Abstract
The environmental impact of the ancient Maya, and subsequent ecological recovery following the Terminal Classic decline, have been the key foci of research into socio-ecological interactions in the Yucatán peninsula. These foci, however, belie the complex pattern of resource exploitation and agriculture associated with post-Classic Maya societies and European colonisation. We present a high-resolution, 1200-year record of pollen and charcoal data from a 52-cm short core extracted from New River Lagoon, near to the European settlement of Indian Church, northern Belize. This study complements and extends a previous 3500-year reconstruction of past environmental change, located 1-km north of the new record and adjacent to the ancient Maya site of Lamanai. This current study shows a mixed crop production and palm agroforestry management strategy of the ancient Maya, which corroborates previous evidence at Lamanai. Comparison of the two records suggests that core agricultural and agroforestry activities shifted southwards, away from the centre of Lamanai, beginning at the post-Classic period. The new record also demonstrates that significant changes in land-use were not associated with drought at the Terminal Classic (ca. CE 1000) or the European Encounter (ca. CE 1500), but instead resulted from social and cultural change in the post-Classic period (CE 1200) and new economies associated with the British timber trade (CE 1680). The changes in land-use documented in two adjacent records from the New River Lagoon underline the need to reconstruct human–environment interactions using multiple, spatially, and temporally diverse records.
1. Introduction
Investigating changing socio-ecological interactions through time is necessary to contextualise modern anthropogenically driven ecosystem change within a known range of disturbance and recovery [1,2,3,4]. These data can, in turn, inform the resilience or sensitivity of ecosystems in response to changing land use practices and resource exploitation associated with social, economic, and environmental change, such as demographic growth, technological change, political instability, and climate change. Furthermore, past anthropogenically driven ecosystem change, in particular, the manipulation of vegetation composition or soil properties, has been shown to leave a legacy in modern ecosystem functioning and composition [5,6,7]. It is therefore necessary for conservation and management strategies to determine the extent to which these ecosystems have been shaped by human influence [8]. In parts of the Americas densely settled in the prehistoric period (e.g., the Maya region of Mexico and Central America), ecological baselines have shifted over thousands of years due to human influence [9], therefore it is necessary to determine legacies of previous impacts (especially amongst longer lived tropical tree species) and trajectories of ecosystem recovery after major disturbances.
The ancient Maya civilisation is arguably one of the most studied past societies in terms of socio-ecological interactions. The much discussed ‘collapse’ of this civilisation at the end of the Classic Period, ca. 750–1000 CE, is argued to have been caused by, amongst other things, environmental degradation and an inability to cope with drought [10,11,12,13]. Numerous palaeoecological studies have demonstrated significant reductions in forest pollen, associated with heightened concentrations of charcoal fragments and crop pollen, signalling extensive forest clearance for agriculture in the lead-up to the Classic period [14,15,16,17]. Forest recovery, also demonstrated in pollen records, follows the decline of the Classic civilisation. This narrative has been replicated throughout the dry forests of the Yucatán peninsula, where the ancient Maya civilisation was centred. Other studies, however, have demonstrated forest management practices during the Classic Maya period, specifically pine and palm cultivation, thereby countering the argument that the Maya unilaterally destroyed forest cover, rather than having promoted a range of management strategies [18,19]. Additionally, historical ecologists have examined the modern forest composition in areas once heavily managed by the ancient Maya and have concluded that the abundance of economically important tree taxa (sources of both industrial and food products) are legacies of past Maya agroforestry practices and reflect a mixed, environmentally conscious land use strategy [7,20,21,22].
A focus on socio-ecological interactions of the ancient Maya and the legacy of their past land use practices on modern ecosystems, however, frequently disregards the more recent post-Classic and post-European environmental impacts. The image of ‘lost’ temples in the jungle following the decline of the ancient Maya civilisation may be enticing, but ignores the palimpsest of land use history during the most recent centuries as the post-Classic Maya reorganised socially and economically, whilst the Europeans brought conflict, disease, new technologies, and land-use practices to the Americas. This study focuses on Belize, a region that features a long history of human influence on its diverse ecology and landscapes. Rather than following a simplistic model of collapse and abandonment, many ancient Maya polities, including Lamanai, continued into the post-Classic period [23,24]. Following European colonisation, the burgeoning Spanish and British empires looked to exploit natural resources, such as logwood, mahogany, and sugar cane. Building on a previous reconstruction of ancient Maya land-use [19], this study uses new pollen data from northern Belize to provide a detailed examination of socio-ecological changes from the ancient Maya period across perhaps the most disruptive events of human history in the Americas: the European Encounter (Table 1).

Table 1.
Key events at Lamanai, Belize, 1500 BCE–1981 CE. Adapted from [25].
2. Materials and Methods
2.1. Site Description
Northern Belize is in the southeast of the Yucatán Peninsula (between approximately 88°45′ and 89°15′ W, and 16°00 and 18°30′ N) bordering Mexico to the north, the Caribbean Sea in the east, and Guatemala to the south and west (Figure 1). Lamanai is on the west side of the New River Lagoon (NRL; approximately at 88°40′ W and 17°40′ N), in the district of Orange Walk. NRL is a freshwater lagoon within the river channel of the New River and is likely a widened valley section situated between the limestone Yaalbac Hills in the west and sandy Pliocene ridges located in the east [26]. The NRL is about 23 km long, 0.75 km wide, and covers approximately 13.5 km2, the largest body of freshwater in Belize [19,26]. Lowland broadleaf forest, lowland savannah and mangrove, and littoral swamps are the predominant features of the vegetation of Northern Belize. The underlying geology guides the ecology, with pine savannah associated with well-drained acidic sand ridges, evergreen forest found on calcareous sediments, herbaceous swamps, seasonally inundated savannah, and marshland in the freshwater lowlands [19,26]. Grass savannahs with infrequent oaks, pine, and Acoelorrhaphe wrightii (palmetto palms) are characteristic of the northern central region, and mangrove swamps occur along the northern coast and river inlets [27]. Key modern economic trees include: Achras sapota (Sapodilla), which is tapped for latex or chicle that forms the basis of chewing-gum; Swietenia macrophylla (Mahogany), and Calophyllum brasiliense (Santa Maria), which are both timber exports [28].
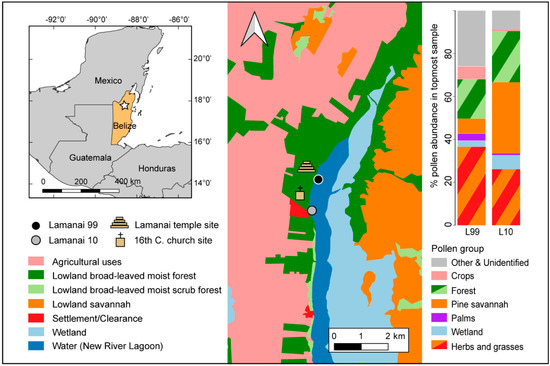
Figure 1.
Location of the Lamanai-99 (black circle, 16Q 324951 E, 1964889 N) and Lamanai-10 (grey circle, 16Q 324746 E, 1963519 N) sediment cores relative to Maya and post-colonial settlement of Indian Church (northernmost red cleared area) along the western shore of NRL. Landcover classifications were obtained from Meerman and Sabido (2011). Inset: Location of the study region (Lamanai) in northern Belize.
2.2. Modern Climate and Vegetation at Lamanai, Northern Belize
The seasonally dry tropical climate of Northern Belize is directed by the movement of the Inter-Tropical Convergence Zone (ITCZ), with a boreal summer wet season. Northern Belize has a drier and more seasonal climate than the south [29], with annual precipitation in the range of 1520–2030 mm [30]. A study of the modern vegetation at Lamanai [31] reported that the predominant arboreal species included Brosimum alicastrum, Protium copal, Talisia oliviformis, and Pimenta dioica, and suggested this was due to the lime rich soil found at the site, which is favoured by these species. At present, a fringe of reeds (with predominant species Cladium jamaicence and Phragmites australis) surrounds the NRL and, in the west, these reeds grade into a band dominated by logwood (Haematoxylon campechianum) and then into tropical evergreen seasonal broadleaved lowland forest [31]. This is the vegetation that surrounds the present-day archaeological site of Lamanai. East of the NRL, the vegetation transitions from a reed fringe to marsh land (dominated by Eleocharis intersticta and C. jamaicence) and then into a band of logwood, before becoming savannah [31]. The savannah has been described [31] as short-grass savannah with infrequent needle-leaved trees, featuring species including Pinus caribaea, Metopium brownei, and Byronsima crassifolia.
2.3. Site History in the Regional Context
The Central Maya Lowlands, including Belize and the Petén region of Northern Guatemala, has extensive archaeological evidence for the presence of the Maya, with crops synonymous with Maya agriculture. The diversity of past Maya land use is observed in pollen records throughout the northern region of Belize, suggesting forest clearance for field-based agriculture occurring c. 2500 BCE, with evidence of crop production as early as 3400 BCE [32,33,34,35,36,37]. Soil erosion is evident in multiple records across the Central Maya Lowlands, soon after the appearance of crops [38,39,40,41,42,43], with some evidence of terracing [40] and wetland agriculture [37,44] during c. 2000–1000 BCE. Lamanai was an important Maya site. Major phases of occupation at Lamanai are described extensively, with Maya settlement beginning at c. 1500 BCE, persisting until the post-Conquest period [23,24,45,46,47,48,49,50], and even into the modern period [51,52,53] (Table 1).
