Bronchial Artery Embolisation in Haemoptysis Management: A Scoping Review with Emphasis on Embolic Materials and Indications
Abstract
Highlights
- Bronchial artery embolisation (BAE) remains a versatile therapeutic option for haemoptysis, with a wide range of embolic materials currently in use.
- Polyvinyl alcohol (PVA), coils, gelatin sponges (GS), and N-butyl-2-cyanoacrylate (NBCA) have distinct profiles that influence their clinical use.
- Clinical decision-making in BAE should be tailored to the patient’s condition and embolic material properties.
- The development of standardised management guidelines for patients requiring bronchial artery embolisation is essential to improve outcomes.
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
- Reports specifying the embolisation agent(s) used (key criterion);
- Studies focusing on embolisation for haemoptysis using conventional embolic agents (NBCA, coils, PVA, microspheres, gelatin sponges);
- Outcomes measured as clinical success (immediate haemoptysis control), haemoptysis-free survival, or recurrence rate;
- Original studies published between 2019 and 2024;
- Articles handling typical haemoptysis aetiologies such as bronchiectasis, tuberculosis, cystic fibrosis, aspergillosis or malignancy;
- Studies available in full text in English.
- Studies not reporting the type or frequency of embolic agent used (key criterion);
- Publications focusing primarily on atypical causes of haemoptysis not listed among the inclusion aetiologies;
- Chemoembolisation or embolisation involving therapeutic (non-occlusive) agents or procedures;
- Articles published outside the 2019–2024 timeframe;
- Editorials, letters, commentaries, or non-peer-reviewed documents;
- Studies focused solely on non-bronchial systemic embolisation;
- Animal or in vitro studies;
- Studies not available in full-text form.
2.3. Study Selection and Data Charting
3. Polyvinyl Alcohol (PVA)
3.1. Short Description
3.2. Advantages
3.3. Disadvantages
3.4. Clinical Use
4. Microspheres
4.1. Short Description
4.2. Advantages
4.3. Disadvantages
4.4. Clinical Use
5. N-Butyl-2-Cyanoacrylate (NBCA)
5.1. Short Description
5.2. Advantages
5.3. Disadvantages
5.4. Clinical Use
6. Gelatin Sponges (GS)
6.1. Short Description
6.2. Advantages
6.3. Disadvantages
6.4. Clinical Use
7. Coils
7.1. Short Description
7.2. Advantages
7.3. Disadvantages
7.4. Clinical Use
8. Practical Implications and Decision Algorithm
8.1. Clinical and Anatomical Considerations in Embolic Agent Selection
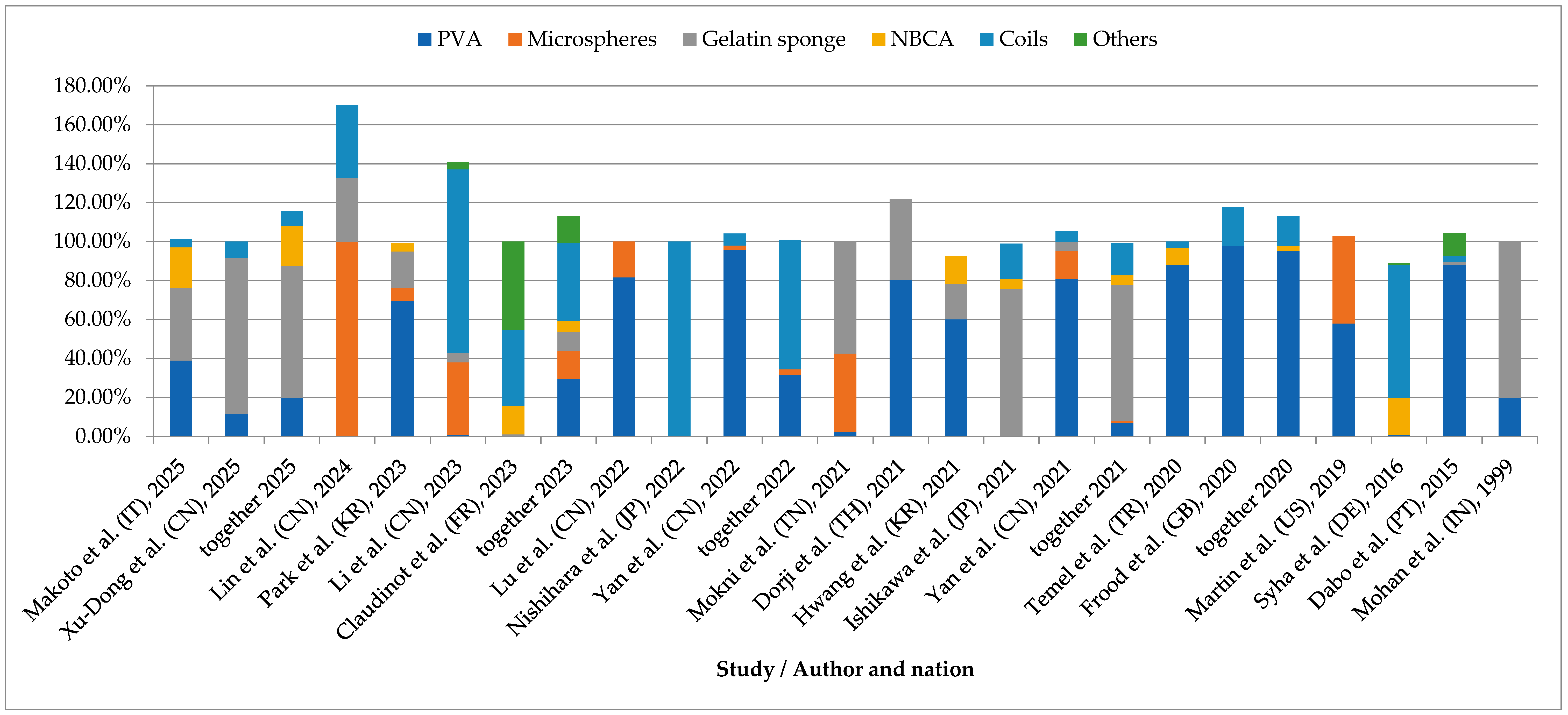
8.2. Recurrence Risk and Material-Specific Outcome
8.3. Emerging Technologies and Materials in Bronchial Artery Embolisation
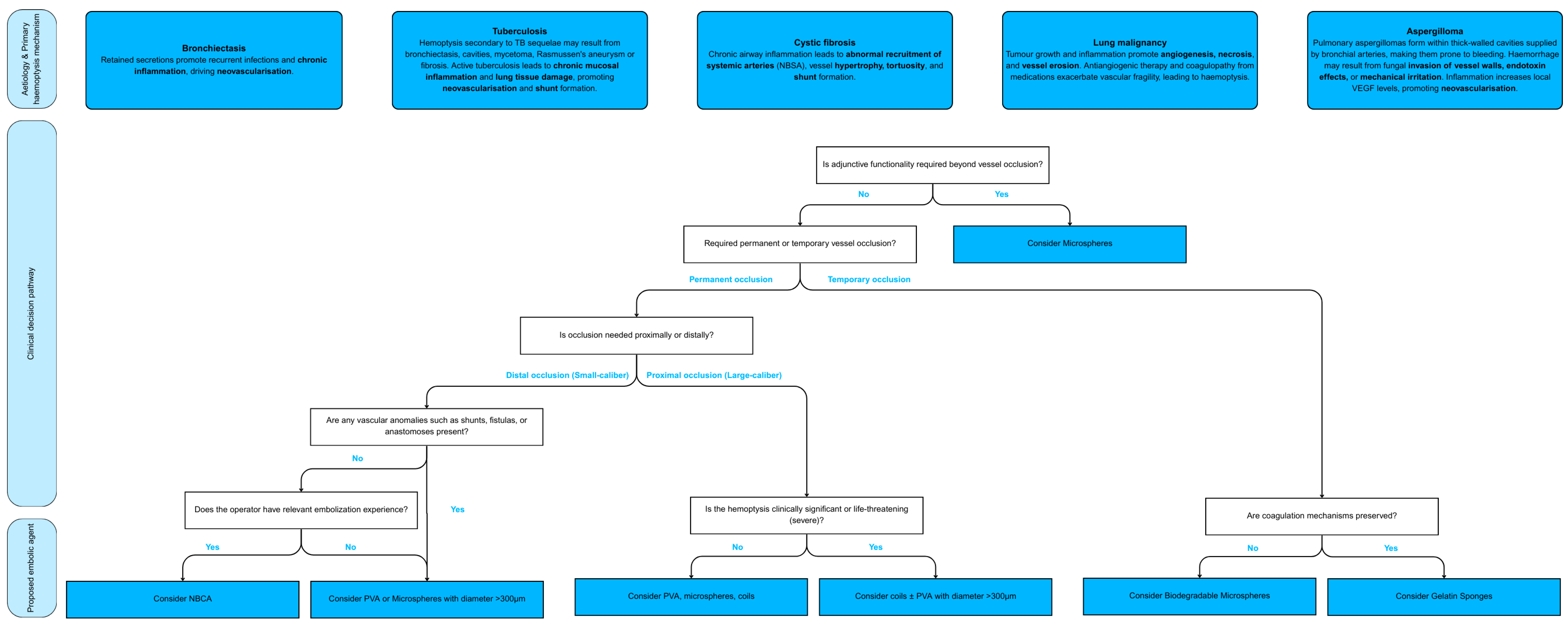
9. Discussion
10. Limitations
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BAE | Bronchial artery embolisation |
| PVA | Polyvinyl alcohol |
| NBCA | N-butyl-2-cyanoacrylate |
| GS | Gelatin sponge |
| BAs | Bronchial arteries |
| NBSAs | Non-bronchial systemic arteries |
| TB | Tuberculosis |
| CF | Cystic fibrosis |
| DEBs | Drug-eluting beads |
| ssBACE | Super selective bronchial artery coil embolisation |
| FEV1 | Forced expiratory volume in 1 s |
| AVMs | Arteriovenous malformations |
| CIRSE | Cardiovascular and Interventional Radiological Society of Europe |
| BPH | Benign Prostatic Hyperplasia |
| BACE | Bronchial Arterial Chemoembolisation |
| BAICE | Bronchial Arterial Infusion Chemoembolisation |
| CBCT | Cone-Beam Computed Tomography |
| VEGF | Vascular Endothelial Growth Factor |
| MDCT | Multidetector Computed Tomography |
| HRAs | Haemoptysis-Related Arteries |
| N/A | Non-Applicable |
| Hb | Haemoglobin |
| Hct | Haematocrit |
| SBP | Systolic Blood Pressure |
Appendix A
| Title | Author | Year | Patients Treated with BAE | Main Aetiologies | Embolic Materials Used in BAE | Recurrences During Follow-Up | |
|---|---|---|---|---|---|---|---|
| Clinical outcomes of cystic fibrosis patients with hemoptysis treated with bronchial artery embolization | Amaral Peixoto da Silveira et al. [23] | 2021 | 17 | Cystic fibrosis | PVA | 56.4% | |
| Exacerbation of chronic pulmonary aspergillosis was associated with a high rebleeding rate after bronchial artery embolization | Ando et al. [9] | 2019 | 41 | Aspergillosis Tuberculosis | Coils | 51.2% (24 mo) | |
| Coil embolisation for massive haemoptysis in cystic fibrosis | Dohna et al. [46] | 2021 | 34 | Cystic fibrosis | Coils | 35.3% (9 mo) | |
| Analysis of recurrence of risk factors after transcatheter bronchial artery embolization for hemoptysis | Dong and An [11] | 2024 | 406 | Malignancy Bronchiectasis Tuberculosis Arteriovenous malformations | PVA Coils | 13.55% (Tumour related: 52.63%; Non-tumour related: 4.55%) | |
| Microspheres present comparable efficacy and safety profiles compared with polyvinyl alcohol for bronchial artery embolization treatment in hemoptysis patients | Fu et al. [17] | 2021 | 152 | Bronchiectasis Tuberculosis NTM infection BA malformation Bronchus tumour Diffuse lesions Aortic dissection stent implantation | Microspheres PVA | Microspheres 9.7% (6 mo) 9.7% (12 mo) | PVA 7.8% (6 mo) 8.9% (12 mo) |
