Influence of a Bleaching Agent on the Color Stability of Indirect Composite Resins Immersed in Dyes
Abstract
1. Introduction
2. Materials and Methods
2.1. Manufacturing of Samples
2.2. Process of Immersion
2.3. Reading of the Color Alteration
2.4. Statistical Analysis
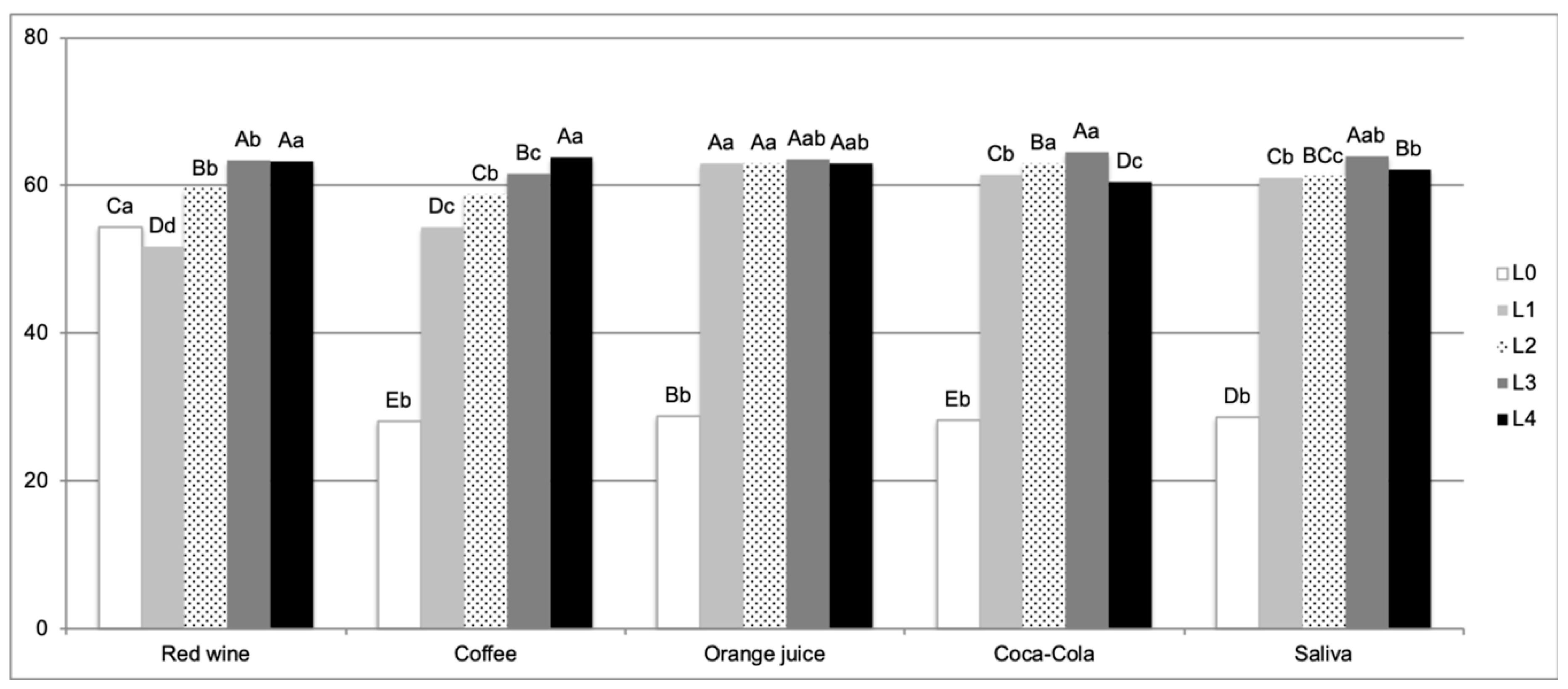
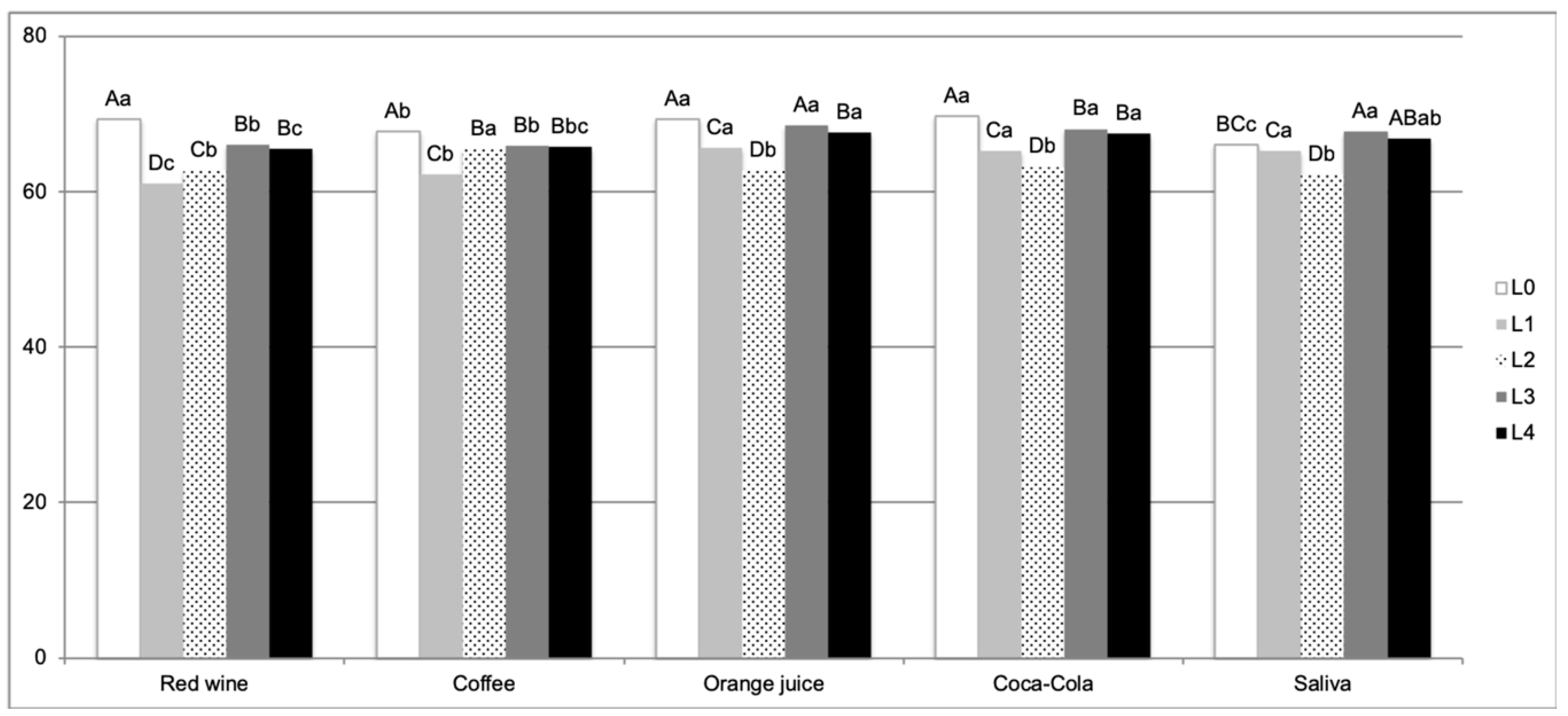
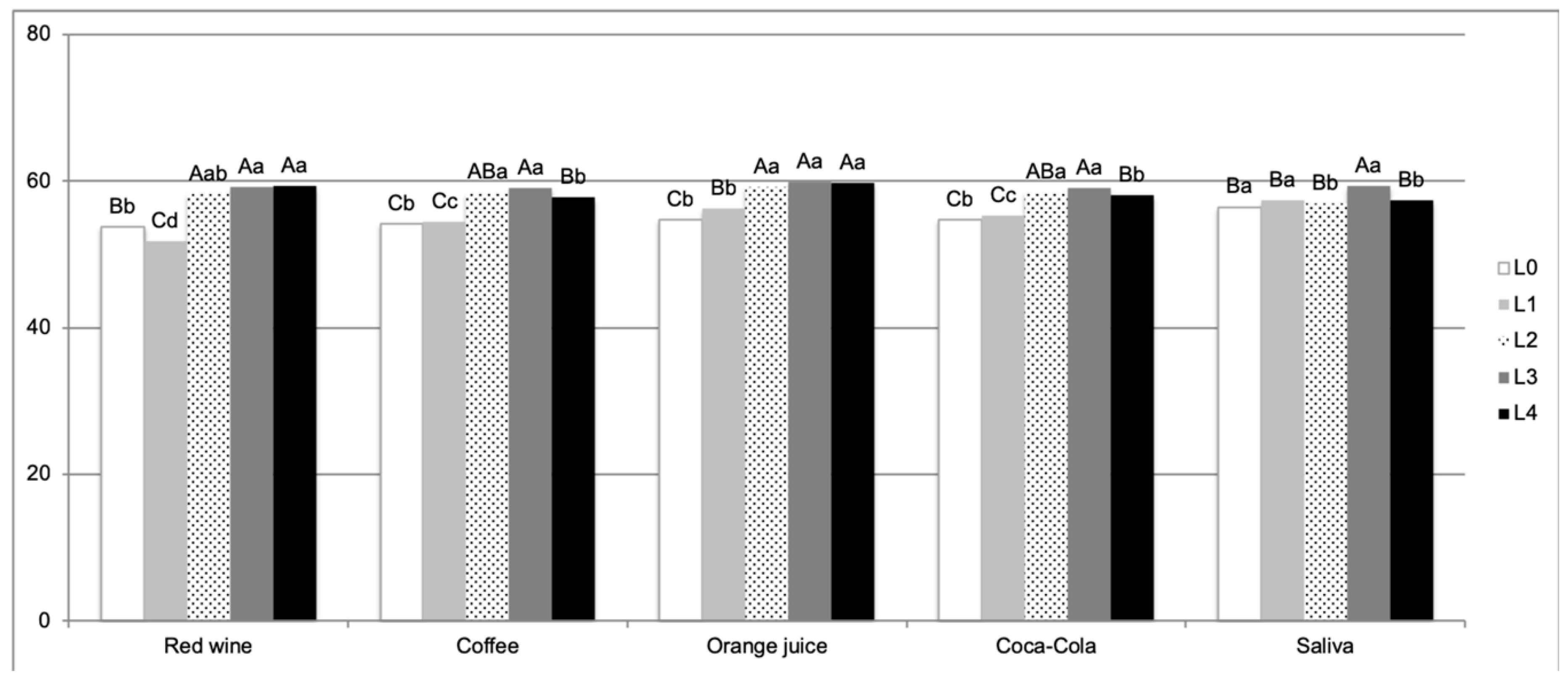
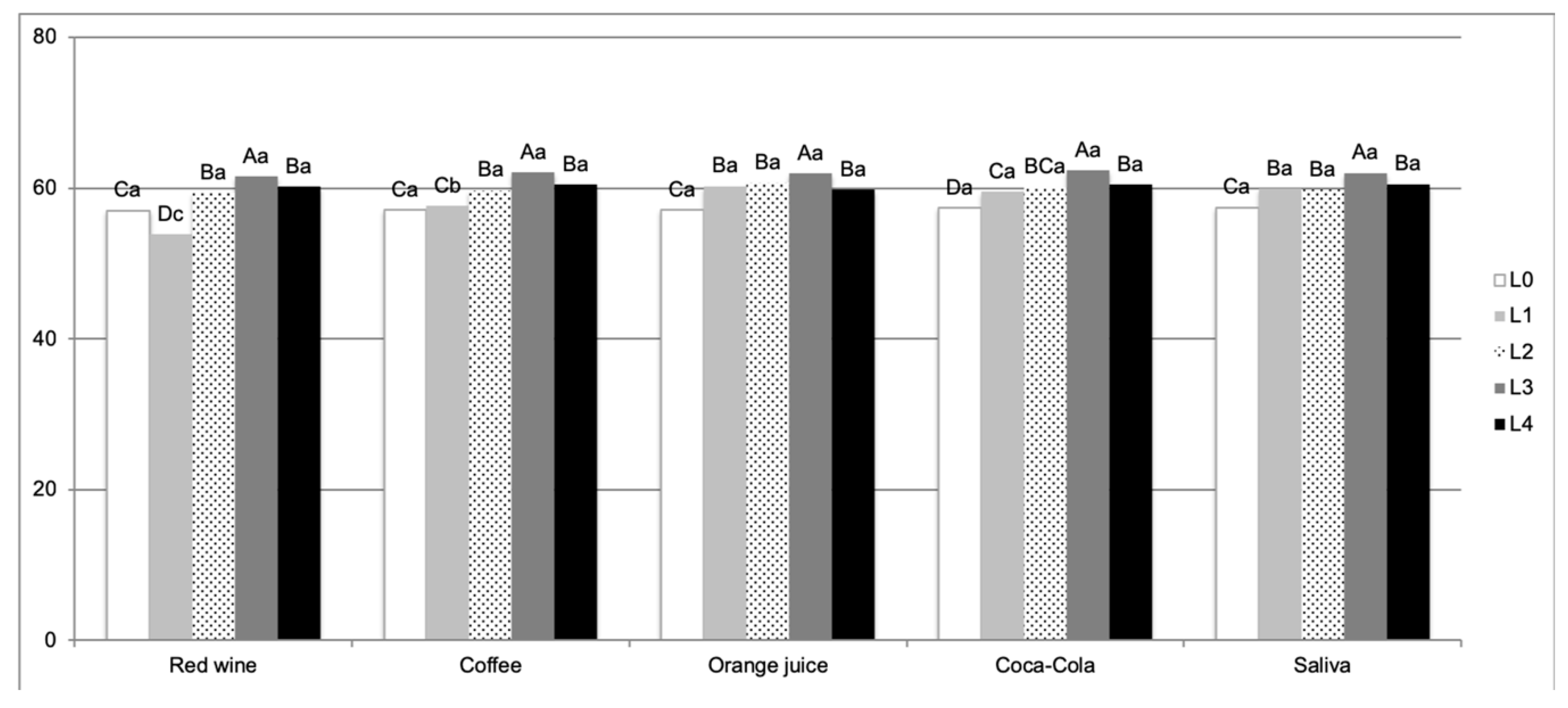
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torres, C.R.G.; Ribeiro, C.F.; Bresciani, E.; Borges, A.B. Influence of hydrogen peroxide bleaching gels of color, opacity, and