Association Between Weight-Adjusted Waist Index and Emphysema in Adults in the United States: A Cross-Sectional Study Involving 44,949 Participants
Abstract
Highlights
- Individuals with higher WWI values have a higher risk of emphysema, and it reveals the impact of visceral fat on lung health.
- The implication of the main findings: The results of the study highlight the importance of using WWI in clinical settings to assess the risk of emphysema, especially for patients with significant visceral fat accumulation.
- WWI can be incorporated into screening programs and guidelines for respiratory disease prevention, providing a basis for personalized risk assessment and management.
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey Description
2.2. Study Population
2.3. Assessment of Emphysema and Weight-Adjusted Waist Index
2.4. Statistical Analysis
- Model 1: Unadjusted model, which evaluated the raw association between WWI and emphysema;
- Model 2: Partially adjusted model, adjusting for potential confounders such as age, gender, and race;
- Model 3: Fully adjusted model, further adjusting for variables such as education level, marital status, household income, smoking status, hypertension, BMI, and family income-to-poverty ratio (PIR).
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Multivariable Logistic Regression Results
- Model 1 (unadjusted) demonstrated that WWI, as a continuous variable, was significantly associated with a higher likelihood of emphysema (OR: 2.1, 95% CI: 1.9–2.3);
- Model 2 (partially adjusted) accounted for basic demographic variables such as age, gender, and race. Even after these adjustments, the association between WWI and emphysema remained significant (OR: 1.7, 95% CI: 1.5–1.8);
- Model 3 (fully adjusted) controlled for additional confounders such as education level, marital status, household income, smoking status, diabetes, hypertension, BMI, and PIR. Even in this model, the association between WWI and emphysema remained significant (OR: 1.4, 95% CI: 1.2–1.5), with each unit increase in WWI raising the likelihood of emphysema by 40%. The prevalence rate of emphysema in the high WWI group was 1.5 times higher than in the low WWI group (OR: 1.5, 95% CI: 1.1–1.9, p for trend = 0.003).
3.3. Subgroup Analysis and Interaction Test Results
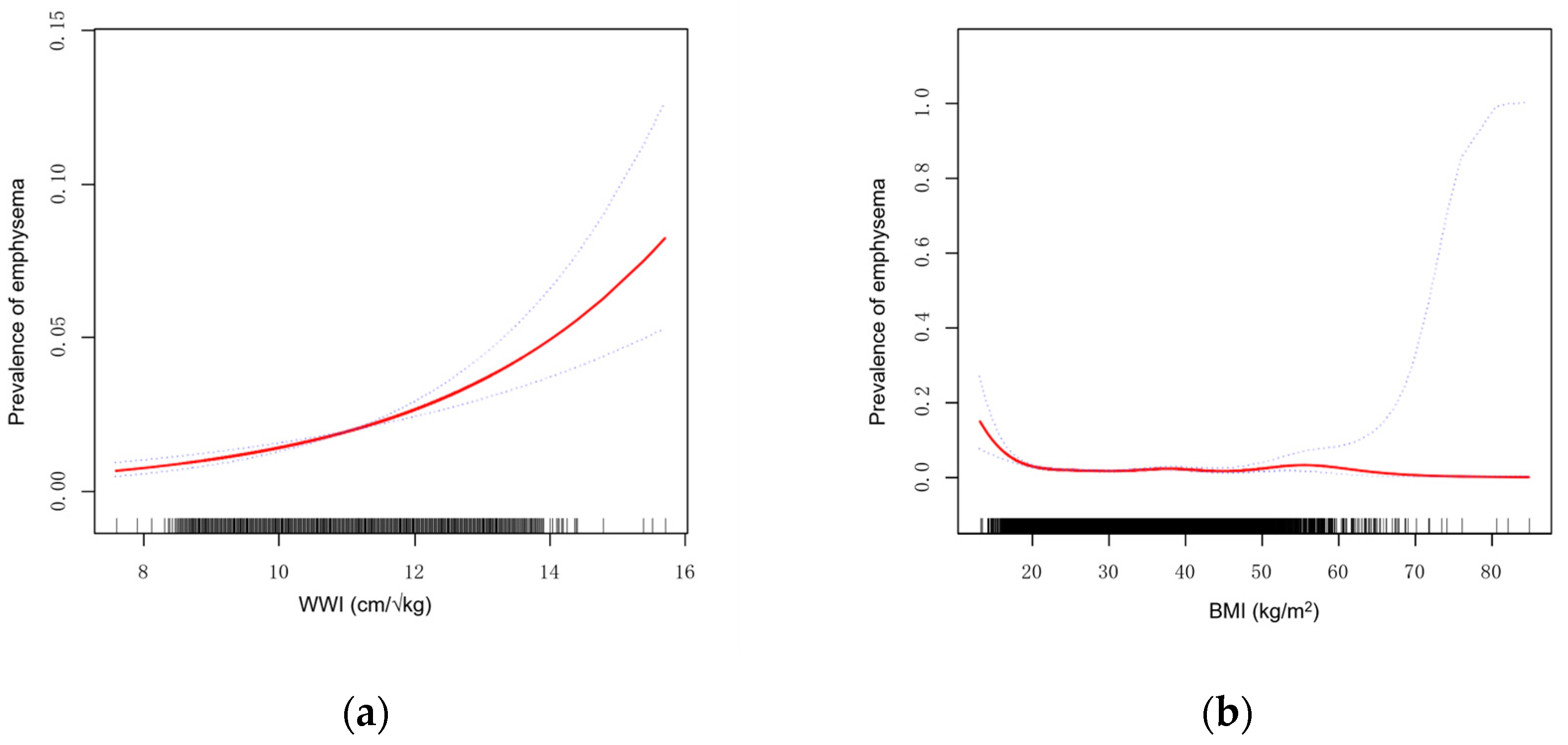
3.4. Smoothing Curve Fitting and Threshold Effect Analysis
4. Discussion
4.1. Strengths
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christenson, S.A.; Smith, B.M.; Bafadhel, M.; Putcha, N. Chronic Obstructive Pulmonary Disease. Lancet 2022, 399, 2227–2242. [Google Scholar] [CrossRef] [PubMed]
- López-Campos, J.L.; Tan, W.; Soriano, J.B. Global Burden of COPD. Respirology 2016, 21, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bacaicoa, C.; Santano-Mogena, E.; Rico-Martín, S.; Rey-Sánchez, P.; Juárez-Vela, R.; Sánchez Muñoz-Torrero, J.F.; López-Espuela, F.; Calderón-García, J.F. Association between Asymptomatic Hyperuricemia with Adiposity Indices: A Cross-Sectional Study in a Spanish Population. Nutrients 2023, 15, 4798. [Google Scholar] [CrossRef]
