The Evolution of Paleolithic Hunting Weapons: A Response to Declining Prey Size
Abstract
:1. Introduction
2. Material and Methods
- Relative abundance of megaherbivores—MNI (megaherbivores)/total MNI—This is close to the common abundance index where, instead of one taxon, we include megaherbivores as a group of taxa.
- MNI-based relative biomass contribution of megaherbivores. We multiplied the mass of each megaherbivore species by its MNI, summed it across all megaherbivore species, and divided it by the total biomass. In the case of the Hadza [75], introducing biomass to the relative MNI index resulted in the closest estimate to the actual relative biomass contribution of the taxon to the total biomass of the assemblage.
3. Results
3.1. Africa
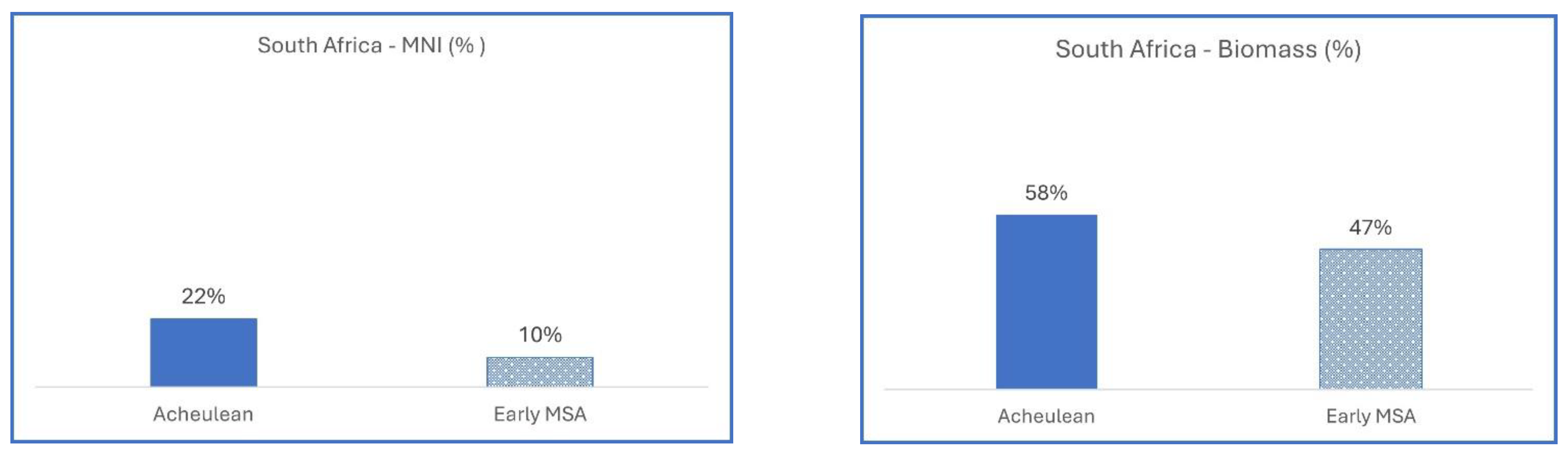
3.1.1. South Africa
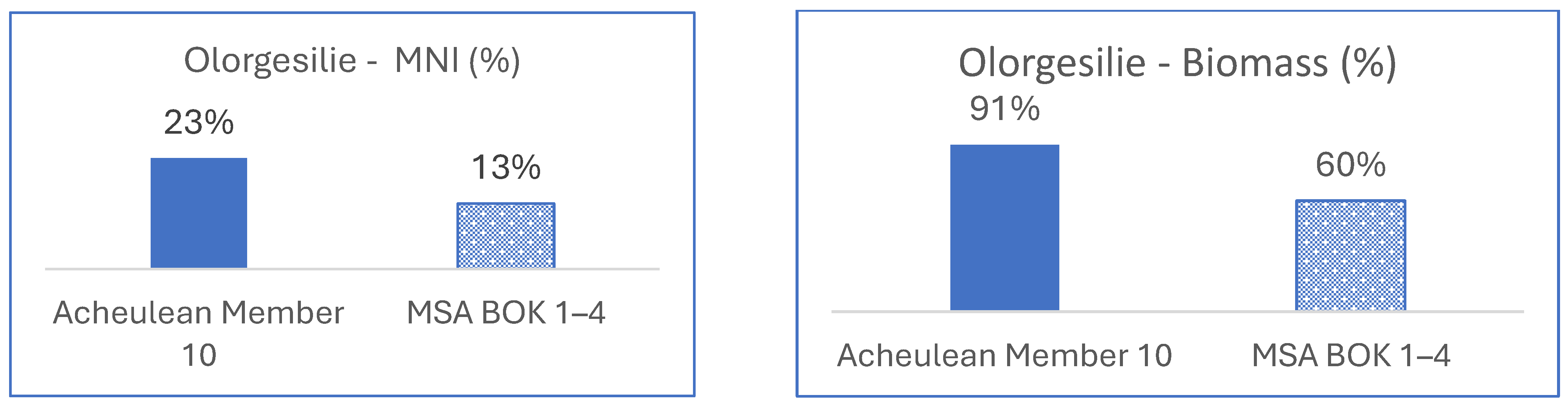
3.1.2. East Africa, Olorgesailie
3.2. Europe
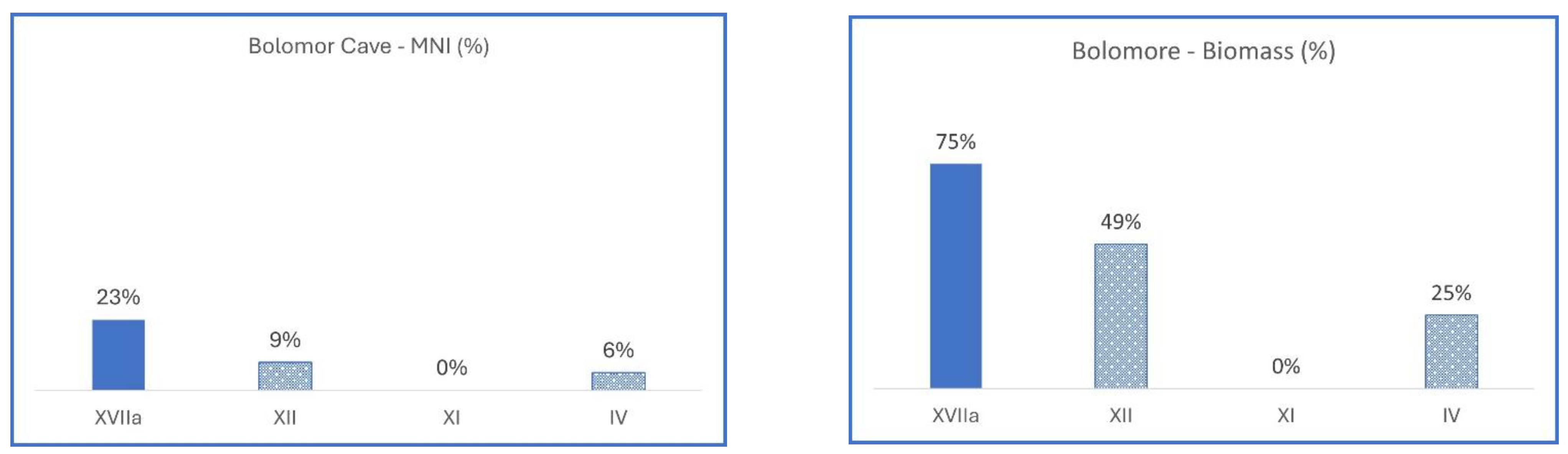
3.2.1. Spain, Bolomor Cave
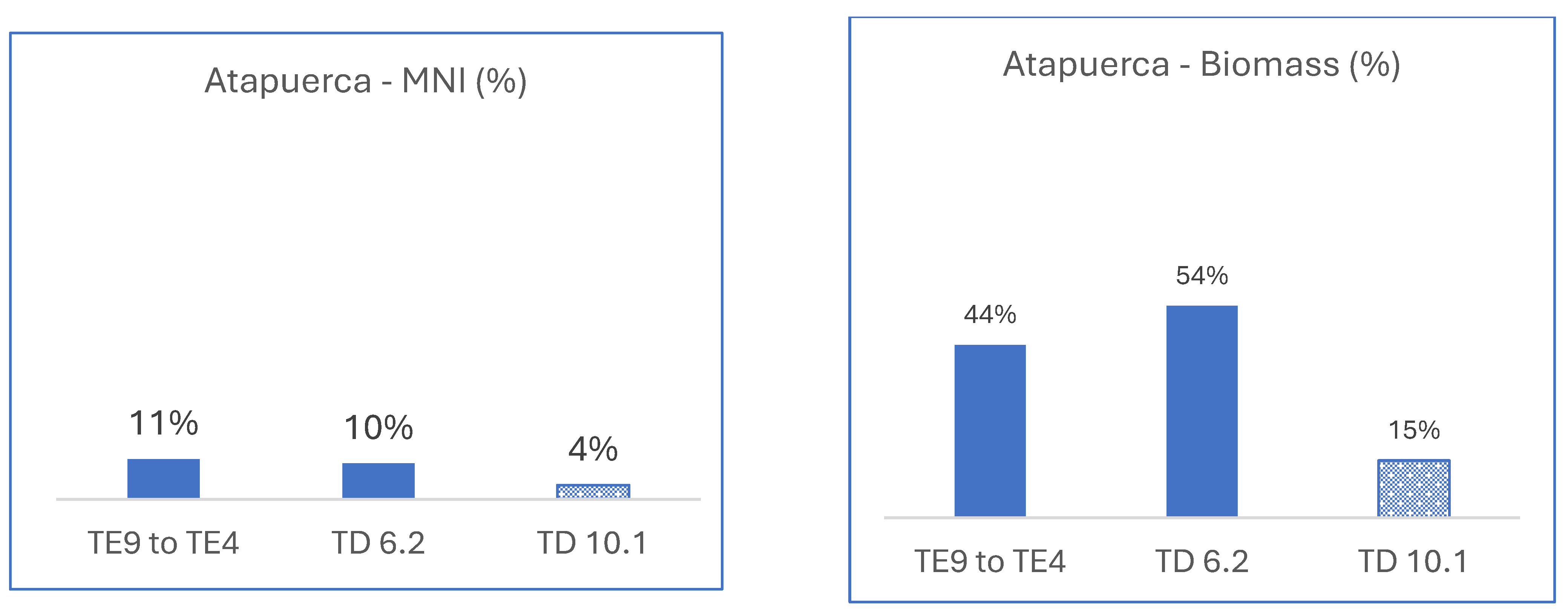
3.2.2. Spain, Atapuerca
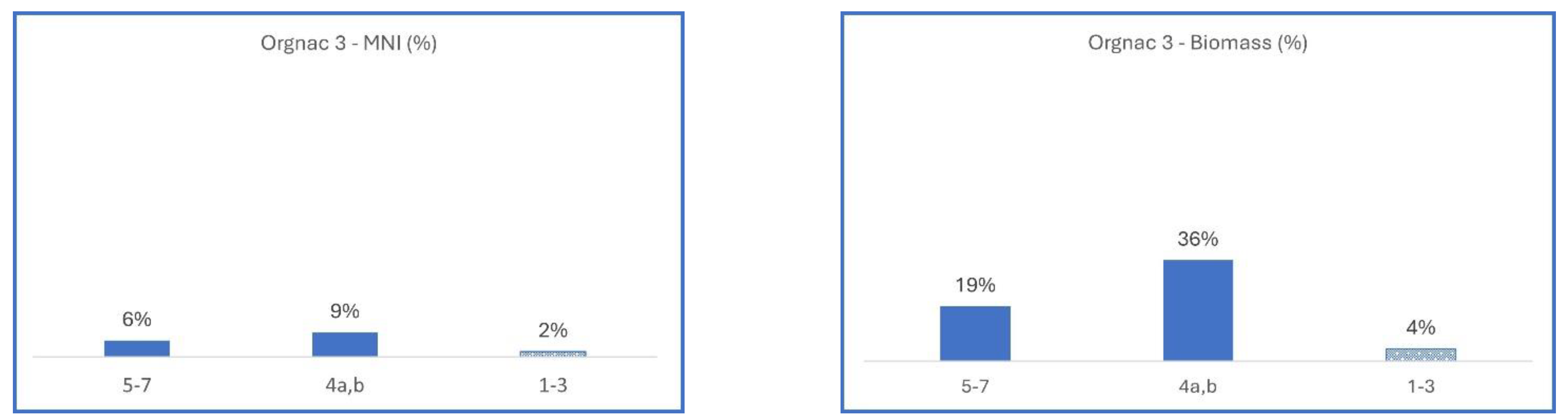
3.2.3. France, Orgnac 3
4. Discussion
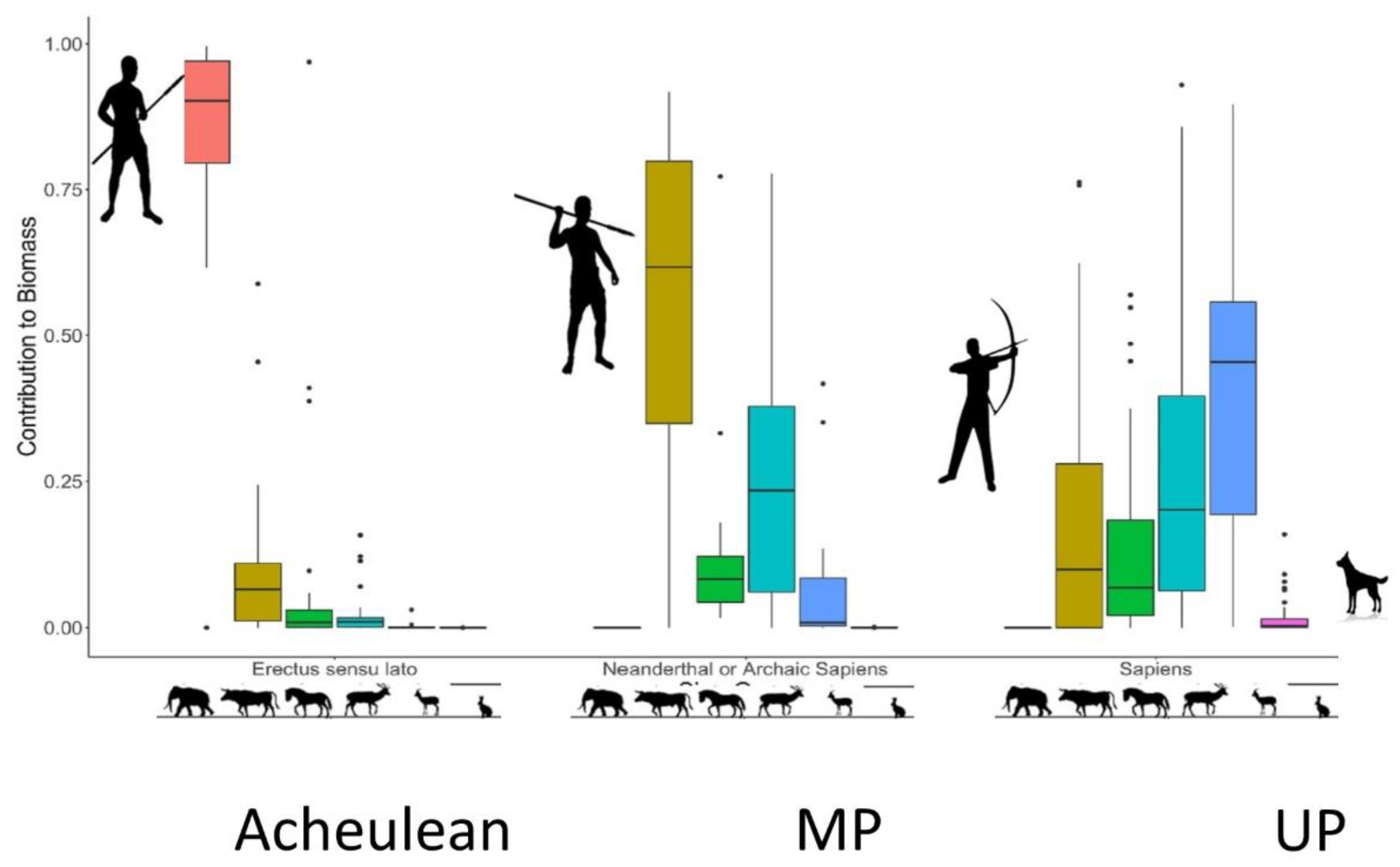
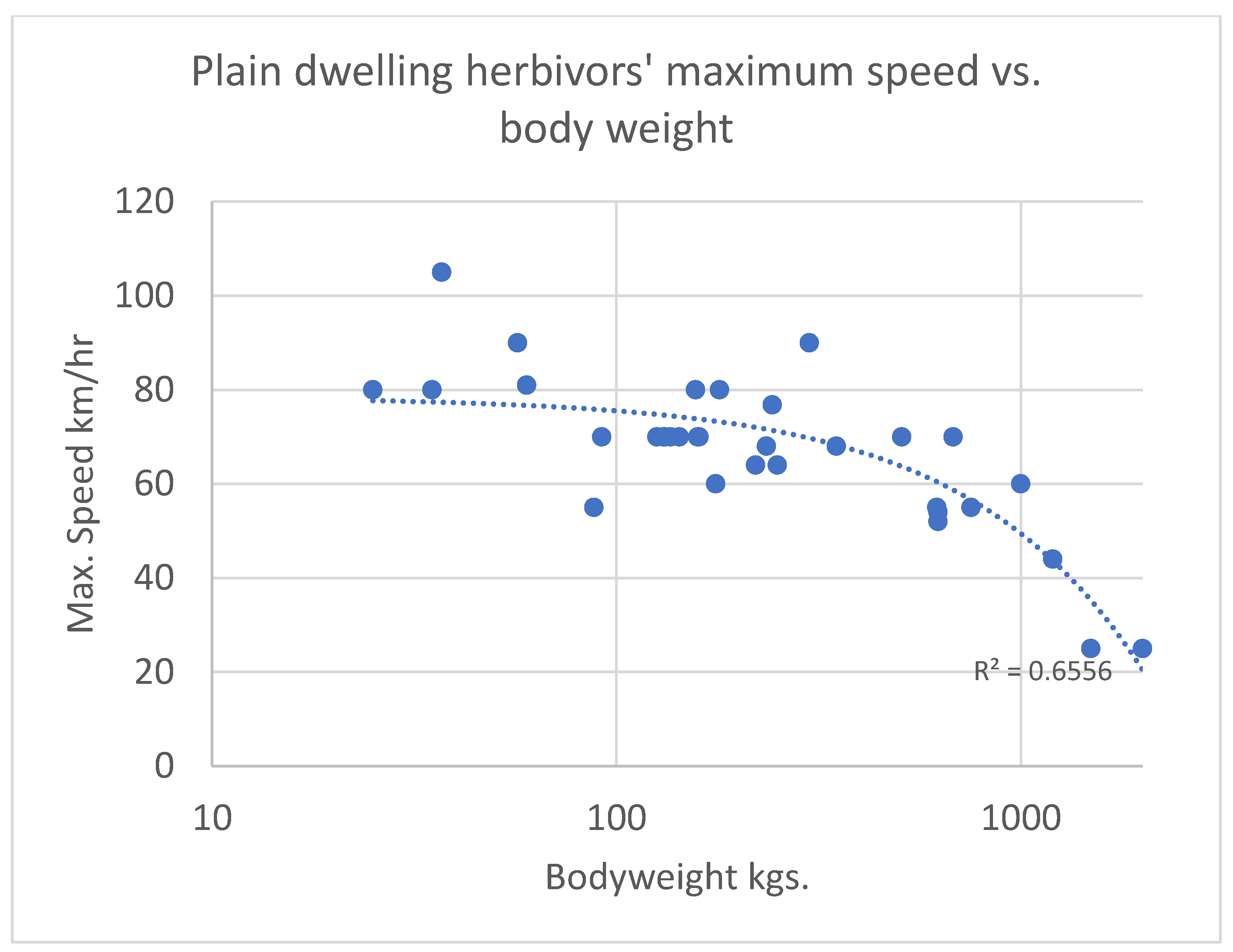
4.1. Energetic Return and Prey Size
4.2. Tipping Spears with Stone Points
4.3. Developing Complex Weapon Systems
4.4. Prey Size Decline and the Evolution of Cognition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ben-Dor, M.; Barkai, R. Prey Size Decline as a Unifying Ecological Selecting Agent in Pleistocene Human Evolution. Quaternary 2021, 4, 7. [Google Scholar] [CrossRef]
- Lombard, M.; Gardenfors, P. Tracking the evolution of causal cognition in humans. J. Anthropol. Sci. 2017, 95, 219–234. [Google Scholar] [CrossRef]
- Pargeter, J.; Khreisheh, N.; Stout, D. Understanding stone tool-making skill acquisition: Experimental methods and evolutionary implications. J. Hum. Evol. 2019, 133, 146–166. [Google Scholar] [CrossRef]
- Wadley, L. Compound-adhesive manufacture as a behavioral proxy for complex cognition in the Middle Stone Age. Curr. Anthropol. 2010, 51, S111–S119. [Google Scholar] [CrossRef]
- Dembitzer, J.; Barkai, R.; Ben-Dor, M.; Meiri, S. Levantine overkill: 1.5 Million Years of Hunting Down the Body Size Distribution in the Paleolithic Southern Levant. Quat. Sci. Rev. 2021, 276, 107316. [Google Scholar] [CrossRef]
- Faith, J.T.; Rowan, J.; Du, A. Early hominins evolved within non-analog ecosystems. Proc. Natl. Acad. Sci. USA 2019, 116, 21478–21483. [Google Scholar] [CrossRef] [PubMed]
- Yravedra, J. Zooarqueología de la Península Ibérica: Implicaciones tafonómicas y paleoecológicas en el debate de los homínidos del Pleistoceno Medio-Superior. BAR Int. Ser. 2001, 979. [Google Scholar] [CrossRef]
- Koch, P.L.; Barnosky, A.D. Late Quaternary Extinctions: State of the Debate. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 215–252. [Google Scholar] [CrossRef]
- Smith, F.A.; Smith, R.E.E.; Lyons, S.K.; Payne, J.L.; Villaseñor, A. The accelerating influence of humans on mammalian macroecological patterns over the late Quaternary. Quat. Sci. Rev. 2019, 211, 1–16. [Google Scholar] [CrossRef]
- Meltzer, D.J. Overkill, glacial history, and the extinction of North America’s Ice Age megafauna. Proc. Natl. Acad. Sci. USA 2020, 117, 28555–28563. [Google Scholar] [CrossRef]
- Faith, J.T.; Rowan, J.; Du, A.; Barr, W.A. The uncertain case for human-driven extinctions prior to Homo sapiens. Quatern. Res. 2020, 96, 88–104. [Google Scholar] [CrossRef]
- Wroe, S.; Field, J.H.; Archer, M.; Grayson, D.K.; Price, G.J.; Louys, J.; Faith, J.T.; Webb, G.E.; Davidson, I.; Mooney, S.D. Climate change frames debate over the extinction of megafauna in Sahul (Pleistocene Australia-New Guinea). Proc. Natl. Acad. Sci. USA 2013, 110, 8777–8781. [Google Scholar] [CrossRef] [PubMed]
- Faurby, S.; Svenning, J.C. Historic and prehistoric human-driven extinctions have reshaped global mammal diversity patterns. Divers. Distrib. 2015, 21, 1155–1166. [Google Scholar] [CrossRef]