fluorescence of composite resins. Oper. Dent. 2012, 37, 526–531. [Google Scholar] [CrossRef]
- Jain, V.; Platt, J.A.; Moore, B.K.; Borges, G.A. In vitro wear of new indirect resin composites. Oper. Dent. 2009, 34, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, Y.K. Changes in color and color coordinates of an indirect resin composite during curing cycle. J. Dent. 2008, 36, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Okulus, Z.; Buchwald, T.; Voelkel, A. Characterization of light-cured, dental-resin-based composites. J. Appl. Polym. Sci. 2015, 132, 42812. [Google Scholar] [CrossRef]
- Leinfelder, K.F. New developments in resin restorative systems. J. Am. Dent. Assoc. 1997, 128, 573–581. [Google Scholar] [CrossRef]
- Peutzfeldt, A.; Asmussen, E. The effect of postcuring on quantity of remaining double bonds, mechanical properties, and in vitro wear of two resin composites. J. Dent. 2000, 28, 447–452. [Google Scholar] [CrossRef]
- Anfe, T.E.; Agra, C.M.; Vieira, G.F. Evaluation of the possibility of removing staning by repolishing composite resins submitted to artificial again. J. Esthet. Restor. Dent. 2011, 23, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Villalta, P.; Lu, H.; Okte, Z.; Garcia-Godoy, F.; Powers, J.M. Effects of staining and bleaching on color change of dental composite resins. J. Prosthet. Dent. 2006, 95, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Türkün, L.S.; Türkün, M. Effect of bleaching and repolishing procedures on coffee and tea stain removal from three anterior composite veneering materials. J. Esthet. Restor. Dent. 2004, 16, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Opalescence Boost. Available online: https://www.ultradent.com/products/categories/whitening/in-office/opalescence-boost (accessed on 10 March 2019).
