Abstract
Phytolith analysis is a well-established archaeobotanical tool, having provided important insights into pre-Columbian crop cultivation and domestication across Amazonia through the Holocene. Yet, its use as a palaeoecological tool is in its infancy in Amazonia and its effectiveness for reconstructing pre-Columbian land-use beyond archaeological sites (i.e., ‘off-site’) has so far received little critical attention. This paper examines both new and previously published soil phytolith data from SW Amazonia to assess the robustness of this proxy for reconstructing pre-Columbian land-use. We conducted the study via off-site soil pits radiating 7.5 km beyond a geoglyph in Acre state, Brazil, and 50 km beyond a ring-ditch in northern Bolivia, spanning the expected gradients in historical land-use intensity. We found that the spatio-temporal patterns in palm phytolith data across our soil-pit transects support the hypothesis that pre-Columbian peoples enriched their forests with palms over several millennia, although phytoliths are limited in their ability to capture small-scale crop cultivation and deforestation. Despite these drawbacks, we conclude that off-site soil phytolith analysis can provide novel insights into pre-Columbian land use, provided it is effectively integrated with other land-use (e.g., charcoal) and archaeological data.
1. Introduction
There is a long-standing controversy over the degree to which different pre-Columbian (pre-AD1492) human societies across Amazonia, over the past several millennia, transformed their rainforest environments from pristine wilderness into domesticated landscapes, through forest clearance, use of fire, and agro-forestry [1,2,3,4,5]. Palaeoecology has the potential to help resolve this debate by providing empirical evidence for vegetation history, especially when closely integrated with archaeology [6,7]. The most common palaeoecological technique is fossil pollen and charcoal analysis of radiocarbon-dated lake/bog sediments, which has provided new insights into the scale of deforestation, use of fire, type of farming/forest management practices, and crop domestication/cultivation in different parts of Amazonia. For example, pollen and charcoal analyses of sediment cores from small lakes in close proximity to archaeological sites have revealed: two millennia of agro-forestry near Santarem, Brazil [8]; crop cultivation and fire use and suppression associated with raised-field agriculture in Amazon savannas [9,10]; and maize cultivation and only small-scale deforestation in Bolivia [11,12]. Sediment cores from tight clusters of small lakes have shown that pre-Columbian rainforest disturbance (burning and maize cultivation) was often highly localised [13], whilst the most recent basin-wide synthesis of lake pollen records [14] shows that the geographic scale and temporal pattern of deforestation, and subsequent reforestation following societal collapse, was highly heterogeneous across Amazonia, and largely preceded European Contact.
However, despite the important advances in understanding provided by these studies, fossil pollen analysis has significant challenges and drawbacks as a palaeoecological tool for Amazonia. The biggest limitation is that pollen is only preserved well in low-oxygen environments, such as lakes or bogs. Because Amazonia is a fluvially dominated landscape, the vast majority of lakes are riverine oxbows, most of which are too young (<500 years) to examine pre-Columbian land use. Of those rare oxbows which do pre-date European Contact, few are conveniently located close to archaeological human settlements, where signatures of human land use are expected to be strongest. Even for those few lakes or bogs close to archaeological sites, the much larger catchment area for pollen (especially for key wind-dispersed taxa such as most of the Moraceae, >25 km2) [15,16,17,18] than for plant macro-remains such as seeds and fruits from archaeological (i.e., ‘on-site’) contexts, means that this mis-match in spatial resolution renders integration between pollen-based palaeoecological data with archaeological data problematic. Furthermore, pollen records from oxbows inevitably provide land-use histories highly skewed toward pre-Columbian settlements near rivers and reveal little or nothing about land use in the interfluves, where major earthworks (e.g., >400 geoglyphs in eastern Acre state alone) have been discovered in recent years [19,20], challenging the old fluvial vs. interfluvial model of pre-Columbian settlement patterns [21,22,23].
These major drawbacks of lake-based pollen analysis as an effective tool for investigating pre-Columbian Amazonian land use have led to increasing attention paid to alternative palaeo-vegetation proxies in recent years, in particular soil phytolith analysis. Phytoliths are microscopic silica bodies found in plant tissue and have long been used as an archaeobotanical tool (alongside plant macro-remains) elsewhere in the Americas [24] to provide information on diet, crop cultivation/domestication, and plant processing, but have only recently been applied to Amazonia, whether for ‘on-site’ archaeobotanical studies of diet/crop cultivation [25,26] or ‘off-site’ palaeoecological reconstruction [20,27,28,29]. (Note: Throughout this paper we use ‘on-site’ vs. ‘off-site’ to refer to relative proximity to sites of archaeological excavation; e.g., human habitation centre, artificial earthwork, ADE.) Phytoliths offer two key advantages over pollen as a palaeo-vegetation proxy with respect to the spatial resolution and taxonomic resolution of key herbaceous land-use indicator taxa. Firstly, unlike pollen, phytoliths preserve well in soils, which means that the choice of sample site is almost limitless. Secondly, unlike pollen, which is dispersed well beyond the parent plant, phytoliths are deposited in situ in the soil where the parent plant grew, following death and decomposition of its vegetal matter. Consequently, soil phytolith analysis offers the potential for palaeoecological reconstructions at far higher spatial resolution than is possible with lake/bog-based pollen analysis. However, the potential biasing of soil phytolith records by internal soil factors, such as dissolution caused by soil pH [30,31,32,33], differential translocation due to differences in soil particle size [34,35], and post-depositional mixing [36], must be accounted for to ensure the palaeoecological interpretations are robust [37].
The second advantage of phytolith analysis over pollen analysis is its greater taxonomic resolution of herbaceous taxa, some of which are important land-use indicators. For example, Heliconia is a key indicator of forest disturbance (i.e., clearings), which is easily identifiable by its phytoliths, but undetectable in pollen records [38,39,40]. Grasses (Poaceae) and sedges (Cyperaceae) can only be identified to the family level by their pollen but can be identified to the sub-family and sometimes genus levels by their phytoliths [38,41,42,43]. Most importantly, with respect to land-use reconstruction, cultigens such as squash (Cucurbita spp.), arrowroot (Maranta arundinacea), leren (Calathea spp.), and rice (Oryza spp.) are readily identifiable by their phytoliths [25,44,45] but are absent or rare in pollen records.
Consequently, on-site soil phytolith analyses have yielded important new discoveries about pre-Columbian land use and plant domestication across Amazonia in recent years; e.g., multi-millennial histories of agro-forestry upon anthropogenic soils (Amazon Dark Earths, ADEs) in eastern Amazonia [8,26,46] and early Holocene centres of squash and manioc domestication associated with anthropic forest islands in southwestern Amazonia [47].
However, drawing inferences of spatio-temporal patterns of pre-Columbian forest management and disturbance (e.g., agro-forestry, forest clearance), based on phytolith records from off-site soil profiles/pits beyond archaeological sites [26,27,28,29], is less straightforward and open to question due to uncertainty over: (a) scale of deforestation, (b) differentiation between ‘natural’ forest vs. ‘cultural/domesticated’ forest, (c) evidence for crop cultivation and anthropogenic burning, and (d) the influence of soil mixing and post-depositional processes upon the stratigraphic integrity and temporal resolution of phytolith records. However, unlike pollen analysis, the limitations of which are well understood and have been documented over many years [48,49,50,51], the reliability of off-site soil phytolith analysis as a palaeo-vegetation proxy for Amazonia has received scant attention.
2. Aims and Approach
This paper seeks to evaluate off-site soil phytolith analysis as an effective palaeoecological tool for reconstructing Amazonian pre-Columbian land use in rainforest ecosystems. Our overarching aim is to determine the extent to which off-site soil phytolith analysis can reveal spatial and temporal gradients in different types of pre-Columbian land use: forest clearance, forest management, fire, and crop cultivation. We carried this out by reference to two study areas in SW Amazonia:
- The ring-ditch region of northern Bolivia, for which we present new data.
- The geoglyph region of eastern Acre state, Brazil, where we examine previously published phytolith data [20].
3. Methods
3.1. Study Areas
3.1.1. Ring Ditches of Northern Bolivia
The Riberalta region of northern Bolivia (Figure 1) has a mean annual precipitation of 1500–2400 mm, with a 2–3-month dry season [52,53], and is dominated by humid evergreen rainforest [54]. The latter comprises over 800 tree species, with Burseraceae, Fabaceae, Moraceae, and Arecaceae being the most abundant arboreal families [55]. Soils in this region are sandy clay ferrasols which are strongly weathered, acidic, and have low agricultural potential [56]. More fertile fluvisols are found along the region’s major rivers—the Beni and Madre de Dios. Several pre-Columbian ‘ring ditch’ earthworks have been identified atop bluffs of the Beni and Madre de Dios rivers, near the town of Riberalta [57]. The large size of these earthworks and the results of preliminary archaeological excavations have identified large, semi-sedentary populations inhabiting these river bluffs. The largest of these ring ditches, covering 125 ha of bluff along the Beni River, is located at the village of Tumichucua, ca. 18 km upstream of the town Riberalta. This pre-Columbian ring-ditch was occupied ca. 2200–1550 cal yr BP [58]. Phytolith residue analysis of ceramics excavated from Tumichucua and other ring ditches in the Riberalta region provides evidence for consumption and processing of palms and other cultigens (maize and squash), demonstrating that the ring-ditch builders cultivated crops and managed nearby forests [59]. Similar ring ditches have been discovered elsewhere in northern Bolivia [60] and much of Brazilian southern Amazonia [61]. Although the functional variation of these sites is still being studied, historic accounts from the Baures region, Llanos de Moxos, suggest that many were enclosed by palisades, serving a defensive function [4].
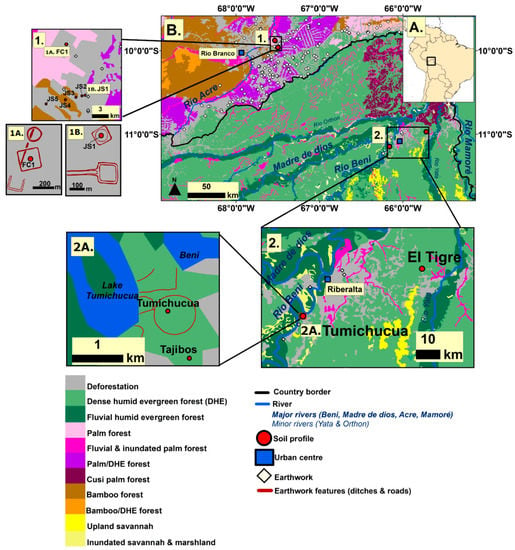
Figure 1.
Location maps. (A) Location of Riberalta region in northern Bolivia and the geoglyph region of eastern Acre, Brazil (Inset). (B) Location of study sites in relation to modern vegetation and earthwork distribution (geoglyphs and ring-ditches). (1) Location of the Acre soil profiles. (1A) Location of the FC1 soil profile in relation to the Fazenda Colorada geoglyph. (1B) Location of the JS1 soil profile in relation to the Jaco Sa geoglyph. (2) Location of the Tumichucua and El Tigre soil profiles. (2A) Locations of the Tumichucua and Tajibos soil profiles in relation to the Tumichucua ring-ditch. Earthwork locations from De Souza et al. [54] and location of Acre soil profiles from Watling et al. [20].
