1. Introduction
The Carthusian Order was part of the reform movement of the Catholic Church in the 11th-century, which involved the combination of manual labour with solitude and contemplation. The order was established by Bruno of Cologne in 1084. The first Carthusian monastery was founded in a mountain place called Chartreuse near Grenoble (France), from which the entire order derived its name [
1,
2].
In May 1366, the foundation of the Charterhouse of Calci was authorised by the Archbishop of Pisa in the so-called Vallis Gratiosa, a secluded place near Pisa (central Italy) to promote the isolation according to the Rule of the Order. By the end of the 14th century, the first nucleus had been completed, but its construction continued for a long time. In the second half of the 15th century, the monastery was expanded by building new monks’ cells and green spaces for gardens. Between the 17th and 18th centuries a major renovation led to its current version. In the 20th century, the Charterhouse experienced different circumstances. It was partially converted into a military hospital and barracks during World War I. After the monastic community closed in 1969, the monks left the Monastery permanently in 1972. The Italian Ministry of Cultural Heritage assumed its management directly, instituting the National Museum of the Monumental Charterhouse of Calci. Finally, in 1979, the architectural complex was partially entrusted to the University of Pisa and to the Museum of Natural History.
Charterhouses were intertwined in a complex network of relationships between people, plants, and the environment. Gardens had an important role in the hermitic life of the monks, and represented individual feelings and their relationships with nature and divinity. Moreover, the agricultural practices carried out by lay brothers inside the Charterhouse, which included the cultivation of fruit trees, grapevines, and crops, as well as the management of surrounding lands and woods, were of primary importance for the economic and religious system of Chartusians.
The present work stems from the archaeological research programme performed in the Charterhouse of Calci since 2018. The research project can be ascribed to Garden Archaeology and has been carried out with the main aim of understanding the garden practices and horticulture over the centuries of occupation. The study includes pollen, anthracological, and carpological analyses. The study of animal bones completed the bioarchaeological analyses [
3,
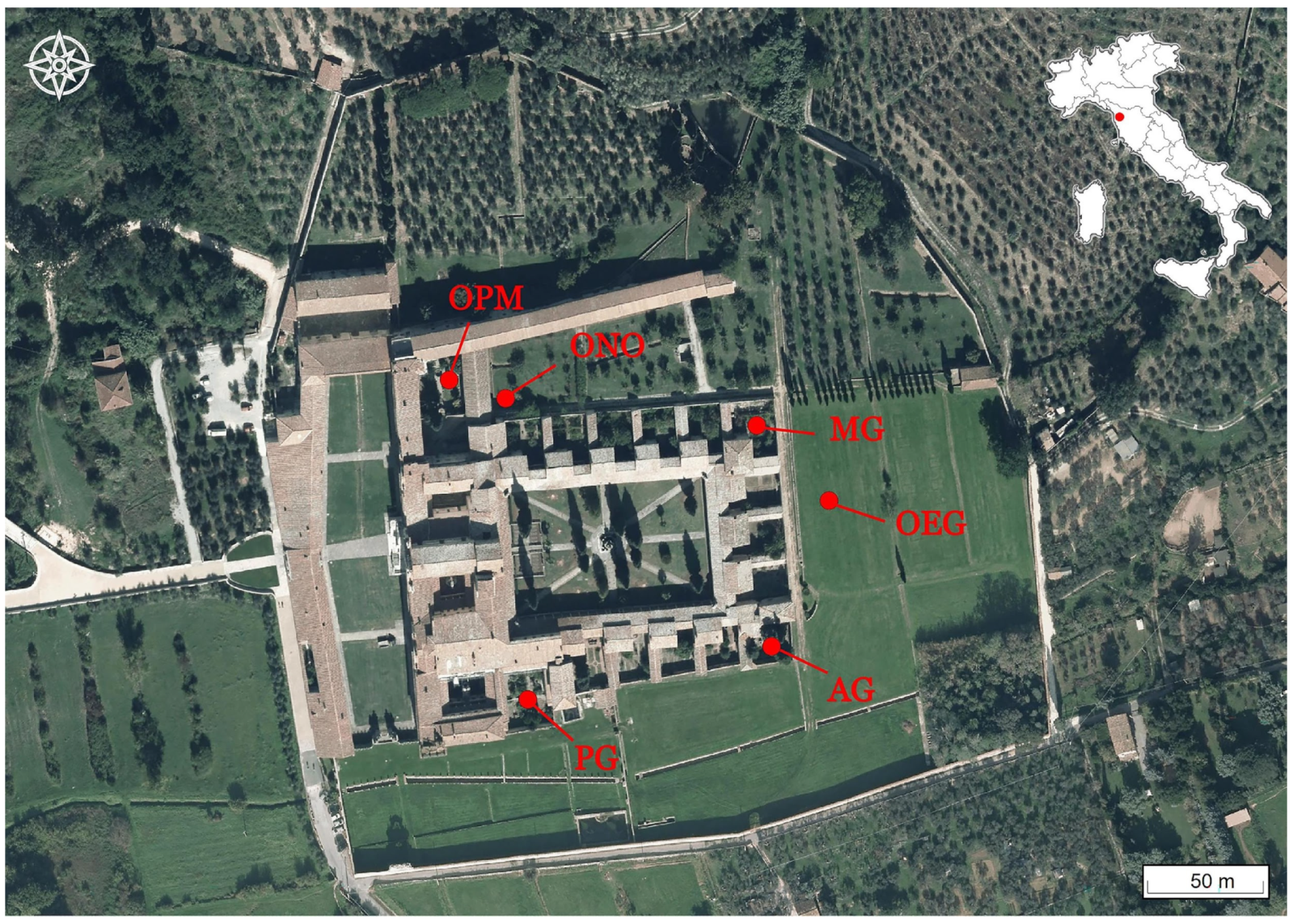
4]. The archaeological investigation has focused on the architectural transformations of the gardens of the Father Prior’s, the Father Apothecary’s, and the Father Master’s cells, and the green spaces outside the cloister walls, consisting of courtyards and orchards (
Figure 1). Special attention has been given to biological disturbances caused, e.g., by roots, animals, and microorganisms, pedological variations and horticultural activities to obtain a detailed picture of planting/explanting, fertilisation, and other cultivation practices.
In this framework, pollen and plant macroremains have been studied to determine which ornamental species were planted in the gardens at different periods of their occupation and their relationships with the local environment. Previous studies of pollen from gardens have revealed the great potentiality of this approach to reconstruct details of horticultural selection during Roman times (Villa Arianna-Stabiae in Italy and gardens of Herod the Great-Judea in Israel [
5]; Casa dei Casti Amanti-Pompeii [
6]) or in Medieval and Renaissance times (Giardino delle Duchesse-Ferrara [
7,
8]). The data obtained from samples taken in different sectors, inside and outside the Charterhouse of Calci, provided information on the specific biodiversity of the different types of gardens and the environmental context closest to the Charterhouse building during the last six centuries.
2. Materials and Methods
The archaeological excavations have been carried out inside and outside the cloister wall of the Charterhouse. Six areas were excavated in three Fathers’ gardens: (i) two areas in the Father Prior’s Garden (PG), a larger one on the lower terrace and a more limited one on the upper terrace, (ii) one area in the Father Apothecary’s Garden (AG), and (iii) three areas in the Father Master’s Garden (MG). Three areas were investigated in the spaces outside (O) the cloister area: one in the so-called Pisan Mountains courtyard (OPM), a small garden amid the buildings belonging to the late 18th-century renovation of the Charterhouse, one in the Northern Orchard (ONO), and one in the Eastern vegetable Garden (OEG). The excavation in the OPM was carried out on a square portion of 5.50 m2 within one of the four flowerbeds of the courtyard. The ONO and OEG areas have been investigated through two small archaeological areas of 4 m2 each.