- Johnson, C.N.; Alroy, J.; Beeton, N.; Bird, M.I.; Brook, B.W.; Cooper, A.; Gillespie, R.; Herrando-Pérez, S.; Jacobs, Z.; Miller, G.H. What caused extinction of the Pleistocene megafauna of Sahul? Proc. R. Soc. Lond. B Biol. Sci. 2016, 283, 20152399. [Google Scholar] [CrossRef]
- Saltré, F.; Rodríguez-Rey, M.; Brook, B.W.; Johnson, C.N.; Turney, C.S.; Alroy, J.; Cooper, A.; Beeton, N.; Bird, M.I.; Fordham, D.A.; et al. Climate change not to blame for late Quaternary megafauna extinctions in Australia. Nat. Commun. 2016, 7, 10511. [Google Scholar] [CrossRef]
- Faith, J.T. Late Pleistocene and Holocene mammal extinctions on continental Africa. Earth-Sci. Rev. 2014, 128, 105–121. [Google Scholar] [CrossRef]
- Barnosky, A.D.; Lindsey, E.L. Timing of Quaternary megafaunal extinction in South America in relation to human arrival and climate change. Quat. Int. 2010, 217, 10–29. [Google Scholar] [CrossRef]
- Yaroshevich, A.; Oron, M.; Sharon, G. Big-game hunting during the late Middle Paleolithic in the Levant: Insights into technology and behavior from Nahal Mahanayeem Outlet, Upper Jordan River, israel. J. Archaeol. Sci. Rep. 2023, 47, 103777. [Google Scholar] [CrossRef]
- Boëda, E.; Geneste, J.-M.; Griggo, C.; Mercier, N.; Muhesen, S.; Reyss, J.L.; Taha, A.; Valladas, H. A Levallois point embedded in the vertebra of a wild ass (Equus africanus): Hafting, projectiles and Mousterian hunting weapons. Antiquity 1999, 73, 394–402. [Google Scholar] [CrossRef]
- Lazuén, T. European Neanderthal stone hunting weapons reveal complex behaviour long before the appearance of modern humans. J. Archaeol. Sci. 2012, 39, 2304–2311. [Google Scholar] [CrossRef]
- Villa, P.; Lenoir, M. Hunting and hunting weapons of the Lower and Middle Paleolithic of Europe. In The Evolution of Hominin Diets; Springer: Berlin/Heidelberg, Germany, 2009; pp. 59–85. [Google Scholar]
- Lombard, M.; Moncel, M.-H. Neanderthal Hunting Weapons Re-Assessed: A Tip Cross-Sectional Area Analysis of Middle Palaeolithic Point Assemblages from South Eastern France. Quaternary 2023, 6, 17. [Google Scholar] [CrossRef]
- Wilkins, J.; Schoville, B.J.; Brown, K.S.; Chazan, M. Evidence for early hafted hunting technology. Science 2012, 338, 942–946. [Google Scholar] [CrossRef]
- Plummer, T.W.; Oliver, J.S.; Finestone, E.M.; Ditchfield, P.W.; Bishop, L.C.; Blumenthal, S.A.; Lemorini, C.; Caricola, I.; Bailey, S.E.; Herries, A.I.; et al. Expanded geographic distribution and dietary strategies of the earliest Oldowan hominins and Paranthropus. Science 2023, 379, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Venditti, F.; Cristiani, E.; Nunziante-Cesaro, S.; Agam, A.; Lemorini, C.; Barkai, R. Animal residues found on tiny Lower Paleolithic tools reveal their use in butchery. Sci. Rep. 2019, 9, 13031. [Google Scholar] [CrossRef] [PubMed]
- Venditti, F.; Agam, A.; Tirillo, J.; Nunziante-Cesaro, S.; Barkai, R. An integrated study discloses chopping tools use from Late Acheulean Revadim (Israel). PLoS ONE 2021, 16, e0245595. [Google Scholar] [CrossRef] [PubMed]
- Assaf, E.; Caricola, I.; Gopher, A.; Rosell, J.; Blasco, R.; Bar, O.; Zilberman, E.; Lemorini, C.; Baena, J.; Barkai, R.; et al. Shaped stone balls were used for bone marrow extraction at Lower Paleolithic Qesem Cave, Israel. PLoS ONE 2020, 15, e0230972. [Google Scholar] [CrossRef] [PubMed]
- Zupancich, A.; Shemer, M.; Barkai, R. Biface use in the Lower Paleolithic Levant: First insights from late acheulean Revadim and jaljulia (Israel). J. Archaeol. Sci. Rep. 2021, 36, 102877. [Google Scholar] [CrossRef]
- Marinelli, F.; Lemorini, C.; Barkai, R. Lower Palaeolithic small flakes and megafauna: The contribution of experimental approach and use-wear analysis to reveal the link. In Human-Elephant Interactions: From Past to Present; Tübingen University Press: Tübingen, Germany, 2021. [Google Scholar]
- Solodenko, N.; Zupancich, A.; Cesaro, S.N.; Marder, O.; Lemorini, C.; Barkai, R. Fat residue and use-wear found on Acheulian biface and scraper associated with butchered elephant remains at the site of Revadim, Israel. PLoS ONE 2015, 10, e0118572. [Google Scholar] [CrossRef]
