Abstract
Background: Cannabis is the most commonly used illicit drug worldwide. An increasing number of jurisdictions are legalising cannabis for both medicinal and recreational use. The changing cannabis market has resulted in both an increase in the number of people consuming these compounds, and an increase in the frequency and quantity of cannabis being used. Endogenous and exogenous cannabinoids act on receptors across the entire body including the genitourinary system; however, there is a paucity of understanding of how cannabinoids affect genitourinary malignancy. Objective: To present a narrative review of the available literature detailing the relationship between cannabis and the incidence, diagnosis, and management of genitourinary malignancy. Methods: A comprehensive search was undertaken using the Ovid MEDLINE, Ovid Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) up to July 2021. Studies included case reports, case series, case-control studies, and in vitro studies. Results: The search identified 40 studies in total: 8 described the relationship between cannabis and testicular carcinoma, 20 related to prostate cancer, 5 to bladder cancer, 5 to renal cancer, 1 to penile cancer, and 1 study examined testicular carcinoma, renal cell carcinoma, bladder cancer, and prostate cancer. Conclusions: Cannabis use has been linked to an increased risk of developing testicular tumours, whilst the evidence for bladder cancer is mixed. There is no apparent increase in risk for prostate cancer, penile cancer, or renal cell carcinoma; however, this evidence was based on a very small number of patients. There remains a lack of understanding of the relationship between cannabis and genitourinary malignancy. With an expected increase in cannabis use, monitoring for testicular tumour plus efforts to further understand its effects upon the genitourinary tract will aid diagnosis and management.
1. Introduction
Cannabis has been used by human populations for thousands of years for multiple reasons, but more recently for its perceived medicinal benefits. The United Nations World Drug Report 2021 estimates that cannabis is used by 4% of the global population between the ages of 15 and 64 years[1]. Cannabis is permitted for medicinal and/or recreational use in many North American jurisdictions, and its increasing acceptance has resulted in an increase in the frequency and number of people seeking to use it[1].
The active compounds in cannabis are referred to as cannabinoids and the primary active molecule is Δ9-tetrahydrocannabinol (THC)[2]. Both exogenous and endogenous cannabinoids exert their effects in humans via 2 main receptors: cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2)[2]. CB1 and CB2 are part of the G-protein-coupled receptor superfamily, which affects downstream signalling pathways. Receptors are located all around the body, including within the male urogenital system. The use of cannabis has been advocated for the treatment of chronic pain, mental health disorders, chemotherapy-induced nausea, and cancer-related pain[3]. There have been many papers looking at quality of life effects of cannabis, but few measuring effects on disease process.
Genitourinary malignancies are a major global health challenge, and there has been an increase in their incidence over the past 30 years[4]. Whilst prostate and bladder cancer generally affect older men, renal and testicular cancer typically affect younger men. Each of these entities represent a challenge to public health globally, and multimodal strategies to minimise their risk and improve their management are required to ease their burden on health systems and societies[4].
Given the increasing use of cannabis for both medical and recreational purposes, greater understanding is needed of the potential effect of cannabis on urogenital malignancies. The aim of this review is to establish the current evidence for cannabinoids as a risk for developing genitourinary malignancy, and to assess their use as an antineoplastic agent in urogenital malignancy.
2. Methods
We intended to complete a systematic review of the effect of cannabis on the incidence of genitourinary malignancy and its use as an antineoplastic agent, with a meta-analysis if appropriate. However, there were insufficient results to permit a quantitative analysis of the outcomes. Therefore, we completed a qualitative narrative review.
2.1. Search strategy
A comprehensive search strategy was undertaken using the Ovid MEDLINE, Ovid Embase, Cochrane Central Register of Controlled Trials (CENTRAL) from inception to present, Google Scholar (first 200 citations relevancy ranked); clinical trial registries; references of included studies up to July 1, 2020. The search was restricted to articles written in English. The following words and MeSH terms were used: “cannabinoid,” “cannabidiol,” “cannabinol,” “dronabinol,” “cannabis,” “medical cannabis,” “(cannabi* or THC or tetrahydro cannabinol or dronabinol or hemp or bhang or marijuana or marihuana or hashish or hash or skunk or marinol or Nabilone or cesamet or sativex or sinsemilla),” which were then crossed with any of the following terms “exp urogenital tract cancer,” “exp kidney cancer,” “([prostate or penis or penile or testicular or germ cell or urothelial or testis or ureteric or ureter or urinary or renal cell or kidney or bladder or genital tract] and [cancer or carcinoma or tumour or tumor or metastasis]).” Studies included observational studies, such as case reports, case series, case-control studies and in vitro studies, and interventional studies such as randomised controlled trials and clinical trials. Search terms were then used in other databases using MEDLINE parameters. We used the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement to report results. The review was prospectively registered on PROSPERO 2020 CRD42020195998.
2.2. Data extraction and screening
Sources were independently obtained and reviewed by 2 individual authors to determine eligibility based upon relevance. Publications’ titles and abstracts were first reviewed for relevance to the topic. Appropriate publications then underwent a full-text review. Articles that passed this screening process were then included in the manuscript based on the full text. Any disagreement was resolved by the corresponding author. The systematic review results are depicted in Figure 1.
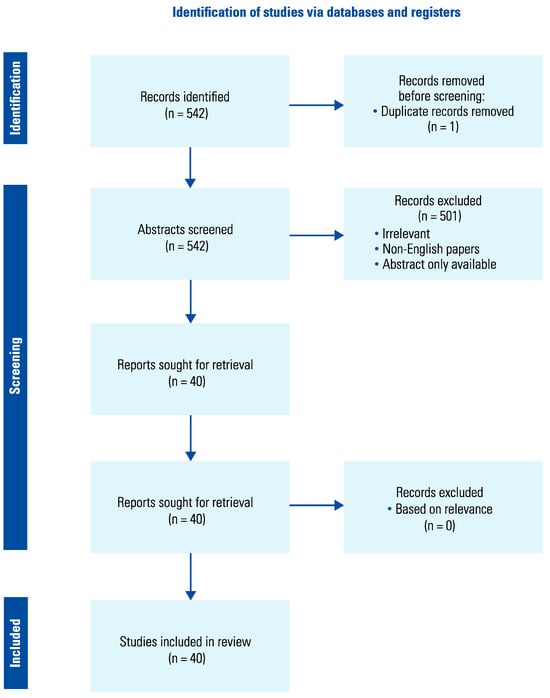
Figure 1.
Data extraction method.
3. Results
The search identified 40 studies in total. Eight described the relationship between cannabis and testicular carcinoma, 5 related to renal cancer, 5 related to bladder cancer, one related to penile cancer, and 20 related to prostate cancer. One study examined testicular carcinoma, renal cell carcinoma, bladder cancer, and prostate cancer. Studies could be broadly categorised into 2 groups: those examining the effect the consumption of cannabis has on the incidence of urogenital cancers, and those examining the effect of cannabis or cannabinoids on the diagnosis, treatment or follow-up of urogenital cancer.