Across the Central Maya Lowlands, most large-scale agricultural terracing and major hydraulic manipulation occurred from c. CE 300–500 [32,54]. Records from Northern Belize suggest that the most intensive land-use occurred c. CE 650–950 [39]. This is consistent with records from Lamanai, where major construction and population peaked during the early Classic period (c. CE 250) [26] and periods of extraction of pine from savannahs adjacent to the east of the New River Lagoon are distinguishable from clearance of seasonal broadleaf forest for agriculture in the early Classic period [19]. Records from across Northern Belize including: Laguna Verde [32,36]; Chan Cahal [32,35]; Cobweb Swamp [34]; and Cob Swamp [34], suggest that the raised fields were abandoned post c. CE 950, with some recovery of forest evident. However, at Lamanai, high palm values in the pollen record persists during 100 BCE–CE 1100, which is perhaps indicative of continued Maya palm cultivation. This inferred cultivation is consistent with the continuous occupation of the Maya at Lamanai until after CE 1500 observed in the archaeological [51,52,53], palaeolimnological [26], and palaeoecological [19] records.
Spanish Christian missionary interaction in Belize was concentrated between CE 1540 and 1570, and extended to Northern Belize during c. CE 1544–1707 and lasted in southern Belize until c. CE 1724 [51]. Lamanai has two 16th century churches built in the Spanish style, dating from c. CE 1544 and CE 1568. A Spanish missionary visit to Lamanai in CE 1641 reported that Lamanai and its churches were abandoned [55]. Archaeological excavations at Lamanai suggest intermittent Maya habitation at Lamanai post CE 1700 [23,50]; however, permanent residence probably ended by the end of the 17th century with the Maya population relocating to the Petén region of Northern Guatemala [55] (Table 1).
Formal British colonial governance in Belize extended from CE 1786–1981, however British colonial records document that European logging industries began over a century earlier, with logwood (H. campechianum) the focus of extraction during CE 1660–1950 from coastal and riverine regions across Northern Belize [25]. During CE 1800–1945, the predominant forestry product was mahogany (S. macrophylla), extracted from the seasonal broadleaf forests [25]. A British venture established a sugar mill at Lamanai c. CE 1837 that was in operation until c. CE 1875, when it was abandoned [47,56] (Table 1).
2.4. Sediment Collection
This study presents new high-resolution data from the last ca. 1200 years and incorporates the results of Rushton et al. [19], which contains the detailed methods for the generation of a long-core pollen sequence from 1700 BCE until present. The Lamanai 1999 core (Lamanai-99), measuring 350 cm, was extracted from NRL from a jetty adjacent to the temple area of Lamanai, using a Livingstone corer. Previously published geochemical- and diatom-inferred palaeoclimate data [26] and pollen- and charcoal-inferred vegetation change [19] show landscape and environmental changes from the Maya pre-Classic era until present. The unconsolidated top sediments, however, were not collected in 1999, and a short sediment–water interface core was extracted in 2010 to complement and extend the previous Livingstone core analysis (Lamanai-99) and to provide a more detailed analysis of land use change from the post-Classic through to the European colonial period. This short core from the NRL was extracted from the end of a jetty adjacent to the post-colonial settlement of Indian Church, approximately 1 km south of the original coring site (Figure 1) at a similar distance to the shore. A Perspex tube Glew corer with a 5 cm diameter was used to retrieve the sediment-water interface intact. Samples were extruded in the field and were shipped in sealed plastic bags. All material was stored at 4°C. Samples for pollen, charcoal, and loss on ignition (LOI) analysis were taken at 1 cm intervals. The top 1 cm surface sediment from the Glew core was analysed for comparison with the modern vegetation.
2.5. Chronology
Given the recent advances in Bayesian modelling of chronologies and the recent publication of IntCal20 [57,58], a new chronology was created for the Lamanai-99 core [19,26], which is based on the original published six radiocarbon dates (Table 2). Due to the limited availability of terrestrial macrofossil material for dating, two horizons were dated with gastropod shells. An offset for the hardwater effect in the gastropod dates (1660 years) was calculated by comparing dates obtained from terrestrial plant material and carbonates (gastropod) in one horizon (see Table 2). The Lamanai-10 core was dated with six radiocarbon dates based on terrestrial macrofossils; of which two dates were rejected as outliers (Table 2). The chronology of the Lamanai-10 core was correlated to the original Lamanai-99 core using one dated pollen ‘tie point’ (i.e., a concomitant rise in the regional pine signal), which allowed one date from the Lamanai-99 sequence to be incorporated into the Lamanai-10 chronology. Independent age models were developed for both cores given the distance between the coring locations. Dates (terrestrial materials) and corrected dates (gastropods) with 1-σ standard deviation were used to create the two age-models in rBACON [58] in R version 3.5 [59] using the IntCal20 calibration curve. The default priors were accepted for accumulation rate; with vertical sections of 5 cm thickness, producing 11 sections for Lamanai-10 and 63 sections for Lamanai-99. Lamanai-10 was run with an acc.mean of 20 year/cm and Lamanai-99 with a faster accumulation rate of 10 year/cm. Ages used are those based on the produced weighted average mean for each sample. Age models are presented in Figure 2.

Table 2.
Radiocarbon dates, hardwater offset, and the pollen tie-point * used to create the two independent age models using Bayesian modelling. ** denotes rejected dates. † indicates the paired dates (organic material and gastropods) for which the 1660-year hardwater offset was calculated. ‘Terrestrial macrofossils’ refer to samples that contained identifiable plant remains recovered from Lamanai-10, whereas Lamanai-99 was dated with undifferentiated ‘organic matter’.
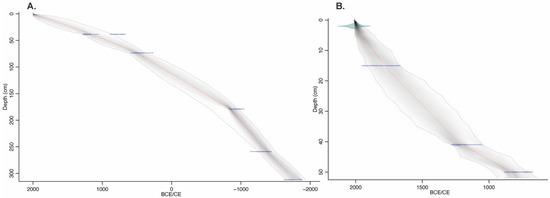
Figure 2.
Bayesian age-models of the Lamanai-99 (A) and Lamanai-10 (B) cores. Grey stippled lines show 95% confidence intervals with the red line representing the single best model based on the mean age for each depth; the shaded area demonstrates most likely age ranges at each horizon. Details of radiocarbon dating, rejected dates, and core correlation are contained in Table 2.
2.6. Pollen and Charcoal Analysis
Pollen and charcoal preparation and analysis followed the same protocol for both cores. Standard chemical digestion was used, including hot 10% NaOH and acetolysis treatments [60,61]. Prior to chemical digestion samples were sieved at 250 µm to remove larger shells, and a cold 10% HCl treatment was applied to remove carbonates. Sodium hexametaphosphate was used to remove clays, and the samples were repeatedly centrifuged and rinsed with distilled water until the supernatant was clear. The methodological protocol described by [62] was used to improve the recovery of Z. mays and other large cultigen pollen grains. Known concentrations of Lycopodium spores were added to each sample to enable the calculation of pollen and charcoal concentrations. Charcoal counts were made from material prepared for pollen analysis, consistent with the description reported in [19]. The charcoal particles counted on the pollen slides for the Lamanai-99 core were <100 µm in size. In the Lamanai-10 core, charcoal concentration is subdivided into macro (>100 µm) and micro charcoal particles (≤100 µm), also counted from the pollen slides, and summed for data presentation here. To account for the different charcoal counting procedures, the charcoal records from each record were standardised to z-scores.
Pollen identification resources described by [19] were also used in the analysis of the Lamanai-10 core. A minimum of 200 and a maximum of 320 fossil terrestrial grains were counted: The pollen sum is exclusive of unidentifiable damaged grains, aquatic taxa, and fungal spores. Cyperaceae was included in the pollen sum because sedges have a significant presence in the herbaceous component of the adjacent savannah communities. Although sedges are also a component of the fringing semi-aquatic vegetation at NRL, these communities are dominant on the eastern shore of NRL, therefore the impact of locally derived pollen of semi-aquatic plants in the pollen sum is likely to be minimal. Rhizophoraceae (mangrove), species of which feature in littoral forest in central Belize, were also included in the pollen sum. Pollen taxa were separated by ecological groups; seasonal forest, savannah, disturbance taxa, Arecaceae (palms), and crop taxa based on the dominant habitat preference of the parent plants associated with each pollen morphotype [31].
