Climate Change, Fire and Human Activity Drive Vegetation Change during the Last Eight Millennia in the Xistral Mountains of NW Iberia
Abstract
1. Introduction
2. Sites, Archaeological Context, and Methods
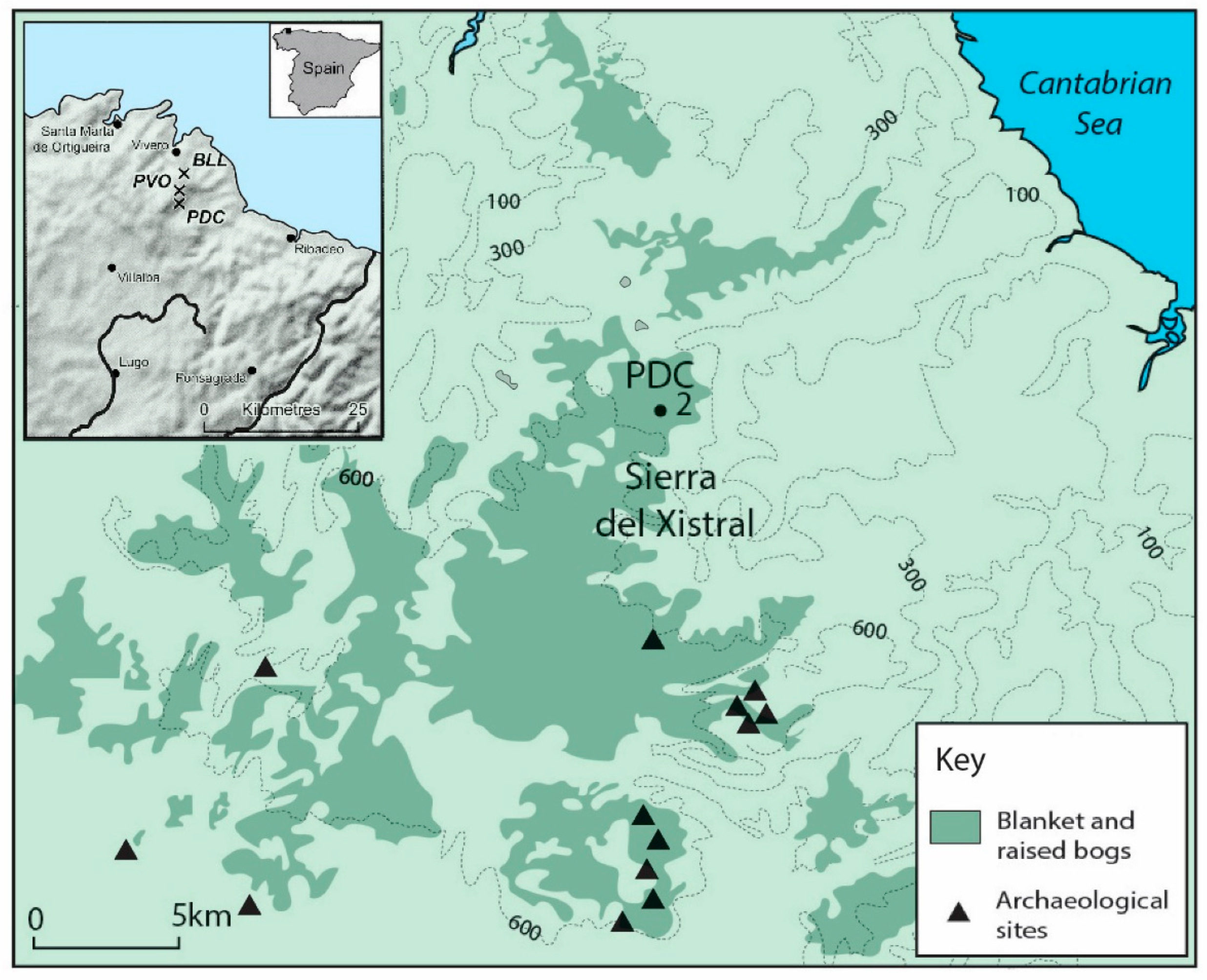
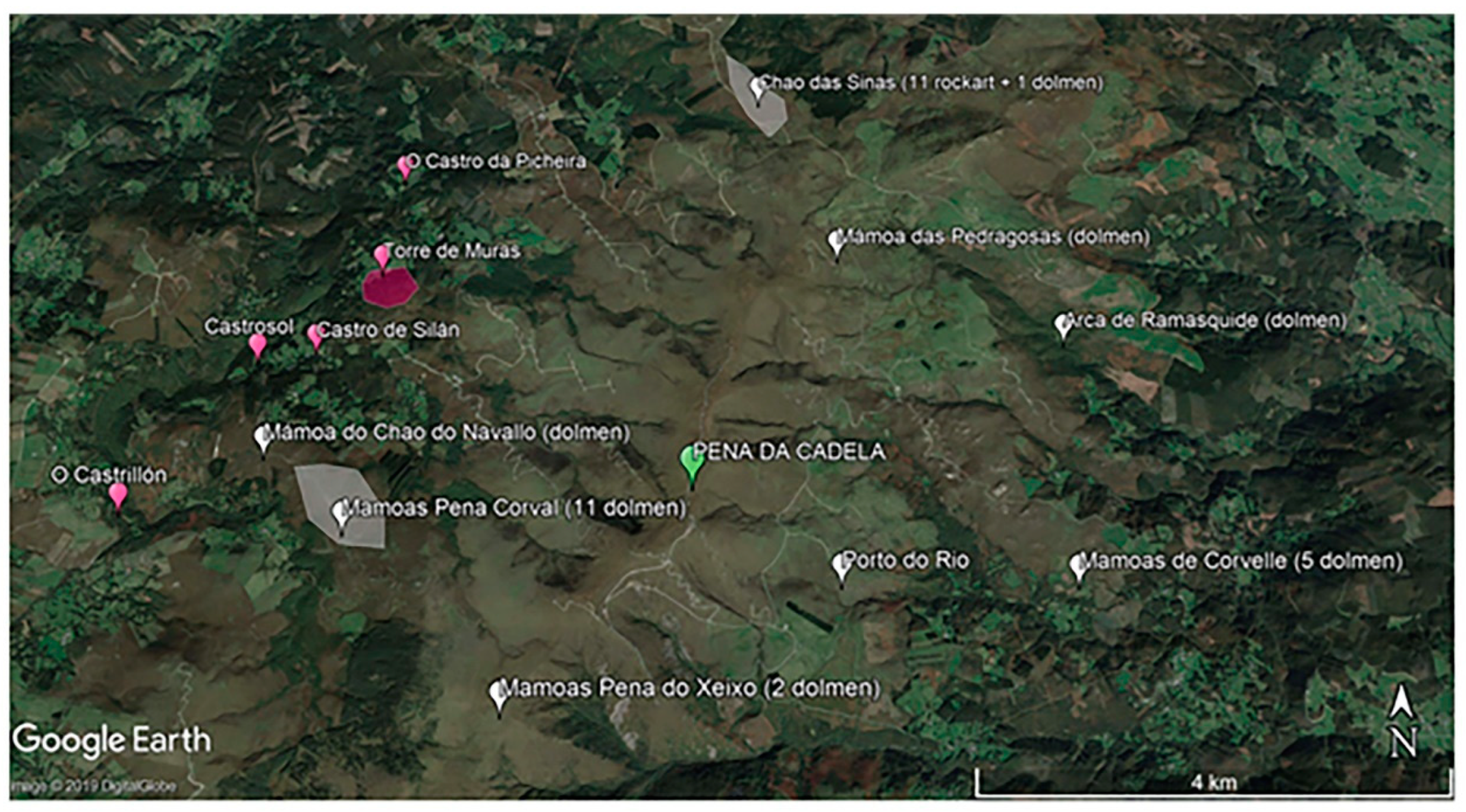
2.1. Site Characteristics and Sampling
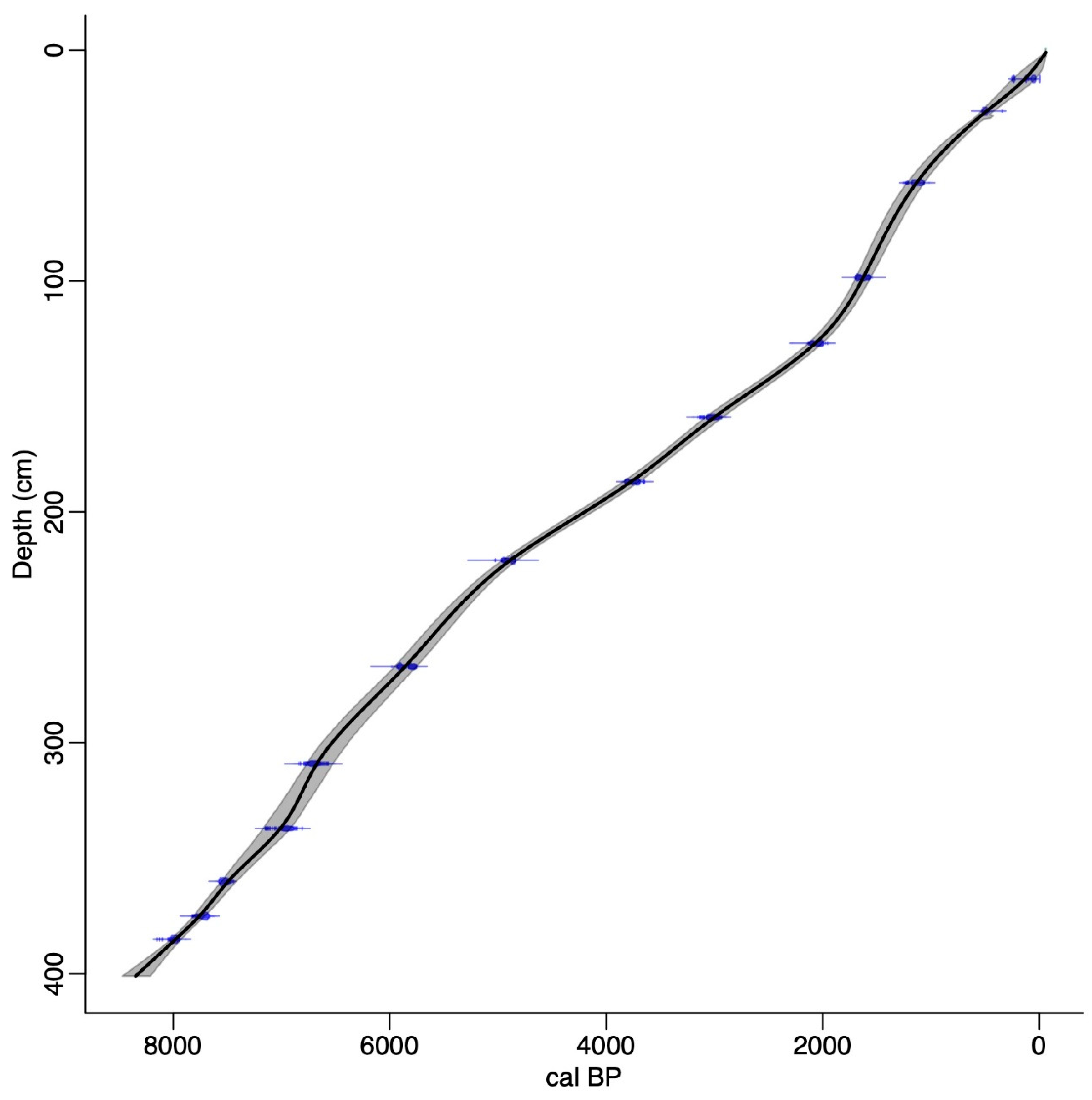
2.2. Radiocarbon Chronology and Age-Depth Model
2.3. Palynological Record
2.4. Geochemistry
2.5. Peat Humification
2.6. Numerical Methods
3. Results
3.1. Changes in Vegetation and Land Use
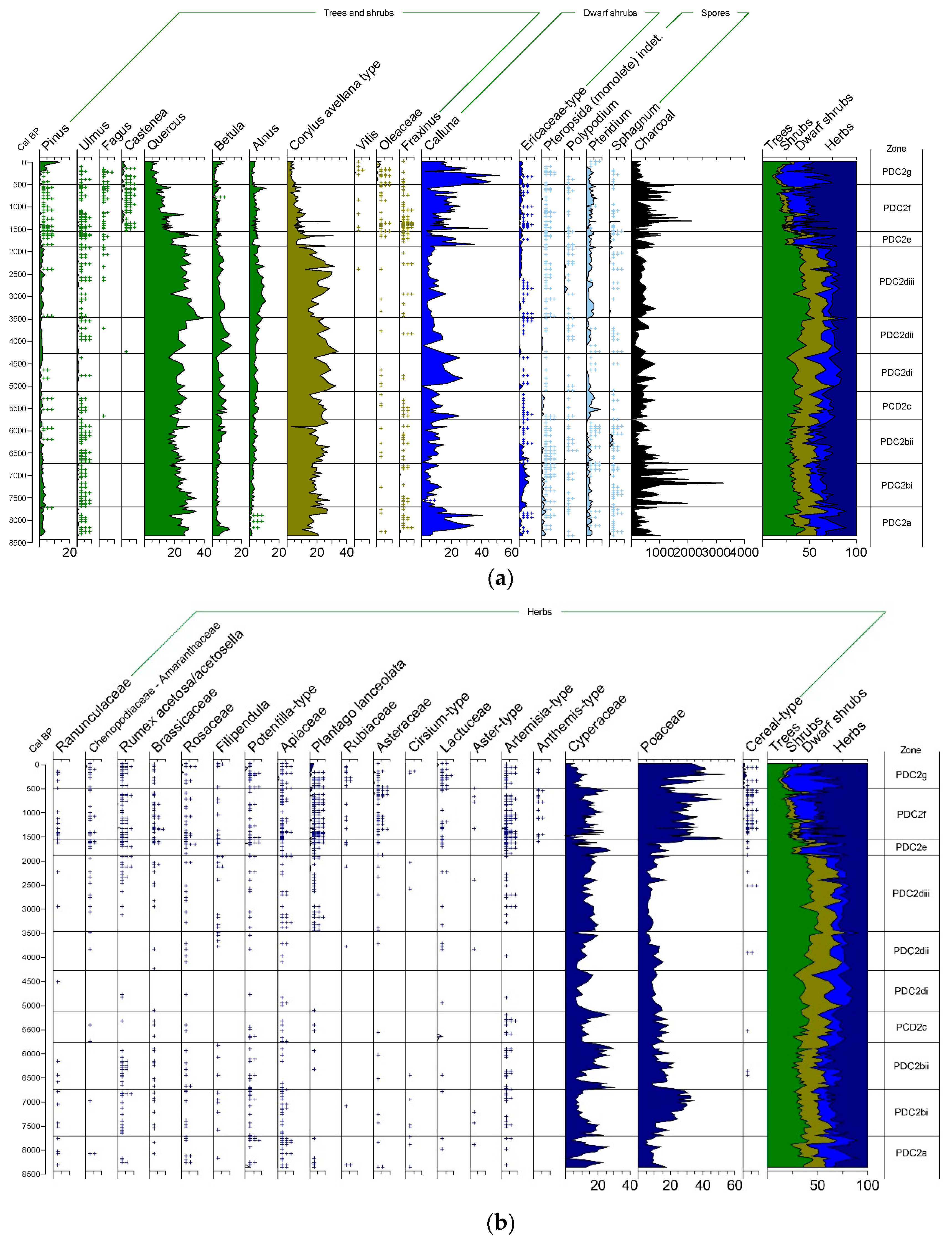
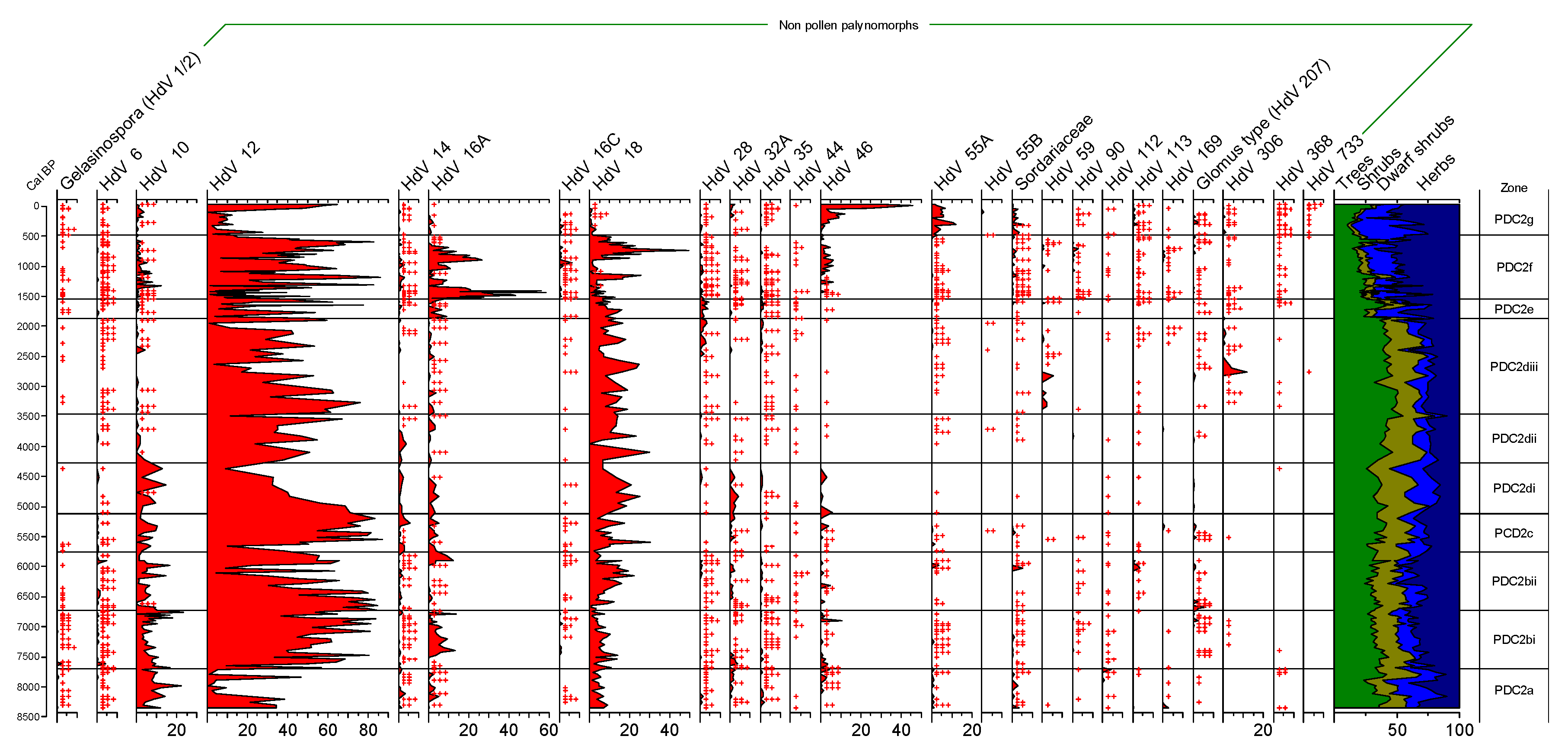
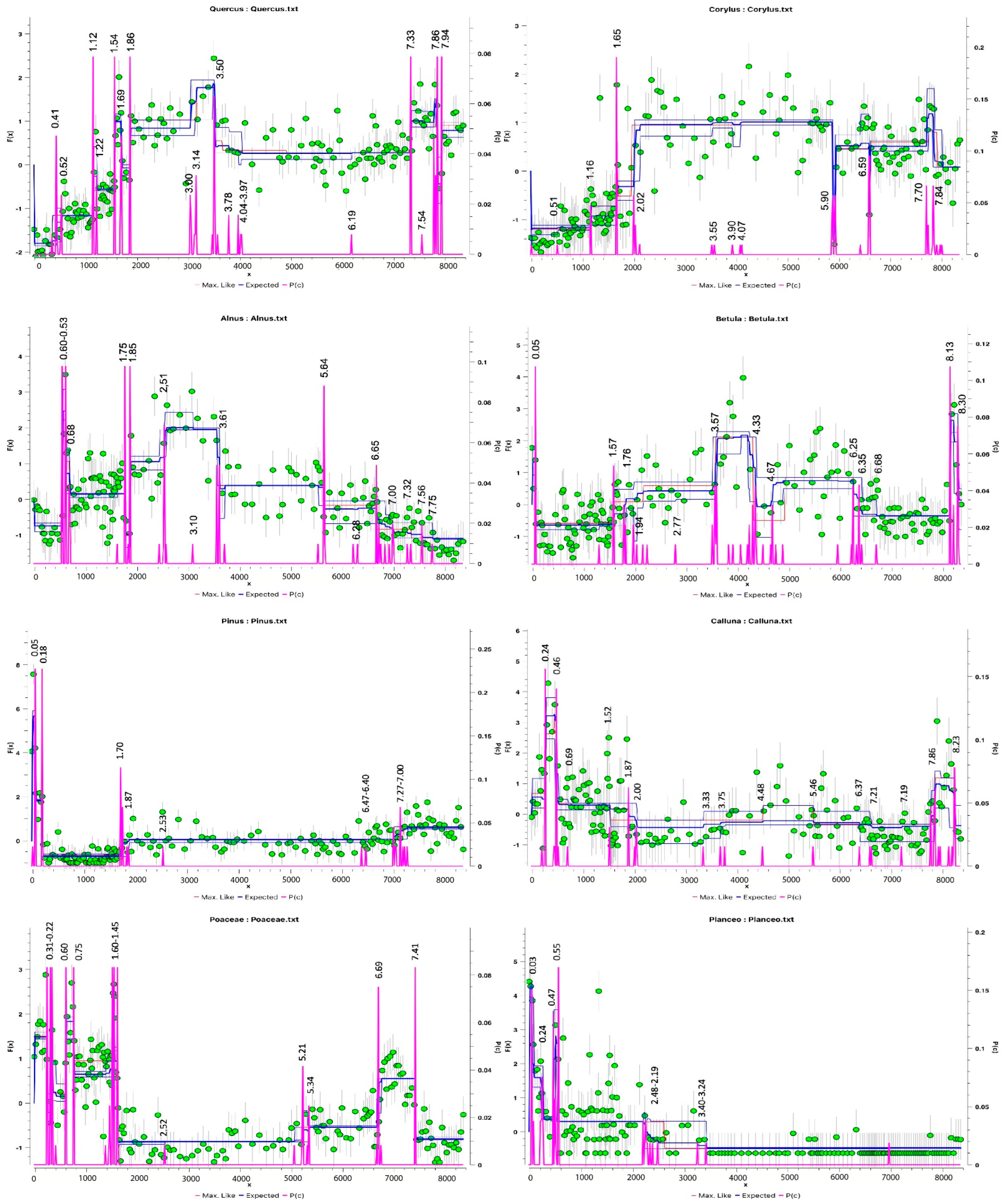
3.1.1. Palynological Records
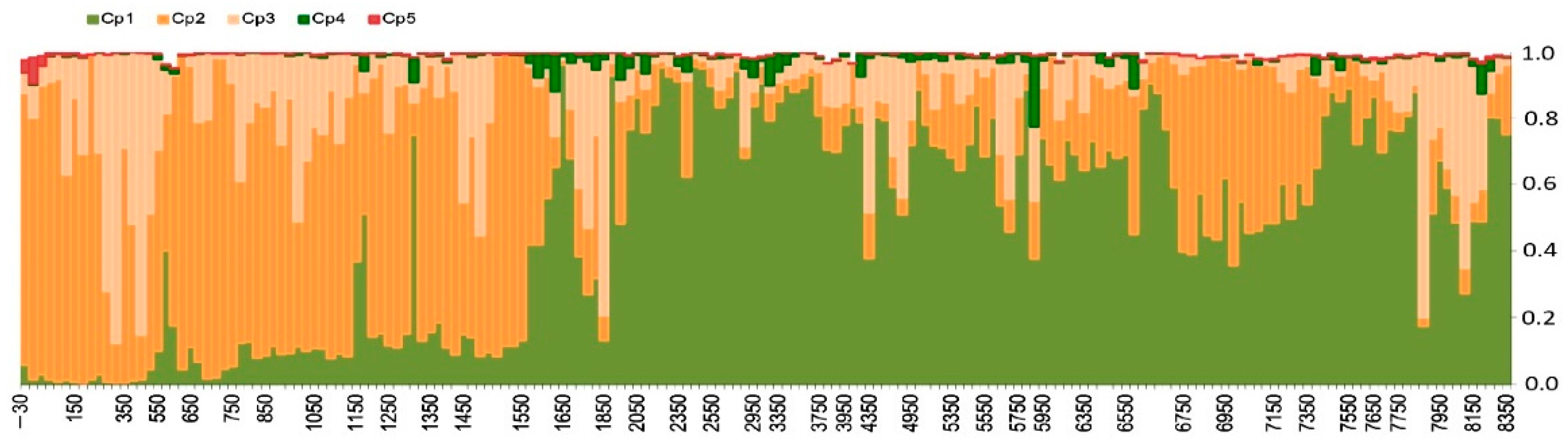
3.1.2. Principal Component Analysis
3.1.3. Changepoint Analysis
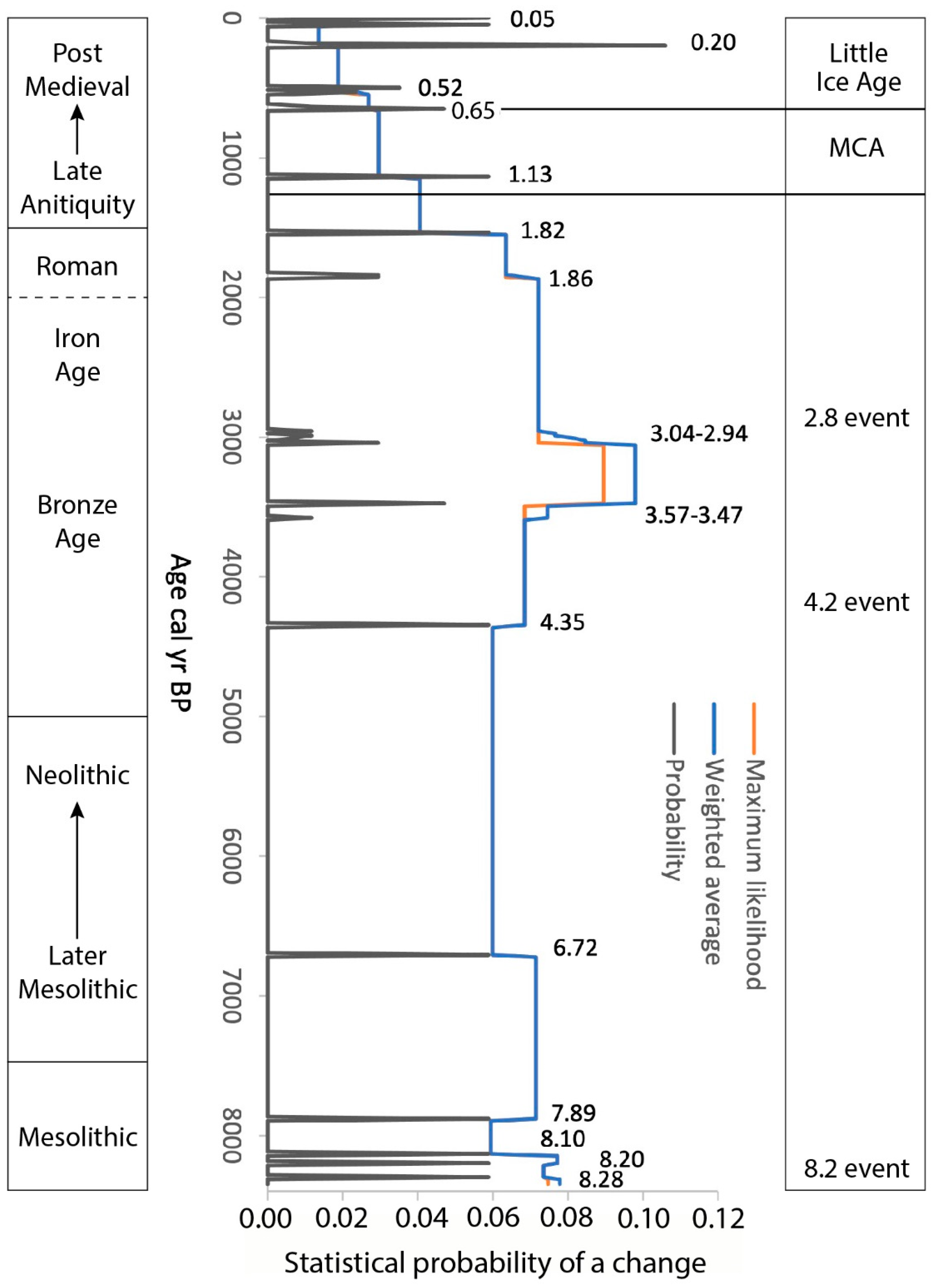
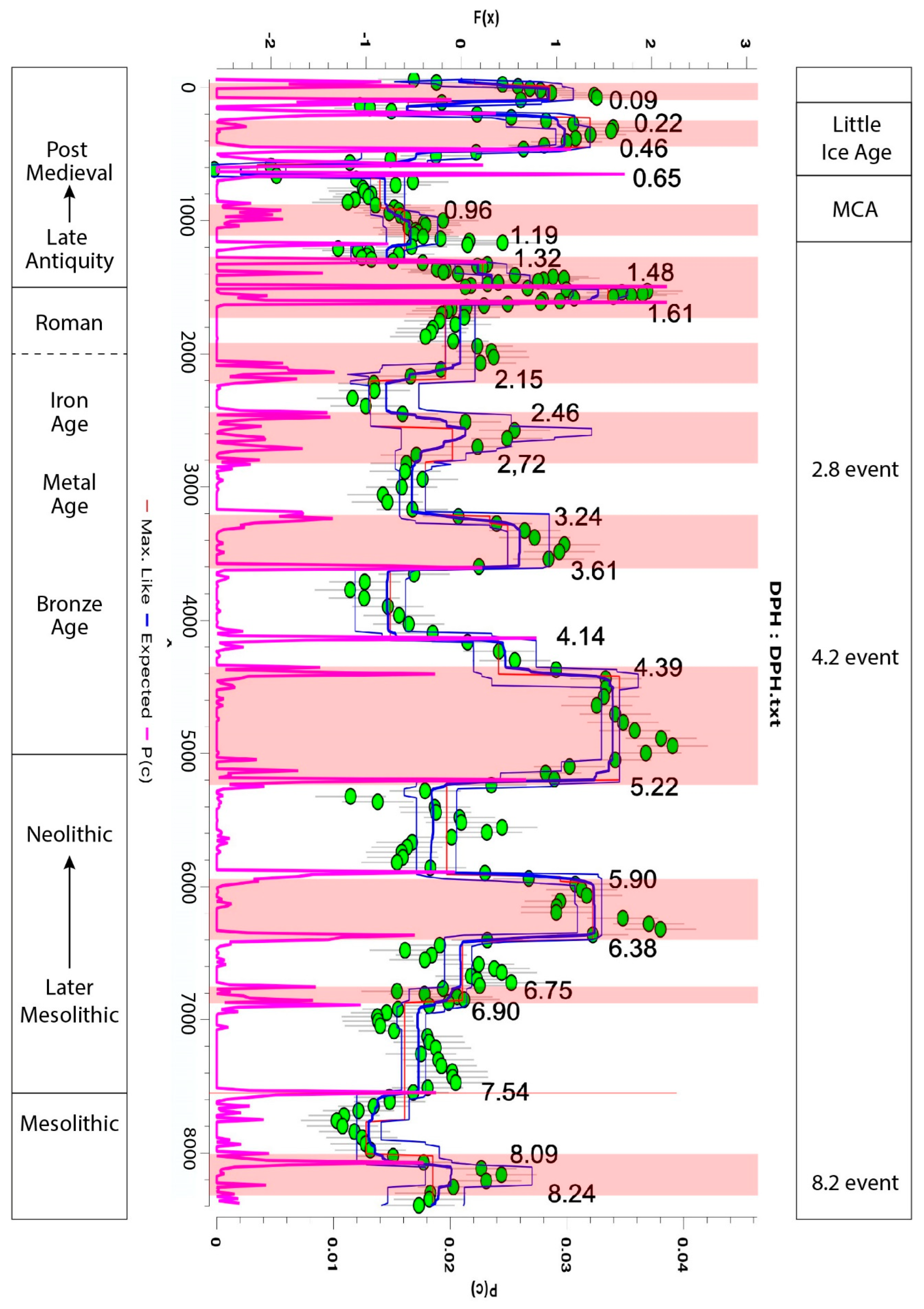
3.2. Geochemistry
3.3. Changes in Humidity in the Palynological and Peat Humification Record
4. Discussion
4.1. Early Holocene (Upper Palaeolithic and Mesolithic, pre 7500 cal. BP)
4.2. Later Mesolithic-Neolithic (c. 7500–5000 cal. BP)
4.3. Metal Ages (c. 5000–2000 cal. BP)
4.4. Iron Age
4.5. Roman Period (c. 2000–1500 cal. BP)
4.6. Late Antiquity, Middle Ages and Post-Medieval Times (1500 cal. BP-Present)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cullen, H.M.; Demenocal, P.B.; Hemming, S.; Hemming, G.; Brown, F.H.; Guilderson, T.; Sirocko, F. Climate change and the collapse of the Akkadian empire: Evidence from the deep sea. Geology 2000, 28, 379–382. [Google Scholar] [CrossRef]
- DeMenocal, P.B. Cultural responses to climate change during the late Holocene. Science 2001, 292, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Drysdale, R.; Zanchetta, G.; Hellstrom, J.; Maas, R.; Fallick, A.; Pickett, M.; Cartwright, I.; Piccini, L. Late Holocene drought responsible for the collapse of Old World civilizations is recorded in an Italian cave flowstone. Geology 2006, 34, 101–104. [Google Scholar] [CrossRef]
- Fiorentino, G.; Caracuta, V. Third millennium B.C. climate crisis and the social collapse in the Middle Bronze Age in Syria highlighted by carbon stable isotope analysis of 14C-AMS dated plant remains. Quat. Int. 2007, 127–128, 19. [Google Scholar]
- Rosen, A.M. Civilizing Climate. Social Responses to Climate Change in the Ancient Near East; Altamira Press: Lanham, MD, USA, 2007. [Google Scholar]
- Bar-Matthews, M.; Ayalon, A. Mid-Holocene climate variations revealed by high-resolution speleothem records from Soreq Cave, Israel and their correlation with cultural changes. Holocene 2011, 21, 163–171. [Google Scholar] [CrossRef]
- Roberts, N.; Eastwood, W.J.; Kuzucuoğlu, C.; Fiorentino, G.; Caracuta, V. Climatic, vegetation and cultural change in the eastern Mediterranean during the mid-Holocene environmental transition. Holocene 2011, 21, 147–162. [Google Scholar] [CrossRef]
- Lillios, K.T.; Blanco-González, A.; Drake, B.L.; López-Sáez, J.A. Mid-late Holocene climate, demography, and cultural dynamics in Iberia: A multi-proxy approach. Quat. Sci. Rev. 2016, 135, 138–153. [Google Scholar] [CrossRef]
- Griffiths, S.; Robinson, E. The 8.2 ka BP climate change event and human population resilience in northwest Atlantic Europe. Quat. Int. 2018, 465, 251–257. [Google Scholar] [CrossRef]
- Caseldine, C.J.; Turney, C. The bigger picture: Towards integrating palaeoclimate and environmental data with a history of societal change. J. Quat. Sci. 2010, 25, 88–93. [Google Scholar] [CrossRef]
- Berglund, B.E. Human impact and climate changes—Synchronous events and a causal link? Quat. Int. 2003, 105, 7–12. [Google Scholar] [CrossRef]
- Magny, M. Holocene climate variability as reflected by mid-European lake-level fluctuations and its probable impact on prehistoric human settlements. Quat. Int. 2004, 113, 65–79. [Google Scholar] [CrossRef]