3.1.2. Geoglyphs of Eastern Acre, Brazil
The greatest known concentration of pre-Columbian geometric ditched earthworks (>400) lies to the north of Bolivia in eastern Acre, Brazil (Figure 1), where they are referred to as ‘geoglyphs’, constructed between ca. 2000–650 cal yr BP [62,63]. These Acre earthworks comprise a greater diversity of geometric shapes than the simple ring-ditch structures of northern Bolivia. Furthermore, unlike the Bolivian ring ditches near Riberalta, most of the Acre geoglyphs are located on interfluves, close to streams but far away from major rivers, and archaeological evidence of occupation is scarce, leading to the suggestion that they may have served a ceremonial, rather than settlement, function [64]. In contrast to northern Bolivia, extensive areas of rainforest of eastern Acre are dominated by bamboo (Guadua spp.), which may have proven easier to clear for geoglyph construction [20,64]. Soils in this region are sandy clay acrisols, which like ferrasols, are weathered and acidic with low agricultural potential, but differ in terms of other morphological, mineralogical, and chemical factors [56]. As with Riberalta, the more fertile fluvisols are found only along the region’s major rivers—the Purus, Juruá, and Acre.
3.2. Site Selection Strategy and Sampling Design
In the eastern Acre study area, Watling et al. [20] dug a 7.5 km transect consisting of five soil pits (each 1.5 m deep), with an on-site pit dug in the centre of the 100 m diameter ‘Jaco Sa’ geoglyph (67°29′51.39″ W, 9°57′38.96″ S) and four off-site pits dug along a linear transect, progressively further away at 0.5, 1.5, 3.5, and 7.5 km. An additional profile was excavated 10 km away from ‘Jaco Sa’ within the ‘Fazenda Colorada’ geoglyph to serve as a comparison. This sampling design sought to quantify the spatial scale of forest clearance associated with geoglyph construction.
We adopted a similar sampling strategy for the Riberalta study area, using a series of three soil pits—an on-site pit dug within the Tumichucua ring ditch (66°9′38.2″ W, 11°8′50.2″ S) and two off-site pits dug 1 km away (at ‘Los Tajibos’ 66°9′24.7″ W, 11°9′19.9″ S) and 50 km away in the rainforest interfluve (a 1-hectare ecological plot, ‘El Tigre’ 65°43′12″ W, 10°58′12″ S) (Figure 1). Tumichucua and Tajibos are both within a modern indigenous territory, where slash-and-burn agriculture is practiced today. The interfluvial ‘El Tigre’ plot is located 20 km east of the Beni River and 8 km west of the Yata river and is part of an Amazon-wide network of 1 ha ecological plots (RAINFOR) [65,66]; it shows no obvious sign of human land use. These three Bolivian plots were chosen for study because they provided an opportunity to test whether phytolith analysis can capture significant spatial gradients in the magnitude of anthropogenic impacts upon rainforest (e.g., clearance, burning, agro-forestry), not only today, but in pre-Columbian times. Although we acknowledge that archaeological investigations have not yet been undertaken in the vicinity of the ‘El Tigre’ plot, or along the Yata river to the east, our underlying assumption is that pre-Columbian human impacts would have been significantly greater at the riverine Tumichucua occupation centre than at the ‘El Tigre’ interfluve because greater population density would be expected for the former compared with the latter (due to labour for ring-ditch construction and easier access to valuable river resources—i.e., drinking water, fish protein, ease of travel via canoe).
We restricted the focus of this paper to Riberalta and eastern Acre because they employ a common sample design and field- and lab-based methodology, enabling direct comparison of data.
3.3. Field and Laboratory Methods
Field and lab methods for the Acre geoglyph study are described in Watling et al. [20]. Similar methods were undertaken for the Riberalta ring-ditch study area as follows:
Soil pits, measuring 1 × 2 m in area, were dug to 1 m depth. Soil samples were collected from the pit profile at consecutive 5 cm intervals and shipped to the University of Reading for cold storage. Phytolith analysis was undertaken at 5–10 cm resolution, following the wet oxidation method [38], with 15 soil samples per profile analysed from Tumichucua and Tajibos and 14 soil samples analysed from El Tigre. Samples were divided into ‘A’ silt (<50 µm) and ‘C’ sand (>50 µm) fractions. For the silt fraction, phytoliths were examined at 500× magnification. A minimum of 200 phytoliths were counted, and the rest of the slide was scanned to identify other diagnostic types. For the sand fraction, the entire slide was scanned at 200× magnification and all diagnostic phytoliths counted. Identifications were made using published atlases [38,40,67,68,69,70], as well as the phytolith reference collection at the University of Reading Tropical Palaeoecology Laboratory. To assist interpretation of the phytolith data, macroscopic charcoal was analysed to reconstruct fire history The latter was based on 3 cm3 soil samples taken at 5 cm increments, using a modified macroscopic sieving method with >250 µm and 125–250 µm size classes to distinguish between local versus extra-local charcoal sources, respectively [20,71,72].
Macroscopic charcoal particles greater than 0.5 cm in size were collected for radiocarbon dating (Table 1) as follows: as the pit was dug, the 2 m2 surface area was levelled and cleaned at 10 cm depth intervals; macroscopic charcoal particles were collected across this 2 m2 area and pooled together into a single sample to obtain a mean age for a given horizon. By pooling charcoal particles in this way, rather than dating individual charcoal particles, the likelihood of age inversions due to anomalously young or old individual particles (e.g., due to bioturbation) is likely to be reduced. Four AMS dates per soil pit were obtained and calibrated to 2σ accuracy using the IntCal 13 calibration curve [73,74].

Table 1.
Radiocarbon dates from the Riberalta region soil pits.
Factors which can potentially bias soil profile phytolith assemblages include soil pH, which affects phytolith dissolution, and soil particle size, which can make phytolith translocation more or less likely [30,34,75]. Both of these factors were therefore measured in the present study. pH was measured using a calibrated pH meter on samples taken at 10 cm intervals. Soil particle size was measured at 5 cm intervals using a Mastersizer 3000 laser diffraction analyser. The volume-based percentages produced by laser diffraction were converted to mass-based percentages using a calibration model [76] since laser diffraction underestimates the proportion of clay particles [77].
Carbon, nitrogen, and exchangeable cations were extracted at 10 cm intervals through each soil profile to analyse soil nutrient availability. These available nutrients indicate the agricultural potential of the soil and may thus provide further insights into pre-Columbian land use. Carbon and nitrogen were measured using a Delta V IRMS at 5 cm increments and expressed as % dry weight of soil. Exchangeable cations were determined using the standard ammonium acetate leaching procedure [78] at 10 cm resolution and expressed as cmolc/kg soil. The major nutrients Ca, Mg, K, and Na were measured, but Al could not be measured due to logistical constraints.
To improve understanding of the relationship between soil phytolith assemblages and parent vegetation, and thus the ability of this proxy to record pre-Columbian forest use and human impacts, the soil-surface phytolith assemblage of the ‘El Tigre’ 1 ha evergreen rainforest RAINFOR plot was compared with its floristic inventory, where every stem > 10 cm d.b.h. (diameter at breast height) was recorded (Table 2).

Table 2.
Comparison of the phytolith soil-surface assemblage with the floristic inventory from the El Tigre rainforest plot, illustrating the limitations of phytoliths in capturing the floristic composition of woody dicotyledons. Of all the vascular plants > 10 cm d.b.h. (diameter at breast height) recorded in the floristic inventory, the only taxon identifiable to family level in the soil-surface phytolith assemblage is Arecaceae. Furthermore, although Phenakospermum guyannense (an arborescent herb which is an early successional disturbance indicator) is identifiable by its phytoliths, it is absent from the soil-surface phytolith assemblage despite comprising 8% of the stems in the floristic inventory. Absence of such taxa from the phytolith record therefore does not signify their absence from the parent vegetation.
4. Results
4.1. Study Area 1—Ring Ditches of Riberalta Region, Northern Bolivia (Figure 2, Figure 3 and Figure 4)
Radiocarbon dates for each of the three Riberalta soil profiles are shown in Table 1 below.
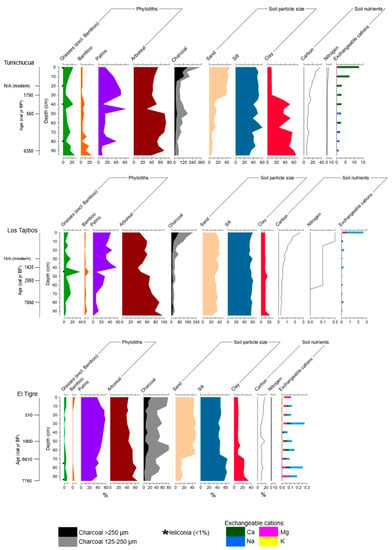
Figure 2.
Summary diagrams of the three Riberalta soil profiles, showing percentage phytolith frequencies (A-fraction), charcoal abundance (particles per cc), soil particle size (% mass), midrange 14C dates (calibrated years before present), C and N (% dry weight of soil), and exchangeable cations (cmolc/kg soil).
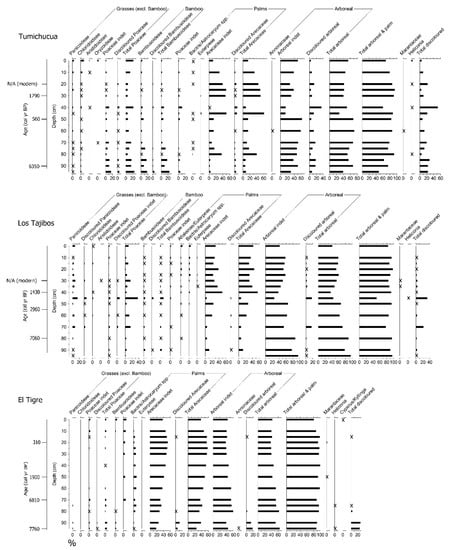
Figure 3.
Full phytolith percentage diagrams (A-fraction) of the three Riberalta soil profiles, with midrange 14C dates (calibrated years before present) plotted. X indicates <2%.
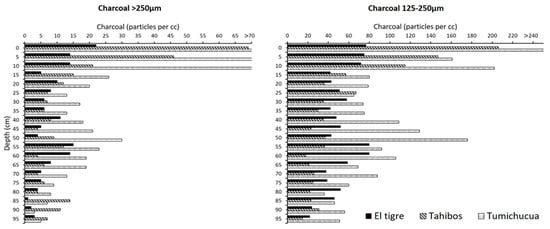
Figure 4.
Charcoal concentrations (particles per cc) for the three Riberalta soil profiles. Note that charcoal concentrations for the uppermost samples are as follows: >250 μm (0–5 cm: 171; 5–10 cm: 105; 10–15 cm: 122); 125–250 μm (0–5 cm: 342).