- Finkel, M.; Barkai, R. Technological persistency following faunal stability during the Pleistocene: A model for reconstructing Paleolithic adaptation strategies based on mosaic evolution. L’Anthropologie 2021, 125, 102839. [Google Scholar] [CrossRef]
- Lombard, M. Mountaineering or ratcheting? Stone Age hunting weapons as proxy for the evolution of human technological, behavioral and cognitive flexibility. In The Nature of Culture; Springer: Berlin/Heidelberg, Germany, 2016; pp. 135–146. [Google Scholar]
- Toth, N.; Schick, K. An overview of the cognitive implications of the Oldowan Industrial Complex. Azania Archaeol. Res. Afr. 2018, 53, 3–39. [Google Scholar] [CrossRef]
- Koerper, H.C.; Stickel, E.G. Cultural drift: A primary process of culture change. J. Anthropol. Res. 1980, 36, 463–469. [Google Scholar] [CrossRef]
- Domínguez-Rodrigo, M.; Pickering, T.R. The meat of the matter: An evolutionary perspective on human carnivory. Azania Archaeol. Res. Afr. 2017, 52, 4–32. [Google Scholar] [CrossRef]
- Linares Matás, G.J.; Yravedra, J. ‘We hunt to share’: Social dynamics and very large mammal butchery during the Oldowan–Acheulean transition. World Archaeol. 2022, 53, 224–254. [Google Scholar] [CrossRef]
- Oliver, J.S.; Plummer, T.W.; Hertel, F.; Bishop, L.C. Bovid mortality patterns from Kanjera South, Homa Peninsula, Kenya and FLK-Zinj, Olduvai Gorge, Tanzania: Evidence for habitat mediated variability in Oldowan hominin hunting and scavenging behavior. J. Hum. Evol. 2019, 131, 61–75. [Google Scholar] [CrossRef]
- Bridgland, D.R.; Field, M.H.; Holmes, J.A.; McNabb, J.; Preece, R.C.; Selby, I.; Wymer, J.J.; Boreham, S.; Irving, B.G.; Parfitt, S.A.; et al. Middle Pleistocene interglacial Thames–Medway deposits at Clacton-on-Sea, England: Reconsideration of the biostratigraphical and environmental context of the type Clactonian Palaeolithic industry. Quat. Sci. Rev. 1999, 18, 109–146. [Google Scholar] [CrossRef]
- Milks, A.G.; Lehmann, J.; Leder, D.; Sietz, M.; Koddenberg, T.; Böhner, U.; Wachtendorf, V.; Terberger, T. A double-pointed wooden throwing stick from Schöningen, Germany: Results and new insights from a multianalytical study. PLoS ONE 2023, 18, e0287719. [Google Scholar] [CrossRef]
- Conard, N.J.; Serangeli, J.; Bigga, G.; Rots, V. A 300,000-year-old throwing stick from Schöningen, northern Germany, documents the evolution of human hunting. Nat. Ecol. Evol. 2020, 4, 690–693. [Google Scholar] [CrossRef]
- Thieme, H. Lower Palaeolithic hunting spears from Germany. Nature 1997, 385, 807–810. [Google Scholar] [CrossRef]
- Richter, D.; Krbetschek, M. The age of the Lower Paleolithic occupation at Schöningen. J. Hum. Evol. 2015, 89, 46–56. [Google Scholar] [CrossRef]
- Shea, J.J. The origins of lithic projectile point technology: Evidence from Africa, the Levant, and Europe. J. Archaeol. Sci. 2006, 33, 823–846. [Google Scholar] [CrossRef]
- Conard, N.J.; Serangeli, J.; Böhner, U.; Starkovich, B.M.; Miller, C.E.; Urban, B.; Van Kolfschoten, T. Excavations at Schöningen and paradigm shifts in human evolution. J. Hum. Evol. 2015, 89, 1–17. [Google Scholar] [CrossRef]
- Bramble, D.M.; Lieberman, D.E. Endurance running and the evolution of Homo. Nature 2004, 432, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Agam, A.; Barkai, R. Elephant and mammoth hunting during the Paleolithic: A Review of the relevant archaeological, ethnographic and ethno-historical records. Quaternary 2018, 1, 3. [Google Scholar] [CrossRef]
- Churchill, S.E. Weapon technology, prey size selection, and hunting methods in modern hunter-gatherers: Implications for hunting in the Palaeolithic and Mesolithic. Archeol. Pap. Am. Anthropol. Assoc. 1993, 4, 11–24. [Google Scholar] [CrossRef]
- Liebenberg, L. Persistence hunting by modern hunter-gatherers. Curr. Anthropol. 2006, 47, 1017–1026. [Google Scholar] [CrossRef]