- Bray, G.A.; Heisel, W.E.; Afshin, A.; Jensen, M.D.; Dietz, W.H.; Long, M.; Kushner, R.F.; Daniels, S.R.; Wadden, T.A.; Tsai, A.G.; et al. The Science of Obesity Management: An Endocrine Society Scientific Statement. Endocr. Rev. 2018, 39, 79–132. [Google Scholar] [CrossRef]
- Silveira, E.A.; Castro, M.C.R.; Rezende, A.T.O.; Dos Santos Rodrigues, A.P.; Delpino, F.M.; Oliveira, E.S.; Corgosinho, F.C.; De Oliveira, C. Body Composition Assessment in Individuals with Class II/III Obesity: A Narrative Review. BMC Nutr. 2024, 10, 142. [Google Scholar] [CrossRef]
- Stefan, N.; Häring, H.-U.; Hu, F.B.; Schulze, M.B. Divergent Associations of Height with Cardiometabolic Disease and Cancer: Epidemiology, Pathophysiology, and Global Implications. Lancet Diabetes Endocrinol. 2016, 4, 457–467. [Google Scholar] [CrossRef]
- Park, Y.; Kim, N.H.; Kwon, T.Y.; Kim, S.G. A Novel Adiposity Index as an Integrated Predictor of Cardiometabolic Disease Morbidity and Mortality. Sci. Rep. 2018, 8, 16753. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Zhou, H.; Xu, H. Association between Weight-Adjusted-Waist Index and Chronic Kidney Disease: A Cross-Sectional Study. BMC Nephrol. 2023, 24, 266. [Google Scholar] [CrossRef]
- Palma, G.; Sorice, G.P.; Genchi, V.A.; Giordano, F.; Caccioppoli, C.; D’Oria, R.; Marrano, N.; Biondi, G.; Giorgino, F.; Perrini, S. Adipose Tissue Inflammation and Pulmonary Dysfunction in Obesity. Int. J. Mol. Sci. 2022, 23, 7349. [Google Scholar] [CrossRef]
- Cao, T.; Xie, R.; Wang, J.; Xiao, M.; Wu, H.; Liu, X.; Xie, S.; Chen, Y.; Liu, M.; Zhang, Y. Association of Weight-Adjusted Waist Index with All-Cause Mortality among Non-Asian Individuals: A National Population-Based Cohort Study. Nutr. J. 2024, 23, 62. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Li, R.; Leader, J.K.; Zheng, B.; Bon, J.; Gur, D.; Sciurba, F.; Jin, C.; Pu, J. Obesity and Extent of Emphysema Depicted at CT. Clin. Radiol. 2015, 70, e14–e19. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S.; Sherrill, D.L.; Bobadilla, A.; Martinez, F.D.; Barbee, R.A. The Relation of Body Mass Index to Asthma, Chronic Bronchitis, and Emphysema. Chest 2002, 122, 1256–1263. [Google Scholar] [CrossRef]
- Yao, D.; Chang, Q.; Wu, Q.-J.; Gao, S.-Y.; Zhao, H.; Liu, Y.-S.; Jiang, Y.-T.; Zhao, Y.-H. Relationship between Maternal Central Obesity and the Risk of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Cohort Studies. J. Diabetes Res. 2020, 2020, 6303820. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Y.; Xu, M.; Li, R.; Zhang, J.; Zhu, S.; He, Z.; Chen, M.; Wang, G. Association of Weight-Adjusted-Waist Index with Asthma Prevalence and the Age of First Asthma Onset in United States Adults. Front. Endocrinol. 2023, 14, 1116621. [Google Scholar] [CrossRef]