- Arbogast, R.M.; Jacomet, S.; Magny, M.; Schibler, J. The significance of climate fluctuations for lake level changes and shifts in subsistence economy during the late Neolithic (4300–2400 BC) in central Europe. Veg. Hist. Archaeobot. 2006, 15, 403–418. [Google Scholar] [CrossRef]
- Turney, C.S.; Baillie, M.; Palmer, J.; Brown, D. Holocene climatic change and past Irish societal response. J. Archaeol. Sci. 2006, 33, 34–38. [Google Scholar] [CrossRef]
- Magny, M.; Haas, J.N. A major widespread climatic change around 5300 cal. yr BP at the time of the Alpine Iceman. J. Quat. Sci. 2004, 19, 423–430. [Google Scholar] [CrossRef]
- Caseldine, C.; Thompson, G.; Langdon, C.; Hendon, D. Evidence for an extreme climatic event on Achill Island, Co. Mayo, Ireland around 5200–5100 cal. yr BP. J. Quat. Sci. 2005, 20, 169–178. [Google Scholar] [CrossRef]
- Schmid, B.V.; Büntgen, U.; Easterday, W.R.; Ginzler, C.; Walløe, L.; Bramanti, B.; Stenseth, N.C. Climate-driven introduction of the Black Death and successive plague reintroductions into Europe. Proc. Natl. Acad. Sci. USA 2015, 112, 3020–3025. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.A.; Kutzbach, J.E.; Coe, M.T.; Levis, S. Feedbacks between climate and boreal forests during the Holocene epoch. Nature 1994, 371, 52–54. [Google Scholar] [CrossRef]
- Tinner, W.; Lotter, A.F. Central European vegetation response to abrupt climate change at 8.2 ka. Geology 2001, 29, 551–554. [Google Scholar] [CrossRef]
- Jalut, G.; Dedoubat, J.J.; Fontugne, M.; Otto, T. Holocene circum-Mediterranean vegetation changes: Climate forcing and human impact. Quat. Int. 2009, 200, 4–18. [Google Scholar] [CrossRef]
- López-Merino, L.; Sánchez, N.S.; Kaal, J.; López-Sáez, J.A.; Cortizas, A.M. Post-disturbance vegetation dynamics during the Late Pleistocene and the Holocene: An example from NW Iberia. Glob. Planet. Chang. 2012, 92, 58–70. [Google Scholar] [CrossRef]
- Silva-Sánchez, N.; Martínez Cortizas, A.; López-Merino, L. Linking forest cover, soil erosion and mire hydrology to late-Holocene human activity and climate in NW Spain. Holocene 2014, 24, 714–725. [Google Scholar] [CrossRef]
- Stefanini, B.; Oksanen, P.O.; Corcoran, J.P.; Mitchell, F.J.G. Appraising the cohesion of palaeoenvironmental reconstructions in north-west Spain since the mid-Holocene from a high temporal resolution multi-proxy peat record. Holocene 2018, 28, 681–694. [Google Scholar] [CrossRef]
- Desprat, S.; Goñi, M.F.S.; Loutre, M.F. Revealing climatic variability of the last three millennia in northwestern Iberia using pollen influx data. Earth Planet. Sci. Lett. 2003, 213, 63–78. [Google Scholar] [CrossRef]
- Carrión, J.S.; Fernández, S.; González-Sampériz, P.; Gil-Romera, G.; Badal, E.; Carrión-Marco, Y.; López Merino, L.; López-Sáez, J.A.; Fierro, E.; Burjachs, F. Expected trends and surprises in the Lateglacial and Holocene vegetation history of the Iberian Peninsula and Balearic Islands. Rev. Palaeobot. Palynol. 2010, 162, 458–475. [Google Scholar] [CrossRef]
- Martínez Cortizas, A.; Pérez Alberti, A. Atlas Climático de Galicia, Consellería de Medio Ambiente; Xunta de Galicia: Santiago de Compostela, Spain, 1999; 250p. [Google Scholar]
- Fraga Vila, M.I.; Sahuquillo, E.; García Tasende, M. Vegetación característica de las turberas de Galicia. In Turberas de Montaña de Galicia; Martínez Cortizas, A., García-Rodeja, E., Eds.; Consellería de Medio Ambiente, Xunta de Galicia: Santiago de Compostela, Spain, 2001; pp. 79–98. [Google Scholar]
- Martínez Cortizas, A.; García-Rodeja, E.; Pontevedra Pombal, E.; Nóvoa Muñoz, J.C.; Weiss, D.; Cheburkin, A. Atmospheric Pb deposition in Spain during the last 4600 years recorded by two ombrotrophic peat bogs and implications for the use of peat as archive. Sci. Total Environ. 2002, 292, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Mighall, T.M.; Cortizas, A.M.; Biester, H.; Turner, S.E. Proxy climate and vegetation changes during the last five millennia in NW Iberia: Pollen and non-pollen palynomorph data from two ombrotrophic peat bogs in the North Western Iberian Peninsula. Rev. Palaeobot. Palynol. 2006, 141, 203–223. [Google Scholar] [CrossRef]
