Hydrothermal Synthesis of Pseudocubic Rutile-Type Titania Particles
Abstract
1. Introduction
2. Materials and Methods
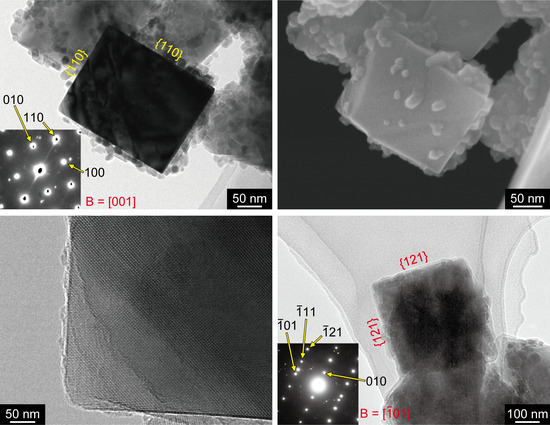
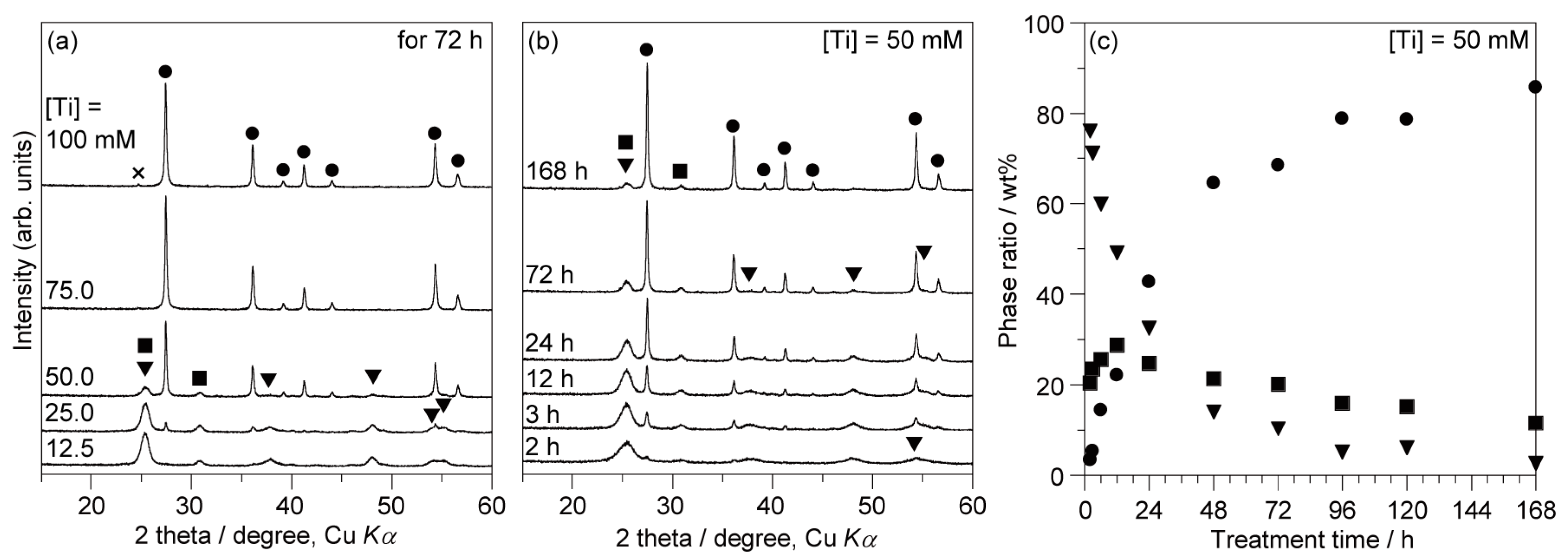
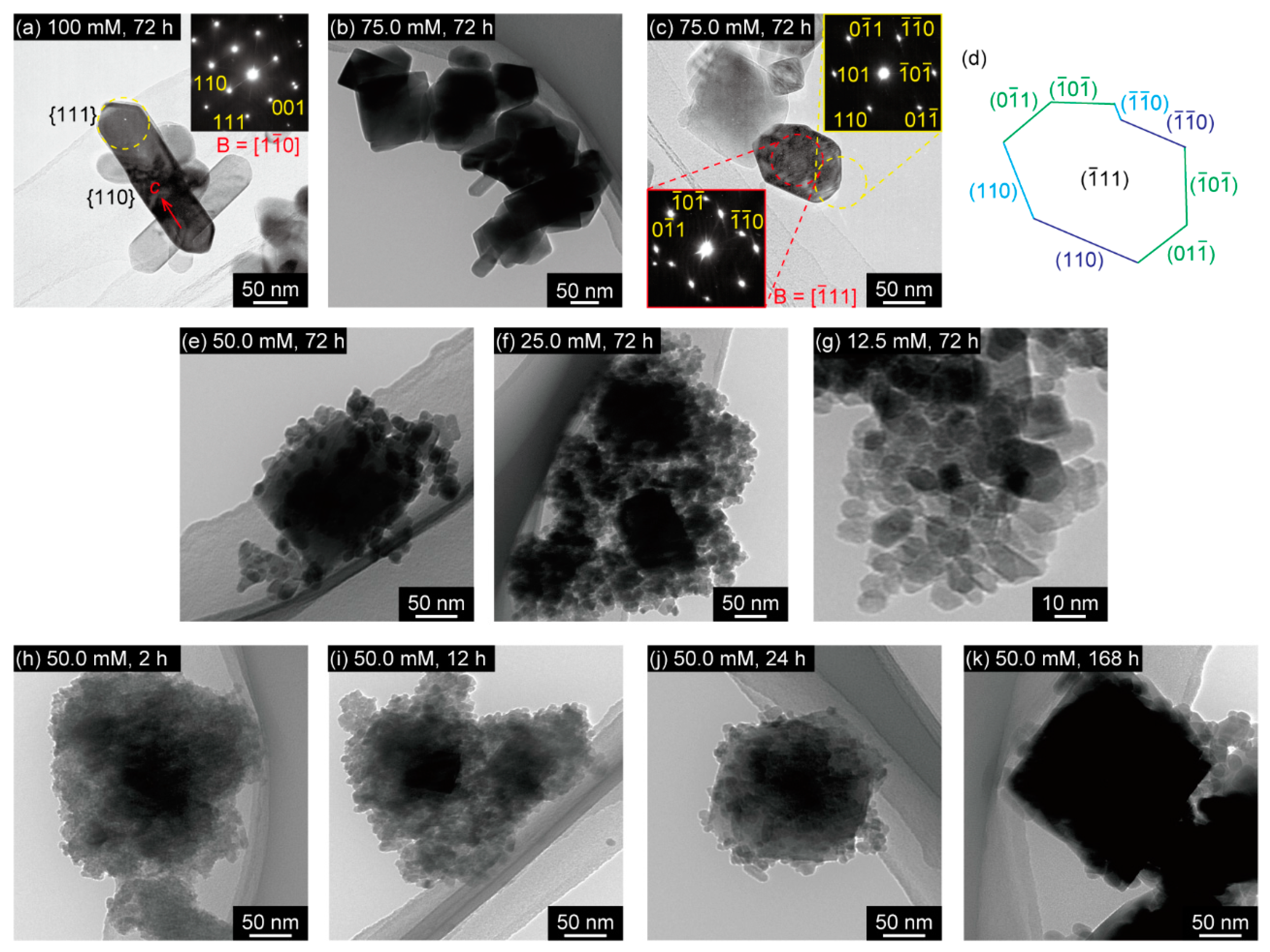
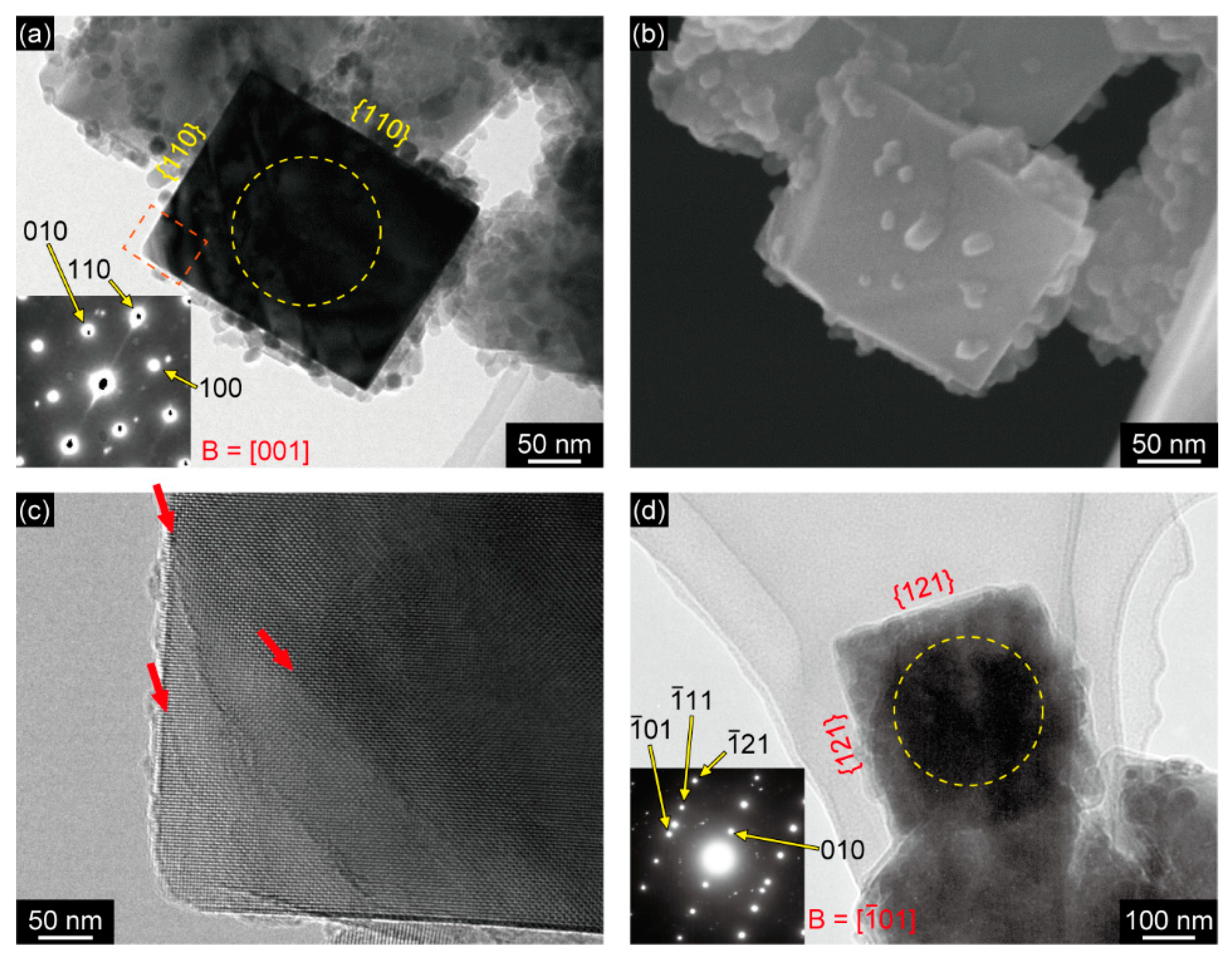
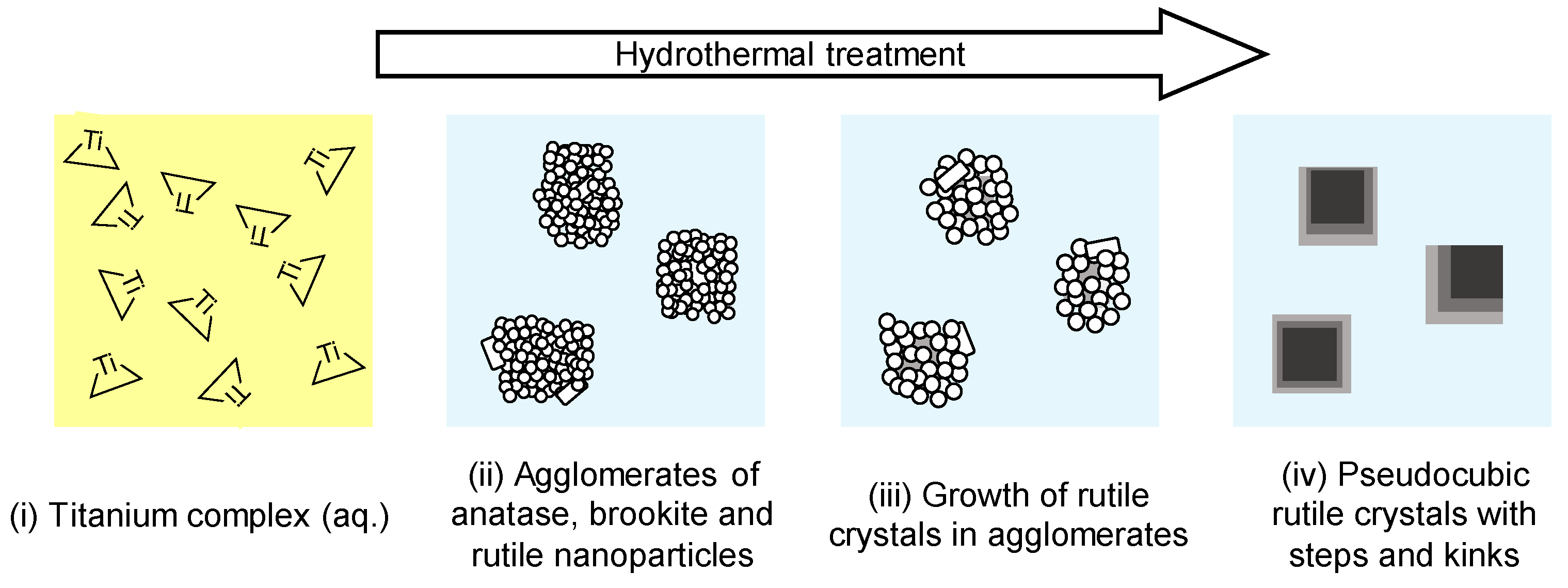
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Yoreo, J.J.; Vekilov, P.G. Principles of Crystal Nucleation and Growth. Rev. Mineral. Geochem. 2003, 54, 57–93. [Google Scholar] [CrossRef]
- Sekerka, R.F. Equilibrium and Growth Shapes of Crystals: How Do They Differ and Why Should We Care? Cryst. Res. Technol. 2005, 40, 291–306. [Google Scholar] [CrossRef]
- Byrappa, K.; Adschiri, T. Hydrothermal Technology for Nanotechnology. Prog. Cryst. Growth Charact. Mater. 2007, 53, 117–166. [Google Scholar] [CrossRef]
- Lazzeri, M.; Vittadini, A.; Selloni, A. Structure and Energetics of Stoichiometric TiO2 Anatase Surfaces. Phys. Rev. B 2001, 63, 155409. [Google Scholar] [CrossRef]
- Oliver, P.M.; Watson, G.W.; Kelsey, E.T.; Parker, S.C. Atomistic Simulation of the Surface Structure of the TiO2 Polymorphs Rutile and Anatase. J. Mater. Chem. 1997, 7, 563–568. [Google Scholar] [CrossRef]