- Bunn, H.T. Large ungulate mortality profiles and ambush hunting by Acheulean-age hominins at Elandsfontein, Western Cape Province, South Africa. J. Archaeol. Sci. 2019, 107, 40–49. [Google Scholar] [CrossRef]
- Kübler, S.; Owenga, P.; Reynolds, S.C.; Rucina, S.M.; King, G.C. Animal movements in the Kenya Rift and evidence for the earliest ambush hunting by hominins. Sci. Rep. 2015, 5, 14011. [Google Scholar] [CrossRef]
- Milks, A. A review of ethnographic use of wooden spears and implications for pleistocene hominin hunting. Open Quat. 2020, 6, 12. [Google Scholar] [CrossRef]
- Wilkins, J.; Schoville, B.J. Edge damage on 500-thousand-year-old spear tips from Kathu Pan 1, South Africa: The combined effects of spear use and taphonomic processes. In Multidisciplinary Approaches to the Study of Stone Age Weaponry; Springer: Berlin/Heidelberg, Germany, 2016; pp. 101–117. [Google Scholar]
- Lombard, M. Variation in hunting weaponry for more than 300,000 years: A tip cross-sectional area study of Middle Stone Age points from southern Africa. Quat. Sci. Rev. 2021, 264, 107021. [Google Scholar] [CrossRef]
- McBrearty, S.; Tryon, C. From Acheulean to middle stone age in the Kapthurin formation, Kenya. In Transitions before the Transition; Springer: Berlin/Heidelberg, Germany, 2006; pp. 257–277. [Google Scholar]
- Mercier, N.; Valladas, H. Reassessment of TL age estimates of burnt flints from the Paleolithic site of Tabun Cave, Israel. J. Hum. Evol. 2003, 45, 401–409. [Google Scholar] [CrossRef]
- Villa, P.; Soriano, S. Hunting weapons of Neanderthals and early modern humans in South Africa: Similarities and differences. J. Anthropol. Res. 2010, 66, 5–38. [Google Scholar] [CrossRef]
- Brooks, A.S.; Nevell, L.; Yellen, J.E.; Hartman, G. Projectile technologies of the African MSA. In Transitions Before the Transition; Springer: Boston, MA, USA, 2006; pp. 233–255. [Google Scholar]
- Backwell, L.; Bradfield, J.; Carlson, K.J.; Jashashvili, T.; Wadley, L.; d’Errico, F. The antiquity of bow-and-arrow technology: Evidence from Middle Stone Age layers at Sibudu Cave. Antiquity 2018, 92, 289–303. [Google Scholar] [CrossRef]
- Lombard, M.; Phillipson, L. Indications of bow and stone-tipped arrow use 64,000 years ago in KwaZulu-Natal, South Africa. Antiquity 2010, 84, 635–648. [Google Scholar] [CrossRef]
- Sano, K.; Arrighi, S.; Stani, C.; Aureli, D.; Boschin, F.; Fiore, I.; Spagnolo, V.; Ricci, S.; Crezzini, J.; Boscato, P.; et al. The earliest evidence for mechanically delivered projectile weapons in Europe. Nat. Ecol. Evol. 2019, 3, 1409–1414. [Google Scholar] [CrossRef]
- Germonpré, M.; Lázničková-Galetová, M.; Sablin, M.V.; Bocherens, H. Could Incipient Dogs Have Enhanced Differential Access to Resources among Upper Palaeolithic Hunter-Gatherers in Europe? McDonald Institute for Archaeological Research: Cambridge, UK, 2020. [Google Scholar]
- Guagnin, M.; Perri, A.R.; Petraglia, M.D. Pre-Neolithic evidence for dog-assisted hunting strategies in Arabia. J. Anthropol. Archaeol. 2018, 49, 225–236. [Google Scholar] [CrossRef]
- Lupo, K.D. When and where do dogs improve hunting productivity? The empirical record and some implications for early Upper Paleolithic prey acquisition. J. Anthropol. Archaeol. 2017, 47, 139–151. [Google Scholar] [CrossRef]
- Yeomans, L.; Martin, L.; Richter, T. Close companions: Early evidence for dogs in northeast Jordan and the potential impact of new hunting methods. J. Anthropol. Archaeol. 2019, 53, 161–173. [Google Scholar] [CrossRef]
- Wadley, L. Were snares and traps used in the Middle Stone Age and does it matter? A review and a case study from Sibudu, South Africa. J. Hum. Evol. 2010, 58, 179–192. [Google Scholar] [CrossRef]
- Sinitsyn, A.; Stepanova, K.; Petrova, E. New direct evidence of mammoth hunting from Kostenki. Prehist. Archaeol. J. Interdiscip. Stud. 2019, 1, 149–158. [Google Scholar] [CrossRef]
- Bergman, C.A. Hafting and use of bone and antler points from Ksar Akil, Lebanon. MOM Ed. 1987, 15, 117–126. [Google Scholar]
- Hughes, S.S. Getting to the point: Evolutionary change in prehistoric weaponry. J. Archaeol. Method Theory 1998, 5, 345–408. [Google Scholar] [CrossRef]