| Safety and efficacy of transcatheter embolization in patients with massive hemoptysis due to intercostal pulmonary venous shunts | Fu et al. [16] | 2019 | 24 | Bronchiectasis Tuberculosis Infections Malignancy Pneumoconiosis | Coils PVA | 8.3% (6 mo) 12.5% (12 mo) 25% (48 mo) | |
| Embolization of bronchial arteries and nonbronchial systemic arteries with n-butyl-cyanoacrylate in patients with hemoptysis: A retrospective single-center study | García Jurado et al. [5] | 2020 | 55 | Bronchiectasis Cryptogenic haemoptysis Malignancy Aspergillosis | NBCA | 9.3% | |
| Transarterial Embolization for the Management of Emergent Hemoptysis in Patients With Primary and Metastatic Lung Tumors | Geevarghese et al. [33] | 2024 | 37 | Malignancy (primary and secondary lung cancer) | Embospheres PVA Coils NBCA GS | 51.35% | |
| Efficacy and safety of particle embolization in bronchial arteries of hemoptysis patients with shunts | Guzelbey et al. [49] | 2024 | 49 | Tuberculosis Bronchiectasis Aspergillosis Bacterial pneumonia Malignancy NTM infection | PVA | 10.2% | |
| Predictive Factors for Recurrent Hemoptysis after Bronchial Artery Embolization in Patients with Lung Cancer | Hanotin et al. [20] | 2024 | 144 | Malignancy (primary lung cancer) | Microspheres Coils NBCA ethylene-vinyl alcohol copolymer (EVOH) | 34.7% (1 mo) | |
| Feasibility and outcomes of bronchial artery embolization in patients with non-massive hemoptysis | Hwang et al. [8] | 2021 | 233 | Tuberculosis Bronchiectasis Aspergillosis NTM infection Pneumonia Malignancy | GS Coils PVA NBCA | 27.47% | |
| Spinal Cord Infarction after Bronchial Artery Embolization for Hemoptysis: A Nationwide Observational Study in Japan | Ishikawa et al. [36] | 2021 | 8563 | Cryptogenic haemoptysis Tuberculosis Malignancy Cystic fibrosis Respiratory infection Bronchiectasis Aspergillosis | Coils GS NBCA | N/A | |
| Combined therapy with bronchial artery embolization and tranexamic acid for hemoptysis | Lee et al. [22] | 2020 | 64 | Tuberculosis Aspergillosis Infection Malignancy Bronchiectasis Pulmonary thromboembolism | PVA | 31.3% | |
| A retrospective study on the management of massive hemoptysis by bronchial artery embolization: risk factors associated with recurrence of hemoptysis | Li et al. [63] | 2023 | 105 | Tuberculosis Lung infections Bronchiectasis | Coils GS Thread PVA | 54.7% (36 mo) | |
| Risk factors for the recurrence in pulmonary tuberculosis patients with massive hemoptysis | Lin et al. [57] | 2023 | 81 | Tuberculosis | Gelatin sponge Coils | 16.18% | |
| The efficacy and safety of combined therapy with endobronchial tamponade and bronchial artery embolization for massive hemoptysis | Lin et al. [62] | 2024 | 67 | Bronchiectasis Necrotising pneumonia Malignancy Tuberculosis | Microspheres GS Coils | BAE + endobronchial tamponade: 3.8% (3 mo) 15.4% (12 mo) | BAE alone: 4.9% (3 mo) 39.0% (12 mo) |
| Endovascular treatment of chronic hemoptysis in patients with pulmonary tuberculosis | Loiudice et al. [50] | 2021 | 12 | Tuberculosis | PVA | 81.82% (6 mo) | |
| Bronchial artery embolization for the management of frequent hemoptysis caused by bronchiectasis | Lu et al. [10] | 2022 | 69 | Bronchiectasis Cystic fibrosis | PVA Embosphere GS | 30.43% | |
| Bronchial Artery Embolization for Hemoptysis in Cystic Fibrosis Patients: A 17-Year Review | Martin et al. [59] | 2019 | 28 | Cystic fibrosis | Microspheres PVA 300–700 μm | 25% | |
| Bronchial artery embolization in patients with life-threatening massive hemoptysis: comparison of the efficacy and safety of particulate embolizing agents and n-2-butyl-cyanoacrylate | Mazıcan et al. [27] | 2024 | 58 | Bronchiectasis Tuberculosis Malignancy Granulomatosis with polyangiitis Churg-Strauss syndrome Aspergillosis Bronchial fistula Dieulafoy’s disease | NBCA Microspheres PVA | NBCA: 3.3% | Microspheres/PVA: 17.9% |
| Outcomes of bronchial artery embolization for the management of hemoptysis | Mokni et al. [51] | 2021 | 46 | Bronchiectasis Malignancy Tuberculosis | GS Microspheres PVA | 12.0% | |
| GS: 17.39% | Embosphere: 6.25% | ||||||
| Prevalence of non-bronchial systemic culprit arteries in patients with hemoptysis with bronchiectasis and chronic pulmonary infection who underwent de novo bronchial artery embolization | Nishihara et al. [4] | 2022 | 83 | Bronchiectasis NTM infection Aspergillosis Tuberculosis | Coils | 8.43% (12 mo) | |
| Effectiveness of bronchial arterial embolization using N-butyl-2cyanoacrylate for local control of pulmonary hilar or mediastinal tumors that are refractory to chemotherapy | Nomori et al. [32] | 2020 | 42 | Malignancy (hilar of mediastinal tumours, multiple metastases, unresectable tumours) | NBCA | 9.52% | |
| Early versus delayed bronchial artery embolization for non-massive hemoptysis | Park et al. [25] | 2022 | 138 | Bronchiectasis Tuberculosis | PVA Microspheres | 14.0% | |
