapid) Spark-Plasma Sintering of Silicon Carbide. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Du, B.; Gucci, F.; Porwal, H.; Grasso, S.; Mahajanb, A.; Reece, M.J. Flash spark plasma sintering of magnesium silicide stannide with improved thermoelectric properties. J. Mater. Chem. C 2017, 5, 1514–1521. [Google Scholar] [CrossRef]
- Matsugi, K.; Hatayama, T.; Yanagisawa, O. Effect of Direct Current Pulse Discharge on Specific Resistivity of Copper and Iron Powder Compacts. J. Jpn. Inst. Met. 1995, 59, 740–745. [Google Scholar] [CrossRef][Green Version]
- Ozaki, K.; Kobayashi, K.; Nishio, T.; Matsumoto, A.; Sugiyama, A. Sintering Phenomena on Initial Stage in Pulsed Current Sintering. J. Jpn. Soc. Powder Powder Metall. 2000, 47, 293–297. [Google Scholar] [CrossRef]
- Omori, M. Sintering consolidation, reaction and crystal growth by the spark plasma system (SPS). Mater. Sci. Eng. 2000, A 287, 183–188. [Google Scholar] [CrossRef]
- Shen, Z.; Johnsson, M.; Zhao, Z.; Nygren, M. Spark Plasma Sintering of Alumina. J. Am. Ceram. Soc. 2002, 85, 1921–1927. [Google Scholar] [CrossRef]
- Schmidt, J.; Niewa, R.; Schmidt, M.; Grin, Y. Spark Plasma Sintering Effect on the Decomposition of MgH2. J. Am. Ceram. Soc. 2005, 88, 1870–1874. [Google Scholar] [CrossRef]
- Song, X.; Liu, X.; Zhang, J. Neck Formation and Self-Adjusting Mechanism of Neck Growth of Conducting Powders in Spark Plasma Sintering. J. Am. Ceram. Soc. 2006, 89, 494–500. [Google Scholar] [CrossRef]
- Chaim, R. Densification mechanisms in spark plasma sintering of nanocrystalline ceramics. Mater. Sci. Eng. 2007, A443, 25–32. [Google Scholar] [CrossRef]
- Misawa, T.; Shikatani, N.; Kawakami, Y.; Enjoji, T.; Ohtsu, Y. Influence of Internal Pulsed Current on the Sintering Behavior of Pulsed Current Sintering Process. Mater. Sci. Forum. 2010, 638, 2109–2114. [Google Scholar] [CrossRef]
- Hulbert, D.M.; Anders, A.; Dudina, D.V.; Andersson, J.; Jiang, D.; Unuvar, C.; Anselmi-Tamburini, U.; Lavernia, E.J.; Mukherjee, A.K. The absence of plasma in “spark plasma sintering”. J. Appl. Phys. 2008, 104, 033305. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Liu, Z.F.; Lu, J.F.; Shen, X.B.; Wang, F.C.; Wang, Y.D. The sintering mechanism in spark plasma sintering–Proof of the occurrence of spark discharge. Scr. Mater. 2014, 81, 56–59. [Google Scholar] [CrossRef]
- Groza, J.R.; Zavaliangos, A. Sintering activation by external electrical field. Mater. Sci. Eng. 2000, A287, 171–177. [Google Scholar] [CrossRef]
- Makino, Y.; Mori, T.; Eguchi, H.; Yoshioka, T.; Saito, H.; Kyoh, B.; Miyake, S. Dense nano-structured and preferentially-oriented anatase synthesized by pulsed high current heating. J. Jpn. Soc. Powder Powder Met. 2005, 52, 805–811. [Google Scholar] [CrossRef]
- Nanko, M.; Maruyama, T.; Tomino, H. Neck Growth on Initial Stage of Pulse Current Pressure Sintering for Coarse Atomized Powder Made of Cast-Iron. J. Jpn. Inst. Metals. 1999, 63, 917–923. [Google Scholar] [CrossRef][Green Version]
