Abstract
Background: Dementia with Lewy bodies (DLBs) often presents with neuropsychiatric symptoms (NPSs), yet the role of hyperglycemia, a common cause of delirium in older adults, as a contributing factor remains under-recognized. This article aims to explore the relationship between hyperglycemia and NPSs. Methods: We report the case of a 71-year-old male with DLBs and type 2 diabetes mellitus (T2DM) who experienced worsening NPSs closely associated with periods of hyperglycemia. Initial pharmacological and nonpharmacological interventions were insufficient, prompting adjustments to insulin therapy and dietary modifications to stabilize blood glucose levels. Results: Improved glycemic control resulted in a clinically significant reduction in NPSs. Conclusions: This case suggests a potential link between hyperglycemia and NPSs in DLB patients, emphasizing the importance of maintaining glycemic control in managing NPSs. Although the exact mechanisms remain incompletely understood, adopting a holistic framework for brain health could offer a comprehensive approach to cognitive care. Further studies are needed to elucidate the biological pathways involved, validate these findings in larger populations, and develop evidence-based clinical guidelines.
1. Case Presentation
A 71-year-old male patient, with a history of polycythemia vera, type 2 diabetes mellitus (T2DM), and a prior diagnosis of dementia with Lewy bodies (DLBs), was admitted to a psychiatric hospital due to behavioral and cognitive symptoms. Upon admission, his Mini-Mental State Examination (MMSE) score was 16 out of 30. He initially sought neurocognitive health services in 2019 for memory impairment, nocturnal dream-enactment behavior, and mild axial parkinsonian signs. DatSCAN neuroimaging revealed deficient dopaminergic active transport in the substantia nigra and striatum. A probable diagnosis of DLBs was established based on the 2017 McKeith criteria [1]. Over the subsequent 3 years, the patient experienced a progressive decline in cognition, along with intermittent visual hallucinations. Quetiapine was introduced and titrated to 25 mg daily, resulting in noted behavioral improvement. His medication also included sertraline, acetylsalicylic acid, hydroxycarbamide, and both rapid and long-acting insulin.
In the 6 months leading up to the hospitalization, the patient exhibited delusions of infidelity, vivid visual hallucinations, mood swings, and increased psychomotor agitation. Upon admission, common causes of delirium, such as pain, infection, constipation, and urinary retention, were ruled out, and brain MRI showed no acute abnormalities. The patient’s medication regimen had remained unchanged prior to admission. Despite non-pharmacological interventions and a switch from quetiapine to clozapine at 6.25 mg twice daily, the symptoms persisted. Rivastigmine was initiated at 4.6 mg daily to address the persisting neuropsychiatric symptoms (NPSs), yet the patient continued to experience cognitive fluctuations, frequent night-time awakenings, visual hallucinations and disruptive behavior. After a week of limited success with the initial pharmacological approach, and prior to escalating treatment, the focus shifted toward identifying potential contributing factors to the neuropsychiatric symptoms. It was observed that most agitation episodes occurred during hyperglycemic states. Consequently, capillary blood glucose monitoring, initially performed three times daily, was adjusted to include additional measurements during behavioral changes. Behavioral symptoms were systematically assessed using the Pittsburgh Agitation Scale [2]. Psychotropic medications for agitation were administered on an as-needed basis only when a moderate Pittsburgh score > 8 was recorded and non-pharmacological interventions, such as multisensory stimulation and reality orientation, proved ineffective, as well as in cases of severe agitation with a score > 13. Despite achieving appropriate glycemic control (glycated hemoglobin of 7.5%), pharmaceutical interventions, such as clomethiazole or benzodiazepines, were consistently administered during episodes of glycemia exceeding 18 mmol/L and on two occasions when glycemia dropped below 3.5 mmol/L (Figure 1). Notably, typical neuroleptics were never utilized in the management. Clomethiazole was administered up to four times daily at a dose of 192 mg, primarily to manage agitation, while clonazepam 0.5 mg was prescribed to address sleep disturbances. The patient did not meet criteria for a hyperglycemic hyperosmolar state or diabetic ketoacidosis. We adapted rapid and long-acting insulin and put the patient on a diabetic diet. Over the course of one week, we noted a gradual improvement in the glycemic peaks, with blood glucose peaks ranging between 4 and 16 mmol/l and glycated hemoglobin of 7.5%. Notably, we subsequently observed a reduction in episodes of psychomotor agitation, along with a decreased need for acute antipsychotic and sedative treatments.