| Efficacy and safety of treating acute haemoptysis using glue embolization: A retrospective observational study and comparison to the literature | Shamseldin et al. [28] | 2024 | 35 | Malignancy Pneumonia Tuberculosis | NBCA Coils Particles | 5.7% | |
| Bronchial artery embolization using small particles is safe and effective: a single center 12-year experience | Sheehan et al. [15] | 2024 | 144 | Bronchiectasis Mycetoma Tuberculosis Sarcoidosis Pulmonary hypertension Malignancy Cystic fibrosis Cryptogenic harmoptysis | PVA (150 μm to 250 μm) | 7.0% (30 days) 28.57% | |
| Bronchial arterial embolization using a gelatin sponge for hemoptysis from pulmonary aspergilloma: comparison with other pulmonary diseases | Shimohira et al. [42] | 2019 | 52 | Aspergillosis Bronchiectasis Tuberculosis Malignancy | GS | 26.92% | |
| Embosphere microspheres size for bronchial artery embolization in patients with hemoptysis caused by bronchiectasis: a retrospective comparative analysis of 500–750 versus 700–900 μm microspheres | Xu et al. [31] | 2024 | 112 | Bronchiectasis | Embospheres 500–750 μm or 700–900 μm | 61.6% | |
| Development of a model to predict recurrence after bronchial artery embolization for non-cancer related hemoptysis | Yan et al. [65] | 2021 | 487 | Bronchiectasis Tuberculosis Chronic pneumonia Cryptogenic haemoptysis Aspergillosis Pneumoconiosis | PVA Microspheres GS Coils | 19.1% | |
| Does the presence of systemic artery-pulmonary circulation shunt during bronchial arterial embolization increase the recurrence of noncancer-related hemoptysis? A retrospective cohort study | Yan et al. [73] | 2023 | 326 | Bronchiectasis Tuberculosis Chronic pneumonia Cryptogenic haemoptysis | PVA Microspheres GS | 23.0% | |
| Embolization with more diluted glue-lipiodol in patients with massive hemoptysis: single center experience results | Kolu et al. [35] | 2022 | 48 | Bronchiectasis Tuberculosis Malignancy Aspergillosis Arteriovenous malformations | NBCA | 8.9% | |
| Author | Volume-Based Criteria of Massive Haemoptysis | Functional/Clinical Criteria of Massive Haemoptysis |
|---|---|---|
| Amaral Peixoto da Silveira et al. [23] | >240 mL/24 h | N/A |
| Ando et al. [9] | >200 mL/24 h | N/A |
| Dohna et al. [46] | >240 mL/24 h or >100 mL/24 h for a few days or weeks | N/A |
| Dong and An [11] | >100 mL in one episode or >500 mL/24 h | N/A |
| García Jurado et al. [5] | >300 mL/24 h | Any volume of haemoptysis leading to fall in: Hb > 1 g/dL Hct > 5% SBP < 90 mmHg with a respiratory failure or hypotension |
| Guzelbey et al. [49] | >300 mL/24 h + respiratory failure or haemodynamic instability | |
| Hanotin et al. [20] | >100 mL/24 h + respiratory failure or haemodynamic instability | |
| Hwang et al. [8] | >300 mL/24 h | N/A |
| Lee et al. [22] | >200 mL/24 h | N/A |
| Li et al. [63] | >300–600 mL/24 h | N/A |
| Lin et al. [57] | >100 mL in one episode or >500 mL/24 h | N/A |
| Loiudice et al. [50] | >240 mL in one episode or >300–600 mL/24 h | N/A |
| Lu et al. [10] | ≥300 mL/24 h | N/A |
| Martin et al. [59] | >240 mL/24 h | N/A |
| Mokni et al. [51] | N/A | Presence of airway obstruction, hypotension, or significant blood loss affecting vital prognosis |
| Nishihara et al. [4] | ≥200 mL/24 h | N/A |
| Sheehan et al. [15] | >300 mL/24 h | N/A |
| Yan et al. [65,73] | ≥300 mL/24 h | N/A |
| Embolisation Agent 1 | Author | Recurrence Rate (%) | Average Recurrence Rate (%) | Clinical Success (%) | Average Clinical Success (%) | Complication 2 Rate (%) | Average Complication 2 Rate (%) |
|---|---|---|---|---|---|---|---|
| PVA | Amaral Peixoto da Silveira et al. [23] | 56.4 | 36.21 | 100 | 96.9 | 20 | 23.7 |
| Guzelbey et al. [49] | 10.2 | 100 | 32.7 | ||||
| Lee et al. [22] | 31.3 | 96.8 | N/A | ||||
| Sheehan et al. [15] | 28.6 | 93 | 11 | ||||
| Fu et al. [17] | 8.9 | 100 | 46.7 | ||||
| Loiudice et al. [50] | 81.9 | 91.6 | 8.3 | ||||
| Coils | Ando et al. [9] | 51.2 | 31.64 | 92.7 | 94.9 | N/A | N/A |
| Dohna et al. [46] | 35.3 | 97 | N/A | ||||
| Nishihara et al. [4] | 8.4 | N/A | N/A | ||||
| NBCA | García Jurado et al. [5] | 9.3 | 7.76 | 98.2 | 97.9 | 10.9 | 14.9 |
| Nomori et al. [32] | 9.5 | 100 | 2.4 | ||||
| Mazıcan et al. [27] | 3.3 | 100 | 10.7 | ||||
| Kolu et al. [35] | 8.9 | 93.8 | 35.4 | ||||
| GS | Shimohira et al. [42] | 26.9 | 22.2 | 100 | 97.5 | 0 | 3.3 |
| Mokni et al. [51] | 17.4 | 6.5 | |||||
| Microspheres | Mokni et al. [51] | 6.3 | 25.9 | 95 | 95.9 | 28.2 | |
| Xu et al. [31] | 61.6 | N/A | 31.3 | ||||
| Fu et al. [17] | 9.7 | 96.8 | 46.7 |
References
- Olsen, K.M.; Manouchehr-pour, S.; Donnelly, E.F.; Henry, T.S.; Berry, M.F.; Boiselle, P.M.; Colletti, P.M.; Harrison, N.E.; Kuzniewski, C.T.; Laroia, A.T.; et al. ACR Appropriateness Criteria® Hemoptysis. J. Am. Coll. Radiol. 2020, 17, S148–S159. [Google Scholar] [CrossRef]
- Singer, E.D.; Faiz, S.A.; Qdaisat, A.; Abdeldaem, K.; Dagher, J.; Chaftari, P.; Yeung, S.-C.J. Hemoptysis in Cancer Patients. Cancers 2023, 15, 4765. [Google Scholar] [CrossRef]
