The Safety and Performance of a Novel Extracorporeal Membrane Oxygenation Device in a Long-Term Ovine Model
Abstract
Highlights
- In this work, we evaluated a novel extracorporeal membrane oxygenation (ECMO) device (Lifemotion®, Chinabridge, China) with veno-nenous (VV) and veno-arterial (VA) ECMO in a ovine model for assessing the safety and performance of the ECMO device and found the ECMO device could support in keeping experimental sheep alive for 14 days and showed a comparable performance as the marketed product (Novalung® Xlung™ kit 230, Xonis, Germany).
- This research also supported the preclinical animal trials of Lifemotion®, the first ECMO device approved in China for market launch.
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics and Animal Groups
2.2. ECMO Device Information
2.3. Trial Process and Post-Surgical Care
2.3.1. Preparation Before Operation
2.3.2. Priming of the ECMO Devices
2.4. Operation Procedures
2.4.1. Anesthesia and Endotracheal Intubation
2.4.2. Constructing Arteriovenous Access for Monitoring Vital Signs
2.5. Standard Management After Operation
2.5.1. Critical Management
2.5.2. General Care and Treatment
2.5.3. The Management of Bleeding and Anticoagulation
2.5.4. Post-Operation Care, Monitoring, and Data Collection
2.5.5. Histological Analysis
2.5.6. Data Record and Collection
2.6. Proteomic Analysis
2.7. Data Statistics
3. Results
3.1. All Sheep Subjects Survived Until the End of the Trial Without Significant Thrombosis
3.2. The Sheep Subjects of the LIFEMOTION Group Showed Stable Vital Signs During ECMO Support
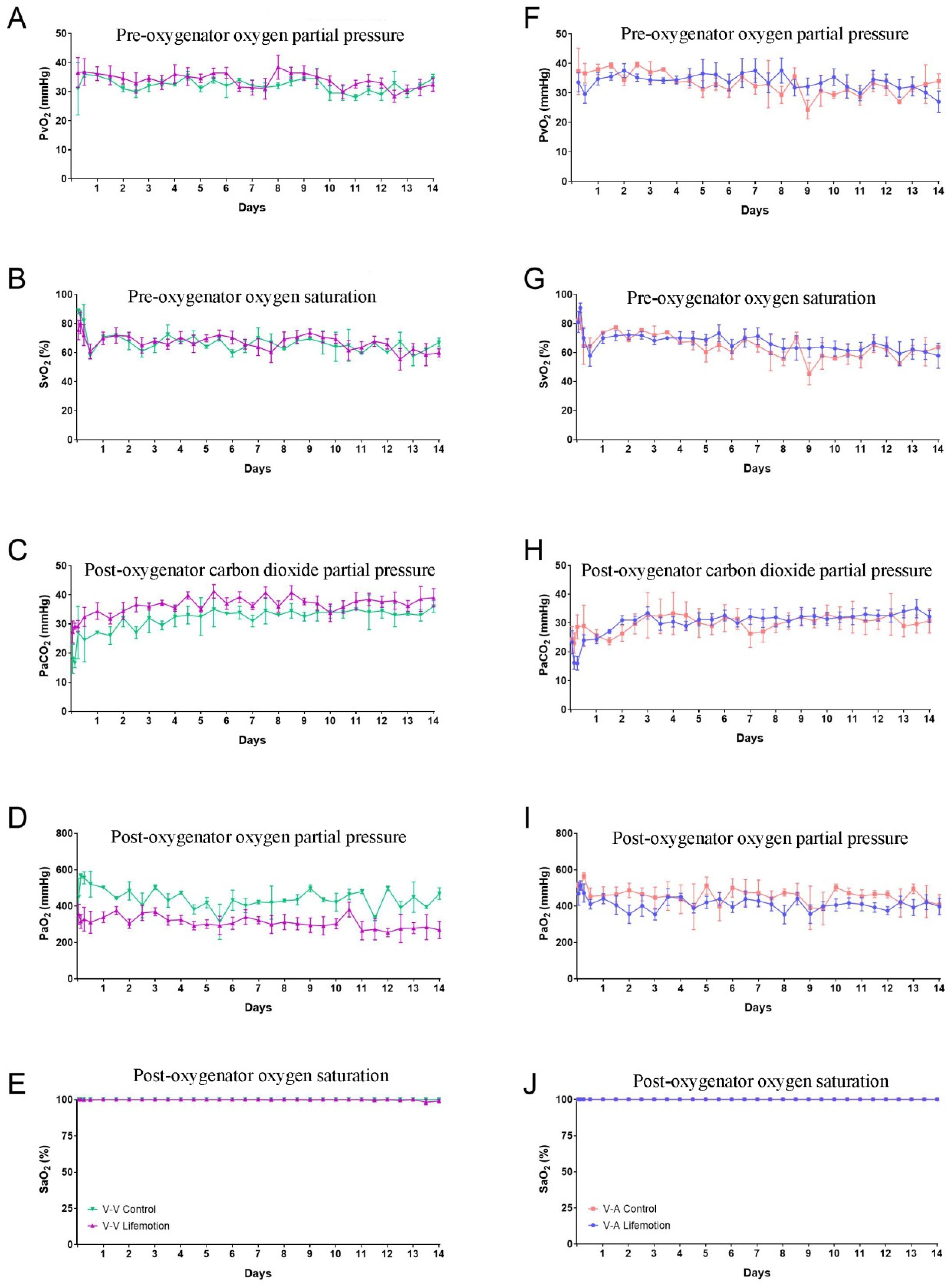
3.3. Sheep Subjects of the LIFEMOTION Group Had Satisfying Oxygenation During ECMO Support
3.4. Sheep Subjects Showed Adequate Anticoagulation Management During ECMO Support
3.5. The Oxygenator of the LIFEMOTION Group Showed Comparable Performance in Oxygenation to the Control Group During ECMO
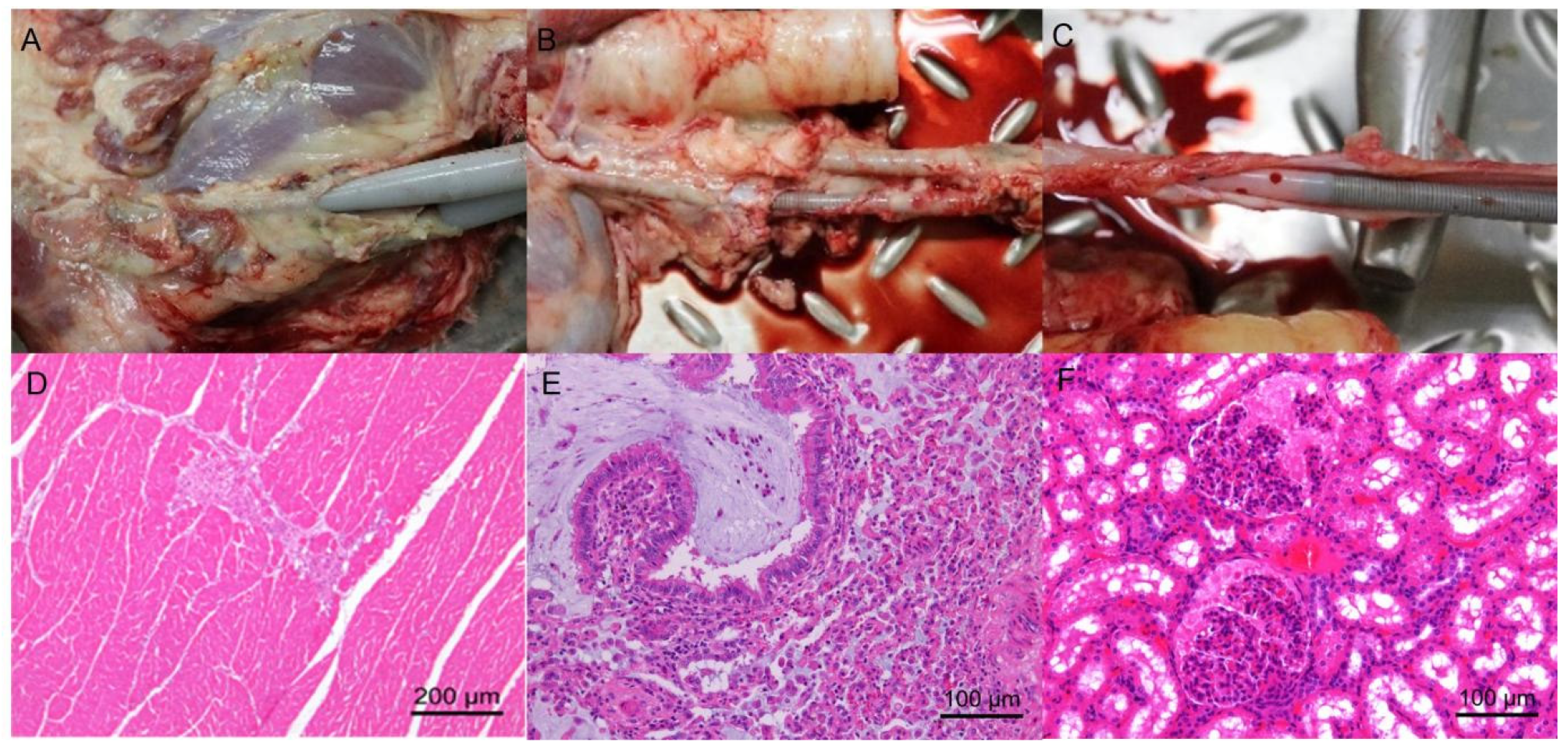
3.6. Pathological Analysis Revealed No Major Complications or Organ Damage
3.7. The Novel Long-Term ECMO Device Had a Negligible Impact on the Animal’s Serum Protein Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qi, J.; Gao, S.; Liu, G.; Yan, S.; Zhang, M.; Yan, W.; Zhang, Q.; Teng, Y.; Wang, J.; Zhou, C.; et al. An Ovine Model of Awake Veno-Arterial Extracorporeal Membrane Oxygenation. Front. Vet. Sci. 2021, 8, 809487. [Google Scholar] [CrossRef]
- Marasco, S.F.; Lukas, G.; McDonald, M.; McMillan, J.; Ihle, B. Review of ECMO (extra corporeal membrane oxygenation) support in critically ill adult patients. Heart Lung Circ. 2008, 17, S41–S47. [Google Scholar] [CrossRef]
- Supady, A.; Combes, A.; Barbaro, R.P.; Camporota, L.; Diaz, R.; Fan, E.; Giani, M.; Hodgson, C.; Hough, C.L.; Karagiannidis, C.; et al. Respiratory indications for ECMO: Focus on COVID-19. Intensive Care Med. 2022, 48, 1326–1337. [Google Scholar] [CrossRef]