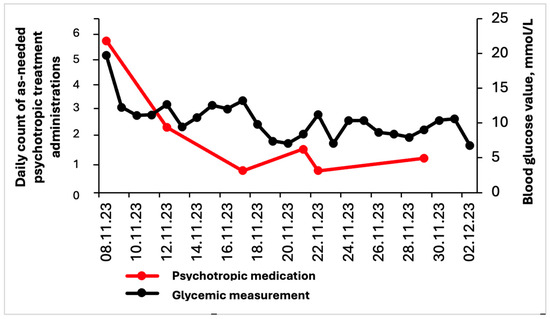
Figure 1.
Evolution of blood glucose levels (mmol/L) and daily as-needed psychotropic medication administrations during hospitalization. As-needed treatment consisted of either clomethiazole 192 mg or clonazepam 0.5 mg. Although the Pittsburgh Agitation Scale score is not depicted in the graph, the reduced frequency of psychotropic medication administration reflects its improvement, which began between November 12 and 14, coinciding with the stabilization of glycemia.
2. Discussion
This case report highlights the importance of considering hyperglycemia as a potential trigger for worsening NPSs in DLB patients with T2DM in the absence of a hyperglycemic hyperosmolar state or diabetic ketoacidosis. To the best of our knowledge, this is the first reported case demonstrating that correcting metabolic imbalances can positively influence a patient’s neurobehavioral profile. The comprehensive diagnostic evaluation excluded all other organic causes, including findings from brain MRI, and delirium secondary to medication effects was also ruled out.
Brain health refers to the optimal development and functioning of the brain throughout an individual’s life [3]. NPSs, also referred to as behavioral and psychological symptoms of dementia, are a common feature of DLBs, which can manifest in the early stages or later in the disease trajectory and can include a wide array of symptoms, such as hallucinations, delusions, agitation, depression, and night-time behavior disturbances, among others [4]. The cause of behavioral changes is often multifactorial, involving neurochemical alterations, psychological factors and environmental contributors. However, their prevention or mitigation in individuals with diagnosed dementia may contribute to slowing cognitive decline and preserving aspects of brain function [4,5]. Studies have suggested that patients with T2DM, such as our own, besides being at a greater risk of developing dementia [6], have an increased risk of NPSs [7,8]. While the precise interrelated pathophysiological mechanisms remain incompletely understood, findings from other neurological conditions provide insights for hypothesizing potential mechanisms in DLBs. There is growing evidence of the role of hyperglycemia in delirium, a symptom that may overlap with the cognitive fluctuations characteristic of DLBs [9]. A recent study involving 487 older hospitalized non-demented patients reported that transient hyperglycemia was independently associated with a 3.7-fold increased risk of delirium [7]. Delirium, in turn, has been shown to significantly contribute to cognitive decline in individuals with dementia, with an observed threefold increased risk of progression in severity [10]. Although the underlying pathophysiological processes are complex, glucose-sensitive neurons may play a crucial role in maintaining energy homeostasis by integrating hormonal, metabolic, neurotransmitter, and peptide signals, thereby potentially supporting essential biological functions and brain health [11]. Elevated blood glucose levels are associated with acute and chronic systemic inflammation and oxidative stress, which compromise the integrity of the blood–brain barrier, leading to neuronal injury and impaired synaptic functioning [12,13]. Furthermore, hyperglycemia may indirectly contribute to delirium by affecting the gut microbiome and increasing intestinal permeability [14,15]. In addition, the dopaminergic pathway, altered in DLB patients, may play a role in the development of delirium. Evidence suggests that delirium could reflect a state of dopaminergic excess, driven by the overstimulation of D2 receptors in the mesolimbic and mesocortical pathways. Clinical observations indicate that dopaminergic drugs, such as levodopa and dopamine agonists, can induce delirium, particularly in older patients. In contrast, dopamine antagonists, including typical antipsychotics, are commonly used in the pharmacological management of delirium [16]. These effects are particularly concerning in DLBs, where dopaminergic and acetylcholinergic systems, critical for cognitive and behavioral regulation, are significantly affected by the disease’s pathology [17]. Studies in animal models further illustrate the impact of glucose dysregulation on these neurotransmission systems. Antony et al. demonstrated that hyperglycemia impairs cholinergic signaling by altering nicotinic acetylcholine receptors in diabetic and non-diabetic rats compared to normoglycemic conditions [18]. In Parkinson’s disease (PD), patients with poorly controlled hyperglycemia exhibit greater motor symptom progression compared to non-diabetic patients [19]. In a prospective study of 379 patients, those with poorly controlled diabetes had a ≥14-point worsening in the Unified Parkinson’s Disease Rating Scale (UPDRS III) compared to non-diabetic patients and those with well-controlled diabetes [20]. Hyperglycemia is proposed to impair PD-related neural pathways, including the mesocorticolimbic and nigrostriatal motor pathways [21]. Additionally, recent findings suggest that T2DM accelerates PD progression by exacerbating pre-existing α-syn pathology [22]. Given that PD and DLBs share the same underlying α-synuclein pathology, recent neuroimaging studies have revealed overlapping yet distinct patterns of progression [23,24]. While these changes correlate with cognitive impairment and spread across brain regions in PD, they are more severe and widespread in DLBs, contributing to its distinct clinical phenotype [25]. Moreover, PD and T2DM share common cellular mechanisms. The insulin receptor, abundantly expressed in substantia nigra neurons, is disrupted by hyperglycemia, which impairs insulin signaling, suppresses dopaminergic neuronal firing, and decreases dopamine turnover [26]. As highlighted by Das et al., appropriate glycemic control represents a potential therapeutic target in PD [26], demonstrated by the use of glucagon-like peptide-1 receptor agonists, which have shown benefits in improving motor function in advanced PD [27]. Building on these findings, which emphasize the metabolic–neurodegenerative interplay between T2DM and neurodegenerative diseases, and consistent with our case, we postulate that hyperglycemia may serve as a potential risk factor for exacerbating NPSs in DLB patients.