A structure-from-Motion (SfM)-based field documentation workflow was applied to produce a high-resolution 3D spatial reconstruction of the archaeological stratigraphy (
Figure 2).
SfM is a photogrammetric technique for producing three-dimensional data from two-dimensional photos, increasingly adopted in archaeology for digitising surfaces, landscapes, and objects (see, for example, [
9]).
No radiocarbon dating has been performed. All the datings come from the analysis of archaeological finds and archaeological stratigraphy.
2.1. Pollen Analysis
Samples from selected Stratigraphic Units (SU) were chosen for pollen analysis and a total of 28 samples were studied: 4 samples from the PG (CerC1-CerC4), 3 from AG (CerC5-CerC7), 5 from MG (CerC8-CerC12), 7 from the OPM courtyard (CerC13-CerC19), 6 from the ONO orchard (CerC20-CerC25), and 3 from the OEG outside area (CerC26-CerC28) (
Table 1).
After weighing the sample, a known quantity of spores (
Lycopodium) was added to each sample; this is useful to calculate pollen concentration (as p/g = pollen grains per gram). The extraction method involved: deflocculation with tetrasodium pyrophosphate decahydrate, sieving with a nylon filter, dissolution of the carbonates with 10% hydrochloric acid, dehydration in acetic acid and acetolysis, enrichment with heavy liquid (Na-metatungstate hydrate), dissolution of the silicates in 40% hydrofluoric acid, passages in alcohol, inclusion in glycerine, and drying in an oven at about 50 °C. After treatment, fixed slides were set up, and stored in the Reference Pollen Collection of the University of Modena’s Laboratory, for reading under a transmitted light optical microscope. Pollen analyses were made at magnifications of 400× and 1000× under oil immersion. Pollen identification was conducted using dichotomous keys, photographic atlases, and the laboratory’s pollen comparison collection. Undetermined pollen was counted in the pollen sum. Non-Pollen Palynomorphs (NPPs) were detected according to van Geel’s methodology [
10]. Both mono- and polyspecific pollen clumps are linked to the presence of flowers in situ, i.e., spontaneous growth or deliberate cultivation/deposition of plants, insect activity or the presence of arthropod or herbivore droppings in the layer.
The pollen diagram was drawn with Tilia 3.0.1 [
11] and shows histograms testifying that samples have been horizontally collected in the different contexts. Pollen data from the entire dataset (CerC1-CerC28) were sorted by Principal Component Analysis (PCA) using XLSTAT software to obtain data on dissimilarities between the contexts in terms of plant composition and biodiversity. PCA elaborations were carried out considering both the complete list of pollen taxa and the sums of broader taxonomic categories (i.e., Family). Based on the very similar results obtained in the different applications, the synthetic version was chosen because it is better from a graphical point of view.
2.2. Plant Macroremains
The analyses of the charcoals and seeds/fruits were carried out on 11 SU from the PG, 8 from the AG, and 8 from the MG contexts (
Table 1). Each sample was obtained by water sieving and sifting with a mesh size greater than 1 mm, with a sampling rate varying between 10 and 25% for the largest SU and the total amount of sediment for those of limited ones (e.g., ash lenses and pit fills).
The anthracological analysis on charcoal fragments larger than 2 mm was performed under a reflected light optical microscope, at a magnification of 100×, 200×, and 500×. Identification was made with reference to wood anatomy atlases [
12,
13,
14]. Charcoal fragments were classified at species or genus level, or with the taxonomic nomenclature used in anthracology. Sometimes, poor preservation or vitrification of the remains resulted in the identification of the family or non-identification at all. The charcoal fragments identified for each taxon in each SU were quantified, calculating the percentage of each taxon, or the taxa quantities grouped by chronological intervals.
Carpological analysis was conducted using a stereomicroscope at magnifications from 0.5× to 20×. The records were distinguished from the fragments, quantifying both groups, and each category was classified by carpological type based on morphological characters. For each type, taxonomic recognition at the level of species, genus, or taxon was carried out under the light optical microscope [
15].
3. Results
A synoptic description of the results obtained from the archaeological and archaeobotanical studies is reported below by considering (i) the Fathers’ gardens, located within the cloister walls, and (ii) the green spaces, with different uses, located out of the cloister walls. Firstly, the archaeobotanical data are described by reporting pollen, charcoal, and seed/fruit evidence obtained by the samples collected from each context. Then, the statistical data elaboration (PCA) of pollen spectra allowed us to compare the different contexts and highlight dissimilarities among them.
In general, 8675 pollen grains were counted, and a mean of about 47,000 p/g was found on average in the samples. All over the spectra, the number of pollen taxa is 135 (39 taxa of trees and shrubs and 96 of non-arboreal plants), ranging between 24 and 57 pollen taxa per sample. Arboreal Pollen is almost ⅕ of the spectra (AP = 21% on average), while Non-Arboreal Pollen is prevalent in all samples (NAP = 79%).
Among trees, pollen of
Olea europaea (2.7% on average), deciduous
Quercus (2.5%),
Pinus (1.4%), and
Ulmus (1.1%) are prevalent, together with shrubs of Ericaceae (3.7%), Cheno-Amaranthaceae (2%), and some herb plants belonging to Asteraceae (19.7%, prevalently Cichorieae), wild Poaceae (7.9%),
Ranunculus acris type (5%), Apiaceae (4.4%, including
Apium), Cyperaceae (4.2%), Brassicaceae (3.5%),
Plantago (3.3%),
Urtica (3.2%), Caryophyllaceae (2.6%), Fabaceae (2.4%), Rosaceae (2.3%, including
Rosa),
Anagallis (1.2%),
Saxifraga hirsuta type (1.2%), and Lamiaceae (1.1%). Cereals are present with
Hordeum and
Avena/
Triticum groups and
Panicum (up to 2.4% in CerC25). Of interest is the presence of
Castanea sativa,
Corylus avellana,
Vitis, and
Cannabis (
Figure 3).
Charcoal analysis counted 2559 charred wood remains. All over the spectra, the number of determined taxa is 30, attributable to trees and shrubs (in particular, 12 deciduous broadleaves, 8 evergreen sclerophyllous plants, 1 conifer, and 6 cultivated/cultivable plants), ranging between 1 and 20 taxa per sample (
Figure 4).
Charcoal of Pinus halepensis/pinaster (11.8% on average), Quercus cf. cerris (11.3%), Castanea sativa (6.5%), deciduous Quercus (5.7%), Fraxinus cf. ornus (5.4%), Olea europaea (3.9%), Quercus cf. ilex (2.9%), and Alnus (2.7%) are prevalent among trees. Erica (9%), Rhamnus/Phillyrea (4.8%), Smilax (2.5%), and Arbutus unedo (1.9%) are the most recorded shrubs. We also highlight the presence of Maloideae (5.6%).
Carpological analysis counted 32 charred seed/fruit remains (
Table 2). The number of taxa is 10, attributable to fruit trees (6 taxa and 21 individual remains), wild hygrophilous trees (1 taxon and 5 remains), cereals (2 taxa and 4 remains), and pulses (1 taxon and 2 remains).
Among fruit trees, Vitis vinifera, Corylus avellana, Malus/Pyrus, and Prunus persica are the most recorded, with genus Triticum (T. aestivum/durum) as cereal.
3.1. The Fathers’ Internal Gardens (PG, AG, MG)
The archaeological investigations inside the cloistered wall focused on the gardens of the Father Prior’s, Father Apothecary’s, and Father Master’s cell [
3,
4]. The gardens were addressed as highly human-made landscapes of limited extent. For this reason, their transformations and relationships with the environment, plant communities, and cultivation inside and outside the monastery were investigated.
3.1.1. Father Prior’s Garden—PG
4 pollen samples (CerC1-CerC4).
17th–second half 19th centuries.
Aquatics, vegetables, and wet lawn with some rocky and ornamental plants.