- Dos Santos, D.M.; De Paula, A.M.; Bonatto, L.d.R.; Da Silva, E.V.; Vechiato Filho, A.J.; Moreno, A.; Goiato, M.C. Influence of colorant solutions in properties of indirect resin composites. Am. J. Dent. 2015, 28, 219–223. [Google Scholar] [PubMed]
- Fontes, S.T.; Fernández, M.R.; Moura, C.M.; Meireles, S.S. Color stability of a nanofill composite: Effect of different immersion media. J. Appl. Oral Sci. 2009, 17, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.M.; Sasaki, R.T.; Florio, F.M.; Basting, R.T. Influence of time on bond strength after bleaching with 35% hydrogen peroxide. J. Contemp. Dent. Pract. 2008, 9, 81–88. [Google Scholar] [PubMed]
- Goiato, M.C.; Zuccolotti, B.C.; Moreno, A.; Dos Santos, D.M.; Pesqueira, A.A.; de Carvalho Dekon, S.F. Colour change of soft denture liners after storage in coffee and coke. Gerondontology 2011, 28, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, D.N.; Goiato, M.C.; Zuccolotti, B.C.R.; Moreno, A.; Dos Santos, D.M. Evaluation of hardness and colour change of soft liners after accelerated ageing. Prim. Dent. Care 2009, 16, 127–130. [Google Scholar] [CrossRef]
- Oğuz, S.; Mutluay, M.M.; Doğan, O.M.; Bek, B. Color change evaluation of denture soft lining materials in coffee and tea. Dent. Mater. J. 2007, 26, 209–216. [Google Scholar] [CrossRef]
- Ruyter, I.E.; Nilner, K.; Moller, B. Color stability of dental composite resin materials for crown and bridge veneers. Dent. Mater. 1987, 3, 246–251. [Google Scholar] [CrossRef]
- Noie, F.; O’Keefe, K.L.; Powers, J.M. Color stability of resin cements after accelerated aging. Int. J. Prosthodont. 1995, 8, 51–55. [Google Scholar] [PubMed]
- Samra, A.P.B.; Pereira, S.K.; Delgado, L.C.; Borges, C.P. Color stability evaluation of aesthetic restorative materials. Braz. Oral Res. 2008, 22, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Festuccia, M.S.; Garcia, L.d.F.; Cruvinel, D.R.; Pires-De-Souza, F.d.C. Color stability, surface roughness and microhardness of composites submitted to mouthrinsing action. J. Appl. Oral Sci. 2012, 20, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Yu, B.; Lim, H.N.; Lim, J.I. Difference in the color stability of direct and indirect resin composites. J. Appl. Oral Sci. 2011, 19, 154–160. [Google Scholar] [CrossRef]
- Nakazawa, M. Color stability of indirect composite materials polymerized with different polymerization systems. J. Oral Sci. 2009, 51, 267–273. [Google Scholar] [CrossRef]
- Domingos, P.A.; Garcia, P.P.; Oliveira, A.L.; Palma-Dibb, R.G. Composite resin color stability: Influence of light sources and immersion media. J. Appl. Oral Sci. 2011, 19, 204–211. [Google Scholar] [CrossRef]
- Fujita, M.; Kawakami, S.; Noda, M.; Sano, H. Color change of newly developed esthetic restorative material immersed in food-simulating solutions. Dent. Mater. J. 2006, 25, 352–359. [Google Scholar] [CrossRef]
- Ertaş, E.; Güler, A.U.; Yücel, A.C.; Köprülü, H.; Güler, E. Color stability of resin composites after immersion in different drinks. Dent. Mater. J. 2006, 25, 371–376. [Google Scholar] [CrossRef]
- Guler, A.U.; Yilmaz, F.; Kulunk, T.; Guler, E.; Kurt, S. Effects of different drinks on stainability of resin composite provisional restorative materials. J. Prosthet. Dent. 2005, 94, 118–124. [Google Scholar] [CrossRef]