- Sisk, M.L.; Shea, J.J. Experimental use and quantitative performance analysis of triangular flakes (Levallois points) used as arrowheads. J. Archaeol. Sci. 2009, 36, 2039–2047. [Google Scholar] [CrossRef]
- Bradfield, J. The Evolution of Bone Points as Hunting Weapons in South Africa. Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2010. [Google Scholar]
- Bocquentin, F.; Bar-Yosef, O. Early Natufian remains: Evidence for physical conflict from Mt. Carmel, Israel. J. Hum. Evol. 2004, 47, 19–23. [Google Scholar] [CrossRef]
- Winterhalder, B. Foraging Strategies in the Boreal Forest: An Analysis of Cree Hunting and Gathering. In Hunter-Gathere Foraging Startegies; Winterhalder, B., Smith, E.A., Eds.; University of Chicago Press: Chicago, IL, USA, 1981. [Google Scholar]
- Villa, P.; D’errico, F. Bone and ivory points in the Lower and Middle Paleolithic of Europe. J. Hum. Evol. 2001, 41, 69–112. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dor, M.; Gopher, A.; Hershkovitz, I.; Barkai, R. Man the fat hunter: The demise of Homo erectus and the emergence of a new hominin lineage in the Middle Pleistocene (ca. 400 kyr) Levant. PLoS ONE 2011, 6, e28689. [Google Scholar] [CrossRef]
- Ben-Dor, M.; Barkai, R. Supersize does matter: The importance of large prey in Paleolithic subsistence and a method for measurement of its significance in zooarchaeological assemblages. In Human-Elephant Interactions: From Past to Present; Konidaris, G., Barkai, R., Tourloukis, V., Harvati, K., Eds.; Tübingen University Press: Tübingen, Germany, 2021. [Google Scholar] [CrossRef]
- Owen-Smith, R.N. Megaherbivores: The Influence of Very Large Body Size on Ecology; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Brammer, J.R.; Menzies, A.K.; Carter, L.S.; Giroux-Bougard, X.; Landry-Cuerrier, M.; Leblanc, M.-L.; Neelin, M.N.; Studd, E.K.; Humphries, M.M. Weighing the importance of animal body size in traditional food systems. FACETS 2022, 7, 286–318. [Google Scholar] [CrossRef]
- Grayson, D.K. Quantitative Zooarchaeology: Topics in the Analysis of Archaelogical Faunas; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Lyman, R.L. Quantitative Paleozoology; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Lyman, R.L. Observations on the history of zooarchaeological quantitative units: Why NISP, then MNI, then NISP again? J. Archaeol. Sci. Rep. 2018, 18, 43–50. [Google Scholar] [CrossRef]
- Domínguez-Rodrigo, M. Critical review of the MNI (minimum number of individuals) as a zooarchaeological unit of quantification. Archaeol. Anthropol. Sci. 2012, 4, 47–59. [Google Scholar] [CrossRef]
- Smith, F.A.; Smith, R.E.E.; Lyons, S.K.; Payne, J.L. Body size downgrading of mammals over the late Quaternary. Science 2018, 360, 310–313. [Google Scholar] [CrossRef]
- Braun, D.R.; Levin, N.E.; Stynder, D.; Herries, A.I.; Archer, W.; Forrest, F.; Roberts, D.L.; Bishop, L.C.; Matthews, T.; Lehmann, S.B.; et al. Mid-Pleistocene hominin occupation at Elandsfontein, Western Cape, South Africa. Quat. Sci. Rev. 2013, 82, 145–166. [Google Scholar] [CrossRef]
- Forrest, F.L. Zooarchaeological and Palaeoenvironmental Reconstruction of Newly Excavated Middle Pleistocene Deposits from Elandsfontein, South Africa; City University of New York: New York, NY, USA, 2017. [Google Scholar]
- Forrest, F.L.; Stynder, D.D.; Bishop, L.C.; Levin, N.E.; Lehmann, S.B.; Patterson, D.B.; Matthews, T.; Braun, D.R. Zooarchaeological reconstruction of newly excavated Middle Pleistocene deposits from Elandsfontein, South Africa. J. Archaeol. Sci. Rep. 2018, 17, 19–29. [Google Scholar] [CrossRef]
- Klein, R.G. Patterns of ungulate mortality and ungulate mortality profiles from Langebaanweg(Early Pliocene) and Elandsfontein(Middle Pleistocene), south-western Cape Province, South Africa. Ann. S. Afr. Mus. 1982, 90, 49–64. [Google Scholar]