4.1.1. Tumichucua Ring-Ditch
The basal date shows that the 1 m soil profile spans the last 6000 years, consistent with previously published records from tropical South America [20,79,80,81,82], although anomalously young ages at 20 cm (modern) and 50 cm (560 cal yr BP) preclude establishment of an accurate age–depth model (Table 1). Raw phytolith counts are shown in the Supplementary Materials. Most of the profile is dominated by arboreal phytoliths (40–70%). Palm phytolith abundance fluctuates considerably through the profile, ranging from 10–60%. Bamboo fluctuates between 5 and 10% through most of the profile, but reaches 10–20% toward the base. Grass abundance fluctuates between 5 and 20%. Discoloured phytoliths [83], which are black- and brown-coloured phytoliths potentially discoloured by burning [84], are present throughout the record, fluctuating between 5 and 50%. Synchronous peaks in Poaceae (20%) and discoloured phytoliths (50%) occur at 40–45 cm depth. Palm abundance then increases to 50% in the subsequent phytolith sample (30–35 cm). Macroscopic charcoal is also present throughout, with a 4-fold increase in concentrations between 15 cm and the surface. There is a marked change in soil properties through the profile—the lower two thirds (100–35 cm) dominated by clay and silt, and the upper third (35–0 cm) dominated by silt and sand. The pH at Tumichucua (average pH 5) is slightly less acidic than at the other two sites and becomes slightly more acidic from 60–65 cm downwards (pH 4). Tumichucua is enriched in the available nutrients (C, N, Ca, Mg, and Na) relative to the other profiles, with particularly high levels of C (40% dry weight) and Ca (13 cmolc/kg) in the surface sample. K is the only nutrient which is lower compared to the other sites (0.2 cmolc).
4.1.2. Los Tajibos (1 km from Ring Ditch)
The four AMS dates show a good age–depth relationship (notwithstanding the modern age at 30 cm) and show that this 1 m soil profile spans at least the last 7000 years (Table 1). Raw phytolith counts are shown in the Supplementary Materials. Arboreal phytoliths dominate the entire profile (40–90%), with a large peak in discoloured arboreal phytoliths (40%) at 45–50 cm. Palm phytoliths are present throughout, but more abundant in the top half of the profile (30–60%) than the bottom half (10–30%). Grass and bamboo fluctuate around 5–10% and <5%, respectively, through most of the profile, with peaks of 40% and 10% at 45–50 cm, synchronous with the peak in discoloured phytoliths. As with Tumichucua, palm then increases in the above horizon (40–45 cm) to 60%. Macroscopic charcoal is present throughout the profile and shows a similar 4-fold increase in concentration between 15 cm and the surface, although charcoal concentrations overall are roughly half those of Tumichucua. In contrast to Tumichucua, soil properties at Tajibos are largely uniform throughout the profile (60% silt and ca. 30% sand), except for an isolated peak in clay (20%) at 90–95 cm. The pH at Tajibos is acidic (average pH 4) and remains stable throughout the profile. All available nutrients are significantly lower compared to Tumichucua. The surface sample peak in Na (2.5 cmolc/kg) is the highest among the exchangeable cations for the site.
4.1.3. El Tigre (50 km from Tumichucua)
The four AMS dates show a good age–depth relationship for this profile, the base of which dates to ca. 7760 cal yr BP. Raw phytolith counts are shown in the Supplementary Materials. Arboreal taxa comprise 40 to 60% of the phytolith assemblages throughout the profile. Palms increase gradually from 20% at the base to ca. 60% at 25–30 cm. Poaceae is present throughout, fluctuating around 5% or less, while bamboo is negligible through most of the profile, except for small peaks of 5% toward the top and bottom. Discoloured phytoliths are largely absent, except for peaks of burnt palm (10%) and arboreal taxa (20%) at the base (90–100 cm). However, macroscopic charcoal is present throughout, at comparable concentrations to the other two sites through most of the profile but without the large peak in the uppermost 15 cm. This site shows a similar change in soil properties to Tumichucua (i.e., up-profile change from clay/silt to silt/sand), although this transition occurs deeper in the profile at El Tigre (65–75 cm). pH at El Tigre is acidic (average pH 3), but slightly less so in the lower profile (pH 4). Nutrient availability is low throughout the profile. Carbon and nitrogen values are comparable with those of Tumichucua but without the corresponding surface sample peaks. The exchangeable cations are even lower than those at Tajibos (ranging from 0.9 cmolc/kg for Ca and 0.01 cmolc/kg for K).
4.2. Study Area 2—Geoglyphs of Eastern Acre, Brazil (Figure 5)
Full details of the phytolith and charcoal data from the 7.5 km transect of five soil pits extending from the ‘Jaco Sa’ geoglyph are presented in Watling et al. [20]. We reproduce the results in Figure 5 and summarise the key patterns and trends below:
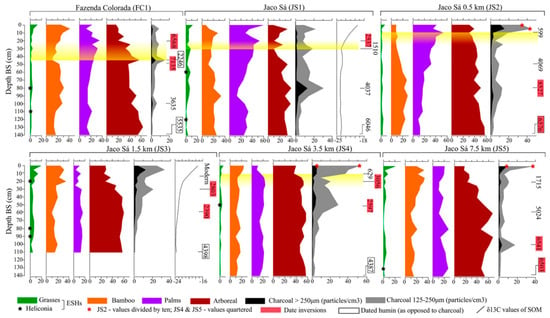
Figure 5.
Summary diagrams for the Acre soil pit transect, showing phytolith percentages (A-fraction), charcoal concentration (particles per cc), stable carbon isotope values (per mile), and midrange 14C dates (calibrated years before present). Yellow shading indicates the approximate time period of geoglyph construction and use (from Watling et al. [20], with permission).
As with the Bolivian study area, basal horizons for the five soil pits date to the middle Holocene, ranging from 4500 to 6500 cal yr BP. Phytolith assemblages are dominated by arboreal taxa, although at somewhat lower abundance (20–60%) than at the Bolivian sites due to the greater abundance of bamboo in the rainforests of eastern Acre. Bamboo occurs throughout all the profiles, varying from 10 to 40%, but with no consistent spatio-temporal pattern along the transect: relatively constant at 0 and 3.5 km; decreasing abundance in the upper half of the profile at 0.5 km, and increasing abundance in the upper half of the profile at 1.5 and 7.5 km. Grasses (non-bamboo) are consistently low throughout the profiles of all five pits (<5–10%), notwithstanding 20% peaks in surface samples at 1.5 and 7.5 km. Palms are present throughout all profiles, typically ranging from 10–40%, but show a clear spatio-temporal pattern, with trends of increasing palm percentages (from <20 to >40%) in the upper halves of the profiles within 0.5 km of the geoglyph, contrasting with uniform lower abundance of palms (10–20%) at the three pits beyond 0.5 km of the geoglyph. The palm peaks (50%) at 0 and 0.5 km roughly correlate with geoglyph archaeological dates in the region. Charcoal is present through all profiles, although in significantly lower concentrations than at the Bolivian sites. Sharp peaks in charcoal occur in the uppermost 15 cm of all 4 off-site profiles (0.5–7.5 km), in common with the Tumichucua and Tajibos Bolivian records, but no such peak occurs at the on-site (0 km) ‘Jaco Sa’ profile, in contrast with the Tumichucua ring-ditch profile.
5. Discussion
5.1. Scale of Deforestation
A critical pre-requisite to inferring pre-Columbian forest clearance from fossil phytolith data is a sound understanding of modern vegetation–phytolith relationships in open- versus closed-canopy ecosystems. The first such study in SW Amazonia was undertaken by Dickau et al. [67], who examined soil-surface phytolith assemblages of surface soils from 1 ha (500 × 20 m) RAINFOR plots in rainforest and savanna ecosystems of Noel Kempff Mercado National Park in NE Bolivia, where rainforests are broadly comparable to those of our Riberalta study area, notwithstanding some floristic differences [85]. Despite considerable intra-plot variation at some sites, they found that, overall, there was a statistically robust difference in phytolith signatures between savanna versus rainforest ecosystems. Savanna soils were characterised by >60% grass (excluding bamboo) and <40% arboreal phytolith taxa, while rainforest soils were characterised by <10% grass and 60–70% arboreal phytolith taxa. These soil-surface savanna phytolith signatures therefore serve as the best available analogue (albeit imperfect) for open, grass-dominated landscapes created by deforestation.
5.1.1. Riberalta Study Area
Given the monumental size of the Tumichucua ring ditch, ca. 100 m diameter with 5 m deep ditches, one would expect that the immediate vicinity would have been deforested to create the open ground needed for its construction and occupation. Grass phytoliths remain <20% throughout the 6000 ring-ditch soil profile (Figure 2 and Figure 3), well below the 60% signature characteristic of open ecosystems such as savanna [67]. We acknowledge, however, that Poaceae percentages alone may not provide conclusive evidence for forest clearance, given that only 7% Poaceae was recorded in archaeological swidden soils in the lower Tapajos [86] and peaks of 20% Poaceae in the surface samples of the Acre soil pits represented ca. 40 years of a completely deforested landscape (see next section). Forest clearings are often dominated by a number of other herbaceous and shrubby species unidentifiable by their phytoliths.
Heliconia phytoliths are scarce in the Tumichucua profile. This herb is typically abundant in forest openings in Brazil, Panama, and Costa Rica, but only make up <1 % of phytolith counts in these studies [40]. Moreover, a surface-soil phytolith study by Watling et al. [83] of different forest types near the Upper Madeira river of SW Amazonia found that herbaceous disturbance indicators, such as Heliconia and Phenakospermum, were highly under-represented in phytolith assemblages compared with the parent vegetation (corroborated by the soil-surface phytolith assemblage from the El Tigre plot, Table 2). Phytolith abundances are normally highest and best preserved in the surface sample, so the chances of these herbaceous taxa being represented lower down the soil profile, where phytoliths are less abundant, is even more unlikely [87,88]. Watling et al. [83] hypothesise that the time-averaging effect of bioturbation in soil profiles could dilute the signal of disturbance taxa, potentially producing false-negative results. Therefore, although high percentages of Heliconia phytoliths are consistent with forest clearance [40], one cannot conclude that low phytolith percentages of this taxon necessarily signify a lack of human disturbance [83,89,90].
Furthermore, it is important to note that abundance of Poaceae and Heliconia phytoliths does not, by itself, necessarily signify anthropogenic forest clearance, as these taxa are also prevalent in openings caused by natural disturbance. Only when considered in the context of other human occupation or land-use indicators from the same soil profile (e.g., charcoal, phytoliths of cultigens) or neighbouring archaeological sites (dated cultural horizons), can one infer whether peaks in these disturbance-indicator taxa likely reflect anthropogenic forest clearance or natural disturbance. In the case of the Riberalta soil pits, there is a clear spatial trend of higher grass phytolith and charcoal concentrations in the Tumichucua archaeological site versus the El Tigre plot which aligns with the expected pattern of land-use intensity. Meanwhile, peaks in Poaceae and the presence of Heliconia sp. around 40 cm depth at Tumichucua and Los Tabijos are strong indicators of disturbance episodes, particularly when considered alongside concomitant trends in the palm and arboreal phytolith curves.