- Catelan, A.; Briso, A.L.; Sundfeld, R.H.; Goiato, M.C.; dos Santos, P.H. Color stability of sealed composite resin restorative materials after ultraviolet artificial aging and immersion in staining solutions. J. Prosthet. Dent. 2011, 105, 236–241. [Google Scholar] [CrossRef]
- Khokhar, Z.A.; Razzoog, M.E.; Yaman, P. Color stability of restorative resins. Quintessence Int. 1991, 22, 733–737. [Google Scholar]
- Crispin, B.; Caputo, A. Color stability of temporary restorative materials. J. Prosthet. Dent. 1979, 42, 27–33. [Google Scholar] [CrossRef]
- Turker, S.B.; Biskin, T. Effect of three bleaching agents on the surface properties of three different esthetic restorative materials. J. Prosthet. Dent. 2003, 89, 466–473. [Google Scholar] [CrossRef]
- Cehreli, Z.C.; Yazici, R.; Garcia-Godoy, F. Effect of home-use bleaching gels on fluoride releasing restorative materials. Oper. Dent. 2003, 28, 605–609. [Google Scholar] [PubMed]
- Bayne, S.C. Correlation of clinical performance with ‘in vitro tests’ of restorative dental materials that use polymer-based matrices. Dent. Mater. 2012, 28, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Malkondu, Ö.; Yurdagüven, H.; Say, E.C.; Kazazoğlu, E.; Soyman, M. Effect of bleaching on microhardness of esthetic restorative materials. Oper. Dent. 2011, 36, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Durner, J.; Stojanovic, M.; Urcan, E.; Spahl, W.; Haertel, U.; Hickel, R.; Reichl, F.X. Effect of hydrogen peroxide on the three-dimensional polymer network in composites. Dent. Mater. 2011, 27, 573–580. [Google Scholar] [CrossRef] [PubMed]


| Brand | Chemical Composition | Polymerization |
|---|---|---|
| Adoro | 17–19% of dimethacrylate, 82–83% copolymers of silicon oxide, and 1% stabilizers, catalysts, and pigments. | Pre-polymerization in a Targis Quick unit (halogen lamp, intensity of 600 mW/cm2). Resin impregnated with glycerin gel. Polymerization in a Lunamat 100 unit, with eight lamps emitting fluorescent light in a mirrored environment for 25 min (10 min with light, 10 min with heat at 1040 °C and 5 min with cooling unit) at a total power of 750 W. |
| Resilab Master | Small particles with a mean size of 0.05 mm, 53% of ceramic filler particles, BisGMA (bisphenol A-glycidyl methacrylate), BisEMA (ethoxylated bisphenol A- dimethacrylate), UDMA (urethane dimethacrylate), TEGMA (tri-ethylene glycol dimethacrylate), aluminum borosilicate, highly dispersible silica, photoinitiators, inhibitors, and pigments. | Pre-polymerization for 4 min in an EDG-Lux unit (400–500 mW/cm2) with temperature not exceeding 50 °C. Final polymerization for 8 min in an EDG-Lux unit (400–500 mW/cm2). |
| Cristobal | 74% pyrogenic silica particles, barium glass, and borosilicate. | Pre-polymerization in an MPa 2000 unit for 90 s (200 mW/cm2) in the first cycle and then for 75 s (800–1000 mW/cm2) in the second cycle. Final polymerization in a Post Cure unit for 8 min at 80 °C. |
| Sinfony | 48% organic matrix (UDMA), 40% strontium glass (macroparticle of 0.6 μm), 5% pyrogenic silica (microparticle of 0.06 μm), 5% glass ionomer cement particles, 1% silane and 1% initiator. | Pre-polymerization for 15 s in Visio Alfa Light and Visio Beta Vario Light units used with a Visio Beta Vacuum pump (470 mW/cm2). Final polymerization in two stages: 1 minute of light emission in air followed by 14 min of light emission in vacuum in Visio Beta. |