The garden of the Father Prior, organised in an upper and lower terrace, was created at the end of the 14th century. Around the mid/second half of the 18th century, the area was reorganised to realise a ‘meditation garden‘ with flowerbeds and an octagonal fountain flanked by two rectangular basins that converged scenically in the grotesque backdrop. In the 19th century, the octagonal basin was converted into a stand-alone ornamental fountain with a fake stony ‘island’ in the centre; above the demolished southern parapets of the side basins, a new flowerbed was created with sandstone curbs along the southern boundary wall. A more recent phase of use of the flowerbed is the intentional burial of a domestic cat (
Felis catus) in a wooden box. Afterwards, the flowerbed was sporadically used as a dump site, and for only occasional horticultural purposes in the present time [
3,
4].
Pollen analysis revealed a large variety of plant species (41–48 pollen taxa per sample), consisting mainly of herbaceous plants (min.88%–max.92%). In addition to the aquatics, such as Nymphaea alba type (white water lily) and Nuphar (yellow pond lily) (present with 0.6% only in sample CerC3), which attest the ornamental function of the fountain system, Cyperaceae (5.4% on average), Sparganium emersum type (European bur-reed) and Typha latifolia type (common cattail) (together 0.6% on average) testify to wet grass cover both in situ and, as suggested by Alnus (alder) (1.1% on average), in the area. Moreover, pollen of cultivated vegetables, such as Allium (garlic), Daucus cf. carota (carrot), Mentha type (mint), and Raphanus (horseradish), altogether totalling about 1.5% on average, was observed in the flowerbed of the upper terrace. In addition, the presence of pollen of the Hordeum and Avena/Triticum groups (about 1.4% on average) is evidence of some transport or cultivation of cereals in the site. Evidence of ornamental plants was also detected, including different genera of Rosaceae such as Rosa (rose), possibly grown planted as hedges. Moreover, there are Aphanes/Alchemilla (parsley-piert/lady’s mantle), Filipendula (meadowsweet), Potentilla (cinquefoils), in the flowerbeds. The garden plants were also represented by ‘pseudo-climbing plants’, such as Solanum (nightshade). Other flowers probably were selected for their different seasonal blooms, for example, Geranium (cranesbills), Papaver (poppy), Adonis (pheasant’s eye summer), Anagallis (pimpernel), Oxalis (common yellow wood sorrel), Polygala (milkworts) blooming in summer; Primula (primrose) and Liliaceae s.l. blooming in spring; Helleborus (hellebores) during winter; Herniaria (ruptureworts), Sedum (stonecrops), and Saxifraga (saxifrages), which include stonecrop and graceful flowering evergreens. The small number of local trees might have included Acer campestre type (field maples), which was explanted in the 19th century from the upper terrace, traces of Corylus avellana (hazel), Juglans (walnut) and Vitis (grapevine), while Hedera helix (ivy) and other evergreens, such as Erica (heather), probably covered the perimeter walls.
11 charcoal samples.
Second half 15th–end 20th centuries.
Mesophilous and thermophilous deciduous broadleaved, sclerophyllous evergreen and coniferous trees and shrubs. Some cultivated fruit trees.
The anthracological study analysed 1017 charcoal remains, of which 944 were determined in 25 taxa (
Figure 4). Anthracological analysis revealed the prevalence of non-cultivated plants (67.5% of determined charcoals) pertaining to woodlands and semi-natural areas. Woodland is primarily attested by evergreen sclerophyllous taxa (22%) and by mesophilous and thermophilous deciduous broadleaved trees (22.4%), followed by conifer (16.4%). Among the cultivated taxa (32.5%),
Castanea sativa (chestnut) predominates (19.8%). Deciduous broadleaves are documented by arboreal species, such as
Fraxinus cf.
ornus (manna ash),
Fagus sylvatica (European beech), and
Quercus cf.
cerris (Turkey oak). Evergreen sclerophyllous formations are mainly shrubs, such as
Erica,
Arbutus unedo (strawberry tree),
Rhamnus/
Phillyrea (buckthorns/phillyrea),
Smilax (sarsaparilla), and trees such as
Quercus cf.
ilex (holm oak). Including both deciduous and evergreen plants, Maloideae (apple subfamily) is rather recorded.
Pinus halepensis/
pinaster (Aleppo/maritime pine) is the only conifer tree. Lowland and riparian environments are poorly evidenced by
Fraxinus cf.
angustifolia (southern ash) and
Alnus. In addition to chestnut, species of agricultural interest,
Olea europaea (olive tree) is considerably documented, combined with
Prunus cf.
dulcis (almond tree),
Juglans regia, and
Vitis vinifera.
The carpological analysis provided identification of cultivated fruit plants (3 taxa;
Table 2). A single endocarp of
Corylus avellana and a pip of
Vitis vinifera come from the late 19th-century stratigraphy and a stone of
Prunus persica (peach) from the end of the 20th century.
3.1.2. Father Apothecary’s Garden—AG
3 pollen samples (CerC5-CerC7).
Late 15th/early 16th–first half 20th centuries.
Medicinal and aromatic plants, ornamental shrubs and trees.
In the Apothecary’s Garden, the oldest traces of anthropic frequentation date back to the 15th century, when layers of debris levelled the sloping natural surface, and agricultural practices are attested. The garden was created in the 17th century and transformed between the 17th and 18th centuries when it reached its present appearance, with sandstone curb flowerbeds covered with a pergola that crossed the garden. Continuous gardening practices are evidenced till the 20th century when the garden was affected by mortar and charcoal dumping and a naturally progressive growth deposit, followed by a layer originating from the decomposition of the roots of the cypress tree still in situ [
3,
4].
The Apothecary’s Garden shows wide floristic diversity (from 42 to 57 pollen taxa per sample), with 78–84% pollen from herbaceous plants and a significant occurence of woody plants. Hazelnut, oak, and alder are represented in all samples, and pollen spectra include Quercus (oak) (ca. 4% on average), Juniperus/Cupressus (cypress), Fraxinus ornus, and Ulmus (elms) (ca. 2% each). The presence of Syringa vulgaris (lilac) (1.1% on average), which is a shrub widely appreciated for its fragrant flowers, is of particular interest. Among the other ornamental and woody plants, Hedera helix (up to 3.0% in CerC6) and Erica are also well-represented, together with Corylus avellana and Vitis (1% each). Interestingly, traces of the pollen of inaperturate Vitis was detected (0.3% and 0.9% in samples CerC6-CerC7). Castanea sativa is rare (3.9% only in sample CerC7) and its presence in the spectra could be due to the transport of acid soils from a hilly/mountain environment rather than a local planting. Wet plants are not highly represented in pollen spectra from this garden (3.6%), although Alnus, Populus (poplar), Salix (willows), and hygrophilous herbs are evident. The relatively high value of cereal pollen (1.4%) testifies to the use of straws in horticultural practices. High values have been identified for medicinal-aromatic plants, such as Hypericum (St. John’s wort), Salvia (sage), Malva (common mallow), and Papaver include food and medicinal plants and can be wild or cultivated. A very frequent find is Apium, which could be identified as the cultivated species (A. graveolens) based on the high percentages found (5.1%). Apium graveolens var. rapaceum (celeriac) is used for medicinal purposes, cooking, and preparing infusions and it can easily be preserved up to 4–5 months. Peucedanum (hog’s fennel) can also be used in the production of medicines and in the preparation of liqueurs. Other herbaceous plants are also present, such as Valerianella (lamb’s lettuce), a variety of salads, and Cirsium (bull thistle), sometimes used in cooking as an artichoke.