- Barnard, A.S.; Curtiss, L.A. Prediction of TiO2 Nanoparticle Phase and Shape Transitions Controlled by Surface Chemistry. Nano Lett. 2005, 5, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.Q.; Selloni, A. First-principles study of the Structures and Energetics of Stoichiometric Brookite TiO2 Surfaces. Phys. Rev. B 2007, 76, 235307. [Google Scholar] [CrossRef]
- Vittadini, A.; Casarin, M.; Selloni, A. Structure and Stability of TiO2-B Surfaces: A Density Functional Study. J. Phys. Chem. C Lett. 2009, 113, 18973–18977. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H.G.; Pan, J.; Yang, Y.Q.; Lu, G.Q.; Cheng, H.M. Titanium Dioxide Crystals with Tailored Facets. Chem. Rev. 2014, 114, 9559–9612. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 Single Crystals with a Large Percentage of Reactive Facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef]
- Yang, H.G.; Liu, G.; Qiao, S.Z.; Sun, C.H.; Jin, Y.G.; Smith, S.C.; Zou, J.; Cheng, H.M.; Lu, G.Q. Solvothermal Synthesis and Photoreactivity of Anatase TiO2 Nanosheets with Dominant {001} Facets. J. Am. Chem. Soc. 2009, 131, 4078–4083. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Chen, P.C.; Tsai, M.C.; Chen, T.T.; Chang, I.C.; Chiu, H.T.; Lee, C.Y. Alkali Metal Ion Assisted Synthesis of Faceted Anatase TiO2. CrystEngComm 2013, 15, 2966–2971. [Google Scholar] [CrossRef]