5.1.2. Acre Study Area
Watling et al. [20] argued for a lack of significant deforestation based on the phytolith profiles of the five soil pits radiating out from the ‘Jaco Sa’ geoglyph in eastern Acre state (Figure 5) because grass (non-bamboo) phytolith percentages are consistently <5–10%. Peak values of 20% grass in the 0–5 cm surface sample likely reflect a mixed forest/pasture assemblage, as it encompasses deforestation and conversion to cattle pasture in the last 40 years. The dominance in all phytolith profiles of bamboo, palm, and other arboreal taxa shows that closed-canopy rainforest, with varying proportions of palm and bamboo, has occupied this area since the middle Holocene, an interpretation corroborated not only by the aforementioned modern phytolith studies of Bolivia [67], but also similar soil-surface studies by Watling et al. [70] across different forest types of the study area. As with the Bolivian Tumichucua ring-ditch, it is surprising that the ‘Jaco Sa’ geoglyph profile (0 km) similarly shows no phytolith evidence for open ground (i.e., high Poaceae (non-bamboo) and Heliconia percentages), despite the vegetation clearance (whether bamboo or tree removal) that must have been needed to construct this large earthwork, as marked by a charcoal peak dated to 1385–1530 cal yr BP [20]. Although there is minimal ceramic evidence for occupation of these geoglyphs, suggesting that they may have functioned as ceremonial/religious centres rather than centres of occupation [63,91,92], one might expect that the centre of this earthwork would have had more open ground than the off-site pits several kilometres away, and yet proportions of Poaceae are consistently low through all pit profiles along the transect and Heliconia is very rare. As with the series of Riberalta profiles, the phytolith data from the Acre transect do not show forest replacement by savanna under drier mid-Holocene climate conditions (i.e., toward the base of the profiles), although this is perhaps less surprising given that this transect is further away (ca. 200 km) from the nearest savanna ecotone.
5.2. Forest Management—Palm Enrichment
Palms (Arecaceae) are readily identifiable by their phytoliths and are especially useful for indigenous peoples—for edible fruits, dyes, drugs, beverages, building material, etc.—and their greater abundance and diversity near sites of present and past human occupation is likely testament to forest management over many years [93,94,95,96]. However, whilst there are numerous other arboreal taxa with economically important species (e.g., Bertholletia excelsa, Pouteria caimito, and Bixa orellana) [94], they are unfortunately indistinguishable from each other and other dicotyledonous tree taxa by their phytoliths (Table 2 and Table 3). Consequently, any phytolith evidence for pre-Columbian enrichment of forests with useful tree species generally comes from palms alone. Given the great number of palm species in Amazonia, and their ecological diversity, attributing changes in Arecaceae phytolith abundance—whether across space or time—to human influence, rather than natural ecological factors, is speculative at best, and must therefore be considered in the spatial context of known archaeological sites. If an abundance of Arecaceae in soil phytolith records does constitute a signature of pre-Columbian forest management, one should find a clear spatio-temporal pattern, whereby: a) palm percentages increase through the late Holocene as human population (i.e., land use) increases, with peak abundance roughly correlative with the chronology of ring-ditch/geoglyph occupation (ca. 2000–650 cal yr BP) [20,62,63], when use of the site is likely to have been highest; and b) palm abundance decreases with increasing distance from archaeological sites of human occupation, concordant with decreasing population density and land-use intensity. This spatio-temporal relationship holds true for the Acre geoglyph transect (Figure 5); i.e., palm percentages are highest within 0.5 km of the geoglyph, where peak abundance (ca. 40%) is roughly co-eval with geoglyph construction and occupation, and consistently lower beyond 0.5 km, fluctuating around 10–20% through the profiles. This positive relationship between palm abundance and pre-Columbian population density bolsters soil auger-based studies by McMichael et al. [29] and floristic studies by Levis et al. [94,97] elsewhere in Amazonia. These data thus lend further support to the hypothesis that pre-Columbian peoples created cultural/domesticated forests via management of economically important palm species [20,94,95,98].

Table 3.
List of useful taxa which have some level of domestication, and are present in the El Tigre plot, but which lack diagnostic phytoliths at any taxonomic level beyond “arboreal indeterminate”, from Levis et al. [94].
The temporal trends in palm phytolith percentages at the Riberalta plots are similar to those of Acre; i.e., the top third/half of the three soil profiles have generally higher palm phytolith percentages than the lower half, consistent with increasing human population through the late Holocene. At Tumichucua and Tajibos, the aforementioned peaks in Poaceae, Bambusoideae, and discoloured phytoliths, alongside the presence of Heliconia at ca. 40–45 cm and 45–50 cm, respectively, mark the horizons at which palm abundances increase significantly from 10 to 50/60%. These changes in phytolith assemblages likely reflect an increase in forest management, whereby economically useful palms were encouraged to grow at the expense of other less useful taxa [95]. No such disturbance event is recorded at the El Tigre interfluvial site.
On the other hand, palm phytolith abundance in our three Riberalta plots show a quite different spatial pattern in palm phytolith abundance from that of the Acre transect, since palm phytolith percentages are highest at the interfluvial ‘El Tigre’ rainforest site, rather than the fluvial Tumichucua ring-ditch site, contradicting the hypothesis that palm abundance is proportional to human population/land-use intensity, i.e., proximity to archaeological sites (Figure 2 and Figure 3). These results highlight that natural factors, such as edaphic conditions, hydrology, and natural disturbance, are also important drivers of palm abundance over large spatial scales. It is also conceivable, however, that the palm phytolith records of the three Riberalta soil profiles may yet reflect pre-Columbian land use. Firstly, although unoccupied today, in the absence of any archaeological surveys in the vicinity of the El Tigre plot, we cannot be certain that our assumption of lower pre-Columbian land use at this site compared with the Tumichucua and Tajibos plots is correct. El Tigre lies 10 km to the west of the archaeologically unexplored River Yata. Although only a minor river, future archaeological excavations may reveal that this river was a significant site of pre-Columbian occupation, given that it is bracketed by the archaeologically rich Beni and Mamore rivers to the west and east, respectively. As it lies only 10 km to the west of River Yata, it is possible that the zone of pre-Columbian forest management extended to the interfluvial forests around El Tigre. Secondly, although normally attributed to secondary succession following recent deforestation [99,100], it is possible that the large expanses of nearby cusi (Attalea speciosa) palm forests (not identifiable to species level by its phytoliths), located only ca. 10 km north of El Tigre (Figure 1), are a legacy of pre-Columbian land use and may have a late Holocene history of periodic range expansion into the El Tigre region. Another issue which may complicate the interpretation of inter-plot differences in palm phytolith abundance between Tumichucua, Tajibos, and El Tigre is potential differences in the proportions of other plant species in these plots that do not produce diagnostic phytoliths, which will influence palm phytolith abundance when expressed as relative percentages.
Surface-soil phytolith assemblages, when integrated with floristic data from ecological plots, can provide important insights into interpretation of fossil phytolith assemblages. Modern phytolith–vegetation studies from NE Bolivia (Noel Kempff Mercado National Park, NKMNP) [67] and eastern Acre [70] (Figure 6) are particularly pertinent. An interesting feature of these two studies is the high intra-plot spatial variability in palm phytolith percentages. For example, within a 1 ha plot of terra firme evergreen rainforest, soil-surface palm phytolith percentages vary by as much as 20–50% (A-fraction) and 15–75% (C-fraction) for NE Bolivia, and 15–60% (A-fraction) for eastern Acre. This intra-plot variability no doubt reflects the varying proximity of the soil phytolith sample to palm trees through a given plot. At first glance, these data might imply that fossil phytolith records from a single soil pit merely capture local scale variability in the distribution of different plant taxa within a plot, and thus capture too local a spatial scale to provide meaningful records of vegetation history. However, such concerns are unwarranted given that much longer periods of phytolith deposition and mixing in soils will create a time-averaging effect through the soil profile and will smooth out this intra-plot spatial variability in phytolith assemblages [41,101].
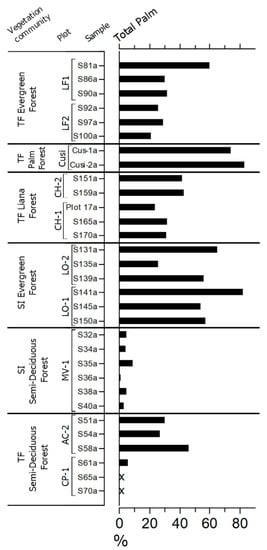
Figure 6.
Percentage diagram of total A-fraction palm phytoliths from soil surface samples in different forest community plots in and around Noel Kempff Mercado National Park, NE Bolivia (modified from Dickau et al. [67]). X signifies <2%. TF: terra firme; SI: seasonally inundated. Plot names: CP, Cerro Pelao; AC, Acuario; MV: Monte Verde; LO: Las Londras; CH: El Chore: Cusi: Attalea phalerata; LF: Los Fierros. Samples collected within 1 ha plots (500 × 20 m) at 50 m intervals.
Analyses of mean percentage phytolith data across 1 ha plots by Dickau et al. [67] and Watling et al. [20] revealed that palms can be markedly over-represented in soil phytolith assemblages relative to their abundance in the parent vegetation, giving a misleading impression of their true abundance in vegetation histories. However, this is not the case for El Tigre (Table 2), where the relative abundance of palms in the surface-soil phytolith assemblage (38%) and the plot vegetation (40%) is similar. Modern phytolith studies also reveal that variations in palm phytolith abundance between vegetation plots do not necessarily reflect a corresponding change in palm tree abundance. For example, a comparison between the soil phytolith data of Dickau et al. [67] and the floristic data for the same 1 ha plots in NKMNP from Burn et al. [15] shows that the percentage of palm in soil-surface phytolith assemblages of seasonally flooded rainforest plots (50–80%) is double that of terra firme rainforest plots (30%), even though its abundance in the vegetation of the former (1.6–4.2%) is lower than that of the latter (8.8–14.0%). These data highlight potential uncertainties in drawing palaeoecological inferences from inter-plot spatial differences in palm phytolith percentages, as discussed earlier with respect to El Tigre versus Tumichucua.
5.3. Crop Cultivation and Burning
Corroborative evidence for pre-Columbian forest enrichment with palm would come from coincident peaks in charcoal and presence of cultigen phytoliths. Such evidence would point to agro-forestry, whereby cultivation of annual/perennial cultigens is combined with forest management (enrichment with palms and use of fire for opening forest canopies). No cultigen phytoliths were found in any of the soil profiles of either the Acre or Riberalta transect. However, absence of evidence is not necessarily evidence of absence. Both maize (Zea mays) and squash (Cucurbita sp.) phytoliths were recovered from cultural horizons at Tumichucua and the Acre geoglyphs [59], so the absence of cultigen phytoliths from our soil-pit profiles does not conclusively show that cultigens were not grown in the vicinity.
This point is corroborated by McMichael et al. [28], who compared lake pollen data with nearby soil phytolith data collected with soil augers to determine spatial scales of pre-Columbian land use near lakes Ayauchi, Gentry, and Parker in western Amazonia. Despite the presence of maize pollen in the sediment cores of each lake, maize phytoliths were only recovered in trace amounts from 7 of the 94 soil augers collected. Likewise, despite the presence of maize pollen in sediments from lakes El Cerrito and Frontera in the Bolivian Llanos de Moxos, Whitney et al. [10] found only a single maize ‘leaf cross’ phytolith in a single sample in a soil profile dug within nearby pre-Columbian raised fields. It is therefore clear that the scarcity of cultigen phytoliths in soil profiles is a significant limitation for this proxy as an effective tool for capturing pre-Columbian crop cultivation.
Key uncertainties regarding cultigen phytolith taphonomy in soils need to be resolved. For example, little is known about the minimum area or duration of cultivation required for crop phytoliths to be visible in phytolith assemblages (using a minimum count of 200 phytoliths). Additionally, the extent to which one should even expect to find cultigen phytoliths in off-site soil profiles is open to question, given that many of the most diagnostic phytoliths from cultigens are restricted to the edible organs, such as maize cobs and squash rinds, which would have been removed from the field to settlement areas for processing and consumption.