The anthracological study involves 751 charcoals (among which 680 determined records) belonging to 24 taxa (
Figure 4). Many records belong to uncultivated plants (20 taxa) and 95.3% are from woodlands and semi-natural areas. The woodland is mainly testified by conifer (16.6%) and evergreen sclerophyllous formation (17.8%), and especially by mesophilous and thermophilous deciduous broadleaved trees (47.2%). Among cultivated taxa (4.7%),
Olea europaea is the most frequent (3.5%). Trees, such as
Fraxinus cf.
ornus,
Quercus cf.
cerris,
Ulmus, and
Ostrya carpinifolia (European hop-hornbeam), provide evidence of deciduous vegetation. Woody taxa also extend to Maloideae (apple subfamily) and undergrowth shrubs, such as cf.
Crataegus (hawthorn) and
Cornus (dogwood). Riparian habitats are testified by
Alnus. Evergreen sclerophyllous flora consists of shrubs, such as
Erica,
Arbutus unedo,
Rhamnus/
Phillyrea,
Smilax, and tree plants, such as
Quercus cf.
ilex and
Pinus halepensis/
pinaster. Among the cultivated/cultural trees,
Olea europaea is the most recorded, followed by
Castanea sativa.
The carpological analysis also identified 5 taxa (
Table 2): two stones of
Prunus persica dated on the basis of the archaeological stratigraphy to the 15th and 19th–20th centuries, two caryopses of
Triticum sp. (wheat) and one
Olea europaea stone between the 19th and 20th centuries. In the 17th century, one pip of
Vitis vinifera and five cones of
Alnus were recorded.
3.1.3. Father Master’s Garden—MG
5 pollen samples (CerC8-CerC12).
19th–20th centuries.
Woody food plants, scented shrubs, aromatic and medicinal plants.
The first phase of the garden, prior to the 19th-century reorganisation, has been identified by the cuts to remove the previous trees. An artesian well, only partially excavated, presumably formed part of the same spatial arrangement. During the 19th century, the garden was redesigned by closing the well and constructing a flowerbed at the central square. The central flowerbed was surrounded by ‘L’ shaped flowerbeds and brick paving. Gardening activities followed until the second half of the 20th century [
3,
4].
In the Master’s Garden, the pollen taxa show a variable diversity (24–48 per sample), indicating the presence of different sectors. Some of them were possibly poorly cared for, as testified by the abundance of wild grasses, and were poorer of species, while others contained a large variety of cultivated plants. The state of preservation of pollen grains was different in the same sample, and this could be related to the transport of soil brought from elsewhere for cultivation practices. For example, the transport of soils from forested areas could explain the presence of coniferous pollen (Pinus-pine; Abies-fir), as well as the frequent records of Castanea sativa pollen (up to 11.1% in CerC12, with some pollen clumps). In the different sectors, relatively high percentages of Alnus (up to 3.7% in CerC8), Corylus avellana, Olea europaea and Vitis were recorded. Syringa vulgaris is quite common among shrubs. Some of the Mediterranean shrubs attested in good quantities in all samples also produce lovely and long-lasting flowers during summer: Cistus (rock rose), Helianthemum (sunrose), and Erica. Finally, common climbing plants in gardens include Hedera helix and Jasminum (the fragrant jasmine). The percentages of plants from wetlands are more remarkable than elsewhere. A mong them aquatic buttercups, sometimes not clearly distinguishable from other types of Ranunculaceae, also including Ranunculus type (buttercups), Helleborus, and Thalictrum (meadow rue) (6.5% on average). The sedge family, Cyperaceae, including Scirpus (club-rush), are also common (ca. 2% on average). A large number of medicinal-aromatic plants have been detected: in addition to those already mentioned in the Apothecary’s Garden, Mentha type and Cannabis (hemp) are listed. The multipurpose Cannabis could be used both for fibres as textiles or ropes and its medicinal properties, and possibly oil could also have been obtained from the seeds. Finally, the graceful flowers of Anagallis and Primula vulgaris could have adorned a flowerbeds, as could Cyclamen (cyclamen), Filipendula and Rosa, present in all samples.
8 charcoal samples.
18th–second half 20th centuries.
Mesophilous and thermophilous deciduous broadleaved, evergreen sclerophyllous and coniferous trees and shrubs.
Anthracological analysis includes 462 charcoals (among which 379 determined records) belonging to 21 taxa (
Figure 4). The data are dominated by 18 taxa of non-cultivated plants (93.7% of the determined charcoals) belonging to woodlands and semi-natural areas. Woodlands, in particular, are evidenced by mesophilous and thermophilous deciduous broadleaved trees (32.5%), evergreen sclerophyllous vegetation (26.1%), and conifer (14.8%). Coniferous plants are once again represented by
Pinus halepensis/
pinaster. Deciduous woods are mainly represented by
Fraxinus cf.
ornus,
Quercus cf.
cerris, and
Fagus sylvatica. Maloideae,
Acer (maple),
Cornus, and
Ostrya carpinifolia are also attested. The evergreen sclerophyllous flora includes shrubs,
Arbutus unedo,
Erica,
Smilax and
Rhamnus/
Phillyrea, then trees such as
Quercus cf.
ilex.
Alnus belongs to riparian environments. Agriculture-related species are the same reported in other contexts:
Olea europaea,
Castanea sativa and, sporadically,
Juglans regia, and their percentage is relatively low in this context (6.3%).
Eighteen macro-remains belong to fruit plants, cultivated and almost all from the second half of the 20th century (
Table 2). The six taxa identified include trees, that are
Corylus avellana,
Malus/
Pyrus (apple/pear),
Prunus cf.
avium (sweet cherry), and
Vitis vinifera, and arable crops, such as
Triticum aestivum/
durum (bread/macaroni wheat). The presence of
Vicia sp. (vetch) can be evidence both of the presence either of a weed or a crop.
3.2. The External Green Spaces: Archaeological Sectors OPM, ONO and OEG
7 pollen samples (CerC13-CerC19) and 3 charcoal samples from OPM courtyard;
6 pollen samples (CerC20-CerC25) and 3 charcoal samples from the ONO orchard;
3 pollen samples (CerC26-CerC28) from the OEG outside area;
14th/15th–19th/20th centuries.
In the OPM courtyard, the oldest evidence refers to the end of the 15th century, when a green open area was created, possibly a garden or cultivated sector outside the original perimeter of the Carthusian monastery building, with at least one tree. Between the end of the 15th century and the beginning of the 16th century, a series of ash dumps, burnt soil, and bricks with traces of combustion, probably related to a neighbouring productive activity, covered the area. Pollen spectra show a significant amount of Olea europaea in this phase (CerC18). Some interesting plants are represented by pollen of medicinal herbs such as Malva and Echium (viper’s bugloss), and the traces of Ribes (gooseberry).
A new levelling occurred in the mid-16th century. The spoliation of an unknown structure placed in the central-northern part of the present courtyard destroyed the 17th and 18th centuries archaeological stratification. In the 19th century, the courtyard was arranged with four flowerbeds built with a brick curb and gardening activities and tree planting followed. Pollen suggests that
Juniperus/
Cupressus, Ericaceae, and
Sambucus nigra (European elder) were among the trees and shrubs planted (CerC14). Anthracological analysis includes 150 charcoals belonging to 15 taxa (
Figure 4). Woodlands are mainly recorded and represented by
Quercus cf.
cerris,
Erica, and
Pinus halepensis/
pinaster. Species of agricultural interest is
Olea europaea.
The ONO green area occupied by the orchard shows the oldest traces of anthropic frequentation back to the 14th century when the first nucleus of the Charterhouse was erected. These traces were possibly connected to horticultural practices that followed one another until the late 18th century, spaced out by colluvia. Pollen samples from this phase (CerC24, CerC25) show a significant amount of Ericaceae, possibly arranged in hedges and cultivated for their beautiful flowers. Interestingly
Olea europaea is well represented, with about 3% in these two samples, suggesting the presence of this important food plant in the orchard. At the end of the 18th century, probably in conjunction with the extensive renovation of the Carthusian Monastery, a new level of soil (CerC22) was added, followed by continuous horticultural activities (CerC20, CerC21) up to the present day. Cereal fields and the presence of Brassicaceae are evidence of cultivation in the area. The anthracological study involves 150 charcoals belonging to 18 taxa (
Figure 4), mainly non-cultivated plants. They include, for example, the conifer
Pinus halepensis/
pinaster, deciduous broadleaves
Quercus cf.
cerris,
Fraxinus cf.
ornus, and
Alnus. The latter tree belongs to riparian environments. Evergreen sclerophyllous vegetation includes shrubs, such as
Erica and
Arbutus unedo. Species of agricultural interest are
Olea europaea and
Castanea sativa.
