Osteogenic Enhancement of Zirconia-Toughened Alumina with Silicon Nitride and Bioglass®
Abstract
1. Introduction
2. Experimental Procedures
2.1. Surface Functionalization Procedures
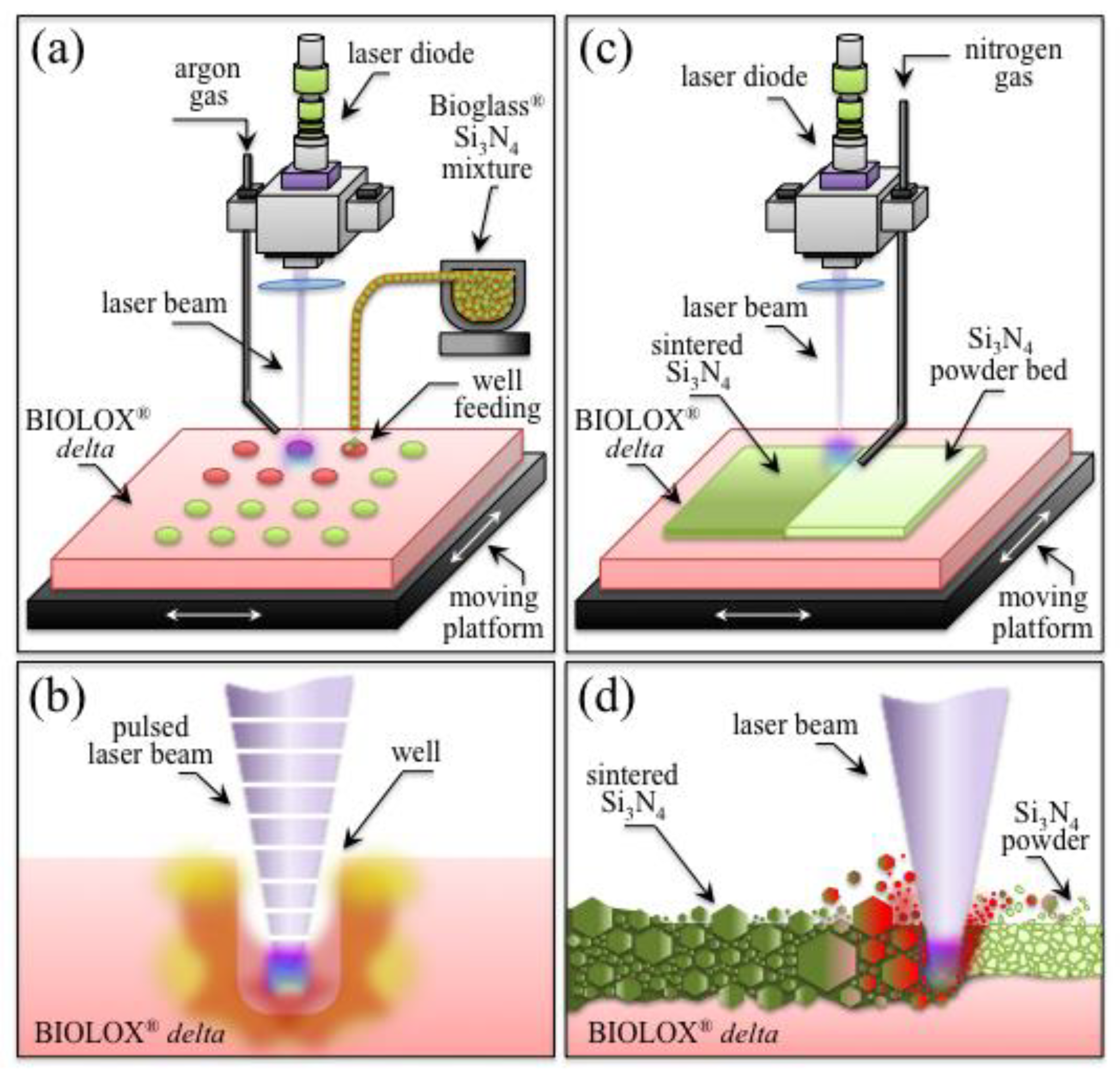
2.1.1. Laser-Patterning Procedure
2.1.2. Laser-Sintering Procedure
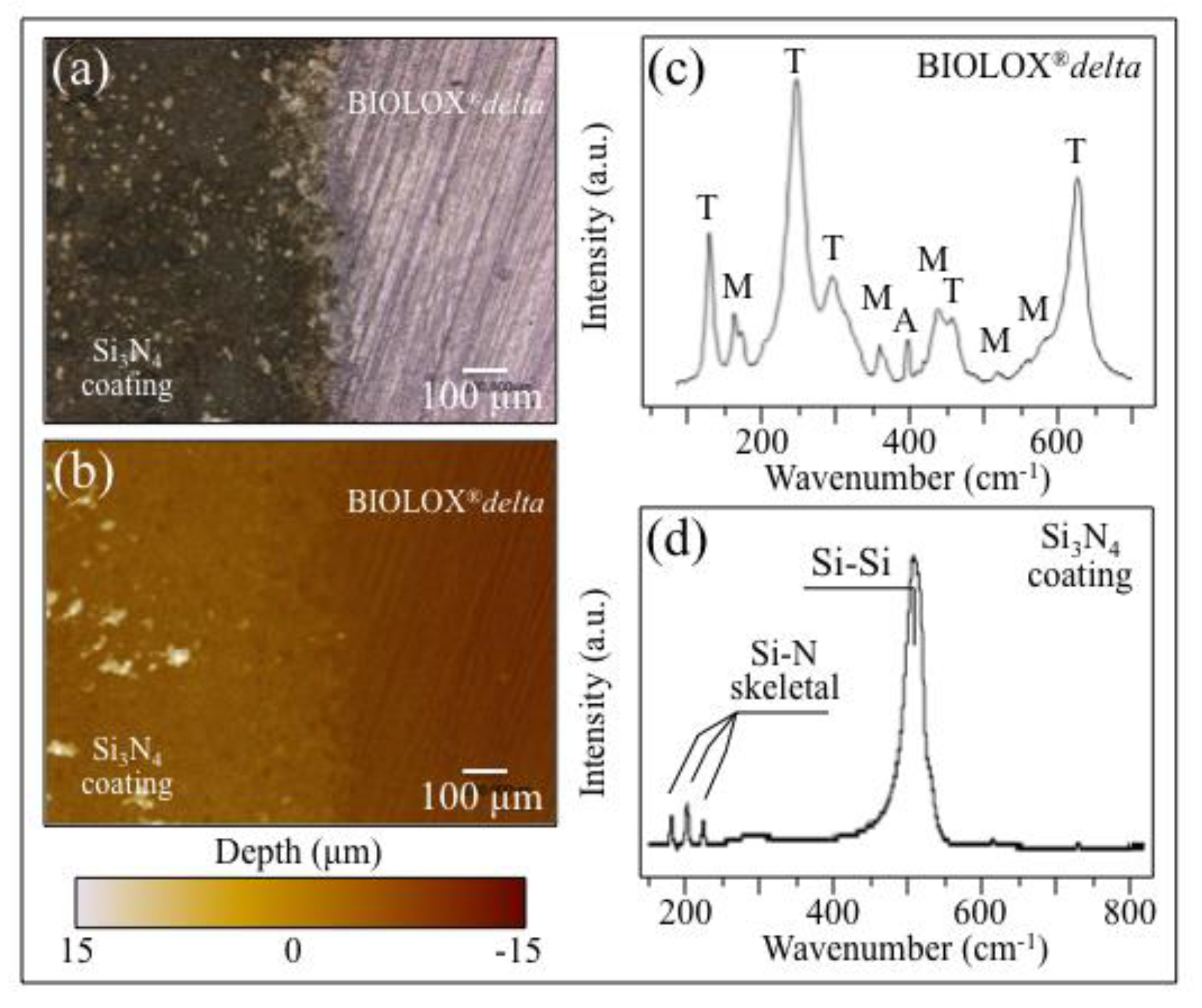
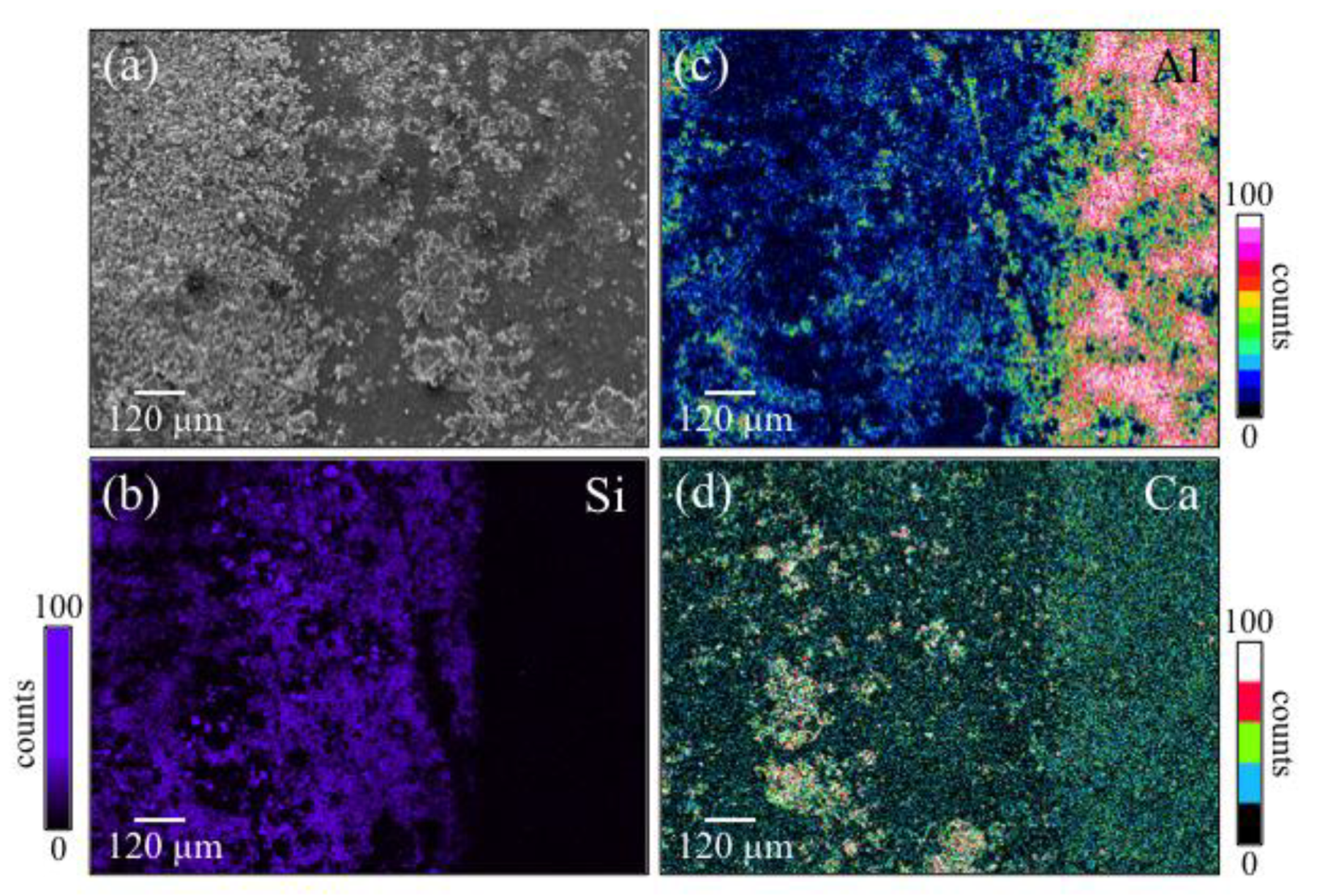
2.2. Surface Characterization