- Milewski, R.C.; Chatterjee, S.; Merritt-Genore, H.; Hayanga, J.W.A.; Grant, M.C.; Roy, N.; Hirose, H.; Moosdorf, R.; Whitman, G.J.; Haft, J.W.; et al. ECMO During COVID-19: A Society of Thoracic Surgeons/Extracorporeal Life Support Organization Survey. Ann. Thorac. Surg. Short Rep. 2023, 1, 168–173. [Google Scholar] [CrossRef]
- Barbaro, R.P.; MacLaren, G.; Boonstra, P.S.; Iwashyna, T.J.; Slutsky, A.S.; Fan, E.; Bartlett, R.H.; Tonna, J.E.; Hyslop, R.; Fanning, J.J.; et al. Extracorporeal membrane oxygenation support in COVID-19: An international cohort study of the Extracorporeal Life Support Organization registry. Lancet 2020, 396, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Hajage, D.; Lebreton, G.; Monsel, A.; Voiriot, G.; Levy, D.; Baron, E.; Beurton, A.; Chommeloux, J.; Meng, P.; et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: A retrospective cohort study. Lancet Respir. Med. 2020, 8, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Hogen, R.; Sedra, A.H.; Motamed, A.; Emamaullee, J. The evolving role of ECMO in liver transplantation. Curr. Opin. Organ Transpl. 2021, 26, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Mou, Z.; He, J.; Guan, T.; Chen, L. Acute kidney injury during extracorporeal membrane oxygenation: VA ECMO versus VV ECMO. J. Intensive Care Med. 2022, 37, 743–752. [Google Scholar] [CrossRef]
- Brodie, D.; Bacchetta, M. Extracorporeal membrane oxygenation for ARDS in adults. N. Engl. J. Med. 2011, 365, 1905–1914. [Google Scholar] [CrossRef]
- Wrisinger, W.C.; Thompson, S.L. Basics of Extracorporeal Membrane Oxygenation. Surg. Clin. N. Am. 2022, 102, 23–35. [Google Scholar] [CrossRef]
- Le Gall, A.; Follin, A.; Cholley, B.; Mantz, J.; Aissaoui, N.; Pirracchio, R. Veno-arterial-ECMO in the intensive care unit: From technical aspects to clinical practice. Anaesth. Crit. Care Pain Med. 2018, 37, 259–268. [Google Scholar] [CrossRef]
- Nasser, M.F.; Jabri, A.; Sharma, S.; Alhuneafat, L.; Omar, Y.A.; Krishnan, V.; Cameron, S.J. Outcomes with use of extra-corporeal membrane oxygenation in high-risk pulmonary embolism: A national database perspective. J. Thromb. Thrombolysis 2023, 55, 499–505. [Google Scholar] [CrossRef]
- Gerfer, S.; Djordjevic, I.; Maier, J.; Movahed, A.; Elskamp, M.; Kuhn, E.; Liakopoulos, O.; Wahlers, T.; Deppe, A.C. Endothelial and Hemodynamic Function in a Large Animal Model in Relation to Different Extracorporeal Membrane Oxygenation Cannulation Strategies and Intra-Aortic Balloon Pumping. J. Clin. Med. 2023, 12, 4038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, R.; Pei, S.; Gao, S.; Sun, Y.; Cheng, G.; Yu, D.; Wang, X.; Gao, Z.; Ji, B. Neutrophil extracellular traps are increased after extracorporeal membrane oxygenation support initiation and present in thrombus: A preclinical study using sheep as an animal model. Thromb. Res. 2023, 221, 173–182. [Google Scholar]