- Kumeta, K.; Nakamura, Y.; Takada, A.; Ishizaki, K. Surface Observation of Pulsed Electric Sintered Alumina Balls. J. Jpn. Cerem. Soc. 1999, 107, 187–189. [Google Scholar] [CrossRef]
- Kim, H.T.; Kawahara, M.; Tokita, M. Specimen Temperature and Sinterability of Ni Powder by Spark Plasma Sintering. J. Jpn. Soc. Powder Powder Metall. 2001, 47, 887–891. [Google Scholar] [CrossRef]
- Hirai, T.; Tokita, M.; Pan, W.; Chen, L.D.; Omori, M. Porous Silicon Carbide Sintered Compacts and Fabrication Method. JP. Patent No. 4,398,027, 30 October 2009. [Google Scholar]
- Zavaliangos, A.; Zhang, J.; Krammer, M.; Groza, J.R. Temperature evolution during field activated sintering. Mater. Sci. Eng. A. 2004, 379, 218–228. [Google Scholar] [CrossRef]
- Matsugi, K.; Kuramoto, H.; Hatayama, T.; Yanagisawa, O. Temperature distribution at steady state under constant current discharge in spark sintering process of Ti and Al2O3 powders. J. Mater. Process. Technol. 2004, 146, 274–281. [Google Scholar] [CrossRef]
- Tiwari, D.; Basu, B.; Biswas, K. Simulation of thermal and electric field evolution during spark plasma sintering. Ceram. Int. 2009, 35, 699–708. [Google Scholar] [CrossRef]
- Dang, K.Q.; Kawahara, M.; Takei, S.; Nanko, M. Effect of Pulsed Current Waveform on Sample Temperature and Sintering Behavior in PECS of Alumina. J. Jpn. Soc. Powder Powder Metall. 2009, 56, 780–789. [Google Scholar] [CrossRef]
- Tokita, M. Apparatus for Multiple Head-Type Spark Plasma Sintering. JP. Patent No.3,618,630, 19 November 2004. [Google Scholar]
- Tokita, M.; Nakagawa, K. Method and System for Rotary Table-type Pulsed Electric Current Sintering. JP. Patent No. 3,645,811, 2007. [Google Scholar]
- Tokita, M.; Nakagawa, K.; Suzuki, S. Method and Apparatus for Shuttle-type Spark Plasma Sintering System. JP. Patent No. 3,822,174, 30 June 2006. [Google Scholar]
- Tokita, M.; Suzuki, S.; Nakagawa, K. Nano-Precision Sintering System. U.S. Patent No. 7,547,204, 16 June 2009. [Google Scholar]
- Tokita, M. Method and Apparatus for Automatically Loading Powder into a Mold. U.S. Patent No. 6,881,048 B1, 19 April 2005. [Google Scholar]
- Nygren, M.; Shen, Z. On the preparation of bio-, nano- and structural ceramics and composites by spark plasma sintering. Solid State Sci. 2003, 5, 125–131. [Google Scholar] [CrossRef]
- Koyanagi, T. Preparation of thermoelectric Material by Spark Plasma Sintering. In Proceedings of the Third Symposium on Spark Plasma Sintering, Kure, Japan, 26–27 November 1998; pp. 54–55. [Google Scholar]
- Tokita, M.; Tamari, N.; Takeuchi, T.; Makino, Y. Consolidation behavior and mechanical properties of SiC with Al2O3 and Yb2O3 consolidated by SPS. J. Jpn. Soc. Powder Powder Metall. 2009, 6, 788–795. [Google Scholar] [CrossRef][Green Version]