Our patient presented with multiple risk factors for developing agitation beyond his inadequately managed T2DM and diagnosis of DLBs. These included a language barrier, as he was primarily Spanish-speaking with limited proficiency in French, and physical limitations due to being wheelchair-bound from a risk of falls. However, the presence of Spanish-speaking nurses and regular involvement in physiotherapy provided supportive interventions. The pharmacological management of NPSs is often of limited effectiveness and must target specific debilitating behavioral symptoms to be more impactful [28]. However, the use of antipsychotics is relatively contraindicated in DLB patients due to the risk of severe hypersensitivity, which is associated with increased morbidity and mortality [1]. Existing best practice guidelines for the management of NPSs consistently promote nonpharmacological interventions as the first-line treatment, particularly for agitation and aggression, and there is a growing evidence base to support the value of this approach [29]. Our decision to search for alternative treatment targets stemmed largely from the limited response to antipsychotics we were witnessing, coupled with the non-negligeable iatrogenic risk of these interventions [30]. In addition to placing a greater emphasis on occupational therapy, we searched for potential systemic precipitating and/or contributing factors. A review of the data from blood glucose monitoring revealed glycemic fluctuations with the extremes coinciding chronologically with episodes of agitation. By prioritizing glycemic control and stability, primarily through dietary changes and insulin therapy adjustments, we observed a decrease in agitation episodes and cognitive fluctuations, as reported by the patient’s caregiver.
While our understanding of brain–behavior relationships remains limited, we believe these findings align with the broader concept of brain health, which should evolve to encompass not only cognitively unimpaired individuals but also those with cognitive impairments. This case highlights how targeting metabolic disorders, particularly hyperglycemia, from prevention to active treatment strategies, can be considered as a crucial aspect of dementia care, particularly in the management of NPSs. We thus propose the concept of brain health as a framework that seeks to apply its principles to the entire spectrum of cognitive function, from maintaining optimal cognition in healthy individuals to managing and mitigating decline in those with neurodegenerative diseases. Central to this framework is the proactive management of risk factors and NPS, even at the most advanced stages of the disease.
3. Conclusions
Our case presentation underscores the importance of recognizing hyperglycemia as a potential trigger for the worsening of the neurobehavioral profile in DLB patients. NPSs remain among the most significant challenges in dementia care, particularly in DLBs, and are frequently under-recognized or inadequately treated. Addressing these factors through targeted interventions could play a crucial role in mitigating the decline associated with dementia. Furthermore, mitigating the burden of NPSs should be regarded as an essential component of preserving residual brain health in individuals with cognitive impairments. We therefore propose that the concept of brain health should be regarded as addressing the entire spectrum of cognitive function, extending from the preservation of optimal cognition in unimpaired individuals to the effective management and mitigation of cognitive and functional decline and NPSs in those with neurodegenerative diseases, regardless of the stage of the disease.