- De Lombera Hermida, A.D. Caves and people. Archaeological research at the eastern margins of NW Iberia. In To the West of Spanish Cantabria: The Palaeolithic Settlement of Galicia; British Archaeological Reports: Oxford, UK, 2011; pp. 111–122. [Google Scholar]
- López Cordeiro del Mar, M. Resultados obtenidos en la construcción de una ‘Nueva Arqueología’ del paleolítico gallego. Cuad. Estud. Gallegos 2004, 51, 133–151. [Google Scholar] [CrossRef]
- Villar Quinteiro, R. La Cueva del Rei Cintolo (Lugo, Galicia): Algunos datos cronoarqueológicos de la galería superior. Gallaecia 2007, 26, 31–53. [Google Scholar]
- Fábregas Valcarce, R.; Lombera Hermida, A. El Paleolítico superior en Galicia a la luz de las últimas investigaciones. In El Paleolítico Superior Peninsular: Novedades del Siglo XXI Jornadas Internacionales sobre el Paleolítico Superior Peninsular; Mangado Llach, X., Ed.; Universtat de Barcelona: Barcelona, Spain, 2010; pp. 255–270. [Google Scholar]
- Ramil Rego, E.; Ramil Soneira, J. El Fin de los Tiempos Glaciares en Galicia. Magdaleniense y Epipalolítico. Os Primeiros Poboadores de Galicia: O Paleolítico; Edicións do Castro: Sada, Spain, 1996; pp. 117–147. [Google Scholar]
- Fábregas Valcarce, R.; Vilaseco Vázquez, X.I. Building forever or just for the time being? A view from NW Iberia. In The Megalithic Architectures of Europe; Laporte, L., Scarre, C., Eds.; Oxbow Books: Oxford, UK, 2016; pp. 101–110. [Google Scholar]
- Sánchez-Pardo, J.C. Castros y aldeas galaicorromanas: Sobre la evolución y transformación del poblamiento indígena en la Galicia romana. Zephyrvs 2010, 65, 129–148. [Google Scholar]
- Stuiver, M.; Reimer, P.J.; Reimer, R.W. CALIB 8.2 (WWW Program). 2021. Available online: http://calib.org (accessed on 28 November 2022).
- Blaauw, M. Methods and code for ‘classical’ age-modelling of radiocarbon sequences. Quat. Geochronol. 2010, 5, 512–518. [Google Scholar] [CrossRef]
- Moore, P.D.; Webb, J.A.; Collinson, M.E. Pollen Analysis, 2nd ed.; Blackwell Scientific Publications: London, UK, 1991. [Google Scholar]
- Barber, K.E. History of vegetation. In Methods in Plant Ecology; Chapman, S.B., Ed.; Blackwell: Oxford, UK, 1976; pp. 5–83. [Google Scholar]
- Stockmarr, J. Tablets with spores used in absolute pollen analysis. Pollen Spores 1971, 13, 615–621. [Google Scholar]
- Fægri, K.; Kaland, P.E.; Krzywinski, K. Textbook of Pollen Analysis, 4th ed.; John Wiley and Sons: Chichester, UK, 1989. [Google Scholar]
- Andersen, S.-T. Identification of wild grass and cereal pollen. Danmarks Geologiske Undersøgelser. Årbog. 1979, 1978, 69–82. [Google Scholar]
- Cushing, E.J. Evidence for differential pollen preservation in Late Quaternary sediments in Minnesota. Rev. Palaeobot. Palynol. 1967, 4, 87–101. [Google Scholar] [CrossRef]
- Edwards, K.J. The separation of Corylus and Myrica pollen in modern and fossil samples. Pollen et Spores 1981, 23, 205–218. [Google Scholar]
- Brown, A.G.; Carpenter, R.G.; Walling, D.E. Monitoring fluvial pollen transport, its relationship to catchment vegetation and implications for palaeoenvironmental studies. Rev. Palaeobot. Palynol. 2007, 147, 60–76. [Google Scholar] [CrossRef]
- van Geel, B. A palaeoecological study of Holocene peat bog sections in Germany and the Netherlands. Rev. Palaeobot. Palynol. 1978, 25, 1–120. [Google Scholar] [CrossRef]
- Grimm, E. TILIA and TILIA.GRAPH; Illinois State Museum: Springfield, IL, USA, 1991. [Google Scholar]
- Stace, C. New Flora of the British Isles; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Martínez Cortizas, A.; Mighall, T.M.; Pontevedra Pombal, X.; Nóvoa Muñoz, J.C.; Peiteado Varela, E.; Piñeiro Rebolo, R. Linking changes in atmospheric dust deposition, vegetation evolution and human activities in NW Spain during the last 5300 years. Holocene 2005, 15, 698–706. [Google Scholar] [CrossRef]