2.3. Cell Culture and Biological Tests
2.4. Statistical Analyses
3. Experimental Results
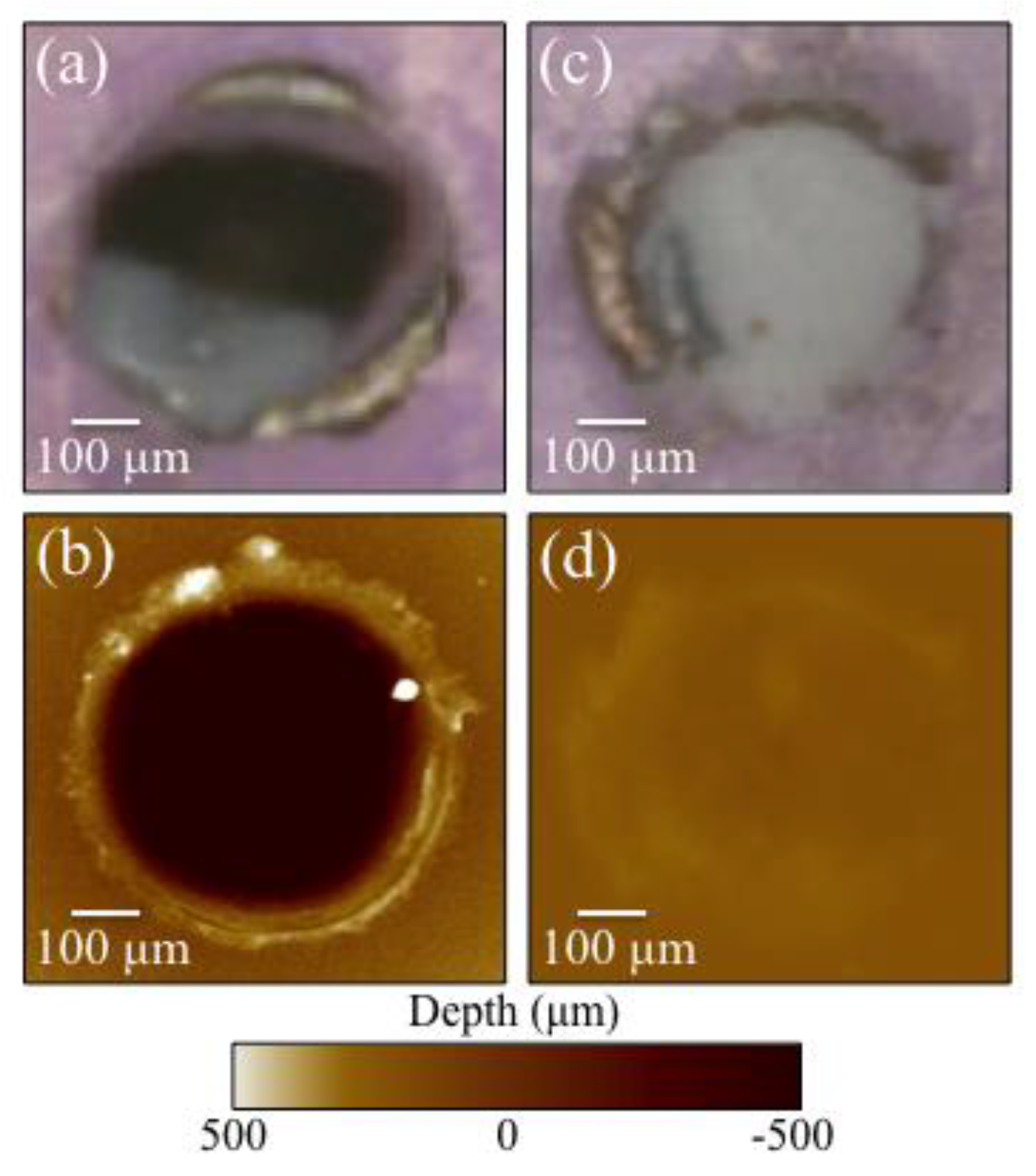
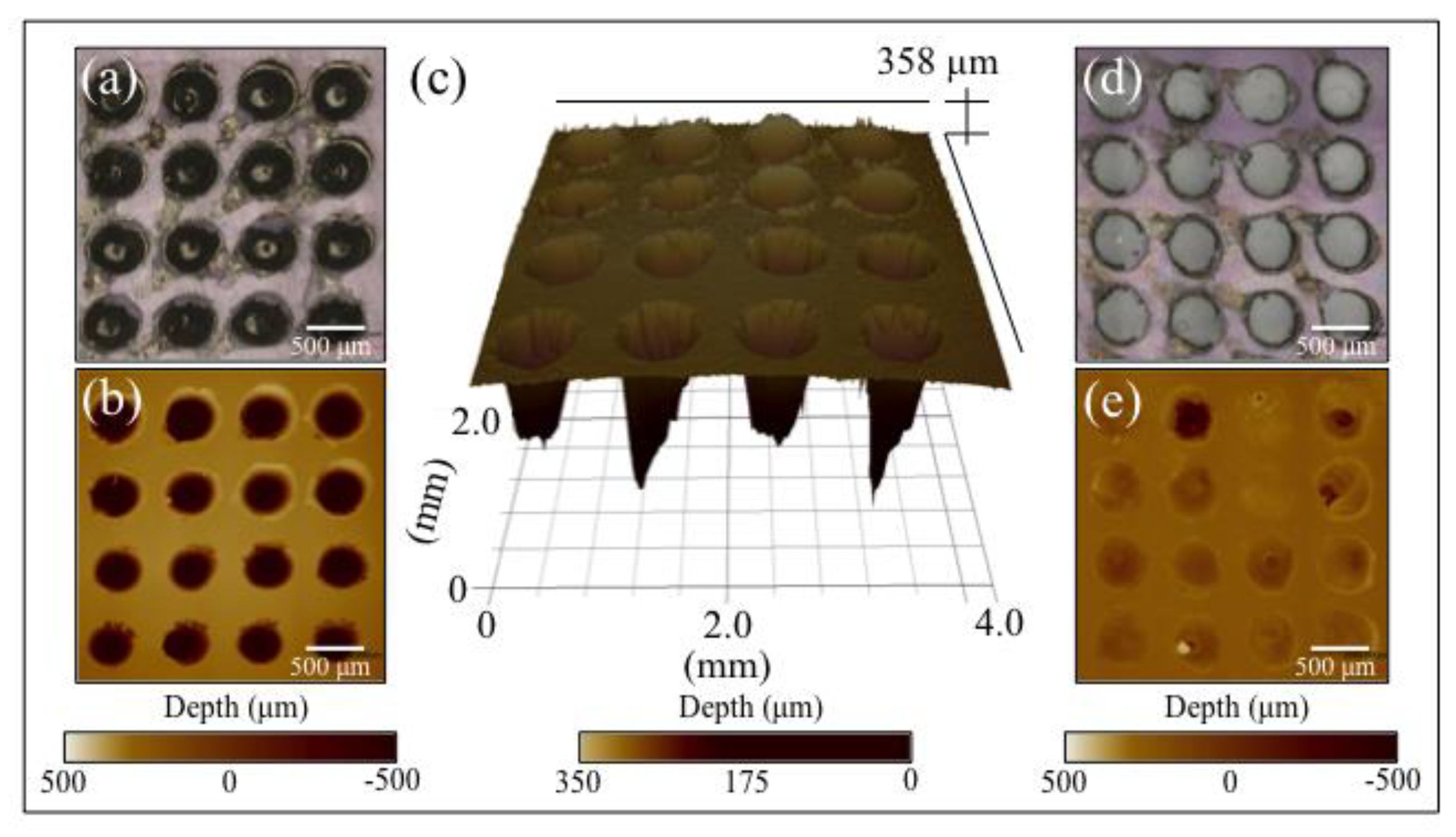