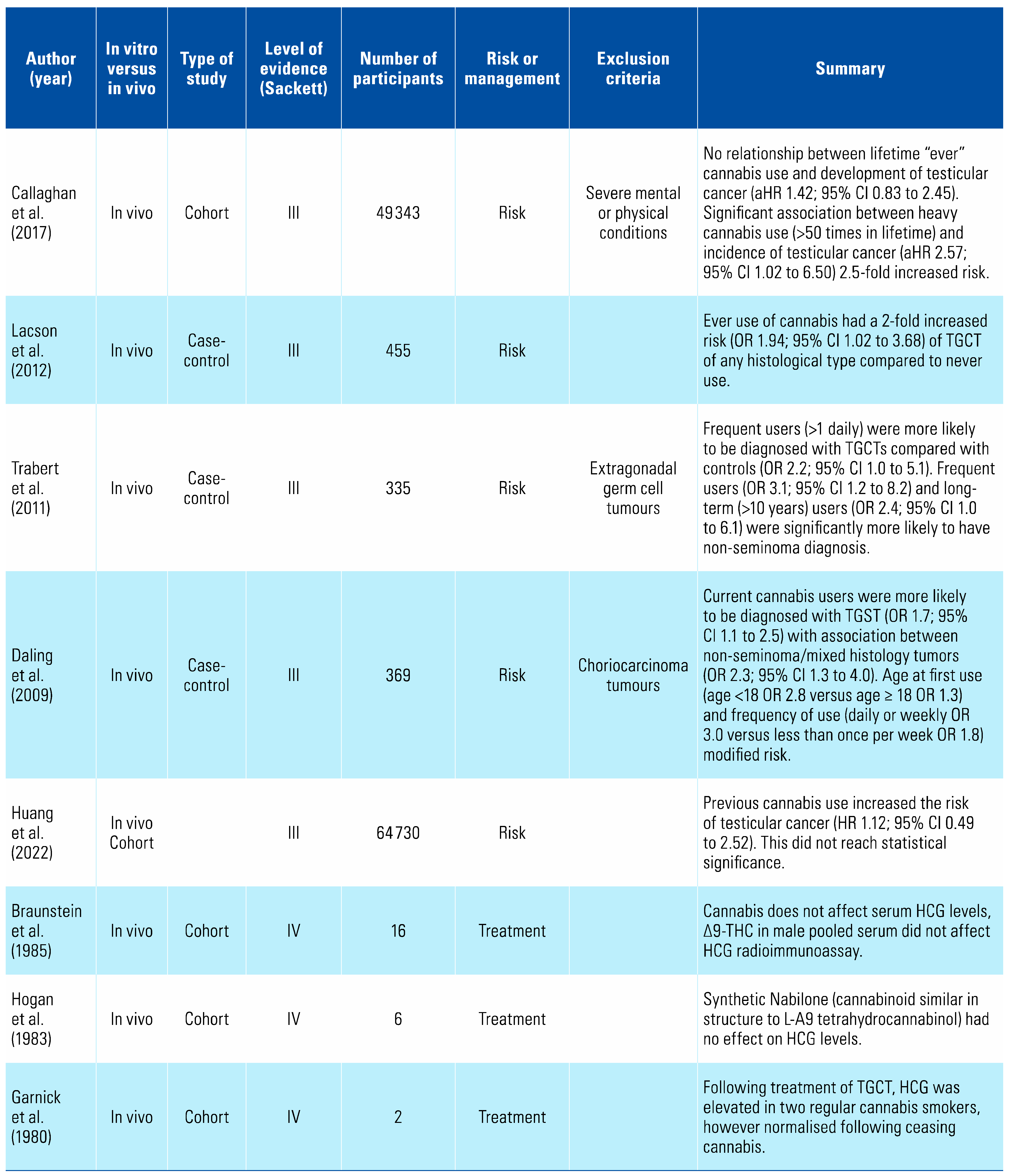
Cannabis consumption is an independent risk factor for the development of non-seminomatous germ cell testicular tumours[5,6,7,8] (Table 1). There is no compelling evidence that it affects β-HCG levels, although this has earlier been suggested in case reports, and therefore its use should not affect protocols for the post-treatment follow-up of these tumours[9,10].

Table 1.
Summary of studies on the relationship between cannabis and testicular cancers.
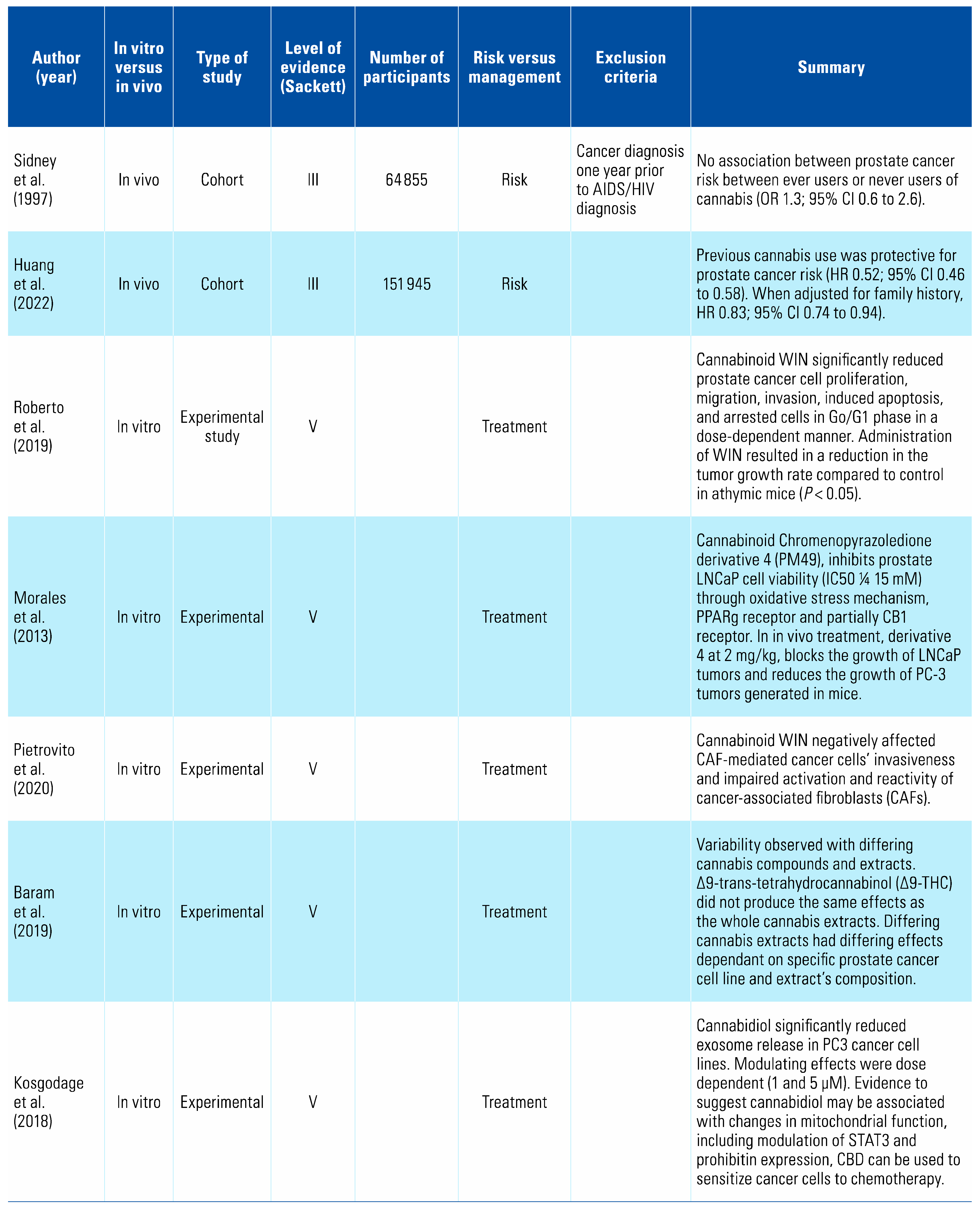
One study examined the effect of cannabis consumption on prostate cancer and found there was no increased risk with its use[11], whilst a second found a decreased risk[8] (Table 2). Nineteen studies examined the effect of cannabinoids on prostate cancer cells and demonstrated pro-apoptotic properties, identifying a potential novel treatment pathway[12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30].