- Schofield, J.E.; Edwards, K.J.; Mighall, T.M.; Cortizas, A.M.; Rodríguez-Racedo, J.; Cook, G. An integrated geochemical and palynological study of human impacts, soil erosion and storminess from southern Greenland since c. AD 1000. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 295, 19–30. [Google Scholar] [CrossRef]
- Silva-Sánchez, N.; Schofield, J.E.; Mighall, T.M.; Cortizas, A.M.; Edwards, K.J.; Foster, I. Climate changes, lead pollution and soil erosion in south Greenland over the past 700 years. Quat. Res. 2015, 84, 159–173. [Google Scholar] [CrossRef]
- Kylander, M.E.; Martínez-Cortizas, A.; Sjöström, J.K.; Gåling, J.; Gyllencreutz, R.; Bindler, R.; Alexanderson, H.; Schenk, F.; Reinardy, B.T.; Chandler, B.M.; et al. Storm chasing: Tracking Holocene storminess in southern Sweden using mineral proxies from inland and coastal peat bogs. Quat. Sci. Rev. 2023, 299, 107854. [Google Scholar] [CrossRef]
- Mighall, T.M.; Abrahams, P.W.; Grattan, J.P.; Hayes, D.; Timberlake, S.; Forsyth, S. Geochemical evidence for atmospheric pollution derived from prehistoric copper mining at Copa Hill, Cwmystwyth, mid-Wales, UK. Sci. Total Environ. 2002, 292, 69–80. [Google Scholar] [CrossRef]
- De Vleeschouwer, F.; Le Roux, G.; Shotyk, W. Peat as an archive of atmospheric contamination and environmental change: A case study of lead in Europe. PAGES 2010, 18, 20–22. [Google Scholar] [CrossRef]
- Cheburkin, A.K.; Shotyk, W. An energy-dispersive miniprobe multielement analyzer (EMMA) for direct analysis of Pb and other trace elements in peats. Fresenius’ J. Anal. Chem. 1996, 354, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Blackford, J.J.; Chambers, F.M. Determining the degree of peat decomposition for peat-based paleoclimatic studies. Int. Peat J. 1993, 5, 7–24. [Google Scholar]
- Hansson, S.V.; Rydberg, J.; Kylander, M.; Gallagher, K.; Bindler, R. Evaluating paleoproxies for peat decomposition and their relationship to peat geochemistry. Holocene 2013, 23, 1666–1671. [Google Scholar] [CrossRef]
- Biester, H.; Knorr, K.-H.; Schellekens, J.; Basler, A.; Hermanns, Y.-M. Comparison of different methods to determine the degree of peat decomposition in peat bogs. Biogeosciences 2013, 11, 2691–2707. [Google Scholar] [CrossRef]
- Broder, T.; Blodau, C.; Biester, H.; Knorr, K.H. Peat decomposition records in three pristine ombrotrophic bogs in southern Patagonia. Biogeosciences 2012, 9, 1479–1491. [Google Scholar] [CrossRef]
- Kylander, M.E.; Bindler, R.; Martínez Cortizas, A.; Gallagher, K.; Mörth, C.-M.; Rauch, S. A novel geochemical approach to paleorecords of dust deposition and effective humidity: 8500 years of peat accumulation at Store Mosse (the “Great Bog”), Sweden. Quat. Sci. Rev. 2013, 69, 69–82. [Google Scholar] [CrossRef]
- Eriksson, L. Introduction to Multi- and Mega-Variate Data Analysis Using Projection Methods (PCA & PLS); Umetrics AB: Umea, Sweden, 1999. [Google Scholar]
- Grimm, E.C. CONISS: A FORTRAN 77 program for stratigraphically constrained cluster analysis by the method of incremental sum of squares. Comput. Geosci. 1987, 13, 13–35. [Google Scholar] [CrossRef]
- Criado-Boado, F.; Parcero-Oubiña, C.; Otero Vilariño, C.; Aboal-Fernández, R.; Ayán Vila, X.; Barreiro, D.; Ballesteros-Arias, P.; Cabrejas, E.; Costa-Casais, M.; Fábrega-Álvarez, P. Atlas Arqueolóxico da Paisaxe Galega; Xerais: Vigo, Spain, 2016. [Google Scholar]
- Cugny, C.; Mazier, F.; Galop, D. Modern and fossil non-pollen palynomorphs from the Basque mountains (western Pyrenees, France): The use of coprophilous fungi to reconstruct pastoral activity. Veg. Hist. Archaeobotany 2010, 19, 391–408. [Google Scholar] [CrossRef]