In the OEG outside area, pollen spectra are characterised by high amounts of olive pollen (up to 15.6% in CerC28), which was also observed as pollen clumps suggesting the local presence of the trees. The agrarian landscape is also testified to the relatively high values of cereals, including Hordeum group (4.9% on average), Avena/Triticum group (0.9% on average) and traces of Panicum (1.3% only in CerC26), with some Fabaceae. In the samples, there is also Vitis (0.4% on average) and Ericaceae (6.3%), reflecting the spreading of gardens and hedges close to this area.
3.3. Principal Component Analysis: Similar but Different Areas
Palynological analysis returned a large and complex dataset consisting of 135 variables (pollen taxa) and 28 samples (CerC1-CerC28). Therefore, it was necessary to apply the Principal Component Analysis (PCA) as a method to bring out strong patterns from the dataset. The PCA proved effective in interpreting information on the ecological and floristic connotations of the different gardens and other contexts outside the cloister walls (
Figure 5). The first PC (=F1: represented by the horizontal axis) explains 11.89% of the total variance and the second PC (=F2: the vertical axis) describes the second most variation in the data (8.63%). The components obtained do not have a distinct ecological-environmental connotation as wet environments, entomophilous species, possibly cultivated herbaceous plants, and fruit-bearing trees are present in all quadrants of the PCA space. This output is plausible as it refers to contexts of similar nature within the same site. However, a general description of flora and vegetation can be assumed for both components. The first PC presents high loadings for “mainly medicinal-aromatic herbs and woody food plants” (e.g.,
Peucedanum,
Apium,
Hypericum,
Valerianella,
Corylus avellana, and
Vitis) and low loadings for “mainly cereal and other cultivated plants” (e.g.,
Hordeum group,
Avena/
Triticum group, and Brassicaceae). The second PC presents high loadings for “mainly hydro-hygrophilous herbs and ornamental plants” (e.g.,
Phragmites australis,
Nuphar,
Nymphaea alba type,
Oxalis, Rosaceae, and
Polygala) and low loadings for “mainly Mediterranean trees and shrubs” (e.g.,
Olea europaea,
Fraxinus ornus, and
Syringa).
Most of the samples are distributed along the first component, along which pollen taxa with high loadings mostly contribute to the Apothecary’s and Master’s gardens, and those with low loadings to the other contexts outside the cloister walls. The four samples from the Prior’s Garden are placed in quadrants I and II, mostly characterised by pollen taxa with high loadings in the second component. The samples cluster within the ordination plot with little overlap between the different contexts. Samples of different chronology but from the same context tend to organize close together in PCA space (contexts separated by different colours in
Figure 5). This is the most significant contribution obtained from the PCA analysis, showing continuity of use for each context that seems to be characterised by a specific floristic and vegetational diversity over time.
4. Discussion
The interdisciplinary archaeological and archaeobotanical data allow us to highlight the garden and horticultural practices putting into evidence the individual (gardens) and collective (green spaces and surrounding woods) practices adopted by the Carthusians. Therefore, archaeological, palynological, anthracological, and carpological data are discussed below to better understand the composition and seasonality of gardens, the collective horticultural practices, and the surrounding landscape during the centuries of occupation of the Charterhouse.
4.1. Individual Agencies: The Composition and Seasonality of the Fathers’ Gardens
The archaeological investigation highlighted the diachronic transformation of the gardens as expressions of monks’ individual personalities and feelings, substantiated by their choice of specific plants and flowers. Indeed, there was no rule or custom on cultivating the gardens. Their figurative picture was rather an expression of the monks’ intimate relationship with nature and divinity [
16] (p. 76). Human agency is evident in the construction choices of the gardens, such as the layout of the flowerbeds and their transformations, and in the selection of ornamental plants. These plants, with their flowers, colours, fragrances, and seasonality, have the capacity to act and create ever-changing gardens, just as water does with its ability to allow the life of water lilies and the interplay of light, sound and refreshment in conjunction with green plants and walls that provide shade. The architecture of the gardens, with their high boundary walls, separated the Fathers from the external world, immersing them in a space tailored to monastic life, not excluding interaction with animals. This is documented by the archaeozoological analyses that unearthed the presence of bones of cats and terrestrial turtles, and also birds that, together with insects, were attracted by flowers, plants, water, and presumably food remains [
3,
4].
Pollen analysis of the Fathers’ garden sediments revealed a high plant diversity and confirms that anthropogenic activities largely influenced the local flora (
Figure 3). In the Father Prior’s Garden, archaeobotanical data provide an outline of the changing features of the garden due to the different plants cultivated over the centuries and their seasonal variability. Daily horticultural activities are also evident in the pollen records, primarily proved by the cultivation of medicinal herbs as well as food and aromatic plants, such as garlic, carrot, horseradish, and mint. From the mid/second half of the 18th century, the garden became a meditation space adorned with flowers and ornamental plants blooming in the different seasons, such as roses, integrated into a symmetrical articulation of paths and flowerbeds. Aquatic plants, including water lilies—
Nymphaea alba and
Nuphar—were related to an octagonal fountain, all converging scenically in a grotesque backdrop. This resulted in a garden wetter than others in the Charterhouse. The diversity of aromatic and medicinal plants is especially rich in the Father Apothecary’s Garden, where sage, mallow, the common Saint-John’s wort, poppy, celery, hog’s fennel, and lamb’s lettuce could be wild or cultivated and selected for their properties. Also, the carpological record could represent some garden plants. Furthermore, in these two gardens, and especially in the Father Master’s Garden, were woody plants in the form of shrubs with beautiful flowers, like lilacs and heaths, autumn fruits like hazelnuts, and trees. Among the latter, oaks, ash, pine, and maples may have been cultivated or, most probably, were also distributed in the area.
4.2. Collective Production, Fertilisation, Manuring, Pruning, Diet, and Other Practices
The dense intertwining of human and non-human agency can be read in the fertilising practices attested by the ash patches and burnt plant remains: here, human agency is linked to that of the ash, its potential to fertilise and enrich the soil, the agency of the plants themselves, and fire. Likewise, soil transport from the external areas to the gardens, well highlighted by pollen analysis and the presence of spores of coprophilous fungi, underlines both the human agency of careful soil selection for its geobotanical characteristics and that of the soil itself and the organisms and microorganisms that make it up and enable its fertility and cultivation. This complex and intricate network of activities creates positive mutual dependences, such as enjoying one’s own garden, meditation, and peace.
Some horticultural practices can be inferred from pollen and archaeobotanical data. In the Father Prior’s Garden, the significant presence of
Hordeum and
Avena/
Triticum cereal pollen can be interpreted as transporting some plant parts to use as ground cover in the garden rather than a small local cultivation of cereals performed inside it for daily use. In the Father Apothecary’s Garden, significant amounts of chestnut pollen may be due to organic acid soils collected in the surrounding hills and transported in the garden for manuring or other horticultural practices. The high presence of chestnut pollen in the Master’s Garden, even higher than in the previous garden and with pollen clumps, can be interpreted as a similar case of acid soils used as fertiliser in gardening practices. The chestnut tree was probably cultivated outside the cloister wall (according to charcoal records since the 15th century,
Figure 6). Almond tree, olive tree, grapevine, and walnut could have been grown in both outdoor and indoor gardens.