- Tamari, N.; Tanaka, T.; Tanaka, K.; Kondo, I.; Kawahara, M.; Tokita, M. Effect of Spark Plasma Sintering on Densification and Mechanical Properties of Silicon Carbide. J. Ceram. Soc. Jpn. 1995, 103, 740–742. [Google Scholar] [CrossRef]
- Gao, L.; Hong, J.S.; Miyamoto, H.; Torre, S. Bending strength and microstructure of Al2O3 ceramics densified by spark plasma sintering. J. Euro. Ceram. Soc. 2000, 20, 2149–2159. [Google Scholar] [CrossRef]
- Wang, S.W.; Chen, L.D.; Hirai, T.; Kang, Y.S. Microstructure inhomogeneity in Al2O3 sintered bodies formed during the plasma-activated sintering process. J. Mater. Sci. Letts. 1999, 18, 1119–1121. [Google Scholar] [CrossRef]
- Zhou, Y.; Hirao, K.; Yamauchi, Y.; Kanzaki, S. Densification and grain growth in pulse electric current sintering of alumina. J. Euro. Ceram. Soc. 2004, 24, 3465–3470. [Google Scholar] [CrossRef]
- Stanciu, L.A.; Kodash, V.Y.; Groza, J.R. Grain Growth during Field Assisted Sintering of α-Al2O3 and MoSi2 Powders. Metall. Trans. 2001, 32A, 2633–2638. [Google Scholar] [CrossRef]
- Kim, H.T.; Kawahara, M.; Tokita, M. Fabrication of Nano-Materials by the Spark Plasma Sintering (SPS) Method. In Proceedings of the PM2000 Powder Metallurgy World Congress, Kyoto, Japan, 12–16 November 2001; pp. 741–744. [Google Scholar]
- Makino, Y.; Sakaguchi, M.; Terada, J.; Akamatsu, K. Consolidation of ultrafine alumina powders with SPS method. J. Jpn. Soc. Powder Powder Metall. 2007, 54, 219–225. [Google Scholar] [CrossRef][Green Version]
- Takeuchi, T.; Tabuchi, M.; Suyama, Y.; Kageyama, H. Preparation of Dense BaTiO3 Ceramics with Submicrometer Grains by Spark Plasma Sintering. J. Am. Ceram. Soc. 1999, 82, 939–943. [Google Scholar] [CrossRef]
- Anselmi-Tamburini, U.; Garay, J.E.; Munir, Z.A. Fast low-temperature consolidation of bulk nanometric ceramic materials. Scr. Mater. 2006, 54, 823–828. [Google Scholar] [CrossRef]
- Ohyanagi, M.; Yamamoto, T.; Kitaura, H.; Kodera, Y.; Ishii, T.; Munir, Z.A. Consolidation of nanostructured SiC with disorder-order transformation. Scr. Mater. 2004, 50, 111–114. [Google Scholar] [CrossRef]
- Tokita, M. Development of Large-Size Ceramic/Metal Bulk FGM Fabricated by Spark Plasma Sintering. Mater. Sci. Forum 1999, 308, 83–88. [Google Scholar] [CrossRef]
- Tokita, M.; Kawahara, M.; Sonoda, M.; Omori, M.; Okubo, A.; Hirai, T. Preparation and Triborogical Characterization of ZrO2(3Y)+20wt%Al2O3/SUS410L Stainless Steel Composite Functionally Graded Material Fabricated by Spark Plasma Sintering Method. J. Jpn. Soc. Powder Powder Metall. 1999, 46, 269–276. [Google Scholar] [CrossRef][Green Version]
- Watari, F.; Kondo, H.; Matsuo, S.; Miyao, R.; Yokoyama, A.; Omori, M.; Hirai, T.; Tamura, Y.; Uo, M.; Ohara, N.; et al. Development of Functionally Graded Implant and Dental Post for Bio-Medical Application. Mater. Sci. Forum. 2003, 423, 321–326. [Google Scholar] [CrossRef]