- Panda, A.; Goyal, A. Bronchial Artery Embolization in Hemoptysis: A Systematic Review. Diagn. Interv. Radiol. 2017, 23, 307–317. [Google Scholar] [CrossRef]
- Nishihara, T.; Ishikawa, H.; Omachi, N.; Yamaguchi, Y.; Kitaguchi, K.; Hattori, T. Prevalence of Non-Bronchial Systemic Culprit Arteries in Patients with Hemoptysis with Bronchiectasis and Chronic Pulmonary Infection Who Underwent de Novo Bronchial Artery Embolization. Eur. Radiol. 2022, 33, 4198–4204. [Google Scholar] [CrossRef] [PubMed]
- García Jurado, P.B.; Pérez Montilla, M.E.; Lombardo Galera, M.S.; Entrenas Castillo, M.; García-Revillo, J.; Espejo Herrero, J.J. Embolization of Bronchial Arteries and Nonbronchial Systemic Arteries with N-Butyl-Cyanoacrylate in Patients with Hemoptysis: A Retrospective Single-Center Study. Radiol. (Engl. Ed.) 2023, 65, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhuang, Z.; Yang, M.; Luo, J.; Zhang, W.; Yan, Z.; Wang, X. Bronchial Artery Embolization for Hemoptysis: A Systematic Review and Meta-Analysis. J. Interv. Med. 2021, 4, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Springer, D.; Cofta, S.; Juszkat, R.; Żabicki, B.; Goździk-Spychalska, J.; Nowicka, A.; Winiarska, H.; Batura-Gabryel, H. The Effectiveness of Bronchial Artery Embolisation in Patients with Haemoptysis. Adv. Respir. Med. 2018, 86, 220–226. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kim, J.H.; Park, S.; Lee, K.H.; Park, S.H. Feasibility and Outcomes of Bronchial Artery Embolization in Patients with Non-Massive Hemoptysis. Respir. Res. 2021, 22, 221. [Google Scholar] [CrossRef]
- Ando, T.; Kawashima, M.; Masuda, K.; Takeda, K.; Okuda, K.; Suzuki, J.; Ohshima, N.; Horibe, M.; Tamura, A.; Nagai, H.; et al. Exacerbation of Chronic Pulmonary Aspergillosis Was Associated with a High Rebleeding Rate after Bronchial Artery Embolization. Respir. Investig. 2019, 57, 260–267. [Google Scholar] [CrossRef]
- Lu, G.-D.; Yan, H.-T.; Zhang, J.-X.; Liu, S.; Shi, H.-B.; Zu, Q.-Q. Bronchial Artery Embolization for the Management of Frequent Hemoptysis Caused by Bronchiectasis. BMC Pulm. Med. 2022, 22, 394. [Google Scholar] [CrossRef]
- Dong, Y.; An, J. Analysis of Recurrence of Risk Factors after Transcatheter Bronchial Artery Embolization for Hemoptysis. Ann. Saudi Med. 2024, 44, 414–421. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for Conducting Systematic Scoping Reviews. Int. J. Evid. -Based Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Woo, S.; Yoon, C.J.; Chung, J.W.; Kang, S.-G.; Jae, H.J.; Kim, H.-C.; Seong, N.J.; Kim, Y.-J.; Woo, Y.-N. Bronchial Artery Embolization to Control Hemoptysis: Comparison of N-Butyl-2-Cyanoacrylate and Polyvinyl Alcohol Particles. Vasc. Interv. Radiol. 2013, 269, 594–602. [Google Scholar] [CrossRef]
- Sheehan, F.; Graham, A.; Tait, N.P.; Ind, P.; Alsafi, A.; Jackson, J.E. Bronchial Artery Embolization Using Small Particles Is Safe and Effective: A Single Center 12-Year Experience. Eur. Radiol. 2024, 34, 7786–7794. [Google Scholar] [CrossRef]
- Fu, Z.; Liang, Y.; Zhao, W.; Tian, J.; Cai, F.; Zhang, X. Safety and Efficacy of Transcatheter Embolization in Patients with Massive Hemoptysis Due to Intercostal Pulmonary Venous Shunts. Radiol. Med. 2019, 124, 588–594. [Google Scholar] [CrossRef]
- Fu, Z.; Li, X.; Cai, F.; Yuan, Y.; Zhang, X.; Qin, J.; Liang, Y. Microspheres Present Comparable Efficacy and Safety Profiles Compared with Polyvinyl Alcohol for Bronchial Artery Embolization Treatment in Hemoptysis Patients. J. Transl. Med. 2021, 19, 422. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Li, J.; Su, R.; Zhou, M.; Zhu, H.; Sun, Z. The Efficacy, Safety, and Related Factors of Bronchial Artery Embolization for Hemoptysis: A Systematic Review and Meta-Analysis with Subgroup Analysis. Cardiovasc. Diagn. Ther. 2024, 14, 859–877. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, R.; Zhang, B.; Tian, Y.; Lu, Y.; An, X.; Shi, X. Preparation and Investigation of a Novel Iodine-Based Visible Polyvinyl Alcohol Embolization Material. J. Interv. Med. 2022, 5, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Hanotin, C.; Salvayre, R.; Lassalle, L.; Fartoukh, M.; Lehrer, R.; Gibelin, A.; Barral, M. Predictive Factors for Recurrent Hemoptysis after Bronchial Artery Embolization in Patients with Lung Cancer. J. Vasc. Interv. Radiol. 2024, 35, 1296–1303. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoon, C.J.; Jung, Y.S.; Choi, W.S.; Lee, C.; Lee, G.M. Comparison of N-Butyl-2-Cyanoacrylate and Polyvinyl Alcohol Particles for Bronchial Artery Embolisation in Primary Lung Cancer: A Retrospective Cohort Study. Respir. Res. 2022, 23, 257. [Google Scholar] [CrossRef]
- Lee, H.N.; Park, H.S.; Hyun, D.; Cho, S.K.; Park, K.B.; Shin, S.W.; Soo Do, Y. Combined Therapy with Bronchial Artery Embolization and Tranexamic Acid for Hemoptysis. Acta Radiol. 2021, 62, 610–618. [Google Scholar] [CrossRef]
- Silveira, M.A.P.D.; Silveira, P.A.P.D.; Beltrami, F.G.; Scaffaro, L.A.; Dalcin, P.D.T.R. Clinical Outcomes of Cystic Fibrosis Patients with Hemoptysis Treated with Bronchial Artery Embolization. J. Bras. Pneumol. 2021, 47, e20200557. [Google Scholar] [CrossRef]