A detailed description of taxon identification, informed by regional pollen diagrams, was used in the analysis of this record [19]. Palms (Arecaceae) have been described as economic taxa [20], however they are presented separately as they are found in multiple formations. Z. mays was separated from other wild grasses using the criteria set out by [63], which included an analysis of the distribution of exine intertectile columnellae using phase contrast at ×1000 magnification. It is not possible, however, to distinguish it from its ancestor Balsas teosinte (Z. mays subsp. parviglumis) [63,64,65] because of morphological similarities, particularly size [63]. However, as teosinte is not native to Belize, it is likely that observed pollen grains morphologically consistent with Zea are domesticated Z. mays.
2.7. Numerical Analyses
Pollen results (expressed as percentages) for both the Lamanai-99 (Figure 3) and Lamanai-10 (Figure 4) cores were zoned using CONISS (stratigraphically constrained cluster analysis by incremental sum of squares) using Euclidean distance as a dissimilarity index. Analysis was conducted in Rioja in R [59,66]. Only pollen grains comprising 1% at one or more horizons in the sediment core were included (this cut-off was also applied to crop pollen) and three zones were delineated from this analysis in both cores. Rarefaction of the pollen data, which standardises palynological richness to a set pollen sum, was performed in Psimpoll [67] on both pollen records as a proxy for past changes in vegetation diversity [68]. Pollen samples were rarefied to a pollen count of 300.
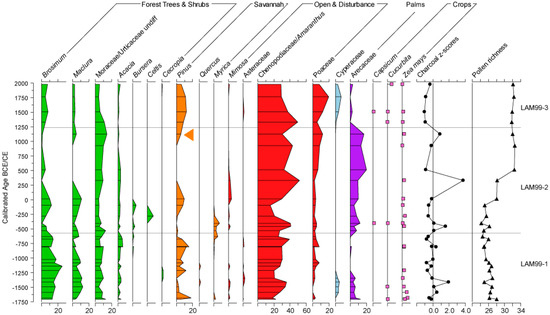
Figure 3.
Pollen percent abundance data, rarefied pollen richness data, and transformed charcoal data (z-scores) from Lamanai-99 plotted against the reanalysed age-model. The arrow marks the horizon (rise in pine pollen) used to correlate the chronologies of the two records.
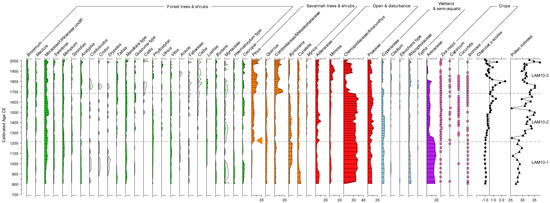
Figure 4.
Pollen percent abundance data, rarefied pollen richness values, and transformed charcoal data (z-scores) from Lamanai-10 plotted against age. The arrow marks the horizon (rise in pine pollen) used to correlate the chronologies of the two records. 5× exaggeration curves are shown for taxa with low abundance.
3. Results
3.1. Modern Pollen and Charcoal Assemblages
The two cores were extracted from different parts of the NRL and therefore reflect related but distinct pollen signals (Figure 1), and due to their near-shore locations, these signals contain a strong local component to the vegetation, which allows a comparison of the temple area and post-colonial settlement. The modern pollen assemblage from Lamanai-99 (Figure 3) is predominantly represented by herbaceous pollen types (ca 50%), the source of which is likely to be a combination of the lagoon marginal wetlands, the savannahs to the east of the lagoon, and disturbed open vegetation from modern clearances and agriculture (Figure 1). Herb taxa include Chenopodiaceae/Amaranthus (9%), Asteraceae (7%), and Poaceae (6%). The pollen signal also captures broadleaf seasonal forest (18%) that is predominantly located on the western shore of the lagoon and pine savannah (ca 10%), which dominates the eastern side of the lagoon. Broadleaf seasonal forest pollen types include Moraceae/Urtiaceae (4%), Brosimum type (3%), Manikara zapote (3%), and S. macrophylla (2%). The pine savannah signal comprised Pinus (16%), and also Mimosa (4%), Byronsima (2.5%), and Quercus (2.5%). Combretaceae/Melastomaceae comprised 12%, and could be representative of both pine savannah species, e.g., Miconia (Melastomaceae) and also seasonally inundated forest species associated with the logwood fringe (H. campechianum, 2%) that surrounds the modern NRL, including Bucida buceras [69]. There are low palm levels recorded (1%) and very low crop taxa, with only Z. mays present (1%). In the modern core top sample, charcoal concentrations are 81 particles/cm3.
The modern pollen signal from Lamanai-10 (Figure 4), extracted from near the western lake shore, contains a lower proportion of herbaceous pollen types (22%), but a higher proportion of pine savannah pollen types (46%) compared to Lamanai-99. The pine savannah signal is comprised mostly of Pinus (16%), Combretaceae/Melastomataceae (Miconia) (9%), Curatella americana (4%), and Myrica (3%), with low abundances of Byronsima (1.5%) and Quercus (1%). The broadleaf seasonal forest comprises (19%) of the pollen signature and is represented by undifferentiated Moraceae/Urticaceae (4%), Brosimum type (3%), Acalypa (3%), S. macrophylla (2.5%), M. zapote (2%), Maclura (1%), and the weedy tree Cecropia (1.5%). Herbaceous pollen types, reflective of the NRL wetlands, pine savannahs, and open disturbed vegetation include Poaceae (8%), Asteraceae (6%), Chenopodiaceae/Amaranthus (6%), Mimosa (5%), and Cyperaceae (3%). H. campachenium (2%), and Rhizophoraceae (2%) (mangrove) pollen are also present, which reflect waterlogged and marginal riverine vegetation, respectively. Freshwater mangroves are known to occur in the inland river systems of Belize [27]. Arecaceae and crop pollen, specifically Cucurbita, are present, but in low abundance (<1%). Charcoal concentrations total 81 cm3 in the modern sample at Lamanai-10.
3.2. Long Core Record (Lamanai-99)
The palynological and charcoal results from this core have been published as a record of vegetation history from Lamanai, NRL for the period 1500 BCE to 1500 CE [19]. Here, we present a summary of the vegetation changes contextualised by the updated age-model that spans 1700 BCE to present (Figure 3). The previous age model was not extrapolated to the surface; the new age model presented here demonstrates that the post-European period is represented by only two horizons in Lamanai-99, thus the previous interpretation is unchanged in that majority of the record represents the pre-European period. The revised age model also highlights the requirement for the new higher resolution record offered here.
The Lamanai-99 pollen record [19] shows three periods of extraction of Pinus from pine savannahs that are distinguishable from clearance of seasonal broadleaf forest for agriculture. Throughout the pollen record, field-based agriculture that is located adjacent to Lamanai is visible in an abundant record of Z. mays grains. In addition, an increased palm signal was observed during 1700–1200 BCE (previously 1630–1150 BCE) and 600 BCE to 1250 CE (previously 100 BCE–1100 CE), which could indicate Maya cultivation. After 1250 CE there is a decline in the palm pollen signal, although the temporal resolution of analysis is coarse and requires further investigation in Lamanai-99. After 1500 CE there is an increase in Pinus extraction, which previous authors [19] suggest could be associated with the European arrival. The short core record (Lamanai-10) presented here, however, enables a more detailed understanding of vegetation change over the last 1000 years.
3.3. Short Core Record (Lamanai-10)
The new pollen record from Lamanai-10 is shown in Figure 4.
Zone One. 52–42 cm: 800 CE to 1200 CE:
Broadleaf forest cover ranges between 14% and 23% (average 19%) with a broadly increasing trend toward the top of the zone. Total Urticaceae/Moraceae (2–6%), which includes Brosimum (1–3%), Metopium type (1–4%), Manilkara type (1–3%), Vitex (1–2%), and Bursera simaruba (0–2%) are the most abundant taxa found in this zone. The following forest taxa are also present, but found in lower amounts, averaging 1% in the zone: Acalypha, Celtis, Luehea type, Spondias type, and Swietenia type. Taxa typical of savannah cover ranges between 25% and 31% (average 28%), but pine values are negligible in this zone (1%). Abundant woody and shrub savannah taxa include Byrsonima type (2–8%), Curatella (1–7%), Quercus (2–3%), and Combretaceae/Melastomaceae (Miconia) (1–4%). Herb taxa are abundant (average 20%), ranging 15–25%. Z. mays pollen is present almost continuously throughout the zone and other crop pollen types are present in less consistent amounts: Capsicum, Cucurbita, and Ipomoea batatas type. Arecaceae pollen values are highest in this zone compared to later periods, ranging 15–20%. Pollen representative of semi-aquatic and wetland communities, including Cyperaceae, Rhizophoraceae, Cladium type, Eleocharis, and Typha are also present (average 7%). Charcoal concentrations are low throughout the zone and pollen richness averages 28 E(T300).