- Kondo, H.; Yokoyama, A.; Omori, M.; Ohkubo, A.; Hirai, T.; Watari, F.; Uo, M.; Kawasaki, T. Fabrication of Titanium Nitride/Apatite Functionally Graded Implants by Spark Plasma Sintering. Mater. Trans. 2004, 45, 3156–3162. [Google Scholar] [CrossRef]
- Casari, F.; Zadra, M.; Girardini, L.; Molinari, A. Design of Layered Metal-Ceramic FGMs Produced by Spark Plasma Sintering. In Proceedings of the Multiscale and Functionally Graded Materials, M&FGM2006, Oahu, HI, USA, 15–18 October 2007; Volume 973, pp. 832–837. [Google Scholar]
- Belmonte, M.; Gonzales-Julian, J.; Miranzo, P.; Osendi, M.I. Continuous in situ functionally graded silicon nitride materials. Acta Mater. 2009, 57, 2607–2612. [Google Scholar] [CrossRef]
- Omori, M.; Okubo, A.; Gilhwan, K.; Hirai, T. Consolidation of Thermosetting Polyimide by the Spark Plasma System. J. Mater. Synth. Process. 1997, 5, 279–282. [Google Scholar]
- Tokita, M. The potential of Spark Plasma Sintering (SPS) method for the fabrication on an industrial scale of Functionally Graded Materials. Adv. Sci. Technol. 2010, 63, 322–331. [Google Scholar] [CrossRef]
- Tokita, M.; Mitsui, Y.; Yoshida, H. Development of ZrO2/Ti alloy FGMs Horn tip Tool for Ultra-sonic Homogenizer by Spark Plasma Sintering (SPS) method. In Proceedings of the ISFGMs2018 15th International Symposium on Functionally Graded Materials, Kitakyushu, Japan, 5–8 August 2018; O-17. pp. 1–6. [Google Scholar]
- Tokita, M. Development of Square-Shaped Large-size WC/Co/Ni system FGM Fabricated by Spark Plasma Sintering (SPS) Method and Its Industrial Applications. Mater. Sci. Forum. 2005, 492, 711–718. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Shi, L.; Jiang, W.; Chen, L.D. Rapid fabrication of Ti3SiC2-SiC nanocomposite using the spark plasma sintering-reactive synthesis (SPS-RS) method. Scr. Mater. 2007, 56, 241–244. [Google Scholar] [CrossRef]
- Muroi, M.; Trotter, G.; McCormick, P.G.; Kawahara, M.; Tokita, M. Preparation of nano-grained zirconia ceramics by low-temperature, low-pressure spark plasma sintering. J. Mater. Sci. 2008, 43, 6376–6384. [Google Scholar] [CrossRef]
- Li, S.F.; Izui, H.; Okano, M.; Zhang, W.H.; Watanabe, T. Mechanical Properties of ZrO2 (Y2O3)-Al2O3 Nanocomposites with Addition of Hydroxyapatite Prepared by Spark Plasma Sintering. Mater. Sci. Forum. 2010, 631, 413–423. [Google Scholar]
- Abderrazak, H.; Schoenstei, F.; Abdellaoui, M.; Jouini, N. Spark plasma sintering consolidation of nanostructured TiC prepared by mechanical alloying. Int. J. Refract. Metals Hard Mater. 2011, 29, 170–176. [Google Scholar] [CrossRef]
- Wu, J.; Chen, F.; Shen, Q.; Schoenung, J.M.; Zhang, L. Spark Plasma Sintering and Densification Mechanisms of Antimony-Doped Tin Oxide Nanoceramics. J. Nanomater. 2013, 1. [Google Scholar] [CrossRef]
- Manière, C.; Durand, L.; Weibel, A.; Estournes, C. Spark-plasma-sintering and finite element method: From the identification of the sintering parameters of a submicronic α-alumina powder to the development of complex shapes. Acta Mater. 2016, 102, 169–175. [Google Scholar] [CrossRef]