- Wu, H.B.; Chen, J.S.; Lou, X.W.; Hng, H.H. Asymmetric Anatase TiO2 Nanocrystals with Exposed High-index Facets and Their Excellent Lithium Storage Properties. Nanoscale 2011, 3, 4082–4084. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zheng, B.; Ouyang, J.; Wang, X.; Kuang, Q.; Jiang, Y.; Xie, Z.; Zheng, L. Control of Anatase TiO2 Nanocrystals with a Series of High-Energy Crystal Facets via a Fuorine-Free Strategy. Chem. Asian J. 2012, 7, 2538–2542. [Google Scholar] [CrossRef] [PubMed]
- Diebold, U. The Surface Science of Titanium Dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Shi, J.; Yu, Y. One-Dimensional Titanium Dioxide Nanomaterials: Nanowires, Nanorods, and Nanobelts. Chem. Rev. 2014, 114, 9346–9384. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, J.; Gong, L.; Wu, M.; Yu, J.C. Cross-Medal Arrays of Ta-Doped Rutile Titania. J. Am. Chem. Soc. 2009, 131, 12048–12049. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Lou, X.W. Unusual RutileTiO2 Nanosheets with Exposed (001) Facets. Chem. Sci. 2011, 2, 2219–2223. [Google Scholar] [CrossRef]
- Truong, Q.D.; Kato, H.; Kobayashi, M.; Kakihana, M. Hierarchical Structures of Rutile Exposing High-index Facets. J. Cryst. Growth 2015, 418, 86–91. [Google Scholar] [CrossRef]