Zone Two. 41–21 cm: 1200 CE to 1680 CE:
Broadleaf forest pollen increases in Zone two, ranging 16–29%. The most abundant seasonal forest taxa are Moraceae/Urticaceae (3–8%) including Brosimum (0–3%), Metopium type (1–3%), B. simaruba (1–3%), Manilkara type (1–3%), Guazuma (0–3%), Talisia (0–3%), Acalypha (0–3%), and Swietenia (0–2%). Pollen of pine savannah increases (29–49%), with woody and shrub taxa represented by Byronsima (2–6%), Quercus (1–4%), Curatella (1–3%), and Combretaceae/Melastomataceae (Miconia) (0–4%), with Pinus values increasing to maximum 6% in this zone. Herb taxa, Asteraceae (6%), and Mimosa (3%) reflecting the open under-storey, are also present. Pollen of the crop plants Z. mays, Cucurbita, and Ipomoea are all consistently present. Poaceae (4–12%), representative of both open disturbed areas, savannahs, and the fringing wetlands are consistently abundant throughout the zone and Chenopodiaceae/Amaranthus pollen (disturbance indicator) ranges 17–30% throughout. Arecaceae pollen declines to 4–15%, but it is consistently abundant still throughout Zone 2. Charcoal rises through the zone, peaking at 1630 CE, after which concentrations decline. Pollen richness averages 34 E(T300).
Zone Three. 20–0 cm: 1680 CE to present:
Seasonal forest pollen remains unchanged in Zone Three, ranging 18–34% and peaking at the top of the Zone (3–2 cm). Urticaceae/Moraceae (3–8%) dominate the seasonal forest signal, with Brosimum comprising 2–4% of the pollen sum. Other abundant seasonal forest taxa include Metopium type (1–5%), Manilkara type (0–6%), Acalypha (1–4%), Swietenia type (0–4%), Bursera type (0–3%), and Talisa type (0–3%). Haematoxylum (0–2%) are also present. Of the woody savannah taxa, Pinus rises in abundance compared to the previous zone (3–16%), with values falling between ca. 1890 and 1960 CE. Also representative of savannah are Combretaceae/Melastomaceae (Miconia) (5–16%), Curatella (1–4%), and Quercus (0–4%). Herb pollen values are higher than the previous zone, ranging 12–33%. Pollen representative of semi-aquatic and wetland communities rise in abundance, specifically Cyperaceae and Rhizophoraceae, which each average 2% abundance. Arecaceae values decline further (0–10%) compared to previous zones, and, fall to negligible levels after 7 cm. Crop types are poorly represented in this zone, with sporadic Zea mays and Capsicum. Cucurbita and Ipomoea are absent. Charcoal concentrations peak at ca. 1800 CE and show high variability over the last two centuries, peaking again at ca. 1940 CE and near the present day. Pollen richness averages 30 E(T300).
4. Discussion
4.1. Pollen and Charcoal Signals in Lamanai-99 and Lamanai-10
Savannahs dominated by P. caribaea are represented in the pollen record by abundant Pinus pollen, reflected in the topmost sediment samples in both cores. Pines are prolific producers of wind-dispersed pollen, thus regional sources, such as high elevations from Mexico and Guatemala, may contribute some pollen to the Pinus signature in the NRL record. However, source plants located nearer to deposition sites contribute a substantially larger proportion of pollen compared with regional or ‘background’ pollen, where vegetation patches in a heterogeneous environment are larger than the lake area, as in the case of Belizean pine savannah [70]. We interpret, therefore, variations in the pine pollen signal to reflect changing abundance of pine in savannahs surrounding NRL and the wider Belizean lowlands. Given this regional signal, the post-colonial rise in pine pollen abundance is used to anchor the chronologies of the two cores (Table 2) and reflects a wider regional pattern of recovering pine resources in the Belizean savannahs.
By contrast, much of the remaining pollen signal in the two cores (Lamanai-99 and Lamanai-10) reflect the local vegetation adjacent to where they were extracted. Both cores were taken from the jetties near the western edge of NRL (Figure 1), but they capture the land-use of two different settlements, specifically the Classic Maya temple site of Lamanai (Lamanai-99) and the post-colonial settlement of Indian Church (Lamanai-10), which are separated in time, as well as space. The higher resolution analysis provided by Lamanai-10 provides insight into the impact of the social and economic changes associated with the post-Classic and subsequent European colonial influences on the area. Given the heterogeneity of the pollen signals, we can be confident that the large pollen types represented in the records, such as those produced by some important crops and palms, capture land use close to the settlements. In particular, multiple field studies have shown that Z. mays are poorly dispersed from the source plants [71,72] and that well-preserved pollen grains are unlikely to have been reworked if they are large and susceptible to mechanical damage (i.e., crumpling and folding) [73], which was not the case in either core. The presence of multiple, undamaged palm and Z. mays grains most likely represent a distinctive signal of changing land use patterns in space and time.
Although charcoal from pollen slides (microscopic pollen) are known to be reflective of regional fires [74], there are differences between the two records that suggest a degree of spatial heterogeneity of land use is also recorded in the charcoal signals. The Lamanai-99 charcoal record declines towards the present day. By contrast, the charcoal concentrations increase rapidly at Lamanai-10 in the post-Classic period and peak in the late 18th century. It is worth commenting, however, that Lamanai-99 was sampled at a coarse resolution, with only six charcoal samples representing the past millennium; thus, some of the differences can be explained by differences in sampling resolution. However, the rising charcoal values at Lamanai-10 in the post-Classic and early colonial periods are not reflected at any point in Lamanai-99, which suggests that there was greater use of fire around the Indian Church settlement.
4.2. Maize and Palm—A Record of Maya Cultivation and Spanish Construction
The revised age model demonstrates pollen evidence of Maya crop cultivation of Z. mays and Cucurbita at Lamanai as early as 1700 BCE [19], which is consistent with the presence of crops in other records from Northern Belize c. 2000–1000 BCE [33,34,37,75]. Throughout the pollen record of Lamanai-99 [19], including the post-Classic period, there is a consistent Z. mays signal, with multiple, pristine grains observed, which is indicative of continuous agriculture adjacent to Lamanai. The Lamanai-10 record also reflects this record of continuous crop production from the Classic and into the post-Classic period. Unlike Lamanai-99, which had only two samples in the post-colonial period, Lamanai-10 confirms the continued production of Z. mays through Spanish, and subsequent British, colonisation (20 samples). This signal, however, weakens in the past two centuries.
Similarly, the Arecaceae pollen signal is consistently high through the Classic and into the post-Classic periods in both records. Palms including Acrocomia aculeata and Attalea cohune form part of the forest cover at the modern site of Lamanai [31] but are present only in low levels (1%) in the modern pollen assemblages. The palm signals during the Classic and post-Classic are upwards of 20× higher and therefore are likely to reflect intentional cultivation of these economically important plants. Palms are recognised as an important vegetation resource used by the Maya to build their domestic dwellings, called ‘palapas’ [76,77]. It is suggested that the modern Belizean Maya prefer Thrinax radiate and Sabal yapa for thatching [77], but frequently use Orbigyna cohune as it is more abundant. Trunks of the Acoelorraphe wrightii palm are used for palapa walls, with T. radiate and S. yapa used as part of roofs and household implements [76]. The period covered by the elevated palm signal (600 BCE–1250 CE) is one of substantial building at Lamanai [46] and as such, may be linked with an increased palm signal. The interpretation of the elevated palm pollen as an intentional management strategy is corroborated in another Belizean study [34] where an observed increase in palm pollen during periods of Maya occupation is attributed to cultivation.
The coeval and consistent maize pollen signal in both records suggests that the Maya cultivated a large area of the western shore of New River Lagoon for maize, palms, and other crops, but that this cultivation de-intensified or shifted location away from the temple site in the post-Classic period following 1240 CE when the palm signal at the temple site of Lamanai (Lamanai-99 core) terminates. Palm cultivation continues around the area that would become the post-colonial settlement of Indian Church, albeit in lower abundances. This shift in palm cover is accompanied by a concomitant and sustained rise in charcoal, suggesting changing or intensifying land use at this more southerly location, ca. 1 km south of the main temple area (Figure 1). The use of fire increases in the post-Classic through to colonial periods and the weedy tree taxon, Cecropia, rises in abundance through this period of intensifying fire use, which implies increasing pressures on broad-leaved forest. This pattern of shifting focus in land use south of the temple site supports the archaeological interpretation of a translocated settlement in the post-Classic period [78]. However, the unvarying abundances of palms and crop pollen suggests continuity in the type of land management and crop production at Indian Church through the post-Classic and into the colonial period until ca. CE 1800 CE. It is likely the Spanish colonial settlement, which needed housing and other infrastructure, would have frequently used palms to construct building, including churches. Lamanai is suggested to have been an important centre for Christianised Maya in Belize [51], and the two 16th-century churches were built between the temple site at Lamanai and the later settlement of Indian Church. Although the two churches had foundations and some stone walls, the majority of the construction would have been thatched with palm [77]. The focus of the Spanish Christian mission at Lamanai further supports the interpretation of an increased palm signal as owing to cultivation, as palms remained an important commodity, used in both the building of dwellings and churches.