- Fan, S.; Cheng, X.; Wang, X.; Liu, Y.; He, W.; Chen, H. Bronchial Artery Embolization versus Conservative Treatment for Hemoptysis: A Systematic Review and Meta-Analysis. BMC Pulm. Med. 2024, 24, 428. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Lee, S.; Lee, H.N.; Cho, Y. Early versus Delayed Bronchial Artery Embolization for Non-Massive Hemoptysis. Eur. Radiol. 2022, 33, 116–124. [Google Scholar] [CrossRef]
- Hu, J.; Albadawi, H.; Chong, B.W.; Deipolyi, A.R.; Sheth, R.A.; Khademhosseini, A.; Oklu, R. Advances in Biomaterials and Technologies for Vascular Embolization. Adv. Mater. 2019, 31, 1901071. [Google Scholar] [CrossRef]
- Mazıcan, M.; Karluka, I.; Fındıkcıoglu, A.; Andıc, C. Bronchial Artery Embolization in Patients with Life-Threatening Massive Hemoptysis: Comparison of the Efficacy and Safety of Particulate Embolizing Agents and n-2-Butyl-Cyanoacrylate. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 310–318. [Google Scholar] [CrossRef]
- Shamseldin, M.; Kluge, J.; Bauer, J.; Puls, R. Efficacy and Safety of Treating Acute Haemoptysis Using Glue Embolization: A Retrospective Observational Study and Comparison to the Literature. J. Med. Imag. Rad. Oncol. 2024, 68, 177–184. [Google Scholar] [CrossRef]
- Baltacıoğlu, F.; Çimşit, N.Ç.; Bostanci, K.; Yüksel, M.; Kodalli, N. Transarterial Microcatheter Glue Embolization of the Bronchial Artery for Life-Threatening Hemoptysis: Technical and Clinical Results. Eur. J. Radiol. 2010, 73, 380–384. [Google Scholar] [CrossRef]
- Kettenbach, J.; Ittrich, H.; Gaubert, J.Y.; Gebauer, B.; Vos, J.A. CIRSE Standards of Practice on Bronchial Artery Embolisation. Cardiovasc. Interv. Radiol. 2022, 45, 721–732. [Google Scholar] [CrossRef]
- Xu, H.-D.; Yang, L.; Hu, S.-B. Embosphere Microspheres Size for Bronchial Artery Embolization in Patients with Hemoptysis Caused by Bronchiectasis: A Retrospective Comparative Analysis of 500–750 versus 700–900 Μm Microspheres. BMC Pulm. Med. 2024, 24, 203. [Google Scholar] [CrossRef] [PubMed]
- Nomori, H.; Yamazaki, I.; Shiraishi, A.; Adachi, T.; Otsuki, A.; Oyama, Y. Effectiveness of Bronchial Arterial Embolization Using N-Butyl-2-Cyanoacrylate for Local Control of Pulmonary Hilar or Mediastinal Tumors That Are Refractory to Chemotherapy. Eur. J. Radiol. 2020, 131, 109160. [Google Scholar] [CrossRef] [PubMed]
- Geevarghese, R.; Petre, E.N.; Ziv, E.; Santos, E.; Rodriguez, L.; Zhao, K.; Sotirchos, V.S.; Solomon, S.B.; Alexander, E.S. Transarterial Embolization for the Management of Emergent Hemoptysis in Patients With Primary and Metastatic Lung Tumors. Clin. Lung Cancer 2024, 26, S1525730424002547. [Google Scholar] [CrossRef]
- n-BCA Trail Investigators. N-Butyl Cyanoacrylate Embolization of Cerebral Arteriovenous Malformations: Results of a Prospective, Randomized, Multi-Center Trial. AJNR Am. J. Neuroradiol. 2002, 23, 748–755. [Google Scholar]
- Kolu, M.; Kurtuluş, Ş.; Dere, O.; Yurttutan, N.; Yıldırım, I.O. Embolization with More Diluted Glue-Lipiodol in Patients with Massive Hemoptysis: Single Center Experience Results. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1543–1548. [Google Scholar] [CrossRef]
- Ishikawa, H.; Ohbe, H.; Omachi, N.; Morita, K.; Yasunaga, H. Spinal Cord Infarction after Bronchial Artery Embolization for Hemoptysis: A Nationwide Observational Study in Japan. Radiology 2021, 298, 673–679. [Google Scholar] [CrossRef]
- Dinc, G.; Oğuz, Ş. The Efficacy of Pelvic Arterial Embolisation for the Treatment in Massive Vaginal Haemorrhage in Obstetric and Gynaecological Emergencies: A Single-Centre Experience. J. Obstet. Gynaecol. 2019, 39, 774–781. [Google Scholar] [CrossRef]
- Roberto Giammalva, G.; Brunasso, L.; Costanzo, R.; Paolini, S.; Umana, G.; Yağmurlu, K.; Chaurasia, B.; Cicero, S.; Scalia, G.; Basile, L.; et al. The Role of Hemostatic Devices in Neurosurgery. A Systematic Review. J. Clin. Neurosci. 2021, 89, 151–157. [Google Scholar] [CrossRef]
- Hawthorn, B.R.; Ratnam, L.A. Role of Interventional Radiology in Placenta Accreta Spectrum (PAS) Disorders. Best. Pract. Res. Clin. Obstet. Gynaecol. 2021, 72, 25–37. [Google Scholar] [CrossRef]
- Fang, Y.; Guo, W.; Ni, P.; Liu, H. Recent Research Advances in Polysaccharide-Based Hemostatic Materials: A Review. Int. J. Biol. Macromol. 2024, 271, 132559. [Google Scholar] [CrossRef]
- Nagano, N.; Suzuki, M.; Yamamoto, S.; Kobayashi, K.; Iikura, M.; Izumi, S.; Hojo, M.; Sugiyama, H. Short- and Long-Term Efficacy of Bronchial Artery Embolization Using a Gelatin Sponge for the Treatment of Cryptogenic Hemoptysis. GHM 2022, 4, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Shimohira, M.; Ohta, K.; Nagai, K.; Sawada, Y.; Nakashima, M.; Maki, H.; Bando, Y.; Shibamoto, Y. Bronchial Arterial Embolization Using a Gelatin Sponge for Hemoptysis from Pulmonary Aspergilloma: Comparison with Other Pulmonary Diseases. Emerg. Radiol. 2019, 26, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Rémy, J.; Arnaud, A.; Fardou, H.; Giraud, R.; Voisin, C. Treatment of Hemoptysis by Embolization of Bronchial Arteries. Radiology 1977, 122, 33–37. [Google Scholar] [CrossRef]