- Koide, M.; Takei, S.; Sato, T.; Matusita, K. Preparation of Silica Glass by Pulsed Electric Current method. J. Ceram. Soc. Jpn. 2002, 110, 867–869. [Google Scholar] [CrossRef]
- Grasso, S.; Kim, B.N.; Hu, C.; Maizza, G.; Sakka, Y. Highly Transparent Pure Alumina Fabricated by High-Pressure Spark Plasma Sintering. J. Am. Ceram. Soc. 2010, 93, 2460–2462. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, B.N.; Morita, K.; Yoshida, H.; Hiraga, K.; Sakka, Y. Fabrication of Transparent Yttria by High-Pressure Spark Plasma Sintering. J. Am. Ceram. Soc. 2011, 94, 3206–3210. [Google Scholar] [CrossRef]
- Nanko, M.; Dang, K.Q. Two-step pulsed electric current sintering of transparent Al2O3 ceramics. J. Adv. Appl. Ceram. 2014, 113, 80–84. [Google Scholar] [CrossRef]
- Mizuuchi, K.; Inoue, K.; Agari, Y.; Yamada, S.; Tanaka, M.; Sugioka, M.; Takeuchi, T.; Tani, J.; Kawahara, M.; Lee, J.H.; et al. Thermal Properties of Diamond-Particle-Dispersed Cu-Matrix-Composites Fabricated by Spark Plasma Sintering (SPS). Mater. Sci. Forum. 2010, 638, 2115–2212. [Google Scholar] [CrossRef]
- Mizuuchi, K.; Inoue, K.; Agari, Y. Trend of the development of metal-based heat dissipative materials. Microelectron. Reliab. 2017, 79, 5–19. [Google Scholar] [CrossRef]
- Izui, H.; Kinbara, S.; Okano, M. Mechanical Properties of Ti-15–3 Alloy Reinforced with SiC Fibers by Spark Plasma Sintering. Pulse Electr. Curr. Synth. Process. Mater. 2006, 289–300. [Google Scholar] [CrossRef]
- Sato, H.; Umaoka, S.; Watanabe, Y.; Kim, I.S.; Kawahara, M.; Tokita, M. Biodegradable Fiber Reinforced Ti Composite Fabricated by Spark Plasma Sintering Method. Mater. Sci. Forum. 2007, 539, 3201–3206. [Google Scholar] [CrossRef]
- Umino, K.; Wakayama, S.; Takenobu Sakai, T.; Umehara, Y.; Akatsu, T. Mechanical Properties of CNF Reinforced Ceramic Composites Sintered with SPS Technique. J. Solid Mech. Mater. Eng. 2011, 5, 866–872. [Google Scholar] [CrossRef][Green Version]
- Thomson, K.E.; Jiang, D.; Yao, W.; Ritchie, R.O.; Mukherjee, A.K. Characterization and mechanical testing of alumina-based nanocomposites reinforced with niobium and/or carbon nanotubes fabricated by spark plasma sintering. Acta Mater. 2012, 60, 622–632. [Google Scholar] [CrossRef]
- Izui, H.; Komaki, S.; Okano, M. Mechanical Properties of TiB/Ti Composites by Spark Plasma Sintering. J. Solid Mech. Mater. Eng. 2008, 2, 234–238. [Google Scholar] [CrossRef][Green Version]
- Licheri, R.; Orrù, R.; Musa, C.; Locci, A.M.; Cao, G. Consolidation via spark plasma sintering of HfB2/SiC and HfB2/HfC/SiC composite powders obtained by self-propagating high-temperature synthesis. J. Alloys Compd. 2009, 478, 572–578. [Google Scholar] [CrossRef]