With respect to charcoal, there is no correlation between palm phytolith abundance and charcoal concentrations in any of the soil profiles (Figure 4). However, there is a clear trend of increasing charcoal abundance from the El Tigre interfluve toward the Tumichucua ring-ditch site on the river bluff. The latter implies that the charcoal profiles reflect pre-Columbian anthropogenic burning and that fire must have been an important land-use tool [8,94,102,103]. This inference is further strengthened by the gradient in surface-soil charcoal between the three Riberalta soil pits, as Tumichucua and Tajibos are part of the territory of an indigenous community today, while the El Tigre interfluvial site is unoccupied. At both Tumichucua and nearby Tajibos, charcoal concentrations peak at the soil surface, suggesting that the use of fire today, via slash-and-burn agriculture, is much greater than in pre-Columbian times. The latter is perhaps unsurprising, given that large-scale slash-and-burn farming may have only become feasible with the introduction of metal axes and machetes by Europeans [104].
Further evidence for land use within the ring-ditch comes from the geochemical data. There is a higher availability of important soil nutrients (C, N, Ca, Na, and Mg) at Tumichucua compared with Tajibos and El Tigre, which have low nutrient availability levels comparable with those of other remote interfluvial ferrasols (Figure 2) [56]. Although these soils are not Amazon Dark Earths (terra preta) [105,106], the association between these elevated nutrient levels with a pre-Columbian settlement site raises the possibility that they reflect a millennial-scale legacy of soil enrichment due to human activities.
5.4. Soil Properties, Temporal Resolution, and Phytolith Preservation and Translocation
A key challenge of soil-based phytolith studies as a tool for investigating pre-Columbian land use is their limited temporal resolution. Numerous prior studies [29,80,81,82,107,108], as well as the two sets of soil pits in this study (Table 1 and Figure 5), clearly show that soil profiles in tropical South America broadly conform to an age-depth relationship, whereby 1 m depth is generally mid-Holocene in age. However, significant mixing of the soil profile due to bioturbation [36] can compromise the robustness of age–depth relationships, even when macroscopic charcoal particles from a given stratigraphic horizon are pooled together into an aggregate sample for dating, as was performed for all soil pits in this study. Consequently, age reversals are not uncommon. Relating down-profile changes in phytolith assemblages and charcoal concentrations to changes in pre-Columbian land use through time is therefore problematic, even at multi-centennial, and in some cases, millennial-scale resolution. At best, the soil-pit phytolith records of Riberalta and Acre reveal broad, multi-millennial-scale trends in forest management through the mid-to-late Holocene, but, unlike lacustrine sequences, cannot offer the temporal resolution necessary for robust correlations of such changes with individual cultural horizons identified in archaeological excavations. Furthermore, this coarse temporal resolution means that short-term (e.g., sub-centennial-scale), isolated, anthropogenic forest impacts, whether deforestation, fire, or crop cultivation, are unlikely to be captured by soil-based phytolith or charcoal analysis, although the cumulative, long-term effects of such impacts should no doubt be apparent.
In addition to physical mixing of the soil profile via bioturbation [36], other factors, such as dissolution and translocation, may potentially compromise the stratigraphic reliability and robustness of soil profile phytolith records. It has been argued by Alexandre et al. [30] and Song et al. [109] that the soil phytolith pool has an average age which can be markedly younger from that of the associated soil due to phytolith dissolution, and that the turnover time of this labile phytolith pool can be as rapid as 400 years in the tropics, over which time up to 90% of the phytoliths may be recycled. This implies that phytolith assemblages assumed to be several thousand years old, based on the dated soil profile, may in fact be only several hundred years old [110]. However, these findings are contentious, as several studies, based on radiocarbon dating of phytoliths, have shown that phytolith assemblages do exhibit stratigraphically robust Holocene age–depth relationships [34,111,112,113,114,115]. Nevertheless, there is strong evidence to show that the rate of phytolith dissolution, and their return to the soil silica pool [37,115,116], is strongly dependent upon soil pH, with minimum dissolution occurring at pH 3 and greatest dissolution under alkaline conditions [38,75]. However, because all the soils in our two study areas are acidic (pH 3–5), we can discount pH as a significant factor negatively impacting phytolith preservation in our soil profiles.
Soil texture has also been identified as a potential influence upon the stratigraphic integrity of phytolith assemblages, as differences in porosity between sandy versus clayey soils have been shown to affect the rate of down-profile phytolith translocation, especially for smaller phytoliths [30,35,88]. There are significant differences in soil texture among some of our Bolivian soil pits (Figure 2), providing an opportunity to test whether this soil property influenced phytolith stratigraphy at our sites. For example, at Tumichucua, the soil comprises a clay/silt mix below 30 cm and a sand/silt mix above 30 cm, while at Tajibos, there is a uniform sand/silt mix throughout the profile. The fact that the phytolith profiles of these neighbouring soil pits are broadly similar, despite their contrasting soil texture profiles, indicates that stratigraphic changes in phytolith assemblages across our study areas likely reflect real floristic changes in the parent vegetation, rather than differential down-profile translocation of different phytolith taxa resulting from differences in soil porosity.
Other factors that may potentially influence soil phytolith profiles, which we have not been able to test for, include phytolith morphotype, i.e., size, shape, and degree of silicification [31,117], and soil moisture regime [32,118].
5.5. Recommendations for Future Work
Although some of the limitations of soil phytolith analysis are intractable (e.g., temporal resolution of age–depth relationships in soil profiles), we suggest several potentially fruitful avenues toward addressing some of the other limitations:
1. Spatial resolution and sample density: A key limitation in our sampling design is the small number of soil pits in our transects—constrained by the time and labour involved, both in their excavation, especially in remote areas difficult to access, as well as the resulting laboratory analyses. McMichael et al. [28,29] have adopted an alternative approach based on soil augers, which have the clear advantage in speed of soil sample collection, enabling far greater density of sampling sites than is possible with soil pits. However, augers cannot provide samples with the same stratigraphic integrity as is possible with soil pits, limiting their use for examining age–depth relationships. Mechanical percussion corers would provide a rapid means of collecting stratigraphically intact soil cores, but the weight of the coring equipment constrains site selection to those accessible by vehicle; a key limitation in much of Amazonia. Perhaps a blended approach involving all three sampling techniques may offer a useful way forward.
2. Taxonomic resolution: The inability to differentiate the phytoliths of different dicotyledonous arboreal taxa, even to family level, is a major limitation of this palaeoecological proxy. As argued by Watling et al. [119] and Piperno and McMichael [120], there is therefore a clear need to improve the modern phytolith reference collections of arboreal taxa as a basis for refining their identification and potentially differentiating some key arboreal taxa, at least to family level, and to test their preservation in soils. Furthermore, since these analyses were carried out, there have been improvements in taxonomic identifications of palm phytoliths below family level [69,83,120]. Knowing which taxa are represented in different locations could facilitate interpretations regarding their abundance.
3. Modern phytolith–vegetation relationships: Significant progress has been made in the last decade in characterising the modern (soil-surface) phytolith assemblages of different types of tropical forest and savanna ecosystems in Amazonia [67,70,83]. However, there is a need to extend these studies to more human-disturbed ecosystems under different types of land use (e.g., polyculture, agro-forestry) in indigenous territories and areas around communities of local people (e.g., caboclos and ribereños) to obtain more realistic modern phytolith analogues of pre-Columbian land use.
4. Post-depositional factors: There is a need for further research to better elucidate the extent to which phytolith assemblages in different types of Amazonian soils are altered by post-depositional processes; in particular, the influence of particle size and bioturbation upon down-profile translocation, and the robustness and representativeness of the stable phytolith pool over time. Comparison of radiocarbon dates from phytoliths, macroscopic charcoal, and the humic soil organic fraction from the same stratigraphic horizons should help quantify the extent to which these translocation and dissolution processes distort phytolith stratigraphies.
6. Conclusions
Phytolith data from the Acre geoglyph transect reveal a clear spatio-temporal pattern in the abundance of palm phytoliths, with palm abundance increasing through the mid-late Holocene, and increasing toward the geoglyph. The latter demonstrates support for Levis et al.’s [94] hypothesis that, in the modern flora, palm abundance and diversity is positively related to proximity to archaeological sites, and thus a legacy of pre-Columbian forest management. This hypothesis of increasing population density and land use through the late Holocene driving an increase in proportion of palms in the vegetation is also supported by temporal trends in the Bolivian soil phytolith data. However, the Bolivian data call into question notions of a strict cause-and-effect relationship between palm abundance and human land-use intensity.
Our analyses have highlighted some of the limitations of off-site soil phytolith analysis as a palaeoecological tool for investigating pre-Columbian land use in Amazonia, particularly with respect to taxonomic resolution and temporal resolution. However, when used in combination with other land-use indicators (e.g., charcoal), and with appropriate site selection (i.e., soil-pit transects radiating from archaeological sites), we have shown that they can provide important insights into pre-Columbian forest management and land-use intensity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/quat6020033/s1, Table S1: Raw Phytolith Counts.
Author Contributions
F.E.M. and J.H. conceived and designed the overall project; F.E.M. led the fieldwork and collected the soil samples; J.H. undertook the laboratory analyses, supervised by F.E.M. and S.B.; S.B. directed the physical and geochemical analyses of soil properties; J.H. wrote the first draft of the paper; J.H., F.E.M., and S.B. jointly contributed to interpretation of the data and writing subsequent drafts; A.A.-M., A.M., R.B. (Rene Boot), R.B. (Roel Brienen), T.F., J.L., S.M., G.P., O.L.P., M.P.-C., M.T., V.V. and P.Z. contributed either to the creation, administration, and/or collection of ecological and floristic data from the El Tigre RAINFOR plot, and are listed alphabetically. V.V. also provided logistical support during fieldwork, while O.L.P., R.B. (Rene Boot), R.B. (Roel Brienen), T.F., M.P.-C. and P.Z. provided comments on later drafts of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a NERC ‘Scenario’ DTP Ph.D. award to J.H. (grant NE/L002566/1). This publication is based on Research Project number 65 of the ForestPlots.net global collaboration ‘Impact of mid-late Holocene climate change and pre-Columbian land use upon Bolivian ecotonal tropical forests’. Radiocarbon dates were funded by the UK NERC Radiocarbon Facility (allocation number 2179.0319 to F.M.).
Data Availability Statement
Raw phytolith counts can be found in the Supplementary Materials section and have been uploaded to Neotoma. https://www.neotomadb.org/.