- Perrotti, A.; van Asperen, E. Dung fungi as a proxy for megaherbivores: Opportunities and limitations for archaeological applications. Veg. Hist. Archaeobotany 2019, 28, 93–104. [Google Scholar] [CrossRef]
- Lundqvist, N. Nordic Sordariaceae. Symb. Bot. Ups. 1972, 20, 1–374. [Google Scholar]
- van Geel, B.; Bohncke, S.J.P.; Dee, H. A palaeoecological study of an upper late glacial and Holocene sequence from ‘De Borchert’, The Netherlands. Rev. Palaeobot. Palynol. 1980, 31, 367–448. [Google Scholar] [CrossRef]
- van Geel, B.; Buurman, J.; Brinkkemper, O.; Schelvis, J.; Aptroot, A.; van Reenen, G.; Hakbijl, T. Environmental reconstruction of a Roman period settlement site in Uitgeest (The Netherlands), with special reference to coprophilous fungi. J. Archaeol. Sci. 2003, 30, 873–884. [Google Scholar] [CrossRef]
- Willemsen, J.; van ‘t Veer, R.; van Geel, B. Environmental change during medieval reclamation of the raised-bog area Waterland (The Netherlands): A palaeophytosociological approach. Rev. Palaeobot. Palynol. 1996, 94, 75–100. [Google Scholar] [CrossRef]
- Martínez Cortizas, A.; López-Costas, O.; Orme, L.; Mighall, T.; Kylander, M.E.; Bindler, R.; Gallego Sala, Á. Holocene atmospheric dust deposition in NW Spain. Holocene 2020, 30, 507–518. [Google Scholar] [CrossRef]
- Bennett, K.D.; Lamb, H.F. Holocene pollen sequences as a record of competitive interactions among tree populations. TREE 1988, 3, 141–144. [Google Scholar] [CrossRef]
- Gallagher, K.; Bodin, T.; Sambridge, M.; Weiss, D.; Kylander, M.; Large, D. Inference of abrupt changes in noisy geochemical records using transdimensional changepoint models. Earth Planet. Sci. Lett. 2011, 311, 182–194. [Google Scholar] [CrossRef]
- Olid, C.; Garcia-Orellana, J.; Martínez-Cortizas, A.; Masqué, P.; Peiteado-Varela, E.; Sanchez-Cabeza, J.A. Multiple site study of recent atmospheric metal (Pb, Zn and Cu) deposition in the NW Iberian Peninsula using peat cores. Sci. Total Environ. 2010, 408, 5540–5549. [Google Scholar] [CrossRef]
- Kylander, M.E.; Weiss, D.J.; Cortizas, A.M.; Spiro, B.; Garcia-Sanchez, R.; Coles, B.J. Refining the pre-industrial atmospheric Pb isotope evolution curve in Europe using an 8000 year old peat core from NW Spain. Earth Planet. Sci. Lett. 2005, 240, 467–485. [Google Scholar] [CrossRef]
- Alley, R.B.; Mayewski, P.A.; Sowers, T.; Stuiver, M.; Taylor, K.C.; Clark, P.U. Holocene climatic instability: A prominent, widespread event 8200 yr ago. Geology 1997, 25, 483–486. [Google Scholar] [CrossRef]
- Ellison, C.R.; Chapman, M.R.; Hall, I.R. Surface and deep ocean interactions during the cold climate event 8200 years ago. Science 2006, 312, 1929–1932. [Google Scholar] [CrossRef] [PubMed]
- González-Sampériz, P.; Utrilla, P.; Mazo, C.; Valero-Garcés, B.; Sopena, M.C.; Morellón, M.; Sebastián, M.; Moreno, A.; Martínez-Bea, M. Patterns of human occupation during the early Holocene in the Central Ebro Basin (NE Spain) in response to the 8.2 ka climatic event. Quat. Res. 2009, 71, 121–132. [Google Scholar] [CrossRef]
- Muñoz Sobrino, C.; Ramil-Rego, P.; Gómez-Orelllana, L.; Díaz Varela, R. Palynological data on major Holocene climatic events in NW Iberia. Boreas 2005, 34, 381–400. [Google Scholar] [CrossRef]
- González-Sampériz, P.; Valero-Garcés, B.L.; Moreno, A.; Jalut, G.; García-Ruiz, J.M.; Martí-Bono, C.; Delgado-Huertas, A.; Navas, A.; Otto, T.; Dedoubat, J.J. Climate variability in the Spanish Pyrenees during the last 30,000 yr revealed by the El Portalet sequence. Quat. Res. 2006, 66, 38–52. [Google Scholar] [CrossRef]
- Martínez-Cortizas, A.; Costa-Casais, M.; López-Sáez, J.A. Environmental change in NW Iberia between 7000 and 500 cal BC. Quat. Int. 2009, 200, 77–89. [Google Scholar] [CrossRef]
- Ramil-Rego, P.; Muñoz-Sobrino, C.; Rodríguez-Guitián, M.; Gómez-Orellana, L. Differences in the vegetation of the Northern Iberian Peninsula during the last 16,000 years. Plant Ecol. 1998, 138, 41–62. [Google Scholar] [CrossRef]