- Hartmund Frederiksen, P.; Linde, L.; Gregers, E.; Udesen, N.; Helgestad, O.; Banke, A.; Dahl, J.; Jensen, L.; Lassen, J.; Larsen, J. Cardiac energetics and end-organ perfusion with veno-arterial extracorporeal membrane oxygenation versus ecmella for cardiogenic shock in a large animal model. Eur. Heart J. 2023, 44, ehad655.1153. [Google Scholar] [CrossRef]
- Millar, J.E.; Bartnikowski, N.; von Bahr, V.; Malfertheiner, M.V.; Obonyo, N.G.; Belliato, M.; Suen, J.Y.; Combes, A.; McAuley, D.F.; Lorusso, R. Extracorporeal membrane oxygenation (ECMO) and the acute respiratory distress syndrome (ARDS): A systematic review of pre-clinical models. Intensive Care Med. Exp. 2019, 7, 18. [Google Scholar] [CrossRef]
- Silva, K.A.S.; Emter, C.A. Large animal models of heart failure: A translational bridge to clinical success. Basic Transl. Sci. 2020, 5, 840–856. [Google Scholar]
- Yates, W.G.; Schaap, R.N.; Baumann, G.C. Effects of filler-free silicone rubber on platelets during bovine extracorporeal membrane oxygenation. Trans. Am. Soc. Artif. Intern. Organs 1978, 24, 644–649. [Google Scholar]
- Djordjevic, I.; Maier-Trauth, J.; Gerfer, S.; Elskamp, M.; Muehlbauer, T.; Maul, A.; Rademann, P.; Ivanov, B.; Krasivskyi, I.; Sabashnikov, A.; et al. Fluid Management in Veno-Arterial Extracorporeal Membrane Oxygenation Therapy-Analysis of an Experimental Pig Model. J. Clin. Med. 2023, 12, 5330. [Google Scholar] [CrossRef] [PubMed]
- Karagiannidis, C.; Joost, T.; Strassmann, S.; Weber-Carstens, S.; Combes, A.; Windisch, W.; Brodie, D. Safety and Efficacy of a Novel Pneumatically Driven Extracorporeal Membrane Oxygenation Device. Ann. Thorac. Surg. 2020, 109, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, K.; Katagiri, N.; Takewa, Y.; Tsukiya, T.; Mizuno, T.; Itamochi, Y.; Kumano, K.; Tatsumi, E. Evaluation of the Novel Centrifugal Pump, CAPIOX SL, in Chronic Large Animal Experiments. Artif. Organs 2018, 42, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Brekke, I.J.; Puntervoll, L.H.; Pedersen, P.B.; Kellett, J.; Brabrand, M. The value of vital sign trends in predicting and monitoring clinical deterioration: A systematic review. PLoS ONE 2019, 14, e0210875. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.A.; Onyskiw, J.E.; Prasad, N.G. Meta-analysis of arterial oxygen saturation monitoring by pulse oximetry in adults. Heart Lung 1998, 27, 387–408. [Google Scholar] [CrossRef]
- Wagner, P.D. The physiological basis of pulmonary gas exchange: Implications for clinical interpretation of arterial blood gases. Eur. Respir. J. 2015, 45, 227–243. [Google Scholar] [CrossRef]
- Arbiol-Roca, A.; Imperiali, C.E.; Dot-Bach, D.; Valero-Politi, J.; Dastis-Arias, M. Stability of pH, Blood Gas Partial Pressure, Hemoglobin Oxygen Saturation Fraction, and Lactate Concentration. Ann. Lab. Med. 2020, 40, 448–456. [Google Scholar] [CrossRef]
- Millar, J.E.; Fanning, J.P.; McDonald, C.I.; McAuley, D.F.; Fraser, J.F. The inflammatory response to extracorporeal membrane oxygenation (ECMO): A review of the pathophysiology. Crit. Care 2016, 20, 387. [Google Scholar] [CrossRef]