Pollen and macroremain records suggest which fruits supplemented the diet of the Charterhouse inhabitants. Cereals, such as bread/macaroni wheat, extend information to arable crops that OEG pollen analyses clearly show externally. Although Maloideae and Prunoideae records were common in all the three gardens, their charcoal was not considered as part of the cultivated taxa. Indeed, their anatomical features do not support the distinction between spontaneous and cultivated plants. Some records could be related to the pruning of local fruit-bearing plants, such as sweet cherry and almond tree [
4]. Moreover, fruit remains attested to the cultivation of apple/pear and peach. The presence of charred wood may be explained by the traditional practice of adding firewood ash to the soil as fertiliser. In the AG samples, also considering the consistency with the anthracological record, carpological material may have been deposited by discharge of firewood residues or burnt waste. In particular, the woody fruits of the alder, such as tiny pinecones, may persist dried on pruned branches for a long time—so they could still burn together with fuelwood. As already mentioned, based on the few pollen found, (
Section 3.1.1 and
Section 3.1.2), alder was most probably collected from riparian environments out of the gardens and transported inside.
Like other monastic Orders, the Carthusian role does not allow animal meat consumption except on very rare occasions. Carthusians could still consume dairy products, eggs, and fish, as well as some other aquatic or amphibian animals, such as otters, molluscs, and tortoises [
17] (pp. 34–44) [
18] (pp. 282–288). The archaeozoological records from the Fathers’ gardens attest to the presence, in addition to
Felis catus (domestic cat), of domestic mammals, such as
Oryctolagus cuniculus (rabbit) and
Bos taurus (cattle), and farmyard animals, such as
Gallus gallus (domestic fowl). However, there is very little evidence within the cell gardens, and our data do not allow us to state that animal husbandry was practised within the cloister boundaries nor that manure was used as fertiliser [
4].
4.3. The Surrounding Landscape: Managing the Wood
The ash patches and charred wood remains represent an important intertwining of human and non-human agency involving the monastic community, plants, and fire. The linking component between these three elements is ash, with its potential to fertilise and enrich the soil. In the ash, the charred wood remains are a tangible expression of the tree and shrub community, but it is the human agency that selects the fuel and consequently shapes the forest resource. The charcoal record clearly shows the use of fuelwood from uncultivated plants of woody and semi-natural lands (
Figure 6). The percentage of fuelwood from these areas is predominant between the 15th and 19th centuries, ranging from 97.3% to 90.5%. It decreased to 37.6% at the beginning of the 19th century, increasing later and becoming dominant in the 20th century.
In the 15th and 17th centuries, the thermophilous deciduous woodland was the most exploited habitat (
Figure 6), mainly represented by the deciduous oak group, with Turkey oak and sporadically downy oak, then manna ash, elm, hop-hornbeam, and also European beech. This forest cover is similar to the present-day mixed deciduous woodland, with significant differences: European hop-hornbeam dominates, whereas mixed Turkey oak forests are fragmented in scattered and occasional clusters [
19,
20]. The mixed deciduous oak forest, dominated by Turkey oak, was, in fact, present for the entire investigated chronology as the pollen spectrum of deciduous
Quercus also shows (
Figure 3) and was the primary source of firewood at least until the end of the 17th century (
Figure 6), probably because it was more available in the area than today and efficient in terms of biomass production.
In both biostratigraphic records, hygrophilous and floodplain trees, testified to by alder, southern ash, and elm (
Figure 3 and
Figure 6), were present but sporadically used as fuelwood. The most remarkable aspect is the marked heliophilous and thermophilous component of frugal and fast-growing deciduous trees, capable of colonising open habitats and lightly wooded areas (manna ash, above all, then hop-hornbeam and elm;
Figure 3 and
Figure 6). This ecological succession is typical of the Turkey oak forest managed by coppicing to harvest firewood [
21] in the Upper Tyrrhenian Tuscany, where deciduous oak woodland extended between the Middle Ages [
22,
23,
24,
25], and then declined due to anthropogenic impact related to crops and pastures [
26].
Since the 15th century, the use of sclerophyllous trees and shrubs from maquis has been continuous in the gathering of firewood, together with wood from Turkey oak forest (
Figure 6). For this period, maquis is representative of forest types altered by anthropogenic activities and can be considered both regressive degradation and restoration series [
19,
20,
21]. Ericaceae pollen (
Figure 3), particularly from the three outside contexts, strongly confirms the presence of open woodlands colonised by sclerophyllous shrubs. In the time interval from the 18th to the end of the 20th century, the most exploited habitat was the Mediterranean evergreen forest. Fuelwood came from high maquis, including mainly sclerophyllous shrubs, such as buckthorn or narrow-leaved mock privet, strawberry tree, and heather.
The concurrent use of
Pinus halepensis/
pinaster shows a close match with the current vegetation. Its high fuelwood percentage, reaching 29.4% in
Figure 6, and pollen presence (
Pinus) in the three outside contexts (
Figure 3) can be attributed to the presence of one of the highest hillside concentrations of
P. pinaster in all of Tuscany, on nearby Mount Pisano [
19,
20]. The supply of maritime pine wood backdates the presence of the species on Mount Pisano (at least to the 15th century) and supports the native species hypothesis. The use of pine wood is chronologically wide (five centuries) and suggests its continuous presence in the area, probably managed by the renewal of the resource.
From the 18th century, the use of sclerophyllous shrubs of maquis and maritime pine became prevalent and exceeded the use of wood from Turkey oak and thermophilous deciduous broadleaved forests (
Figure 6). The supply of firewood reflected the present spread of maquis and maritime pine forests due to the continuous consumption and degradation of tree cover and substratum by ongoing agrarian and silvo-pastoral activities [
19,
20].
The presence of beech in the anthracological record (
Figure 6) testifies to the use as a fuel of a local woody resource, attested by a slight pollen signal (
Figure 3). Beech is a typically mountainous species whose canonical distribution in Tuscany is from ca. 1000 m to 1500/1700 m a.s.l. However, few and spotty beech stands are located in Mount Pisano (ca. 400 and 700 m a.s.l.) [
19], and plant remains are attested between the 1st and 3rd centuries near Pisa and the Charterhouse [
27]. In the last 4000 years, beech was typical of woodlands below 400 m a.s.l. along the Italic peninsula in the Mediterranean macrobioclimate [
28]. Only forestry exploitation degraded its habitat within the local thermophilous plant communities.
4.4. The Use of Cultivated Areas: Ecology and Economics
Cultivated lands are suggested from the 15th century, and the long-standing and used plants are olive and chestnut trees (
Figure 6). The olive tree, and particularly its derived product, olive oil, plays an important role in religious activities. The archaeobotanical evidence indicates a very scant presence of the olive tree and oil until the late Middle Ages in Tuscany [
29]. Only between the 12th and 13th centuriesthe great expansion of olive growing occurred when towns and urban communities aspired to have access to olive oil, a food connected to higher social classes, such as the religious ones and considered a luxury [
25]. The higher values of
Olea europaea and cereals outside the walls (
Figure 3) represent the typical pollen picture of the open agrarian landscape characterised by olive groves and cereal fields, especially outside the OEG area (10.1% and 8.3% on average, respectively, in samples CerC26-CerC28). Contrarily, the lowest values of
Corylus avellana and aromatic-medicinal plants like Apiaceae are further evidence that these plants were cultivated in the gardens.
According to archaeobotanical research, chestnut fruit cultivation in Tuscany began in the middle of the 11th century, when the post-Carolingian aristocracy turned a profit on woodlands [
30]. On Mount Pisano, this cultivation was established in the 9th and 10th centuries [
19]. An increase of chestnut cultivation in the mountain and hilly areas in Italy was also recorded between the 10th and 14th centuries [
31,
32], a phenomenon that led to the coining of the term ‘chestnut civilisation’ [
33]. Nowadays, chestnut stands, maquis, and maritime pine forests largely replaced the potential vegetation of mixed deciduous broadleaved woods and represent the most widespread forest coenosis of the hilly area, from 25/50 m to ca. 900 m a.s.l. [
19,
20]. The scarcity of
Castanea sativa pollen outside further supports the interpretation that the chestnut was transported into the gardens with the acid soils taken from the hills. Moreover, the lowest values of Cichorieae pollen in the outside contexts may be partly due to the different soils, which were more organic-rich sediments in the cultivation areas.