- del Mar LÓPEZ Cordeiro, Mª. El yacimiento epipaleolítico de Chan da Cruz (Valadouro, Lugo): Síntesisde los primeros resultados. The Mesolithic site of Chan da Cruz (Valadouro, Lugo). Complutum 2003, 14, 39–54. [Google Scholar]
- Criado-Boado, F.; Bonilla Rodríguez, A.; Cerqueiro Landín, D.; Díaz Vázquez, M.; González Méndez, M.; Infante Roura, F.; Méndez Fernández, F.; Penedo Romero, R.; Rodríguez Puentes, E.; Vaquero Lastres, J. Paisajes, Arqueología del Paisaje. El área Bocelo-Furelos. Entre los Tiempos Paleolíticos y Medievales (Campañas de 1987, 1988 y 1989); Arqueoloxía-Investigación, Santiago Xunta de Galicia: Santiago, Spain, 1991; Volume 6, p. 294. [Google Scholar]
- de Pablo, J.F.L.; Jochim, M.A. The impact of the 8,200 cal BP climatic event on human mobility strategies during the Iberian Late Mesolithic. J. Anthropol. Res. 2010, 66, 39–68. [Google Scholar] [CrossRef]
- de Pablo, J.F.L.; Puche, M.G. Climate change and population dynamics during the Late Mesolithic and the Neolithic transition in Iberia. Doc. Praehist. 2009, 36, 67–96. [Google Scholar] [CrossRef]
- Sweeney, L.; Harrison, S.P.; Vander Linden, M. Assessing anthropogenic influence on fire history during the Holocene in the Iberian Peninsula. Quat. Sci. Rev. 2022, 287, 107562. [Google Scholar] [CrossRef]
- Kaal, J.; Cortizas, A.M.; Eckmeier, E.; Casais, M.C.; Estévez, M.S.; Boado, F.C. Holocene fire history of black colluvial soils revealed by pyrolysis-GC/MS: A case study from Campo Lameiro (NW Spain). J. Archaeol. Sci. 2008, 35, 2133–2143. [Google Scholar] [CrossRef]
- Muñoz-Sobrino, C.; Ramil-Rego, P.; Rodríguez, M. Upland vegetation in the north-west Iberian Península after the last glaciation: Forest history and deforestation dynamics. Veg. Hist. Archaeobotany 1997, 6, 215–233. [Google Scholar] [CrossRef]
- Muñoz Sobrino, C.; Ramil-Rego, P.; Gómez-Orellana, L. Vegetation of the Lago de Sanabria area (NW Iberia) since the end of the Pleistocene: A palaeoecological reconstruction on the basis of two new pollen sequences. Veg. Hist. Archaeobotany 2004, 13, 1–22. [Google Scholar] [CrossRef]
- López-Merino, L. Paleoambiente y Antropización en Asturias Durante el Holoceno. Unpublished Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2009. [Google Scholar]
- Balsera, V.; Díaz-del-Río, P.; Gilman, A.; Uriarte, A.; Vicent, J.M. Approaching the demography of late prehistoric Iberia through summed calibrated date probability distributions (7000–2000 cal BC). Quat. Int. 2015, 386, 208–211. [Google Scholar] [CrossRef]
- Drake, B.L.; Blanco-González, A.; Lillios, K.T. Regional demographic dynamics in the Neolithic transition in Iberia: Results from summed calibrated date analysis. J. Archaeol. Method Theory 2017, 24, 796–812. [Google Scholar] [CrossRef]
- Kołaczek, P.; Zubek, S.; Błaszkowski, J.; Mleczko, P.; Margielewski, W. Erosion or plant succession—How to interpret the presence of arbuscular mycorrhizal fungi (Glomeromycota) spores in pollen profiles collected from mires. Rev. Palaeobot. Palynol. 2013, 189, 29–37. [Google Scholar] [CrossRef]
- Valcarce, R.F.; Fernández, T.L.; de Lombera Hermida, A.; Alonso, J.P.; Alberti, A.P. Novos achados paleolíticos no interior de Galicia: A depresión de Monforte de Lemos e as súas industrias líticas. Gallaecia: Revista de Arqueoloxía e Antigüidade 2007, 26, 7–30. [Google Scholar]
- Prieto Martínez, M.P.; López-Costas, O.; Vázquez Liz, P.; Lantes Suárez, O. The Cist of Praia da Rola (Mugueimes, Ourense, NW Iberia Peninsula); XVIIIe Congrès Mondial UISPP: Paris, France, 2018. [Google Scholar]
- Arias, P.; Cubas, M. Muerte y ritual en el Neolítico del noroeste ibérico: El megalitismo y otras manifestaciones del comportamiento funerario de las sociedades de los milenios V y IV aC en la región cantábrica y Galicia. In De Gibraltar aos Pirenéus-Megalitismo, Vida e Morte na Fachada Atlântica Peninsular; 2018; pp. 133–154. Available online: repositorio.ul.pt (accessed on 1 October 2022).