- Gao, S.; Wang, W.; Qi, J.; Liu, G.; Wang, J.; Yan, S.; Teng, Y.; Zhou, C.; Wang, Q.; Yan, W.; et al. Safety and Efficacy of a Novel Centrifugal Pump and Driving Devices of the OASSIST ECMO System: A Preclinical Evaluation in the Ovine Model. Front. Med. 2021, 8, 712205. [Google Scholar] [CrossRef]
- Hou, X. Anticoagulation monitoring in extracorporeal membrane oxygenation. Perfusion 2021, 36, 438–439. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmi, M.H.; Tiede, A.; Teebken, O.E.; Bisdas, T.; Haverich, A.; Mischke, R. Ovine blood: Establishment of a list of reference values relevant for blood coagulation in sheep. ASAIO J. 2012, 58, 79–82. [Google Scholar] [CrossRef]
- Madhani, S.P.; Frankowski, B.J.; Burgreen, G.W.; Antaki, J.F.; Kormos, R.; D’Cunha, J.; Federspiel, W.J. In vitro and in vivo evaluation of a novel integrated wearable artificial lung. J. Heart Lung Transpl. 2017, 36, 806–811. [Google Scholar] [CrossRef]
- Zeibi Shirejini, S.; Carberry, J.; McQuilten, Z.K.; Burrell, A.J.; Gregory, S.D.; Hagemeyer, C.E. Current and future strategies to monitor and manage coagulation in ECMO patients. Thromb. J. 2023, 21, 11. [Google Scholar] [CrossRef]
- Levy, J.H.; Staudinger, T.; Steiner, M.E. How to manage anticoagulation during extracorporeal membrane oxygenation. Intensive Care Med. 2022, 48, 1076–1079. [Google Scholar] [CrossRef]
- Kato, C.; Oakes, M.; Kim, M.; Desai, A.; Olson, S.R.; Raghunathan, V.; Shatzel, J.J. Anticoagulation strategies in extracorporeal circulatory devices in adult populations. Eur. J. Haematol. 2021, 106, 19–31. [Google Scholar] [CrossRef]
- He, B.; Huang, Z.; Huang, C.; Nice, E.C. Clinical applications of plasma proteomics and peptidomics: Towards precision medicine. Proteom.–Clin. Appl. 2022, 16, 2100097. [Google Scholar] [CrossRef]
- Li, L.; Sun, C.; Sun, Y.; Dong, Z.; Wu, R.; Sun, X.; Zhang, H.; Jiang, W.; Zhou, Y.; Cen, X. Spatially resolved proteomics via tissue expansion. Nat. Commun. 2022, 13, 7242. [Google Scholar] [CrossRef] [PubMed]
- Gerdle, B.; Wåhlén, K.; Gordh, T.; Bäckryd, E.; Carlsson, A.; Ghafouri, B. Plasma proteins from several components of the immune system differentiate chronic widespread pain patients from healthy controls–an exploratory case-control study combining targeted and non-targeted protein identification. Medicine 2022, 101, e31013. [Google Scholar] [CrossRef]
- Cheng, R.; Hachamovitch, R.; Kittleson, M.; Patel, J.; Arabia, F.; Moriguchi, J.; Esmailian, F.; Azarbal, B. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: A meta-analysis of 1,866 adult patients. Ann. Thorac. Surg. 2014, 97, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Zhang, L.; Bao, L. Complications and mortality of venovenous extracorporeal membrane oxygenation in the treatment of neonatal respiratory failure: A systematic review and meta-analysis. BMC Pulm. Med. 2020, 20, 124. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-Y.; Ko, W.-J.; Tsai, P.-R.; Sun, C.-C.; Chang, Y.-Y.; Lee, C.-W.; Chen, Y.-C. Infections occurring during extracorporeal membrane oxygenation use in adult patients. J. Thorac. Cardiovasc. Surg. 2010, 140, 1125–1132.e1122. [Google Scholar] [CrossRef]