Acknowledgments
We are grateful to: the Museo de Historia Natural ‘Noel Kempff Mercado’ in Santa Cruz, Bolivia, for kindly providing herbarium material for the phytolith reference collection at the University of Reading; Daniel Soto for logistical support and botanical expertise; the local community at Tumichucua for permission to collect soil samples from the pre-Columbian ring ditch and the ‘Los Tajibos’ plot; and to John Carson, Richard Smith, and Heather Plumpton for assistance in fieldwork. We thank Lourens Poorter for his contribution to the El Tigre plot ecological data, as well as his comments on an earlier draft of the manuscript. We thank the handling editor and three reviewers whose comments improved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gross, D.R. Protein Capture and Cultural Development in the Amazon Basin. Am. Anthr. 1975, 77, 526–549. [Google Scholar] [CrossRef]
- Meggers, B.J. Environmental Limitation on the Development of Culture. Am. Anthr. 1954, 56, 801–824. [Google Scholar] [CrossRef]
- Denevan, W.M. Cultivated Landscapes of Native Amazonia and the Andes; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Erickson, C.L. The Transformation of Environment into Landscape: The Historical Ecology of Monumental Earthwork Construction in the Bolivian Amazon. Diversity 2010, 2, 618–652. [Google Scholar] [CrossRef]
- Erickson, C.L.; Balée, W. (Eds.) The historical ecology of a complex landscape in Bolivia. In Time and Complexity in Historical Ecology: Studies in the Neotropical Lowlands; Columbia University Press: New York, NY, USA, 2006; pp. 187–233. [Google Scholar]
- Mayle, F.E.; Iriarte, J. Integrated palaeoecology and archaeology—A powerful approach for understanding pre-Columbian Amazonia. J. Archaeol. Sci. 2014, 51, 54–64. [Google Scholar] [CrossRef]
- Iriarte, J.; Elliott, S.; Maezumi, S.Y.; Alves, D.; Gonda, R.; Robinson, M.; de Souza, J.G.; Watling, J.; Handley, J. The origins of Amazonian landscapes: Plant cultivation, domestication and the spread of food production in tropical South America. Quat. Sci. Rev. 2020, 248, 106582. [Google Scholar] [CrossRef]
- Maezumi, S.Y.; Alves, D.; Robinson, M.; de Souza, J.G.; Levis, C.; Barnett, R.L.; de Oliveira, E.A.; Urrego, D.; Schaan, D.; Iriarte, J. The legacy of 4,500 years of polyculture agroforestry in the eastern Amazon. Nat. Plants 2018, 4, 540–547. [Google Scholar] [CrossRef]
- Iriarte, J.; Power, M.J.; Rostain, S.; Mayle, F.E.; Jones, H.; Watling, J.; Whitney, B.S.; McKey, D.B. Fire-free land use in pre-1492 Amazonian savannas. Proc. Natl. Acad. Sci. USA 2012, 109, 6473–6478. [Google Scholar] [CrossRef]
- Whitney, B.S.; Dickau, R.; E Mayle, F.; Walker, J.H.; Soto, J.D.; Iriarte, J. Pre-Columbian raised-field agriculture and land use in the Bolivian Amazon. Holocene 2014, 24, 231–241. [Google Scholar] [CrossRef]
- Carson, J.F.; Whitney, B.S.; Mayle, F.E.; Iriarte, J.; Prümers, H.; Soto, J.D.; Watling, J. Environmental impact of geometric earthwork construction in pre-Columbian Amazonia. Proc. Natl. Acad. Sci. USA 2014, 111, 10497–10502. [Google Scholar] [CrossRef]
- Whitney, B.S.; Dickau, R.; Mayle, F.E.; Soto, J.D.; Iriarte, J. Pre-Columbian landscape impact and agriculture in the Monumental Mound region of the Llanos de Moxos, lowland Bolivia. Quat. Res. 2013, 80, 207–217. [Google Scholar] [CrossRef]
- Bush, M.B.; Silman, M.R.; De Toledo, M.B.; Listopad, C.; Gosling, W.D.; Williams, C.; De Oliveira, P.E.; Krisel, C. Holocene fire and occupation in Amazonia: Records from two lake districts. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 209–218. [Google Scholar] [CrossRef]
- Bush, M.B.; Nascimento, M.N.; Åkesson, C.M.; Cárdenes-Sandí, G.M.; Maezumi, S.Y.; Behling, H.; Correa-Metrio, A.; Church, W.; Huisman, S.N.; Kelly, T.; et al. Widespread reforestation before European influence on Amazonia. Science 2021, 372, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Burn, M.J.; Mayle, F.E.; Killeen, T.J. Pollen-based differentiation of Amazonian rainforest communities and implications for lowland palaeoecology in tropical South America. Palaeogeogr. Palaeoclim. Palaeoecol. 2010, 295, 1–18. [Google Scholar] [CrossRef]
- Gosling, W.D.; Mayle, F.E.; Tate, N.J.; Killeen, T.J. Differentiation between Neotropical rainforest, dry forest, and savanna ecosystems by their modern pollen spectra and implications for the fossil pollen record. Rev. Palaeobot. Palynol. 2009, 153, 70–85. [Google Scholar] [CrossRef]
- Jacobson, G.L.; Bradshaw, R. The Selection of Sites for Paleovegetational Studies. Quat. Res. 1981, 16, 80–96. [Google Scholar] [CrossRef]
- Lane, C.S.; Cummings, K.E.; Clark, J.J. Maize pollen deposition in modern lake sediments: A case study from Northeastern Wisconsin. Rev. Palaeobot. Palynol. 2010, 159, 177–187. [Google Scholar] [CrossRef]
- Pärssinen, M.; Schaan, D.; Ranzi, A. Pre-Columbian geometric earthworks in the upper Purús: A complex society in western Amazonia. Antiquity 2009, 83, 1084–1095. [Google Scholar] [CrossRef]
- Watling, J.; Iriarte, J.; Mayle, F.E.; Schaan, D.; Pessenda, L.C.R.; Loader, N.J.; Street-Perrott, F.A.; Dickau, R.E.; Damasceno, A.; Ranzi, A. Impact of pre-Columbian “geoglyph” builders on Amazonian forests. Proc. Natl. Acad. Sci. USA 2017, 114, 1868–1873. [Google Scholar] [CrossRef]
- Lathrap, D.W. Aboriginal Occupation and Changes in River Channel on the Central Ucayali, Peru. Am. Antiq. 1968, 33, 62–79. [Google Scholar] [CrossRef]
- Lathrap, D.W. The Upper Amazon; Thames and Hudson: Southampton, UK, 1970. [Google Scholar]
- Meggers, B.J. Cultural evolution in Amazonia. In Profiles in Cultural Evolution; Rambo, A.T., Gillogly, K., Eds.; Anthropological Papers, 85; Museum of Anthropology, University of Michigan: Ann Arbor, MI, USA, 1991; pp. 191–216. [Google Scholar]
- Pearsall, D.M. Paleoethnobotany: A Handbook of Procedures; Academic Press: San Diego, CA, USA, 1989. [Google Scholar]
- Hilbert, L.; Neves, E.G.; Pugliese, F.; Whitney, B.S.; Shock, M.; Veasey, E.; Zimpel, C.A.; Iriarte, J. Evidence for mid-Holocene rice domestication in the Americas. Nat. Ecol. Evol. 2017, 1, 1693–1698. [Google Scholar] [CrossRef]
- Watling, J.; Shock, M.P.; Mongeló, G.Z.; Almeida, F.O.; Kater, T.; De Oliveira, P.E.; Neves, E.G. Direct archaeological evidence for Southwestern Amazonia as an early plant domestication and food production centre. PLoS ONE 2018, 13, e0199868. [Google Scholar] [CrossRef]
- McMichael, C.H.; Piperno, D.R.; Bush, M.B.; Silman, M.R.; Zimmerman, A.R.; Raczka, M.F.; Lobato, L.C. Sparse Pre-Columbian Human Habitation in Western Amazonia. Science 2012, 336, 1429–1431. [Google Scholar] [CrossRef] [PubMed]
- McMichael, C.H.; Bush, M.; Piperno, D.R.; Silman, M.R.; Zimmerman, A.; Anderson, C. Spatial and temporal scales of pre-Columbian disturbance associated with western Amazonian lakes. Holocene 2011, 22, 131–141. [Google Scholar] [CrossRef]
- McMichael, C.H.; Piperno, D.R.; Neves, E.G.; Bush, M.B.; Almeida, F.O.; Mongeló, G.; Eyjolfsdottir, M.B. Phytolith Assemblages Along a Gradient of Ancient Human Disturbance in Western Amazonia. Front. Ecol. Evol. 2015, 3, 141. [Google Scholar] [CrossRef]
- Alexandre, A.; Meunier, J.-D.; Colin, F.; Koud, J.-M. Plant impact on the biogeochemical cycle of silicon and related weathering processes. Geochim. Cosmochim. Acta 1997, 61, 677–682. [Google Scholar] [CrossRef]
- Cabanes, D.; Weiner, S.; Shahack-Gross, R. Stability of phytoliths in the archaeological record: A dissolution study of modern and fossil phytoliths. J. Archaeol. Sci. 2011, 38, 2480–2490. [Google Scholar] [CrossRef]
- Bartoli, F.; Wilding, L.P. Dissolution of Biogenic Opal as a Function of its Physical and Chemical Properties. Soil Sci. Soc. Am. J. 1980, 44, 873–878. [Google Scholar] [CrossRef]
- Osterrieth, M.; Madella, M.; Zurro, D.; Alvarez, M.F. Taphonomical aspects of silica phytoliths in the loess sediments of the Argentinean Pampas. Quat. Int. 2007, 193, 70–79. [Google Scholar] [CrossRef]
- Fishkis, O.; Ingwersen, J.; Lamers, M.; Denysenko, D.; Streck, T. Phytolith transport in soil: A field study using fluorescent labelling. Geoderma 2010, 157, 27–36. [Google Scholar] [CrossRef]
- Kaczorek, D.; Puppe, D.; Busse, J.; Sommer, M. Effects of phytolith distribution and characteristics on extractable silicon fractions in soils under different vegetation—An exploratory study on loess. Geoderma 2019, 356, 113917. [Google Scholar] [CrossRef]
- Hart, D.M. The influence of soil fauna on phytolith distribution in a soil. In Phytoliths and Starch Research in the Australian-Pacific-Asian Regions: The State of the Art, Terra Australis 19; Hart, D.M., Wallis, L.A., Eds.; Pandanus Books: Canberra, Australia, 2003; pp. 83–91. [Google Scholar]
- Madella, M.; Lancelotti, C. Taphonomy and phytoliths: A user manual. Quat. Int. 2012, 275, 76–83. [Google Scholar] [CrossRef]
- Piperno, D.R. Phytoliths: A Comprehensive Guide for Archaeologists and Paleoecologists; AltaMira Press: Oxford, UK, 2006. [Google Scholar]
- Piperno, D.R. Phytolith Analysis: An Archaeological and Geological Perspective; Academic Press: San Diego, CA, USA, 1984. [Google Scholar]
- Piperno, D.R.; Pearsall, D.M. The Origins of Agriculture in the Lowland Neotropics; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Fredlund, G.G.; Tieszen, L.T. Modern Phytolith Assemblages from the North American Great Plains. J. Biogeogr. 1994, 21, 321. [Google Scholar] [CrossRef]