- Ding, C.; Shi, Y.; Li, J.; Li, M.; Hu, L.; Rao, J.; Liu, L.; Zhao, P.; Xie, C.; Zhan, B.; et al. Association of Weight-Adjusted-Waist Index with All-Cause and Cardiovascular Mortality in China: A Prospective Cohort Study. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1210–1217. [Google Scholar] [CrossRef]
- Fang, H.; Xie, F.; Li, K.; Li, M.; Wu, Y. Association between Weight-Adjusted-Waist Index and Risk of Cardiovascular Diseases in United States Adults: A Cross-Sectional Study. BMC Cardiovasc. Disord. 2023, 23, 435. [Google Scholar] [CrossRef]
- Zheng, D.; Zhao, S.; Luo, D.; Lu, F.; Ruan, Z.; Dong, X.; Chen, W. Association between the Weight-Adjusted Waist Index and the Odds of Type 2 Diabetes Mellitus in United States Adults: A Cross-Sectional Study. Front. Endocrinol. 2023, 14, 1325454. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity Is Associated with Macrophage Accumulation in Adipose Tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Ibrahim, M.M. Subcutaneous and Visceral Adipose Tissue: Structural and Functional Differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Tanimura, K.; Sato, S.; Fuseya, Y.; Hasegawa, K.; Uemasu, K.; Sato, A.; Oguma, T.; Hirai, T.; Mishima, M.; Muro, S. Quantitative Assessment of Erector Spinae Muscles in Patients with Chronic Obstructive Pulmonary Disease. Novel Chest Computed Tomography-Derived Index for Prognosis. Ann. Am. Thorac. Soc. 2016, 13, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Addissouky, T.A.; El Sayed, I.E.T.; Ali, M.M.A.; Wang, Y.; El Baz, A.; Elarabany, N.; Khalil, A.A. Oxidative Stress and Inflammation: Elucidating Mechanisms of Smoking-Attributable Pathology for Therapeutic Targeting. Bull. Natl. Res. Cent. 2024, 48, 16. [Google Scholar] [CrossRef]
- Albano, G.D.; Gagliardo, R.P.; Montalbano, A.M.; Profita, M. Overview of the Mechanisms of Oxidative Stress: Impact in Inflammation of the Airway Diseases. Antioxidants 2022, 11, 2237. [Google Scholar] [CrossRef]
- Aoshiba, K.; Nagai, A. Oxidative Stress, Cell Death, and Other Damage to Alveolar Epithelial Cells Induced by Cigarette Smoke. Tob. Induc. Dis. 2003, 1, 219. [Google Scholar] [CrossRef]
- Demedts, I.K.; Demoor, T.; Bracke, K.R.; Joos, G.F.; Brusselle, G.G. Role of Apoptosis in the Pathogenesis of COPD and Pulmonary Emphysema. Respir. Res. 2006, 7, 53. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic Inflammation in Fat Plays a Crucial Role in the Development of Obesity-Related Insulin Resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Vega, G.L.; Adams-Huet, B.; Peshock, R.; Willett, D.; Shah, B.; Grundy, S.M. Influence of Body Fat Content and Distribution on Variation in Metabolic Risk. J. Clin. Endocrinol. Metab. 2006, 91, 4459–4466. [Google Scholar] [CrossRef]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-Induced Hypertension: Interaction of Neurohumoral and Renal Mechanisms. Circ. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef]