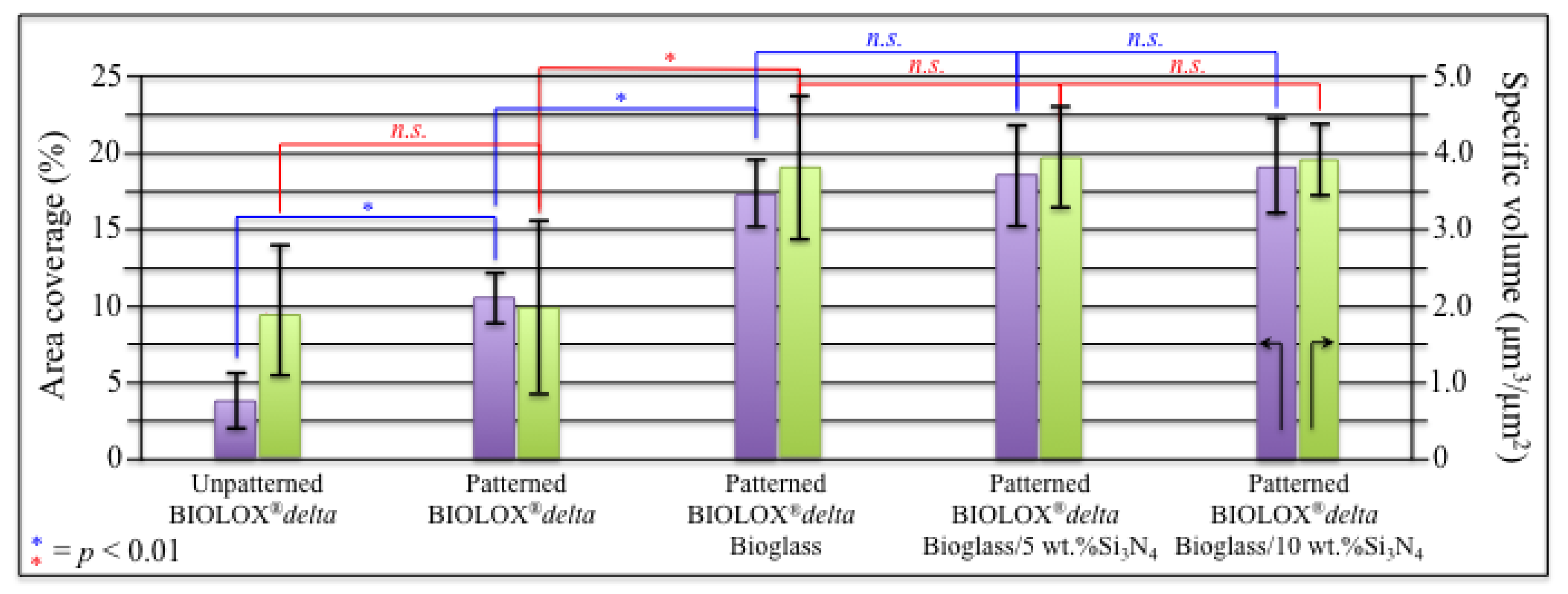
3.1. Laser Patterned and Bioglass®/Si3N4-Filled ZTA Surfaces
3.2. Laser-Sintering of Si3N4-Coated ZTA Surfaces
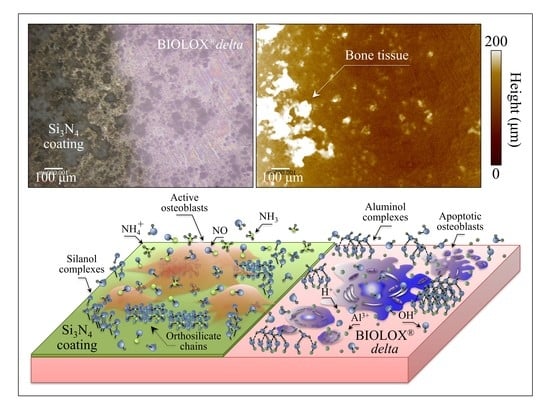