| Epricord | 53% ceramic filler content, 25% multifunctional polymers and 22% conventional resin photoinitiators. The mean particle size is 0.6 μm. | Pre-polymerization for 30 s in a Kota unit. Final polymerization for 180 s with a halogen lamp (600 mW/cm2) in a Kota unit. |
| Solution | Brand | Chemical Composition |
|---|---|---|
| Red wine | Periquita dry red wine, José Maria Da Fonseca Vinhos S.A., Azeitão, Portugal | Red grape varieties, conservative INS 220 (sulfur dioxide, SO2), sulphurous acid, and 12.7% alcohol. |
| Coffee | Coffee Pilão, Sara Lee, Jundiaí, São Paulo, Brazil | Roasted and ground coffee. |
| Orange juice | Coca-Cola, Ribeirão Preto, Brazil | Orange juice, water, sugar, orange pulp, natural flavors, ascorbic acid, and citric acid. |
| Coca-Cola | Coca-Cola, Ribeirão Preto, Brazil | Carbonated water, sugar, cola nut extract, yellow dye IV, acidulant INS 338, and natural flavors. |
| Artificial saliva | Farmácia de Manipulação Apothicário, Araçatuba, Brazil | [KCl (0.4 g·L−1), NaCl (0.4 g·L−1), CaCl2·2H2O (0.906 g·L−1), NaH2PO4·2H2O (0.690 g·L−1), Na2S·9H2O (0.005 g·L−1), and urea (1 g·L−1)]. |
| SS | df | MS | F | P | |
|---|---|---|---|---|---|
| Resin | 6566.386 | 4 | 1641.597 | 2142.478 | <0.001 |
| Solution | 131.647 | 4 | 32.912 | 42.954 | <0.001 |
| Resin × Solution | 458.558 | 16 | 28.660 | 37.404 | <0.001 |
| Between subjects | 172.398 | 225 | 0.766 | ||
| Period | 19,564.573 | 2.845 | 6877.370 | 5664.649 | <0.001 |
| Period × Resin | 12,870.394 | 11.379 | 1131.055 | 931.611 | <0.001 |
| Period × Solution | 930.291 | 11.379 | 81.754 | 67.338 | <0.001 |
| Period × Resin × Solution | 3266.354 | 45.516 | 71.762 | 59.108 | <0.001 |
| Within subjects | 777.105 | 675 | 1.151 | ||
| P < 0.05 denotes statistically significant difference. | |||||
| SS | df | MS | F | P | |
|---|---|---|---|---|---|
| Resin | 23,185.790 | 4 | 5796.447 | 1693.562 | <0.001 |
| Solution | 200.716 | 4 | 50.179 | 14.661 | <0.001 |
| Resin × Solution | 829.049 | 16 | 51.816 | 15.139 | <0.001 |
| Between subjects | 770.093 | 225 | 3.423 | ||
| Period | 8636.929 | 3.071 | 2812.801 | 1324.969 | <0.001 |
| Period × Resin | 25,959.112 | 12.282 | 2113.535 | 995.580 | <0.001 |
| Period × Solution | 2326.537 | 12.282 | 189.422 | 89.227 | <0.001 |
| Period × Resin × Solution | 4517.111 | 49.129 | 91.943 | 43.310 | <0.001 |
| Within subjects | 1466.683 | 900 | 1.630 | ||
| P < 0.05 denotes statistically significant difference. | |||||
| Resin | Staining Solution | |||||
|---|---|---|---|---|---|---|
| Red Wine | Coffee | Orange Juice | Coca-Cola | Saliva | ||
| Adoro | ∆E1 | 6.59 Aa | 5.99 Aab | 5.42 Ab | 6.14 Aab | 0.57 Bc |
| ∆E2 | 2.79 Bb | 5.05 Ba | 1.89 Bc | 2.20 Bbc | 0.45 Bd | |
| ∆E3 | 3.40 Ba | 2.47 Cb | 1.89 Bb | 2.47 Bb | 1.96 Ab | |
| ∆E4 | 0.76 Ca | 0.66 Da | 1.20 Ba | 1.22 Ca | 0.85 Ba | |
| Means followed by the same capital letter in column do not differ (P < 0.05; Tukey). Means followed by the same lowercase letter in the line do not differ (P < 0.05; Tukey). | ||||||
| Resin | Staining Solution | |||||
|---|---|---|---|---|---|---|
| Red Wine | Coffee | Orange Juice | Coca-Cola | Saliva | ||