- Klein, R.G.; Avery, G.; Cruz-Uribe, K.; Halkett, D.; Hart, T.; Milo, R.G.; Volman, T.P. Duinefontein 2: An Acheulean site in the western Cape province of South Africa. J. Hum. Evol. 1999, 37, 153–190. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Uribe, K.; Klein, R.G.; Avery, G.; Avery, M.; Halkett, D.; Hart, T.; Milo, R.G.; Sampson, C.G.; Volman, T.P. Excavation of buried late Acheulean (mid-quaternary) land surfaces at Duinefontein 2, Western Cape Province, South Africa. J. Archaeol. Sci. 2003, 30, 559–575. [Google Scholar] [CrossRef]
- Hutson, J.M. The faunal remains from Bundu Farm and Pniel 6: Examining the problematic Middle Stone Age archaeological record within the southern African interior. Quat. Int. 2018, 466, 178–193. [Google Scholar] [CrossRef]
- Hutson, J.M. A Comparative Study of Life and Death at Middle Stone Age Open-Air Sites within the Southern African Interior; University of Nevada, Reno: Reno, NV, USA, 2012. [Google Scholar]
- Brink, J.S. The Archaeozoology of Florisbad, Orange Free State; Stellenbosch University: Stellenbosch, South Africa, 1987. [Google Scholar]
- Haradon, C.M. The Ecological Context of the Acheulean to Middle Stone Age Transition in Africa. Ph.D. Thesis, The George Washington University, Washington, DC, USA, 2010. [Google Scholar]
- Potts, R.; Behrensmeyer, A.K.; Faith, J.T.; Tryon, C.A.; Brooks, A.S.; Yellen, J.E.; Deino, A.L.; Kinyanjui, R.; Clark, J.B.; Haradon, C.M.; et al. Environmental dynamics during the onset of the Middle Stone Age in eastern Africa. Science 2018, 360, 86–90. [Google Scholar] [CrossRef]
- Blasco, R.; Fernández Peris, J. A uniquely broad spectrum diet during the Middle Pleistocene at Bolomor Cave (Valencia, Spain). Quat. Int. 2012, 252, 16–31. [Google Scholar] [CrossRef]
- Blasco, R.; Rosell, J.; Fernandez Peris, J.; Luis Arsuaga, J.; Maria Bermudez de Castro, J.; Carbonell, E. Environmental availability, behavioural diversity and diet: A zooarchaeological approach from the TD10-1 sublevel of Gran Dolina (Sierra de Atapuerca, Burgos, Spain) and Bolomor Cave (Valencia, Spain). Quat. Sci. Rev. 2013, 70, 124–144. [Google Scholar] [CrossRef]
- Huguet, R.; Saladié, P.; Cáceres, I.; Díez, C.; Rosell, J.; Bennàsar, M.; Blasco, R.; Esteban-Nadal, M.; Gabucio, M.J.; Rodríguez-Hidalgo, A.; et al. Successful subsistence strategies of the first humans in south-western Europe. Quat. Int. 2013, 295, 168–182. [Google Scholar] [CrossRef]
- Moncel, M.-H.; Moigne, A.-M.; Combier, J. Towards the Middle Palaeolithic in western Europe: The case of Orgnac 3 (southeastern France). J. Hum. Evol. 2012, 63, 653–666. [Google Scholar] [CrossRef]
- Bahain, J.-J.; Mercier, N.; Valladas, H.; Falguères, C.; Masaoudi, H.; Joron, J.-L.; Froget, L.; Moigne, A.-M.; Combier, J.; Moncel, M.-H. Reappraisal of the chronology of Orgnac 3 Lower-to-Middle Paleolithic site (Ardèche, France), a regional key sequence for the Middle Pleistocene of southern France. J. Hum. Evol. 2022, 162, 103092. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.M.; Ruebens, K.; Gaudzinski-Windheuser, S.; Steele, T.E. Subsistence strategies throughout the African Middle Pleistocene: Faunal evidence for behavioral change and continuity across the Earlier to Middle Stone Age transition. J. Hum. Evol. 2019, 127, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kuman, K.; Inbar, M.; Clarke, R. Palaeoenvironments and cultural sequence of the Florisbad Middle Stone Age hominid site, South Africa. J. Archaeol. Sci. 1999, 26, 1409–1425. [Google Scholar] [CrossRef]
- Faith, J.T.; Potts, R.; Plummer, T.W.; Bishop, L.C.; Marean, C.W.; Tryon, C.A. New perspectives on middle Pleistocene change in the large mammal faunas of East Africa: Damaliscus hypsodon sp. nov.(Mammalia, Artiodactyla) from Lainyamok, Kenya. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 361, 84–93. [Google Scholar] [CrossRef]
- Potts, R.; Dommain, R.; Moerman, J.W.; Behrensmeyer, A.K.; Deino, A.L.; Riedl, S.; Beverly, E.J.; Brown, E.T.; Deocampo, D.; Kinyanjui, R.; et al. Increased ecological resource variability during a critical transition in hominin evolution. Sci. Adv. 2020, 6, eabc8975. [Google Scholar] [CrossRef]