- Wang, L.; Gao, T.; Li, Y.; Xie, Y.; Zeng, S.; Tai, C.; Feng, Y.; Shen, P.; Wang, B. A Long-Term Anti-Inflammation Markedly Alleviated High-Fat Diet-Induced Obesity by Repeated Administrations of Overexpressing IL10 Human Umbilical Cord-Derived Mesenchymal Stromal Cells. Stem Cell Res. Ther. 2022, 13, 259. [Google Scholar] [CrossRef]
- Yida, Z.; Imam, M.U.; Ismail, M.; Ismail, N.; Ideris, A.; Abdullah, M.A. High Fat Diet-Induced Inflammation and Oxidative Stress Are Attenuated by N-Acetylneuraminic Acid in Rats. J. Biomed. Sci. 2015, 22, 96. [Google Scholar] [CrossRef]
- Bouchi, R.; Takeuchi, T.; Akihisa, M.; Ohara, N.; Nakano, Y.; Nishitani, R.; Murakami, M.; Fukuda, T.; Fujita, M.; Minami, I.; et al. High Visceral Fat with Low Subcutaneous Fat Accumulation as a Determinant of Atherosclerosis in Patients with Type 2 Diabetes. Cardiovasc. Diabetol. 2015, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Emamat, H.; Jamshidi, A.; Farhadi, A.; Ghalandari, H.; Ghasemi, M.; Tangestani, H. The Association between the Visceral to Subcutaneous Abdominal Fat Ratio and the Risk of Cardiovascular Diseases: A Systematic Review. BMC Public Health 2024, 24, 1827. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, X.; Deng, M.; Yin, Y.; Li, Y.; Zhang, Q.; Bian, Y.; Miao, J.; Li, J.; Hou, G. Clinical Impacts of Sarcopenic Obesity on Chronic Obstructive Pulmonary Disease: A Cross-Sectional Study. BMC Pulm. Med. 2023, 23, 394. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S. (Ed.) Metabolic Syndrome: A Comprehensive Textbook; Springer International Publishing: Cham, Switzerland, 2023; ISBN 978-3-031-40115-2. [Google Scholar]
- Power, M.L.; Schulkin, J. Sex Differences in Fat Storage, Fat Metabolism, and the Health Risks from Obesity: Possible Evolutionary Origins. Br. J. Nutr. 2008, 99, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.K.; Kang, H.Y.; Lee, Y.; Choi, S.H.; Kim, H.J.; Kim, J.S. The Longitudinal Association between Changes in Lung Function and Changes in Abdominal Visceral Obesity in Korean Non-Smokers. PLoS ONE 2018, 13, e0193516. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Li, F. Nonlinear Relationship between Visceral Adiposity Index and Lung Function: A Population-Based Study. Respir. Res. 2021, 22, 161. [Google Scholar] [CrossRef]
- Mafort, T.T.; Rufino, R.; Costa, C.H.; Lopes, A.J. Obesity: Systemic and Pulmonary Complications, Biochemical Abnormalities, and Impairment of Lung Function. Multidiscip. Respir. Med. 2016, 11, 28. [Google Scholar] [CrossRef]
- Agbim, U.; Carr, R.M.; Pickett-Blakely, O.; Dagogo-Jack, S. Ethnic Disparities in Adiposity: Focus on Non-Alcoholic Fatty Liver Disease, Visceral, and Generalized Obesity. Curr. Obes. Rep. 2019, 8, 243–254. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, H.; Xia, N. The Interplay Between Adipose Tissue and Vasculature: Role of Oxidative Stress in Obesity. Front. Cardiovasc. Med. 2021, 8, 650214. [Google Scholar] [CrossRef]

| Characteristics | Weight-Adjusted Waist Index(cm/√kg) | p-Value | |
|---|---|---|---|
| G1 (7.59~10.46) N = 11,178 | G2 (10.46~15.70) N = 33,771 | ||
| Age, year (SD) | 37.8 (14.4) | 52.9 (17.5) | <0.001 |