Author Contributions
P.S. drafted the manuscript and created the figures. D.J. and S.G. performed the clinical assessment, assisted in the patient’s clinical assessment and edited the manuscript. L.S. and F.A. performed the critical revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding author due to privacy and confidentiality concerns.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- McKeith, I.G.; Boeve, B.F.; Dickson, D.W.; Halliday, G.; Taylor, J.-P.; Weintraub, D.; Aarsland, D.; Galvin, J.; Attems, J.; Ballard, C.G.; et al. Diagnosis and Management of Dementia with Lewy Bodies. Neurology 2017, 89, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.; Burgio, L.; Kollar, M.; Cain, M.; Allison, M.; Fogleman, M.; Michael, M.; Zubenko, G.S. The Pittsburgh Agitation Scale: A User-Friendly Instrument for Rating Agitation in Dementia Patients. Am. J. Geriatr. Psychiatry 1994, 2, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, C.L.A.; Endres, M.; Sander, A.; Crean, M.; Subramaniam, S.; Carvalho, V.; Di Liberto, G.; Franco, O.H.; Pijnenburg, Y.; Leonardi, M.; et al. The European Academy of Neurology Brain Health Strategy: One Brain, One Life, One Approach. Eur. J. Neurol. 2022, 29, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Borda, M.G.; Brønnick, K.K.; Garcia-Cifuentes, E.; Jaramillo-Jimenez, A.; Reyes-Ortiz, C.; Patricio-Baldera, J.; Soennesyn, H.; Pérez-Zepeda, M.U.; Vik-Mo, A.O.; Aarsland, D. Specific Neuropsychiatric Symptoms Are Associated with Functional Decline Trajectories in Alzheimer’s Disease and Lewy Body Dementia: A Five-Year Follow-up Study. Front. Med. 2023, 10, 1267060. [Google Scholar] [CrossRef]
- Carrarini, C.; Russo, M.; Dono, F.; Barbone, F.; Rispoli, M.G.; Ferri, L.; Di Pietro, M.; Digiovanni, A.; Ajdinaj, P.; Speranza, R.; et al. Agitation and Dementia: Prevention and Treatment Strategies in Acute and Chronic Conditions. Front. Neurol. 2021, 12, 644317. [Google Scholar] [CrossRef]
- De La Monte, S.M.; Tong, M.; Wands, J.R. The 20-Year Voyage Aboard the Journal of Alzheimer’s Disease: Docking at ‘Type 3 Diabetes’, Environmental/Exposure Factors, Pathogenic Mechanisms, and Potential Treatments. J. Alzheimers Dis. 2018, 62, 1381–1390. [Google Scholar] [CrossRef]
- Song, J. Amygdala Activity and Amygdala-Hippocampus Connectivity: Metabolic Diseases, Dementia, and Neuropsychiatric Issues. Biomed. Pharmacother. 2023, 162, 114647. [Google Scholar] [CrossRef]
- van Reedt Dortland, A.K.; Giltay, E.J.; Van Veen, T.; Zitman, F.G.; Penninx, B.W. Penninx, Metabolic syndrome abnormalities are associated with severity of anxiety and depression and with tricyclic antidepressant use. Acta Psychiatr. Scand. 2010, 122, 30–39. [Google Scholar] [CrossRef]
- Gore, R.L.; Vardy, E.R.L.C.; O’Brien, J.T. Delirium and Dementia with Lewy Bodies: Distinct Diagnoses or Part of the Same Spectrum? J. Neurol. Neurosurg. Psychiatry 2014, 86, 50–59. [Google Scholar] [CrossRef]
- Davis, D.H.J.; Terrera, G.M.; Keage, H.; Rahkonen, T.; Oinas, M.; Matthews, F.E.; Cunningham, C.; Polvikoski, T.; Sulkava, R.; MacLullich, A.M.J.; et al. Delirium Is a Strong Risk Factor for Dementia in the Oldest-Old: A Population-Based Cohort Study. Brain 2012, 135, 2809–2816. [Google Scholar] [CrossRef]
- López-Gambero, A.J.; Martínez, F.; Salazar, K.; Cifuentes, M.; Nualart, F. Brain Glucose-Sensing Mechanism and Energy Homeostasis. Mol. Neurobiol. 2018, 56, 769–796. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Friedman, B.A.; Etxeberria, A.; Huntley, M.A.; Van Der Brug, M.P.; Foreman, O.; Paw, J.S.; Modrusan, Z.; Beach, T.G.; Serrano, G.E.; et al. Alzheimer’s Patient Microglia Exhibit Enhanced Aging and Unique Transcriptional Activation. Cell Rep. 2020, 31, 107843. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Halliwell, B. Oxidative Stress, Dysfunctional Glucose Metabolism and Alzheimer Disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia Drives Intestinal Barrier Dysfunction and Risk for Enteric Infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef]