- Xiao, N.; Lewandowski, R.J. Embolic Agents: Coils. Semin. Interv. Radiol. 2022, 39, 113–118. [Google Scholar] [CrossRef] [PubMed]
- White, J.B.; Ken, C.G.M.; Cloft, H.J.; Kallmes, D.F. Coils in a Nutshell: A Review of Coil Physical Properties. AJNR Am. J. Neuroradiol. 2008, 29, 1242–1246. [Google Scholar] [CrossRef]
- Dohna, M.; Renz, D.M.; Stehling, F.; Dohna-Schwake, C.; Sutharsan, S.; Neurohr, C.; Wirtz, H.; Eickmeier, O.; Grosse-Onnebrink, J.; Sauerbrey, A.; et al. Coil Embolisation for Massive Haemoptysis in Cystic Fibrosis. BMJ Open Resp. Res. 2021, 8, e000985. [Google Scholar] [CrossRef]
- Ishikawa, H.; Hara, M.; Ryuge, M.; Takafuji, J.; Youmoto, M.; Akira, M.; Nagasaka, Y.; Kabata, D.; Yamamoto, K.; Shintani, A. Efficacy and Safety of Super Selective Bronchial Artery Coil Embolisation for Haemoptysis: A Single-Centre Retrospective Observational Study. BMJ Open 2017, 7, e014805. [Google Scholar] [CrossRef]
- Ryuge, M.; Hara, M.; Hiroe, T.; Omachi, N.; Minomo, S.; Kitaguchi, K.; Youmoto, M.; Asakura, N.; Sakata, Y.; Ishikawa, H. Mechanisms of Recurrent Haemoptysis after Super-Selective Bronchial Artery Coil Embolisation: A Single-Centre Retrospective Observational Study. Eur. Radiol. 2019, 29, 707–715. [Google Scholar] [CrossRef]
- Guzelbey, T.; Arslan, M.F.; Cingoz, M.; Erdim, C.; Altun, O.; Mutlu, I.N.; Kilickesmez, O. Efficacy and Safety of Particle Embolization in Bronchial Arteries of Hemoptysis Patients with Shunts. Clin. Radiol. 2024, 79, 704–710. [Google Scholar] [CrossRef]
- Loiudice, G.; Catelli, A.; Corvino, A.; Quarantelli, M.; Venetucci, P. Endovascular Treatment of Chronic Hemoptysis in Patients with Pulmonary Tuberculosis. Acta Biomed. Atenei Parm. 2021, 92, e2021201. [Google Scholar] [CrossRef]
- Mokni, A.; Abid, N.; Loukil, M.; Laouini, I.; Bouzaidi, K.; Ghrairi, H. Apport de l’embolisation Artérielle Bronchique Pour Le Traitement de l’hémoptysie. Rev. Des. Mal. Respir. Actual. 2021, 13, 150. [Google Scholar] [CrossRef]
- Higuchi, S.; Horinouchi, H.; Nakayama, S.; Aoki, T.; Kotoku, A.; Ueda, J.; Tsuji, A.; Fukuda, T.; Ogo, T. Feasibility of Revascularization after Gelatin Sponge Embolization for Hemoptysis during Balloon Pulmonary Angioplasty. Int. J. Cardiol. 2024, 413, 132343. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-J.; Yu, H.; Wang, Y.; Jiang, F.-M.; Wang, W.; Li, X.-O.; Wang, Y.; Liang, Z.-A. Multidetector Computed Tomography Angiography Prior to Bronchial Artery Embolization Helps Detect Culprit Ectopic Bronchial Arteries and Non-Bronchial Systemic Arteries Originating from Subclavian and Internal Mammary Arteries and Improve Hemoptysis-Free Early Survival Rate in Patients with Hemoptysis. Eur. Radiol. 2019, 29, 1950–1958. [Google Scholar] [CrossRef]
- Nezami, N.; Georgiades, C.; Hong, K.K.; Buethe, J. Bronchial Artery Chemoembolization With Radiopaque Doxorubicin Eluding Beads in Patients With Malignant Hemoptysis from Metastatic Lung Cancer. Technol. Cancer Res. Treat. 2022, 21, 15330338221131167. [Google Scholar] [CrossRef]
- Xiaobing, L.; Meipan, Y.; Pengfei, X.; Yue, Z.; Ying, L.; Xiangnan, L.; Yu, Q.; Yaozhen, M.; Chunxia, L.; Gang, W. Bronchial Artery Chemoembolization for Hemoptysis in Advanced Primary Lung Cancer. Clin. Lung Cancer 2022, 23, e203–e209. [Google Scholar] [CrossRef]
- Liu, M.Y.; Rose, S.C.; Loh, A.; Taddonio, M.; Redmond, J.W.; Meisinger, Q.C.; Minocha, J.; Berman, Z.T. Utility of Cone-Beam CT for Bronchial Artery Embolization and Chemoinfusion: A Single-Institution Retrospective Case Series. Cardiovasc. Interv. Radiol. 2022, 45, 834–840. [Google Scholar] [CrossRef]
- Lin, Q.; Chen, J.; Yu, T.; Gao, B.; Kuang, K.; Fan, Y.; Xu, J.; Li, X.; Lin, X.; Xu, L. Risk Factors for the Recurrence in Pulmonary Tuberculosis Patients with Massive Hemoptysis. Clin. Respir. J. 2023, 17, 663–671. [Google Scholar] [CrossRef]
- Hwang, H.-G.; Lee, H.-S.; Choi, J.-S.; Seo, K.-H.; Kim, Y.-H.; Na, J.-O. Risk Factors Influencing Rebleeding after Bronchial Artery Embolization on the Management of Hemoptysis Associated with Pulmonary Tuberculosis. Tuberc. Respir. Dis. 2013, 74, 111. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.N.; Higgins, L.; Mohabir, P.; Sze, D.Y.; Hofmann, L.V. Bronchial Artery Embolization for Hemoptysis in Cystic Fibrosis Patients: A 17-Year Review. J. Vasc. Interv. Radiol. 2020, 31, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Makoto, T.T.; Daniele, P.; Francesco, P.; Lorenzo, B.; Sara, Z.; Antonio, B.; Francesco, M.; Cristina, M. Bronchial Artery Embolization for the Treatment of Hemoptysis: Permanent versus Temporary Embolic Materials, a Single Center Study. CVIR Endovasc. 2025, 8, 40. [Google Scholar] [CrossRef]
- Xu-Dong, J.; Qi-Fan, W.; Ya-Rong, S.; Yun-Hua, L. Analysis of Risk Factors for Recurrence within 6 Months after Bronchial Artery Embolization for Massive Hemoptysis Due to Pulmonary Tuberculosis. Medicine 2025, 104, e41734. [Google Scholar] [CrossRef]