- Fernández Peris, J.; Barciela, V.; Blasco, R.; Cuartero Monteagudo, F.; Sañudo Die, P. El Paleolítico Medio en el territorio valenciano y la variabilidad tecno-económica de la Cova del Bolomor. Treballs d'Arqueologia 2008, 14, 141–169. [Google Scholar]
- Rodríguez, J.; Burjachs, F.; Cuenca-Bescós, G.; García, N.; Van der Made, J.; González, A.P.; Blain, H.-A.; Expósito, I.; López-García, J.M.; Antón, M.G. One million years of cultural evolution in a stable environment at Atapuerca (Burgos, Spain). Quat. Sci. Rev. 2011, 30, 1396–1412. [Google Scholar] [CrossRef]
- García-Medrano, P.; Ollé, A.; Mosquera, M.; Cáceres, I.; Carbonell, E. The nature of technological changes: The Middle Pleistocene stone tool assemblages from Galería and Gran Dolina-subunit TD10. 1 (Atapuerca, Spain). Quat. Int. 2015, 368, 92–111. [Google Scholar] [CrossRef]
- Lindstedt, S.L.; Boyce, M.S. Seasonality, fasting, endurance, and body size in mammals. Am. Nat. 1985, 125, 873–878. [Google Scholar] [CrossRef]
- Rosenberg-Yefet, T.; Shemer, M.; Barkai, R. Lower Paleolithic winds of change: Prepared core technologies and the onset of the Levallois method in the Levantine late Acheulian. Front. Earth Sci. 2022, 10, 847358. [Google Scholar] [CrossRef]
- Ellis, C.J. Factors influencing the use of stone projectile tips. In Projectile Technology; Springer: New York, NY, USA, 1997; pp. 37–74. [Google Scholar]
- Hitchcock, R.; Bleed, P. Each according to need and fashion. In Projectile Technology; Springer: New York, NY, USA, 1997; pp. 345–368. [Google Scholar]
- Morin, E.; Bird, D.; Winterhalder, B.; Bird, R.B. Deconstructing Hunting Returns: Can We Reconstruct and Predict Payoffs from Pursuing Prey? J. Archaeol. Method Theory 2022, 29, 561–623. [Google Scholar] [CrossRef]
- Kilby, J.D.; Surovell, T.A.; Huckell, B.B.; Ringstaff, C.W.; Hamilton, M.J.; Haynes, C.V., Jr. Evidence supports the efficacy of Clovis points for hunting proboscideans. J. Archaeol. Sci. Rep. 2022, 45, 103600. [Google Scholar] [CrossRef]
- Moore, C.R.; Kimball, L.R.; Goodyear, A.C.; Brooks, M.J.; Daniel, I.R., Jr.; West, A.; Taylor, S.G.; Weber, K.J.; Fagan, J.L.; Walker, C.M. Paleoamerican exploitation of extinct megafauna revealed through immunological blood residue and microwear analysis, North and South Carolina, USA. Sci. Rep. 2023, 13, 9464. [Google Scholar] [CrossRef] [PubMed]
- Eren, M.I.; Story, B.; Perrone, A.; Bebber, M.; Hamilton, M.; Walker, R.; Buchanan, B. North American Clovis point form and performance: An experimental assessment of penetration depth. Lithic Technol. 2020, 45, 263–282. [Google Scholar] [CrossRef]
- Prates, L.; Rivero, D.; Perez, S.I. Changes in Projectile design and size of prey reveals the role of Fishtail points in megafauna hunting in South America. Sci. Rep. 2022, 12, 16964. [Google Scholar] [CrossRef]
- Buchanan, B.; Collard, M.; Hamilton, M.J.; O’Brien, M.J. Points and prey: A quantitative test of the hypothesis that prey size influences early Paleoindian projectile point form. J. Archaeol. Sci. 2011, 38, 852–864. [Google Scholar] [CrossRef]
- Anderson, D. How to make a bigger hole: An experimental analysis of projectile point morphology in wound creation. In Furthering Perspectives: Anthropological Views of the World Volume 4: 2010; Colorado State University: Fort Collins, CO, USA, 2010. [Google Scholar]
- Davidson, D.S. Australian spear-traits and their derivations. J. Polyn. Soc. 1934, 43, 41–72. [Google Scholar]
- Wood, J.; Fitzhugh, B. Wound ballistics: The prey specific implications of penetrating trauma injuries from osseous, flaked stone, and composite inset microblade projectiles during the Pleistocene/Holocene transition, Alaska U.S.A. J. Archaeol. Sci. 2018, 91, 104–117. [Google Scholar] [CrossRef]
- Frison, G.C. Prehistoric Hunters of the High Plains; Academic Press: New York, NY, USA, 1978; Volume 1. [Google Scholar]
- Shea, J.J. Middle Paleolithic spear point technology. In Projectile Technology; Knecht, H., Ed.; Springer: New York, NY, USA, 1997; pp. 79–106. [Google Scholar]