- Kakihana, M.; Kobayashi, M.; Tomita, K.; Petrykin, V. Application of Water-soluble Titanium Complexes as Precursors for Synthesis of Titanium-containing Oxides via Aqueous Solution Processes. Bull. Chem. Soc. Jpn. 2011, 83, 1285–1308. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kato, H.; Kakihana, M. Synthesis of Titanium Dioxide Nanocrystals with Controlled Crystal- and Micro-structures from Titanium Complexes. Nanomater. Nanotechnol. 2013, 3, 3–23. [Google Scholar] [CrossRef]
- Kobayashi, M. Synthesis and Development of Titania with Controlled Structures. J. Ceram. Soc. Jpn. 2016, 124, 863–869. [Google Scholar] [CrossRef]
- Sato, J.; Kobayashi, M.; Kato, H.; Miyazaki, T.; Kakihana, M. Hydrothermal Synthesis of Magnetite Particles with Uncommon Crystal Facets. J. Asian Ceram. Soc. 2014, 2, 258–264. [Google Scholar] [CrossRef]
- Neravathu, D.G.; Bushiri, M.J. High Index Facets Bounded α-Fe2O3 Pseudocubic Nanocrystals with Enhanced Electrochemical Properties: Zn2+ Ions Assisted Solvo-Hydrothermal Synthesis. CrystEngComm 2019, in press. [Google Scholar] [CrossRef]
- Han, X.; Li, L.; Wang, C. Synthesis of Tin Dioxide Nanooctahedra with Exposed High-Index {332} Facets and Enhanced Selective Gas Sensing Properties. Chem. Asian J. 2012, 7, 1572–1575. [Google Scholar] [CrossRef] [PubMed]