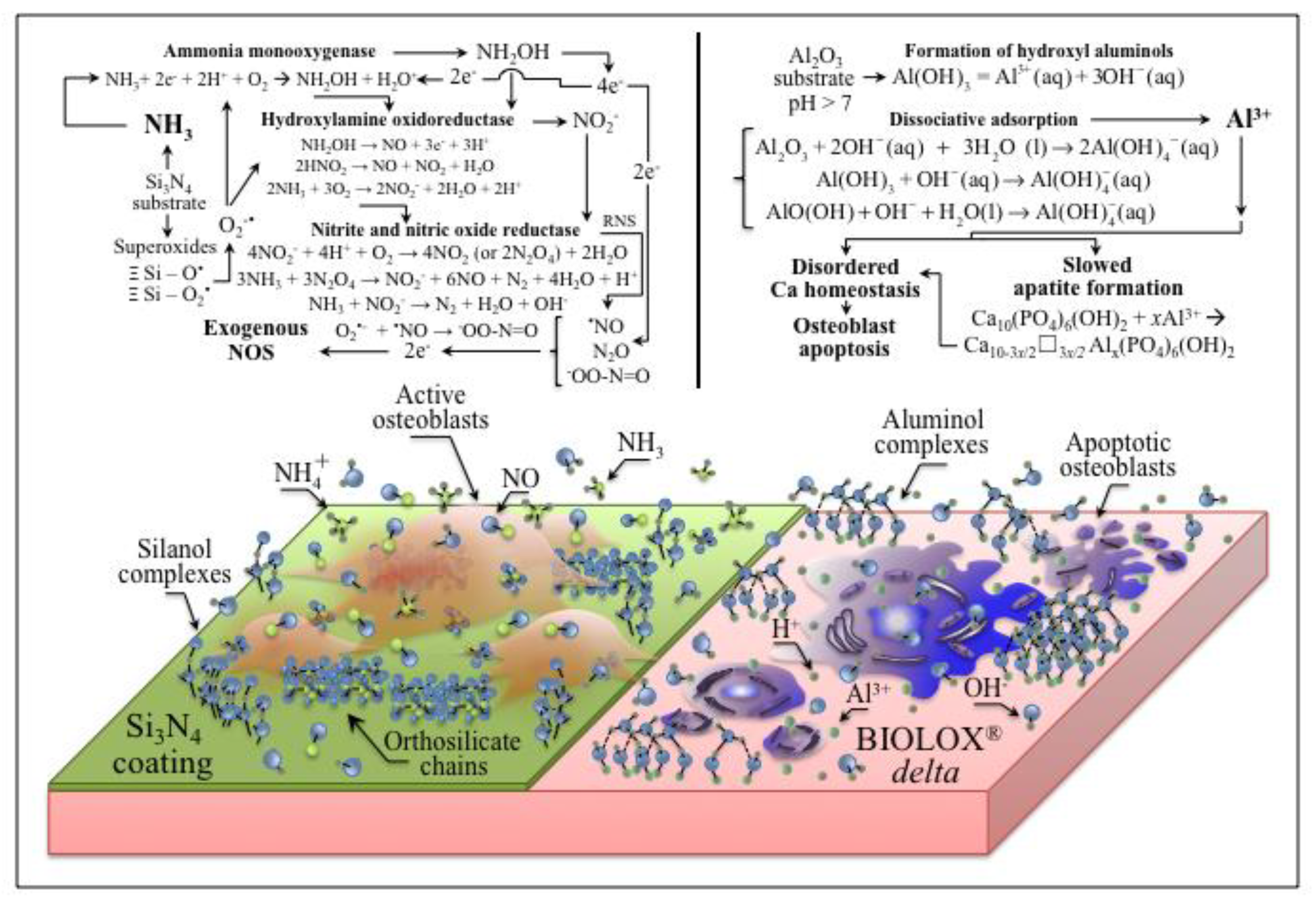
4. Discussion
5. Conclusions
- Osteoblasts cultured on ZTA underwent apoptosis from the surface chemistry of ZTA, such that no osteogenic properties were manifest on ZTA.