Between the 19th century and the transition to the 20th century, the use of wood from cultivated areas, notably chestnut groves, rose (
Figure 6). This occurred immediately after the increase in the use of maquis and could therefore confirm the deterioration of thermophilous deciduous forests to spread cultivable areas. Prunings and infertile individuals of these fruit tree plantations became a source of firewood, shifting chestnut groves towards coppice. This process degraded the pre-existing chestnut fruit high forest stands [
34]. Currently, on Mount Pisano, chestnut trees are rare, while coppice has prevailed [
19,
20].
5. Conclusions
At the end of our discussion, we would like to emphasise some key points. The first one is that this research has been developed from the beginning as an interdisciplinary investigation project involving three scientific research teams with specific and complementary expertise. Only this collaborative approach has permitted an in-depth analysis of the data and the results achieved. On the other hand, this distributed work took five years from the first time the trowel dug the ground to this publication.
The second key point is that our research has been based on a solid theoretical approach, which started from collected and scientifically investigated data. We have proposed to interpret archaeological and archaeobotanical data not only in terms of the presence/absence or within the well-established categories of alimentation, productive activities or embellishment of the gardens but rather in relation to the intricate network of human and non-human connections, rejecting an exclusively anthropocentric view. Reading the complex archaeological record composed of soil, pottery, stones, pollen, plant macroremains, and faunal bones starting from their agencies allowed us to avoid the pre-eminence of human agency.
Based on the data from the gardens and contexts outside the cloister walls, a considerable local variability of contexts was responsible for the different available resources and landscape. Main details on the local biodiversity and land use can be summarised as follows:
(i) the specific composition and seasonality of the gardens and the choice of plants and flowers adopted by the Carthusians during the centuries of occupation of the Charterhouse; in particular, palynological analysis provide information on the floristic and vegetational dissimilarities of the three gardens, characterised by the presence of ornamental plants (Father Prior’s Garden), aromatic and medicinal plants (Father Apothecary’s Garden), and fruit trees (Father Master’s Garden);
(ii) the use of fertilising practices, attested by the ash patches, burnt plant remains, and probably by careful sorting of soil in the surrounding hills and its transport within the gardens;
(iii) pollen and macroremain records suggest that the inhabitants of the Charterhouse based their diet on cereals (bread/macaroni wheat), other possible cultivated herbs (vetch and species belonging to Brassicaceae), wild and cultivated woody plants (e.g., chestnut, olive tree, almond tree, and grapevine);
(iv) the management of local hilly woods: in the 15th and 17th centuries, the thermophilous deciduous woodland was the most exploited habitat, while later, until the end of the 20th century, the Mediterranean evergreen forest was more exploited as fuelwood. Between the 19th century and the 20th century, the use of chestnut wood increased.
Moreover, the archaeozoological records attest to the presence of domestic animals such as domestic cat, cattle, rabbits, and domestic fowl. There is very little evidence to suggest that animal husbandry was practised within the cloister boundaries.
In the gardens, orchards and woods of the Charterhouse of Calci, humans and non-humans acquire centrality and peripherality simultaneously because, at the centre, there is precisely the network of agencies with their mutual dependencies. In our study, the non-humans—the plants and things—play a role as important as that of the humans—Carthusian Fathers and the lay brothers—in a sort of symmetry. Humans did not merely plant flowers, vegetables, and trees or exploit woods; they planted seeds, managed woods or created a garden to the extent that the non-human agency of the plants (and also of things) afforded it to them.
In this way, the garden represents a co-creation made together by human and non-human agencies that afforded, among other things, silence, shade, colour, perfume, the presence of butterflies, turtles, cats, and even microorganisms such as coprophilous fungi that in turn performed their affordance, and so forth. This complex network has produced dependencies [
35] (p. 17) in which each monk was entrapped, for example, between the fragrance of flowers and the necessity to take care of them, dedicating time and effort to gardening activities in order to enjoy the garden itself.
Author Contributions
Conceptualization, G.G. and A.M.M.; methodology, G.G., A.C., F.A., M.B. and E.R.; validation, M.B. and A.M.M.; formal analysis, M.B. and E.R.; investigation, F.A., A.C., G.G., M.B, M.R. and E.R.; resources, M.B.; data curation, A.C. and F.A.; writing—original draft preparation, G.G., A.C., A.M.M., E.C., M.B. and E.R.; writing—review and editing, G.G., A.C., M.B., A.M.M. and E.C.; visualization, E.C. and M.B.; supervision, G.G. and A.M.M.; funding acquisition, G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by University of Pisa inside the project “Studi conoscitivi e ricerche per la conservazione e la valorizzazione del Complesso della Certosa di Calci e dei suoi Poli Museali”. The position of E.C. was funded by the project “National Biodiversity Future Center—NBFC”.
Data Availability Statement
Acknowledgments
This paper comes from an archaeological research programme performed since 2018 and directed by the MAPPA lab of the University of Pisa in collaboration with the Museum of Natural History of the University of Pisa, the Palynology and Paleobotany Laboratory at the University of Modena and Reggio Emilia, the Laboratory of Topography of the Mining Territories of the University of Siena. The project was dedicated to understanding the garden and horticultural practices implemented over the centuries of occupation of the Charterhouse of Calci, Pisa (Italy). Research project implemented under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4—Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union—Next Generation EU. Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP E93C22001090001, Project title “National Biodiversity Future Center—NBFC”. We thank the anonymous reviewers, whose constructive remarks helped improving the clarity of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Giusti, M.A.; Lazzarini, M.T. La Certosa di Pisa a Calci; Pacini Editore: Pisa, Italy, 1993. [Google Scholar]
- Manghi, A. La Certosa di Pisa. Storia (1366–1866) e Descrizione; Tip. Editrice del Cav. F. Mariotti: Pisa, Italy, 1911. [Google Scholar]
- Anichini, F.; Buonincontri, M.; Campus, A.; Gattiglia, G.; Mercuri, A.M.; Rattighieri, E.; Rossi, M. I giardini dei padri. Garden Archaeology alla Certosa di Calci (Pisa). FOLD&R FastiOnLine Doc. Res. 2022, 543, 1–24. [Google Scholar]