- Iriarte, J. Assessing the feasibility of identifying maize through the analysis of cross-shaped size and three-dimensional morphology of phytoliths in the grasslands of southeastern South America. J. Archaeol. Sci. 2003, 30, 1085–1094. [Google Scholar] [CrossRef]
- Ollendorf, A.L. Toward a classification scheme of sedge (Cyperaceae) phytoliths. In Phytolith Systematics: Emerging Issues; Rapp, G., Mulholand, S.C., Eds.; Plenum Press: New York, NY, USA, 1992; pp. 91–111. [Google Scholar]
- Bozarth, S.R. Diagnostic Opal Phytoliths from Rinds of Selected Cucurbita Species. Am. Antiq. 1987, 52, 607–615. [Google Scholar] [CrossRef]
- Piperno, D.R.; Ranere, A.J.; Holst, I.; Iriarte, J.; Dickau, R. Starch grain and phytolith evidence for early ninth millennium B.P. maize from the Central Balsas River Valley, Mexico. Proc. Natl. Acad. Sci. USA 2009, 106, 5019–5024. [Google Scholar] [CrossRef] [PubMed]
- Bozarth, S.R.; Price, K.; Woods, W.I.; Neves, E.G.; Rebellato, R. Phytoliths and terra preta: The Hatahara site example. In Amazonian Dark Earths: Wim Sombroek’s Vision; Woods, W.I., Teixeira, W.G., Lehmann, J., Steiner, C., Winkler Prins, A., Rebellato, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 85–98. [Google Scholar]
- Lombardo, U.; Iriarte, J.; Hilbert, L.; Ruiz-Pérez, J.; Capriles, J.M.; Veit, H. Early Holocene crop cultivation and landscape modification in Amazonia. Nature 2020, 581, 190–193. [Google Scholar] [CrossRef]
- Godwin, H. Pollen Analysis. An outline of the problems and potentialities of the method. New Phytol. 1934, 33, 278–304. [Google Scholar] [CrossRef]
- Larson, B.M.H.; Barrett, S.C.H. A comparative analysis of pollen limitation in flowering plants. Biol. J. Linn. Soc. 2000, 69, 503–520. [Google Scholar] [CrossRef]
- Macdonald, G.M. Methodological falsification and the interpretation of palaeoecological records: The cause of the early Holocene birch decline in western Canada. Rev. Palaeobot. Palynol. 1993, 79, 83–97. [Google Scholar] [CrossRef]
- Seppä, H.; Bennett, K. Quaternary pollen analysis: Recent progress in palaeoecology and palaeoclimatology. Prog. Phys. Geogr. Earth Environ. 2003, 27, 548–579. [Google Scholar] [CrossRef]
- Beekma, J.; Zonta, A.; Keijzer, B. Base Ambiental para el Dessarrollo; Departamento de Pando y la Provincia Vaca Diez, Servicio Holandes de Cooperación al Desarrollo SNV: Pichincha, Ecuador, 1996; p. 91. [Google Scholar]
- Myers, G.P.; Newton, A.C.; Melgarejo, O. The influence of canopy gap size on natural regeneration of Brazil nut (Bertholletia excelsa) in Bolivia. For. Ecol. Manag. 2000, 127, 119–128. [Google Scholar] [CrossRef]
- Wasson, J.G.; Barrera, S.; Barrère, B.; Binet, D.; Collomb, D.; Gonzales, I.; Gourdin, F.; Guyot, J.L.; Rocabado, G. Hydro-ecoregions of the Bolivian Amazon: A geographical framework for the functioning of river ecosystems. In The Ecohydrology of South American Rivers and Wetlands; McClain, M.E., Ed.; International Association of Hydrological Sciences: Wallingford, UK, 2002; pp. 69–91. [Google Scholar]
- Ibisch, P.L.; Beck, S.G.; Gerkmann, B.; Carretero, A. Ecoregions and ecosystems. In Biodiversity: The Richness of Bolivia; Ibisch, P.L., Mérida, G., Eds.; Editorial FAN: Santa Cruz, CA, USA, 2004; pp. 47–88. [Google Scholar]
- Quesada, C.A.; Lloyd, J.; Anderson, L.O.; Fyllas, N.M.; Schwarz, M.; Czimczik, C.I. Soils of Amazonia with particular reference to the RAINFOR sites. Biogeosciences 2011, 8, 1415–1440. [Google Scholar] [CrossRef]
- Saunaluoma, S. Pre-Columbian Earthworks in the Riberalta Region of the Bolivian Amazon. Amaz.-Rev. Antropol. 2010, 2, 104–138. [Google Scholar] [CrossRef]
- Arnold, D.E.; Pendery, S.R.; Prettol, K.A.; Webster, G.S.; Greenfield, H.J. News and Short Contributions. J. Field Archaeol. 1988, 15, 457. [Google Scholar] [CrossRef]
- Watling, J.; Saunaluoma, S.; Pärssinen, M.; Schaan, D. Subsistence practices among earthwork builders: Phytolith evidence from archaeological sites in the southwest Amazonian interfluves. J. Archaeol. Sci. Rep. 2015, 4, 541–551. [Google Scholar] [CrossRef]
- Prümers, H.; Betancourt, C.J. 100 años de investigación arqueológica en los Llanos de Mojos. Arqueoantropol. Año 2014, 4, 11–54. [Google Scholar]
- de Souza, J.G.; Schaan, D.P.; Robinson, M.; Barbosa, A.D.; Aragão, L.E.; Marimon, B.H., Jr.; Marimon, B.S.; da Silva, I.B.; Khan, S.S.; Nakahara, F.R.; et al. Pre-Columbian earth-builders settled along the entire southern rim of the Amazon. Nat. Commun. 2018, 9, 1125. [Google Scholar] [CrossRef]
- Schaan, D.P. Sacred Geographies of Ancient Amazonia: Historical Ecology of Social Complexity; Left Coast Press: Walnut Creek, CA, USA, 2012. [Google Scholar]
- Saunaluoma, S.; Schaan, D. Monumentality in Western Amazonian formative societies: Geometric ditched enclosures in the Brazilian state of Acre. Antiqua 2012, 2, e1. [Google Scholar] [CrossRef]
- McMichael, C.H.; Palace, M.W.; Golightly, M. Bamboo-dominated forests and pre-Columbian earthwork formations in south-western Amazonia. J. Biogeogr. 2014, 41, 1733–1745. [Google Scholar] [CrossRef]
- Lopez-Gonzalez, G.; Lewis, S.L.; Burkitt, M.; Phillips, O. ForestPlots.net Database. 2009. Available online: www.forestplots.net (accessed on 12 September 2017).
- ForestPlots.net.; Blundo, C.; Carilla, J.; Grau, R.; Malizia, A.; Malizia, L.; Osinaga-Acosta, O.; Bird, M.; Bradford, M.; Catchpole, D.; et al. Taking the pulse of Earth’s tropical forests using networks of highly distributed plots. Biol. Conserv. 2021, 260, 108849. [Google Scholar] [CrossRef]
- Dickau, R.; Whitney, B.S.; Iriarte, J.; Mayle, F.E.; Soto, J.D.; Metcalfe, P.; Street-Perrott, F.A.; Loader, N.J.; Ficken, K.J.; Killeen, T.J. Differentiation of neotropical ecosystems by modern soil phytolith assemblages and its implications for palaeoenvironmental and archaeological reconstructions. Rev. Palaeobot. Palynol. 2013, 193, 15–37. [Google Scholar] [CrossRef]
- Iriarte, J.; Paz, E.A. Phytolith analysis of selected native plants and modern soils from southeastern Uruguay and its implications for paleoenvironmental and archeological reconstruction. Quat. Int. 2007, 193, 99–123. [Google Scholar] [CrossRef]
- Morcote-Ríos, G.; Bernal, R.; Raz, L. Phytoliths as a tool for archaeobotanical, palaeobotanical and palaeoecological studies in Amazonian palms. Bot. J. Linn. Soc. 2016, 182, 348–360. [Google Scholar] [CrossRef]
- Watling, J.; Iriarte, J.; Whitney, B.; Consuelo, E.; Mayle, F.; Castro, W.; Schaan, D.; Feldpausch, T. Differentiation of neotropical ecosystems by modern soil phytolith assemblages and its implications for palaeoenvironmental and archaeological reconstructions II: Southwestern Amazonian forests. Rev. Palaeobot. Palynol. 2016, 226, 30–43. [Google Scholar] [CrossRef]
- Clark, J.S. Particle Motion and the Theory of Charcoal Analysis: Source Area, Transport, Deposition, and Sampling. Quat. Res. 1988, 30, 67–80. [Google Scholar] [CrossRef]
- Whitlock, C.; Larsen, C. Charcoal as a fire proxy. In Tracking Environmental Change Using Lake Sediments, Volume 3: Terrestrial, Algal and Siliceous Indicators; Smol, J.P., Birks, H.J.B., Last, W.M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 75–98. [Google Scholar]
- McCormac, F.G.; Hogg, A.G.; Blackwell, P.G.; E Buck, C.; Higham, T.F.G.; Reimer, P.J. Shcal04 Southern Hemisphere Calibration, 0–11.0 Cal Kyr BP. Radiocarbon 2004, 46, 1087–1092. [Google Scholar] [CrossRef]
- Reimer, P.J.; Bard, E.; Bayliss, A.; Beck, J.W.; Blackwell, P.G.; Ramsey, C.B.; Buck, C.E.; Cheng, H.; Edwards, R.L.; Friedrich, M.; et al. IntCal13 and Marine13 radiocarbon age calibration curves 0–50,000 years cal BP. Radiocarbon 2013, 55, 1869–1887. [Google Scholar] [CrossRef]
- Fraysse, F.; Pokrovsky, O.S.; Schott, J.; Meunier, J.-D. Surface properties, solubility and dissolution kinetics of bamboo phytoliths. Geochim. Cosmochim. Acta 2006, 70, 1939–1951. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Q.; Li, X.; Jia, X.; Wei, X.; Shao, M. Determination of Soil Texture by Laser Diffraction Method. Soil Sci. Soc. Am. J. 2015, 79, 1556–1566. [Google Scholar] [CrossRef]
- Campbell, J.R. Limitations in the laser particle sizing the soils. In Advances in Regolith; Roach, I.C., Ed.; Cooperative Research Centre for Landscape Environments and Mineral Exploration (CRC LEME): Canberra, Australia, 2003; pp. 38–42. [Google Scholar]
- Rodwell, D.L. Soil Science: Methods and Applications; Longman Group UK Ltd.: London, UK, 1994; pp. 139–141. [Google Scholar]
- Gouveia, S.; Pessenda, L.; Aravena, R.; Boulet, R.; Scheel-Ybert, R.; Bendassoli, J.; Ribeiro, A.; Freitas, H. Carbon isotopes in charcoal and soils in studies of paleovegetation and climate changes during the late Pleistocene and the Holocene in the southeast and centerwest regions of Brazil. Glob. Planet. Chang. 2002, 33, 95–106. [Google Scholar] [CrossRef]
- Pessenda, L.C.; Gomes, B.M.; Aravena, R.; Ribeiro, A.S.; Boulet, R.; Gouveia, S.E. The carbon isotope record in soils along a forest-cerrado ecosystem transect: Implications for vegetation changes in the Rondonia state, southwestern Brazilian Amazon region. Holocene 1998, 8, 599–603. [Google Scholar] [CrossRef]