- Surface patterning of ZTA surface with a cylindrical well grid (500 μm in diameter and depth) failed to improve cell proliferation and osteogenesis.
- Filling the patterned wells with Bioglass® alone increased the amount of mineralized apatite on the ZTA surface, with no impact on cell proliferation.
- Enhanced osteoblast proliferation and a favorable bone mineral/matrix ratio could be obtained by adding a 10 wt.% fraction Si3N4 to the Bioglass® mixture.
- Coating ZTA with a few μm thick sintered Si3N4 layer enhanced osteogenesis by imparting the osteogenic properties of Si3N4 onto ZTA.
Author Contributions
Funding
Conflicts of Interest
References
- Jayakumar, P.; Di Silvio, L. Osteoblasts in bone tissue engineering. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2011, 224, 1415–1440. [Google Scholar] [CrossRef] [PubMed]
- Hessle, L.; Johnson, K.A.; Clarke Anderson, H.; Narisawa, S.; Sali, A.; Goding, J.W.; Terkeltaub, R.; Millan, J.L. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc. Natl. Acad. USA 2002, 99, 9445–9449. [Google Scholar] [CrossRef] [PubMed]
- Phadke, A.; Varghese, S. Synthetic biomaterials and stem cells for connective tissue engineering. In Molecular, Cellular, and Tissue Engineering; Bronzino, J.D., Peterson, D.R., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 146–163. [Google Scholar]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Piconi, C. Bioinert Ceramics: State-of-the-Art. Key Eng. Mater. 2017, 758, 3–13. [Google Scholar] [CrossRef]
- Webster, T.J.; Patel, A.A.; Rahaman, M.N.; Bal, B.S. Anti-infective and osteointegration properties of silicon nitride, poly(ether ether ketone), and titanium implants. Acta Biomater. 2012, 8, 4447–4454. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signaling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell. Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Pezzotti, G.; Bock, R.M.; Adachi, T.; Rondinella, A.; Boschetto, F.; Zhu, W.; Marin, E.; McEntire, B.J.; Bal, B.S.; Mazda, O. Silicon nitride surface chemistry: A potent regulator of mesenchymal progenitor cell activity in bone formation. Appl. Mater. Today 2017, 9, 82–95. [Google Scholar] [CrossRef]
- Pezzotti, G.; McEntire, B.J.; Bock, R.; Boffelli, M.; Zhu, W.; Vitale, E.; Puppulin, L.; Adachi, T.; Yamamoto, T.; Kanamura, N.; et al. Silicon nitride: A synthetic mineral for vertebrate biology. Sci. Rep. 2016, 6, 31717. [Google Scholar] [CrossRef]
- Pezzotti, G.; Marin, E.; Adachi, T.; Rondinella, A.; Boschetto, F.; Zhu, W.; Sugano, N.; Bock, R.M.; McEntire, B.; Bal, B.S. Bioactive silicon nitride: A new therapeutic material for osteoarthropathy. Sci. Rep. 2017, 7, 44848. [Google Scholar] [CrossRef]
- Pezzotti, G.; Oba, N.; Zhu, W.; Marin, E.; Rondinella, A.; Boschetto, F.; McEntire, B.; Yamamoto, K.; Bal, B.S. Human osteoblasts grow transitional Si/N apatite in quickly osseointegrated Si3N4 cervical insert. Acta Biomat. 2017, 64, 411–420. [Google Scholar] [CrossRef]
- Lu, Y.-H.; Chen, H.-T. Hydrogen generation by the reaction of H2O with Al2O3-based materials: A computational analysis. Phys. Chem. Chem. Phys. 2015, 17, 6834–6843. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, N.C.; Posner, A.S. In vitro model of aluminum-induced osteomalacia: Inhibition of hydroxyapatite formation and growth. Calcif. Tissue Int. 1984, 36, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.; Becaria, A.; Lahiri, D.K.; Sharman, K.; Bondy, S.C. Chronic exposure to aluminum in drinking water increases inflammatory parameters selectively in the brain. J. Neurosci. Res. 2004, 75, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.W.; LoGrasso, P.V. Mitochondrial c-Jun N-terminal kinase (JNK) signaling initiates physiological changes resulting in amplification of reactive oxygen species generation. J. Biol. Chem. 2011, 286, 16052–16062. [Google Scholar] [CrossRef] [PubMed]
- Tournier, C.; Hess, P.; Yang, D.D.; Xu, J.; Turner, T.K.; Nimnual, A.; Bar-Sagi, D.; Jones, S.N.; Flavell, R.A.; Davis, R.J. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science 2000, 288, 870–874. [Google Scholar] [CrossRef]
- Li, X.; Han, Y.; Guan, Y.; Zhang, L.; Bai, C.; Li, Y. Aluminum induces osteoblast apoptosis through the oxidative stress-mediated JNK signaling pathway. Biol. Trace Elem. Res. 2012, 150, 502–508. [Google Scholar] [CrossRef]
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef]
- Zayzafoon, M.; Fulzele, K.; McDonald, J.M. Calmodulin and calmodulin-dependent kinase II α regulate osteoblast differentiation by controlling c-fos expression. J. Biol. Chem. 2005, 280, 7049–7059. [Google Scholar] [CrossRef]
- Yeo, H.; McDonald, J.M.; Zayzafoon, M. NFATcl: A novel anabolic therapeutic target for osteoporosis. Ann. NY Acad. Sci. 2006, 1068, 564–567. [Google Scholar] [CrossRef]
- Yeo, H.; Beck, L.H.; McDonald, J.M.; Zayzafoon, M. Cyclosporin A elicits dose-dependent biphasic effects on osteoblast differentiation and bone formation. Bone 2007, 40, 1502–1516. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www. R-project.org/ (accessed on 2 October 2019).