4.3. Exploitation of Pine by the Maya, Spanish and British
The revised age model has pushed the identified periods of pine clearance for construction earlier than originally presented [19]. The chronology now suggest that clearances began as early as 650–300 BCE (previously 170 BCE–CE 150) and intensified from 280 to 900 CE (previously 600–980 CE), therefore extending a period of intensive disturbance at Lamanai to encompass the Classic period. These periods of substantial reduction in the pine pollen signal are consistent with phases of construction and development at Lamanai. The Maya selectively used Pinus for construction and fuel, with vast quantities of pine needed to produce the limestone required to build and decorate the temples, stelae, and ball courts [18,79]. It has been suggested that, at the Maya city of Copán, Honduras, pine (P. caribea) was used for torches, with similar use of pine by the modern Maya [77].
Archaeologists have demonstrated the ubiquity of Pinus found as pine needles and/or charcoal in assemblages from cave and surface sites across Northern Belize [80]. Such frequency, geographical, and contextual variety of Pinus present in the record demonstrates the importance of pine as a resource. The Maya had two central uses of Pinus: Domestic (e.g., fuel, torches, building material) and ritual (e.g., ceremonial burning) [81]. The utilitarian function of Pinus as a torch is evident by the association of Pinus charcoal assemblages and ceramic torch holders in cave sites [82,83,84,85]. However, pine torches were probably also an important aspect of ritual [81], with the burning of pine wood and resin as an offering to deities a common motif in Classic Maya iconography [86,87,88].
Although Pinus is a common feature of charcoal assemblages from the Maya Lowlands, thus demonstrating its widespread use, Pinus is not a resource that is often local to Maya sites, as the ecological distribution of Pinus is broadly restricted to upland regions and pine savannahs [27]. The widespread use of pine across the Maya Lowlands, whilst also having well-defined ecological distribution, further emphasises the cultural significance of Pinus for the Maya [81]. The site at Lamanai had the advantage of a substantial and local source of pine, growing in the savannah, east of the NRL. Prevalent pine charcoal has been found [80] in the analysis of the contexts of caches (intentionally gathered and ritually buried objects) from three high status structures in the Central Precinct of Lamanai, dated until the early post-Classic period.
In both the Lamanai-99 and Lamanai-10 cores, Pinus shows an initial increase during the post-Classic period, which we have interpreted to represent a regional, concomitant rise in pine resources, given the highly dispersed and prolific nature of pine pollen. The higher temporal resolution Lamanai-10 core, however, demonstrates that whilst pine pollen initially increases following 1200 CE (Zone Two, Figure 4), pressures remain on pine resources from the post-Classic through to the colonial period. The continuous extraction of pine from savannahs in the post-Classic may reflect the sustained demand for pine as an important part of ceremonial practice across the Maya Lowlands, even as some settlements across the region faced ‘collapse’ through periods of Late Classic/Terminal Classic drought [10,11,89,90,91,92]. The archaeological record corroborates the pattern of decline in ceremonial pine use during the Post-Classic [80].
The continued low abundance of Pinus pollen, observed in Zone Two of Lamanai-10 core from CE 1500 to 1700, could indicate the continued use of pine (like palms) as a source of fuel and building materials by European settlers. Despite this continuous presence of pine during the colonial period, the higher sampling resolution of Lamanai-10 demonstrates the pressures on pine resources in the 20th century and provides evidence of further pine resource exploitation linked to British colonial activity in Belize. Although European settlement at Lamanai began during the early Spanish colonial period (c. CE 1544–1641) with the construction of two churches [47,51], it appears from the pollen record that the Spanish colonists did not utilise pine as a source of timber or fuel to the same extent as previous Maya settlement and subsequent British industry. As documented by the archival and archaeological records, the 19th century was a period of British-led clearance and construction at Lamanai, as the first known British-owned sugar mill in Belize was built within the modern-day archaeological site during the mid-1850s and was in operation c. 1860–1875 [93]. A Plantation Grant issued by the British Government in 1837 indicates land clearance for sugar cane planation occurred at Lamanai by at least the 1830s [47,93]. The historical record demonstrates the continued use of pine as torches during the 19th century, describing their use to illuminate the overnight transport of mahogany logs from the forest to the riverbank [94]. The increased Pinus signal in the Lamanai-10 core from 5 cm indicates that as the population of the Lamanai area reduced to a small hamlet, the pine savannah was no longer a significant source of timber for construction and/or fuel, and consequently, regrew.
4.4. The Impact of Land Management and Crop Production on Biodiversity
The new data from Lamanai-10 demonstrate that spatial, as well as temporal, resolution is an important consideration in reconstructing past land use. Without the additional spatial information provided by the Lamanai-10 core, the easing of land-use pressures around the temple area could be inferred to reflect depopulation, rather than shifting focus to an area just 1 km away (Indian Church) (Figure 1). As well as spatial differences in land use, the two cores provide a comparison of patterns of biodiversity in the context of past management practices, which is required to understand the current and future status of Neotropical diversity [95]. The two cores combined provide a 3500-year-old palaeoecological record of the environs of Lamanai to enable a comparison of such different management practices, considering those of the Maya with the Spanish and British cultures who utilised vegetation resources post 1500 CE.
The relatively low pollen richness in the Lamanai-99 core, prior to ca. 300 CE, indicates that the highest pressures on both pine savannah and seasonal forest occurred during the early Classic period. Pollen richness, a proxy for diversity in vegetation communities, rises after ca. 300 CE around the time that palm agroforestry occurs adjacent to the temple area. The diversity index remains high through the rest of the core, albeit with coarse temporal resolution, which suggests the land use change that had the greatest impact on ecosystem composition and diversity occurred in the pre-Columbian period and is not associated with either the terminal Classic or the Spanish colonisation. In addition to socio-political drivers, there is no clear impact of climate variability [12,43,96], specifically drought associated with the Maya terminal ‘collapse’ on the land management systems [26], although we recognise that the temporal resolution is coarse in Lamanai-99.
In the more temporally resolved record of Lamanai-10, a similar pattern emerges where changes in pollen richness are not associated necessarily with ‘collapse’ or colonisation. Instead, the lowest pollen richness occurs in the period leading up to the post-Classic, but increases at the point we infer that the Maya communities intensify land use in the area that would become Indian Church. The decline in palm pollen, indicating perhaps a reduction in the requirement for palm building materials compared to the period prior to 1240 CE, occurs alongside increasing use of fire, continuing crop production and rising weedy tree abundances rise after CE 1480. This pattern suggests that the Maya communities shifted to a ‘forest garden’ style of land use [97], which is promoted in modern conservation as a useful tool to maintain seasonal forest cover whilst growing field-based crops, palms, and arboreal resources. Advocates of the ‘forest garden’ model of Maya management [20] suggest that modern conservation approaches should consider those of the late Classic Maya, however the evidence presented here is that the Maya seem to have applied this model in the post-Classic period away from the temple sites. This land use is associated with the high pollen richness, and hence high plant diversity, in the Lamanai-10 record. This pattern of land use continues uninterrupted through the colonial period, including the period under which the Spanish churches were constructed. Instead, the largest change in both land use, and reduction in plant diversity, occurs in the late 17th century, associated with British colonial timber extraction (predominately logwood and mahogany) and attempts to establish industrial sugar cane production during the 19th century [93].
Recent post-colonial forest conservation may be reflected in the period of forest recovery that is evident at the top (3–2 cm) of the Lamanai-10 core, with the highest seasonal forest value across both cores occurring at 3 cm, accompanied with low herb, palm, and crop signals. This is probably due to the small population found in the village of Indian Church and the archaeological site under Government legislative protection. Rarefaction analysis suggests that the top of the Lamanai-10 core has the highest palynological richness across both cores. This indicates that the biodiversity of the land surrounding Lamanai is higher in the modern era compared to previous periods, including that of the forest gardens of the post-Classic Maya.
5. Conclusions
Here, we have described how past land use patterns shifted in space and time through a period of significant cultural and political change in Belize using complementary records of changing vegetation and fire use, extracted from sediment cores located ca.1 km apart. The spatial differences in past land use show that whilst sediment records taken adjacent to temple areas, which remain the focus of many palaeoenvironmental studies, are excellent indicators of past land use among the Classic Maya, palaeoecologists and archaeologists need to analyse past change at a variety of historical settings to understand the full range of changing socio-ecological interactions through time that includes both considerations of industrial resources (e.g., Pinus) as well as agricultural crops (e.g., Z. mays). This is especially true of palaeoecological investigations in Belize, which is known for its multi-layered cultural history, as well as biological diversity.
A key rationale for investigating past human–environment interactions is to understand the impact of various land uses on ecosystem composition relative to its ‘natural’ state. There is no pre-anthropogenic ecological baseline in our records, however, as forests in the Yucatán have undergone significant alterations for millennia [9]. Instead, we examined relative differences in past biodiversity and forest cover at Lamanai, and demonstrate that it is highest during recent decades, the result of government-enforced protection of the archaeological reserve. The sustainable exploitation of Belize’s natural capital, however, also requires a consideration of cultural and economic dimensions of land use, as well as offering protection to provisioning and supporting ecosystem services. Our results demonstrate that biodiversity and forest cover are next highest in the post-Classic era when we infer that the Maya used a forest garden model of cultivation and food production, which has been promoted as a means of sustaining human populations whilst balancing environmental protection. It is important to note, however, the past Maya exhibited a range of land management strategies, including more exploitative practices, and we need avoid typifying past human–environment interactions according to cultural group. This is particularly true of the ancient Maya, who, in polarised debates, have been portrayed as ‘good’ forest gardeners or ‘bad’ environmental destroyers, when instead, the environmental impact of their land use practices is far more nuanced and pragmatic.