- Pessenda, L.C.R.; Ribeiro, A.D.S.; Gouveia, S.E.M.; Aravena, R.; Boulet, R.; Bendassolli, J.A. Vegetation dynamics during the late Pleistocene in the Barreirinhas region, Maranhão State, northeastern Brazil, based on carbon Isotopes in soil organic matter. Quat. Res. 2004, 62, 183–193. [Google Scholar] [CrossRef]
- Pessenda, L.C.; Ledru, M.-P.; Gouveia, S.E.; Aravena, R.; Ribeiro, A.S.; Bendashsollil, J.A.; Boulet, R. Holocene palaeoenvironmental reconstruction in northeastern Brazil inferred from pollen, charcoal and carbon isotope records. Holocene 2004, 15, 812–820. [Google Scholar] [CrossRef]
- Watling, J.; Castro, M.T.; Simon, M.F.; Rodrigues, F.O.; de Medeiros, M.B.; De Oliveira, P.E.; Neves, E.G. Phytoliths from native plants and surface soils from the Upper Madeira river, SW Amazonia, and their potential for paleoecological reconstruction. Quat. Int. 2020, 550, 85–110. [Google Scholar] [CrossRef]
- Parr, J.F. Effect of fire on phytolith coloration. Geoarchaeology 2006, 21, 171–185. [Google Scholar] [CrossRef]
- Toledo, M.; Poorter, L.; Peña-Claros, M.; Alarcón, A.; Balcázar, J.; Chuviña, J.; Leaño, C.; Licona, J.C.; Ter Steege, H.; Bongers, F. Patterns and Determinants of Floristic Variation across Lowland Forests of Bolivia. Biotropica 2010, 43, 405–413. [Google Scholar] [CrossRef]
- Alves, D.T. Dark Earth Plant Management in the Lower Tapajos. Ph.D. Thesis, University of Exeter, Exeter, UK, 2017. [Google Scholar]
- Blecker, S.W.; McCulley, R.L.; Chadwick, O.A.; Kelly, E.F. Biologic cycling of silica across a grassland bioclimosequence. Glob. Biogeochem. Cycles 2006, 20, GB3023. [Google Scholar] [CrossRef]
- Fishkis, O.; Ingwersen, J.; Streck, T. Phytolith transport in sandy sediment: Experiments and modeling. Geoderma 2009, 151, 168–178. [Google Scholar] [CrossRef]
- Piperno, D.R.; McMichael, C.; Bush, M.B. Further evidence for localized, short-term anthropogenic forest alterations across pre-Columbian Amazonia. Proc. Natl. Acad. Sci. USA 2017, 114, E4118–E4119. [Google Scholar] [CrossRef]
- Watling, J.; Iriarte, J.; Mayle, F.E.; Schaan, D.; Pessenda, L.C.R.; Loader, N.J.; Street-Perrott, F.A.; Dickau, R.E.; Damasceno, A.; Ranzi, A. Reply to Piperno et al.: It is too soon to argue for localized, short-term human impacts in interfluvial Amazonia. Proc. Natl. Acad. Sci. USA 2017, 114, E4120–E4121. [Google Scholar] [CrossRef]
- Saunaluoma, S. Geometric Earthworks in the State of Acre, Brazil: Excavations at the Fazenda Atlântica and Quinauá Sites. Lat. Am. Antiq. 2012, 23, 565–583. [Google Scholar] [CrossRef]
- Saunaluoma, S.; Pärssinen, M.; Schaan, D. Diversity of Pre-colonial Earthworks in the Brazilian State of Acre, Southwestern Amazonia. J. Field Archaeol. 2018, 43, 362–379. [Google Scholar] [CrossRef]
- Balée, W. Contingent Diversity on Anthropic Landscapes. Diversity 2010, 2, 163–181. [Google Scholar] [CrossRef]
- Levis, C.; Costa, F.R.C.; Bongers, F.; Peña-Claros, M.; Clement, C.R.; Junqueira, A.B.; Neves, E.G.; Tamanaha, E.K.; Figueiredo, F.O.G.; Salomão, R.P.; et al. Persistent effects of pre-Columbian plant domestication on Amazonian forest composition. Science 2017, 355, 925–931. [Google Scholar] [CrossRef]
- Clement, C.R.; Denevan, W.M.; Heckenberger, M.J.; Junqueira, A.B.; Neves, E.G.; Teixeira, W.G.; Woods, W.I. The domestication of Amazonia before European conquest. Proc. R. Soc. B Boil. Sci. 2015, 282, 20150813. [Google Scholar] [CrossRef]
- Levis, C.; Flores, B.M.; Moreira, P.A.; Luize, B.G.; Alves, R.P.; Franco-Moraes, J.; Lins, J.; Konings, E.; Peña-Claros, M.; Bongers, F.; et al. How People Domesticated Amazonian Forests. Front. Ecol. Evol. 2018, 5, 171. [Google Scholar] [CrossRef]
- Levis, C.; de Souza, P.F.; Schietti, J.; Emilio, T.; Pinto, J.L.P.D.V.; Clement, C.R.; Costa, F.R.C. Historical Human Footprint on Modern Tree Species Composition in the Purus-Madeira Interfluve, Central Amazonia. PLoS ONE 2012, 7, e48559. [Google Scholar] [CrossRef]
- de Oliveira, E.A.; Marimon-Junior, B.H.; Marimon, B.S.; Iriarte, J.; Morandi, P.S.; Maezumi, S.Y.; Nogueira, D.S.; Aragao, L.E.; da Silva, I.B.; Feldpausch, T.R. Legacy of Amazonian Dark Earth soils on forest structure and species composition. Glob. Ecol. Biogeogr. 2020, 29, 1458–1473. [Google Scholar] [CrossRef]
- Gehring, C.; Zelarayán, M.C.; Luz, R.L.; Almeida, R.B.; Boddey, R.M.; Leite, M.F.A. Babassu palm (Attalea speciosa Mart.) super-dominance shapes its surroundings via multiple biotic, soil chemical, and physical interactions and accumulates soil carbon: A case study in eastern Amazonia. Plant Soil 2020, 454, 447–460. [Google Scholar] [CrossRef]
- Rocha, G.P.; Vieira, D.L.; Simon, M.F. Fast natural regeneration in abandoned pastures in southern Amazonia. For. Ecol. Manag. 2016, 370, 93–101. [Google Scholar] [CrossRef]
- Aleman, J.; Leys, B.; Apema, R.; Bentaleb, I.; Dubois, M.A.; Lamba, B.; Lebamba, J.; Martin, C.; Ngomanda, A.; Truc, L.; et al. Reconstructing savanna tree cover from pollen, phytoliths and stable carbon isotopes. J. Veg. Sci. 2011, 23, 187–197. [Google Scholar] [CrossRef]
- Maezumi, S.Y.; Robinson, M.; De Souza, J.; Urrego, D.H.; Schaan, D.; Alves, D.; Iriarte, J. New Insights from Pre-Columbian Land Use and Fire Management in Amazonian Dark Earth Forests. Front. Ecol. Evol. 2018, 6, 111. [Google Scholar] [CrossRef]
- Maezumi, S.Y.; Whitney, B.S.; Mayle, F.E.; de Souza, J.G.; Iriarte, J. Reassessing climate and pre-Columbian drivers of paleofire activity in the Bolivian Amazon. Quat. Int. 2018, 488, 81–94. [Google Scholar] [CrossRef]
- Denevan, W.M. Stone versus metal axes: The ambiguity of shifting cultivation in prehistoric Amazonia. J. Steward Anthrop. Soc. 1992, 20, 153–165. [Google Scholar]
- Glaser, B.; Birk, J.J. State of the scientific knowledge on properties and genesis of Anthropogenic Dark Earths in Central Amazonia (terra preta de Índio). Geochim. Cosmochim. Acta 2012, 82, 39–51. [Google Scholar] [CrossRef]
- Robinson, M.; Jaimes-Betancourt, C.; Elliott, S.; Maezumi, S.Y.; Hilbert, L.; Alves, D.; de Souza, J.G.; Iriarte, J. Anthropogenic soil and settlement organisation in the Bolivian Amazon. Geoarchaeology 2020, 36, 388–403. [Google Scholar] [CrossRef]
- Pessenda, L.C.R.; Gouveia, S.E.M.; Aravena, R. Radiocarbon Dating of Total Soil Organic Matter and Humin Fraction and Its Comparison with 14C Ages of Fossil Charcoal. Radiocarbon 2001, 43, 595–601. [Google Scholar] [CrossRef]
- Balesdent, J.; Basile-Doelsch, I.; Chadoeuf, J.; Cornu, S.; Derrien, D.; Fekiacova, Z.; Hatté, C. Atmosphere–soil carbon transfer as a function of soil depth. Nature 2018, 559, 599–602. [Google Scholar] [CrossRef]
- Song, Z.; McGrouther, K.; Wang, H. Occurrence, turnover and carbon sequestration potential of phytoliths in terrestrial ecosystems. Earth-Sci. Rev. 2016, 158, 19–30. [Google Scholar] [CrossRef]
- Strömberg, C.A.; Dunn, R.E.; Crifò, C.; Harris, E.B. Phytoliths in paleoecology: Analytical considerations, current use, and future directions. In Methods in Paleoecology: Reconstructing Cenozoic Terrestrial Environments and Ecological Communities, Vertebrate Paleobiology and Paleoanthropology; Croft, D.A., Su, D.F., Simpson, S.W., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 235–287. [Google Scholar]
- Grave, P.; Kealhofer, L. Assessing Bioturbation in Archaeological Sediments using Soil Morphology and Phytolith Analysis. J. Archaeol. Sci. 1999, 26, 1239–1248. [Google Scholar] [CrossRef]
- Kerns, B.K.; Moore, M.M.; Hart, S.C. Estimating forest-grassland dynamics using soil phytolith assemblages and d13C of soil organic matter. Écoscience 2001, 8, 478–488. [Google Scholar] [CrossRef]
- McClaran, M.P.; Umlauf, M. Desert grassland dynamics estimated from carbon isotopes in grass phytoliths and soil organic matter. J. Veg. Sci. 2000, 11, 71–76. [Google Scholar] [CrossRef]
- Morris, L.R.; West, N.E.; Ryel, R.J. Testing soil phytolith analysis as a tool to understand vegetation change in the sagebrush steppe and pinyon-juniper woodlands of the Great Basin Desert, USA. Holocene 2010, 20, 697–709. [Google Scholar] [CrossRef]
- Piperno, D.R.; Becker, P. Vegetational History of a Site in the Central Amazon Basin Derived from Phytolith and Charcoal Records from Natural Soils. Quat. Res. 1996, 45, 202–209. [Google Scholar] [CrossRef]
- Iler, R. The Chemistry of Silica. Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry; John Wiley and Sons: Chichester, UK, 1979. [Google Scholar]
- Cabanes, D.; Shahack-Gross, R. Understanding Fossil Phytolith Preservation: The Role of Partial Dissolution in Paleoecology and Archaeology. PLoS ONE 2015, 10, e0125532. [Google Scholar] [CrossRef] [PubMed]
- Shoji, S.; Nanzyo, M.; Dahlgren, R. Volcanic Ash Soils: Genesis, Properties, and Utilization; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1994; Volume 21. [Google Scholar]
- Watling, J.; Almeida, F.; Kater, T.; Zuse, S.; Shock, M.P.; Mongeló, G.; Bespalez, E.; Santi, J.R.; Neves, E.G. Arqueobotânica de ocupações ceramistas na Cachoeira do Teotônio. Boletim do Museu Paraense Emílio Goeldi. Ciênc. Hum. 2020, 15, 2178–2547. [Google Scholar] [CrossRef]
- Piperno, D.R.; McMichael, C. Phytoliths in modern plants from amazonia and the neotropics at large: Implications for vegetation history reconstruction. Quat. Int. 2020, 565, 54–74. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).