| Gender, % | <0.001 | ||
| Male | 58.4 | 45.2 | |
| Female | 41.6 | 54.8 | |
| Race, % | <0.001 | ||
| Mexican American | 9.3 | 19.2 | |
| Other Hispanic | 6.2 | 9.2 | |
| Non-Hispanic White | 44.1 | 43.9 | |
| Non-Hispanic Black | 29.3 | 18.4 | |
| Other Race | 11.1 | 9.3 | |
| Education level, % | <0.001 | ||
| <high school | 16.4 | 28.8 | |
| high school | 21.4 | 23.8 | |
| >high school | 62.2 | 47.4 | |
| Marital status, % | <0.001 | ||
| Married | 44.2 | 55.5 | |
| Unmarried | 55.8 | 44.5 | |
| Annual family income, % | <0.001 | ||
| USD 0 to USD 24,999 | 29.6 | 32.2 | |
| USD 25,000 to USD 74,999 | 38.2 | 40.0 | |
| USD 75,000 and over | 32.2 | 27.8 | |
| PIR (SD) | 2.8 (1.7) | 2.5 (1.6) | <0.001 |
| BMI (SD) | 24.9 (4.8) | 30.3 (6.7) | <0.001 |
| Smoking, % | <0.001 | ||
| Ever | 42.1 | 46.4 | |
| Never | 57.9 | 53.6 | |
| Diabetes, % | <0.001 | ||
| Yes | 3.0 | 15.5 | |
| No | 97.0 | 84.5 | |
| Hypertension, % | <0.001 | ||
| Yes | 15.6 | 40.8 | |
| No | 84.4 | 59.2 | |
| Emphysema, % | <0.001 | ||
| Yes | 0.7 | 2.4 | |
| No | 99.3 | 97.6 | |
| WWI | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| OR (95%CI) | OR (95%CI) | OR (95%CI) | |
| Continuous | 2.1 (1.9, 2.3) | 1.7 (1.5, 1.8) | 1.4 (1.2, 1.5) |
| Categories | |||
| ≤10.46 | 1.0 | 1.0 | 1.0 |
| >10.46 | 3.7 (2.9, 4.7) | 1.9 (1.4, 2.4) | 1.5 (1.1, 1.9) |
| p for trend | <0.001 | <0.001 | 0.003 |
| OR (95% CI) | p-Value | |
|---|---|---|
| Model 1 a | 1.4 (1.3, 1.6) | <0.001 |
| Model 2 b | ||
| Breakpoint (K) | 12.5 | |
| OR1 (<12.5) | 1.5 (1.4, 1.7) | <0.001 |
| OR2 (>12.5) | 0.8 (0.5, 1.4) | 0.394 |
| OR2/OR1 | 0.5 (0.3, 0.9) | 0.028 |
| Log likelihood ratio | 0.020 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Polish Respiratory Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Yang, Z.; Guo, J.; Zu, Y.; Li, F.; Zhao, B. Association Between Weight-Adjusted Waist Index and Emphysema in Adults in the United States: A Cross-Sectional Study Involving 44,949 Participants. Adv. Respir. Med. 2024, 92, 472-484. https://doi.org/10.3390/arm92060043
Cheng H, Yang Z, Guo J, Zu Y, Li F, Zhao B. Association Between Weight-Adjusted Waist Index and Emphysema in Adults in the United States: A Cross-Sectional Study Involving 44,949 Participants. Advances in Respiratory Medicine. 2024; 92(6):472-484. https://doi.org/10.3390/arm92060043
Chicago/Turabian StyleCheng, Hui, Ziheng Yang, Jiateng Guo, Yukun Zu, Fan Li, and Bo Zhao. 2024. "Association Between Weight-Adjusted Waist Index and Emphysema in Adults in the United States: A Cross-Sectional Study Involving 44,949 Participants" Advances in Respiratory Medicine 92, no. 6: 472-484. https://doi.org/10.3390/arm92060043
APA StyleCheng, H., Yang, Z., Guo, J., Zu, Y., Li, F., & Zhao, B. (2024). Association Between Weight-Adjusted Waist Index and Emphysema in Adults in the United States: A Cross-Sectional Study Involving 44,949 Participants. Advances in Respiratory Medicine, 92(6), 472-484. https://doi.org/10.3390/arm92060043