In agreement with previous archaeological investigations, the pollen and charcoal data demonstrate that the focus of land use pressures shifted south of the temple site in the post-Classic era, but there is no evidence of an alteration in land use patterns associated with drought in the terminal Classic period. The pollen record from Lamanai-10 also demonstrates a remarkable continuity in inferred land management associated with the early Spanish colonial era. Thus, two of the more significant socio-cultural disruptions covered by this study, the terminal Classic and initial European colonisation, do not coincide with the largest shifts in land use. Instead, intensifying agroforestry in the Classic period, potentially to support building activities, and timber extraction in the British colonial era, are associated with the largest variations in the palaeoecological record. These results show that, in spite of the seemingly heterogenous modern landscape, we cannot assume that socio-cultural and environmental changes coincided in time and space, and that discussions of past human–environment interactions requires a more nuanced consideration of land use change.
Author Contributions
E.A.C.R. and S.E.M. designed the study and conducted the fieldwork in 2010 and 1999, respectively. E.A.C.R. performed the laboratory analyses and selected material for radiocarbon dating. B.S.W. co-directed the pollen and charcoal laboratory and numerical methods. All authors contributed to the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by an Arts and Humanities Research Council PhD studentship (AH/1014764/1) (Elizabeth AC Rushton). Core Lamanai-99 was collected during fieldwork funded by a Leverhulme Trust research grant (F/158/BQ) (awarded to Peter Furley and Sarah E Metcalfe). Radiocarbon dating was carried out under Natural Environment Research Council allocations 761.1298 and 1624.0312 to Sarah E Metcalfe.
Acknowledgments
With fond remembrance, we acknowledge our friend and colleague Peter Furley, who enabled and inspired palaeoecological research in Belize. Thanks also to David Millard for his assistance with fieldwork in 2010.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Adolf, C.; Tovar, C.; Kühn, N.; Behling, H.; Berrío, J.C.; Dominguez-Vázquez, G.; Figueroa-Rangel, B.; Gonzalez-Carranza, Z.; Islebe, G.A.; Hooghiemstra, H.; et al. Identifying drivers of forest resilience in long-term records from the Neotropics. Biol. Lett. 2020, 16, 20200005. [Google Scholar] [CrossRef]
- Chazdon, R.L. Tropical forest recovery: Legacies of human impact and natural disturbances. Perspect. Plant Ecol. Evol. Syst. 2003, 6, 51–71. [Google Scholar] [CrossRef]
- Loughlin, N.J.D.; Gosling, W.D.; Mothes, P.; Montoya, E. Ecological consequences of post-Columbian indigenous depopulation in the Andean–Amazonian corridor. Nat. Ecol. Evol. 2018, 2, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Willis, K.J.; Bailey, R.M.; Bhagwat, S.A.; Birks, H.J.B. Biodiversity baselines, thresholds and resilience: Testing predictions and assumptions using palaeoecological data. Trends Ecol. Evol. 2010, 25, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Montoya, E.; Lombardo, U.; Levis, C.; Aymard, G.A.; Mayle, F.E. Human Contribution to Amazonian Plant Diversity: Legacy of Pre-Columbian Land Use in Modern Plant Communities. In Neotropical Diversification: Patterns and Processes (Fascinating Life Sciences); Rull, V., Carnaval, A.C., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 495–520. [Google Scholar]
- De Oliveira, E.A.; Marimon-Junior, B.H.; Marimon, B.S.; Iriarte, J.; Morandi, P.S.; Maezumi, S.Y.; Nogueira, D.S.; Aragão, L.E.; da Silva, I.B.; Feldpausch, T.R. Legacy of Amazonian Dark Earth soils on forest structure and species composition. Glob. Ecol. Biogeogr. 2019. [Google Scholar] [CrossRef]
- White, D.A.; Hood, C.S. Vegetation patterns and environmental gradients in tropical dry forests of the northern Yucatan Peninsula. J. Veg. Sci. 2004, 15, 151–160. [Google Scholar] [CrossRef]
- Perring, M.P.; De Frenne, P.; Baeten, L.; Maes, S.L.; Depauw, L.; Blondeel, H.; Carón, M.M.; Verheyen, K. Global environmental change effects on ecosystems: The importance of land-use legacies. Glob. Chang. Biol. 2016, 22, 1361–1371. [Google Scholar] [CrossRef]
- Islebe, G.A.; Torrescano-Valle, N.; Aragón-Moreno, A.A.; Vela-Peláez, A.A.; Valdez-Hernández, M. The Paleoanthropocene of the Yucatán Peninsula: Palynological evidence of environmental change. Boletín Soc. Geol. Mex. 2018, 70, 49–60. [Google Scholar] [CrossRef]
- Haug, G.H.; Günther, D.; Peterson, L.C.; Sigman, D.M.; Hughen, K.A.; Aeschlimann, B. Climate and the collapse of Maya civilization. Science 2003, 299, 1731–1735. [Google Scholar] [CrossRef]
- Hodell, D.A.; Curtis, J.H.; Brenner, M. Possible role of climate in the collapse of Classic Maya civilization. Nature 1995, 375, 391–394. [Google Scholar] [CrossRef]
- Kennett, D.J.; Breitenbach, S.F.M.; Aquino, V.V.; Asmerom, Y.; Awe, J.; Baldini, J.U.L.; Bartlein, P.; Culleton, B.J.; Ebert, C.; Jazwa, C.; et al. Development and Disintegration of Maya Political Systems in Response to Climate Change. Science 2012, 338, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Kennett, D.J.; Beach, T.P. Archeological and environmental lessons for the Anthropocene from the Classic Maya collapse. Anthropocene 2013, 4, 88–100. [Google Scholar] [CrossRef]
- Franco-Gaviria, F.; Correa-Metrio, A.; Cordero-Oviedo, C.; López-Pérez, M.; Cárdenes-Sandí, G.M.; Romero, F.M. Effects of late Holocene climate variability and anthropogenic stressors on the vegetation of the Maya highlands. Quat. Sci. Rev. 2018, 189, 76–90. [Google Scholar] [CrossRef]
- Hansen, R.D.; Bozarth, S.; Jacob, J.; Wahl, D.; Schreiner, T. Climatic and environmental variability in the rise of Maya civilization: A preliminary perspective from northern Petén. Anc. Mesoam. 2002, 13, 273–295. [Google Scholar] [CrossRef]
- Leyden, B.W. Pollen evidence for climatic variability and cultural disturbance in the Maya Lowlands. Anc. Mesoam. 2002, 13, 85–101. [Google Scholar] [CrossRef]
- Wahl, D.; Byrne, R.; Schreiner, T.; Hansen, R. Holocene vegetation change in the northern Peten and its implications for Maya prehistory. Quat. Res. 2006, 65, 380–389. [Google Scholar] [CrossRef]
- McNeil, C.L.; Burney, D.A.; Burney, L.P. Evidence disputing deforestation as the cause for the collapse of the ancient Maya polity of Copan, Honduras. Proc. Natl. Acad. Sci. USA 2010, 107, 1017–1022. [Google Scholar] [CrossRef]
- Rushton, E.A.; Metcalfe, S.E.; Whitney, B.S. A late-Holocene vegetation history from the Maya lowlands, Lamanai, Northern Belize. Holocene 2013, 23, 485–493. [Google Scholar] [CrossRef]
- Ford, A.; Nigh, R. Origins of the Maya Forest Garden: Maya Resource Management. J. Ethnobiol. 2009, 29, 213–236. [Google Scholar] [CrossRef]
- Ford, A.; Nigh, R. The Maya Forest Garden: Eight Millennia of Sustainable Cultivation of the Tropical Woodlands; Routledge: Abingdon, UK, 2016. [Google Scholar]
- Ross, N.J. Modern tree species composition reflects ancient Maya “forest gardens” in northwest Belize. Ecol. Appl. 2011, 21, 75–84. [Google Scholar] [CrossRef]
- Graham, E. Collapse, conquest and Maya survival at Lamanai, Belize. Archaeol. Int. 2000, 4, 52–56. [Google Scholar] [CrossRef]
- Graham, E.A. Lamanai Reloaded: Alive and well in the early post-Classic. In Archaeological Investigations in the Eastern Maya Lowlands: Papers of the 2003 Belize Archaeology Symposium; Institute of Archaeology National Institute of Culture and History: Belmopan, Belize, 2004; Volume 1, p. 223. [Google Scholar]
- Rushton, E.A. “Under the Shade I Flourish”: An Environmental History of Northern Belize over the Last Three Thousand Five Hundred Years. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2014. [Google Scholar]
- Metcalfe, S.; Breen, A.; Murray, M.; Furley, P.; Fallick, A.; McKenzie, A. Environmental change in northern Belize since the latest Pleistocene. J. Quat. Sci. 2009, 24, 627–641. [Google Scholar] [CrossRef]
- Bridgewater, S. A Natural History of Belize: Insights from the Chiquibul Forest and Las Cuevas Research Station, 1st ed.; University of Texas Press: Austin, TX, USA, 2012. [Google Scholar]