- Desco, M.; Cano, M.J.; Duarte, J.; Rodriguez, F.; Fernández-Caleya, D.; Alvarez-Valdivielso, M.; Antoranz, J.C.; Rubio, M.A.; García-Barreno, P.; del Cañizo, J.F. Blood biochemistry values of sheep (Ovis aries ligeriensis). Comp. Biochem. Physiol A Comp. Physiol. 1989, 94, 717–719. [Google Scholar] [CrossRef]
- Weiss-Tessbach, M.; Ratzinger, F.; Obermueller, M.; Burgmann, H.; Staudinger, T.; Robak, O.; Schmid, M.; Roessler, B.; Jilma, B.; Kussmann, M.; et al. Biomarkers for differentiation of coronavirus disease 2019 or extracorporeal membrane oxygenation related inflammation and bacterial/fungal infections in critically ill patients: A prospective observational study. Front. Med. 2022, 9, 917606. [Google Scholar] [CrossRef]
- Schopka, S.; Philipp, A.; Müller, T.; Lubnow, M.; Lunz, D.; Unterbuchner, C.; Rupprecht, L.; Keyser, A.; Schmid, C. The impact of interleukin serum levels on the prognosis of patients undergoing venoarterial extracorporeal membrane oxygenation. Artif. Organs 2020, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Kuo, S.W.; Ko, W.J.; Tsai, P.R.; Wu, S.W.; Lai, C.H.; Wang, C.-H.; Chen, Y.-S.; Chen, P.-L.; Liu, T.-T.; et al. Early measurement of IL-10 predicts the outcomes of patients with acute respiratory distress syndrome receiving extracorporeal membrane oxygenation. Sci. Rep. 2017, 7, 1021. [Google Scholar] [CrossRef] [PubMed]
- Veraar, C.; Kirschner, E.; Schwarz, S.; Jaksch, P.; Hoetzenecker, K.; Tschernko, E.; Dworschak, M.; Ankersmit, H.J.; Moser, B. Follistatin-like 1 and Biomarkers of Neutrophil Activation Are Associated with Poor Short-Term Outcome after Lung Transplantation on VA-ECMO. Biology 2022, 11, 1475. [Google Scholar] [CrossRef]
- Li, J.; Yu, Z.; Zeng, J.; Liu, Z.; Zhao, Z.; Zhang, Y.; Li, G. Circular RNA UBAP2 (hsa_circ_0007367) Correlates with Microcirculatory Perfusion and Predicts Outcomes of Cardiogenic Shock Patients Undergoing Extracorporeal Membrane Oxygenation Support. Shock 2022, 57, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Coletti, K.; Griffiths, M.; Nies, M.; Brandal, S.; Everett, A.D.; Bembea, M.M. Cardiac Dysfunction Biomarkers are Associated with Potential for Successful Separation from Extracorporeal Membrane Oxygenation in Children. Asaio J. 2022, 69, 198–204. [Google Scholar] [CrossRef]
- Tsuchida, T.; Wada, T.; Gando, S. Coagulopathy Induced by Veno-Arterial Extracorporeal Membrane Oxygenation Is Associated With a Poor Outcome in Patients With Out-of-Hospital Cardiac Arrest. Front. Med. 2021, 8, 651832. [Google Scholar]
- Siegel, P.M.; Orlean, L.; Bojti, I.; Kaier, K.; Witsch, T.; Esser, J.S.; Trummer, G.; Moser, M.; Peter, K.; Bode, C.; et al. Monocyte Dysfunction Detected by the Designed Ankyrin Repeat Protein F7 Predicts Mortality in Patients Receiving Veno-Arterial Extracorporeal Membrane Oxygenation. Front. Cardiovasc. Med. 2021, 8, 689218. [Google Scholar] [CrossRef]