- Marin, E.; Horiguchi, S.; Zanocco, M.; Boschetto, F.; Rondinella, A.; Zhu, W.; Bock, R.M.; McEntire, B.J.; Adachi, T.; Bal, B.S.; et al. Bioglass functionalization of laser-patterned bioceramic surfaces and their enhanced bioactivity. Heliyon 2018, 4, e01016. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaðolds and orthopaedic implants: A review. Biomater 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.A.; Mestres, G. Role of pore size and morphology in musculo-skeletal tissue regeneration. Mater. Sci. Eng. C 2016, 61, 922–939. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.-T.; Yang, Y.; Tian, T.; Stanford, C.; Zhang, X.-P.; Zhao, K. Effect of pore size and porosity on the biomechanical properties and cytocompatibility of porous NiTi alloys. PLoS ONE 2015, 10, e0128138. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, Y.; Liu, R.; Wu, S.; Fang, J.; Huang, B.; Li, Z.; Chen, Z.; Chen, Z. Tuning surface properties of bone biomaterials to manipulate osteoblastic cell adhesion and the signaling pathways for the enhancement of early osseointegration. Colloids Surf. B Biointerfaces 2018, 164, 58–69. [Google Scholar] [CrossRef]
- Boskey, A.L. Bone composition: Relationship to bone fragility and antiosteoporotic drug effects. BoneKEy Rep. 2013, 2, 447. [Google Scholar] [CrossRef]
- Pezzotti, G.; Yamada, K.; Sakakura, S.; Pitto, R.P. Raman spectroscopic analysis of advanced ceramic composite for hip prosthesis. J. Am. Ceram. Soc. 2008, 91, 1199–1206. [Google Scholar] [CrossRef]
- Sergo, V.; Pezzotti, G.; Katagiri, G.; Muraki, N.; Nishida, T. Stress dependence of the Raman spectrum of β-silicon nitride. J. Am. Ceram. Soc. 1996, 79, 781–784. [Google Scholar] [CrossRef]
- Chappard, D.; Bizot, P.; Mabilleau, G.; Hubert, L. Aluminum and bone: Review of new clinical circumstances associated with Al3+ deposition in the calcified matrix of bone. Morphologie 2016, 100, 95–105. [Google Scholar] [CrossRef]
- Savarino, L.; Cenni, E.; Stea, S.; Donati, M.E.; Paganetto, G.; Moroni, A.; Toni, A.; Pizzoferrato, A. X-ray diffraction of newly formed bone close to alumina- or hydroxyapatite-coated femoral stem. Biomaterials 1993, 14, 900–905. [Google Scholar] [CrossRef]
- Frayssinet, P.; Tourenne, F.; Rouquet, N.; Bonel, G.; Conte, P. Biological effects of aluminium diffusion from plasma-sprayed alumina coatings. J. Mater. Sci. Mater. Med. 1994, 5, 491–494. [Google Scholar] [CrossRef]
- Rodriguez, M.; Felsenfeld, A.J.; Llach, F. Aluminum administration in the rat separately affects the osteoblast and bone mineralization. J. Bone Miner. Res. 1990, 5, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, G.; Adachi, T.; Boschetto, F.; Zhu, W.; Zanocco, M.; Marin, E.; Bal, B.S.; McEntire, B.J. Off-stoichiometric reactions at the cell/substrate biomolecular interface: In situ and ex situ monitoring of cell proliferation, differentiation, and bone tissue formation. Int. J. Mol. Sci. 2019, 17, 4080. [Google Scholar] [CrossRef] [PubMed]
- Notingher, I.; Green, C.; Dyer, C.; Perkins, E.; Hopkins, N.; Lindsay, C.; Hench, L.L. Discrimination between ricin and sulphur mustard toxicity in vitro using Raman spectroscopy. J. R. Soc. Interface 2004, 1, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Brauchle, E.; Thude, S.; Bruker, S.Y.; Schenke-Layland, K. Cell death stages in single apoptotic and necrotic cells monitored by Raman microspectroscopy. Sci. Rep. 2014, 4, 4698. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Howard, G.A. Effects of aluminum on bone in vitro. Clin. Res. 1984, 32, 50A. [Google Scholar]
- Severson, A.R.; Haut, C.F.; Firling, C.E.; Huntley, T.E. Influence of short-term aluminum exposure on demineralized bone matrix induced bone formation. Arch. Toxicol. 1992, 66, 706–712. [Google Scholar] [CrossRef]