- Campus, A.; Anichini, F.; Gattiglia, G.; Buonincontri, M.; Naime, Y.; Rossi, M. Multiproxy archaeological investigations and Garden Archaeology in the Charterhouse of Calci (Tuscany, central Italy) and its territory: Results and research data. ArcheoLogica Data 2023, 3, 167–189. [Google Scholar] [CrossRef]
- Langgut, D. Prestigious early Roman gardens across the Empire: The significance of gardens and horticultural trends evidenced by pollen. Palynology 2022, 46, 1–17. [Google Scholar] [CrossRef]
- Ciarallo, A.; Mariotti Lippi, M. The garden of “casa dei casti amanti” (Pompeii, Italy). Gard. Hist. 1993, 21, 110–116. [Google Scholar] [CrossRef]
- Bosi, G.; Mazzanti, M.; Mercuri, A.M.; Torri, P.; Trevisan Grandi, G.; Accorsi, C.A.; Guarnieri, C.; Vallini, C.; Scafuri, F. Il Giardino delle Duchesse del Palazzo Ducale Estense di Ferrara da Ercole I (XV sec.) ad oggi: Basi archeobotaniche e storico-archeologiche per la ricostruzione del giardino. In The Archaeology of Crop Fields and Gardens; Morel, J.-P., Tresserras Juan, J., Matamala, J.C., Eds.; EDIPUGLIA: Lecce, Italy, 2006; pp. 103–128. [Google Scholar]
- Bosi, G.; Mercuri, A.M.; Guarnieri, C.; Bandini Mazzanti, M. Luxury food and ornamental plants at the 15th century A.D. Renaissance court of the Este family (Ferrara, Northern Italy). Veg. Hist. Archaeobot. 2009, 18, 389–402. [Google Scholar] [CrossRef]
- Jones, C.A.; Church, E. Photogrammetry is for everyone: Structure-from-motion software user experiences in archaeology. J. Archaeol. Sci. Rep. 2020, 30, 102261. [Google Scholar] [CrossRef]
- Van Geel, B. Application of fungal and algal remains and other microfossils in palynological analyses. In Handbook of Holocene Palaeoecology and Palaeohydrology; Berglund, B.E., Ed.; Wiley: Chichester, UK, 1986; pp. 497–505. [Google Scholar]
- Grimm, E.C. TILIA and TGView; Illinois State Museum: Springfield, IL, USA, 2004. [Google Scholar]
- Abbate Edlmann, M.L.; de Luca, L.; Lazzeri, S. Atlante Anatomico Degli Alberi ed Arbusti Della Macchia Mediterranea; Istituto Agronomico per l’Oltremare: Firenze, Italy, 1994. [Google Scholar]
- Schweingruber, F.H. Anatomy of European Woods; Haupt: Stuttgart, Switzerland, 1990. [Google Scholar]
- Vernet, J.-L.; Ogereau, P.; Figueiral, I.; Machado Yanes, C.; Uzquiano Ollero, P. Guide D’identification des Charbons de Bois Préhistoriques et Récents: Sud-Ouest de l’Europe, France, Péninsule Ibérique Etîles Canaries; CNRS Editions: Paris, France, 2001. [Google Scholar]
- Neef, R.; Cappers, R.T.; Bekker, R.M.; Boulos, L. Digital Atlas of Economic Plants in Archaeology; Barkhuis: Eelde, The Nederlands, 2012. [Google Scholar]
- Venturi Ferriolo, M. Simbologia dei giardini nella tradizione monastica. Note per una lettura veterotestamentaria. In Certose e Certosini in Europa. Atti del Convegno alla Certosa di San Lorenzo, Padula 22, 23, 24 Settembre 1988; Sergio Civita Editore: Napoli, Italy, 1990; Volume 1, pp. 75–81. [Google Scholar]
- De Grossi Mazzorin, J.; Miniti, C. Diet and Religious Practices: The Example of two Monastic Orders in Rome between the XVIth and XVIIIth Centuries. Anthropozoologica 1999, 30, 33–50. [Google Scholar]
- De Grossi Mazzorin, J.; Minniti, C. L’analisi dei resti faunistici: Alcune osservazioni sull’alimentazione dei Minimi di San Francesco di Paola. In Pincio. I. La Villa Médicis et le Couvent de la Trinité-des-Monts à Rome. Réinvestir un Site Antique; Broise, H., Jolivet, V., Eds.; École Française de Rome: Rome, Italy, 2009; pp. 277–291. [Google Scholar]
- Bertacchi, A.; Sani, A.; Tomei, P.E. La Vegetazione del Monte Pisano; Felici: Pisa, Italy, 2004. [Google Scholar]
- Pierini, B.; Garbari, F.; Peruzzi, F. Flora vascolare del Monte Pisano (Toscana nord-occidentale). Inf. Bot. It. 2009, 41, 147–213. [Google Scholar]
- Mondino, G.P.; Bernetti, G. Serie Boschi e Macchie di Toscana. 2. I tipi Forestali; Edizioni Regione Toscana: Firenze, Italy, 1998. [Google Scholar]
- Colombaroli, D.; Marchetto, A.; Tinner, W. Long-term interactions between Mediterranean climate, vegetation and fire regime at Lago di Massaciuccoli (Tuscany, Italy). J. Ecol. 2007, 95, 755–770. [Google Scholar] [CrossRef]
- Vannière, B.; Colombaroli, D.; Chapron, E.; Leroux, A.; Tinner, W.; Magny, M. Climate versus human-driven fire regimes in Mediterranean landscapes: The Holocene record of Lago dell’Accesa (Tuscany, Italy). Quat. Sci. Rev. 2008, 27, 1181–1196. [Google Scholar] [CrossRef]
- Allevato, E.; Arobba, D.; Di Pasquale, G.; Pappalardo, M.; Ribecai, C. Indicazioni paleovegetazionali dai carotaggi MAPPA. MapPapers 2013, 3, 107–118. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Buonincontri, M.P.; Allevato, E.; Saracino, A. Human-derived landscape changes on the northern Etruria coast (western Italy) between Roman times and the late Middle Ages. Holocene 2014, 24, 1491–1502. [Google Scholar] [CrossRef]
- Buonincontri, M.P.; Pieruccini, P.; Susini, D.; Lubritto, C.; Ricci, P.; Rey, F.; Tinner, W.; Colombaroli, D.; Drescher-Schneider, R.; Dallai, L.; et al. Shaping Mediterranean landscapes: The cultural impact of anthropogenic fires in Tyrrhenian southern Tuscany during the Iron and Middle Ages (800–450 BC/AD 650–1300). Holocene 2020, 30, 1420–1437. [Google Scholar] [CrossRef]
- Bertacchi, A.; Lombardi, T.; Sani, A.; Tomei, P.E. Plant macroremains from the Roman harbour of Pisa (Italy). Environ. Archaeol. 2008, 13, 181–188. [Google Scholar] [CrossRef]
- Buonincontri, M.P.; Bosso, L.; Smeraldo, S.; Chiusano, M.L.; Pasta, S.; Di Pasquale, G. Shedding light on the effects of climate and anthropogenic pressures on the disappearance of Fagus sylvatica in the Italian lowlands: Evidence from archaeo-anthracology and spatial analyses. Sci. Total Environ. 2023, 877, 162893. [Google Scholar] [CrossRef] [PubMed]
- Buonincontri, M.P.; Pecci, A.; Di Pasquale, G.; Ricci, P.; Lubritto, C. Multiproxy approach to the study of Medieval food habits in Tuscany (central Italy). Archaeol. Anthropol. Sci. 2017, 9, 653–671. [Google Scholar] [CrossRef]
- Buonincontri, M.; Moser, D.; Allevato, E.; Basile, B.; Di Pasquale, G. Farming in a rural settlement in central Italy: Cultural and environmental implications of crop production through the transition from Lombard to Frankish influence (8th–11th centuries a.d.). Veg. Hist. Archaeobot. 2014, 23, 775–788. [Google Scholar] [CrossRef]
- Mercuri, A.M.; Bandini Mazzanti, M.; Florenzano, A.; Montecchi, M.C.; Rattighieri, E. Olea, Juglans and Castanea: The OJC group as pollen evidence of the development of human-induced environments in the Italian peninsula. Quat. Int. 2013, 303, 24–42. [Google Scholar] [CrossRef]
- Buonincontri, M.P.; Saracino, A.; Di Pasquale, G. The transition of chestnut (Castanea sativa Miller) from timber to fruit tree: Cultural and economic inferences in the Italian peninsula. Holocene 2015, 25, 1111–1123. [Google Scholar] [CrossRef]
- Cherubini, G. La «civiltà» del castagno in Italia alla fine del Medioevo. Archeol. Mediev. 1981, 8, 247–280. [Google Scholar]
- Bianchi, L.; Maltoni, A.; Mariotti, B.; Paci, M. La Selvicoltura dei Castagneti da Frutto Abbandonati Della Toscana; Arsia: Firenze, Italy, 2009. [Google Scholar]
- Hodder, I. Entangled. An Archaeology of the Relationships between Humans and the Things; Wiley-Blackwell: Oxford, UK, 2012. [Google Scholar]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).