- Ghaleb, S.; Reagor, J.A.; Tarango, C.; Benscoter, A.; Smith, R.; Byrnes, J.W. Correlation among Hemolysis Biomarkers in Pediatric Patients Undergoing Extracorporeal Membrane Oxygenation. J. Extra Corpor. Technol. 2021, 53, 125–129. [Google Scholar] [CrossRef]
- Pais, P.; Robinson, S.; Majithia-Beet, G.; Lotto, A.; Kumar, T.; Westrope, C.; Sullo, N.; Eagle, H.; Bryony, B.; Joel-David, L.; et al. Biomarkers of Inflammation and Lung Recovery in Extracorporeal Membrane Oxygenation Patients With Persistent Pulmonary Hypertension of the Newborn: A Feasibility Study. Pediatr. Crit. Care Med. 2020, 21, 363–372. [Google Scholar] [CrossRef]
- Lee, A.E.; Pandiyan, P.; Liu, M.M.; Williams, M.A.; Everett, A.D.; Mueller, G.P.; Morriss, M.C.; Raman, L.; Carlson, D.; Gatson, J.W. Tau Is Elevated in Pediatric Patients on Extracorporeal Membrane Oxygenation. Asaio J. 2020, 66, 91–96. [Google Scholar] [CrossRef]
- Yang, L.; Fan, Y.; Lin, R.; He, W. Blood Lactate as a Reliable Marker for Mortality of Pediatric Refractory Cardiogenic Shock Requiring Extracorporeal Membrane Oxygenation. Pediatr. Cardiol. 2019, 40, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, C.S.; Yuan, X.L.; Ling, J.Y.; Zhang, Q.; Liang, Y.; Liu, B.; Zhao, L.X. Association of serum biomarkers with outcomes of cardiac arrest patients undergoing ECMO. Am. J. Emerg. Med. 2018, 36, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, S.P.; Kim, J.B.; Jung, S.H.; Choo, S.J.; Chung, C.H.; Lee, J.W. Blood lactate level during extracorporeal life support as a surrogate marker for survival. J. Thorac. Cardiovasc. Surg. 2014, 148, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Schrage, B.; Rübsamen, N.; Becher, P.M.; Roedl, K.; Söffker, G.; Schwarzl, M.; Dreher, A.; Schewel, J.; Ghanem, A.; Grahn, H.; et al. Neuron-specific-enolase as a predictor of the neurologic outcome after cardiopulmonary resuscitation in patients on ECMO. Resuscitation 2019, 136, 14–20. [Google Scholar] [CrossRef]
- Wilm, J.; Philipp, A.; Müller, T.; Bredthauer, A.; Gleich, O.; Schmid, C.; Lehle, K. Leukocyte Adhesion as an Indicator of Oxygenator Thrombosis During Extracorporeal Membrane Oxygenation Therapy? Asaio J. 2018, 64, 24–30. [Google Scholar] [CrossRef]
- Tan, V.E.; Moore, W.S.; Chopra, A.; Cies, J.J. Association of procalcitonin values and bacterial infections in pediatric patients receiving extracorporeal membrane oxygenation. Perfusion 2018, 33, 278–282. [Google Scholar]
- McVey, M.J.; Kuebler, W.M. Extracellular vesicles: Biomarkers and regulators of vascular function during extracorporeal circulation. Oncotarget 2018, 9, 37229–37251. [Google Scholar] [CrossRef][Green Version]
- Chung, J.H.; Yeo, H.J.; Kim, D.; Lee, S.M.; Han, J.; Kim, M.; Cho, W.H. Changes in the levels of beta-thromboglobulin and inflammatory mediators during extracorporeal membrane oxygenation support. Int. J. Artif. Organs 2017, 40, 575–580. [Google Scholar] [CrossRef]