- Setzerkorn, W.D. Formerly British Honduras: A Profile of The New Nation of Belize; Ohio University Press: Athens, OH, USA, 1981. [Google Scholar]
- Esselman, P.; Boles, E. Status and future needs of limnological research in Belize. Limnol. Dev. Ctries. 2001, 3, 35–68. [Google Scholar]
- Walker, S.H. Summary of Climatic Records for Belize; Land Resources Division, Overseas Development Administration: Surrey, UK, 1973. [Google Scholar]
- Meerman, J.; Sabido, W. Central American Ecosystems Map: Belize. In Ecosystem Map and Descriptions; The World Bank: Washington, DC, USA, 2001; Volume II. [Google Scholar]
- Beach, T.; Luzzadder-Beach, S.; Dunning, N.; Jones, J.; Lohse, J.; Guderjan, T.; Bozarth, S.; Millspaugh, S.; Bhattacharya, T. A review of human and natural changes in Maya Lowland wetlands over the Holocene. Quat. Sci. Rev. 2009, 28, 1710–1724. [Google Scholar] [CrossRef]
- Hansen, B.; Pohl, M.D. Pollen stratigraphy of Laguna de Cocos. In Ancient Maya Wetland Agriculture: Excavations on Albion Island, Northern Belize; Pohl, M., Ed.; Westview Press: Boulder, CO, USA, 1990; pp. 155–186. [Google Scholar]
- Jones, J.G. Pollen evidence for early settlement and agriculture in northern Belize. Palynology 1994, 18, 205–211. [Google Scholar] [CrossRef]
- Luzzadder-Beach, S.; Beach, T.P.; Dunning, N.P. Wetland fields as mirrors of drought and the Maya abandonment. Proc. Natl. Acad. Sci. USA 2012, 109, 3646–3651. [Google Scholar] [CrossRef]
- Morse, M.L. Pollen from Laguna Verde, Blue Creek, Belize: Implications for Paleoecology, Paleoethnobotany, Agriculture, and Human Settlement. Master’s Thesis, Texas A&M University, College Station, TX, USA, 2009. [Google Scholar]
- Pohl, M.D.; Pope, K.O.; Jones, J.G.; Jacob, J.S.; Piperno, D.R.; DeFrance, S.D.; Lentz, D.L.; Gifford, J.A.; Danforth, M.E.; Josserand, J.K. Early Agriculture in the Maya Lowlands. Lat. Am. Antiq. 1996, 7, 355–372. [Google Scholar] [CrossRef]
- Adams, R.E.; Robichaux, H.R.; Valdez, F., Jr.; Houk, B.A.; Mathews, R. Transformations, periodicity, and urban development in the Three Rivers region. In The Terminal Classic in the Maya Lowlands: Collapse, Transition, and Transformation; Demarest, A.A., Rice, P.M., Rice, D.S., Eds.; University Press of Colorado: Boulder, CO, USA, 2004; pp. 324–341. [Google Scholar]
- Anselmetti, F.S.; Hodell, D.A.; Ariztegui, D.; Brenner, M.; Rosenmeier, M.F. Quantification of soil erosion rates related to ancient Maya deforestation. Geology 2007, 35, 915–918. [Google Scholar] [CrossRef]
- Beach, T.; Luzzadder-Beach, S.; Dunning, N.; Cook, D. Human and natural impacts on fluvial and karst depressions of the Maya Lowlands. Geomorphology 2008, 101, 308–331. [Google Scholar] [CrossRef]
- Deevey, E.S.; Rice, D.S.; Rice, P.M.; Vaughan, H.H.; Brenner, M.; Flannery, M.S. Mayan Urbanism: Impact on a Tropical Karst Environment. Science 1979, 206, 298–306. [Google Scholar] [CrossRef]
- Rice, D.S. Paleolimnological analysis in the central Peten, Guatemala. In The Managed Mosaic: Ancient Maya Agricultural and Resource Use; Fedick, S.L., Ed.; University of Utah Press: Salt Lake City, UT, USA, 1996; pp. 193–206. [Google Scholar]
- Rosenmeier, M.F.; Hodell, D.A.; Brenner, M.; Curtis, J.H.; Guilderson, T.P. A 4000-Year Lacustrine Record of Environmental Change in the Southern Maya Lowlands, Petén, Guatemala. Quat. Res. 2002, 57, 183–190. [Google Scholar] [CrossRef]
- Berry, K.A.; McAnany, P.A. Reckoning with the wetlands and their role in ancient Maya society. In The Political Economy of Ancient Mesoamerica: Transformations during the Formative and Classic Periods; Scarborough, V.L., Clark, J.E., Eds.; University of New Mexico Press: Albuquerque, NM, USA, 2007; pp. 149–162. [Google Scholar]
- Pendergast, D.M. The church in the jungle: The ROM’s first season at Lamanai. Rotunda 1975, 8, 32–40. [Google Scholar]
- Pendergast, D.M. Lamanai, Belize: Summary of excavation results, 1974–1980. J. Field Archaeol. 1981, 8, 29–53. [Google Scholar]
- Pendergast, D.M. The 19th-century sugar mill at Indian Church, Belize. J. Field Archaeol. 1982, 8, 1–5. [Google Scholar]
- Pendergast, D.M. Ancient Maya mercury. Science 1982, 217, 533–535. [Google Scholar] [CrossRef]
- Pendergast, D.M. Intercessions with the Gods: Caches and their significance at Altun Ha and Lamanai, Belize. In The Sowing and the Dawning: Termination, Dedication, and Transformation in the Archaeological and Ethnographic Record of Mesoamerica; Boteler-Mock, S., Ed.; University of New Mexico Press: Albuquerque, NM, USA, 2002; pp. 55–63. [Google Scholar]
- Pendergast, D.M.; Sabloff, J.; Andrews, E.W. Stability through Change: Lamanai, Belize from the Ninth to the Seventeenth Century. In Late Lowland Maya Civilization: Classic to Postclassic; Sabloff, A., Andrews, E.W., Eds.; University of New Mexico Press: Albuquerque, NM, USA, 1986; pp. 223–250. [Google Scholar]
- Graham, E. Maya Christians and Their Churches in Sixteenth-Century Belize; University Press of Florida: Gainesville, FL, USA, 2011. [Google Scholar]
- Pendergast, D.M. The southern Maya lowlands contact experience: The view from Lamanai, Belize. In Columbian Consequences: The Spanish Borderlands in Pan-American Perspective; Thomas, D.E., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1991; pp. 336–354. [Google Scholar]
- Pendergast, D.M.; Jones, G.D.; Graham, E. Locating Maya lowlands Spanish colonial towns: A case study from Belize. Lat. Am. Antiq. 1993, 4, 59–73. [Google Scholar] [CrossRef]
- Scarborough, V.L.; Gallopin, G.G. A water storage adaptation in the Maya lowlands. Science 1991, 251, 658–662. [Google Scholar] [CrossRef]
- Jones, G.D. Maya Resistance to Spanish Rule: Time and History on a Colonial Frontier; University of New Mexico Press: Albuquerque, NM, USA, 1989. [Google Scholar]
- Mayfield, T. Ceramics, Landscape, and Colonialism: Archaeological Analysis of the British Settlement at Lamanai, Belize. Master’s Thesis, Illinois State University, Normal, IL, USA, 2009. [Google Scholar]
- Reimer, P.J.; William, E.N.; Austin, E.B.; Bayliss, A.; Blackwell, P.G.; Bronk Ramsey, C.; Butzin, M.; Cheng, H.; Edwards, R.L.; Friedrich, M.; et al. The IntCal20 Northern Hemisphere radiocarbon age calibration curve (0–55 cal kBP). Radiocarbon 2020, 62, 725–757. [Google Scholar] [CrossRef]
- Blaauw, M.; Christen, J. rbacon: Age-Depth Modelling Using Bayesian Statistics; R Package Version 2; R Package: R Development Core Team: Vienna, Austria, 2018. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Bennett, K.D.; Willis, K.J. Pollen. In Tracking Environmental Change Using Lake Sediments: Vol 3. Terrestrial, Algal, and Siliceous Indicators; Smol, J.P., Birks, J.B., Last, W.M., Eds.; Springer: Berlin, Germany, 2002; pp. 5–32. [Google Scholar]
- Faegri, K.; Iversen, J. Textbook of Pollen Analysis, 4th ed.; John Wiley: Chichester, UK, 1989. [Google Scholar]
- Whitney, B.S.; Rushton, E.A.; Carson, J.F.; Iriarte, J.; Mayle, F.E. An improved methodology for the recovery of Zea mays and other large crop pollen, with implications for environmental archaeology in the Neotropics. Holocene 2012, 22, 1087–1096. [Google Scholar] [CrossRef]
- Holst, I.; Moreno, J.E.; Piperno, D.R. Identification of teosinte, maize, and Tripsacum in Mesoamerica by using pollen, starch grains, and phytoliths. Proc. Natl. Acad. Sci. USA 2007, 104, 17608–17613. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Vigouroux, Y.; Goodman, M.M.; Sanchez, J.; Buckler, E.; Doebley, J. A single domestication for maize shown by multilocus microsatellite genotyping. Proc. Natl. Acad. Sci. USA 2002, 99, 6080–6084. [Google Scholar] [CrossRef] [PubMed]
- Van Heerwaarden, J.; Doebley, J.; Briggs, W.H.; Glaubitz, J.C.; Goodman, M.M.; Gonzalez, J.D.; Ross-Ibarra, J. Genetic signals of origin, spread, and introgression in a large sample of maize landraces. Proc. Natl. Acad. Sci. USA 2011, 108, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Juggins, S. Rioja: Analysis of Quaternary Science Data, R package version (0.9-21). 2017. Available online: http://cran.r-project.org/package=rioja (accessed on 12 October 2020).