Table 2.
Summary of studies on the relationship between cannabis and prostate cancers.
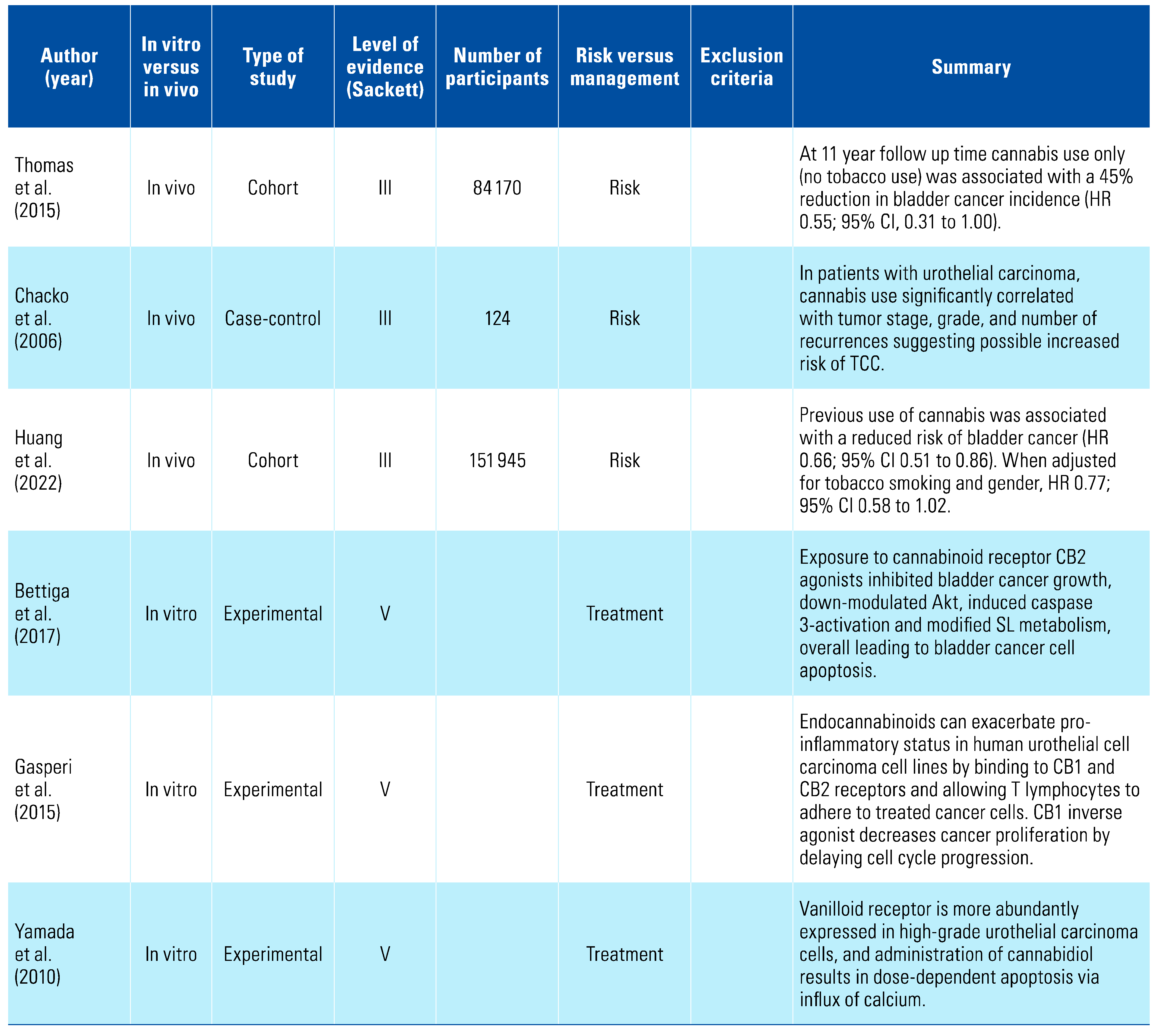
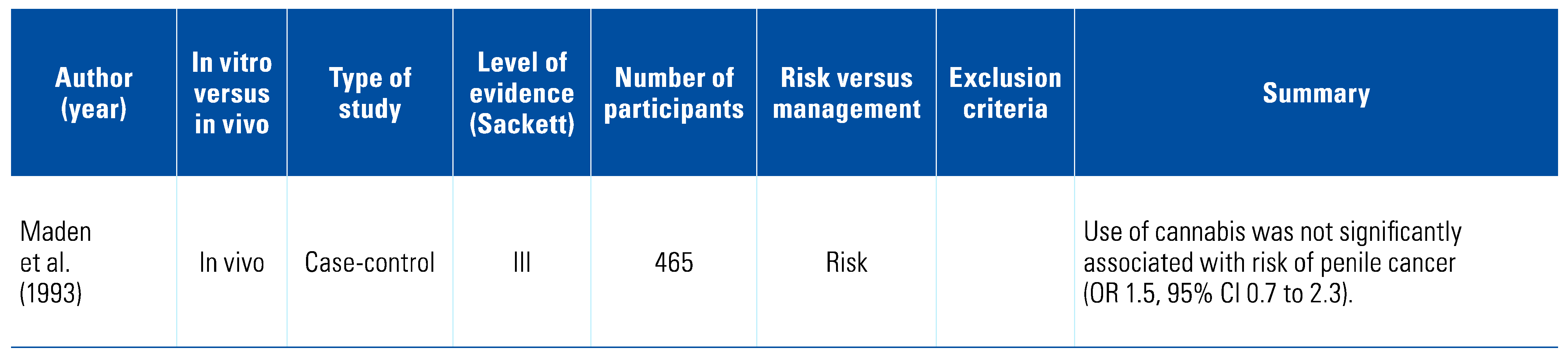
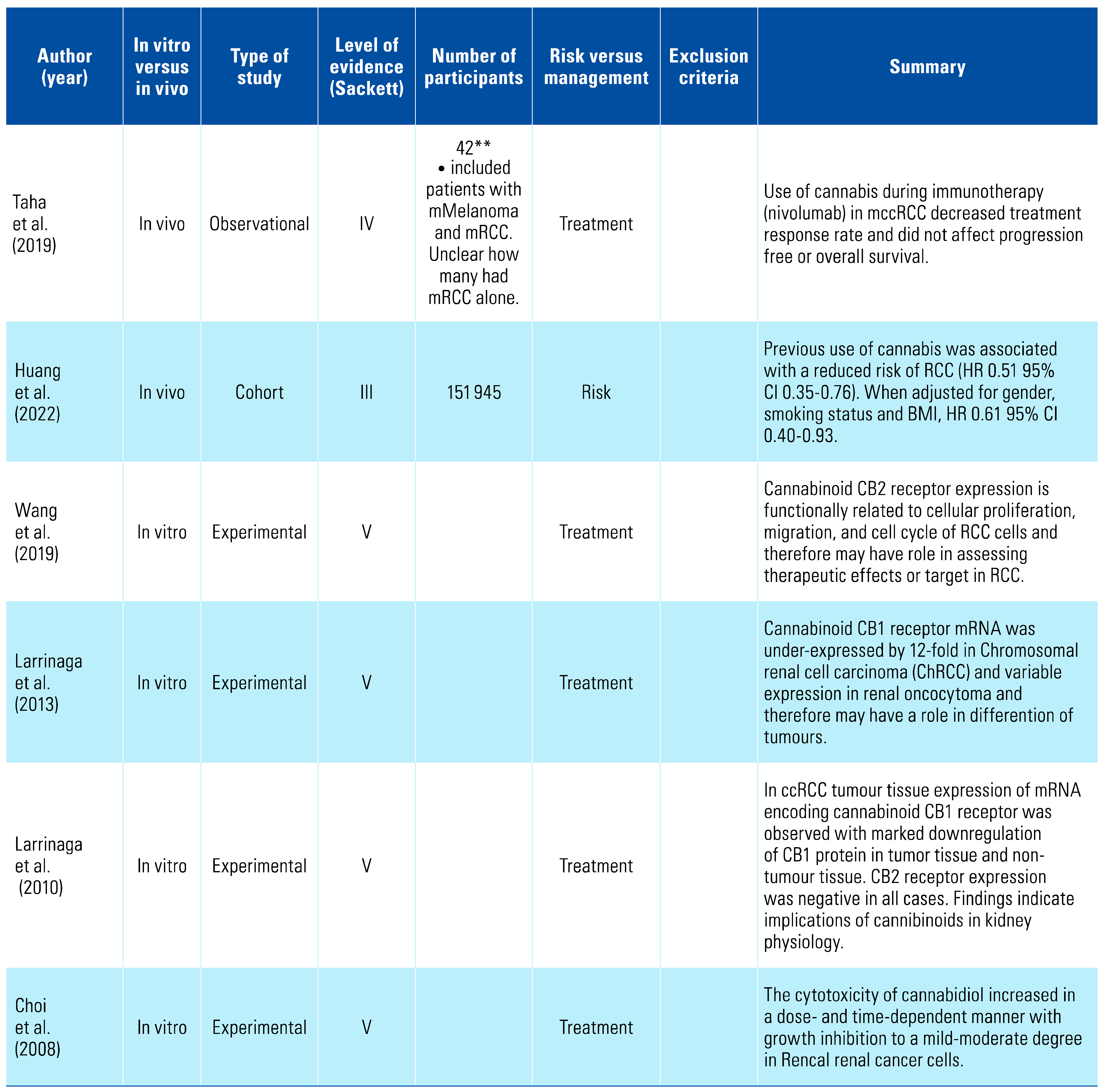
Three studies indicated that cannabis appears to be protective against bladder cancer, and this was further strengthened by the results of 3 in vitro studies showing that cannabis had pro-apoptotic effects on bladder cancer cell lines[8,31,32,33,34,35] (Table 3). There has been no relationship suggested between cannabis consumption and penile cancer[36] (Table 4). One study has examined the relationship between renal cell carcinoma (RCC) and cannabis consumption and found a decreased risk[37]; however, in vitro studies demonstrated marked downregulation of CB1 compared with normal renal tissue, potentially defining a new diagnostic marker[38,39,40,41,42] (Table 5).

Table 3.
Summary of studies on the relationship between cannabis and bladder cancer.

Table 4.
Summary of studies on the relati on ship between cannabis and penile cancer.

Table 5.
Summary of studies on the relationship between cannabis and renal cancer.
4. Discussion
4.1. Testicular Cancer
Testicular cancer is the most common cancer in young men, and for reasons not yet understood, the incidence is increasing in the Western world[43]. A 2009 population-based case-control study of 369 patients aged 18 to 44 found that men with testicular germ cell tumours (TGCT) were almost twice as likely to be current cannabis smokers (OR 1.7; 95% CI 1.1 to 2.5)[5]. When further stratified by histological type, there was a stronger association between non-seminomatous or mixed tumours (OR 2.3; 95% CI 1.4 to 4.0). Younger age at first use and frequency of use appear to increase risk (age < 18 years [OR 2.8] versus age ≥ 18 years [OR 1.3]) as does daily or weekly use (OR 3.0) versus less than once per week use (OR 1.8)[5].
Trabert et al. compared males diagnosed with TGCT and male friend controls, finding that patients with TGCT were more likely to be frequent cannabis users compared with controls (OR 2.2; 95% CI 1.0 to 5.1). Additionally, they found that patients with non-seminoma were significantly more likely than controls to be frequent and long-term users (OR 3.1; 95%CI 1.2 to 8.2 and PR 2.4 and 95% CI 1.0 to 6.1)[7].
A further population-based case-control study from 2012 including 163 patients with 269 age and ethnicity-matched controls demonstrated that compared with no THC use, previous THC use increased the risk of TGCT (OR 1.94; 95% CI 1.02 to 3.68). When stratified by histological sub-type, there was a specific association between non-seminoma and mixed histology tumours (OR 2.42; 95% CI 1.08 to 2.42)[6].