- Lyu, L.; Long, C.; Hei, F.; Ji, B.; Liu, J.; Yu, K.; Chen, L.; Yao, J.; Hu, Q.; Hu, J.; et al. Plasma Free Hemoglobin Is a Predictor of Acute Renal Failure During Adult Venous-Arterial Extracorporeal Membrane Oxygenation Support. J. Cardiothorac. Vasc. Anesth. 2016, 30, 891–895. [Google Scholar] [CrossRef]
- Omar, H.R.; Mirsaeidi, M.; Socias, S.; Sprenker, C.; Caldeira, C.; Camporesi, E.M.; Mangar, D. Plasma Free Hemoglobin Is an Independent Predictor of Mortality among Patients on Extracorporeal Membrane Oxygenation Support. PLoS One 2015, 10, e0124034. [Google Scholar] [CrossRef]
- Liu, C.H.; Kuo, S.W.; Hsu, L.M.; Huang, S.C.; Wang, C.H.; Tsai, P.R.; Chen, Y.S.; Jou, T.S.; Ko, W.J. Peroxiredoxin 1 induces inflammatory cytokine response and predicts outcome of cardiogenic shock patients necessitating extracorporeal membrane oxygenation: An observational cohort study and translational approach. J. Transl. Med. 2016, 14, 114. [Google Scholar] [CrossRef] [PubMed]
- Bembea, M.M.; Rizkalla, N.; Freedy, J.; Barasch, N.; Vaidya, D.; Pronovost, P.J.; Everett, A.D.; Mueller, G. Plasma Biomarkers of Brain Injury as Diagnostic Tools and Outcome Predictors After Extracorporeal Membrane Oxygenation. Crit. Care Med. 2015, 43, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Astoria, M.T.; Karam, S.E.; Moores, R.R., Jr.; Rozycki, H.J. Cardiac Troponin Levels in Neonates Who Require ECMO for Noncardiac Indications Are Elevated in Nonsurvivors. Am. J. Perinatol. 2015, 32, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Lubnow, M.; Philipp, A.; Dornia, C.; Schroll, S.; Bein, T.; Creutzenberg, M.; Lehle, K. D-dimers as an early marker for oxygenator exchange in extracorporeal membrane oxygenation. J. Crit. Care 2014, 29, 473.e1–473.e5. [Google Scholar] [CrossRef]

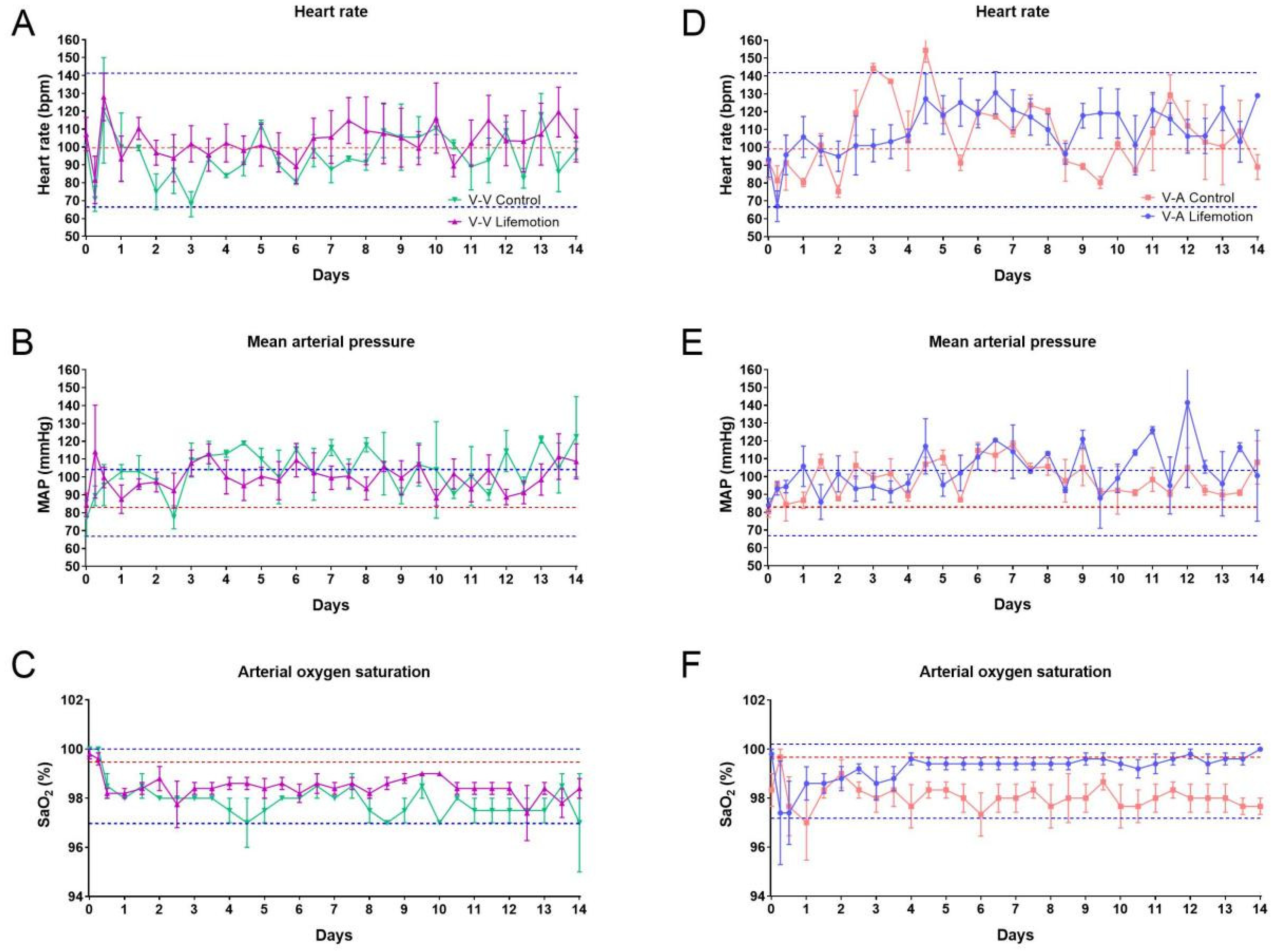
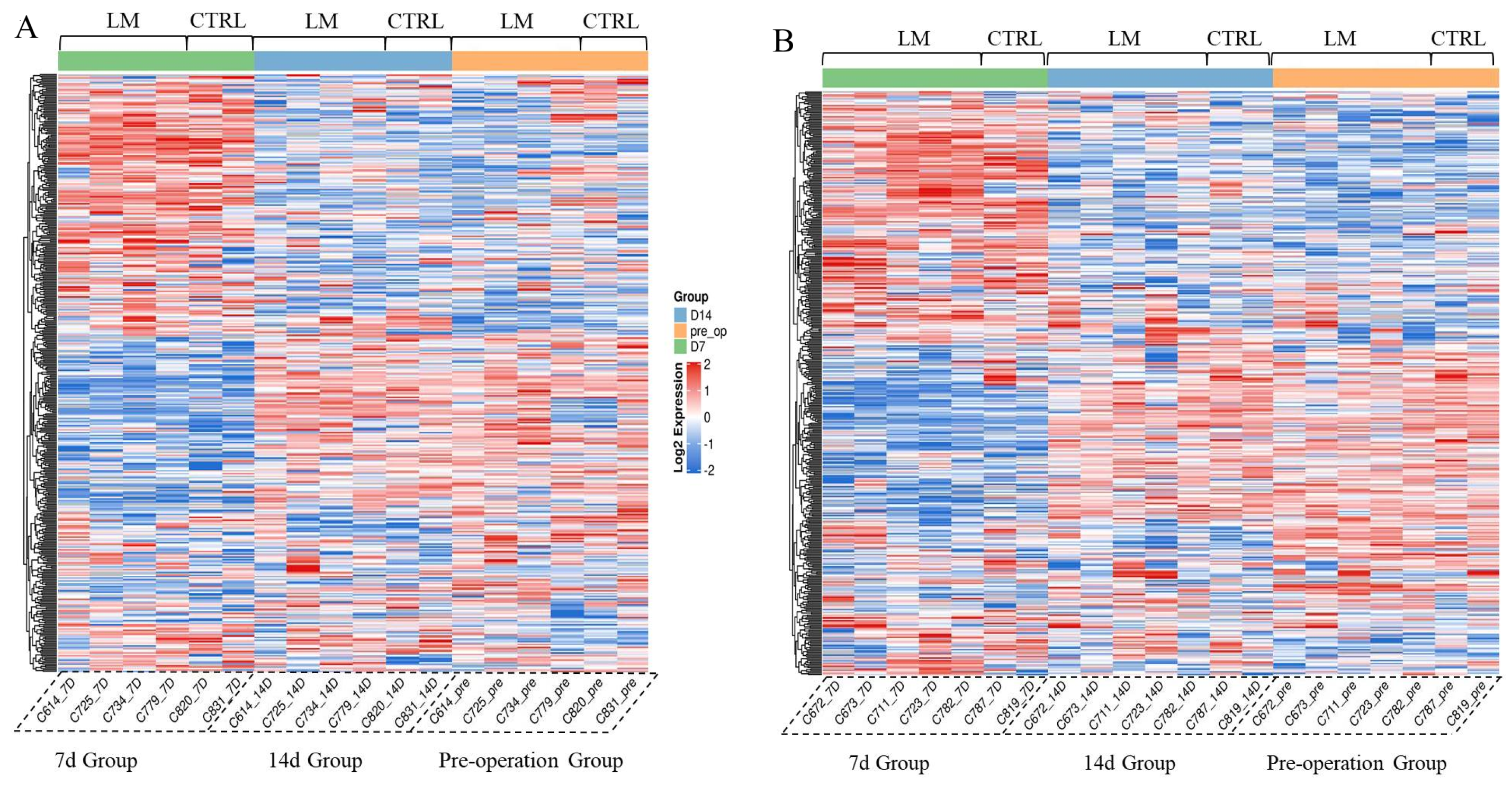

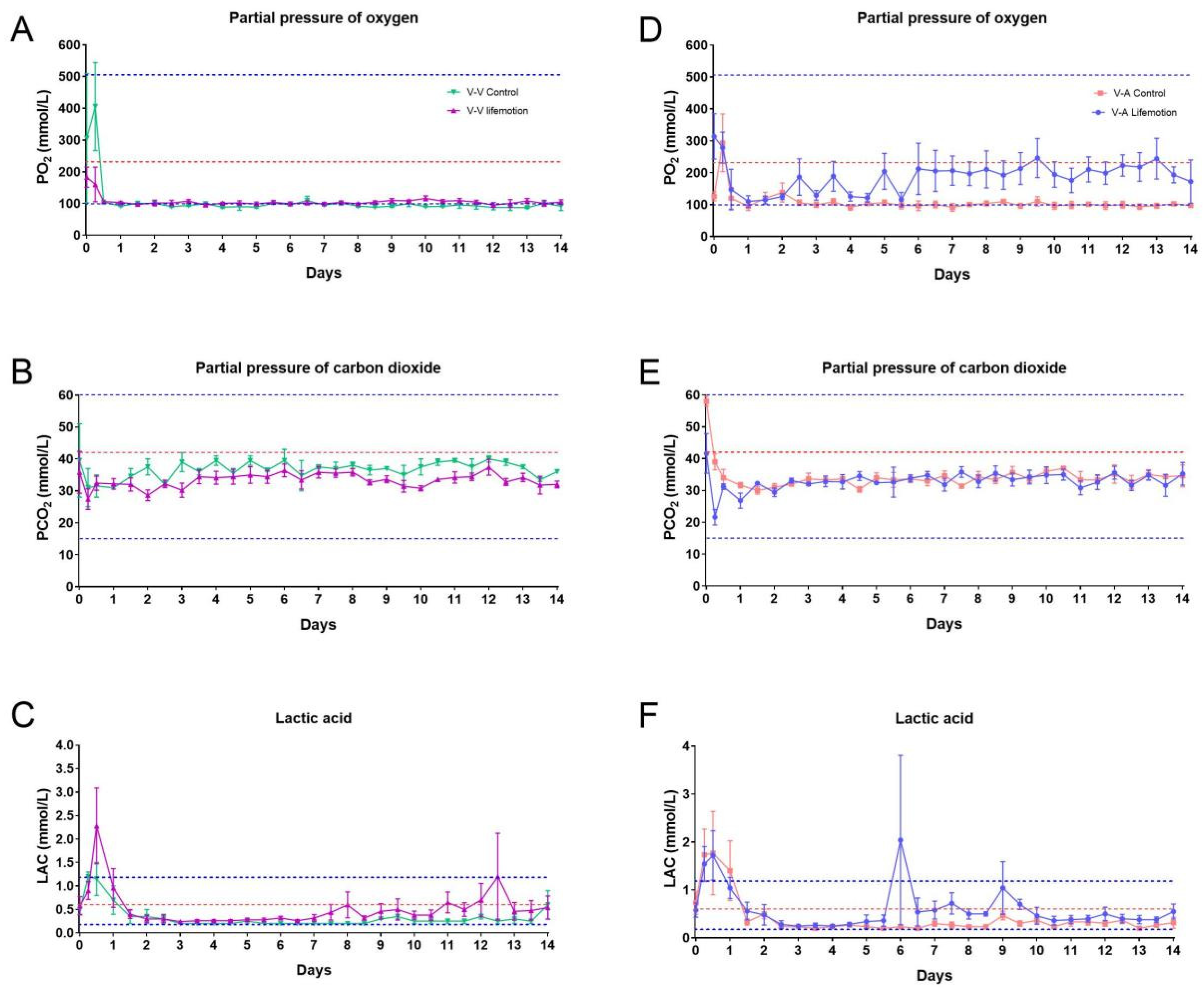
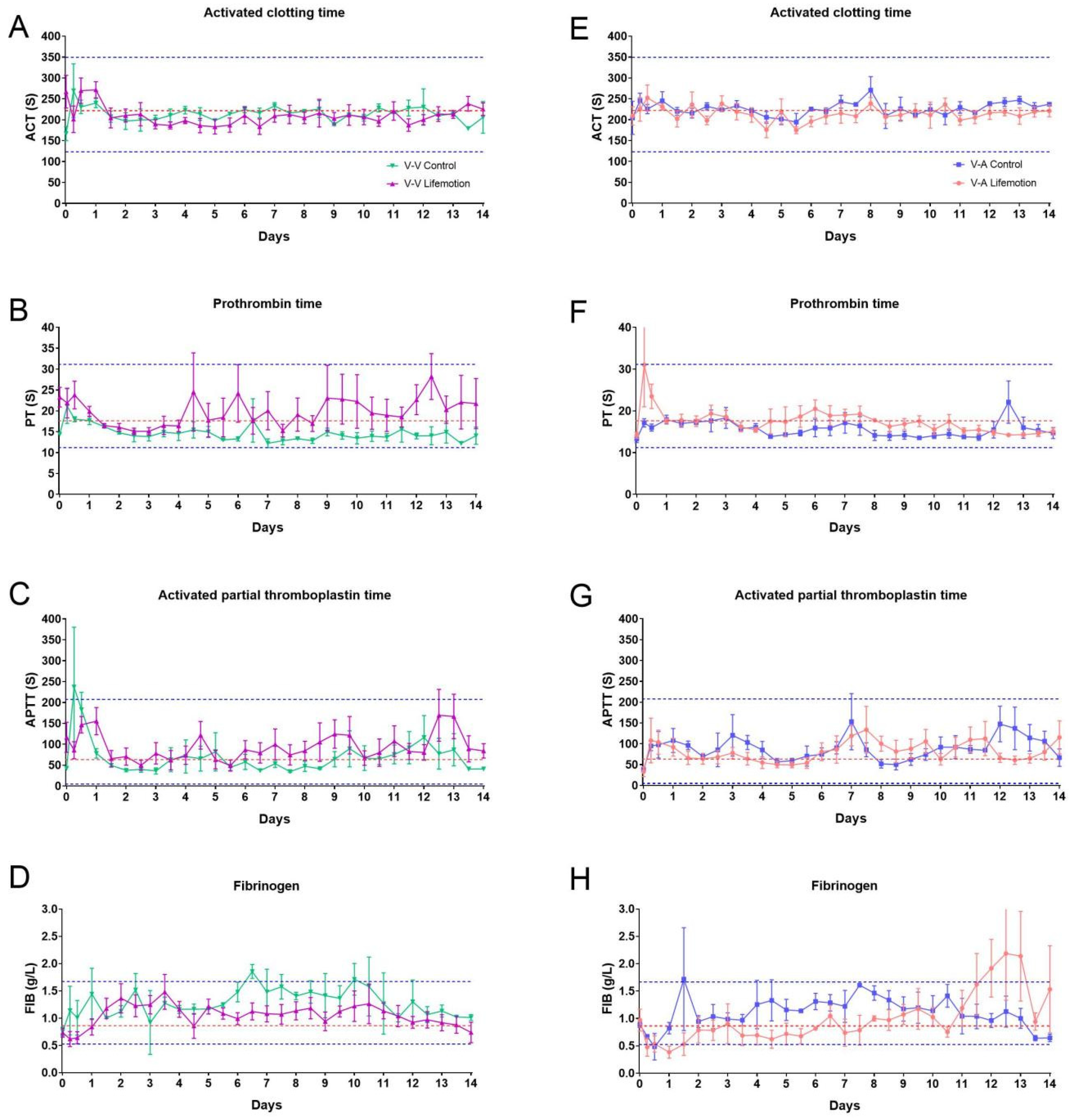
| No | Gender | ECMO Mode | Groups | Duration (Days) | Termination |
|---|---|---|---|---|---|
| S1 | Male | VV | Control (NOVALUNG XLUNG kit 230) | 14 | Scheduled |
| S2 | Male | VV | Control (NOVALUNG XLUNG kit 230) | 14 | Scheduled |
| S3 | Male | VV | LIFEMOTION | 14 | Scheduled |
| S4 | Male | VV | LIFEMOTION | 14 | Scheduled |
| S5 | Male | VV | LIFEMOTION | 14 | Scheduled |
| S6 | Male | VV | LIFEMOTION | 14 | Scheduled |
| S7 | Male | VV | LIFEMOTION | 14 | Scheduled |
| S8 | Male | VA | Control (NOVALUNG XLUNG kit 230) | 14 | Scheduled |
| S9 | Male | VA | Control (NOVALUNG XLUNG kit 230) | 14 | Scheduled |
| S10 | Male | VA | Control (NOVALUNG XLUNG kit 230) | 14 | Scheduled |
| S11 | Male | VA | LIFEMOTION | 14 | Scheduled |
| S12 | Male | VA | LIFEMOTION | 14 | Scheduled |
| S13 | Male | VA | LIFEMOTION | 14 | Scheduled |
| S14 | Male | VA | LIFEMOTION | 14 | Scheduled |
| S15 | Male | VA | LIFEMOTION | 14 | Scheduled |