- Taslicukur, Z.; Sahin, F.C.; Goller, G.; Yucel, O.; Kuskonmaz, N. Reactive Spark Plasma Sintering of Si3N4 Based Composites. Adv. Sci. Technol. 2010, 62, 185–190. [Google Scholar] [CrossRef]
- Elissalde, C.; Chung, U.C.; Artemenko, A.; Estournes, C.; Costes, R.; Paté, M.; Ganne, J.P.; Waechter, S.; Maglione, M. Stoichiometry and Grain Boundaries Control by Spark Plasma Sintering in Ba0.6Sr0.4TiO3: Mn/MgO Composites. J. Am. Ceram. Soc. 2012, 95, 3239–3245. [Google Scholar] [CrossRef]
- Zhou, M.; Rodrigo, D.; Cheng, Y.B. Effects of the electric current on conductive Si3N4/TiN composites in spark plasma sintering. J. Alloys Compd. 2013, 547, 51–58. [Google Scholar] [CrossRef]
- Zou, J.; Liu, J.; Zhao, J.; Zhang, G.J.; Huang, S.; Qian, B.; Vleugels, J.; Van der Biest, O.; Shen, J.Z. A top-down approach to densify ZrB2–SiC–BN composites with deeper homogeneity and improved reliability. Chem. Eng. J. 2014, 249, 93–101. [Google Scholar] [CrossRef]
- Rehman, S.S.; Ji, W.; Fu, Z.; Wang, W.; Wang, H.; Asif, M.; Zhang, J. In situ synthesis and sintering of B4C/ZrB2 composites from B4C and ZrH2 mixtures by spark plasma sintering. J. Eur. Ceram. Soc. 2015, 35, 1139–1145. [Google Scholar] [CrossRef]
- Bai, Y.; Bai, Y.; Gao, J.; Ma, W.; Su, J.; Jia, R. Preparation and characterization of reduced grapheme oxide/fluorhydroxyapatite composites for medical implants. J. Alloys Compd. 2016, 688, 657–667. [Google Scholar] [CrossRef]
- Li, Y.; Katsui, H.; Goto, T. Phase decomposition of (Ti, Zr)(C, N) solid solutions prepared by spark plasma sintering. J. Euro. Ceram. Soc. 2019, 39, 4588–4594. [Google Scholar] [CrossRef]
- Zhang, J.X.; Lu, Q.M.; Liu, K.G.; Zhang, L.; Zhou, M.L. Synthesis and thermoelectric properties of CoSb3 compounds by spark plasma sintering. Mater. Lett. 2004, 58, 1981–1984. [Google Scholar] [CrossRef]
- Chen, L.D.; Huang, X.Y.; Zhou, M.; Shi, X.; Zhang, W.B. The high temperature thermoelectric performances of Zr0.5Hf0.5Ni0.8Pd0.2Sn0.99Sb0.01 alloy with nanophase inclusions. J. Appl. Phys. 2006, 99. [Google Scholar] [CrossRef]
- Ito, M.; Ohira, N. Transport properties of thermoelectric SrTiO3 synthesized by polymerized complex method and spark plasma sintering. J. Phys. Conf. Ser. 2010, 232. [Google Scholar] [CrossRef]
- Maki, R.S.S.; Mitani, S.; Mori, T. Effect of spark plasma sintering (SPS) on the thermoelectric properties of magnesium ferrite. Mater Renew Sustain. Energy. 2017, 6. [Google Scholar] [CrossRef]
- Son, H.W.; Guo, Q.; Suzuki, Y.; Kim, B.N.; Mori, T. Thermoelectric properties of MgTi2O5 /TiN conductive composites prepared via reactive spark plasma sintering for high temperature functional applications. Scri. Mater. 2020, 178, 44–50. [Google Scholar] [CrossRef]
- Orlova, A.I.; Volgutov, V.Y.; Mikhailov, D.A.; Bykov, D.M.; Skuratov, V.A.; Chuvil’deev, V.N.; Nokhrin, A.V.; Boldin, M.S.; Sakharov, N.V. Phosphate Ca1/4Sr1/4Zr2(PO4)3 of the NaZr2(PO4)3 structure type: Synthesis of a dense ceramic material and its radiation testing. J. Nucl. Mater. 2014, 446, 232–239. [Google Scholar] [CrossRef]