- Ramil-Rego, P. La Vegetación Cuaternaria de las Sierras Septentrionales de Lugo a Través del Análisis Polínico. Ph.D. Dissertation, Universidade de Santiago, Assomada, Santiago, 1992; 356p. [Google Scholar]
- Martínez Cortizas, A.; Pontevedra Pombal, X.; García-Rodeja, E.; Nóvoa Muñoz, J.C.; Shotyk, W. Mercury in a Spanish peat bog: Archive of climate change and atmospheric metal deposition. Science 1999, 284, 939–942. [Google Scholar] [CrossRef]
- Ramil-Rego, P.; Aira, M.J.; Taboada, M.T. Análisis polínico y sedimentológico de dos turberas en las sierras septentrionales de Galicia (NW de España). Revue de Paléobiologie 1994, 13, 9–28. [Google Scholar]
- Orme, L.C.; Davies, S.J.; Culler, G.A.T. Reconstructed centennial variability of Late Holocene storminess from Cors Fochno, Wales, UK. J. Quat. Sci. 2015, 30, 478–488. [Google Scholar] [CrossRef]
- Orme, L.C.; Charman, D.J.; Reinhardt, L.; Jones, R.T.; Mitchell, F.J.G.; Stefanini, B.S.; Barkwith, A.; Ellis, M.A.; Grosvenor, M. Past changes in the North Atlantic storm track driven by insolation and sea-ice forcing. Geology 2017, 45, 335–338. [Google Scholar] [CrossRef]
- Ran, M.; Chen, L. The 4.2 ka BP climatic event and its cultural responses. Quat. Int. 2019, 521, 158–167. [Google Scholar] [CrossRef]
- Ortiz, J.E.; Gallego, J.L.R.; Torres, T.; Díaz-Bautista, A.; Sierra, C. Palaeoenvironmental reconstruction of Northern Spain during the last 8000 cal yr BP based on the biomarker content of the Roñanzas peat bog (Asturias). Org. Geochem. 2010, 41, 454–466. [Google Scholar] [CrossRef]
- Castro, D.; Souto, M.; Garcia-Rodeja, E.; Pontevedra-Pombal, X.; Fraga, M.I. Climate change records between the mid-and late Holocene in a peat bog from Serra do Xistral (SW Europe) using plant macrofossils and peat humification analyses. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 420, 82–95. [Google Scholar] [CrossRef]
- Schellekens, J.; Buurman, P.; Fraga, I.; Martínez-Cortizas, A. Holocene vegetation and hydrologic changes inferred from molecular vegetation markers in peat, Penido Vello (Galicia, Spain). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 299, 56–69. [Google Scholar] [CrossRef]
- Wang, T.; Surge, D.; Mithen, S. Seasonal temperature variability of the Neoglacial (3300–2500 BP) and Roman Warm Period (2500–1600 BP) reconstructed from oxygen isotope ratios of limpet shells (Patella vulgata), Northwest Scotland. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 317, 104–113. [Google Scholar] [CrossRef]
- Tereso, J.P.; Bettencourt, A.M.S.; Ramil-Rego, P.; Teira-Brión, A.; López-Dóriga, I.; Lima, A.; Almeida, R. Agriculture in NW Iberia during the Bronze Age: A review of archaeobotanical data. J. Archaeol. Sci. Rep. 2016, 10, 44–58. [Google Scholar] [CrossRef]
- Martín-Seijo, M.; Pedro Tereso, J.; Bettencourt, A.M.S.; Sampaio, H.A.; Abad Vidal, E.; Vidal Caeiro, L. Socio-ecology of Early and Middle Bronze Age communities in the northwest Atlantic region of Iberia: Wood resources procurement and forest management. Quat. Int. 2017, 437, 90–101. [Google Scholar] [CrossRef]
- Bettencourt, A.M.S. A.M.S. A Pré-história do Minho: Do Neolítico à Idade do Bronze. In Minho Traços de Identidade; Pereira, P., Ed.; Conselho Cultural da Universidade do Minho: Braga, Portugal, 2009; pp. 70–120. [Google Scholar]
- Bradley, R. Rock Art and the Prehistory of Atlantic Europe Signing the Land; Routledge: London, UK, 1997. [Google Scholar]
- González-Ruibal, A. Facing two seas: Mediterranean and Atlantic contacts in the north-west of Iberia in the first millennium BC. Oxf. J. Archaeol. 2004, 23, 287–317. [Google Scholar] [CrossRef]
- Martínez Cortizas, A. What is natural: The role of palaeoenvironmental research in reconstructing the history of continental ecosystems. Sagvntvm 2011, 11, 55–56. [Google Scholar]
- Aira Rodríguez, M.J.; Ramil Rego, P.; Alvarez Núñez, A. Estudio paleocarpológico realizado en el Castro de Penalba (Campolameiro, Pontevedra. España). Botánica Complut. 1990, 16, 81–89. [Google Scholar]
- Pardo, J.J.; Castiñeira, J.R.; Platas, I.P.; Vidal, E.A.; Suárez, C.M. Radiocarbon and chronology of the Iron Age hillforts of Northwestern Iberia. 2009. Available online: academia.edu (accessed on 1 October 2022).
- Pérez-Obiol, R.; García-Codron, J.C.; Pelachs, A.; Pérez-Haase, A.; Soriano, J.M. Landscape dynamics and fire activity since 6740 cal yr BP in the Cantabrian region (La Molina peat bog, Puente Viesgo, Spain). Quat. Sci. Rev. 2016, 135, 65–78. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, X.; Liang, S.; Zhou, T.; Huang, K.; Tang, B.; Zhao, W. Time-lag effects of global vegetation responses to climate change. Glob. Change Biol. 2015, 21, 3520–3531. [Google Scholar] [CrossRef]
- Amesbury, M.J.; Charman, D.J.; Fyfe, R.M.; Langdon, P.G.; West, S. Bronze Age upland settlement decline in southwest England: Testing the climate change hypothesis. J. Archaeol. Sci. 2008, 35, 87–98. [Google Scholar] [CrossRef]
- Armit, I.; Swindles, G.T.; Becker, K.; Plunkett, G.; Blaauw, M. Rapid climate change did not cause population collapse at the end of the European Bronze Age. Proc. Natl. Acad. Sci. USA 2014, 111, 17045–17049. [Google Scholar] [CrossRef]
- Turney, C.S.; Jones, R.T.; Thomas, Z.A.; Palmer, J.G.; Brown, D. Extreme wet conditions coincident with Bronze Age abandonment of upland areas in Britain. Anthropocene 2016, 13, 69–79. [Google Scholar] [CrossRef]
- Bettencourt, A.M.S. Estruturas e práticas funerárias do Bronze Inicial e Médio do Noroeste Peninsular. In Arqueología, Sociedad, Territorio y Paisaje; Bueno, P., Gilman, A., Martín Morales, C., Sánchez-Palencia, F.J., Eds.; CSIC: Madrid, Spain, 2011; pp. 115–139. [Google Scholar]
- López-Costas, O.; Müldner, G.; Martínez Cortizas, A. Diet and lifestyle in Bronze Age Northwest Spain: The collective burial of Cova do Santo. J. Archaeol. Sci. 2015, 55, 209–218. [Google Scholar] [CrossRef]
- van Geel, B.; Buurman, J.; Waterbolk, H.T. Archaeological and palaeoecological indications of an abrupt climate change in The Netherlands, and evidence for climatological teleconnections around 2650 BP. J. Quat. Sci. 1996, 11, 451–460. [Google Scholar] [CrossRef]
- van Geel, B.; Heijnis, H.; Charman, D.J.; Thompson, G.; Engels, S. Bog burst in the eastern Netherlands triggered by the 2.8 kyr BP climate event. Holocene 2014, 24, 1465–1477. [Google Scholar]
- Plunkett, G.; Swindles, G.T. Determining the Sun’s influence on Lateglacial and Holocene climates: A focus on climate response to centennial-scale solar forcing at 2800 cal. BP. Quat. Sci. Rev. 2008, 27, 175–184. [Google Scholar] [CrossRef]
- Parcero Oubiña, C. Síntesis de los Trabajos de Excavación en el Yacimiento Castreño de Alto do Castro (Cuntis, Pontevedra); Boletín do Museu Arqueolóxico e Histórico da Coruña: Brigantium, Spain, 2000; Volume 12, pp. 161–174. [Google Scholar]
- Aira Rodríguez, M.J.; Saá Otero, P.; Taboada Castro, T. Estudios Paleobotánicos y Edafológicos en Yacimientos Arqueológicos de Galicia; Arqueoloxía/Investigación: Galicia, Spain, 1989; Volume 4, Available online: http://www.aranzadi.eus/fileadmin/docs/Munibe/1993165174AA.pdf (accessed on 1 November 2022).