A meta-analysis of the above studies found that for current, chronic, and frequent users, there is an association with the development of TGCT, compared with those who have never used[44]. Previous use of cannabis increased the odds of TGCT development by 62% (OR 1.62; 95% CI 1.13 to 2.31) and a use frequency of weekly or more appeared to double the odds of TGCT development (OR 1.92; 95% CI 1.35 to 2.72). Duration of cannabis use (> 10 years versus never used) increased likelihood of TGCT development (OR 1.5; 95% CI 1.08 to 2.09)[44].
A cohort study by Huang et al. has examined United Kingdom biobank specimens of individuals with information on cannabis use[8]. After examining 64 730 samples, it demonstrated a minimally increased risk of developing testicular carcinoma (HR 1.12; 95% CI 0.49 to 2.52), this was not statistically significant (P = 0.793).
A cohort study of 49 343 Swedish males born between 1949 and 1951 found that there was no evidence of a significant relationship between previous cannabis use and the subsequent development of testicular cancer (119 testicular cancer cases, adjusted HR 1.42; 95% CI 0.83 to 2.45)[45]. However, heavy cannabis use (> 50 times in a lifetime) was associated with an increased incidence of testicular cancer (HR 2.57; 95% CI 1.08 to 5.42)[45].
In a 2-patient case series by Garnick et al. elevated serum β-hCG levels returned to normal following cessation of cannabis use[10]. As β-hCG is a tumour marker for some testicular cancers, its implications in follow-up were believed to be significant. Two subsequent small series examining a combined 22 patients determined that THC did not affect β-hCG levels[10,46]. Further investigation is warranted in this younger population where reported cannabis use rates are increasing.
Cannabis use appears to be an important risk factor for TGCT. Whilst research to date demonstrates an apparent effect on incidence, more should be done to understand the mechanism of action and to potentially generate novel targets for treatment.
4.2. Penile Cancer
Penile cancer accounts for < 1% of cancers in men annually[43]. A 2003 single case-control study by Maden et al. found no relationship between cannabis use and penile cancer[36]. At the time of this review there were no publications examining cannabis as a treatment arm for penile cancers. This highlights the need for further research and understanding in this area.