| Resilab | ∆E1 | 13.49 Ad | 29.44 Ac | 35.64 Aa | 34.53 Ab | 33.68 Ab |
| ∆E2 | 8.15 Ba | 4.61 Bb | 1.18 Dc | 1.87 Cc | 1.34 Cc | |
| ∆E3 | 5.13 Ca | 3.19 Cb | 2.26 Ccd | 1.77 Cd | 2.93 Bbc | |
| ∆E4 | 4.56 Dab | 3.87 BCab | 3.53 Bb | 4.77 Ba | 1.98 BCc | |
| Means followed by the same capital letter in column do not differ (P < 0.05; Tukey). Means followed by the same lowercase letter in the line do not differ (P < 0.05; Tukey). | ||||||
| Resin | Staining Solution | |||||
|---|---|---|---|---|---|---|
| Red Wine | Coffee | Orange Juice | Coca-Cola | Saliva | ||
| Epricord | ∆E1 | 12.78 Ab | 13.84 Aa | 11.54 Ac | 11.54 Ac | 11.53 Ac |
| ∆E2 | 5.52 Bab | 6.16 Ba | 4.05 Cc | 4.20 Cc | 5.09 Cb | |
| ∆E3 | 6.32 Ba | 5.93 Ba | 6.35 Ba | 5.94 Ba | 6.28 Ba | |
| ∆E4 | 2.50 Ca | 0.95 Cb | 1.08 Db | 0.84 Db | 0.88 Db | |
| Means followed by the same capital letter in column do not differ (P < 0.05; Tukey). Means followed by the same lowercase letter in the line do not differ (P < 0.05; Tukey). | ||||||
| Resin | Staining Solution | |||||
|---|---|---|---|---|---|---|
| Red Wine | Coffee | Orange Juice | Coca-Cola | Saliva | ||
| Cristobal | ∆E1 | 8.57 Aab | 9.27 Aa | 8.54 Aab | 8.58 Aab | 8.39 Ab |
| ∆E2 | 6.51 Ba | 4.10 Bb | 2.91 Bc | 3.32 Bc | 0.82 Cc | |
| ∆E3 | 1.31 Cbc | 1.85 Cab | 0.74 Cc | 0.77 Cc | 2.40 Ba | |
| ∆E4 | 0.69 Cbc | 2.02 Ca | 0.58 Cc | 1.69 Cab | 2.06 Ba | |
| Means followed by the same capital letter in column and do not differ (P < 0.05; Tukey). Means followed by the same lowercase letter in the line do not differ (P < 0.05; Tukey). | ||||||
| Resin | Staining Solution | |||||
|---|---|---|---|---|---|---|
| Red Wine | Coffee | Orange Juice | Coca-Cola | Saliva | ||
| Sinfony | ∆E1 | 11.22 Aa | 9.95 Ab | 8.83 Ac | 8.06 Ac | 8.25 Ac |
| ∆E2 | 5.87 Ba | 2.76 Bb | 0.84 Cc | 1.39 Cc | 1.01 Cc | |
| ∆E3 | 2.24 Cab | 2.40 Ba | 1.39 Cb | 2.34 Ba | 2.48 Ba | |
| ∆E4 | 1.55 Cb | 2.02 Bab | 2.83 Ba | 1.89 BCab | 1.83 BCab | |
| Means followed by the same capital letter in column do not differ (P < 0.05; Tukey). Means followed by the same lowercase letter in the line do not differ (P < 0.05; Tukey). | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, D.M.; da Silva, E.V.F.; Mendonça, J.B.; Cetrangolo, D.; Caxias, F.P.d.; Goiato, M.C. Influence of a Bleaching Agent on the Color Stability of Indirect Composite Resins Immersed in Dyes. Ceramics 2019, 2, 235-245. https://doi.org/10.3390/ceramics2020019
dos Santos DM, da Silva EVF, Mendonça JB, Cetrangolo D, Caxias FPd, Goiato MC. Influence of a Bleaching Agent on the Color Stability of Indirect Composite Resins Immersed in Dyes. Ceramics. 2019; 2(2):235-245. https://doi.org/10.3390/ceramics2020019
Chicago/Turabian Styledos Santos, Daniele M., Emily V. F. da Silva, Juliani B. Mendonça, Denis Cetrangolo, Fernanda P. de Caxias, and Marcelo C. Goiato. 2019. "Influence of a Bleaching Agent on the Color Stability of Indirect Composite Resins Immersed in Dyes" Ceramics 2, no. 2: 235-245. https://doi.org/10.3390/ceramics2020019
APA Styledos Santos, D. M., da Silva, E. V. F., Mendonça, J. B., Cetrangolo, D., Caxias, F. P. d., & Goiato, M. C. (2019). Influence of a Bleaching Agent on the Color Stability of Indirect Composite Resins Immersed in Dyes. Ceramics, 2(2), 235-245. https://doi.org/10.3390/ceramics2020019