- Ticinesi, A.; Parise, A.; Nouvenne, A.; Cerundolo, N.; Prati, B.; Meschi, T. The Possible Role of Gut Microbiota Dysbiosis in the Pathophysiology of Delirium in Older Persons. Microbiome Res. Rep. 2023, 2, 19. [Google Scholar] [CrossRef]
- Leonard, M.; Spiller, J.; Keen, J.; MacLullich, A.; Kamholtz, B.; Meagher, D. Symptoms of Depression and Delirium Assessed Serially in Palliative-Care Inpatients. Psychosomatics 2009, 50, 506–514. [Google Scholar] [CrossRef]
- Beck, B.J. Neuropsychiatric Manifestations of Diffuse Lewy Body Disease. J. Geriatr. Psychiatry Neurol. 1995, 8, 189–196. [Google Scholar] [CrossRef]
- Antony, S.; Kumar, T.P.; Mathew, J.; Anju, T.; Paulose, C. Hypoglycemia Induced Changes in Cholinergic Receptor Expression in the Cerebellum of Diabetic Rats. J. Biomed. Sci. 2010, 17, 7. [Google Scholar] [CrossRef]
- Chohan, H.; Senkevich, K.; Patel, R.K.; Bestwick, J.P.; Jacobs, B.M.; Ciga, S.B.; Gan-Or, Z.; Noyce, A.J. Type 2 Diabetes as a Determinant of Parkinson’s Disease Risk and Progression. Mov. Disord. 2021, 36, 1420–1429. [Google Scholar] [CrossRef]
- Ou, R.; Wei, Q.; Hou, Y.; Zhang, L.; Liu, K.; Lin, J.; Jiang, Z.; Song, W.; Cao, B.; Shang, H. Effect of Diabetes Control Status on the Progression of Parkinson’s Disease: A Prospective Study. Ann. Clin. Transl. Neurol. 2021, 8, 887–897. [Google Scholar] [CrossRef]
- Renaud, J.; Bassareo, V.; Beaulieu, J.; Pinna, A.; Schlich, M.; Lavoie, C.; Murtas, D.; Simola, N.; Martinoli, M.-G. Dopaminergic Neurodegeneration in a Rat Model of Long-Term Hyperglycemia: Preferential Degeneration of the Nigrostriatal Motor Pathway. Neurobiol. Aging 2018, 69, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.-Q.; Yuan, L.; Sun, Y.; Dou, H.-W.; Su, J.-H.; Hou, Z.-P.; Li, J.-Y.; Li, W. Long-Term Hyperglycemia Aggravates Œ±-Synuclein Aggregation and Dopaminergic Neuronal Loss in a Parkinson’s Disease Mouse Model. Transl. Neurodegener. 2022, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Berman, S.B.; Miller-Patterson, C. PD and DLB: Brain Imaging in Parkinson’s Disease and Dementia with Lewy Bodies. Prog Mol. Biol. Transl. Sci. 2019, 165, 167–185. [Google Scholar] [CrossRef]
- Gomperts, S.N. Lewy Body Dementias. Continuum 2016, 22, 435–463. [Google Scholar] [CrossRef]
- Yousaf, T.; Dervenoulas, G.; Valkimadi, P.-E.; Politis, M. Neuroimaging in Lewy Body Dementia. J. Neurol. 2018, 266, 1–26. [Google Scholar] [CrossRef]
- Das, R.R.; Unger, M.M. Diabetes and Parkinson Disease. Neurology 2018, 90, 869–870. [Google Scholar] [CrossRef]
- Athauda, D.; Maclagan, K.; Skene, S.S.; Bajwa-Joseph, M.; Letchford, D.; Chowdhury, K.; Hibbert, S.; Budnik, N.; Zampedri, L.; Dickson, J.; et al. Exenatide Once Weekly versus Placebo in Parkinson’s Disease: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2017, 390, 1664–1675. [Google Scholar] [CrossRef]
- Ayhan, Y.; Yoseph, S.A.; Miller, B.L. Management of Psychiatric Symptoms in Dementia. Neuro Clin. 2022, 41, 123–139. [Google Scholar] [CrossRef]
- Tampi, R.R.; Jeste, D.V. Dementia Is More Than Memory Loss: Neuropsychiatric Symptoms of Dementia and Their Nonpharmacological and Pharmacological Management. Am. J. Psychiatry 2022, 179, 528–543. [Google Scholar] [CrossRef]
- Rogowska, M.; Thornton, M.; Creese, B.; Velayudhan, L.; Aarsland, D.; Ballard, C.; Tsamakis, K.; Stewart, R.; Mueller, C. Implications of Adverse Outcomes Associated with Antipsychotics in Older Patients with Dementia: A 2011–2022 Update. Drugs Aging 2022, 40, 21–32. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Swiss Federation of Clinical Neuro-Societies. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).