4.3. Prostate Cancer
Prostate cancer is the second most commonly diagnosed cancer in men worldwide, accounting for approximately 15% of male cancer diagnoses each year[43]. Only one observational study has examined the relationship between cannabis use and prostate cancer incidence. This retrospective study examined several different malignancies, and found that when adjusted for confounding factors, previous use of cannabis did not confer a higher risk of prostate cancer in comparison with non-use (RR 1.3, 95% CI 0.6 to 2.6)[11]. Huang et al. demonstrated a reduction in incidence with previous use of cannabis (HR 0.52; 95% CI 0.46 to 0.58), which was statistically significant (P < 0.001). When adjusted for family history, HR 0.83; 95% CI 0.74 to 0.94)[8].
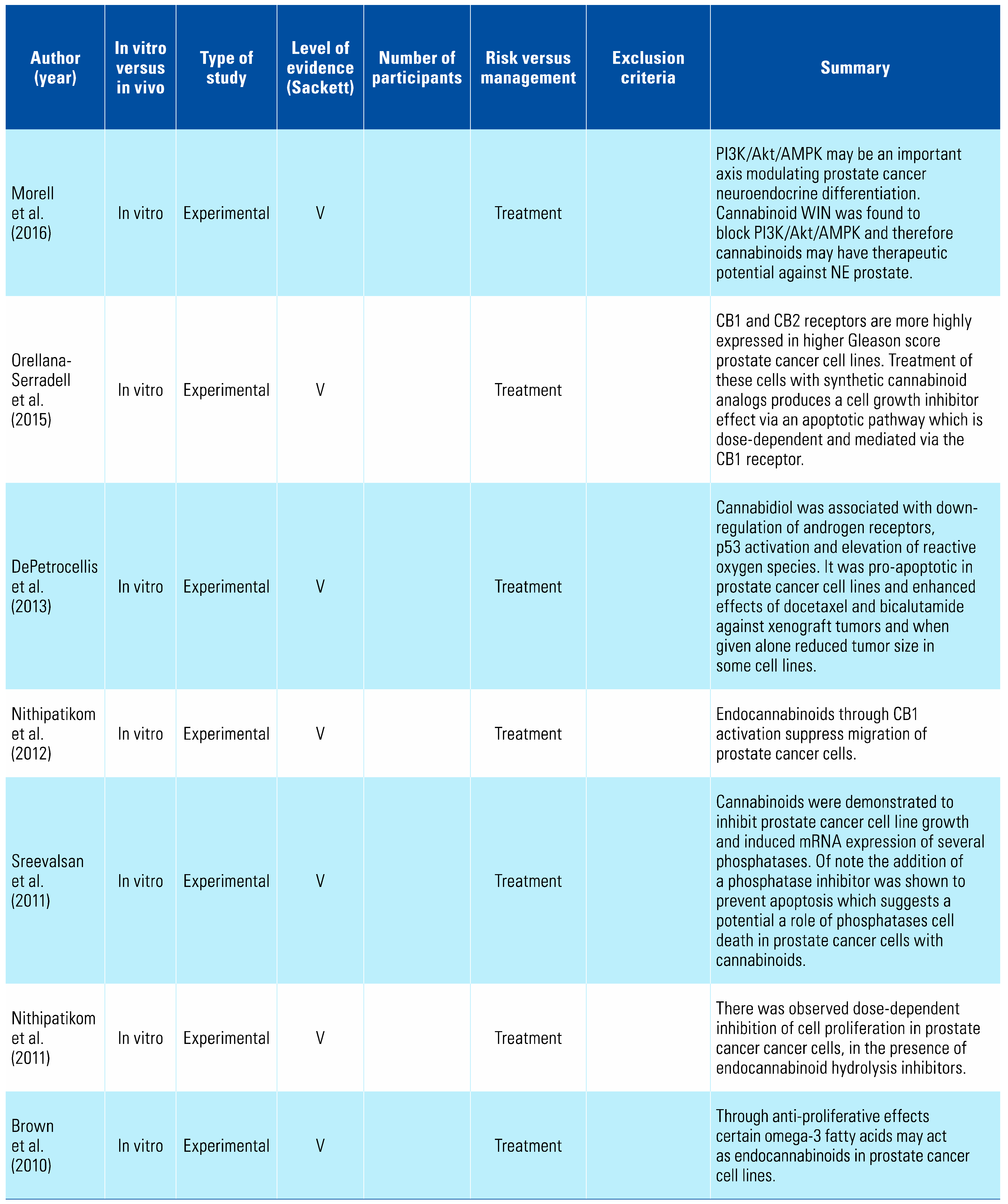
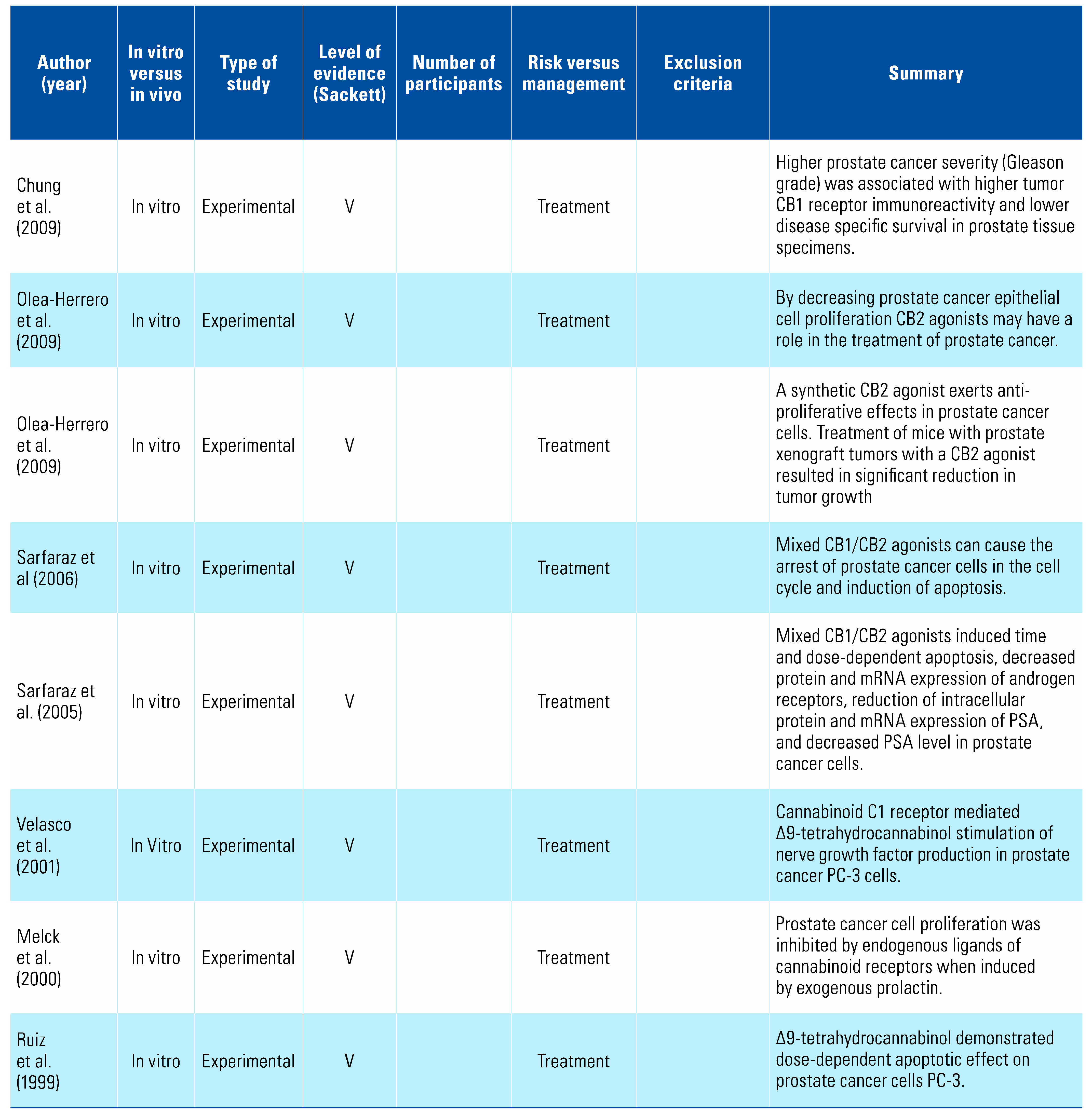
A number of studies have examined cannabis and its derivatives, as well as cannabinoid receptors, as potential treatment targets for prostate cancer. Synthetic cannabinoid WIN 55-212,2 (WIN) has been shown to reduce prostate cancer cell proliferation, migration, and invasion, and to induce apoptosis and arrest cells in Go/G1 phase in a dose-dependent manner by acting as an agonist on receptors CB1 and CB2[15,16,17,18,19,20,21,22,27,29,30]. Mechanistic studies revealed these effects were mediated through a pathway involving cell cycle regulators p27, Cdk4, and pRb[12,13,25,26,28]. However, it should be noted that in vitro studies demonstrated significant variability between differing cannabis compounds and extracts, with effects dependent on specific prostate cancer cell lines in addition to the extract’s composition[14,24].