- Elliott, J.C. Structure and chemistry of the apatites and other calcium orthophosphates. In Studies in Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; Volume 18. [Google Scholar]
- Shapovalov, V.; Truong, T.N. Ab Initio study of water adsorption on α- Al2O3 (0001) crystal surface. J. Phys. Chem. B 2000, 104, 9859–9863. [Google Scholar] [CrossRef]
- Fernández, E.M.; Eglitis, R.I.; Borstel, G.; Balbás, L.C. Ab initio calculations of H2O and O2 adsorption on Al2O3 substrates. Comput. Mater. Sci. 2007, 39, 587–592. [Google Scholar] [CrossRef]
- Kaur, G.; Pandey, O.P.; Singh, K.; Homa, D.; Scott, B.; Pickrell, G. A review of bioactive glasses: Their structure, properties, fabrication and apatite formation. J. Biomed. Mater. Res. Part A 2014, 102, 254–274. [Google Scholar] [CrossRef]
- Kajdas, C. General approach to mechanochemistry and its relation to tri- biochemistry. In Tribology in Engineering; InTech: Rijeka, Croatia, 2013; pp. 209–240. [Google Scholar]
- Mezzasalma, S.; Baldovino, D. Characterization of silicon nitride surface in water and acid environment: A general approach to the colloidal suspensions. J. Colloid Interface Sci. 1996, 180, 413–420. [Google Scholar] [CrossRef]
- Sonnefeld, J. Determination of surface charge density parameters of silicon nitride. Colloids Surf. A 1996, 108, 27–31. [Google Scholar] [CrossRef]
- Fedor, M.J.; Williamson, J.R. The Catalytic Diversity of RNAs. Nat. Rev. Mol. Cell Biol. 2005, 6, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Schierano, G.; Mussano, F.; Faga, M.G.; Menicucci, G.; Manzella, C.; Sabione, C.; Genova, T.; von Degerfeld, M.M.; Peirone, B.; Cassenti, A.; et al. An alumina toughened zirconia composite for dental implant application: In vivo animal results. Biomed. Res. Int. 2015, 2015, 157360. [Google Scholar] [CrossRef] [PubMed]
- Piconi, C.; Sandri, M. New materials for dental implantology. Key Eng. Mater. 2017, 750, 189–194. [Google Scholar] [CrossRef]
- Kohal, R.-J.; Att, W.; Baechle, M.; Bitz, F. Ceramic abutments and ceramic oral implants. An update. Periodontology 2000 2008, 47, 224–243. [Google Scholar] [CrossRef]
- Oda, Y.; Miyatake, S.; Tokuriki, Y.; Handa, H. Alumina-ceramics (Bioceram) as the implant material in anterior cervical fusion. Nihon Geka Hokan 1981, 50, 352–357. [Google Scholar]
- Mostofi, K.; Moghaddam, B.G.; Peyravi, M.; Khouzani, R.K. Preliminary results of anterior cervical arthroplasty by porous alumina ceramic cage for cervical disc herniation surgery. J. Craniovertebral Junction Spine 2018, 9, 223–226. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pezzotti, G.; Marin, E.; Zanocco, M.; Boschetto, F.; Zhu, W.; McEntire, B.J.; Bal, B.S.; Adachi, T.; Yamamoto, T.; Kanamura, N.; et al. Osteogenic Enhancement of Zirconia-Toughened Alumina with Silicon Nitride and Bioglass®. Ceramics 2019, 2, 554-567. https://doi.org/10.3390/ceramics2040043
Pezzotti G, Marin E, Zanocco M, Boschetto F, Zhu W, McEntire BJ, Bal BS, Adachi T, Yamamoto T, Kanamura N, et al. Osteogenic Enhancement of Zirconia-Toughened Alumina with Silicon Nitride and Bioglass®. Ceramics. 2019; 2(4):554-567. https://doi.org/10.3390/ceramics2040043
Chicago/Turabian StylePezzotti, Giuseppe, Elia Marin, Matteo Zanocco, Francesco Boschetto, Wenliang Zhu, Bryan J. McEntire, B. Sonny Bal, Tetsuya Adachi, Toshiro Yamamoto, Narisato Kanamura, and et al. 2019. "Osteogenic Enhancement of Zirconia-Toughened Alumina with Silicon Nitride and Bioglass®" Ceramics 2, no. 4: 554-567. https://doi.org/10.3390/ceramics2040043
APA StylePezzotti, G., Marin, E., Zanocco, M., Boschetto, F., Zhu, W., McEntire, B. J., Bal, B. S., Adachi, T., Yamamoto, T., Kanamura, N., & Mazda, O. (2019). Osteogenic Enhancement of Zirconia-Toughened Alumina with Silicon Nitride and Bioglass®. Ceramics, 2(4), 554-567. https://doi.org/10.3390/ceramics2040043