- Yue, M.; Zhang, J.X.; Liu, W.Q.; Wang, G.P. Chemical stability and microstructure of Nd-Fe-B magnet prepared by spark plasma sintering. J. Magn. Magn. Mater. 2004, 271, 364–368. [Google Scholar] [CrossRef]
- Wang, T.; Yue, M.; Li, Y.; Tokita, M.; Wu, Q.; Zhang, D.; Zhang, J. Tuning of Microstructure and Magnetic Properties of Nanocrystalline Nd–Fe–B Permanent Magnets Prepared by Spark Plasma Sintering. IEEE Magn. Lett. 2015, 6. [Google Scholar] [CrossRef]
- Schmidt, J.; Schnell, W.; Grin, Y.; Kniep, R. Pulse plasma synthesis and chemical bonding in magnesium diboride. Solid State Sci. 2003, 5, 535–539. [Google Scholar] [CrossRef]
- Kakegawa, K.; Kawai, Y.; Wu, Y.; Uekawa, N.; Sasaki, Y. Sintering of Lead Titanate Using a Spark-Plasma-Sintering Technique. J. Am. Ceram. Soc. 2004, 87, 541–545. [Google Scholar] [CrossRef]
- Taminato, S.; Okumura, T.; Takeuchi, T.; Kobayashi, H. Fabrication and charge-discharge reaction of all solid-state lithium battery using Li4-2xGe1-xSxO4 electrolyte. Solid State Ionics. 2018, 326, 52–57. [Google Scholar] [CrossRef]
- Grasso, S.; Sakka, Y.; Maizza, G. Effects of Initial Punch-Die Clearance in Spark Plasma Sintering Process. Mater. Trans. 2008, 49, 2899–2906. [Google Scholar] [CrossRef]
- Maizza, G.; Grasso, S.; Sakka, Y. Moving finite-element mesh model for aiding spark plasma sintering in current control mode of pure ultrafine WC powder. Mater. Sci. 2009, 44, 1219–1236. [Google Scholar] [CrossRef]
- Makino, Y.; Mizuuchi, K.; Tokita, M.; Agari, Y.; Kawahara, M.; Inoue, K. Synthesis of new structural and functional materials by SPS processing. Mater. Sci. Forum. 2010, 638, 2091–2096. [Google Scholar] [CrossRef]
- Li, W.; Olevsky, E.A.; McKittrick, J.; Maximenko, A.L.; German, R.M. Densification mechanisms of spark plasma sintering: Multi-step pressure dilatometry. J. Mater Sci. 2012. [Google Scholar] [CrossRef]
- Epherre, R.; Lesseur, J.; Albino, M.; Veber, P.; Weibel, A.; Chevallier, G.; Maglione, M.; Bernard, D.; Elissalde, C.; Estournès, C. Adjustable dielectric properties of BaTiO3 containing MgO inclusions deformable under Spark Plasma Sintering. Scr. Mater. 2016, 110, 82–86. [Google Scholar] [CrossRef]
- Grasso, S.; Saunders, T.; Porwal, H.; Milsom, B.; Tudball, A.; Reece, M. Flash Spark Plasma Sintering (FSPS) of α and β SiC. J. Am. Ceram. Soc. 2016, 99, 1534–1543. [Google Scholar] [CrossRef]
- Maniere, C.; Torresani, E.; Olevsky, E.A. Simultaneous Spark Plasma Sintering of Multiple Complex Shapes. Materials 2019, 12, 557. [Google Scholar] [CrossRef]




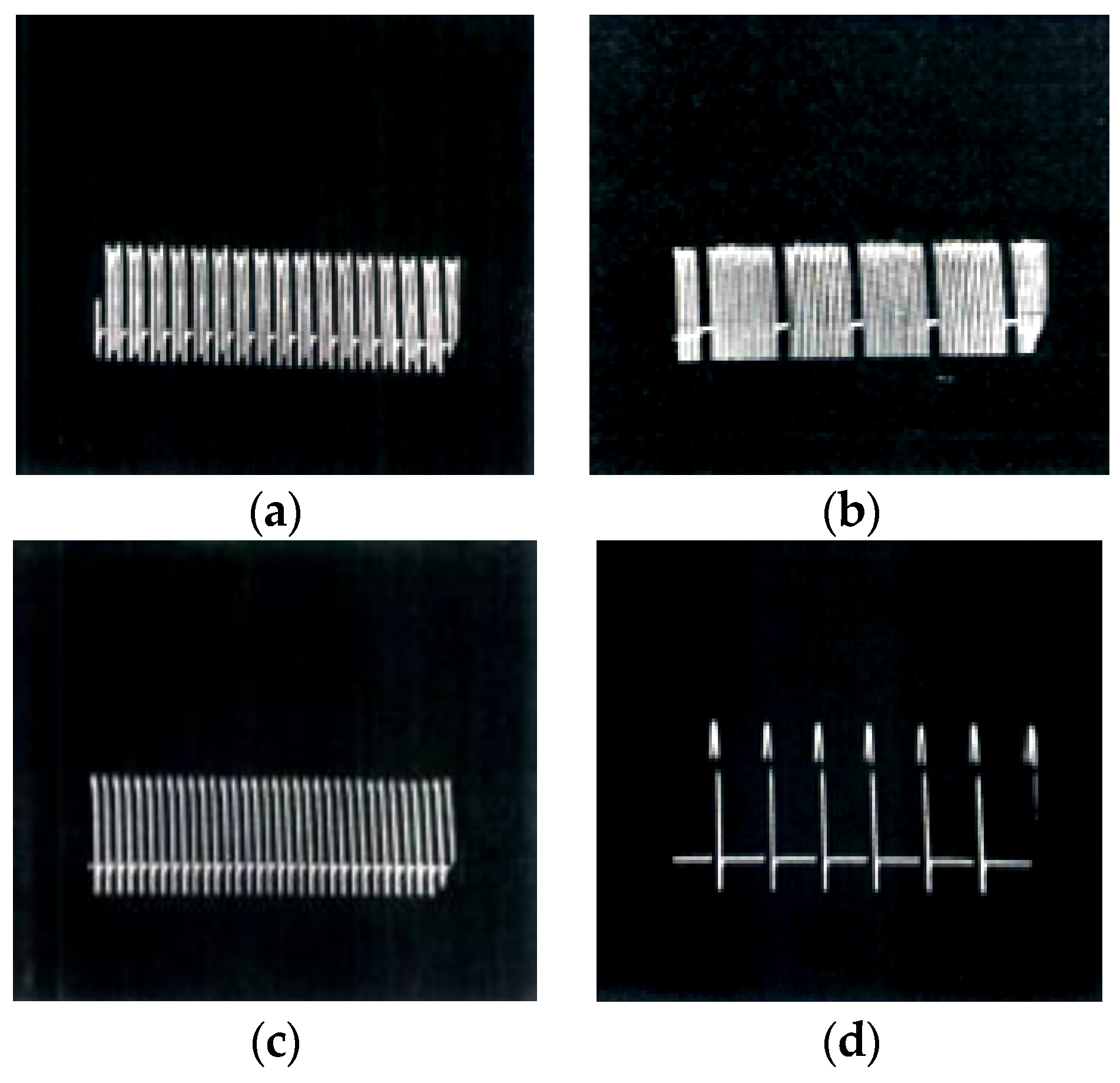


















| Classification | Materials for SPS Processing | |
|---|---|---|
| Metals | ||
| Ceramics | Oxides | Al2O3, Mulite, ZrO2, MgO, SiO2, TiO2, HfO2 |
| Carbides | SiC, B4C, TaC, TiC, WC, ZrC, VC | |
| Nitrides | Si3N4, TaN, TiN, AlN, ZrN, VN | |