- Morales-Molino, C.; Antón, M.G.; Morla, C. Late Holocene vegetation dynamics on an Atlantic–Mediterranean mountain in NW Iberia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 302, 323–337. [Google Scholar] [CrossRef]
- Silva-Sánchez, N. Mining and metallurgical activities in N Iberia and their link to forest evolution using environmental archives (centuries AD V to XI). Estudos do Quaternário/Quat. Stud. 2015, 12, 15–26. [Google Scholar] [CrossRef]
- Martínez Cortizas, A.; López-Merino, L.; Bindler, R.; Mighall, T.; Kylander, M. Atmospheric Pb pollution in N Iberia during the late Iron Age/Roman times reconstructed using the high-resolution record of La Molina mire (Asturias, Spain). J. Paleolimnol. 2013, 50, 71–86. [Google Scholar] [CrossRef]
- López-Merino, L.; Martínez Cortizas, A.; Reher, G.S.; López-Sáez, J.A.; Mighall, T.M.; Bindler, R. Reconstructing the impact of human activities in a NW Iberian Roman mining landscape for the last 2500 years. J. Archaeol. Sci. 2014, 50, 208–218. [Google Scholar] [CrossRef]
- Gallego, J.L.R.; Ortiz, J.E.; Sierra, C.; Torres, T.; Llamas, J.F. Multivariate study of trace element distribution in the geological record of Roñanzas Peat Bog (Asturias, N. Spain). Paleoenvironmental evolution and human activities over the last 8000 cal yr BP. Sci. Total Environ. 2013, 454, 16–29. [Google Scholar] [CrossRef]
- Pontevedra-Pombal, X.; Mighall, T.M.; Nóvoa-Muñoz, J.C.; Peiteado-Varela, E.; Rodríguez-Racedo, J.; García-Rodeja, E.; Martínez-Cortizas, A. Five thousand years of atmospheric Ni, Zn, As, and Cd deposition recorded in bogs from NW Iberia: Prehistoric and historic anthropogenic contributions. J. Archaeol. Sci. 2013, 40, 764–777. [Google Scholar] [CrossRef]
- García-Alix, A.; Jimenez-Espejo, F.J.; Lozano, J.A.; Jiménez-Moreno, G.; Martinez-Ruiz, F.; García Sanjuán, L.; Aranda Jiménez, G.; García Alfonso, E.; Ruiz-Puertas, G.; Scott Anderson, R. Anthropogenic impact and lead pollution throughout the Holocene in Southern Iberia. Sci. Total Environ. 2013, 449, 451–460. [Google Scholar] [CrossRef]
- Martín-Seijo, M.; Vila, M.C. Oak, ash and pine: The role of firewood in funerary rituals at the Roman site of Reza Vella (Ourense, Spain). Archaeol. Anthropol. Sci. 2019, 11, 1911–1926. [Google Scholar] [CrossRef]
- Sánchez Pardo, J.C. Power and rural landscapes in early medieval Galicia (400–900 ad): Towards a re-incorporation of the archaeology into the historical narrative. Early Medieval Europe 2013, 21, 140–168. [Google Scholar] [CrossRef]
- Bond, G.; Kromer, B.; Beer, J.; Muscheler, R.; Evans, M.N.; Showers, W.; Hoffman, S.; Lotti-Bond, R.; Hadjas, I.; Bonani, G. Persistent solar influence of North Atlantic climate during the Holocene. Science 2001, 294, 2130–2136. [Google Scholar] [CrossRef]
- De Jong, R.; Schoning, K.; Björck, S. Increased aeolian activity during climatic regime shifts as recorded in a raised bog in south-west Sweden during the past 1700 years. Clim. Past Discuss. 2007, 3, 383–408. [Google Scholar]
- Sjöström, J.K.; Cortizas, A.M.; Hansson, S.V.; Sánchez, N.S.; Bindler, R.; Rydberg, J.; Mörth, C.M.; Ryberg, E.E.; Kylander, M.E. Paleodust deposition and peat accumulation rates–Bog size matters. Chem. Geol. 2020, 554, 119795. [Google Scholar] [CrossRef]
- Massa, C.; Bichet, V.; Gauthier, É.; Perren, B.B.; Mathieu, O.; Petit, C.; Monna, F.; Giraudeau, J.; Losno, R.; Richard, H. A 2500-year record of natural and anthropogenic soil erosion in South Greenland. Quat. Sci. Rev. 2012, 32, 119–130. [Google Scholar] [CrossRef]
- Silva-Sánchez, N.; Martínez Cortizas, A.; Abel-Schaad, D.; López-Sáez, J.A.; Mighall, T.M. Influence of climate change and human activities on the organic and inorganic composition of peat during the ‘Little Ice Age’ (El Payo mire, W Spain). Holocene 2016, 26, 1290–1303. [Google Scholar] [CrossRef]
- Hölzer, A.; Hölzer, A. Silicon and titanium in peat profiles as indicators of human impact. Holocene 1998, 8, 685–696. [Google Scholar] [CrossRef]
- Oliva, M.; Ruiz-Fernández, J.; Barriendos, M.; Benito, G.; Cuadrat, J.M.; García-Ruiz, J.M.; Giralt, S.; Gómez-Ortiz, A.; Hernández, A.; López-Costas, O.; et al. The Little Ice Age in the Iberian Mountains. Earth-Sci. Rev. 2018, 177, 175–208. [Google Scholar] [CrossRef]
- López-Costas, O.; Müldner, G. Boom and bust at a medieval fishing port: Dietary preferences of fishers and artisan families from Pontevedra (Galicia, NW Spain) during the Late Medieval and Early Modern Period. Archaeol. Anthropol. Sci. 2018, 11, 3717–3731. [Google Scholar] [CrossRef]
- Dopazo Martínez, A.; Fernández Rodríguez, C.; Ramil Rego, P. Arqueometría aplicada a yacimientos galaico-romanos del NW peninsular, valoración de la actividad agrícola y ganadera. In Biogeografía Pleistocena-Holocena de la Península Ibérica; Ramil Rego, P., Fernández Rodríguez, C., Rodríguez Guitián, M., Eds.; Consellería de Cultura: Santiago de Compostela, Spain, 1996; pp. 317–332. [Google Scholar]
- Vázquez Varela, J.M.; Garcia Quintela, M.V. A Vida Cotiá na Galicia Castrexa; Biblioteca de Divulgación: Santiago de Compostela, Spain, 1998. [Google Scholar]
- Saavedra, P. Economía, Política y Sociedad en Galicia: La Provincia de Mondoñedo, 1480–1830; Xunta de Galicia (Consellería da Presidencia): Santiago, Spain, 1985. [Google Scholar]
- López-Costas, O. Antropología de los Restos Óseos Humanos de Galicia: Estudio de la Población Romano y Medieval Gallega. Doctoral Thesis, University of Granada, Granada, Spain, 2012; 555p. [Google Scholar]
- Cal Pardo, E. Breve historia de la diócesis de Mondoñedo. Rudesindus: Miscelánea de Arte e Cultura 2007, 1, 105–123. [Google Scholar]
- Portela Silva, M.X. (Ed.) Documentos da Catedral de Lugo. Século XIV. Tomo I; Consello da Cultura Galega, Sección de Patrimonio Histórico: Santiago de Compostela, Spain, 2007. [Google Scholar]

| Depth (cm) | Radiocarbon Age (14C Year BP) | Laboratory Code | 2 Sigma cal. BP |
|---|---|---|---|
| 14–15 | 50 ± 30 | B-307603 | recent |
| 28–29 | 440 ± 30 | B-307604 | 455–530 |
| 58–59 | 1210 ± 30 | B-307605 | 1060–1180 |
| 90–91 | 1720 ± 30 | B-307606 | 1540–1700 |
| 126–128 | 2080 ± 30 | B-307607 | 1980–2125 |
| 158–160 | 2890 ± 30 | B-307608 | 2930–3080 |
| 186–188 | 3470 ± 30 | B-307609 | 3685–3835 |
| 220–222 | 4320 ± 40 | B-307610 | 4830–4975 |
| 266–268 | 5120 ± 40 | B-307611 | 5750–5940 |
| 308–310 | 5880 ± 50 | B-307612 | 6620–6795 |
| 336–338 | 6090 ± 40 | B-307613 | 6845–7030 |
| 358–360 | 6640 ± 40 | B-307614 | 7460–7575 |
| 380–382 | 6890 ± 40 | B-307615 | 7660–7800 |
| 402–404 | 7180 ± 40 | B-307616 | 8930–8040 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mighall, T.M.; Martínez Cortizas, A.; Silva-Sánchez, N.; López-Costas, O.; López-Merino, L. Climate Change, Fire and Human Activity Drive Vegetation Change during the Last Eight Millennia in the Xistral Mountains of NW Iberia. Quaternary 2023, 6, 5. https://doi.org/10.3390/quat6010005
Mighall TM, Martínez Cortizas A, Silva-Sánchez N, López-Costas O, López-Merino L. Climate Change, Fire and Human Activity Drive Vegetation Change during the Last Eight Millennia in the Xistral Mountains of NW Iberia. Quaternary. 2023; 6(1):5. https://doi.org/10.3390/quat6010005
Chicago/Turabian StyleMighall, Tim M., Antonio Martínez Cortizas, Noemí Silva-Sánchez, Olalla López-Costas, and Lourdes López-Merino. 2023. "Climate Change, Fire and Human Activity Drive Vegetation Change during the Last Eight Millennia in the Xistral Mountains of NW Iberia" Quaternary 6, no. 1: 5. https://doi.org/10.3390/quat6010005
APA StyleMighall, T. M., Martínez Cortizas, A., Silva-Sánchez, N., López-Costas, O., & López-Merino, L. (2023). Climate Change, Fire and Human Activity Drive Vegetation Change during the Last Eight Millennia in the Xistral Mountains of NW Iberia. Quaternary, 6(1), 5. https://doi.org/10.3390/quat6010005