- Lin, C.; Chen, Y.; Cai, D.; Chen, Z.; Peng, Z.; Lai, H.; Liu, D. The Efficacy and Safety of Combined Therapy with Endobronchial Tamponade and Bronchial Artery Embolization for Massive Hemoptysis. BMC Pulm. Med. 2024, 24, 314. [Google Scholar] [CrossRef]
- Li, H.; Ding, X.; Zhai, S.; Gao, K. A Retrospective Study on the Management of Massive Hemoptysis by Bronchial Artery Embolization: Risk Factors Associated with Recurrence of Hemoptysis. BMC Pulm. Med. 2023, 23, 87. [Google Scholar] [CrossRef] [PubMed]
- Claudinot, A.; Douane, F.; Morla, O.; Perret, C.; Neveu, M.; Thouveny, F.; Bouvier, A.; Hureaux, J.; Le Guen, A.; Jouan, J.; et al. Pulmonary Artery Embolization in the Management of Hemoptysis Related to Lung Tumors. JPM 2023, 13, 1597. [Google Scholar] [CrossRef]
- Yan, H.-T.; Lu, G.-D.; Huang, X.-Z.; Zhang, D.-Z.; Ge, K.-Y.; Zhang, J.-X.; Liu, J.; Liu, S.; Shi, H.-B.; Zu, Q.-Q. Development of a Model to Predict Recurrence after Bronchial Artery Embolization for Non-Cancer Related Hemoptysis. BMC Pulm. Med. 2021, 21, 419. [Google Scholar] [CrossRef]
- Dorji, K.; Hongsakul, K.; Jutidamrongphan, W.; Oofuvong, M.; Geater, S. Bronchial Artery Embolization in Life-Threatening Hemoptysis: Outcome and Predictive Factors. J. Belg. Soc. Radiol. 2021, 105, 5. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.-T.; Lu, G.-D.; Zhang, J.-X.; Zhou, C.-G.; Liu, J.; Liu, S.; Shi, H.-B.; Zu, Q.-Q. Comparison of Bronchial Artery Embolisation Versus Conservative Treatment for Bronchiectasis-Related Nonmassive Haemoptysis: A Single-Centre Retrospective Study. Cardiovasc. Interv. Radiol. 2023, 46, 369–376. [Google Scholar] [CrossRef]
- Temel, U. Bronchial Artery Embolization, an Increasingly Used Method for Hemoptysis; Treatment and Avoidance Bronchial Artery Embolization for Hemoptysis Management. Sisli Etfal 2020, 54, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Frood, R.; Karthik, S.; Mirsadraee, S.; Clifton, I.; Flood, K.; McPherson, S.J. Bronchial Artery Embolisation for Massive Haemoptysis: Immediate and Long-Term Outcomes—A Retrospective Study. Pulm. Ther. 2020, 6, 107–117. [Google Scholar] [CrossRef]
- Syha, R.; Benz, T.; Hetzel, J.; Spengler, W.; Kohlhäufl, M.; Gatidis, S.; Grözinger, G.; Horger, M.; Nikolaou, K.; Ketelsen, D. Bronchial Artery Embolization in Hemoptysis: 10-Year Survival and Recurrence-Free Survival in Benign and Malignant Etiologies—A Retrospective Study. Fortschr. Röntgenstr. 2016, 188, 1061–1066. [Google Scholar] [CrossRef]
- Dabó, H.; Gomes, R.; Marinho, A.; Madureira, M.; Paquete, J.; Morgado, P. Bronchial Artery Embolisation in Management of Hemoptysis—A Retrospective Analysis in a Tertiary University Hospital. Rev. Port. De Pneumol. (Engl. Ed.) 2016, 22, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.; Sangameswaran, K.; Prasad, B.; Sabhikhi, G.; D’Souza, J. BRONCHIAL ARTERIOGRAPHY AND TRANSCATHETER EMBOLIZATION IN MANAGEMENT OF SEVERE HAEMOPTYSIS. Med. J. Armed Forces India 1999, 55, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.-T.; Lu, G.-D.; Liu, J.; Liu, S.; Shi, H.-B.; Zhou, C.-G.; Zu, Q.-Q. Does the Presence of Systemic Artery–Pulmonary Circulation Shunt during Bronchial Arterial Embolization Increase the Recurrence of Noncancer-Related Hemoptysis? A Retrospective Cohort Study. Respir. Res. 2023, 24, 119. [Google Scholar] [CrossRef] [PubMed]


| PVA | Microspheres | NBCA | GS | Coils | |
|---|---|---|---|---|---|
| Group of embolic agents | Particulate—irregular; 300–500 µm is most often selected | Particulate—standardised size range of 100–1300 μm and spherical shape | Liquid | Particulate—irregular; the range of 0.5–2 mm is commonly used | Mechanical Occlusion Devices—irregular; range of size is commonly designated in three numbers |
| Mechanism of action | When released into the bloodstream from a microcatheter, particles expand and occlude the targeted vessel | Mechanical occlusion of vessels, conforming to their size | Polymerises upon contact with tissues, forming a permanent occlusion | Uptake large amounts of physiological fluids, initiation of clotting by providing a mechanical matrix. GS acts as a scaffold to promote cell adhesion and tissue regeneration | Catheter-provided embolisation, in contact with blood instantly forms thrombi, achieving excellent haemostasis |
| Main advantages | Low cost; availability; customizable fit; well-documented safety in BAE | Aggregation-resistant; compressible; wide range of sizes; low cost | Low recanalisation rate; short time of procedure; radiopaque; more distal-embolisation | Widely available; relatively inexpensive; safe; easily modified to the desired sizes and accumulated years of medical use; no impact on haemodynamics | Wide range of sizes, shapes, deployment methods and coating structures; fluoroscopic visibility; safe; used independently or in combination with other embolisation agents |