- Bennett, K. Psimpoll and Pscomb. Software, Queen’s University Belfast. 2008. Available online: http://chrono.qub.ac.uk/psimpoll/psimpoll.html (accessed on 12 October 2020).
- Birks, H.J.B.; Line, J. The use of rarefaction analysis for estimating palynological richness from Quaternary pollen-analytical data. Holocene 1992, 2, 1–10. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Beach, T.; Wahl, D. An analysis of modern pollen rain from the Maya lowlands of northern Belize. Rev. Palaeobot. Palynol. 2011, 164, 109–120. [Google Scholar] [CrossRef]
- Sugita, S. Pollen Representation of Vegetation in Quaternary Sediments: Theory and Method in Patchy Vegetation. J. Ecol. 1994, 82, 881–897. [Google Scholar] [CrossRef]
- Jarosz, N.; Loubet, B.; Durand, B.; McCartney, A.; Foueillassar, X.; Huber, L. Field measurements of airborne concentration and deposition rate of maize pollen. Agric. For. Meteorol. 2003, 119, 37–51. [Google Scholar] [CrossRef]
- Raynor, G.S.; Ogden, E.C.; Hayes, J.V. Dispersion and Deposition of Corn Pollen from Experimental Sources 1. Agron. J. 1972, 64, 420–427. [Google Scholar] [CrossRef]
- Twiddle, C.L.; Bunting, M.J. Experimental investigations into the preservation of pollen grains: A pilot study of four pollen types. Rev. Palaeobot. Palynol. 2010, 162, 621–630. [Google Scholar] [CrossRef]
- Whitlock, C.; Larsen, C. Charcoal as a fire proxy. In Tracking Environmental Change Using Lake Sediments: Vol 3. Terrestrial, Algal, and Siliceous Indicators; Smol, J.P., Birks, J.B., Last, W.M., Eds.; Springer: Berlin, Germany, 2002; pp. 75–97. [Google Scholar]
- Krause, S.; Beach, T.; Luzzadder-Beach, S.; Guderjan, T.H.; Valdez, F.; Eshleman, S.; Doyle, C.; Bozarth, S.R. Ancient Maya wetland management in two watersheds in Belize: Soils, water, and paleoenvironmental change. Quat. Int. 2019, 502, 280–295. [Google Scholar] [CrossRef]
- La Torre, M.D.L.A.; Islebe, G.A. Others Traditional ecological knowledge and use of vegetation in southeastern Mexico: A case study from Solferino, Quintana Roo. Biodivers. Conserv. 2003, 12, 2455–2476. [Google Scholar]
- Schlesinger, V. Animals and Plants of the Ancient Maya: A Guide; University of Texas Press: Austin, TX, USA, 2001. [Google Scholar]
- Pendergast, D.M. Lamanai, Belize: An updated view. In Lowland Maya Postclassic; University of Texas Press: Austin, TX, USA, 1985; pp. 91–103. [Google Scholar]
- Rue, D.J. Early agriculture and early Postclassic Maya occupation in western Honduras. Nature 1987, 326, 285–286. [Google Scholar] [CrossRef]
- Lentz, D.L.; Graham, E.; Vinaja, X.; Slotten, V.; Jain, R. Agroforestry and ritual at the ancient Maya center of Lamanai. J. Archaeol. Sci: Rep. 2016, 8, 284–294. [Google Scholar] [CrossRef][Green Version]
- Morehart, C.T.; Lentz, D.L.; Prufer, K.M. Wood of the gods: The ritual use of pine (Pinus spp.) by the ancient lowland Maya. Lat. Am. Antiq. 2005, 16, 255–274. [Google Scholar] [CrossRef]
- Brady, J.E. An Investigation of Maya Ritual Cave Use with Special Reference to Naj Tunich, Guatemala. Ph.D. Thesis, Department of Anthropology, University of California, Los Angeles, CA, USA, 1989. [Google Scholar]
- Graham, E.; MeNatt, L.; Gutehen, M.A. Excavations in Footprint Cave, Caves Branch, Belize. J. Field Archaeol. 1980, 7, 153–172. [Google Scholar]
- Reents-Budet, D.; MacLeod, B. The Archaeology of Petroglyph Cave, Cayo District, Belize; Royal Ontario Museum: Toronto, ON, Canada, 1997. [Google Scholar]
- Stone, A.J. Images from the Underworld: Naj Tunich and the Tradition of Maya Cave Painting; University of Texas Press: Austin, TX, USA, 2010. [Google Scholar]
- Martin, S.; Grube, N. Chronicle of the Maya Kings and Queens: Deciphering the Dynasties of the Ancient Maya; Thames and Hudson: London, UK, 2008. [Google Scholar]
- Schele, L.; Miller, J.H. The Mirror, the Rabbit, and the Bundle: “Accession” Expressions from the Classic Maya Inscriptions. In Studies in Pre-Columbian Art and Archaeology No. 25; Dumbarton Oaks: Washington, DC, USA, 1983; pp. 1–99. [Google Scholar]
- Stuart, D. “The fire enters his house” Architecture and ritual in Classic Maya texts. In Function and Meaning in Classic Maya Architecture; Houston, S.D., Ed.; Dumbarton Oaks: Washington, DC, USA, 1998; pp. 373–425. [Google Scholar]
- Curtis, J.H.; Hodell, D.A.; Brenner, M. Climate variability on the Yucatan Peninsula (Mexico) during the past 3500 years, and implications for Maya cultural evolution. Quat. Res. 1996, 46, 37–47. [Google Scholar] [CrossRef]
- Hodell, D.A.; Brenner, M.; Curtis, J.H. Terminal Classic drought in the northern Maya lowlands inferred from multiple sediment cores in Lake Chichancanab (Mexico). Quat. Sci. Rev. 2005, 24, 1413–1427. [Google Scholar] [CrossRef]
- Hodell, D.A.; Brenner, M.; Curtis, J.H. Climate and cultural history of the northeastern Yucatan Peninsula, Quintana Roo, Mexico. Clim. Chang. 2007, 83, 215–240. [Google Scholar] [CrossRef]
- Neff, H.; Pearsall, D.M.; Jones, J.G.; de Pieters, B.A.; Freidel, D.E. Climate change and population history in the Pacific lowlands of southern Mesoamerica. Quat. Res. 2006, 65, 390–400. [Google Scholar] [CrossRef]
- Mayfield, T.; Graham, E.; Pendergast, D. Cane and Consumerism. In Technology and Tradition in Mesoamerica after the Spanish Invasion: Archaeological Perspectives; Alexander, R.T., Ed.; University of New Mexico Press: Albuquerque, NM, USA, 2019; pp. 147–165. [Google Scholar]
- Morris, D. The Colony of British Honduras: Its Resources and Prospects; with Particular Reference to Its Indigenous Plants and Economic Productions; E. Stanford: London, UK, 1883. [Google Scholar]
- Chazdon, R.L.; Peres, C.A.; Dent, D.; Sheil, D.; Lugo, A.E.; Lamb, D.; Stork, N.E.; Miller, S.E. The potential for species conservation in tropical secondary forests. Conserv. Biol. 2009, 23, 1406–1417. [Google Scholar] [CrossRef]
- Akers, P.D.; Brook, G.A.; Railsback, L.B.; Cherkinksy, A.; Liang, F.; Ebert, C.E.; Hoggarth, J.A.; Awe, J.J.; Cheng, H.; Edwards, R.L. Integrating U-Th, 14C, and 210Pb methods to produce a chronologically reliable isotope record for the Belize River Valley Maya from a low-uranium stalagmite. Holocene 2019, 29, 1234–1248. [Google Scholar] [CrossRef]
- Ford, A. Dominant plants of the Maya forest and gardens of El Pilar: Implications for paleoenvironmental reconstructions. J. Ethnobiol. 2008, 28, 179–199. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).