| Sampling Time | VA-ECMO | VV-ECMO | ||
|---|---|---|---|---|
| Control Group | LIFEMOTION Group | Control Group | LIFEMOTION Group | |
| Pre-operation | 2 | 4 | 2 | 5 |
| 7 days post-surgery | ||||
| 14 days post-surgery | ||||
| Model | Comparable Group |
|---|---|
| VA-ECMO | C_VA_D7/C_VA_pre |
| C_VA_D14/C_VA_pre | |
| F_VA_D7/F_VA_pre | |
| F_VA_D14/F_VA_pre | |
| VV-ECMO | C_VV_D7/C_VV_pre |
| C_VV_D14/C_VV_pre | |
| F_VV_D7/F_VV_pre | |
| F_VV_D14/F_VV_pre |
| Classify | Protein Description | Gene Name | Fold Change | |
|---|---|---|---|---|
| 7 Days | 14 Days | |||
| Coagulation | Coagulation factor XIII A chain | F13A1 | 3.72 (up) | Normal |
| Hemolysis | Hemoglobin subunit beta | HBB | Normal | 4.41 (up) |
| Infection and Inflammation | Complement C3 | C3 | 4.97 (up) | Normal |
| Complement C4-like | C4-like | 3.49 (up) | Normal | |
| Complement factor D | CFD | 3.29 (up) | Normal | |
| Complement C4-A | C4-A | 0.09 (down) | Normal | |
| Interleukin 6 signal transducer | IL6ST | 3.49 (up) | Normal | |
| Organic damage | L-lactate dehydrogenase | LDHA | 4.01 (up) | Normal |
| Creatine kinase | CKM | 3.80 (up) | 5.65 (up) | |
| Classify | Protein Description | Gene Name | Fold Change | |
|---|---|---|---|---|
| 7 Days | 14 Days | |||
| Coagulation | Thrombospondin 1 | THBS1 | 3.03 (up) | Normal |
| Infection and Inflammation | Complement factor D | CD | 5.55 (up) | Normal |
| Complement C3 | C3 | 4.75 (up) | Normal | |
| Complement C4-like | C4-like | 3.57 (up) | Normal | |
| Complement C1s | C1s | 3.02 (up) | Normal | |
| Complement C4-A | C4A | 0.11 (down) | Normal | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Polish Respiratory Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Cai, L.; Huang, J.; Gao, H.; Huang, Z.; Guan, Y.; Li, Y.; Liu, S.; Liang, S.; Li, S.X.; et al. The Safety and Performance of a Novel Extracorporeal Membrane Oxygenation Device in a Long-Term Ovine Model. Adv. Respir. Med. 2025, 93, 34. https://doi.org/10.3390/arm93050034
Li Y, Cai L, Huang J, Gao H, Huang Z, Guan Y, Li Y, Liu S, Liang S, Li SX, et al. The Safety and Performance of a Novel Extracorporeal Membrane Oxygenation Device in a Long-Term Ovine Model. Advances in Respiratory Medicine. 2025; 93(5):34. https://doi.org/10.3390/arm93050034
Chicago/Turabian StyleLi, Yongchao, Lei Cai, Jia Huang, Hongbin Gao, Zhongqiang Huang, Yalun Guan, Yunfeng Li, Shuhua Liu, Shi Liang, Summer Xiatian Li, and et al. 2025. "The Safety and Performance of a Novel Extracorporeal Membrane Oxygenation Device in a Long-Term Ovine Model" Advances in Respiratory Medicine 93, no. 5: 34. https://doi.org/10.3390/arm93050034
APA StyleLi, Y., Cai, L., Huang, J., Gao, H., Huang, Z., Guan, Y., Li, Y., Liu, S., Liang, S., Li, S. X., Lu, H., Li, G., Li, Y., & Zhang, Y. (2025). The Safety and Performance of a Novel Extracorporeal Membrane Oxygenation Device in a Long-Term Ovine Model. Advances in Respiratory Medicine, 93(5), 34. https://doi.org/10.3390/arm93050034