| Main disadvantages | Poor adhesion to the endothelium; Aggregation within the catheter; Radiolucent | Radiolucent; Immunogenic | Requires significant experience; Increased risk of non-target embolisation and tissue necrosis | Degradable nature, poor haemostatic effectiveness in patients with pulmonary aspergilloma and increase injury infection | Higher length of hospitalisation and total health care cost; Requires experienced interventionists |
| Control over embolisation | Low—size selection only; irregular shape may lead to unpredictable aggregation | High—uniform size and shape allow better control | Moderate—NBCA-Lipidol ratio determines polymerisation speed | Moderate—wide range of size, impermanence of the process | Moderate—customizable fit |
| Ability to embolise small vessels | Limited-aggregation may block larger vessels | High-size selection allows embolisation of both small and large vessels | High-reaches the most distal vessels | High-customizable size can be used in smaller calibre vessels | Limited-metallic structure provides a more proximal blockage |
| Risk of reflux/non-target embolisation | Moderate-mainly when particle sizes are below 300 µm or in the presence of shunts | Moderate-mainly when particle sizes are below 300 µm, in the presence of shunts and after adding a contrast agent | High-especially due to technical errors such as wedge positioning of the microcatheter, injecting an excessive volume of the mixture, or prematurely withdrawing the microcatheter | Moderate-diameters smaller than 300 μm may pass through | Low-due to embolisation at the intended position |
| Use in BAE | Frequently used in standard cases (e.g., bronchiectasis) | Haemoptysis associated with malignancies; Other similar to PVA | Haemoptysis associated with malignancies; Effective in massive haemoptysis; Efficacy similar to particulate agents in chronic, progressive diseases | Mostly for cryptogenic haemoptysis; Severe persistent haemoptysis | Used for massive haemoptysis, especially in CF, aneurysms, AVMs |
| Durability of effect | Permanent, although recanalisation frequently occur | Permanent or temporary (depend of material biodegradability) | Permanent embolisation | Temporary embolisation | Permanent occlusion |
| Additional functions | No additional functions | Might contain medical preparations or radioactive isotopes | May help prevent tumour growth by permanent cutting off blood supply | No additional function | No additional functions |
| Possible complications | Premature embolisation due to aggregation within the catheter; Non-target embolisation; | Non-target embolisation; Foreign-body reactions | Non-target embolisation; Risk of catheter adhesion; Tissue necrosis | Non-target embolisation; Risk of compression of approximal structures; Increase wound infection | Complicate the embolisation of the distal part of the same vessel; Increase wound infection; Unintended embolisation |
| Aggregated Outcomes Across Included Studies [4,5,9,15,17,22,23,27,31,32,35,42,46,49,50,51] 1 | |||||
| Average Complications Rate | 23.7% | 28.2% | 14.9% | 3.25% | N/A |
| Average Clinical Success | 96.9% | 95.9% | 97.9% | 97.5% | 94.9% |
| Recurrence Rates (Range) | 8.9–81.9% | 6.3–61.6% | 3.3–9.5% | 17.4–26.9% | 8.4–51.2% |
| Average Recurrence Rate | 36.2% | 25.9% | 7.8% | 22.2% | 31.6% |
| Mechanisms of Recurrence—Based on Selected Individual Studies | |||||
| Leading Recurrence Mechanisms | Incomplete embolisation Disease progression Collateral recruitment Premature embolisation Recanalisation due to lack of adhesion [17,19,31] | Incomplete embolisation Disease progression Collateral recruitment [17,31] | Incomplete identification of HRAs [5,28] | Recanalisation due to resorption [52] | Recanalisation due to coil compaction Incomplete occlusion Collateral recruitment [46,48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Polish Respiratory Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziętarska, A.; Dobek, A.; Sawina, A.; Białek, P.; Majewski, S.; Stefańczyk, L. Bronchial Artery Embolisation in Haemoptysis Management: A Scoping Review with Emphasis on Embolic Materials and Indications. Adv. Respir. Med. 2025, 93, 35. https://doi.org/10.3390/arm93050035
Ziętarska A, Dobek A, Sawina A, Białek P, Majewski S, Stefańczyk L. Bronchial Artery Embolisation in Haemoptysis Management: A Scoping Review with Emphasis on Embolic Materials and Indications. Advances in Respiratory Medicine. 2025; 93(5):35. https://doi.org/10.3390/arm93050035
Chicago/Turabian StyleZiętarska, Anna, Adam Dobek, Anna Sawina, Piotr Białek, Sebastian Majewski, and Ludomir Stefańczyk. 2025. "Bronchial Artery Embolisation in Haemoptysis Management: A Scoping Review with Emphasis on Embolic Materials and Indications" Advances in Respiratory Medicine 93, no. 5: 35. https://doi.org/10.3390/arm93050035
APA StyleZiętarska, A., Dobek, A., Sawina, A., Białek, P., Majewski, S., & Stefańczyk, L. (2025). Bronchial Artery Embolisation in Haemoptysis Management: A Scoping Review with Emphasis on Embolic Materials and Indications. Advances in Respiratory Medicine, 93(5), 35. https://doi.org/10.3390/arm93050035