There is currently a paucity of high-level evidence for the risks of cannabis use and potential for developing prostate cancer, although promising models for cannabinoids as a targeted treatment for prostate cancer do exist.
4.4. Bladder Cancer
Urothelial carcinoma (UC) of the bladder is the tenth most commonly diagnosed cancer worldwide and the seventh most common in men[43]. A case-control study by Chacko et al. (2006) of Vietnam-era veterans found that 88.5% of participants with UC reported habitual use of cannabis, compared with 69.2% of age-matched controls (P = 0.008)[32]. Importantly, however, this study’s small sample size (n = 124) could not be adjusted to remove tobacco use — the most common risk factor for UC — as a confounder. The authors postulated that THC had a carcinogenic effect due to metabolites remaining in the urine for up to 60 hours post consumption, as opposed to 12 hours for nicotine metabolites.
In a separate cohort study of 47 092 men examining those who used cannabis (41%), tobacco (57%), both (27%), or neither (29%), only tobacco use was associated with an increased risk of bladder cancer (HR 1.52; 95% CI 1.12 to 2.07)[31]. Cannabis use was in fact associated with a 45% reduction in bladder cancer incidence (HR 0.55; 95% CI 0.21 to 1.00)[31].
In the study of United Kingdom biobank specimens, bladder cancer risk was again reduced in the setting of previous cannabis use (HR 0.66;95% CI 0.51 to 0.86)[8]. When adjusted for tobacco smoking and sex, HR 0.77; 95% CI 0.58 to 1.02. However, current use of cannabis seemed to be associated with an increased risk. This was difficult to interpret, as the investigators did not have access to the timing of use, and therefore may have been confounded by cannabis use in the setting of managing the symptoms of malignancy.
A number of in vitro studies also have shown cannabinoids to be protective against UC. Yamada et al. showed that villinoid receptors are expressed more in high grade UC. They also demonstrated a dose-dependent relationship between cannabis administration and apoptosis mediated by calcium influx, via villinoid receptors[35]. This was supported by another study examining the activation of CB2 in primary UC of the bladder, where it was found to lead to cell death[33]. These principles were further expanded upon by Gasperi et al. (2015), who demonstrated that endocannabinoids create a pro-inflammatory state in human UC by binding to CB1 and CB2 receptors therefore decreasing cancer proliferation by delaying cell cycle progression[34].
There appears to be an apparent protective effect of THC against the development of bladder cancer. In view of the high proportion of THC users who concurrently use tobacco, care must be taken, however, in interpretation of any studies that do not stratify for these 2 variables.
4.5. Renal Cancer
Renal cell carcinoma accounts for 3% of cancer diagnoses each year, and there has been an increase of 2% in cases each year over the past 20 years; however, mortality rates have remained stable or are decreasing[43].
The examination of biobank specimens by Huang et al. was the first study to look at the association between cannabis and RCC[8]. They found that previous cannabis use has a significant inverse association with developing RCC (HR 0.52; 95% CI 0.35 to 0.76, P < 0.001).
Taha et al. (2019) performed an observational study of 42 patients with either metastatic RCC, non-small cell lung cancer or metastatic melanoma who were undergoing immunotherapy and found that in these patients, the use of cannabis reduced the response rate to treatment. However, cannabis did not affect the progression-free survival or overall survival[38]. It should be noted that the data from this study did not stratify patients based on cancer type and involved only small numbers.
Several in vitro studies have been performed to examine the effect of cannabis and its derivatives on receptors of RCC cell lines. Choi et al. in 2008 reported that RCC cells exhibited mild-to-moderate dose and time-dependent growth inhibition in response to cannabidiol[42]. Another study found that CB2 expression on clear cell RCC cells was functionally related to cellular proliferation, migration, and the cell cycle. Expression of CB2 was up-regulated in RCC compared with normal surrounding tissue, and was an independent prognostic factor for patients, and therefore represents a potential therapeutic target[39].
Conversely, CB1 was under-expressed in RCC tissue as compared with surrounding normal tissue. Given the difficulty in establishing a clear differential diagnosis of RCC in clinical practice, expression of CB1 and CB2 represent a potential diagnostic tool for RCC[40].
Ultimately, there is a lack of understanding of the effect of cannabis use on the incidence of RCC, however CB1 and CB2 present potential targets for diagnostic and therapeutic purposes.
4.6. Confounding effect of tobacco
Tobacco smoke is a known significant carcinogenic substance for the development of many cancers[47]. Cannabis and tobacco use commonly occur together. It was estimated that 57.9% of cigarette smokers reported a lifetime history of cannabis use, but 90% of cannabis users reported a history of smoking cigarettes[48]. Adjustment for tobacco cigarette smoking was reported in all but one of the studies that examined the incidence of genitourinary malignancy. Given their likely association, adjusting for tobacco cigarette smoking is both difficult and important, and should be considered as a key endeavour to ensure study validity in future research.
4.7. Effect of THC delivery
The delivery of THC is changing. Whilst previously cannabis was almost exclusively smoked, novel delivery methods are being explored by consumers, including vaping and oral intake, including capsules and liquid mucosal sprays, as well as being combined in food or liquid forms[49]. Alternative delivery methods can alter both the amount of THC being delivered to a consumer and the potential contaminates that reach the body. Although vaping is thought to avoid toxic components of smoking such as tar, there are still contaminates, including pesticides, heavy metals, and ammonia potentially acting as carcinogens. As the cannabis industry continues to grow, it will be critical for manufacturers to work towards minimising contaminants of the cultivation process, as well as to standardise the quality of products being delivered to consumers[49].
4.8. Limitations
This review highlighted several limitations in the available literature. Studies that have examined the risk of genitourinary malignancy and cannabis use are observational in nature, with no high-level evidence available. Also, there is a large variation in the number of patients included, with many studies having very few patients, making it difficult to generalise results to the population. Additionally, the studies that examine the use of cannabis in the diagnosis, treatment, and follow-up of genitourinary malignancy are still in the experimental phase, limiting their ability to be translated into practical use at this point.