- Leng, M.; Liu, M.; Zhang, Y.; Wang, Z.; Yu, C.; Yang, X.; Zhang, H.; Wang, C. Polyhedral 50-Facet Cu2O Microcrystals Partially Enclosed by {311} High-Index Planes: Synthesis and Enhanced Catalytic CO Oxidation Activity. J. Am. Chem. Soc. 2010, 132, 17084–17087. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Petrykin, V.; Kobayashi, M.; Shiro, M.; Yoshimura, M.; Kakihana, M. A Water-soluble Titanium Complex for the Selective Synthesis of Nanocrystalline Brookite, Rutile, and Anatase by a Hydrothermal Method. Angew. Chem. Int. Ed. 2006, 45, 2378–2381. [Google Scholar] [CrossRef]
- Kobayashi, M.; Petrykin, V.; Kakihana, M.; Tomita, K. Morphology Control of Rutile Nanoparticles in a Hydrothermal Synthesis from Water-soluble Titanium Complex Aqueous Solution. J. Ceram. Soc. Jpn. 2007, 115, 835–839. [Google Scholar] [CrossRef]
- Kobayashi, M.; Petrykin, V.; Kakihana, M.; Tomita, K. Hydrothermal Synthesis and Photocatalytic Activity of Whisker-like Rutile-type Titanium Dioxide. J Am. Ceram. Soc. 2009, 92, S21–S26. [Google Scholar] [CrossRef]
- Truong, Q.D.; Kato, H.; Kobayashi, M.; Kakihana, M. Growth of TiO2 Microspheres with Radially Oriented Configuration. CrystEngComm 2017, 19, 4832–4837. [Google Scholar] [CrossRef]