| Borides | TiB2, HfB2, LaB6, ZrB2, VB2, MgB2 | |
| Fluorides | LiF, CaF2, MgF2 | |
| Cermets | Si3N4+Ni, Al2O3+Ni, ZrO2+Ni | |
| Al2O3+Ti, ZrO2+SUS, Al2O3+SUS | ||
| WC+Co, WC+Ni, TiC+TiN+Ni, BN+Fe, | ||
| Intermetalic compounds | TiAl, MoSi2, Si3Zr5, NiAl | |
| NbCo, Nb3Al, LaBaCuSO4, Sm2Co17 | ||
| Other materials | Organic materials (polymide, etc.), FRM, FRC, CNT composite materials | |
| SPS Sintering | HP Sintering | ||
|---|---|---|---|
| Temperature gradient sintering | ◎ | × | |
| Grain boundary controlled sintering | ◎ | × | |
| Fine crystalline structure controlled sintering | ◎ | × | |
| Temperature rise rate | ◎ | × | |
| Sintering time | Temperature rise time | Fast | Slow |
| Holding time | Short | Long | |
| Homogeneous sintering | ○ | ○ | |
| Expandability | ◎ | △ | |
| Productivity | ◎ | △ | |
| Investment in equipment | ○ | △ | |
| Running cost | ◎ | △ | |
| Al2O3 Starting Powder Materials | SPS Sintering Pressure (MPa) | SPS Sintering Temperature (K) | Sintering Holding Time (s) | Sintering Heating-Up Time (s) |
|---|---|---|---|---|
| γ-Al2O3 | 49 | 1173 | 0 | 60 |
| 1273 | 0 | 60 | ||
| 1373 | 0 | 60 | ||
| 1473 | 0 | 60 | ||
| 60 | 540 | |||
| 600 | 540 | |||
| 1573 | 0 | 60 | ||
| 60 | 540 | |||
| 600 | 540 | |||
| 1673 | 0 | 60 | ||
| 60 | 540 | |||
| 600 | 540 | |||
| 1773 | 0 | 60 | ||
| 60 | 540 | |||
| 600 | 540 | |||
| 690 | 873 | 180 | 600 | |
| 923 | 180 | 600 | ||
| 973 | 180 | 600 | ||
| 1073 | 180 | 600 | ||
| 1173 | 180 | 600 | ||
| α-Al2O3 (0.5 μm) (0.2 μm) | 49 | 1473 | 60 | 540 |
| 1573 | 60 | 540 | ||
| 1673 | 60 | 540 | ||
| 1773 | 60 | 540 |
| Product Code Name | Co Content wt% | WC pdr. Grain Size μm | Density g/cm3 | Hardness mHv | TransverseRupture Strength MPa | Fracture Toughness K1C |
|---|---|---|---|---|---|---|
| TC-05 | <2 | <0.5 | 15.2 | 2350 | 2300 | 6.2 |
| TC-10 | <4 | <0.5 | 15.0 | 2150 | 2640 | 6.5 |
| TC-20 | <6 | <0.5 | 14.8 | 2050 | 2940 | 7.3 |
| M78 | 0 | <0.2 | 15.4 | 2600 | 1500 | 5.1 |
| WC100 | 0 | <0.08 | 15.6 | 2700 | 1470 | 5.6 |
| NC100 | 0 | <0.5 | 15.4 | 2570 | 1180 | 5.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokita, M. Progress of Spark Plasma Sintering (SPS) Method, Systems, Ceramics Applications and Industrialization. Ceramics 2021, 4, 160-198. https://doi.org/10.3390/ceramics4020014
Tokita M. Progress of Spark Plasma Sintering (SPS) Method, Systems, Ceramics Applications and Industrialization. Ceramics. 2021; 4(2):160-198. https://doi.org/10.3390/ceramics4020014
Chicago/Turabian StyleTokita, Masao. 2021. "Progress of Spark Plasma Sintering (SPS) Method, Systems, Ceramics Applications and Industrialization" Ceramics 4, no. 2: 160-198. https://doi.org/10.3390/ceramics4020014
APA StyleTokita, M. (2021). Progress of Spark Plasma Sintering (SPS) Method, Systems, Ceramics Applications and Industrialization. Ceramics, 4(2), 160-198. https://doi.org/10.3390/ceramics4020014