This review has several limitations. The search was conducted of the data bases Ovid MEDLINE, Ovid Embase, Cochrane Central Register of Controlled Trials (CENTRAL) from inception to present, Google Scholar (first 200 citations relevancy ranked), and clinical trial registries. Although it is unlikely, it is possible that other articles may have been identified if other databases had been searched. Additionally, only articles published in English were included, meaning that some relevant studies may have been excluded. Finally, given that no further papers were excluded following full text review, it remains possible that the abstract screening was too narrow and therefore some relevant papers may not have been included.
4.9. Future directions
This review has highlighted the lack of evidence about the effect of cannabis on the risk and management of genitourinary malignancy. Further studies that explore the effect of cannabis, including in different forms such as oils and edibles, should be designed to better understand this area. It may help clinicians to stratify patients’ risk of having malignancy given their history of cannabis use. Additionally, it is important for the policymakers to understand the unintended consequences of the ingestion of these substances, as there remains a lack of evidence surrounding the harm of using cannabis.
Likewise, further understanding of the effects of cannabis on the diagnosis, management, and follow-up of genitourinary malignancy will be important for translation of this research into in vivo models and the development of practical uses for this information.
5. Conclusion
With the increasing legalisation of cannabis for both recreational and medical uses, it is imperative to understand its effect upon the incidence of and potential use in the management of genitourinary malignancies. Cannabis has been associated with both increased and decreased risk of different genitourinary malignancies. The use of cannabis and cannabinoids for the investigation, treatment, and follow-up of these malignancies is not fully understood. This review demonstrates the incomplete nature of evidence that exists in this area. Further research is required to be able to understand and potentially harness the use of cannabis.
Conflicts of Interest
None declared.
Abbreviations
| CB | cannabinoid receptor |
| hCG | human chorionic gonadotropin |
| RCC | renal cell carcinoma |
| TGCT | testicular germ cell tumours |
| THC | tetrahydrocannabinol |
| UC | urothelial carcinoma |
References
- United Nation Office on Drugs and Crime. World Drug Report 2021: Cannabis and opioids [Internet]. 2021. Available online: https://www.unodc.org/res/wdr2021/field/WDR21_Booklet_4.pdf https://www.unodc.org/res/wdr2021/field/WDR21_Booklet_3.pdf (accessed on 09 December 2022).
- Rossato, M.; Pagano, C.; Vettor, R. The cannabinoid system and male reproductive functions. J Neuroendocrinol. 2008, 20 (Suppl. 1), 90–93. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. National Drug Strategy Household Survey 2019. Canberra; 2020.
- Zi, H.; He, S.H.; Leng, X.Y.; Xu, X.F.; Huang, Q.; Weng, H.; et al. Global, regional, and national burden of kidney, bladder, and prostate cancers and their attributable risk factors, 1990–2019. Mil Med Res. 2021, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Daling, J.R.; Doody, D.R.; Sun, X.; Trabert, B.L.; Weiss, N.S.; Chen, C.; et al. Association of marijuana use and the incidence of testicular germ cell tumors. Cancer. 2009, 115, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Lacson, J.C.A.; Carroll, J.D.; Tuazon, E.; Castelao, E.J.; Bernstein, L.; Cortessis, V.K. Population-based case-control study of recreational drug use and testis cancer risk confirms an association between marijuana use and nonseminoma risk. Cancer. 2012, 118, 5374–5383. [Google Scholar] [CrossRef] [PubMed]
- Trabert, B.; Sigurdson, A.J.; Sweeney, A.M.; Strom, S.S.; Mcglynn, K.A. Marijuana use and testicular germ cell tumors. Cancer. 2011, 117, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Huang, D.; Ruan, X.; Huang, J.; Xu, D.; Heavey, S.; et al. Association between cannabis use with urological cancers: a population-based cohort study and a mendelian randomization study in the UK biobank. Cancer Med. 2022. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Garnick, M.B. Spurious rise in human chorionic gonadotropin induced by marihuana in patients with testicular cancer. N Engl J Med. 1980, 303, 1177. [Google Scholar]
- Braustein, G.; Thompson, R.; Gross, S.; Soares, J. Marijuana use does not spuriously elevate serum human chorionic gonadotropin levels. Urology. 1985, 25. [Google Scholar]
- Sidney, S.; Quesenberry, C.P.; Friedman, G.D.; Tekawa, I.S. Marijuana use and cancer incidence (California, United States). Cancer Causes Control. 1997, 8, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Roberto, D.; Klotz, L.H.; Venkateswaran, V. Cannabinoid WIN 55,212-2 induces cell cycle arrest and apoptosis, and inhibits proliferation, migration, invasion, and tumor growth in prostate cancer in a cannabinoid-receptor 2 dependent manner. Prostate. 2019, 79, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Vara, D.; Goméz-Cañas, M.; Zúñiga, M.C.; Olea-Azar, C.; Goya, P.; et al. Synthetic cannabinoid quinones: Preparation, in vitro antiproliferative effects and in vivo prostate antitumor activity. Eur J Med Chem. 2013, 70, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.; Cascio, M.G.; Wahle, K.W.J.; Smoum, R.; Mechoulam, R.; Ross, R.A.; et al. Cannabinoid receptor-dependent and -independent anti-proliferative effects of omega-3 ethanolamides in androgen receptor-positive and -negative prostate cancer cell lines. Carcinogenesis 2010, 31, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.C.; Hammarsten, P.; Josefsson, A.; Stattin, P.; Granfors, T.; Egevad, L.; et al. A high cannabinoid CB1 receptor immunoreactivity is associated with disease severity and outcome in prostate cancer. Eur J Cancer. 2009, 45, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Olea-Herrero, N.; Vara, D.; Malagarie-Cazenave, S.; Díaz-Laviada, I. Inhibition of human tumour prostate PC-3 cell growth by cannabinoids R()-Methanandamide and JWH-015: Involvement of CB 2. Br J Cancer. 2009, 101, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Olea-Herrero, N.; Vara, D.; Malagarie-Cazenave, S.; Díaz-Laviada, I. The cannabinoid R()methanandamide induces IL-6 secretion by prostate cancer PC3 cells. J Immunotoxicol. 2009, 6, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Sarfaraz, S.; Afaq, F.; Adhami, V.M.; Mukhtar, H. Cannabinoid receptor as a novel target for the treatment of prostate cancer. Cancer Res. 2005, 65, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Sarfaraz, S.; Afaq, F.; Adhami, V.M.; Malik, A.; Mukhtar, H. Cannabinoid receptor agonist-induced apoptosis of human prostate cancer cells LNCaP proceeds through sustained activation of ERK1/2 leading to G 1 cell cycle arrest. J Biol Chem. 2006, 281, 39480–36491. [Google Scholar] [CrossRef] [PubMed]