- Kobayashi, M.; Lee, S.; Kato, H.; Kakihana, M. Effect of Hydroxy and Carboxy Groups on Anisotropic Growth of Rutile-type Titania Under Hydrothermal Conditions. J. Asian Ceram. Soc. 2017, 5, 320–325. [Google Scholar] [CrossRef]
| Treatment Time/h | Crystalline Size/nm 1 | ||
|---|---|---|---|
| Anatase | Brookite | Rutile | |
| 2 | 4.9 | 7.7 | 29.1 |
| 3 | 5.7 | 9.7 | 34.8 |
| 12 | 8.6 | 14.0 | 51.7 |
| 24 | 10.0 | 15.6 | 57.4 |
| 72 | 12.9 | 19.8 | 63.7 |
| 168 | 16.6 | 23.5 | 67.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, M.; Kato, H.; Miyazaki, T.; Kakihana, M. Hydrothermal Synthesis of Pseudocubic Rutile-Type Titania Particles. Ceramics 2019, 2, 56-63. https://doi.org/10.3390/ceramics2010005
Kobayashi M, Kato H, Miyazaki T, Kakihana M. Hydrothermal Synthesis of Pseudocubic Rutile-Type Titania Particles. Ceramics. 2019; 2(1):56-63. https://doi.org/10.3390/ceramics2010005
Chicago/Turabian StyleKobayashi, Makoto, Hideki Kato, Takamichi Miyazaki, and Masato Kakihana. 2019. "Hydrothermal Synthesis of Pseudocubic Rutile-Type Titania Particles" Ceramics 2, no. 1: 56-63. https://doi.org/10.3390/ceramics2010005
APA StyleKobayashi, M., Kato, H., Miyazaki, T., & Kakihana, M. (2019). Hydrothermal Synthesis of Pseudocubic Rutile-Type Titania Particles. Ceramics, 2(1), 56-63. https://doi.org/10.3390/ceramics2010005