- Lombard, M. Quartz-tipped arrows older than 60 ka: Further use-trace evidence from Sibudu, KwaZulu-Natal, South Africa. J. Archaeol. Sci. 2011, 38, 1918–1930. [Google Scholar] [CrossRef]
- Hirt, M.R.; Jetz, W.; Rall, B.C.; Brose, U. A general scaling law reveals why the largest animals are not the fastest. Nat. Ecol. Evol. 2017, 1, 1116. [Google Scholar] [CrossRef]
- Odell, G.H.; Cowan, F. Experiments with spears and arrows on animal targets. J. Field Archaeol. 1986, 13, 195–212. [Google Scholar]
- Germonpré, M.; Sablin, M.V.; Lázničková-Galetová, M.; Després, V.; Stevens, R.E.; Stiller, M.; Hofreiter, M. Palaeolithic dogs and Pleistocene wolves revisited: A reply to Morey (2014). J. Archaeol. Sci. 2015, 54, 210–216. [Google Scholar] [CrossRef]
- Lombard, M.; Parsons, I. What happened to the human mind after the Howiesons Poort? Antiquity 2011, 85, 1433–1443. [Google Scholar] [CrossRef]
- Clark, J.L. The Howieson’s poort fauna from Sibudu cave: Documenting continuity and change within Middle Stone Age industries. J. Hum. Evol. 2017, 107, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Janetski, J.C. Fremont hunting and resource intensification in the eastern Great Basin. J. Archaeol. Sci. 1997, 24, 1075–1088. [Google Scholar] [CrossRef]
- Smith, C.S. The bow and arrow, population, environment, and seeds: Intensification in southwest Wyoming. J. Anthropol. Archaeol. 2021, 62, 101300. [Google Scholar] [CrossRef]
- Bettinger, R.L.; Eerkens, J. Point typologies, cultural transmission, and the spread of bow-and-arrow technology in the prehistoric Great Basin. Am. Antiq. 1999, 64, 231–242. [Google Scholar] [CrossRef]
- Ambrose, S.H. Paleolithic technology and human evolution. Science 2001, 291, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T. Hafted spears and the archaeology of mind. Proc. Natl. Acad. Sci. USA 2009, 106, 9544–9545. [Google Scholar] [CrossRef]
- Coolidge, F.L.; Haidle, M.N.; Lombard, M.; Wynn, T. Bridging theory and bow hunting: Human cognitive evolution and archaeology. Antiquity 2016, 90, 219–228. [Google Scholar] [CrossRef]
- Gärdenfors, P.; Lombard, M. Causal cognition, force dynamics and early hunting technologies. Front. Psychol. 2018, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Liebenberg, L. The Origin of Science-The Evolutionary Roots of Scientific Reasoning and Its Implications for Citizen Science; CyberTracker: Cape Town, South Africa, 2013. [Google Scholar]

| Country | Site | Period | Total MNI | Reference |
|---|---|---|---|---|
| South Africa | Elandsfontein | ESA | 1140 | [83,84,85,86] |
| South Africa | Duinefontain | ESA | 225 | [87,88] |
| South Africa | Bundu Farm | Early MSA | 29 | [89,90] |
| South Africa | Pniel 6 | Early MSA | 48 | [89,90] |
| South Africa | Florisbad | Early MSA | 29 | [91] |
| East Africa | Olorgesailie Member 10 | Acheulean | 31 | [92,93] (Supplementary Materials) |
| East Africa | Olorgesailie BOK layers | Early MSA | 45 | [92,93] (Supplementary Materials) |
| Europe, Spain | Bolomor Cave | Ancient MP | 92 | [94,95] |
| Europe, Spain | Sima del Elefante | Early Pleistocene | 46 | [96] |
| Europe, Spain | Gran Dolina | Early Pleistocene, Late Acheulean | 57 | [96] |
| Europe, France | Orgnac 3 | Upper Acheulean, Early MP | 410 | [97,98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben-Dor, M.; Barkai, R. The Evolution of Paleolithic Hunting Weapons: A Response to Declining Prey Size. Quaternary 2023, 6, 46. https://doi.org/10.3390/quat6030046
Ben-Dor M, Barkai R. The Evolution of Paleolithic Hunting Weapons: A Response to Declining Prey Size. Quaternary. 2023; 6(3):46. https://doi.org/10.3390/quat6030046
Chicago/Turabian StyleBen-Dor, Miki, and Ran Barkai. 2023. "The Evolution of Paleolithic Hunting Weapons: A Response to Declining Prey Size" Quaternary 6, no. 3: 46. https://doi.org/10.3390/quat6030046
APA StyleBen-Dor, M., & Barkai, R. (2023). The Evolution of Paleolithic Hunting Weapons: A Response to Declining Prey Size. Quaternary, 6(3), 46. https://doi.org/10.3390/quat6030046