- Velasco, L.; Ruiz, L.; Sánchez, M.G.; Díaz-Laviada, I. Δ9-tetrahydrocannabinol increases nerve growth factor production by prostate PC-3 cells: Involvement of CB1 cannabinoid receptor and Raf-1. Eur J Biochem. 2001, 268, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Melck, D.; De Petrocellis, L.; Orlando, P.; Bisogno, T.; Laezza, C.; Bifulco, M.; et al. Suppression of nerve growth factor Trk receptors and prolactin receptors by endocannabinoids leads to inhibition of human breast and prostate cancer cell proliferation. Endocrinology. 2000, 141, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.; Miguel, A.; Díaz-Laviada, I. Δ9-Tetrahydrocannabinol induces apoptosis in human prostate PC-3 cells via a receptor-independent mechanism. FEBS Lett. 1999, 458, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Pietrovito, L.; Iozzo, M.; Bacci, M.; Giannoni, E.; Chiarugi, P. Treatment with cannabinoids as a promising approach for impairing fibroblast activation and prostate cancer progression. Int J Mol Sci. 2020, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Baram, L.; Peled, E.; Berman, P.; Yellin, B.; Besser, E.; Benami, M.; et al. The heterogeneity and complexity of Cannabis extracts as antitumor agents. Oncotarget. 2019, 10, 4091–4106. [Google Scholar] [CrossRef] [PubMed]
- Kosgodage, U.S.; Mould, R.; Henley, A.B.; Nunn, A.V.; Guy, G.W.; Thomas, E.L. Cannabidiol (CBD) is a novel inhibitor for exosome and microvesicle (EMV) release in cancer. Front Pharmacol. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Morell, C.; Bort, A.; Vara, D.; Ramos-Torres, A.; Rodríguez-Henche, N.; Díaz-Laviada, I. The cannabinoid WIN 55,212-2 prevents neuroendocrine differentiation of LNCaP prostate cancer cells. Prostate Cancer Prostatic Dis. 2016, 19, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Orellana-Serradell, O.; Poblete, C.E.; Sanchez, C.; Castellón, E.A.; Gallegos, I.; Huidobro, C.; et al. Proapoptotic effect of endocannabinoids in prostate cancer cells. Oncol Rep. 2015, 33, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Ligresti, A.; Schiano Moriello, A.; Iappelli, M.; Verde, R.; Stott, C.G.; et al. Non-THC cannabinoids inhibit prostate carcinoma growth in vitro and in vivo: pro-apoptotic effects and underlying mechanisms. Br J Pharmacol. 2013, 168, 79–102. [Google Scholar] [CrossRef] [PubMed]
- Nithipatikom, K.; Gomez-Granados, A.D.; Tang, A.T.; Pfeiffer, A.W.; Williams, C.L.; Campbell, W.B. Cannabinoid receptor type 1 (CB1) activation inhibits small GTPase RhoA activity and regulates motility of prostate carcinoma cells. Endocrinology. 2012, 153, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Sreevalsan, S.; Joseph, S.; Jutooru, I.; Chadalapaka, G.; Safe, S.H. Induction of apoptosis by cannabinoids in prostate and colon cancer cells is phosphatase dependent. Anticancer Res. 2011, 31, 3799–3807. [Google Scholar] [PubMed]
- Thomas, A.A.; Wallner, L.P.; Quinn, V.P.; Slezak, J.; Van Den Eeden, S.K.; Chien, G.W.; et al. Association between cannabis use and the risk of bladder cancer: results from the California men’s health study. Urology. 2015, 85, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Chacko, J.A.; Heiner, J.G.; Siu, W.; Macy, M.; Terris, M.K. Association between marijuana use and transitional cell carcinoma. Urology. 2006, 67, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Bettiga, A.; Aureli, M.; Colciago, G.; Murdica, V.; Moschini, M.; Lucianò, R.; et al. Bladder cancer cell growth and motility implicate cannabinoid 2 receptor-mediated modifications of sphingolipids metabolism. Sci Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Gasperi, V.; Evangelista, D.; Oddi, S.; Florenzano, F.; Chiurchiù, V.; Avigliano, L.; et al. Regulation of inflammation and proliferation of human bladder carcinoma cells by type-1 and type-2 cannabinoid receptors. Life Sci. 2015; 138, 41–51. [Google Scholar] [CrossRef]
- Yamada, T.; Ueda, T.; Shibata, Y.; Ikegami, Y.; Saito, M.; Ishida, Y.; et al. TRPV2 activation induces apoptotic cell death in human T24 bladder cancer cells: a potential therapeutic target for bladder cancer. Urology. 2010, 76, 509.e1–509.e7. [Google Scholar] [CrossRef] [PubMed]
- Maden, C.; Sherman, K.J.; Beckmann, A.M.; Hislop, T.G.; Teh, C.Z.; Ashley, R.L.; et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst. 1993, 85, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Woythal, N.; Arsenic, R.; Kempkensteffen, C.; Miller, K.; Janssen, J.C.; Huang, K.; et al. Immunohistochemical validation of PSMA expression measured by 68 Ga-PSMA PET/CT in primary prostate cancer. J Nucl Med. 2018, 59, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Taha, T.; Meiri, D.; Talhamy, S.; Wollner, M.; Peer, A.; Bar-Sela, G. Cannabis impacts tumor response rate to nivolumab in patients with advanced malignancies. Oncologist. 2019, 24, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, Y.; Zhu, L.; Zou, Y.; Kong, W.; Dong, B.; et al. Cannabinoid receptor 2 as a novel target for promotion of renal cell carcinoma prognosis and progression. J Cancer Res Clin Oncol. 2018, 144, 39–52. [Google Scholar] [CrossRef]
- Larrinaga, G.; Sanz, B.; Blanco, L.; Perez, I.; Candenas, M.L.; Pinto, F.M.; et al. Cannabinoid CB1 receptor is expressed in chromophobe renal cell carcinoma and renal oncocytoma. Clin Biochem 2013, 46, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Larrinaga, G.; Varona, A.; Perez, I.; Sanz, B.; Ufalde, A.; Candenas, M.L.; et al. Expression of cannabinoid receptors in human kidney. Histol Histopathol. 2010, 25, 1133–1138. [Google Scholar]
- Choi, W.H.; Park HDo Baek, S.H.; Chu, J.P.; Kang, M.H.; Mi, Y.J. Cannabidiol induces cytotoxicity and cell death via apoptotic pathway in cancer cell lines. Biomol Ther. 2008, 16, 87–94. [Google Scholar]
- EAU Renal Cell Cancer Guidelines. 2021. Available online: https://uroweb.org/guidelines (accessed on 20 December 2022).
- Gurney, J.; Shaw, C.; Stanley, J.; Signal, V.; Sarfati, D. Cannabis exposure and risk of testicular cancer: A systematic review and meta-analysis. BMC Cancer. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, R.C.; Allebeck, P.; Akre, O.; McGlynn, K.A.; Sidorchuk, A. Cannabis use and incidence of testicular cancer: a 42-year follow-up of Swedish men between 1970 and 2011. Cancer Epidemiol Biomarkers Prev. 2017, 26, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Hogan, P.; Sharpe, M.; Smedley, H.; Sikora, K. Cannabinoids and hCG levels in patients with testicular cancer. Lancet. 1983, 1144. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Botteri, E.; Iodice, S.; Boniol, M.; Lowenfels, A.B.; Maisonneuve, P.; et al. Tobacco smoking and cancer: a meta-analysis. Int J Cancer. 2008, 122, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Budney, A.J.; Lynskey, M.T. The co-occurring use and misuse of cannabis and tobacco: A review. Addiction. 2012, 107, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Dryburgh, L.M.; Bolan, N.S.; Grof, C.P.L.; Galettis, P.; Schneider, J.; Lucas, C.J.; et al. Cannabis contaminants: sources, distribution, human toxicity and pharmacologic effects. Br J Clin Pharmacol. 2018, 84, 2468–2476. [Google Scholar] [CrossRef]
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2023 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.